Abstract
Bombesin receptor subtype-3 (BRS-3) regulates energy homeostasis, with Brs3 knockout (Brs3−/y) mice being hypometabolic, hypothermic, and hyperphagic and developing obesity. We now report that the reduced body temperature is more readily detected if body temperature is analyzed as a function of physical activity level and light/dark phase. Physical activity level correlated best with body temperature 4 min later. The Brs3−/y metabolic phenotype is not due to intrinsically impaired brown adipose tissue function or in the communication of sympathetic signals from the brain to brown adipose tissue, since Brs3−/y mice have intact thermogenic responses to stress, acute cold exposure, and β3-adrenergic activation, and Brs3−/y mice prefer a cooler environment. Treatment with the BRS-3 agonist MK-5046 increased brown adipose tissue temperature and body temperature in wild-type but not Brs3−/y mice. Intrahypothalamic infusion of MK-5046 increased body temperature. These data indicate that the BRS-3 regulation of body temperature is via a central mechanism, upstream of sympathetic efferents. The reduced body temperature in Brs3−/y mice is due to altered regulation of energy homeostasis affecting higher center regulation of body temperature, rather than an intrinsic defect in brown adipose tissue.
Keywords: bombesin receptor subtype-3, obesity, sympathetic nervous system, brown adipose tissue, thermoregulation, CL316243, MK-5046
obesity, an imbalance between energy intake and energy expenditure, is associated with several comorbidities, including diabetes mellitus and cardiovascular disease. Current treatments for obesity are inadequate, stimulating research to better understand the physiology of energy homeostasis and to develop safe and effective pharmacologic treatments. The study of bombesin receptor subtype-3 (BRS-3) can advance both of these objectives.
BRS-3 is a G protein-coupled receptor for which the natural ligand is unknown, and despite its name, BRS-3 has a low affinity for bombesin and related natural peptides (7, 17, 21). In addition to other locations, BRS-3 is present in the central nervous system, including the hypothalamus and caudal brainstem (10, 16, 26, 37), regions involved in the regulation of feeding, energy expenditure, and body weight (32). Genetic and pharmacologic studies demonstrate a role for BRS-3 in energy homeostasis. Brs3 knockout (Brs3−/y) mice exhibit hyperphagia, reduced metabolic rate, and obesity (18, 27), and pharmacological blockade of BRS-3 in rats increases food intake and body weight (10). Conversely, BRS-3 agonists reduced food intake, increased metabolic rate and body temperature (Tb), and reduced body weight in mice, rats, and dogs (10, 11, 22).
Currently, the mechanisms underlying Tb regulation by BRS-3 are unclear. Brown adipose tissue (BAT) is the major site of facultative thermogenesis, dissipating chemical energy via uncoupling protein 1 (UCP1), thereby generating heat and maintaining Tb (2). BAT activity is regulated by sympathetic neural input, with upstream regulation from hypothalamic, preoptic, and other brain regions (1, 23). Thus the lower Tb in Brs3−/y mice could arise from intrinsic defects at a number of sites including BAT (5), sympathetic neuronal transmission (35), or the brain sites at the core of thermal regulation (20). Alternatively, the mild hypothermia could be due to an altered input into what is otherwise a normally responding thermal regulatory system.
We now evaluate the consequences of disrupting BRS-3 signaling on BAT. We also examine the effects of BRS-3 agonism on BAT thermogenesis and the involvement of the central nervous system. Together these approaches allow us to suggest a mechanism for how the BRS-3 system affects Tb.
MATERIALS AND METHODS
Compounds.
MK-5046 (33) was generously provided by Merck Research Laboratories (Rahway, NJ), and CL316243 and lipopolysaccharide were purchased from Sigma-Aldrich (St. Louis, MO).
Mice.
C57BL/6J mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Brs3−/y mice were provided by Dr. James Battey (18) and back-crossed at least eight generations onto a C57BL/6J background. Mice were housed at ∼21–22°C in a humidity-controlled environment with a 12:12-h light-dark cycle and with water and chow (NIH-07 diet) available ad libitum. All experiments used male mice, typically 12–16 wk of age. Pentobarbital sodium (80 mg/kg ip) was used for anesthesia for physiological measurements. Survival surgery anesthesia used ketamine (100 mg/kg) and xylazine (10 mg/kg ip), with Banamine analgesia (2.2 mg/kg sc daily for 3 days). At least 7 days were allowed for recovery. All animal studies were approved by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)/National Institutes of Health (NIH) Animal Care and Use Committee.
Body and interscapular brown adipose tissue temperature measurement in anesthetized mice.
Mice were anesthetized (pentobarbital) and placed on a heated (35°C) table, and a thermistor probe (YSI 427; Measurement Specialties, Shrewsbury, MA) was sutured into place underneath the interscapular brown adipose tissue (iBAT) via an incision caudal to the fat pad (4). A rectal temperature probe (YSI 455; Measurement Specialties) was inserted and secured with tape. The mouse was then positioned supinely, and a tracheal catheter was inserted, allowing free movement of room air. At least 30 min were allowed for stabilization before drug infusion.
