Abstract
A high-calorie diet accompanied by low levels of physical activity (PA) accounts for the widespread prevalence of obesity today, and yet some people remain lean even in this obesogenic environment. Here, we investigate the cause for this exception. A key trait that predicts high PA in both humans and laboratory rodents is intrinsic aerobic capacity. Rats artificially selected as high-capacity runners (HCR) are lean and consistently more physically active than their low-capacity runner (LCR) counterparts; this applies to both males and females. Here, we demonstrate that HCR show heightened total energy expenditure (TEE) and hypothesize that this is due to higher nonresting energy expenditure (NREE; includes activity EE). After matching for body weight and lean mass, female HCR consistently had heightened nonresting EE, but not resting EE, compared with female LCR. Because of the dominant role of skeletal muscle in nonresting EE, we examined muscle energy use. We found that lean female HCR had higher muscle heat dissipation during activity, explaining their low economy of activity and high activity EE. This may be due to the amplified skeletal muscle expression levels of proteins involved in EE and reduced expression levels of proteins involved in energy conservation in HCR relative to LCR. This is also associated with an increased sympathetic drive to skeletal muscle in HCR compared with LCR. We find little support for the hypothesis that resting metabolic rate is correlated with maximal aerobic capacity if body size and composition are fully considered; rather, the critical factor appears to be activity thermogenesis.
Keywords: nonexercise activity thermogenesis, high- and low-capacity runners, energy expenditure, obesity
obesity is increasing worldwide and underlies innumerable associated health problems that lead to decreased quality of life and increased mortality (3, 19, 20, 25, 28, 75). Although the contribution of diet to the obesogenic environment is unquestioned (69), a sedentary lifestyle also has deleterious effects on body weight (BW) and health (8, 15, 33). An individual's level of daily physical activity (PA) is a biologically regulated heritable trait that tends to be associated with leanness (31, 47, 48). We have found that a key feature predicting high PA is intrinsic aerobic capacity; this holds true for both humans and laboratory rodents (35, 37, 39, 56, 63, 96). Rats that have been selectively bred for high aerobic endurance treadmill running capacity [high-capacity runners (HCR)] are consistently more physically active than their low-endurance counterparts selectively bred as low-capacity runners (LCR). Aerobic capacity is a strong predictor of early morbidity and mortality in both men and women (39, 56); in fact, after age, it is the strongest predictor of longevity in men (56). This provides support for the theory that greater capacity for energy transfer at all levels of biological organization underlies reduced risk for complex disease and diminished longevity (aerobic hypothesis). Consistent with this, HCR have a low risk of obesity and associated metabolic disorders as well as enhanced longevity (36, 94); although they were not selected for leanness or obesity per se, HCR and LCR reliably display lean and obese phenotypes, and this is likely to stem from bioenergetic mechanisms underlying complex disease (34). We exploit these traits of a lean, high-endurance phenotype to uncover the mechanisms through which a significant fraction of the human population (roughly one-third) (18) avoids becoming obese even in the present-day obesogenic environment.
The preponderance of evidence points to an individual's “spontaneous” daily activity as an important predictor of obesity resistance (30, 46, 48, 67). Energy expenditure (EE) associated with both voluntary exercise and nonexercise activity thermogenesis (NEAT) is a vital contributor to both weight maintenance and weight loss (46). NEAT accounts for the bulk of activity-associated EE in most individuals (47), and the ability to increase NEAT in the face of additional caloric intake has been shown to be the key trait distinguishing people who resist gaining fat (46). A person's NEAT is determined by their amount of PA, the load they carry, and the fuel cost of that activity. Although NEAT plays an important role in overall EE, little is known about the mechanisms that might explain the variance in NEAT among individuals. Human NEAT is equivalent to all activity thermogenesis in animals in the absence of exercise (61).
LCR are known to have elevated adiposity and low PA, even in the absence of dietary challenge, whereas HCR resist becoming obese (62, 63). Here, we tested the hypothesis that heightened PA and, therefore, NEAT meaningfully augment total energy expenditure (TEE). The dominant contributors to TEE are body size and composition; differences in body sizes between lean and obese phenotypes have hampered accurate comparisons of EE and how this relates to obesity propensity (9, 90). To determine the role of NEAT in this lean, high-capacity phenotype and rule out the confounding effect of BW and composition (49), we measured TEE in male rats as well as a population of female rats of the lean, high-capacity (i.e., HCR) and obesity-prone, low-capacity (i.e., LCR) phenotypes that were equivalent in BW or lean mass. Next, using statistical multilinear modeling, we determined which component(s) of TEE, resting or nonresting EE, differed between lean vs. obesity-prone rats. Heightened NEAT implicates altered skeletal muscle energy use. Therefore, we tested the hypothesis that calories are dissipated during PA as heat energy and investigated several potential molecular mechanisms that could account for this additional EE. Finally, we explored the possibility that differences in activity thermogenesis were accompanied by altered sympathetic nervous system (SNS) outflow to skeletal muscle.
MATERIALS AND METHODS
Adult female HCR/LCR rats (selection generation 25, n = 13/group, 10–12 mo of age and overlapping in body weights) and adult males from generation 27 (n = 16) were obtained from the University of Michigan. Each rat was housed individually on a 12:12-h light-dark cycle with lights on at 0700 eastern standard time. Rats received rodent chow (5P00 MRH 3000; T. R. Last) and water ad libitum. All studies were conducted according to the rules and approval of the Kent State University Institutional Animal Care and Use Committee.
We verified that the weight-matched females selected for this study were generally representative of the phenotypes of generation 25 female rats by comparing results of the aerobic capacity treadmill phenotyping analysis conducted for each rat at 11 wk of age, including body weight and best time, distance and speed, and work performed. T-tests were used to compare the females studied here relative to all females of generation 25.
Body composition.
We measured body composition using Echo MRI-700 (Echo Medical Systems, Houston, TX) to determine the fat and lean mass (in g) of each rat the day before test. This did not interfere with temperature transponder function (see transponder implantation below).
Measurement of TEE and its components.
After body composition determination, adult female HCR and LCR rats were measured for TEE and 24-h PA using small animal indirect calorimetry (4-chamber Oxymax FAST system; Columbus Instruments, Columbus, OH). Although there are sex- and estrous cycle-related differences in metabolism, the phenotypic differences in activity we documented are robust and seen regardless of sex (62) or any variance related to the estrous cycle, which has minimal effects on either resting or nonresting EE (22). Rats were acclimated to the calorimetry chamber (7.5 × 12 × 9 in.) and room for 24–48 h prior to testing; this is sufficient to avoid novelty-induced increases in PA (data not shown). On the day of calorimetry, rats were weighed and placed in the chamber with food and water; the chamber was then sealed. The calorimeter was calibrated using primary gas standards. Air was pumped into the chamber at 1.9–3.1 liters/min, depending on the weight of the rat, and chamber air was sampled at 0.4 liters/min. Measurement of gas exchange took place every 30 s throughout the 24-h period, except for a 3.5-min room air reference and settle period after each 60-sample interval. PA data were collected, using infrared beam-break counts, every 10 s uninterrupted throughout the 24-h period in the x- and z-axes; the 1st hour of data was not included in the analysis. Data collected from 1200 (EST) on day 1 through 1200 on day 2 were analyzed. EE data (V̇o2, V̇co2, respiratory exchange ratio, kcal/h) were averaged, and PA data were expressed as mean beam breaks per minute. BW and food intake (FI) data indicate that rats were in homeostatic energy balance during EE measurements. For male rats, FI was similar during the EE measurement (20.58 ± 0.64; HCR, 21.41 ± 0.77; LCR, 19.19 ± 1.02) compared with normal daily FI (20.53 ± 0.69; unpaired 2-tailed t-test P value = 0.96; HCR, 20.86 ± 0.66; LCR, 19.98 ± 1.55), and BW did not change significantly compared with home cage days (unpaired 2-tailed t-test P value = 0.972). For females, average BW was slightly lower after calorimetry; this is not surprising given that their initial BW is measured at the peak of the daily rhythm (early light phase) and the second measurement is taken at the nadir (late light phase). Change in BW did not differ between groups (HCR, −1.84 ± 1.13 g; LCR, −1.94 ± 0.094 g; t-test P value = 0.95). Female FI was normal for their size and sex, and HCR ate significantly more than LCR for the duration of calorimetry (HCR, 19.93 ± 1.24 g; LCR, 15.60 ± 0.86 g; t-test, P value = 0.01).
