Abstract
Breast cancer is the second leading cause of cancer mortality in women, estimated at nearly 40,000 deaths and more than 230,000 new cases diagnosed in the U.S. this year alone. One of the defining characteristics of breast cancer is the radiographic presence of microcalcifications. These palpable mineral precipitates are commonly found in the breast after formation of a tumor. Since free Ca2+ plays a crucial role as a second messenger inside cells, we hypothesize that these chelated precipitates may be a result of dysregulated Ca2+ secretion associated with tumorigenesis. Transient and sustained elevations of intracellular Ca2+ regulate cell proliferation, apoptosis and cell migration, and offer numerous therapeutic possibilities in controlling tumor growth and metastasis. During lactation, a developmentally determined program of gene expression controls the massive transcellular mobilization of Ca2+ from the blood into milk by the coordinated action of calcium transporters, including pumps, channels, sensors and buffers, in a functional module that we term CALTRANS. Here we assess the evidence implicating genes that regulate free and buffered Ca2+ in normal breast epithelium and cancer cells and discuss mechanisms that are likely to contribute to the pathological characteristics of breast cancer.
Keywords: breast cancer, lactation, mammary epithelium, secretory pathway, SPCA2
DIRECTIONAL REGULATION OF CALCIUM BY CALTRANS: A CALCIUM TRANSPORTING MODULE
ionized Ca2+ is a ubiquitous second messenger, regulating a wide swathe of cellular events ranging in time scale from nanoseconds (in vesicle fusion) through hours (in cell proliferation) (11). Unlike other second messengers, however, elemental Ca2+ can neither be synthesized nor degraded. Hence, regulation of cytosolic Ca2+ (Ca2+cyt) occurs by dynamic compartmentalization and buffering.
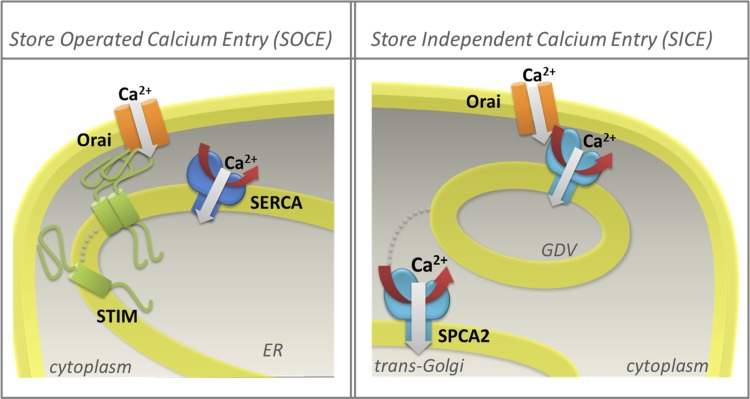
An array of membrane-embedded transport proteins move Ca2+ into and out of the cytoplasm. Active transport mechanisms drive the ion uphill, generating large transmembrane Ca2+ gradients of up to 10,000-fold. These include primary active transporters, or calcium pumps, that directly harness energy from ATP, and secondary active transporters that couple uphill movement of Ca2+ to the energetically favorable transport of Na+ or H+ down their electrochemical gradients. Although there is scarce evidence for the role of secondary transporters such as the Na+/Ca2+ exchanger in mammary epithelia, considerable attention has focused on the expression and regulation of Ca2+-ATPases distributed in the membranes of the endoplasmic reticulum (ER), secretory pathway, and plasma membrane. They belong to the superfamily of P-type ATPases, so-named after a conserved mechanism involving a catalytic phosphorylated intermediate (87). Working in concert, they establish pools of stored Ca2+ within cellular organelles and actively pump Ca2+ out of the cell, maintaining baseline Ca2+cyt levels in the low submicromolar range. These external and organellar reservoirs drive influx of Ca2+ through multiple classes of ion channels, activated by voltage, ligands, or store depletion. Multiple modes of store-dependent and -independent pathways for Ca2+ influx have been described in nonexcitable cells. For example, depletion of ER Ca2+ stores activates the STIM1,2 sensors that aggregate and signal to the Orai1 channel on the plasma membrane via localized, direct interactions (Fig. 1). This canonical mode of signaling, termed SOCE, for store-operated calcium entry, has been the subject of intensive study in recent years (reviewed by 68, 109). More recently, we have described an unconventional interaction between a secretory pathway Ca2+-ATPase, SPCA2, residing on Golgi-derived vesicles, and the Orai1 channel, that elicits Ca2+ influx independent of stores, that we term SICE for store independent calcium entry (24, 40; Fig. 1). The resultant temporal and spatial elevations in Ca2+cyt activate effectors including calmodulin, Ca2+ dependent kinases, and phosphatases that decode the signal and, in turn, act on secondary effectors such as transcription factors that mediate gene regulation. The master regulator of systemic Ca2+ is the calcium sensing receptor, CaSR, a member of the large family of G protein coupled receptors (class C), that monitors extracellular free Ca2+ in serum and is the principal regulator of expression and secretion of parathyroid hormone (PTH) and the related protein (PTHrP) (117). The induction of mammary PTHrP seems to be under the control of serotonin (52).
Fig. 1.
Multiple modes of Ca2+ entry occur in mammary epithelial cells. Left: store-operated mechanisms are triggered by aggregation of STIM proteins that sense luminal Ca2+ in endoplasmic reticulum (ER) stores. Direct interaction with plasma membrane Orai1 channels elicits Ca2+ entry, localized increase in Ca2+cyt, and refilling of stores by the sarcoendoplasmic reticulum Ca2+-ATPase (SERCA) pump. SOCE, store-operated Ca2+ entry. Right: store-independent mechanisms include a direct interaction between the secretory pathway Ca2+-ATPase-2 (SPCA2) pump located in Golgi-derived vesicles (GDV) and Orai1 channels resulting in Ca2+ influx and potential refilling of Golgi stores by fusion of Ca2+-loaded vesicles. Red arrows indicate ATP utilization by the SERCA and SPCA2 pumps; dotted lines show movement of STIM or SPCA2, required for Ca2+ entry mechanisms. SICE, store-independent Ca2+ entry.
In the breast, an exquisitely orchestrated program of changes in the activity and expression of these Ca2+ transporters and modulators commences during pregnancy, culminates with the initiation of lactation at parturition, and concludes with the process of involution (reviewed in 62). Activation of a so-called “alveolar switch” induces proliferation and differentiation of the secretory alveolar compartment (84). Many of the proteins in the secretory pathway, including milk proteins, are transcriptionally upregulated prior to parturition (62, 67). Key members of the CALTRANS module are upregulated during alveolar differentiation and have been identified in microarray studies of expressed transcripts during pregnancy, lactation, and involution (reviewed in 63, 118). Conversely, abnormal expression of several Ca2+ transporters and ion channels has been documented in breast cancer, resulting in oncogenic Ca2+ signaling that drives tumorigenesis. Therefore, we propose that the CALTRANS module is coordinately regulated in normal breast development and lactation, but components of the same module are dysregulated upon transformation of some breast cancer cells to the malignant phenotype. Subsets of CALTRANS members could be linked to tumor phenotype, with differences between primary and metastatic cancers. Further, we propose that CALTRANS module interactions with the altered signaling environment in transformed cells can lead to localized, inappropriate secretion of calcium in the absence of calcium buffers, resulting in microcalcifications which are diagnostic of some breast cancers. Here, we will discuss the background and evidence that supports this hypothesis and consider how differential expression of CALTRANS members may be exploited in cancer therapy.
MOBILIZATION OF CALCIUM IN BREAST DEVELOPMENT AND LACTATION
Calcium is a key nutrient in milk, essential for the development of teeth and bone in the neonate. Total milk calcium can be as high as 80 mM in some mammals, such as mice, although it is typically less than 10 mM in humans (83). Much of this calcium is bound, variably to milk proteins (casein, α-lactalbumin) or to anionic carriers such as phosphate, citrate, and bicarbonate. Free Ca2+ levels are not much above 3 mM in milk, limiting the osmotic stress on mammary epithelial cells. To cope with this extra demand, Ca2+ absorption from the intestine is stimulated, resorption by the kidneys is increased, and bone Ca2+ is mobilized to the extent that bone density declines by ∼5% over the course of nursing. Efficient delivery into milk must be accompanied by synthesis, transport, and secretion of milk proteins, triglycerides, phosphates, and other anions that complex with Ca2+. There are mechanisms in place to promote substantial transcytosis of Ca2+ across the mammary epithelium, beginning with elevated Ca2+ influx at the blood (basal) side and active secretion across apical (luminal) membrane (Fig. 2). Despite increased transcellular Ca2+ flux, free Ca2+cyt must be maintained in the submicromolar range to minimize Ca2+ mediated apoptosis. Finally, Ca2+ may be a trigger for regulated cell death during the process of mammary involution, accompanied by extensive tissue remodeling. First, we consider the mechanisms underlying massive transcytosis of Ca2+ across the mammary epithelium into milk.
Fig. 2.
Calcium movement by the calcium transporter (CALTRANS) module during lactation. During lactation, the coordinated induction of milk proteins (β/κ-casein) and calcium pumps [SPCA2, plasma membrane Ca2+-ATPase-2 (PMCA2), SERCA2] and channels (TRPC6, Orai1) ensures the efficient transcytosis of Ca2+ ions from the blood (basolateral side) to the lumen (apical side) of the mammary gland. Interaction between SPCA2, localized to Golgi-derived vesicles, and Orai1 (and possibly TRPC6) elicits Ca2+ influx at the basal membrane. Ca2+ forms complexes with casein and anions within secretory vesicles, which fuse with the apical membrane. Direct pumping of Ca2+ ions into the lumen by the PMCA2 pump also elevates calcium to total levels of 40–60 mM (ionized and bound) in milk.
Coordinated induction of Ca2+ transporters and channels.