Body and iBAT temperature measurement by telemetry in ambulatory mice.
Mice were anesthetized, and an IPTT-300 transponder (BioMedic Data Systems, Seaford, DE) was sutured into place under the iBAT depot. Another IPTT-300 was inserted intraperitoneally via a midline abdominal incision and sutured to the omentum. Body and iBAT temperatures were recorded with a scanner (DAS-7007; BioMedic Data Systems). To avoid confounding temperature increases due to handling stress, all readings were taken within 60 s of initially handling a mouse with at least 1 h (and typically 2 h) between serial measurements. Alternatively, Tb and activity were continuously measured by telemetry (Mini Mitter/Philips Respironics, Bend, OR) using ER4000 energizer/receivers, G2 E-mitters implanted intraperitoneally, and VitalView software with data collected each minute. The Tb vs. activity cross-correlation analysis was performed with NeuroExplorer (Nex Technologies, Madison, AL).
Ambulatory tests of thermal regulation.
Temperature preference was measured by placing mice in a stainless steel pan (64 × 15 × 20 cm, length × width × height) spanning two hot/cold plates set at 20 and 45 °C. Ninety-minute sessions were conducted on two successive days, with the last 60 min of the second session used for analysis. Position was tracked with an overhead camera and video tracking software (Ethovision 9.0; Noldus, Leesburg, VA). In the cage-switch assay, stress was induced by placing a mouse in a cage previously occupied by a different male mouse (19) with Tb and activity monitored by Mini Mitter. The febrile response at 21°C ambient temperature caused by lipopolysaccharide (10 μg/kg ip) (28) was measured by Mini Mitter. Indirect calorimetry was performed with a 12-chamber Environment Controlled CLAMS (Columbus Instruments, Columbus, OH) with ad libitum access to food and water during the entire testing period.
Intravenous infusions.
Mice were anesthetized and the jugular vein catheterized using PE-10 tubing. CL316243 and MK-5046 were dissolved in saline or 5% dimethylacetamide in saline, respectively, and delivered in a volume of 1 ml/kg.
Intrahypothalamic infusions.
Mice were anesthetized, and cannulas (5.25 mm, 26 gauge; Plastics One, Roanoke, VA) stereotaxically were implanted bilaterally (1.34 mm posterior, 0.75 mm lateral to bregma, 4.75 mm below the surface of the skull; Ref. 8), and secured with dental cement (Parkell, Edgewood, NY). Intrahypothalamic infusions used saline as vehicle and were given via a 33-gauge internal cannula protruding 0.5 mm past the tip of the guide cannula using a syringe pump (KD Scientific, Holliston, MA) infusing 1 μl per cannula over 60 s. The infusion targets the entire hypothalamus. The cannula position was verified by postmortem histological analysis.
Statistics.
Data are means ± SE. Two-way ANOVA with or without repeated measures followed by Holm-Šídák's posttest was used for comparing genotypes vs. the treatment groups. Student's t-test was used when two groups were compared. Analyses used two-tailed P < 0.05 as statistically significant.
RESULTS
Thermal biology in Brs3−/y mice.
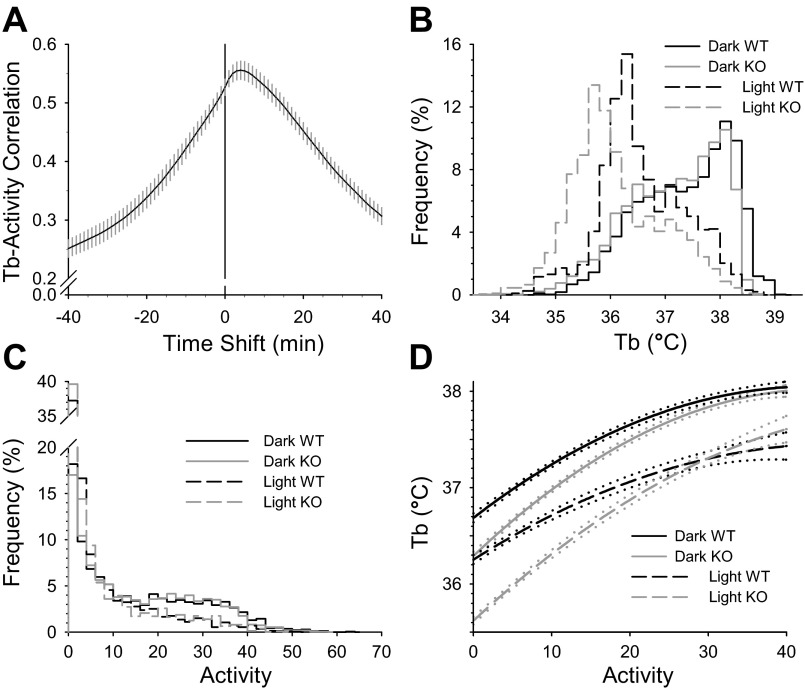
Tb in Brs3−/y mice was slightly lower than in controls, reaching statistical significance in the light but not the dark phase; there was no difference in physical activity levels (Table 1). To better understand the relationship between physical activity and Tb, we quantified the timing of the effect of activity on Tb by looking for the lag time that gives the best correlation between activity and Tb in a cohort of wild-type C57BL/6 mice. This cross-correlation analysis revealed that increased activity causes Tb increases with a 4-min (range, 2- to 6-min) lag (Fig. 1A). Based on this result we analyzed the Brs3−/y Tb and activity data using 10-min averages. The Tb histogram showed the lower Tb in the light phase and the slight shift in Brs3−/y mice but a similar overall pattern to the controls (Fig. 1B). The activity histogram showed the expected higher level in dark than light phase with no difference between Brs3−/y and control mice (Fig. 1C). Next, we examined Tb as a function of activity and observed a clear difference between Brs3−/y and control mice, with a greater difference at lower physical activity levels (Fig. 1D).