Next, we separated the nonresting EE and resting EE components of TEE data described above using Columbus Instruments software (CLAX). Using the data set described above, resting EE was defined as the lowest level of EE, excluding the five lowest episodes (i.e., short sequences of consecutive data points) to prevent potential bias due to variation in gas exchange values that can occur during the switch from sample to reference air measurement. We did not use a consistent time period to define resting EE, as others have done (11, 22, 52), because the high-resolution activity (every 10 s) and EE (every 30 s) recordings revealed that rats were rarely inactive for 30 consecutive minutes or more and that these periods did not reliably fall at the same time of day among rats. Moreover, these sedentary periods were often marked by heightened residual EE from recent PA. This same method was used to define resting EE during the treadmill test (see below). Because each rat had two separate resting EE values calculated within a short period of time (one for 24-h EE, one before treadmill walking), we compared the two values for each rat to validate our method. The two resting EE measurements were very similar: resting EE from the 24-h calorimetry for HCR was 1.23 ± 0.04 kcal/h, and resting EE from the 2-h measurement was 1.29 ± 0.04 kcal/h; for LCR, the values were 1.19 ± 0.05 and 1.18 ± 0.06 kcal/h, respectively. Nonresting EE is defined as (TEE) − (resting EE).
As reported previously (62), resting and PA EE were directly assessed by measuring gas exchange once every 10 s during a treadmill activity test. At least 1 day after a 15-min treadmill acclimation period, rats were placed in the treadmill and allowed to acclimate without food for 2 h. Given the time of day, 2 h without food is likely to be sufficient to avoid the thermic effect of food from what little the rats may have eaten prior to this time during the light phase of the cycle (29); however, thermic effect of food could not be quantified during the 24-h EE measurement. Over the course of this 2-h period, rats showed a predictable pattern of behavior; PA and EE rise markedly then fall gradually, reaching a steady state. After the 2-h resting period, the treadmill was started at 7 m/min for 30 min, during which time steady-state activity EE data were collected. Rats that did not show adequate resting or activity (walking backward on the treadmill, sitting on the treadmill or shocker) were measured at a later date.
All EE data from both males and females were analyzed using t-tests, ANOVA, and analysis of covariance (ANCOVA); however, males and females were not compared directly with each other. Because our previous studies suggested the hypothesis that EE was elevated in HCR, we compared resting and nonresting EE between weight-matched or lean mass-matched HCR and LCR using a one-tailed paired t-test. An unpaired one-tailed t-test was used to compare treadmill gas exchange values between groups based on our previous results. Finally, for both male and female rats, TEE, resting EE, and nonresting EE were analyzed using ANCOVA with either body mass or lean mass as the covariate; interaction terms were removed from the analysis if not significant. Although lean mass is regarded as the best covariate for analyzing resting EE or total EE (90), activity EE is better evaluated using body weight (74). Thus resting and nonresting EE were analyzed using lean mass and body weight as covariates in separate analyses to allow full comparison.
Statistical modeling.
EE data were subjected to modeling using multiple linear regression to determine what parameters/factor(s) best predicted TEE, resting EE, and nonresting EE in HCR compared with LCR. For each of the six combinations of phenotype (HCR, LCR) and EE (TEE, resting EE, nonresting EE), we included BW, fat mass, lean mass, and activity level (horizontal activity counts) as predictors in the initial analysis. To more effectively narrow the relevant predictors of EE, and to avoid overfitting of the data as well as overlap in predictors (i.e., fat and lean mass as components of BW), the data were then reanalyzed after any nonsignificant factors were removed from the analysis. Analysis was done using MS Excel 2010 (www.microsoft.com).
Transponder implantation.
Adult female HCR/LCR rats (n = 7/group, a subset of actual group mentioned above) were anesthetized using isoflurane. A short incision was made on both hindlegs. Sterile temperature transponders (IPTT-300; Bio Medic Data Systems) were implanted adjacent to the gastrocnemius (gastroc) muscle group of both hindlimbs to measure the heat generated by skeletal muscle during activity. Care was taken to place the transponders so as not to disrupt locomotor function. Rats were allowed to recover for 1 wk before the graded treadmill test was performed. Implant placement was examined during tissue harvest, and data from inaccurately placed transponders were omitted from the final analyses.
Graded treadmill test.
To determine skeletal muscle heat dissipation during controlled PA, we measured the temperatures of the gastroc muscle group using a graded treadmill exercise test. The rats were acclimated to the treadmill for 10 min in the days prior to the test as well as immediately before the test. Gastroc temperatures in each leg were recorded at baseline and at set intervals during a five-level graded treadmill test. Starting at 7 m/min, 0° incline, temperature was measured at 2, 5, and 10 min, 15 min (9 m/min at 0° incline), 20 min (9 m/min, 10° incline), 25 min (11 m/min, 10° incline), and 30 min (11 m/min, 20° incline). The test was stopped at end of 35 min; however, some LCR were not able to complete all five levels of activity.
Muscle gene expression.
Skeletal muscle [gastroc and quadriceps (quad)] was collected from adult female HCR/LCR rats (from a separate group, generation 25; n = 8 or 7/group) after euthanization by rapid decapitation without anesthetic agents after several weeks without exercise. Muscle samples were homogenized, and total mRNA was extracted using the Ambion ribopure kit, following the manufacturer's instructions. The purity of mRNA was measured using Nanodrop (ND-1000; Nanodrop Technologies) and A260/280 ratio ranging from 1.8 to 2.1. This mRNA was used to prepare cDNA using an Applied Biosystems kit and thermal cycling at 25°C for 10 min, 48°C for 30 min, 95°C for 5 min, and holding at 4°C. The cDNA was used for quantifying the expression of uncoupling proteins (UCP) 2 and 3, ATP-dependent potassium channel (K+ATP subunits Kir6.1, Kir6.2), mediator of RNA polymerase II transcription subunit 1 (MED1), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH; used as a control). The relative expression was calculated using comparative CT method (ΔCT). Data are expressed as a percent expression, using the HCR as the reference value (defined as 100%), and groups were compared using a two-tialed t-test.
Western blotting.