Early evidence pointed to a role for the Golgi and secretory pathway for delivery of Ca2+ to milk through the fusion of secretory vesicles with the apical membrane (reviewed in 83). Critical steps involved pumping of Ca2+ into the lumen of the Golgi by Ca2+-ATPases, against a gradient of 3 orders in magnitude, along with sequestration of citrate and phosphate and the formation of Ca2+ casein micelles within the secretory pathway (Fig. 2). The secretory pathway Ca2+-ATPase (SPCA), an ortholog of the yeast Golgi Ca2+-ATPase (PMR1), is induced 1 wk before parturition, and steadily increases as lactation progresses (94, 95). Subsequent studies demonstrated two distinct SPCA pump isoforms: the ubiquitous isoform SPCA1 increased modestly upon lactation, while the SPCA2 transcript was dramatically upregulated by ∼35-fold (37) and ∼100-fold at the protein level (24). Furthermore, whereas SPCA1 was ubiquitously distributed to the Golgi organelle in all cells of the mammary gland, including myoepithelial and stromal cells, SPCA2 expression was confined to luminal epithelial cells of the mouse lactating mammary gland where it was found to be predominantly in vesicles (24, 37). Recently, using a three-dimensional cell culture model of lactating mouse mammary glands embedded in extracellular matrix (“mammospheres”), we demonstrated a critical role for SPCA2 in mobilizing Ca2+ by store-independent and store-dependent mechanisms. Knockdown of SPCA2 resulted in biosynthetic arrest of Orai1 in a circumnuclear, ER-like compartment, drastically reduced Ca2+ influx at the basal membrane, and reduced formation of mammary organoids. Ectopic expression of either full-length or the COOH-terminal domain of SPCA2 reconstituted Orai1 trafficking and Ca2+ influx. These findings were consistent with the interaction of the SPCA2 tail domain with Orai1 previously observed in breast cancer cells (40). Targeted, mammary-specific knockout of SPCA2 in a mouse model is needed to confirm the role of secretory pathway pumps in Ca2+ secretion into milk.
The molecular identity of the Ca2+ influx channel(s) at the basolateral membrane of lactating mammary epithelium that contribute to Ca2+ transcytosis has recently been identified. Although several voltage-gated and TRP-type Ca2+ channels are expressed in mammary epithelia (118), none is as dramatically induced upon lactation as the Orai1 channel (24, 79). Consistent with a role in mediating Ca2+ influx, endogenous Orai1 localizes to the basolateral membrane of lactating mouse mammary epithelia and differentiated mammospheres cultured in vitro. Finally, knockdown of Orai1 in vitro drastically blocked Ca2+ influx and mammosphere development (24). Although Orai1 has been dubbed the store-operated channel (SOC) because of its activation by the Ca2+ sensor STIM1 in response to ER store depletion, its role as the major Ca2+ influx pathway on the basolateral membrane in mammary epithelia is largely store independent, consistent with the Ca2+-replete status of stores during lactation (Fig. 1). Store-independent activation of Orai1 during lactation likely commences upon induction of SPCA2, which appears to have a dual function both as a chaperone for Orai1 trafficking as well as in eliciting Ca2+ influx at the basal plasma membrane (24, 40) (Fig. 2).
At the apical membrane, there is abundant evidence pointing to a critical role for ATP-driven calcium pumps in mediating Ca2+ efflux into milk (Fig. 2). Plasma membrane Ca2+-ATPases (PMCAs) are major contributors to the homeostasis of Ca2+ in the breast, through development, lactation, and/or the onset of cancer. PMCA1 is the ubiquitous isoform and has a housekeeping function in Ca2+cyt homeostasis, while other PMCA isoforms show limited tissue distribution and specialized functions in lactation and tumorigenesis. PMCA2 has the highest affinity for Ca2+ and a specific splice variant, PMCA2bw, containing a unique 45 amino acid insert, is upregulated by 200-fold at the peak of lactation (94). PMCA2bw is targeted to the apical membrane of the secretory cell via its unique insert (20), from where it is secreted in the milk fat globule membrane (94). Mice completely lacking the PMCA2 isoform have a 60% reduction in the amount of Ca2+ in milk, when compared with heterozygous littermates (97). During lactation, PMCA2 extrudes Ca2+ directly into the milk duct in its ionic form, rather than bound to phosphates or milk caseins.
Physiological regulation of mammary gland Ca2+ secretion.
Vitamin D is a prohormone that the body converts to 25-hydroxyvitamin D (25OHD) in the liver. 25OHD, the inactive circulating form of vitamin D, is converted to the steroid hormone 1,25-dihydroxyvitamin D [1,25(OH)2D] in the kidney by the enzyme CYP27B1. In its endocrine role, 1,25(OH)2D travels from the kidney to sites of action where it binds to the transcription factor, vitamin D receptor (VDR), in intestine, bone, and kidney, resulting in specific gene activation and the regulation of organismal calcium homeostasis (1, 98, 125). In other cell types, like mammary cells, 1,25(OH)2D is produced by conversion of 25OHD directly in the specific cells, as needed, by locally expressed CYP27B1 (125). 1,25(OH)2D then acts in an intracrine/autocrine fashion through VDR, mediating specific gene activation that regulates cell growth, differentiation, and death (120, 121, 124, 125).
In normal mammary tissue VDR expression is upregulated with mammary development and 1,25(OH)2D inhibits estrogen-induced ductal proliferation/branching (124, 126). Mammary glands from VDR null mice experience accelerated development during puberty and pregnancy. VDR null mouse mammary glands also have reduced apoptosis, thus delaying mammary involution after cessation of lactation. These data indicate that VDR, following 1,25(OH)2D binding, regulates mammary proliferation, differentiation, and apoptosis (121, 125).
CaSR is expressed in the basolateral membrane of the lactating alveolus and regulates parathyroid hormone-related peptide (PTHrP) secretion (114). In a haploinsufficiency model of CaSR (CaSR+/−), an increase in PTHrP and decrease in calcium transport into milk was observed, confirming the important role of CaSR in lactation (2). Mammary-specific knockdown of CaSR was recently achieved by mating CaSRflox/flox mice with mice expressing Cre driven by the β-lactoglobin gene, which leads to CaSR knockdown in late pregnancy and during lactation (74). These studies demonstrated that CaSR is not required for mammary cell differentiation or lactation, but mammary-specific CaSR knockout during lactation significantly decreased the calcium content in milk without reducing PMCA2 expression, leading to attenuated calcium accrual in the pups. Independent studies have shown that CaSR does not regulate PMCA2 expression, but increases PMCA2 activity (52, 116). CaSR is also involved in regulation of systemic calcium homeostasis during lactation, regulating PTHrP content in milk and maternal circulation, and PTH secretion, modulating maternal bone mineralization and calcium excretion (15, 106). CaSR in the parathyroid gland and kidneys compensated for loss of mammary CaSR, since global knockout of CaSR in CaSR−/− × PTH−/− mice caused persistent hypercalcemia throughout lactation due to reduced calcium excretion. Overall, the study suggests that CaSR in breast coordinates maternal and fetal calcium metabolism by regulating both calcium and PTHrP levels.
INTRACELLULAR CALCIUM OVERLOAD AS AN EARLY SIGNAL FOR MAMMARY INVOLUTION
Mammary gland involution has been extensively studied and many comprehensive reviews have described what is known at the transcriptional level (8, 50, 110, 111). During involution, there is a reversible stage in the first 48 h following abrupt cessation of nursing, with widespread apoptosis. This is followed by an irreversible stage accompanied by extensive tissue remodeling. The primary signal(s) that initiate mammary involution following weaning appear to be local factors of elusive identity (8, 69). Much of the research in this area has focused on endocrine and transcription factors as primary regulators of mammary gland involution and ignored the potential for regulation by intracellular Ca2+ ions. We note that following abrupt cessation of lactation expression of SPCA1, SPCA2 and PMCA2 is reduced by ∼90% resulting in mammary tissue accumulation of calcium that peaks at 24 h and remains high through 48 h (96). This suggests that a precipitous loss of the major Ca2+-ATPases required by the mammary gland to regulate the large amount of calcium associated with milk production could lead to accumulation of cell calcium, mitochondria Ca2+ overload, and calcium-mediated cell death.
Consistent with this hypothesis, Vanhouten et al. (115) showed that the loss of PMCA2 causes a “cellular calcium crisis” leading to apoptosis and accelerated mammary involution. In the deafwaddler-2 J (dfw-2J) mouse, which carries a null mutation for PMCA2, there was increased apoptosis and reduced mammary mass in lactating glands compared with wild-type controls. Mammospheres derived from dfw-2J mice were more sensitive to ionomycin-induced Ca2+ release, as indicated by increased apoptosis, and accumulated more Ca2+ relative to control mammospheres. These studies suggest that Ca2+ may be one of the hypothesized local factor(s) (69) in early signaling of mammary involution.
Recent studies have suggested that mammary involution is the result of a nonclassical lysosomal-mediated pathway of cell death resulting from induced lysosomal membrane permeabilization (LMP) (60). The process requires Stat3, and cell death differs from classical apoptosis. Postweaning, the rise in Ca2+cyt elicits both the transcription and enzymatic activation of calpains, which are Ca2+-dependent cysteine proteases that translocate to the lysosomes and mitochondria where they initiate LMP and mitochondrial permeabilization (3). In contrast, PMCA2 overexpression prevents calpain activation, by lowering intracellular calcium, thereby preventing cell death in a T47D breast cancer cell model (115). These data together provide additional evidence for a central role of calcium in involution/cell death for both normal and cancerous breast tissue. The interplay and expression of all the major breast calcium channels and Ca2+-ATPases in the regulation of intracellular calcium will be key to understanding involution and cell death in both normal and cancerous breast tissue (24, 40, 96).