Table 1.
Body temperature and physical activity
| Dark Tb, °C | Dark Activity | Light Tb, °C | Light Activity | |
|---|---|---|---|---|
| Wild type | 37.36 ± 0.14 | 15.7 ± 0.9 | 36.57 ± 0.17 | 8.0 ± 0.7 |
| Brs3−/y | 37.13 ± 0.07 | 15.4 ± 0.7 | 36.11 ± 0.09 | 8.0 ± 0.4 |
| n | 10 | 10 | 10 | 10 |
| P | 0.17 | NS | 0.03 | NS |
Values are means ± SE. Body temperature (Tb) and activity were measured by Mini Mitter telemetry for 4 days, omitting data when mice were handled or treated. The means of 2,760 (dark phase) or 1,970 (light phase) min of Tb and activity data were calculated for each of 10 mice. Activity is in arbitrary units.
Fig. 1.
Effect of activity and light phase on Tb in Brs3−/y mice. A: cross-correlation analysis of body temperature (Tb) and activity in C57BL/6 mice was performed with a dataset from ten C57BL/6 mice, each monitored for 140 h in 1-min intervals. A positive value in the time shift axis means the Tb data are shifted later relative to the activity data by the indicted number of minutes. Data are means ± SE. B–D: mean Tb and activity in 10-min intervals was calculated for wild-type (WT) and Brs3−/y [knockout (KO)] mice; n = 10/group (n = 2,760 dark phase and n = 1,970 light phase data pairs per genotype). The frequency histograms for Tb (B) and activity (C) and the quadratic fit (D) of the Tb to the activity were calculated separately for the dark and light periods. Dotted lines show the 95% confidence intervals of the fitted curves.
To further understand Tb regulation in Brs3−/y mice, a number of interventions that change Tb were tested. Brs3−/y mice increased Tb normally during the first hour in response to the stress of being handled and had a comparable febrile response to lipopolysaccharide, with the hyperthermia resolving more rapidly in the Brs3−/y mice (Fig. 2A). There was a normal circadian rhythm and Tb and physical activity increase in response to the social stress of a cage switch (19) (Fig. 2B). When Brs3−/y mice were placed in a thermal gradient, they dispersed over the gradient much more widely than the controls and chose cooler environmental temperatures (Fig. 2C). The lower preferred environmental temperature was not explained by the slightly higher body weight.
Fig. 2.
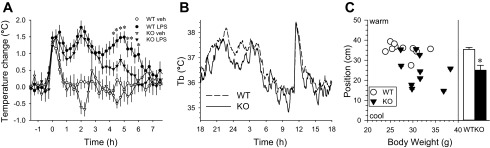
Regulation of Tb in Brs3−/y mice. A: fever elicited by lipopolysaccharide (LPS). WT and Brs3−/y (KO) mice were treated with LPS (10 μg/kg ip) or vehicle (saline) at 9 am (time 0). Data are change from baseline (−90 to −30 min) means ± SE; n = 9–12/group. *P < 0.05 WT LPS vs. KO LPS of 10-min averages. Baseline Tb were 36.1 ± 0.2 (WT veh), 36.1 ± 0.3 (WT LPS), 35.9 ± 0.2 (KO veh), and 35.9 ± 0.2 °C (KO LPS). B: cage-switch. WT and KO mice were individually placed in a cage vacated by another male. Time is clock time; switch was at 11 am. Data are mean; SEs are omitted for visual clarity; n = 8–10/group. C: choice of environmental temperature. Mice were placed in a thermal gradient (nominally 20 to 45°C) during the light phase with position monitored continuously by video (n = 10/group). Bar graph shows means ± SE. *P = 0.001. Mean body weights were 27.8 ± 1.0 and 31.5 ± 1.1 g in WT and KO, respectively (P = 0.03). Ambient temperature was 21.6°C. The WT and Brs3−/y mice moved similar distances.
Brs3−/y mice have intact, functioning BAT.