UCP2, UCP3, and the K+ATP subunits Kir6.1 and Kir6.2 (found predominantly in skeletal muscle) and MED1 were examined in skeletal muscle (gastroc and quad) from adult female HCR/LCR rats (same group of generation 25 rats used for muscle gene expression; n = 5 or 4/group), using actin as a loading control for protein expression. Skeletal muscle samples were homogenized with ice-cold RIPA buffer (Thermo Scientific) containing a protease inhibitor cocktail (Roche Diagnostics). The supernatant from the homogenization and subsequent centrifugation was used for the analysis. Equal quantities of supernatant and sample buffer (150 mM Tris·HCl, pH 6.8, Trizma base for pH, 6% SDS, 30% glycerol, 0.03% pyronin-Y, DTT) were mixed, and tubes were heated at 90°C for 3 min. Samples containing equal quantities of protein were loaded on to a gradient gel (4–15%; Bio Rad) and electrophoresed using SDS running buffer (0.384 M glycine, 0.05 M Trizma base, 0.1% SDS) at constant voltage (150 V) for 30 min. The gel was blotted on to a PVDF membrane using semi-wet blotting apparatus and Otter et al. (64a) transfer buffer (49.6 mM Trizma base, 384mM glycine, 17.5% methanol, 0.01% SDS) at constant current (400 mAmp). The blot was incubated overnight in a blocking solution of 5% milk (Blotto) in 1× PBST (phosphate-buffered saline; 84 mM sodium hydrogen phosphate, 16 mM sodium dihydrogen phosphate, 100 mM sodium chloride, and Tween-20) and then rinsed using 1× PBST. Primary antibodies for UCP2, UCP3, actin, and MED1 (Abcam ab67241, ab3477, ab1801, and ab60950 respectively) and for Kir6.1 and Kir6.2 (sc-11224 and sc-11230, respectively; Santa Cruz Biotechnology) were diluted in blocking solution at a ratio of 1:1,000 (Kir6.2, Kir6.1, and MED1 at 1:500) and incubated with the blot overnight. Secondary antibodies were diluted in blocking solution at a ratio of 1:5,000 and incubated for 1 h at room temperature. After washing, the blots were developed with a chemiluminiscence detector, using an Amersham kit (GE Healthcare). The expression levels relative to actin were plotted as a percent of the reference value (with HCR as 100%), and groups were compared using a two-tailed t-test.
Norepinephrine turnover.
Adult male HCR/LCR rats (generation 30; n = 13 HCR and 12 LCR) were used to assess sympathetic drive to skeletal muscle (including quad, lateral and medial gastroc, and soleus) and brown adipose tissue (BAT). They were individually housed on a 12:12-h light-dark cycle with ad libitum access to standard rodent chow and water and acclimated to daily handling for 1 wk. Level of sympathetic drive to peripheral tissues was determined by norepinephrine (NE) turnover (NETO) method using α-methyl-p-tyrosine (aMPT) (76). aMPT is a competitive inhibitor of tyrosine hydroxylase, the rate-limiting enzyme in catecholamine biosynthesis. After aMPT administration, the endogenous tissue levels of NE decline at a rate proportional to the initial NE concentrations. The rats were divided into two subgroups per group (aMPT and control). On the day of study, rats receiving aMPT were injected with aMPT (125 mg aMPT/kg of BW, 25 mg/ml) and with a booster dose at same concentration 2 h later. All rats were euthanized by rapid decapitation between 1200 and 1500, 4 h after first aMPT injection. Tissues were rapidly dissected and snap-frozen in liquid nitrogen.
Briefly, tissue was thawed and homogenized in a solution containing dihydroxybenzylamine (internal standard) in 0.2 M perchloric acid (PCA) with 1 mg/ml ascorbic acid (AA). Following centrifugation for 15 min at 7,500 g at 4°C, catecholamines were extracted from the homogenate with alumina and eluted into the PCA/AA. The catecholamines were assayed using an HPLC system with electrochemical detection (Coulochem III), MDTM mobile phase, and a reverse-phase MD 150 × 3.2 column. NETO in quad, lateral and medial gastroc, BAT, and soleus were calculated using the following formula (76): k = (lg[NE]0 − lg[NE]4)/(0.434 × 4), K = k[NE]0, where k is the constant rate of NE efflux (also known as fractional turnover rate), [NE]0 is the initial NE concentration or from 0-h group (control), [NE]4 is the final NE concentration or from 4-h group (aMPT), and K = NETO.
Difference in NETO of HCR and LCR tissues were compared using a one-tailed, unpaired t-test for each tissue.
RESULTS
Rats of the lean, high-activity phenotype have higher TEE and nonresting EE but not consistently high resting EE.
Results of t-tests supported the assertion that both the weight-matched female HCR and LCR measured in this study were representative of the larger populations (all other female HCR and LCR from generation 25). Compared with other generation 25 female HCR, the female HCR in this study weighed significantly more (+9.6 g, P = 0.03) but showed equivalent performance on all aerobic capacity variables (best time, distance, and speed, P > 0.30 for all), with the exception of work (Joules), which was greater in the weight-matched HCR (P < 0.05), a consequence of their larger BW. The LCR in this study weighed significantly less than the other female LCR of generation 25 (−13.7 g, P < 0.01), but there were no other differences between this group and other female LCR in generation 25 in work, best time, distance, or speed during the treadmill test.
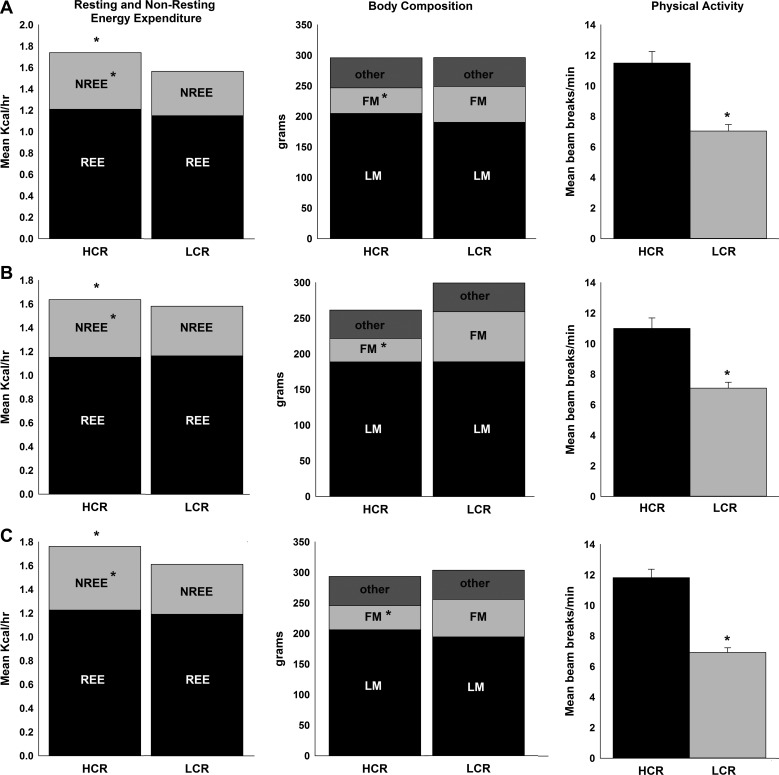
As shown in Fig. 1, female HCR had significantly higher TEE when rats were matched for either BW (Fig. 1A) or lean mass (Fig. 1B) or when they were analyzed as a group (Fig. 1C). To parse the components of TEE (i.e., resting EE + nonresting EE), we first validated our method to estimate resting EE from the 24-h calorimetry dataset using the 2-h treadmill test described above (62). The two methods of calculating resting EE did not differ significantly (t = −0.64, P = 0.53), supporting the validity and accuracy of our resting EE calculation from TEE data. After matching for BW, total energy cost of activity (treadmill walking) was higher in HCR (3.09 ± 0.10 kcal/h) compared with the LCR (2.88 ± 0.08 kcal/h).