MICROCALCIFICATIONS IN BREAST CANCER: DIAGNOSTIC EVIDENCE OF DYSREGULATED CALCIUM TRANSPORT
Microcalcifications have been used extensively to diagnose and characterize breast (23, 81) and other cancers including thyroid (7) and renal (4). The mechanism(s) producing them remain largely undefined, although aberrant secretion and necrosis have been hypothesized to contribute to their formation (93). Two types of microcalcifications—calcium oxalate and calcium apatite—can be distinguished by their radiographic “signatures” on mammograms, with the latter type correlating more closely with malignant transformation (14, 42, 46; reviewed in 101). We propose that microcalcifications result from abnormal expression of bone matrix proteins (osteonectin, osteopontin, bone sialoprotein, bone matrix proteins) in breast cancer cells (10) coupled with an inappropriate upregulation of calcium secretion (9, 16) in the absence of caseins and other calcium buffers (80). Abnormal secretion of Ca2+ may arise by dysregulation of CALTRANS components and signal an early event in tumorigenesis. As seen in subsequent sections, inappropriate and/or sustained elevation of secretory pathway Ca2+-ATPases is typical of luminal subtypes of breast cancer and may lead to extracellular, insoluble deposits of secreted Ca2+. High levels of PMCA2 seen in breast cancer cells (115) would also be conducive to elevation of extracellular Ca2+ and formation of extracellular microcalcifications. Select components of the pathway(s) regulating normal bone mineralization are upregulated in soft tissue calcifications in breast cancer, particularly those regulating phosphate transport and extracellular matrix composition (23). The combined effect of increased extracellular Ca2+ and phosphates plus matrix remodeling is conducive to mineralization. A mechanistic understanding of the source(s) of Ca2+ and implications of distinct microcalcification “signatures” will improve diagnosis and stratification of breast cancer metastatic risk.
DYSREGULATION OF CALTRANS IN BREAST CANCER
Ca2+ is a double-edged sword, promoting both cell growth and cell death, and hence Ca2+cyt levels must be tightly regulated and finely tuned. This rationalizes the complex modulation of Ca2+ transporters accompanying oncogenic transformation and the seemingly contradictory effects of inactivation and activation of specific CALTRANS members in protecting against or promoting tumorigenesis.
CALCIUM PUMPS
In general, elevation of Ca2+-ATPases serves to remove Ca2+ ions from the cytoplasm, clamping Ca2+cyt to resting levels. However, at least one isoform, SPCA2, also elevates Ca2+ entry via a novel interaction with plasma membrane Ca2+ channels. In cancer, abnormal expression of several Ca2+ pumps and channels results in dysregulated Ca2+ homeostasis that drives tumor growth and migration on the one hand and on the other, confers resistance to apoptosis and poor prognosis for survival.
Secretory pathway Ca2+-ATPases (SPCA).
Homozygous deletion of the ATP2C1 gene encoding the ubiquitously expressed, housekeeping isoform SPCA1 is not viable in mouse models, but the aged heterozygotes exhibit squamous tumors in keratinized epithelia and esophagus cells (85). Although the same genotype causes the ulcerative skin disorder Hailey-Hailey disease in humans, it is interesting that some studies show an association with preneoplastic lesions and malignancy (43). SPCA1 levels were found to be elevated in basal-like breast cancers compared with luminal types, and knockdown of SPCA1 in MDA-MB-231 cells delays the Golgi processing of pro-IGF1 receptor, whose expression corresponds with poor prognosis in breast cancer (48).
A second isoform, SPCA2, was highly expressed in luminal-type breast cancer lines, with highest levels in ERBB2+ breast tumors (40). Knockdown of SPCA2 in MCF7 cells attenuated cell proliferation, colony formation in soft agar, and tumor formation in nude mice. Ectopic expression of SPCA2 in the nontumorigenic cell line MCF10A conferred increased cell proliferation and soft agar colony formation, which could be abrogated by knockdown of the Orai1 channel. SPCA2 was partially localized to vesicles near the plasma membrane where it interacted with and elicited opening of Orai1 Ca2+ channels, resulting in the elevation of Ca2+cyt and activation of ERK1/2 signaling pathways. Thus inappropriate expression of SPCA2 confers oncogenic potential via store-independent Ca2+ entry (SICE). Since activation of Ca2+ influx by SPCA2 was independent of its Ca2+-ATPase and transport function (40), active site inhibitors of the pump would not be effective as a cancer therapeutic. However, a 40-amino acid fragment of the NH2-terminal domain of SPCA2 functioned as a dominant negative blocker of the SPCA2-Orai1 interaction, suggesting that a peptide-based approach may be an option. Of note, SPCA2 was not expressed in the highly metastatic cell line, MDA-MB-231, where STIM1/Orai1-mediated SOCE was required for cell migration (122). Understanding the specific molecular signature of dysregulated CALTRANS components would thus be important in directing treatment approaches.
Plasma membrane Ca2+-ATPases (PMCA).
Isoform-specific upregulation of PMCA has been observed in a variety of breast cancer tissues and cell lines, with up to 100-fold elevation of PMCA2 in some cancer cell lines relative to nontransformed cells (64). In comparison, PMCA1 levels were only modestly increased, and a small decrease in the PMCA4 isoform was observed (64). The latter was confirmed in colon cancer cells, where PMCA4 is upregulated accompanying differentiation postconfluence, but downregulated in proliferating cancer cells (5). Silencing of all PMCA isoforms in MCF7 cells at levels that did induce cell death was shown to slow cell cycle progression through G2/M phase (65). It has been suggested that PMCA remodeling in cancer recapitulates the changes in lactation to counter Ca2+-mediated apoptotic pathways (25). Indeed, high expression of PMCA2 inhibits apoptosis in breast cancer cells and is correlated with a poor prognosis in breast cancer outcome (115). These observations suggest that isoform-specific inhibitors may be useful in cancer therapy. In one approach, a random peptide phage display library was screened against an extracellular domain of PMCA4 to generate a series of specific inhibitors with micromolar affinity (112). Such approaches applied to PMCA2 may yield novel drugs to modulate Ca2+ handling and induce apoptosis in breast cancers.
Sarcoendoplasmic reticulum Ca2+-ATPases (SERCA).
A highly specific noncompetitive inhibitor of SERCA pumps is thapsigargin, a sesquiterpene lactone from the roots of Thapsia garganica. Thapsigargin was initially recognized as a tumor promoter in a mouse model of skin cancer (51). Additionally, a null mutation in one copy of the SERCA2 gene leads to squamous cell carcinomas in the murine model (91). This phenotype was demonstrated in cells which had no ras or p53 mutations and showed that haploinsufficiency of SERCA2 can predispose to a cancer phenotype even when tumor-suppressing genes have a normal genotype. However, thapsigargin has been shown to inhibit cancer cell proliferation and migration in multiple cell types, including breast cancer (56). Because thapsigargin is a nonselective cytotoxin, therapeutic efforts via clinical trials (NCT01056029 and NCT01734681) focus on the use of a prodrug form that specifically targets cytotoxicity to cancer cells, exemplified by coupling to prostate specific antigen (PSA) in the treatment of prostate cancer (33). While SERCA has not been specifically evaluated in breast cancer tumors or cell culture models, it is reasonable to conclude that the effects of thapsigargin may also implicate SERCAs in breast cancer.
CALCIUM CHANNELS
Ca2+cyt elevations in mammary epithelia derive from two sources: extracellular via a variety of ion channels, and intracellular, primarily from the ER and secretory pathway via the IP3-gated receptor channel (IP3R).
IP3-receptor channels.
The inositol 1,4,5-trisphosphate receptor (IP3R) is the main Ca2+ release channel in the ER. There are three isoforms of IP3R, and two, IP3R1 and IP3R3, are upregulated and hyperphosphorylated in the breast cancer cell line, T47D (108). Further, IP3R1 and IP3R3 functionally interacted with cy/cdk complexes (cyclin/cyclin-dependent kinases) in T47D cells, suggesting that cy/cdk complexes not only regulate cell proliferation, but also cellular Ca2+ (108).
TRP channels.
Transient receptor potential (TRP)-mediated channels are a large superfamily of Ca2+ channels, including TRPC, TRPV, and TRPM subtypes, that play important roles in several cancers (reviewed in 41, 66, 86). Isoforms of the canonical, nonselective, and lipid-regulated TRPC family have been implicated in tumor formation, breast cancer cell proliferation, and migration. In MCF7 cells, activation of CaSR by extracellular Ca2+ turned on PLC/PKC signaling pathways to elicit Ca2+ influx via TRPC1. Silencing of TRPC1 blocked downstream phosphorylation of ERK1/2 and proliferation of MCF7 cells. Furthermore, TRPC1 expression itself was elevated by ERK1/2 phosphorylation in MCF7 cells (35). TRPC6 was not detected in normal breast tissue (6), but was consistently elevated in biopsies of breast tumors, albeit without correlation to tumor grade, estrogen receptor expression, and lymph node metastasis (49). Whereas TRPC3 and TRPC6 isoforms were upregulated in MCF-7 cells, in the highly metastatic breast cancer cell line MDA-MB-231 both isoforms showed intracellular localization. Despite differences in localization, hyperforin, an activator of TRPC6 and component of St. John's wort, inhibited proliferation of these cancer cell lines but not of the control MCF10A cells, and silencing of TRPC6 reduced cell proliferation without an effect on cell viability (6). The TRPM (melastatin-related TRP) family includes cold-activated TRP channels that are Ca2+ and Mg2+ permeable. They appear to play a significant role in cancers involving the reproductive organs. Specifically, TRPM7 was expressed at high levels in grade III breast tumors and plays a role in uptake of intracellular Ca2+ into breast cancer cells (49). TRPM8 expression is regulated by the estrogen receptor ER alpha and correlates with the ER+ state of tumors (22). TRPV (vanilloid-activated) channels are typically expressed in epithelial cells, and comprise six isoforms. TRPV1 is associated with sensing of menstrual breast pain, raising the possibility that pain associated with breast cancer may be mediated by TRPV1 nociception (47). Not much is known about the localization and specificity of the TRPV channels in mammary tissue, except in the case of TRPV6, which is upregulated at the mRNA level in T47D cells and can be regulated by vitamin D and estrogen/progesterone. Furthermore, TRPV6 mediates the action of tamoxifen on breast cancer cells (12, 13), suggesting that the therapeutic effects of tamoxifen and protein kinase C inhibitors in breast cancer therapy may be related to modulation of TRPV6-mediated calcium entry.
Orai channels.