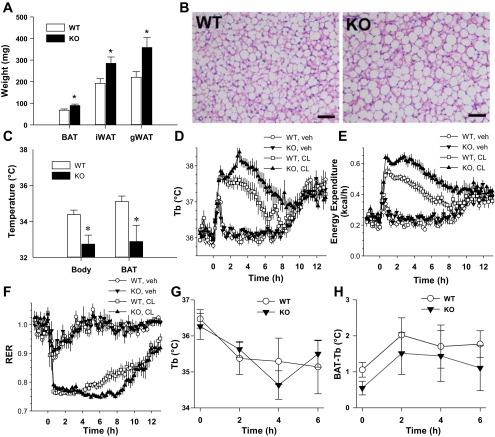
We next examined BAT function. Brs3−/y mice (12–16 wk old), while of comparable body weight to controls (wild type, 22.0 ± 0.8 g and Brs3−/y 22.6 ± 0.4 g), showed increased iBAT, inguinal white adipose tissue (WAT), and gonadal WAT weights (Fig. 3A). The increased iBAT weight is likely due to increased triglyceride content as Brs3−/y mice have larger lipid droplets (Fig. 3B). Ucp1 mRNA and protein levels were similar between Brs3−/y and control mice (data not shown). Body and iBAT temperatures in overnight fasted Brs3−/y mice were reduced compared with wild-type mice (Fig. 3C). The iBAT was slightly warmer than the body core, but this was not statistically different in this experiment.
Fig. 3.
Intact BAT function in Brs3−/y mice. WT and Brs3−/y (KO) mice were studied at 12–16 wk of age. A: interscapular brown adipose tissue (iBAT), inguinal white adipose tissue (iWAT), and gonadal WAT (gWAT) weights. B: histology of iBAT. Scale bar = 50 μm. C: ambulatory telemetry (BioMedic) BAT and body temperature at 9 am after a 16-h fast. D–F: effect of CL316243 on body temperature (D), energy expenditure (E), and respiratory exchange ratio (RER; F). Mice (body weight: WT, 25.0 ± 0.4 g and KO, 26.5 ± 0.8 g) were acclimatized to the indirect calorimetry chambers at 22°C for 4 days and then at 30°C for one day with vehicle or CL316243 treatment (0.1 mg/kg ip) on the 6th or 7th day in a crossover design. Body temperature (G) and difference in temperature between BAT and core body temperature upon exposure to 4°C (H). Time is time since onset of cold exposure. Data are means ± SE; n = 5–6/group. *P < 0.05.
To measure thermogenic capacity, mice were treated with a maximal dose of a β3-adrenoreceptor agonist, CL316243 (2). The Brs3−/y mice showed a greater increase in Tb, energy expenditure, and fat oxidation (indicated by the reduced respiratory exchange ratio) than the control mice (Fig. 3, D–F). Thus the intrinsic thermogenic capacity in response to a β3-adrenoreceptor agonist is clearly intact in Brs3−/y mice.
To assess an integrated thermogenic response (sensory information to brain to BAT), the body and BAT temperature response to cold exposure was measured. The slight reduction in Tb was similar in Brs3−/y and control mice (Fig. 3G) as was the rise in iBAT temperature (Fig. 3H). Together, these results demonstrate that Brs3-deficient mice have functional, inducible BAT, despite their lower body and BAT temperatures, and point to a possible reduction in neural activation of BAT.
BRS3 agonist activates BAT via a central mechanism.
BRS3 agonists can increase Tb (10, 22), so we next examined the effect of a peripherally administered, brain-penetrant BRS3 agonist, MK-5046 (11), on iBAT temperature, using anesthetized mice to eliminate the effect of changes in physical activity. Mice were treated with MK-5046, CL316243, or vehicle. MK-5046 increased iBAT temperature in wild-type but not Brs3−/y mice (Fig. 4A). In contrast, as a positive control, CL316243 increased iBAT temperature in both wild-type and Brs3−/y mice (Fig. 4, B and C). Similar results were obtained measuring rectal temperature (Fig. 4D). These data demonstrate that MK-5046 has a thermogenic effect on BAT and that it is acting via BRS-3.
Fig. 4.
MK-5046 increases iBAT and body temperature. Effects of intravenous treatments were studied in pentobarbital-anesthetized WT and Brs3−/y (KO) mice at 12–16 wk of age. The change in iBAT temperature in response to MK-5046 [MK; 1 mg/kg iv, equivalent exposure to 10 mg/kg orally (33), which causes a maximal body temperature increase (11); A] and CL316243 [CL; 0.1 mg/kg iv; B], compared with vehicle (Veh) in WT and Brs3−/y (KO) mice. For visual clarity, the vehicle data are repeated in A and B. C: mean change in BAT temperature, average of the 5- to 30-min data from A and B. Baseline iBAT temperatures were 35.2 ± 0.3 (WT Veh), 35.8 ± 0.2 (WT CL), 35.0 ± 0.3 (WT MK), 35.0 ± 0.2 (KO Veh), 34.9 ± 0.3 (KO CL), and 35.9 ± 0.2°C (KO MK). D: mean change in body temperature, average of the 5–30 min data from the same experiment. Data are means ± SE; n = 6/group. *P < 0.05.