Fig. 1.
High-capacity runners (HCR) have heightened total energy expenditure (TEE) and nonresting energy expenditure (NREE), but not resting energy expenditure (REE), when matched for body weight (BW; A), for lean mass (LM; B), and/or by group (C). Daily physical activity was higher in HCR when matched for BW, for LM, or by group. HCR and low-capacity runners (LCR) show significant differences in fat mass (FM) but not in LM or BW when analyzed by group (C) or when weight matched (A). *P < 0.05 (HCR ≠ LCR). All data are means ± SE (n = 8/group for BW and LM matched; n = 13/group for TEE by group).
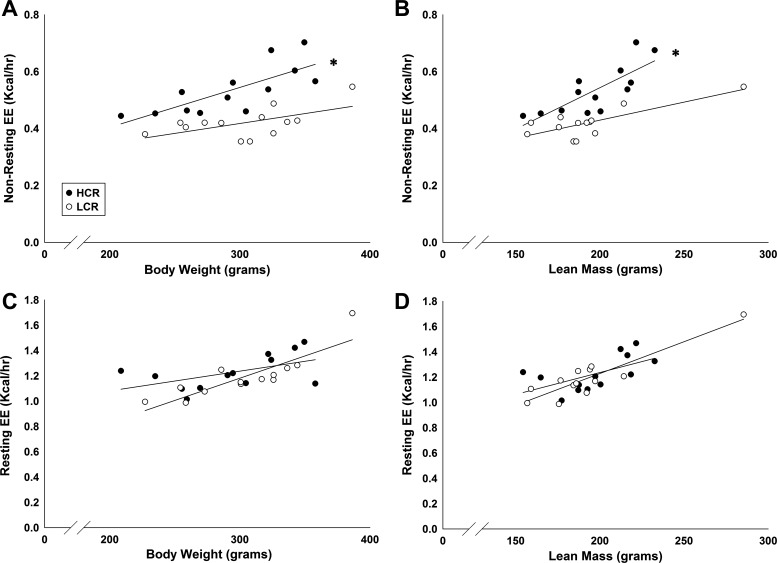
Resting EE over the 24-h test did not differ between female HCR and LCR either as a group (without matching; Fig. 1C) or when matched for lean mass (Fig. 1B). Nonresting EE was significantly higher in female HCR in every analysis performed after matching for either lean mass or BW or without matching (Fig. 1). The heightened nonresting EE in the lean, high-capacity phenotype (HCR) was not secondary to a higher workload, as fat mass and/or total mass were higher in the LCR in each analysis. PA, both horizontal and ambulatory activity, was also higher in female HCR than LCR in every analysis performed (Fig. 1). ANCOVA analyses of EE data from female HCR/LCR revealed that BW or lean mass were the dominant determinants of resting EE, as resting EE was consistently higher in rats with greater BW or lean mass (Fig. 2). Finally, nonresting EE increased with higher BW or lean mass, and HCR consistently had higher nonresting EE compared with LCR, even after BW or composition were considered using ANCOVA (Fig. 2).
Fig. 2.
In all rats, nonresting energy expenditure (EE) increased along with BW (A) and LM (B); NREE was significantly higher in the lean phenotype (HCR) compared with LCR, even after LM and BW were factored out. REE also increased with BW (C) and LM (D) but did not differ significantly between female HCR and LCR. (n = 13/group). *P < 0.05, HCR > LCR.
Table 2 contains the results of the multiple linear regression analyses of TEE and its components in female HCR and LCR. In general, lean mass was the dominant predictor of all EE variables; however, lean mass tended to be a better predictor in LCR than in HCR. PA also contributed significantly to predicting TEE and nonresting EE in both lines of rats. Overall, there was more unaccounted-for variance in TEE and resting EE in HCR compared with LCR.
Table 2.
Results of statistical modeling using multiple linear regression analysis to determine which factors (body mass, lean mass, fat mass, or physical activity) best predicted energy expenditure
| LCR |
HCR |
|||||
|---|---|---|---|---|---|---|
| Predictive factors | r2 | Significance of model or factor (P) | Best predictor | r2 | Significance of model or factor (P) | |
| TDEE | ||||||
| Best predictive model | Lean mass + physical activity | 0.878 | <0.001 | Lean mass + physical activity | 0.637 | 0.006 |
| Within model | Lean mass | <0.001 | Lean mass | 0.093 | ||
| Physical activity | 0.099 | Physical activity | 0.091 | |||
| REE | ||||||
| Best predictive model | All factors | 0.880 | <0.001 | Lean mass | 0.347 | 0.034 |
| Best single predictor | Lean mass | 0.819 | <0.001 | |||
| NREE | ||||||
| Best predictive model | Lean mass + physical activity | 0.791 | <0.001 | Body weight + physical activity | 0.782 | <0.001 |
| Within model | Lean mass | <0.001 | Body weight | 0.010 | ||
| Physical activity | 0.012 | Physical activity | 0.011 | |||
TDEE, total daily energy expenditure; REE, resting energy expenditure; NREE, nonresting energy expenditure.
Each component was modeled separately for each of the lean (HCR) and obesity-prone (LCR) phenotypes.
As illustrated in Fig. 2, ANCOVA analysis of EE data from male HCR/LCR revealed that, as in females, both BW and lean mass strongly affected TEE, resting EE, and nonresting EE. As with the females, male HCR showed greater TEE relative to LCR with either BW or lean mass as the covariate. Nonresting EE was also higher in male HCR compared with male LCR with either BW or lean mass as the covariate. However, unlike in females, the male HCR also showed a slight but significant elevation in resting EE compared with LCR.
High-activity EE is accompanied by skeletal muscle energy dissipation as heat.
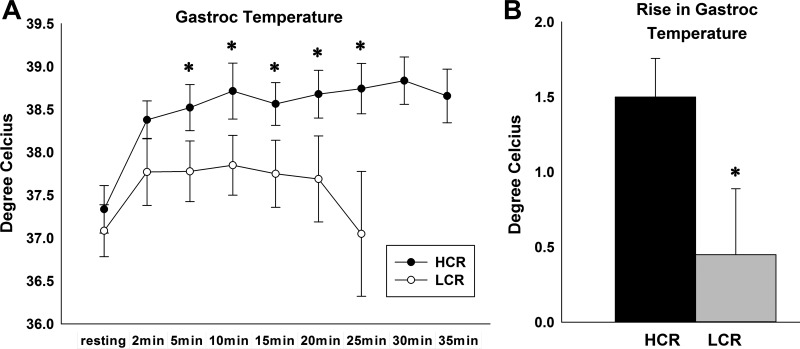
Our analysis of EE clearly implicated nonresting EE (composed primarily of activity EE) more than resting EE as the predominant contributor to the heightened TEE seen in the lean phenotype. This, along with previous data showing decreased economy of activity in HCR (62), strongly implicated differential skeletal muscle energy use as an underlying cause of differences in nonresting EE and, therefore, TEE. The question remained as to how the HCR dispose of these additional calories. Here, we tested the hypothesis that HCR ultimately expend calories through heat dissipation in skeletal muscle. This hypothesis was tested by measuring gastroc temperature during a graded exercise treadmill test equalizing workload between rats (matched by BW). HCR showed significantly higher gastroc temperatures, and their maximal rise in temperature was significantly higher in HCR compared with LCR (Fig. 3), demonstrating that HCR have heightened skeletal muscle heat dissipation compared with LCR.
Fig. 3.