The store-operated Ca2+ entry (SOCE) pathway is regulated by the ER localized single-pass protein, STIM1. When ER stores are low, STIM1 forms puncta at ER/plasma membrane junctions to open the Ca2+ selective, plasma membrane channel, Orai1. The coupling of STIM1 with Orai1 is dependent upon the absence of Ca2+ from the EF-hand of the luminal portion of STIM1, which signals store depletion. Subsequent refilling of the ER store through the SERCA pump reloads Ca2+ in the EF-hand domain of STIM1, uncoupling STIM1 from Orai1. In the lactating breast, ER and Golgi complex volumes are increased to maximize Ca2+ transcytosis and secretory capacity for milk production. However, in breast cancer, abnormal elevation of SPCA2 allows Orai channels to open independent of the ER store Ca2+ concentrations (40). During lactation, there is a significant increase in the amount of Orai1, but not Orai2 or -3 (79). On the pathological end of the spectrum, Orai1 is dramatically increased in basal-type tumor samples and in the mammary tumor cell lines MCF-7 and MDA-MB-231 (79).
Estrogen receptor positive mammary cancer cells (ER+) use the Orai1/STIM1 pathways while estrogen receptor negative (ER−) mammary cancer cell lines may use the elusive Orai3 coupling mechanism, thought to have the same or a similar mechanism as the canonical Orai1/STIM1 pathway (82). The specific STIM isoform partner for Orai3 and its direct mechanism of coupling, however, remain to be identified. siRNA inhibition of Orai3 in MCF-7 cells arrested cell cycle progression at the G1 phase, inhibiting cell proliferation (39). Silencing of Orai3 also reduced the cyclin-dependent kinases (CDKs 2/4), cyclin E and cyclin D1 coupled with the accumulation of p21 and p53. In the normal, immortalized cell line, MCF-10A, the silencing of Orai3 had no significant effect on cell proliferation, viability, or [Ca2+cyt]. The altered regulation of Ca2+ in breast cancer may optimize proliferation and apoptosis resistance, which are hallmarks of the cancerous state (38).
CALCIUM REGULATORS AND BINDING PROTEINS
Calcium channels and transporters play a significant role in controlling compartment-specific Ca2+ concentrations. The critical importance of adequate regulation of Ca2+ is underscored, however, by an additional level of sensing and modulation afforded by the CaSR, which monitors changes in extracellular Ca2+, and calcium buffering proteins, which allow increases in net cellular Ca2+ without catastrophic changes in free Ca2+cyt.
Ca2+ sensing receptor (CaSR).
CaSR is expressed in normal, fibrocystic, and cancerous human breast epithelium (19). Given the roles of CaSR in normal mammary gland/breast, it is not surprising that CaSR is also expressed in many breast cancer cell lines, where it regulates parathyroid hormone-related peptide (PTHrP) secretion (102). Studies of CaSR signaling in mammary epithelium have focused primarily on alterations associated with breast cancer. In MCF-7 cells, activation of CaSR by either extracellular calcium or neomycin, an allosteric activator of CaSR, led to increases in Ca2+cyt, and increased colocalization of calbindin-D28k and CaSR, although the mechanism and significance of the association remains to be explored (89). The estrogen receptor expression status of breast cancers has implications for the likelihood of bone metastases and treatment outcomes. MCF-7 cells treated with elevated extracellular calcium (up to 20 mM, reflecting the bone resorptive niche) or CaSR allosteric agonists show a decrease in estrogen receptor protein but increase in transcriptional activation (58). If these results are verified in vivo, they suggest CaSR serves as a link between estrogen receptor status and bone metastases. CaSR activation by extracellular calcium triggers breast cancer cell migration (100), providing independent evidence for a second role for CaSR in bone targeting. CaSR undergoes unique alterations in signaling preference in malignant breast cells, shifting from Gq/11/Gi/G12/13 in normal breast epithelium to Gs in malignant cells (75). This shift converts the normal downregulation of PTHrP secretion upon CaSR activation into increased secretion, contributing to hypercalcemia of malignancy (75). In breast cancer cell lines (MDA-MB-231 or MCF-7) but not in nonmalignant lines (Hs 578Bst or MCF-10A), CaSR activates choline kinase through a Gα12- and Rho-dependent pathway (54). The mechanism(s) contributing to these shifts in CaSR signaling preference are not known, but likely include a combination of altered expression and/or subcellular targeting of CaSR and/or differential expression of interacting proteins.
Differences in CaSR expression and/or signaling have been explored as potential targets in treatment of breast cancer and/or attenuation of bone targeting. CaSR stimulation leads to increased proliferation of MCF-7 cells, through pathway(s) involving extracellular signal-regulated kinases 1 and 2, and transient receptor potential channel TRPC1 (35, 36). Other studies, however, argue that activation of CaSR reduces breast cancer cell proliferation, decreases expression of survivin, and sensitizes cells to the cytotoxic agent paclitaxel, suggesting CaSR acts as a tumor suppressor in breast epithelium (72, 92). Sorting out the discriminators which determine whether CaSR promotes or hinders proliferation will determine whether it represents a viable target in anti-breast cancer therapies. BRCA1 mutations which inactivate the protein represent a major risk factor for breast cancer. BRCA1 activity is required for CaSR expression in MCF-7/MDA-MB-231 cells, which leads to suppression of survivin expression (92). Ectopic expression of CaSR in BRCA1 defective cells restores survivin suppression and attenuates proliferation (92). If these mechanisms operate in vivo, modulation of CaSR expression may present a novel treatment opportunity. Our understanding of the contributions of CaSR to normal physiology and its derangements in cancer is at present rudimentary, although the weight of evidence argues that targeting CaSR in combinatorial cancer therapies may prove beneficial (17, 100, 107).
Calcium buffering proteins.
Several small molecular weight proteins with EF hand domains bind Ca2+ in the cytosol, effectively buffering intracellular concentrations, and altering the balance of cellular progression to apoptosis or proliferation. The interaction between Calbindin-D28k and CaSR in MCF-7 breast cancer cells was described above (89). Members of the S100 family of calcium binding proteins function intra- and extracellularly to affect metastatic potential (57, 119). The calcium binding protein sorcin was found to be upregulated in a paclitaxel-resistant ovarian carcinoma line, and forced overexpression in breast cancer cells also conferred paclitaxel resistance, although the mechanism remained unknown (88).
Annexins.
This large family of calcium-dependent phospholipid binding proteins are known to promote cancer cell invasion and migration in a variety of cancers. By providing a membrane scaffold, annexins link intracellular Ca2+ levels to a wide variety of membrane events ranging from vesicle exo- and endocytosis to cell differentiation and migration (reviewed in 45). Although annexins show voltage-dependent Ca2+-selective ion channel activity in artificial lipid bilayers, their in vivo function as bona fide channels is less defined. In complex with S100A10 protein, annexin A2 is required for biosynthetic trafficking of several ion channels to the plasma membrane. Annexins A2, A4 and A5 are upregulated in breast cancer (32) and annexin A3 levels were correlated with poor prognosis for survival (123).
SMALL MOLECULE REGULATORS OF CALCIUM HOMEOSTASIS
Because of their small size and permeability, chemical modulators of Ca2+ homeostasis, both endogenous and extrinsic, offer therapeutic potential to control the efficiency of lactation as well as inhibit tumor growth and migration. Here, we briefly summarize evidence implicating some small molecule Ca2+ regulators in mammary biology.
Vitamin D.
It is well established that vitamin D deficiency in the pregnant and lactating mother predisposes the breastfed infant to rickets, still a major public health concern in large parts of the world (90). Several studies report that calcium and dietary vitamin D play key roles in the prevention of breast cancer (21, 44, 71, 98, 105) in pre- and postmenopausal women. In breast cancer 1,25(OH)2D promotes cell differentiation, inhibits proliferation, and induces apoptosis (73, 98, 103, 104, 120, 121). The amount of 1,25(OH)2D needed to mediate these anticancer effects is above physiological/endocrine levels. Therefore, the positive effects of 1,25(OH)2D on cancer are believed to be mediated by intracrine/autocrine pathways following intramammary production of 1,25(OH)2D from dietary derived 25OHD (61, 98, 121).
The difference in pathology for many breast cancers lies in the balance of intracellular and plasma membrane calcium transporters. 1,25(OH)2D increases Ca2+cyt via both plasma membrane entry and ER Ca2+ release (78, 103, 104). Thus in breast cancer cells, with less calcium buffering capacity than normal breast cells, 1,25(OH)2D increases Ca2+cyt to a concentration needed to promote apoptosis in several breast cancer cell lines, resulting in caspase-independent and caspase-dependent cell death (78, 104). Both the amount of VDR and the expression of CYP27B1 in breast cancer cells will determine their sensitivity to 1,25(OH)2D-induced, calcium-mediated apoptosis (61, 121).
Nitric oxide (NO).
During lactation, NO enhances blood flow to mammary glands and improves lactation performance through dietary supplementation with arginine (59). NO also promotes intracellular Ca2+ release from mitochondria as well as induces Ca2+ influx in a variety of cell types, including HeLa cells (34, 55), where it may promote tumorigenicity through constitutive activation of Wnt/β-catenin signaling (34). Increased nitric oxide synthase (NOS) activity, both constitutive and inducible, was associated with highly metastatic tumors (113). Ductal carcinomas showed significant differences in NO biosynthesis in grade II vs. grade III tumors, suggesting a correlation between tumor grade and NO synthase. Additionally, tumor-associated activated macrophages have significant NO synthase activity, which may work synergistically with NO synthase activity in the myoepithelium and vascular endothelial cells (113).
Cannabinoids.