To determine if the thermogenic effect of MK-5046 was centrally mediated, we measured the effect of intrahypothalamic infusion of MK-5046 on Tb. MK-5046 increased Tb by 0.42 ± 0.11°C (P < 0.001; Fig. 5, A and B), demonstrating that MK-5046 is acting centrally to increase Tb.
Fig. 5.
Effect of bilateral intrahypothalamic MK-5046 treatment (1 μg × 2) in pentobarbital-anesthetized C57BL/6 mice. Body temperature change from time baseline (A) and average of the 5–30 min data (B). Baseline temperatures were 35.7 ± 0.2 (Veh) and 35.3 ± 0.1°C (MK). Experiment is a crossover design and data are means ± SE; n = 9/group. *P < 0.05.
DISCUSSION
We have investigated in detail the reduced Tb of Brs3−/y mice. The results show no intrinsic defects in BAT or in the ability of the central nervous system (CNS) to engage sympathetic efferents to BAT. Rather we propose that the reduced Tb is a secondary effect of altered energy homeostasis affecting higher center regulation of Tb, as discussed below. Peripherally administered BRS-3 agonists increase Tb and BAT temperatur, and small doses administered directly to the hypothalamus increase Tb, suggesting that CNS BRS-3 contributes to sympathetic outflow to BAT.
Intact control of Tb in Brs3−/y mice.
The Tb reduction in Brs3−/y mice studied at room temperature is small, averaging ∼0.34 °C below control mice. Tb differences of this magnitude are difficult to detect, so experimental manipulations are often used to increase their size. For example, in Brs3−/y mice fasting amplifies the Tb reduction (22). However, such manipulations have other effects on physiology, confounding interpretation of the results. Here we measured Tb by telemetry and analyzed Tb as a function of physical activity level and light/dark phase. This approach permits monitoring in the home cage without potentially confounding manipulations, makes use of the full dataset, and markedly increases the ability to detect Tb changes.
To our knowledge, Tb kinetics after physical activity have not been reported previously in mice. While the quantitative details are likely to depend on the level and type of activity and methods of Tb and activity measurement, only a brief lag time is expected due to the small body size and thus rapid heat loss. We observed a 4-min lag between activity and the Tb response.
The energy cost of defending Tb is ∼2.0 kcal·day−1·°C−1 in 27 g mice (Abreu-Vieira G, unpublished observations). Thus the ∼0.34°C Tb reduction in Brs3−/y mice saves ∼0.70 kcal/day. It is not known how this energy savings partitions to fat storage vs. reduced food intake. There are no reports characterizing Brs3−/y mice housed chronically at thermoneutrality, which would erase their Tb reduction, and it is unknown if thermoneutrality would exacerbate the increased Brs3−/y adiposity, as it does in other mouse models (3, 25, 31).
What is the mechanistic basis for the Tb lowering in Brs3−/y mice? Direct stimulation of the thermogenesis effector tissues, WAT and BAT, with a β3 adrenergic agonist demonstrated full functionality. In fact, the increases in metabolic rate and Tb were actually greater in Brs3−/y mice. Possible explanations for the augmented Brs3−/y thermogenic response are increased fatty acid availability from the larger WAT and/or BAT stores, providing more fuel for greater heat generation in BAT and more responsive adipose tissues due to reduced prior stimulation. More upstream, the neural sympathetic control of heat generation by BAT is intact in Brs3−/y mice, as demonstrated by the response to handling stress, cage switch stress, and lipopolysaccharide. Notably, the response to cold exposure is similar in Brs3−/y and wild-type mice, demonstrating intact sensing of environmental temperature. Besides the lower Tb, the Brs3−/y mice prefer a cooler environment, indicating that the lower Tb is targeted and is not due to an “inability” to defend a higher Tb.
These data strongly suggest that in Brs3−/y mice there is no defect intrinsic to the BAT itself or to the circuitry determining sympathetic signals efferents to BAT. A unified way to view the thermal physiology of Brs3−/y mice is that the endogenous BRS-3 ligand is a signal of the “energy replete” state. In this sense, Brs3−/y mice are similar to Leprdb/db mice (12). The inability of Brs3−/y mice to sense the putative endogenous ligand (or of Leprdb/db mice to sense leptin; Ref. 34) elicits a physiologic drive to increase energy stores and reduce energy expenditure. Tb reduction is a normal component of that response; small mammals expend large amounts of energy to stay warm, and a reduction in Tb is a common, effective energy-conserving strategy (9). The recent identification of a Drosophila BRS-3 homolog and two ligand peptides (14) may facilitate discovery of the elusive mammalian endogenous BRS-3 ligand(s). Other examples of mutant mice that likely have reduced Tb secondary to altered energy homeostasis include Lepob/ob (29), Mc4r−/− (unpublished observations), and Fgf21 overexpressors (15). It is probably a general rule in small-sized mammals that modifiers of the regulation of energy homeostasis can affect Tb.
BAT and Tb effect of a BRS-3 agonist, MK-5046.