HCR have heightened muscle heat dissipation during physical activity. A: mean gastrocnemius (gastroc) temperature during a graded treadmill exercise test. HCR show higher gastroc temperatures throughout the test period compared with LCR, with the exception of resting. B: maximal rise in gastroc temperature was significantly higher in HCR compared with LCR during the graded treadmill test. *P < 0.05 (HCR > LCR). All data are means ± SE (n = 7/group).
Lean, high-activity rats have higher expression of mRNA and proteins involved in energy expenditure and lower expression of energy-conserving processes in both quadriceps and gastrocnemius.
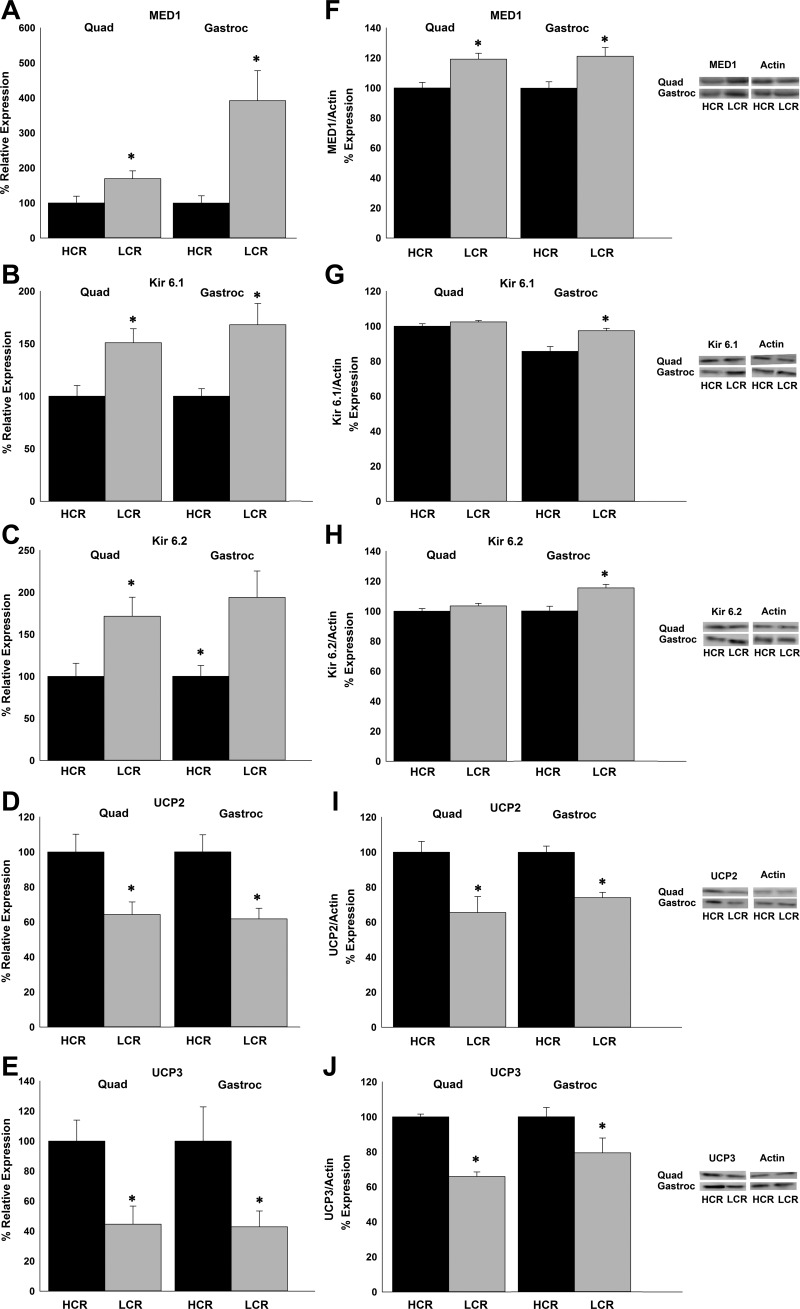
To determine the source of the calorie use and heat dissipation in HCR skeletal muscle, we examined mRNA and protein expression of molecular end points known to alter energy use in quad and gastroc of HCR and LCR; for energy conservation, we examined MED1 (12) and K+ATP (subunits Kir6.1 and Kir6.2) (2, 59); for energy expenditure, we examined UCP2 and UCP3 (71). The mRNA content of UCP2 and UCP3 was found to be higher in HCR compared with LCR; that of potassium channel subunits Kir6.1 and Kir6.2, as well as MED1, was higher in LCR compared with HCR (Fig. 4). Protein expression levels of proteins involved in energy expenditure (UCP2 and -3) were found to be higher in HCR compared with LCR in both quad and gastroc. Protein expression levels of MED1 were higher in LCR compared with HCR in both quad and gastroc (Fig. 4). No differences were found in protein levels of K+ATP subunits Kir6.1 and Kir6.2 in the quad.
Fig. 4.
Compared with LCR, HCR have lower expression of molecular mediators of energy conservation in skeletal muscle [quadriceps (quad) and gastroc], including mediator of RNA polymerase II transcription subunit 1 (MED1; A and F) and subunits of the ATP-gated K+ channels (K+ATP) Kir6.1 (B) and Kir6.2 (C). G and H: Kir6.1 and Kir6.2 protein levels were also higher in gastroc but not quad. Uncoupling protein (UCP)2 and UCP3 mRNA expression (D and E) and protein levels (I and J) were significantly higher in skeletal muscle of HCR compared with LCR. *P < 0.05 (HCR > LCR for UCP2 and UCP3; HCR < LCR for Kir6.1, Kir6.2, and MED1). All data are means ± SE (n = 7–8 for mRNA expression; n = 4–5 for protein expression).
Lean rats have elevated SNS drive to skeletal muscle.
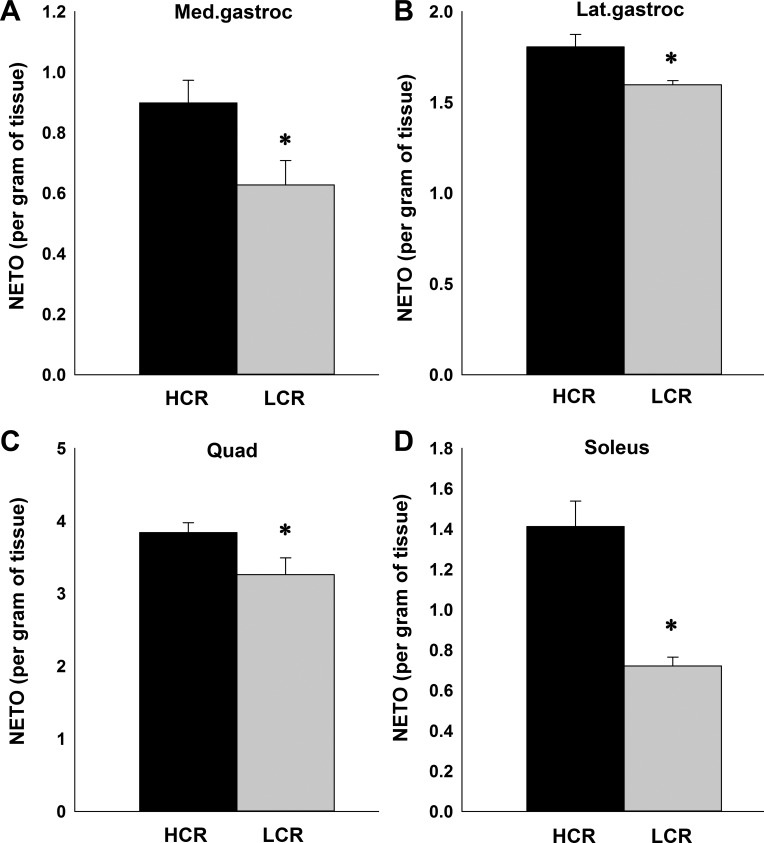
As illustrated in Fig. 5, compared with LCR, HCR had higher NETO in several skeletal muscle groups, including quad (Fig. 5C), medial (Fig. 5A) and lateral gastroc (Fig. 5B), and soleus (Fig. 5D), indicating higher sympathetic drive to skeletal muscle, potentially modulating their economy of activity. Higher NETO was also found in interscapular BAT in HCR (104.08 ± 9.8) compared with LCR (29.17 ± 2.35; 1-tailed, unpaired t-test, P value < 0.0001).