Endocannabinoids have been proposed to play a key role in the suckling response and in mother-infant bonds, based on rodent studies (76). There is growing evidence that cannabinoid compounds from the Cannabis plant also mediate calcium-dependent inhibition of proliferation or induction of apoptosis in mammary tumors (reviewed in 29). The endogenous cannabinoid anandamide and several exogenous cannabinoid mimetics were shown to arrest the proliferation of human breast cancer cells, including MCF-7 (31). Submicromolar levels of anandamide suppressed the mitogenic action of prolactin by suppression of prolactin receptor synthesis and resulting in downregulation of the oncogene product brca1 (31). In MDA-MB-231 cells, cannabidiol elicited both rapid and sustained elevation of Ca2+ that was independent of extracellular Ca2+ (70) suggesting release from intracellular stores, possibly the mitochondria (99). Although TRPV channels have been implicated in the pathway, direct inhibition of TRPV1 did not alter Ca2+ release (70). A systematic analysis of the ability of 11 purified compounds to elicit Ca2+ elevations in HEK293 cells showed that cannabinoids activate TRPA1, TRPV1, and TRPV2 channels, and antagonize TRPM8 (30). Given the resurgent interest in the therapeutic use of Cannabis compounds, further mechanistic studies on their antitumor properties, particularly by the nonpsychotropic cannabinoids, are warranted.
CALCIUM SIGNATURES IN PHYSIOLOGY AND PATHOPHYSIOLOGY: LESSONS LEARNED AND ANTICIPATED
Thus far, we have documented how Ca2+ compartmentalization, mobilization, sensing, and signaling are physiologically regulated under normal conditions of cell growth and differentiation, and in mammary gland development and lactation. It is also clear that these same processes are hijacked in maintaining and promoting breast cancers. However, we are only beginning to appreciate the specificity of such changes associated with various tumor types. A better understanding of how distinct calcium signatures may be driving different stages and phenotypes of cancer development is an essential prerequisite to tailoring personalized treatment and in predicting patient outcome.
The differential expression and cellular phenotypes of two secretory pathway Ca2+-ATPases is illustrative of this exquisite specificity. Both SPCA isoforms have similar transport characteristics, and are expressed in breast where they localize to Golgi compartments, although SPCA2 appears to have a more prominent extra-Golgi vesicular localization. However, elevated SPCA2 levels are associated with luminal, rather than basal, breast cancers, with highest levels found in ERBB2+ cancers, of five transcriptional subtypes that have been defined (40). This fits well with observations that SPCA2 expression is restricted to luminal epithelial cells of the mammary gland, whereas SPCA1 is found in both basal and luminal cell types (37). Consistent with this distinct expression pattern, SPCA1 was found to be elevated in the more aggressive basal-like breast cancers (48). Whereas SPCA2 elevation resulted in a rise in Cacyt, through activation of Ca2+ influx channels, and activation of signaling pathways (pERK), the effect of SPCA1 appeared to be via processing and sorting of insulin-like growth factor receptor, IGF-1, independent of alterations in global Ca2+ (48). Furthermore, the observation that SPCA2 levels are high in MCF-7, a human breast adenocarcinoma line that forms rapidly growing, well defined tumors, but low in the non-tumor-forming, highly metastatic cancer line MDA-MB-231, could hint at underlying mechanistic differences in Ca2+ signaling between these cancer phenotypes. Thus we speculate that SPCA2 elevation may be characteristic and permissive for early stages in tumor formation where cell growth and proliferation dominate. Under these conditions, stores are Ca2+ filled, and Ca2+ influx occurs largely through store-independent mechanisms (SICE), as depicted in Fig. 3A. MAP kinase and cyclin D levels are constitutively activated, leading to high levels of cell proliferation. Although cell polarity and tight junctions may be lost, adhesion between cells must be largely intact, as evidenced by expression of E-cadherin and the ability to form “tumorspheres” in extracellular matrix or soft agar (77). We propose a different scenario of Ca2+ dynamics that accompanies the epithelial to mesenchymal transition (EMT), and acquisition of cell migratory capability (Fig. 3B). As characterized by the MDA-MB-231 cell line, SPCA2 levels are low, Ca2+ release channels are elevated and stores are Ca2+-depleted, and as a consequence, store-operated Ca2+ entry mechanisms are constitutively active, possibly due to increased Ca2+ signaling at the cell surface and release of ER Ca2+ stores. Consistent with our hypothesis, when MCF7 cells were induced to undergo an EMT-like transition by activation with TGFβ, both Orai1 and STIM1 levels were elevated, resulting in higher SOCE as shown in Fig. 3B (53). Blocking SOCE mediated by STIM1 and Orai1 in MDA-MB-231 cells inhibits cell migration (122), and STIM1 overexpression significantly promoted tumor growth, metastasis and angiogenesis in human cervical cancer xenograft experiments (reviewed in 18). Consistent with the observation of SOCE, cell migration in metastatic cells is mediated by activation of aberrant CaSR signaling and directed to Ca2+ rich microenvironments, such as bone (75). Furthermore, cell junctions have been lost and focal adhesions strengthened, as evidenced by loss of E-cadherin expression (77). Although speculative, such a scenario would account for distinct alterations of Ca2+ channels, transporters, and other homeostatic mechanisms in different stages or types of breast cancer.
Fig. 3.
Distinct modes of calcium remodeling in cancer. A: we hypothesize that in rapidly proliferating, primary tumors intracellular Ca2+ stores are replete due to elevated expression of SERCA2 and SPCA2, and Ca2+ entry occurs by SICE. B: in cancer cells that have undergone epithelial to mesenchymal transition, ER Ca2+ stores are released under stimulation by increased GPCR signaling at the plasma membrane, and migration requires SOCE.
Recently, there has been growing emphasis on deciphering the role of Ca2+ signaling in the transition from epithelial to the more aggressive mesenchymal type associated with breast cancer metastasis, with emerging support for some aspects of the model shown in Fig. 3, although some inconsistencies remain likely due to differences in cancer cell models used. Davis and colleagues investigated EGF-induced EMT in MDA-MB-468 cells, a model of mammary cells considered more epithelial compared with MDA-MB-231. Consistent with our hypothesis, they observed store-independent Ca2+ influx (also termed nonstimulated Ca2+ influx) to be significantly greater in MDA-MB-468 cells relative to MDA-MB-231, and diminished following EMT conversion (28). Following selective knockdown, they concluded that both TRPC1 and Orai1 contributed to SICE, whereas Orai1 had a prominent role in SOCE, as expected. EGF-induced EMT was accompanied by an elevation of Ca2+cyt which was required for induction of many EMT-related genes including vimentin, Twist and N-cadherin (26). Curiously, although TRPM7 was required for vimentin expression, no difference was detected in EGF-mediated elevation of Ca2+cyt following silencing of TRPM7 (26). As depicted in Fig. 3B, there was increased expression of Ca2+ release channels, including IP3 receptors (IP3R1, IP3R3) and ryanodine receptor, RYR2, and SOCE (Orai1 and STIM1) following EMT transition (27, 53). It remains to be determined how subtle changes in isoform specificity, as was observed for SERCA pumps in response to EGF stimulation in MBA-MD-468 cells (27), contribute toward remodeling of Ca2+ dynamics to impact tumor initiation, proliferation, and progression. A more comprehensive understanding of these changes will allow therapeutic options to be better tailored to the individual, improving efficacy and mitigating unwanted side effects in the treatment of breast cancers.
GRANTS
This work was supported by grants from the National Institutes of Health (GM-062142) to R. Rao, Funds from Weis Center for Research, Geisinger Clinic to G. E. Breitwieser, and United States Department of Agriculture/Agricultural Research Service intramural funding (CRIS 3625-32000-102-00D) to T. A. Reinhardt.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: B.M.C. prepared figures; B.M.C., G.E.B., T.A.R., and R.R. drafted manuscript; B.M.C., G.E.B., T.A.R., and R.R. edited and revised manuscript; G.E.B., T.A.R., and R.R. approved final version of manuscript.