BRS-3 agonists can increase Tb, particularly in the fasted state and the Tb increase is reduced by propranolol, supporting a sympathetic mechanism of action (10, 22). Here, we directly show that the Tb increase occurs via BAT activation. Intrahypothalamic injection of MK-5046 increased Tb, demonstrating a role for CNS BRS-3. BAT thermogenesis is controlled at multiple levels in the CNS. Thermal sensory inputs, some routed via the lateral parabrachial nucleus (LPB), and other signals are integrated in the preoptic area, transmitted to the dorsomedial hypothalamus (DMH), rostral raphe pallidus, and on to preganglionic sympathetic neurons (23). Other regions contributing to regulating sympathetic tone to BAT include the paraventricular hypothalamus (PVH), orexinergic lateral hypothalamus (LH), and the nucleus of the solitary tract (NTS). BRS-3 binding activity (10) is found in many of these areas (e.g., DMH, PVH, LH, LPB, and NTS) as is BRS-3 mRNA (e.g., medial and median preoptic area, DMH, PVH, and LPB) (37). Further studies (such as injection of smaller volumes of agonist and localized ablation of Brs3) will be required to localize more precisely which neurons and nuclei are driving the Tb increase and their upstream and downstream interactions.
BRS-3 agonism for the treatment of obesity?
How does mechanistic understanding of BRS-3's role in thermal biology inform the prospects of BRS-3 agonism for the treatment of obesity? First, it is important to recognize that smaller homeotherms such as mice have larger surface area to volume ratios, generate much heat in BAT, and strive to conserve body heat (9). In contrast large homeotherms such as adult humans get most (but not all; Ref. 24) of their heat from BAT-independent metabolism and have developed mechanisms to facilitate heat loss. Due to these physiological differences, mice, unlike adult humans, vary their target Tb greatly in response to environmental changes such as cold exposure or food deprivation. As noted, the effects of the BRS-3 system on Tb are likely secondary to its primary role in energy homeostasis. This makes the concern that BRS-3 agonists will produce clinical hyperthermia unlikely, and is consistent with the lack of observed Tb changes in men treated with MK-5046 (30). The realization that adult humans can and do expend energy via BAT activation has brought attention to BAT (24) and highlighted the potential for drugs that activate BAT as a treatment for obesity (36). BRS-3 agonists fit this paradigm; they increase energy expenditure by BAT. BRS-3 agonists also inhibit food intake, which is crucial since increased food intake often accompanies BAT activation and thereby impairs weight loss due to BAT activation. However, caution is warranted. Leptin is another ligand that reduces food intake and activates BAT, and leptin is a near-miraculous drug when given to treat leptin deficiency (6). However, >99.99% of obese people have a high leptin level, not a deficiency, and treatment with leptin in these patients is not effective (13). Until an endogenous BRS-3 ligand is identified, it is unknown if obese people have low or high BRS-3 ligand levels and this may determine how effective a BRS-3 agonist will be for treating obesity.
GRANTS
This research was supported by the Intramural Research Program (NIDDK Grants ZIA DK-075057 and ZIA DK-075063). The visit of G. Abreu-Vieira to NIH was supported within a grant from the Swedish Research Council to Jan Nedergaard and from institutional funds from the Department of Molecular Biosciences, Wenner-Gren Institute, Stockholm University.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: D.M.L., G.A.-V., C.X., and M.L.R. conception and design of research; D.M.L., G.A.-V., and C.X. performed experiments; D.M.L., G.A.-V., C.X., and M.L.R. analyzed data; D.M.L., G.A.-V., C.X., and M.L.R. interpreted results of experiments; D.M.L., G.A.-V., and M.L.R. prepared figures; D.M.L. and M.L.R. drafted manuscript; D.M.L., G.A.-V., C.X., and M.L.R. edited and revised manuscript; D.M.L., G.A.-V., C.X., and M.L.R. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Alexxai Kravitz for advice and the Tb activity cross-correlation analysis and Oksana Gavrilova and Margalit Goldgof for intellectual and technical contributions.