Fig. 5.
Compared with LCR, HCR have elevated sympathetic drive indicated by higher norepinephrine turnover (NETO) in the following skeletal muscle groups: medial gastrocnemius (A), lateral gastrocnemius (B), quadriceps (C), and soleus (D). *P < 0.05 (HCR > LCR). All data are means ± SE (n = 7 HCR and 6 LCR).
DISCUSSION
It is known that lean people and lean animals have higher daily PA levels (30, 48, 63, 64) and that this is a biologically regulated trait (31, 47). However, it is not known whether or not this physical activity energy expenditure, or NEAT, meaningfully contributes to TEE. Here, we use a rat model of a lean phenotype to demonstrate that lean rats show heightened TEE and that this is due primarily to elevated nonresting EE. Taken together with previous studies (62, 63), we have shown that the amplified NEAT characteristic of this lean phenotype is due predominantly to increased daily activity levels combined with increased fuel cost of that activity. The increased calorie use may be secondary to heightened function of muscle UCPs combined with decreased function of K+ATP and MED1 potentially driven by sympathetic outflow to skeletal muscle.
Comparing EE in animals where the phenotypes differ in BW or composition has been an area of disagreement, causing some confusion due to overcorrection for BW or misinterpretation of data (9, 65, 90). One strategy to compare EE between lean vs. obesity-prone groups is to obtain individuals of each phenotype that overlap in BW or lean mass. Here, we used this strategy to compare TEE and its components in HCR and LCR. This necessitated using female rats, as the male HCR and LCR have minimal overlap in weight between groups; the weight-matched females accurately represented their phenotype except for BW. Relative to female LCR, female rats of the lean, high-capacity phenotype (HCR) did not show significantly elevated resting EE. Instead, the lean, high-capacity rats consistently displayed significantly heightened nonresting EE (Fig. 2). This persisted when the groups were matched for BW or lean mass or without matching (Fig. 1 and Table 1). This was consistent with ANCOVA analyses, which demonstrated that nonresting EE was consistently higher in female HCR, even after BW and lean mass were considered as covariates, without any detectable difference in resting EE (Fig. 2).
Table 1.
Physical activity and energy expenditure in rats of the lean (HCR) and obesity-prone (LCR) phenotype
| All rats |
Matched by Body Weight |
Matched by Lean Mass |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Body weight, g | Lean mass, g | Fat mass, g | Total counts | Ambulatory counts | Body weight, g | Lean mass, g | Fat mass, g | Total counts | Ambulatory counts | Stereotypical counts | Body weight, g | Lean mass, g | Fat mass, g | Total counts | Ambulatory counts | |
| Female | ||||||||||||||||
| HCR | 293.4 ± 12.8 | 206.27 ± 7.92 | 39.39 ± 4.29* | 11.82 ± 0.55* | 4.93 ± 0.35* | 295.99 ± 11.5 | 204.96 ± 6.91* | 41.85 ± 5.58* | 11.49 ± 0.75* | 4.86 ± 0.5* | 1.88 ± 0.01* | 264.74 ± 11.44* | 188.95 ± 6.3 | 32.77 ± 3.97* | 10.99 ± 0.69* | 4.44 ± 0.42* |
| LCR | 303.3 ± 12.0 | 194.26 ± 8.32 | 61.32 ± 6.16 | 6.92 ± 0.31 | 2.7 ± 0.23 | 296.06 ± 11.9 | 190.33 ± 5.73 | 58.66 ± 6.7 | 7.04 ± 0.43 | 2.9 ± 0.34 | 0.77 ± 0.14 | 307.89 ± 12.13 | 189.12 ± 6.11 | 70.2 ± 7.74 | 7.09 ± 0.38 | 2.94 ± 0.33 |
| Male | ||||||||||||||||
| HCR | 414.7 ± 12.2* | 292.6 ± 9.2* | 59.2 ± 6.1* | 6.4 ± 0.17* | 2.11 ± 0.09* | |||||||||||
| LCR | 610.5 ± 42.6 | 339.5 ± 20.3 | 193.4 ± 19.4 | 4.7 ± 0.28 | 1.53 ± 0.09 | |||||||||||
Data are means ± SE.
HCR, high-capacity runners; LCR, low-capacity runners.
P < 0.05, HCR ≠ LCR.
Although we were not able to compare weight-matched male HCR and LCR, the results of ANCOVA analysis from male rat EE data were similar to females. BW and lean mass are the strongest determinants of TEE and each of its components (resting and nonresting EE), and male HCR had higher nonresting EE than LCR even after lean mass and BW were considered in the analyses. However, unlike the results from female rats, male HCR had slightly but significantly elevated resting EE compared with male LCR even after lean mass was factored into the analysis. Overall, this gives little support for the hypothesis that there is a meaningful deficiency in resting metabolic rate in the obese. Rather, our results support the assertion that nonresting EE, including physical activity EE, is consistently elevated in the lean phenotype associated with high aerobic capacity, regardless of sex, even after individual differences in body mass and composition are taken into account. This is in line with elevated activity EE observed in other models of leanness (40).
Although ANCOVA can be used to account for group differences in BW or lean mass (90), even this method is not universally accepted (53, 97). We used regression analyses to model the contribution of body mass and its components, as well as PA, to TEE as well as to resting and nonresting EE in female HCR and LCR. As shown in Table 2, the predictive factors more effectively modeled EE in LCR compared with HCR; models that included lean mass and PA best predicted TEE in both HCR and LCR, although lean mass appeared to be more important in LCR, and activity in HCR. Lean mass was the single best predictor of resting EE in both HCR and LCR. PA level was a more important predictive factor for nonresting EE. In LCR, the ability of PA to predict nonresting EE was dependent partially on lean mass, which appeared to be the critical factor determining nonresting EE in LCR. This was not the case for HCR, where PA alone significantly predicted nonresting EE, and this was augmented by considering workload (i.e., BW). This suggests the possibility of an additional factor that contributes to nonresting EE in HCR that was not accounted for in our analyses, a factor that alters the efficiency or economy of locomotion (62), making activity EE less predictable in HCR than in LCR. It is conceivable that economy of activity is differentially modulated in HCR than in LCR, potentially by sympathetic outflow, resulting in greater variance in activity EE in HCR. The ability to use PA levels to predict nonresting EE was likely to be hampered by the uniquely low variance in daily activity when considering only HCR or only LCR (62, 63). Differences in thermic effect of food may also contribute to inter-individual variance in EE.