REFERENCES
- 1.Adams JS, Hewison M. Update in vitamin D. J Clin Endocrinol Metab 95: 471–478, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ardeshirpour L, Dann P, Pollak M, Wysolmerski J, VanHouten J. The calcium-sensing receptor regulates PTHrP production and calcium transport in the lactating mammary gland. Bone 38: 787–793, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Arnandis T, Ferrer-Vicens I, Garcia-Trevijano ER, Miralles VJ, Garcia C, Torres L, Vina JR, Zaragoza R. Calpains mediate epithelial-cell death during mammary gland involution: mitochondria and lysosomal destabilization. Cell Death Differ 19: 1536–1548, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asanuma H, Nakai H, Takeda M, Shishido S, Tajima E, Kawamura T, Hara H, Morikawa Y. Renal cell carcinoma in children: experience at a single institution in Japan. J Urol 162: 1402–1405, 1999 [PubMed] [Google Scholar]
- 5.Aung CS, Ye W, Plowman G, Peters AA, Monteith GR, Roberts-Thomson SJ. Plasma membrane calcium ATPase 4 and the remodeling of calcium homeostasis in human colon cancer cells. Carcinogenesis 30: 1962–1969, 2009 [DOI] [PubMed] [Google Scholar]
- 6.Aydar E, Yeo S, Djamgoz M, Palmer C. Abnormal expression, localization and interaction of canonical transient receptor potential ion channels in human breast cancer cell lines and tissues: a potential target for breast cancer diagnosis and therapy. Cancer Cell Int 9: 23, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bastin S, Bolland MJ, Croxson MS. Role of ultrasound in the assessment of nodular thyroid disease. J Med Imaging Radiat Oncol 53: 177–187, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Baxter FO, Neoh K, Tevendale MC. The beginning of the end: death signaling in early involution. J Mammary Gland Biol Neoplasia 12: 3–13, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Bellahcene A, Castronovo V. Expression of bone matrix proteins in human breast cancer: potential roles in microcalcification formation and in the genesis of bone metastases. Bull Cancer 84: 17–24, 1997 [PubMed] [Google Scholar]
- 10.Bellahcene A, Castronovo V. Increased expression of osteonectin and osteopontin, two bone matrix proteins, in human breast cancer. Am J Pathol 146: 95–100, 1995 [PMC free article] [PubMed] [Google Scholar]
- 11.Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nature Rev Mol Cell Biol 4: 517–529, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Bolanz KA, Hediger MA, Landowski CP. The role of TRPV6 in breast carcinogenesis. Mol Cancer Therap 7: 271–279, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Bolanz KA, Kovacs GG, Landowski CP, Hediger MA. Tamoxifen inhibits TRPV6 activity via estrogen receptor-independent pathways in TRPV6-expressing MCF-7 breast cancer cells. Mol Cancer Res 7: 2000–2010, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Burnside ES, Ochsner JE, Fowler KJ, Fine JP, Salkowski LR, Rubin DL, Sisney GA. Use of microcalcification descriptors in BI-RADS 4th edition to stratify risk of malignancy. Radiology 242: 388–395, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Cao G, Gu Z, Ren Y, Shu L, Tao C, Karaplis A, Goltzman D, Miao D. Parathyroid hormone contributes to regulating milk calcium content and modulates neonatal bone formation cooperatively with calcium. Endocrinology 150: 561–569, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Castronovo V, Bellahcene A. Evidence that breast cancer associated microcalcifications are mineralized malignant cells. Int J Oncol 12: 305–308, 1998 [DOI] [PubMed] [Google Scholar]
- 17.Chakravarti B, Dwivedi SK, Mithal A, Chattopadhyay N. Calcium-sensing receptor in cancer: good cop or bad cop? Endocrine 35: 271–284, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Chen YF, Chen YT, Chiu WT, Shen MR. Remodeling of calcium signaling in tumor progression. J Biomed Sci 20: 23, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng I, Klingensmith ME, Chattopadhyay N, Kifor O, Butters RR, Soybel DI, Brown EM. Identification and localization of the extracellular calcium-sensing receptor in human breast. J Clin Endocrinol Metab 83: 703–707, 1998 [DOI] [PubMed] [Google Scholar]
- 20.Chicka MC, Strehler EE. Alternative splicing of the first intracellular loop of plasma membrane Ca2+-ATPase isoform 2 alters its membrane targeting. J Biol Chem 278: 18464–18470, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Chlebowski RT, Johnson KC, Kooperberg C, Pettinger M, Wactawski-Wende J, Rohan T, Rossouw J, Lane D, O'Sullivan MJ, Yasmeen S, Hiatt RA, Shikany JM, Vitolins M, Khandekar J, Hubbell FA. Calcium plus vitamin D supplementation and the risk of breast cancer. J Natl Cancer Inst 100: 1581–1591, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chodon D, Guilbert A, Dhennin-Duthille I, Gautier M, Telliez MS, Sevestre H, Ouadid-Ahidouch H. Estrogen regulation of TRPM8 expression in breast cancer cells. BMC Cancer 10: 212, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cox RF, Morgan MP. Microcalcifications in breast cancer: lessons from physiological mineralization. Bone 53: 437–450, 2013 [DOI] [PubMed] [Google Scholar]
- 24.Cross BM, Hack A, Reinhardt TA, Rao R. SPCA2 regulates Orai1 trafficking and store independent Ca2+ entry in a model of lactation. PLoS One 8: e67348, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Curry MC, Roberts-Thomson SJ, Monteith GR. Plasma membrane calcium ATPases and cancer. Biofactors 37: 132–138, 2011 [DOI] [PubMed] [Google Scholar]
- 26.Davis FM, Azimi I, Faville RA, Peters AA, Jalink K, Putney JW, Jr, Goodhill GJ, Thompson EW, Roberts-Thomson SJ, Monteith GR. Induction of epithelial-mesenchymal transition (EMT) in breast cancer cells is calcium signal dependent. Oncogene 2013. May 20. 10.1038/onc.2013.187 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davis FM, Parsonage MT, Cabot PJ, Parat MO, Thompson EW, Roberts-Thomson SJ, Monteith GR. Assessment of gene expression of intracellular calcium channels, pumps and exchangers with epidermal growth factor-induced epithelial-mesenchymal transition in a breast cancer cell line. Cancer Cell Int 13: 76, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davis FM, Peters AA, Grice DM, Cabot PJ, Parat MO, Roberts-Thomson SJ, Monteith GR. Non-stimulated, agonist-stimulated and store-operated Ca2+ influx in MDA-MB-468 breast cancer cells and the effect of EGF-induced EMT on calcium entry. PLoS One 7: e36923, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Petrocellis L, Di Marzo V. Role of endocannabinoids and endovanilloids in Ca2+ signalling. Cell Calcium 45: 611–624, 2009 [DOI] [PubMed] [Google Scholar]
- 30.De Petrocellis L, Ligresti A, Moriello AS, Allara M, Bisogno T, Petrosino S, Stott CG, Di Marzo V. Effects of cannabinoids and cannabinoid-enriched Cannabis extracts on TRP channels and endocannabinoid metabolic enzymes. Br J Pharmacol 163: 1479–1494, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Petrocellis L, Melck D, Palmisano A, Bisogno T, Laezza C, Bifulco M, Di Marzo V. The endogenous cannabinoid anandamide inhibits human breast cancer cell proliferation. Proc Natl Acad Sci USA 95: 8375–8380, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deng S, Wang J, Hou L, Li J, Chen G, Jing B, Zhang X, Yang Z. Annexin A1, A2, A4 and A5 play important roles in breast cancer, pancreatic cancer and laryngeal carcinoma, alone and/or synergistically. Oncol Lett 5: 107–112, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Denmeade SR, Isaacs JT. The SERCA pump as a therapeutic target: making a “smart bomb” for prostate cancer. Cancer Biol Ther 4: 14–22, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Du Q, Zhang X, Liu Q, Bartels C, Geller DA. Nitric oxide production upregulates Wnt/beta-catenin signaling by inhibiting Dickkopf-1. Cancer Res 73: 6526–6537, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.El Hiani Y, Ahidouch A, Lehen'kyi V, Hague F, Gouilleux F, Mentaverri R, Kamel S, Lassoued K, Brule G, Ouadid-Ahidouch H. Extracellular signal-regulated kinases 1 and 2 and TRPC1 channels are required for calcium-sending receptor-stimulated MCF-7 breast cancer cell proliferation. Cell Physiol Biochem 23: 335–346, 2009 [DOI] [PubMed] [Google Scholar]
- 36.El Hiani Y, Lehen'kyi V, Ouadid-Ahidouch H, Ahidouch A. Activation of the calcium-sensing receptor by high calcium induced breast cancer cell proliferation and TRPC1 cation channel over-expression potentially through EGFR pathways. Arch Biochem Biophys 486: 58–63, 2009 [DOI] [PubMed] [Google Scholar]
- 37.Faddy HM, Smart CE, Xu R, Lee GY, Kenny PA, Feng M, Rao R, Brown MA, Bissell MJ, Roberts-Thomson SJ, Monteith GR. Localization of plasma membrane and secretory calcium pumps in the mammary gland. Biochem Biophys Res Commun 369: 977–981, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Faouzi M, Hague F, Potier M, Ahidouch A, Sevestre H, Ouadid-Ahidouch H. Downregulation of Orai3 arrests cell-cycle progression and induces apoptosis in breast cancer cells but not in normal breast epithelial cells. J Cell Physiol 226: 542–551, 2011 [DOI] [PubMed] [Google Scholar]
- 39.Faouzi M, Kischel P, Hague F, Ahidouch A, Benzerdjeb N, Sevestre H, Penner R, Ouadid-Ahidouch H. ORAI3 silencing alters cell proliferation and cell cycle progression via c-myc pathway in breast cancer cells. Biochim Biophys Acta 1833: 752–760, 2013 [DOI] [PubMed] [Google Scholar]
- 40.Feng M, Grice DM, Faddy HM, Nguyen N, Leitch S, Wang Y, Muend S, Kenny PA, Sukumar S, Roberts-Thomson SJ, Monteith GR, Rao R. Store-independent activation of Orai1 by SPCA2 in mammary tumors. Cell 143: 84–98, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fiorio Pla A, Avanzato D, Munaron L, Ambudkar IS. Ion channels and transporters in cancer. 6. Vascularizing the tumor: TRP channels as molecular targets. Am J Physiol Cell Physiol 302: C9–C15, 2012 [DOI] [PubMed] [Google Scholar]