REFERENCES
- 1.Bamshad M, Song CK, Bartness TJ. CNS origins of the sympathetic nervous system outflow to brown adipose tissue. Am J Physiol Regul Integr Comp Physiol 276: R1569–R1578, 1999 [DOI] [PubMed] [Google Scholar]
- 2.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev 84: 277–359, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Castillo M, Hall JA, Correa-Medina M, Ueta C, Kang HW, Cohen DE, Bianco AC. Disruption of thyroid hormone activation in type 2 deiodinase knockout mice causes obesity with glucose intolerance and liver steatosis only at thermoneutrality. Diabetes 60: 1082–1089, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Jesus LA, Carvalho SD, Ribeiro MO, Schneider M, Kim SW, Harney JW, Larsen PR, Bianco AC. The type 2 iodothyronine deiodinase is essential for adaptive thermogenesis in brown adipose tissue. J Clin Invest 108: 1379–1385, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Enerback S, Jacobsson A, Simpson EM, Guerra C, Yamashita H, Harper ME, Kozak LP. Mice lacking mitochondrial uncoupling protein are cold-sensitive but not obese. Nature 387: 90–94, 1997 [DOI] [PubMed] [Google Scholar]
- 6.Farooqi IS, Matarese G, Lord GM, Keogh JM, Lawrence E, Agwu C, Sanna V, Jebb SA, Perna F, Fontana S, Lechler RI, DePaoli AM, O'Rahilly S. Beneficial effects of leptin on obesity, T cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. J Clin Invest 110: 1093–1103, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fathi Z, Corjay MH, Shapira H, Wada E, Benya R, Jensen R, Viallet J, Sausville EA, Battey JF. BRS-3: a novel bombesin receptor subtype selectively expressed in testis and lung carcinoma cells. J Biol Chem 268: 5979–5984, 1993 [PubMed] [Google Scholar]
- 8.Franklin KB, Paxinos G. The Mouse Brain in Sterotaxic Coordinates. New York, NY: Elsevier, 2008 [Google Scholar]
- 9.Gordon CJ. Thermal physiology of laboratory mice: defining thermoneutrality. J Therm Biol 37: 654–685, 2012 [Google Scholar]
- 10.Guan XM, Chen H, Dobbelaar PH, Dong Y, Fong TM, Gagen K, Gorski J, He S, Howard AD, Jian T, Jiang M, Kan Y, Kelly TM, Kosinski J, Lin LS, Liu J, Marsh DJ, Metzger JM, Miller R, Nargund RP, Palyha O, Shearman L, Shen Z, Stearns R, Strack AM, Stribling S, Tang YS, Wang SP, White A, Yu H, Reitman ML. Regulation of energy homeostasis by bombesin receptor subtype-3: selective receptor agonists for the treatment of obesity. Cell Metab 11: 101–112, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Guan XM, Metzger JM, Yang L, Raustad KA, Wang SP, Spann SK, Kosinski JA, Yu H, Shearman LP, Faidley TD, Palyha O, Kan Y, Kelly TM, Sebhat I, Lin LS, Dragovic J, Lyons KA, Craw S, Nargund RP, Marsh DJ, Strack AM, Reitman ML. Antiobesity effect of MK-5046, a novel bombesin receptor subtype-3 agonist. J Pharmacol Exp Ther 336: 356–364, 2011 [DOI] [PubMed] [Google Scholar]
- 12.Harris RB. Parabiosis between db/db and ob/ob or db/+ mice. Endocrinology 140: 138–145, 1999 [DOI] [PubMed] [Google Scholar]
- 13.Heymsfield SB, Greenberg AS, Fujioka K, Dixon RM, Kushner R, Hunt T, Lubina JA, Patane J, Self B, Hunt P, McCamish M. Recombinant leptin for weight loss in obese and lean adults: a randomized, controlled, dose-escalation trial. JAMA 282: 1568–1575, 1999 [DOI] [PubMed] [Google Scholar]
- 14.Ida T, Takahashi T, Tominaga H, Sato T, Sano H, Kume K, Ozaki M, Hiraguchi T, Shiotani H, Terajima S, Nakamura Y, Mori K, Yoshida M, Kato J, Murakami N, Miyazato M, Kangawa K, Kojima M. Isolation of the bioactive peptides CCHamide-1 and CCHamide-2 from Drosophila and their putative role in appetite regulation as ligands for G protein-coupled receptors. Front Endocrinol (Lausanne) 3: 177, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Inagaki T, Dutchak P, Zhao G, Ding X, Gautron L, Parameswara V, Li Y, Goetz R, Mohammadi M, Esser V, Elmquist JK, Gerard RD, Burgess SC, Hammer RE, Mangelsdorf DJ, Kliewer SA. Endocrine regulation of the fasting response by PPARalpha-mediated induction of fibroblast growth factor 21. Cell Metab 5: 415–425, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Jennings CA, Harrison DC, Maycox PR, Crook B, Smart D, Hervieu GJ. The distribution of the orphan bombesin receptor subtype-3 in the rat CNS. Neuroscience 120: 309–324, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Jensen RT, Battey JF, Spindel ER, Benya RV. International Union of Pharmacology. LXVIII. Mammalian bombesin receptors: nomenclature, distribution, pharmacology, signaling, and functions in normal and disease states. Pharmacol Rev 60: 1–42, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ladenheim EE, Hamilton NL, Behles RR, Bi S, Hampton LL, Battey JF, Moran TH. Factors contributing to obesity in bombesin receptor subtype-3-deficient mice. Endocrinology 149: 971–978, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee DL, Webb RC, Brands MW. Sympathetic and angiotensin-dependent hypertension during cage-switch stress in mice. Am J Physiol Regul Integr Comp Physiol 287: R1394–R1398, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Magoun HW, Harrison F, Broceck JR, Ranson SW. Activation of heat loss mechanisms by local heating of the brain. J Neurophysiol 1: 101–114, 1938 [Google Scholar]