Several investigations have addressed the extent to which EE, particularly basal metabolic rate (BMR) or related measurements of resting or sleeping metabolic rates, differs among individuals and how this contributes to the tendency to become obese (67, 93). Answering this question requires accurate measurement of BMR as well as precise consideration of the well-known major determinants of BMR, namely body mass (primarily lean mass) and age (11, 23, 24, 66, 87). There is no consensus regarding exactly how much BMR varies in the population after accounting for these factors, but most studies estimate that lean mass alone accounts for 72% or more of interindividual variance in BMR; fat mass and age also independently predict BMR (17). Therefore, it is not a surprise that studies meticulously measuring BMR and body composition find little unaccounted-for variance in BMR that could explain obesity propensity (67, 93). Our statistical modeling of EE reaffirmed the dominance of lean mass as determinants of TEE and resting EE (Table 2); obesity-resistant rats had minor (males) or undetectable (females) differences in resting EE (Figs. 1 and 2). Nonresting EE, on the other hand, is consistently elevated in lean rats. These analyses also supported the importance of PA level as a determinant of TEE and nonresting EE, particularly nonresting EE in HCR. As we have established previously, in both males and females, HCR are consistently more active than LCR (62, 63). We now show that this difference persists even when BW and composition are nearly identical (in female rats; Fig. 1), indicating that lower daily PA levels in LCR are not due to the any difficulty in locomotion incurred by greater body mass but are inherent to the obesity-prone, low-aerobic capacity phenotype. This is true of both ambulatory and stereotypical PA (Table 1). This reflects what many studies have established regarding leanness in humans, namely the link between high aerobic capacity, high PA and NEAT, and favorable metabolic health (16, 32, 46, 48, 62, 63, 80).
NEAT (i.e., activity EE) is the component of TEE that is consistently different between lean and obesity-prone individuals, and this is elevated in obesity-resistant rats through higher levels of PA combined with decreased fuel economy of activity (Fig. 1 and Table 1) (47, 62). Skeletal muscle is the major contributor to activity EE and also significantly impacts resting EE (98). Therefore, the logical next step was to investigate cellular and molecular mechanisms that differentially regulate NEAT in skeletal muscle of these phenotypes. Specifically, what is the fate of the “extra” calories being burned during NEAT in HCR (62)? In an attempt to answer this question, we hypothesized that excess calories used during the less-economical NEAT in the lean, high-capacity phenotype are being dissipated as heat, which is analogous to the fuel inefficiency that occurs during BAT thermogenesis (43, 70, 83). Hindlimb gastroc muscle temperature was significantly higher in HCR than LCR during physical activity of equivalent workload (treadmill speed and incline, similar BW; Fig. 3). This supports the hypothesis that the “wasted” calories utilized for nonresting EE are being used by muscle, specifically for NEAT, and at least some of the energy is being dissipated as heat. This is consistent with the work of others demonstrating relatively inefficient coupling in HCR mitochondria specifically in HCR skeletal muscle (44, 45, 58, 82, 91). This is also consistent with reports that, relative to LCR, HCR have enhanced muscle glucose uptake, glucose and lipid oxidation, and higher muscle glycogen (68). Obesity-related differences in walking or running economy in humans is a matter of some contention. However, studies in both athletes and nonathletes support the idea that those with higher V̇o2 max (i.e., aerobic capacity) have lower economy of activity (50, 73). This relative inefficiency may be a trade-off for enhanced aerobic capacity.
We predicted that the lean vs. obesity-prone phenotypes would exhibit differential expression of molecular mediators of energy consumption in myocytes. We examined K+ATP channels, which are important in determining the metabolic state of the cell by maintaining potential gradient for ATP synthesis (2, 59). Given that mice deficient in K+ATP channels are lean and have lower fuel economy of activity (2), similar to HCR, we predicted that lean, high-NEAT HCR would show low expression of K+ATP channels in skeletal muscle compared with obesity-prone LCR. Consistent with this hypothesis, Kir6.2, the predominant subtype of the K+ATP channel in muscle (1, 13, 59, 77), showed dampened levels of expression in gastroc of HCR (Fig. 4). In addition, a component of the mediator cofactor complex, MED1 (38, 95), also showed lower levels of expression in the muscle of HCR than LCR, particularly in gastroc (Fig. 4). This component of mediator cofactor complex is associated with several nuclear receptors involved in the transcription of genes involved in fatty acid oxidation (38, 95) and is hypothesized to mediate myocyte fuel conservation (12). Low levels of both MED1 and K+ATP channels are consistent with the compromised economy of activity and increased heat generation of HCR muscle, likely through altered control of fatty acid oxidation (2); however, assessments of channel function are needed to directly test this hypothesis.
Similarly to BAT, skeletal myocytes express UCPs (UCP2 and -3). UCPs play an important role in uncoupling ATP generation and in proton leak across mitochondrial membranes, opposing the function of K+ATP channels in the cell. Here, we found that lean rats have consistently heightened expression of both UCP2 and UCP3 in skeletal muscle (both quad and gastroc) compared with the obesity-prone LCR (Fig. 4), consistent with what has been reported by others in male rats (44, 45, 82). There is no consensus on the role of UCPs, especially UCP3, in skeletal muscle, although they are hypothesized to facilitate fatty acid translocation or mitigation of reactive oxygen species (6, 14, 26, 27, 57, 72). It is unknown whether the uncoupling affects efficiency or thermogenesis. Altogether, the data support a theoretical model in which the myocytes of the lean phenotype have increased use of metabolic fuels, particularly fatty acids, through heightened UCP function along with reduced ability to conserve fuel through MED1 and K+ATP channels.
Our findings using a contrasting genetic model system support a role for differential activity-related skeletal muscle thermogenesis in maintaining leanness and identify potential molecular mechanisms underlying this; the data also identify a potential source of these differences. It is possible that the central nervous system modulates muscle fuel efficiency through the SNS in a fashion analogous to what has been documented in other systems, such as BAT (10). As illustrated in Fig. 5, compared with LCR, HCR were found to have significantly higher skeletal muscle NETO, an indicator of SNS drive (4). It is possible that muscle fuel uptake and utilization are modulated through the SNS and controlled by central nervous system effectors (60, 78), possibly including brain orexigenic and anorexigenic peptides that are known to act in the paraventricular nucleus to affect muscle uncoupling protein levels (41, 42, 92). Brain melanocortins have been found to impact muscle lipid mobilization and glucose uptake (60, 85, 89), and the lean and obesity-prone rats show differences in central melanocortins (79), a system recognized to modulate SNS drive (55, 81). Taken together with previous evidence, these data are consistent with a model in which brain systems (e.g., melanocortins) modulate SNS outflow and myocyte β-adrenoreceptors to increase myocyte glucose and fatty acid uptake and utilization, amplifying energy expenditure of activity (i.e., NEAT) (54, 78, 85, 88, 89). Similar to skeletal muscle, we also found that NETO was higher in BAT of HCR compared with LCR. However, effects of BAT thermogenesis on EE would not be expected to preferentially affect nonresting EE, as was found in our female HCR.
The data described here support a theoretical model where the modulation of metabolic fuel use of skeletal myocytes, potentially through enhanced SNS drive, results in increased or decreased fuel efficiency during locomotion. This predominantly impacts nonresting EE, specifically NEAT, rather than resting or basal metabolism, particularly in female rats (Fig. 3). This has implications for how we consider metabolism when attempting to prevent or treat obesity. Targeting of pathways maximizing skeletal muscle energy use during physical activity may take advantage of already existing mechanisms that are endogenously employed to a greater extent in naturally lean people.