- 42.Frappart L, Boudeulle M, Boumendil J, Lin HC, Martinon I, Palayer C, Mallet-Guy Y, Raudrant D, Bremond A, Rochet Y, et al. Structure and composition of microcalcifications in benign and malignant lesions of the breast: study by light microscopy, transmission and scanning electron microscopy, microprobe analysis, and X-ray diffraction. Hum Pathol 15: 880–889, 1984 [DOI] [PubMed] [Google Scholar]
- 43.Gaertner EM. Incidental cutaneous reaction patterns: epidermolytic hyperkeratosis, acantholytic dyskeratosis, and hailey-hailey-like acantholysis: a potential marker of premalignant skin change. J Skin Cancer 2011: 645743, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garland CF, Garland FC, Gorham ED, Lipkin M, Newmark H, Mohr SB, Holick MF. The role of vitamin D in cancer prevention. Am J Public Health 96: 252–261, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gerke V, Creutz CE, Moss SE. Annexins: linking Ca2+ signalling to membrane dynamics. Nature Rev Mol Cell Biol 6: 449–461, 2005 [DOI] [PubMed] [Google Scholar]
- 46.Going JJ, Anderson TJ, Crocker PR, Levison DA. Weddellite calcification in the breast: eighteen cases with implications for breast cancer screening. Histopathology 16: 119–124, 1990 [DOI] [PubMed] [Google Scholar]
- 47.Gopinath P, Wan E, Holdcroft A, Facer P, Davis JB, Smith GD, Bountra C, Anand P. Increased capsaicin receptor TRPV1 in skin nerve fibres and related vanilloid receptors TRPV3 and TRPV4 in keratinocytes in human breast pain. BMC Womens Health 5: 2, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grice DM, Vetter I, Faddy HM, Kenny PA, Roberts-Thomson SJ, Monteith GR. Golgi calcium pump secretory pathway calcium ATPase 1 (SPCA1) is a key regulator of insulin-like growth factor receptor (IGF1R) processing in the basal-like breast cancer cell line MDA-MB-231. J Biol Chem 285: 37458–37466, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guilbert A, Dhennin-Duthille I, Hiani YE, Haren N, Khorsi H, Sevestre H, Ahidouch A, Ouadid-Ahidouch H. Expression of TRPC6 channels in human epithelial breast cancer cells. BMC Cancer 8: 125, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hadsell D, George J, Torres D. The declining phase of lactation: peripheral or central, programmed or pathological? J Mammary Gland Biol Neoplasia 12: 59–70, 2007 [DOI] [PubMed] [Google Scholar]
- 51.Hakii H, Fujiki H, Suganuma M, Nakayasu M, Tahira T, Sugimura T, Scheuer PJ, Christensen SB. Thapsigargin, a histamine secretagogue, is a non-12-O-tetradecanoylphorbol-13-acetate (TPA) type tumor promoter in two-stage mouse skin carcinogenesis. J Cancer Res Clin Oncol 111: 177–181, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Horseman ND, Hernandez LL. New concepts of breast cell communication to bone. Trends Endocrinol Metab 2013. September 18 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 53.Hu J, Qin K, Zhang Y, Gong J, Li N, Lv D, Xiang R, Tan X. Downregulation of transcription factor Oct4 induces an epithelial-to-mesenchymal transition via enhancement of Ca2+ influx in breast cancer cells. Biochem Biophys Res Commun 411: 786–791, 2011 [DOI] [PubMed] [Google Scholar]
- 54.Huang C, Hydo LM, Liu S, Miller RT. Activation of choline kinase by extracellular Ca2+ is Ca2+-sensing receptor, Galpha12 and Rho-dependent in breast cancer cells. Cell Signal 21: 1894–1900, 2009 [DOI] [PubMed] [Google Scholar]
- 55.Huang Y, Zheng L, Yang H, Chen J, Wang Y, Li H, Xie S. Calcium mobilization in HeLa cells induced by nitric oxide. Scanning 2013. June 5 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 56.Jackisch C, Hahm HA, Tombal B, McCloskey D, Butash K, Davidson NE, Denmeade SR. Delayed micromolar elevation in intracellular calcium precedes induction of apoptosis in thapsigargin-treated breast cancer cells. Clin Cancer Res 6: 2844–2850, 2000 [PubMed] [Google Scholar]
- 57.Jiang H, Hu H, Tong X, Jiang Q, Zhu H, Zhang S. Calcium-binding protein S100P and cancer: mechanisms and clinical relevance. J Cancer Res Clin Oncol 138: 1–9, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Journe F, Dumon JC, Kheddoumi N, Fox J, Laios I, Leclercq G, Body JJ. Extracellular calcium downregulates estrogen receptor alpha and increases its transcriptional activity through calcium-sensing receptor in breast cancer cells. Bone 35: 479–488, 2004 [DOI] [PubMed] [Google Scholar]
- 59.Kim SW, Wu G. Regulatory role for amino acids in mammary gland growth and milk synthesis. Amino Acids 37: 89–95, 2009 [DOI] [PubMed] [Google Scholar]
- 60.Kreuzaler PA, Staniszewska AD, Li W, Omidvar N, Kedjouar B, Turkson J, Poli V, Flavell RA, Clarkson RW, Watson CJ. Stat3 controls lysosomal-mediated cell death in vivo. Nat Cell Biol 13: 303–309, 2011 [DOI] [PubMed] [Google Scholar]
- 61.Krishnan AV, Swami S, Feldman D. Equivalent anticancer activities of dietary vitamin D and calcitriol in an animal model of breast cancer: importance of mammary CYP27B1 for treatment and prevention. J Steroid Biochem Mol Biol 136: 289–295, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lanigan F, O'Connor D, Martin F, Gallagher WM. Molecular links between mammary gland development and breast cancer. Cell Mol Life Sci 64: 3159–3184, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee WJ, Monteith GR, Roberts-Thomson SJ. Calcium transport and signaling in the mammary gland: targets for breast cancer. Biochim Biophys Acta 1765: 235–255, 2006 [DOI] [PubMed] [Google Scholar]
- 64.Lee WJ, Roberts-Thomson SJ, Monteith GR. Plasma membrane calcium-ATPase 2 and 4 in human breast cancer cell lines. Biochem Biophys Res Commun 337: 779–783, 2005 [DOI] [PubMed] [Google Scholar]
- 65.Lee WJ, Robinson JA, Holman NA, McCall MN, Roberts-Thomson SJ, Monteith GR. Antisense-mediated Inhibition of the plasma membrane calcium-ATPase suppresses proliferation of MCF-7 cells. J Biol Chem 280: 27076–27084, 2005 [DOI] [PubMed] [Google Scholar]
- 66.Lehen'kyi V, Prevarskaya N. Oncogenic TRP channels. Adv Exp Med Biol 704: 929–945, 2011 [DOI] [PubMed] [Google Scholar]
- 67.Lemay DG, Neville MC, Rudolph MC, Pollard KS, German JB. Gene regulatory networks in lactation: identification of global principles using bioinformatics. BMC Systems Biol 1: 56, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lewis RS. Store-operated calcium channels: new perspectives on mechanism and function. Cold Spring Harb Perspect Biol 3: 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li M, Liu X, Robinson G, Bar-Peled U, Wagner KU, Young WS, Hennighausen L, Furth PA. Mammary-derived signals activate programmed cell death during the first stage of mammary gland involution. Proc Natl Acad Sci USA 94: 3425–3430, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ligresti A, Moriello AS, Starowicz K, Matias I, Pisanti S, De Petrocellis L, Laezza C, Portella G, Bifulco M, Di Marzo V. Antitumor activity of plant cannabinoids with emphasis on the effect of cannabidiol on human breast carcinoma. J Pharmacol Exp Ther 318: 1375–1387, 2006 [DOI] [PubMed] [Google Scholar]
- 71.Lin J, Manson JE, Lee IM, Cook NR, Buring JE, Zhang SM. Intakes of calcium and vitamin D and breast cancer risk in women. Arch Intern Med 167: 1050–1059, 2007 [DOI] [PubMed] [Google Scholar]
- 72.Liu G, Hu X, Chakrabarty S. Calcium sensing receptor down-regulates malignant cell behavior and promotes chemosensitivity in human breast cancer cells. Cell Calcium 45: 216–225, 2009 [DOI] [PubMed] [Google Scholar]
- 73.Lopes N, Paredes J, Costa JL, Ylstra B, Schmitt F. Vitamin D and the mammary gland: a review on its role in normal development and breast cancer. Breast Cancer Res 14: 211, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mamillapalli R, Vanhouten J, Dann P, Bikle D, Chang W, Brown E, Wysolmerski J. Mammary-specific ablation of the calcium-sensing receptor during lactation alters maternal calcium metabolism, milk calcium transport, and neonatal calcium accrual. Endocrinology 154: 3031–3042, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mamillapalli R, VanHouten J, Zawalich W, Wysolmerski J. Switching of G-protein usage by the calcium-sensing receptor reverses its effect on parathyroid hormone-related protein secretion in normal versus malignant breast cells. J Biol Chem 283: 24435–24447, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Manduca A, Campolongo P, Trezza V. Cannabinoid modulation of mother-infant interaction: is it just about milk? Rev Neurosci 23: 707–722, 2012 [DOI] [PubMed] [Google Scholar]
- 77.Manuel Iglesias J, Beloqui I, Garcia-Garcia F, Leis O, Vazquez-Martin A, Eguiara A, Cufi S, Pavon A, Menendez JA, Dopazo J, Martin AG. Mammosphere formation in breast carcinoma cell lines depends upon expression of E-cadherin. PLoS One 8: e77281, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mathiasen IS, Sergeev IN, Bastholm L, Elling F, Norman AW, Jaattela M. Calcium and calpain as key mediators of apoptosis-like death induced by vitamin D compounds in breast cancer cells. J Biol Chem 277: 30738–30745, 2002 [DOI] [PubMed] [Google Scholar]
- 79.McAndrew D, Grice DM, Peters AA, Davis FM, Stewart T, Rice M, Smart CE, Brown MA, Kenny PA, Roberts-Thomson SJ, Monteith GR. ORAI1-mediated calcium influx in lactation and in breast cancer. Mol Cancer Therap 10: 448–460, 2011 [DOI] [PubMed] [Google Scholar]
- 80.Monaco ME, Bronzert DA, Tormey DC, Waalkes P, Lippman ME. Casein production by human breast cancer. Cancer Res 37: 749–754, 1977 [PubMed] [Google Scholar]
- 81.Morgan MP, Cooke MM, McCarthy GM. Microcalcifications associated with breast cancer: an epiphenomenon or biologically significant feature of selected tumors? J Mammary Gland Biol Neoplasia 10: 181–187, 2005 [DOI] [PubMed] [Google Scholar]