- 21.Mantey SA, Weber HC, Sainz E, Akeson M, Ryan RR, Pradhan TK, Searles RP, Spindel ER, Battey JF, Coy DH, Jensen RT. Discovery of a high affinity radioligand for the human orphan receptor, bombesin receptor subtype 3, which demonstrates that it has a unique pharmacology compared with other mammalian bombesin receptors. J Biol Chem 272: 26062–26071, 1997 [DOI] [PubMed] [Google Scholar]
- 22.Metzger JM, Gagen K, Raustad KA, Yang L, White A, Wang SP, Craw S, Liu P, Lanza T, Lin LS, Nargund RP, Guan XM, Strack AM, Reitman ML. Body temperature as a mouse pharmacodynamic response to bombesin receptor subtype-3 agonists and other potential obesity treatments. Am J Physiol Endocrinol Metab 299: E816–E824, 2010 [DOI] [PubMed] [Google Scholar]
- 23.Morrison SF, Madden CJ, Tupone D. Central control of brown adipose tissue thermogenesis. Front Endocrinol (Lausanne) 3: pii: 00005, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nedergaard J, Bengtsson T, Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab 293: E444–E452, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Nikonova L, Koza RA, Mendoza T, Chao PM, Curley JP, Kozak LP. Mesoderm-specific transcript is associated with fat mass expansion in response to a positive energy balance. FASEB J 22: 3925–3937, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ohki-Hamazaki H, Wada E, Matsui K, Wada K. Cloning and expression of the neuromedin B receptor and the third subtype of bombesin receptor genes in the mouse. Brain Res 762: 165–172, 1997 [DOI] [PubMed] [Google Scholar]
- 27.Ohki-Hamazaki H, Watase K, Yamamoto K, Ogura H, Yamano M, Yamada K, Maeno H, Imaki J, Kikuyama S, Wada E, Wada K. Mice lacking bombesin receptor subtype-3 develop metabolic defects and obesity. Nature 390: 165–169, 1997 [DOI] [PubMed] [Google Scholar]
- 28.Oka T, Oka K, Kobayashi T, Sugimoto Y, Ichikawa A, Ushikubi F, Narumiya S, Saper CB. Characteristics of thermoregulatory and febrile responses in mice deficient in prostaglandin EP1 and EP3 receptors. J Physiol 551: 945–954, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, Collins F. Effects of the obese gene product on body weight regulation in ob/ob mice. Science 269: 540–543, 1995 [DOI] [PubMed] [Google Scholar]
- 30.Reitman ML, Dishy V, Moreau A, Denney WS, Liu C, Kraft WK, Mejia AV, Matson MA, Stoch SA, Wagner JA, Lai E. Pharmacokinetics and pharmacodynamics of MK-5046, a bombesin receptor subtype-3 (BRS-3) agonist, in healthy patients. J Clin Pharmacol 52: 1306–1316, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rippe C, Berger K, Boiers C, Ricquier D, Erlanson-Albertsson C. Effect of high-fat diet, surrounding temperature, and enterostatin on uncoupling protein gene expression. Am J Physiol Endocrinol Metab 279: E293–E300, 2000 [DOI] [PubMed] [Google Scholar]
- 32.Schwartz MW, Porte D., Jr. Diabetes, obesity, and the brain. Science 307: 375–379, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Sebhat IK, Franklin C, Lo MC, Chen D, Jewell JP, Miller R, Pang J, Palyha O, Kan Y, Kelly TM, Guan XM, Marsh DJ, Kosinski JA, Metzger JM, Lyons K, Dragovic J, Guzzo PR, Henderson AJ, Reitman ML, Nargund RP, Wyvratt MJ, Lin LS. Discovery of MK-5046, a potent, selective bombesin receptor subtype-3 agonist for the treatment of obesity. ACS Med Chem Lett 2: 43–47, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tartaglia LA, Dembski M, Weng X, Deng N, Culpepper J, Devos R, Richards GJ, Campfield LA, Clark FT, Deeds J, Muir C, Sanker S, Moriarty A, Moore KJ, Smutko JS, Mays GG, Woolf EA, Monroe CA, Tepper RI. Identification and expression cloning of a leptin receptor, OB-R. Cell 83: 1263–1271, 1995 [DOI] [PubMed] [Google Scholar]
- 35.Thomas SA, Palmiter RD. Thermoregulatory and metabolic phenotypes of mice lacking noradrenaline and adrenaline. Nature 387: 94–97, 1997 [DOI] [PubMed] [Google Scholar]
- 36.Whittle A, Relat-Pardo J, Vidal-Puig A. Pharmacological strategies for targeting BAT thermogenesis. Trends Pharmacol Sci 34: 347–355, 2013 [DOI] [PubMed] [Google Scholar]
- 37.Zhang L, Parks GS, Wang Z, Wang L, Lew M, Civelli O. Anatomical characterization of bombesin receptor subtype-3 mRNA expression in the rodent central nervous system. J Comp Neurol 521: 1020–1039, 2013 [DOI] [PubMed] [Google Scholar]