- 82.Motiani RK, Abdullaev IF, Trebak M. A novel native store-operated calcium channel encoded by Orai3: selective requirement of Orai3 versus Orai1 in estrogen receptor-positive versus estrogen receptor-negative breast cancer cells. J Biol Chem 285: 19173–19183, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Neville MC. Calcium secretion into milk. J Mammary Gland Biol Neoplasia 10: 119–128, 2005 [DOI] [PubMed] [Google Scholar]
- 84.Oakes SR, Hilton HN, Ormandy CJ. The alveolar switch: coordinating the proliferative cues and cell fate decisions that drive the formation of lobuloalveoli from ductal epithelium. Breast Cancer Res 8: 207, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Okunade GW, Miller ML, Azhar M, Andringa A, Sanford LP, Doetschman T, Prasad V, Shull GE. Loss of the Atp2c1 secretory pathway Ca2+-ATPase (SPCA1) in mice causes Golgi stress, apoptosis, and midgestational death in homozygous embryos and squamous cell tumors in adult heterozygotes. J Biol Chem 282: 26517–26527, 2007 [DOI] [PubMed] [Google Scholar]
- 86.Ouadid-Ahidouch H, Dhennin-Duthille I, Gautier M, Sevestre H, Ahidouch A. TRP channels: diagnostic markers and therapeutic targets for breast cancer? Trends Mol Med 19: 117–124, 2013 [DOI] [PubMed] [Google Scholar]
- 87.Palmgren MG, Nissen Ptype ATPases P. P-type ATPases. Annu Rev Biophys 40: 243–266, 2011 [DOI] [PubMed] [Google Scholar]
- 88.Parekh HK, Deng HB, Choudhary K, Houser SR, Simpkins H. Overexpression of sorcin, a calcium-binding protein, induces a low level of paclitaxel resistance in human ovarian and breast cancer cells. Biochem Pharmacol 63: 1149–1158, 2002 [DOI] [PubMed] [Google Scholar]
- 89.Parkash J, Chaudhry MA, Rhoten WB. Calbindin-D28k and calcium sensing receptor cooperate in MCF-7 human breast cancer cells. Int J Oncol 24: 1111–1119, 2004 [PubMed] [Google Scholar]
- 90.Pettifor JM. Nutritional rickets: pathogenesis and prevention. Pediatr Endocrinol Rev 10, Suppl 2: 347–353, 2013 [PubMed] [Google Scholar]
- 91.Prasad V, Boivin GP, Miller ML, Liu LH, Erwin CR, Warner BW, Shull GE. Haploinsufficiency of Atp2a2, encoding the sarco(endo)plasmic reticulum Ca2+-ATPase isoform 2 Ca2+ pump, predisposes mice to squamous cell tumors via a novel mode of cancer susceptibility. Cancer Res 65: 8655–8661, 2005 [DOI] [PubMed] [Google Scholar]
- 92.Promkan M, Liu G, Patmasiriwat P, Chakrabarty S. BRCA1 suppresses the expression of survivin and promotes sensitivity to paclitaxel through the calcium sensing receptor (CaSR) in human breast cancer cells. Cell Calcium 49: 79–88, 2011 [DOI] [PubMed] [Google Scholar]
- 93.Radi MJ. Calcium oxalate crystals in breast biopsies. An overlooked form of microcalcification associated with benign breast disease. Arch Pathol Lab Med 113: 1367–1369, 1989 [PubMed] [Google Scholar]
- 94.Reinhardt TA, Filoteo AG, Penniston JT, Horst RL. Ca2+-ATPase protein expression in mammary tissue. Am J Physiol Cell Physiol 279: C1595–C1602, 2000 [DOI] [PubMed] [Google Scholar]
- 95.Reinhardt TA, Horst RL. Ca2+-ATPases and their expression in the mammary gland of pregnant and lactating rats. Am J Physiol Cell Physiol 276: C796–C802, 1999 [DOI] [PubMed] [Google Scholar]
- 96.Reinhardt TA, Lippolis JD. Mammary gland involution is associated with rapid down regulation of major mammary Ca2+-ATPases. Biochem Biophys Res Commun 378: 99–102, 2009 [DOI] [PubMed] [Google Scholar]
- 97.Reinhardt TA, Lippolis JD, Shull GE, Horst RL. Null mutation in the gene encoding plasma membrane Ca2+-ATPase isoform 2 impairs calcium transport into milk. J Biol Chem 279: 42369–42373, 2004 [DOI] [PubMed] [Google Scholar]
- 98.Rosen CJ, Adams JS, Bikle DD, Black DM, Demay MB, Manson JE, Murad MH, Kovacs CS. The nonskeletal effects of vitamin D: an Endocrine Society scientific statement. Endocr Rev 33: 456–492, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ryan D, Drysdale AJ, Lafourcade C, Pertwee RG, Platt B. Cannabidiol targets mitochondria to regulate intracellular Ca2+ levels. J Neurosci 29: 2053–2063, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Saidak Z, Mentaverri R, Brown EM. The role of the calcium-sensing receptor in the development and progression of cancer. Endocr Rev 30: 178–195, 2009 [DOI] [PubMed] [Google Scholar]
- 101.Sakka E, Prentza A, Koutsouris D. Classification algorithms for microcalcifications in mammograms (Review). Oncol Rep 15: 1049–1055, 2006 [DOI] [PubMed] [Google Scholar]
- 102.Sanders JL, Chattopadhyay N, Kifor O, Yamaguchi T, Butters RR, Brown EM. Extracellular calcium-sensing receptor expression and its potential role in regulating parathyroid hormone-related peptide secretion in human breast cancer cell lines. Endocrinology 141: 4357–4364, 2000 [DOI] [PubMed] [Google Scholar]
- 103.Sergeev IN. Calcium as a mediator of 1,25-dihydroxyvitamin D3-induced apoptosis. J Steroid Biochem Mol Biol 89–90: 419–425, 2004 [DOI] [PubMed] [Google Scholar]
- 104.Sergeev IN. Vitamin D and cellular Ca2+ signaling in breast cancer. Anticancer Res 32: 299–302, 2012 [PubMed] [Google Scholar]
- 105.Shin MH, Holmes MD, Hankinson SE, Wu K, Colditz GA, Willett WC. Intake of dairy products, calcium, and vitamin D and risk of breast cancer. J Natl Cancer Inst 94: 1301–1311, 2002 [DOI] [PubMed] [Google Scholar]
- 106.Shu L, Ji J, Zhu Q, Cao G, Karaplis A, Pollak MR, Brown E, Goltzman D, Miao D. The calcium-sensing receptor mediates bone turnover induced by dietary calcium and parathyroid hormone in neonates. J Bone Miner Res 26: 1057–1071, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Singh N, Promkan M, Liu G, Varani J, Chakrabarty S. Role of calcium sensing receptor (CaSR) in tumorigenesis. Best Pract Res Clin Endocrinol Metab 27: 455–463, 2013 [DOI] [PubMed] [Google Scholar]
- 108.Soghoian D, Jayaraman V, Silane M, Berenstein A, Jayaraman T. Inositol 1,4,5-trisphosphate receptor phosphorylation in breast cancer. Tumour Biol 26: 207–212, 2005 [DOI] [PubMed] [Google Scholar]
- 109.Srikanth S, Gwack Y. Orai1-NFAT signalling pathway triggered by T cell receptor stimulation. Mol Cells 35: 182–194, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Stein T, Salomonis N, Gusterson BA. Mammary gland involution as a multi-step process. J Mammary Gland Biol Neoplasia 12: 25–35, 2007 [DOI] [PubMed] [Google Scholar]
- 111.Sutherland KD, Lindeman GJ, Visvader JE. The molecular culprits underlying precocious mammary gland involution. J Mammary Gland Biol Neoplasia 12: 15–23, 2007 [DOI] [PubMed] [Google Scholar]
- 112.Szewczyk MM, Pande J, Grover AK. Caloxins: a novel class of selective plasma membrane Ca2+ pump inhibitors obtained using biotechnology. Pflügers Arch 456: 255–266, 2008 [DOI] [PubMed] [Google Scholar]
- 113.Thomsen LL, Miles DW, Happerfield L, Bobrow LG, Knowles RG, Moncada S. Nitric oxide synthase activity in human breast cancer. Br J Cancer 72: 41–44, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.VanHouten J, Dann P, McGeoch G, Brown EM, Krapcho K, Neville M, Wysolmerski JJ. The calcium-sensing receptor regulates mammary gland parathyroid hormone-related protein production and calcium transport. J Clin Invest 113: 598–608, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.VanHouten J, Sullivan C, Bazinet C, Ryoo T, Camp R, Rimm DL, Chung G, Wysolmerski J. PMCA2 regulates apoptosis during mammary gland involution and predicts outcome in breast cancer. Proc Natl Acad Sci USA 107: 11405–11410, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.VanHouten JN, Neville MC, Wysolmerski JJ. The calcium-sensing receptor regulates plasma membrane calcium adenosine triphosphatase isoform 2 activity in mammary epithelial cells: a mechanism for calcium-regulated calcium transport into milk. Endocrinology 148: 5943–5954, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Vanhouten JN, Wysolmerski JJ. The calcium-sensing receptor in the breast. Best Pract Res Clin Endocrinol Metab 27: 403–414, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.VanHouten JN, Wysolmerski JJ. Transcellular calcium transport in mammary epithelial cells. J Mammary Gland Biol Neoplasia 12: 223–235, 2007 [DOI] [PubMed] [Google Scholar]
- 119.Wang L, Wang X, Liang Y, Diao X, Chen Q. S100A4 promotes invasion and angiogenesis in breast cancer MDA-MB-231 cells by upregulating matrix metalloproteinase-13. Acta Biochim Pol 59: 593–598, 2012 [PubMed] [Google Scholar]
- 120.Welsh J. Cellular and molecular effects of vitamin D on carcinogenesis. Arch Biochem Biophys 523: 107–114, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Welsh J. Vitamin D metabolism in mammary gland and breast cancer. Mol Cell Endocrinol 347: 55–60, 2011 [DOI] [PubMed] [Google Scholar]
- 122.Yang S, Zhang JJ, Huang XY. Orai1 and STIM1 are critical for breast tumor cell migration and metastasis. Cancer Cell 15: 124–134, 2009 [DOI] [PubMed] [Google Scholar]
- 123.Zeng C, Ke Z, Song Y, Yao Y, Hu X, Zhang M, Li H, Yin J. Annexin A3 is associated with a poor prognosis in breast cancer and participates in the modulation of apoptosis in vitro by affecting the Bcl-2/Bax balance. Exp Mol Pathol 95: 23–31, 2013 [DOI] [PubMed] [Google Scholar]
- 124.Zinser GM, Sundberg JP, Welsh J. Vitamin D(3) receptor ablation sensitizes skin to chemically induced tumorigenesis. Carcinogenesis 23: 2103–2109, 2002 [DOI] [PubMed] [Google Scholar]
- 125.Zinser GM, Welsh J. Accelerated mammary gland development during pregnancy and delayed postlactational involution in vitamin D3 receptor null mice. Mol Endocrinol 18: 2208–2223, 2004 [DOI] [PubMed] [Google Scholar]
- 126.Zinser GM, Welsh J. Vitamin D receptor status alters mammary gland morphology and tumorigenesis in MMTV-neu mice. Carcinogenesis 25: 2361–2372, 2004 [DOI] [PubMed] [Google Scholar]