PERSPECTIVES
Apart from the implications regarding obesity and human health, these data can also be viewed form an evolutionary perspective. The lean, high-NEAT rats were derived originally through artificial selection for intrinsic aerobic capacity (35). This selective breeding was based on the hypothesis that differences in aerobic capacity underlie the root causes of complex disease (7). Our results not only support the link between oxygen use and disease (specifically obesity and metabolic disease) but also speak to the hypothesis that endothermy evolved through selection for maximal aerobic capacity and that resting or basal metabolism is necessarily elevated in association with aerobic capacity (5, 84). Our data do not support the supposition that maximal and resting metabolic rates are inexorably linked, aside from the fact that both resting and maximal metabolic rates increase along with body size and lean mass. The HCR were selected specifically for high maximal aerobic capacity and LCR for low capacity (35), yet HCR and LCR had nearly identical resting metabolic rates once differences in lean mass were effectively factored out using lean mass-matched female rats (Fig. 2). Maximal aerobic capacity and resting metabolism must be at least somewhat independently modulated (21). Finally, it is conceivable that the original selection for maximal aerobic capacity was associated with heightened resting metabolism, but the two aspects of metabolism have since been dissociated.
GRANTS
This work was supported by National Institutes of Health (NIH) Grants R01-NS-055859 and R15-DK-097644 and American Heart Association (AHA) Grant GIA 410805 to C. M. Novak and AHA Grant 11PRE7320029 to C. Shukla. The LCR/HCR rat model system was funded by National Center for Research Resources Grant R24-RR-017718 and is presently supported by the Office of Research Infrastructure Programs/OD Grant ROD012098A (to L. G. Koch and S. L. Britton) from the NIH. S. L. Britton was also supported by NIH Grant RO1-DK-077200.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.K.G., H.S., and C.M.N. conception and design of research; C.K.G., S.M., and C.S. performed experiments; C.K.G., S.M., C.S., and C.M.N. analyzed data; C.K.G., S.M., C.S., and C.M.N. interpreted results of experiments; C.K.G. and C.M.N. prepared figures; C.K.G. and C.M.N. drafted manuscript; C.K.G., S.L.B., L.G.K., and C.M.N. edited and revised manuscript; C.K.G., S.M., C.S., S.L.B., L.G.K., H.S., and C.M.N. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge Dr. Werner Geldenhuys for assistance with the statistical modeling and associated text and Antonio A. Nunez for a critical reading of the manuscript. We also acknowledge the expert care of the rat colony provided by Molly Kalahar and Lori Gilligan.
L. G. Koch (lgkoch@med.umich.edu) or S. L. Britton (brittons@umich.edu) may be contacted for information on the LCR and HCR rats; these rat models are maintained as an international collaborative resource at the University of Michigan, Ann Arbor, MI.
REFERENCES
- 1.Aguilar-Bryan L, Bryan J. Molecular biology of adenosine triphosphate-sensitive potassium channels. Endocr Rev 20: 101–135, 1999 [DOI] [PubMed] [Google Scholar]
- 2.Alekseev AE, Reyes S, Yamada S, Hodgson-Zingman DM, Sattiraju S, Zhu Z, Sierra A, Gerbin M, Coetzee WA, Goldhamer DJ, Terzic A, Zingman LV. Sarcolemmal ATP-sensitive K+ channels control energy expenditure determining body weight. Cell Metab 11: 58–69, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allison DB, Fontaine KR, Manson JE, Stevens J, Van Itallie TB. Annual deaths attributable to obesity in the United States. JAMA 282: 1530–1538, 1999 [DOI] [PubMed] [Google Scholar]
- 4.Bartness TJ, Vaughan CH, Song CK. Sympathetic and sensory innervation of brown adipose tissue. Int J Obes (Lond) 34, Suppl 1: S36–S42, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bennet AF, Ruben JA. Endothermy and activity in vertebrates. Science 206: 649–654, 1979 [DOI] [PubMed] [Google Scholar]
- 6.Bézaire V, Seifert EL, Harper ME. Uncoupling protein-3: clues in an ongoing mitochondrial mystery. FASEB J 21: 312–324, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Bray MS. Genomics, genes, and environmental interaction: the role of exercise. J Appl Physiol 88: 788–792, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Brown DW, Brown DR, Heath GW, Balluz L, Giles WH, Ford ES, Mokdad AH. Associations between physical activity dose and health-related quality of life. Med Sci Sports Exerc 36: 890–896, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Butler AA, Kozak LP. A recurring problem with the analysis of energy expenditure in genetic models expressing lean and obese phenotypes. Diabetes 59: 323–329, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev 84: 277–359, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Chappell MA, Rezende EL, Hammond KA. Age and aerobic performance in deer mice. J Exp Biol 206: 1221–1231, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Chen W, Zhang X, Birsoy K, Roeder RG. A muscle-specific knockout implicates nuclear receptor coactivator MED1 in the regulation of glucose and energy metabolism. Proc Natl Acad Sci USA 107: 10196–10201, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coetzee WA, Amarillo Y, Chiu J, Chow A, Lau D, McCormack T, Moreno H, Nadal MS, Ozaita A, Pountney D, Saganich M, Vega-Saenz de Miera E, Rudy B. Molecular diversity of K+ channels. Ann NY Acad Sci 868: 233–285, 1999 [DOI] [PubMed] [Google Scholar]
- 14.Costford SR, Seifert EL, Bézaire V, Gerrits MF, Bevilacqua L, Gowing A, Harper ME. The energetic implications of uncoupling protein-3 in skeletal muscle. Appl Physiol Nutr Metab 32: 884–894, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Danaei G, Ding EL, Mozaffarian D, Taylor B, Rehm J, Murray CJ, Ezzati M. The preventable causes of death in the United States: comparative risk assessment of dietary, lifestyle, and metabolic risk factors. PLoS Med 6: e1000058, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ekelund U, Brage S, Franks PW, Hennings S, Emms S, Wareham NJ. Physical activity energy expenditure predicts progression toward the metabolic syndrome independently of aerobic fitness in middle-aged healthy Caucasians: the Medical Research Council Ely Study. Diabetes Care 28: 1195–1200, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Ferraro R, Ravussin E. Fat mass in predicting resting metabolic rate. Am J Clin Nutr 56: 460–461, 1992 [DOI] [PubMed] [Google Scholar]
- 18.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA 307: 491–497, 2012 [DOI] [PubMed] [Google Scholar]
- 19.Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999–2000. JAMA 288: 1723–1727, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Fontaine KR, Redden DT, Wang C, Westfall AO, Allison DB. Years of life lost due to obesity. JAMA 289: 187–193, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Gêbczyñski AK, Konarzewski M. Metabolic correlates of selection on aerobic capacity in laboratory mice: a test of the model for the evolution of endothermy. J Exp Biol 212: 2872–2878, 2009 [DOI] [PubMed] [Google Scholar]
- 22.Giles ED, Jackman MR, Johnson GC, Schedin PJ, Houser JL, MacLean PS. Effect of the estrous cycle and surgical ovariectomy on energy balance, fuel utilization, and physical activity in lean and obese female rats. Am J Physiol Regul Integr Comp Physiol 299: R1634–R1642, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goldberg AP, Dengel DR, Hagberg JM. Exercise physiology and aging. In: Handbook of the Biology of Aging (4th ed.), edited by Schneider EL, Rowe JW. San Diego, CA: Academic, 1996, p. 331–354 [Google Scholar]
- 24.Gonzalez E, Messi ML, Delbono O. The specific force of single intact extensor digitorum longus and soleus mouse muscle fibers declines with aging. J Membr Biol 178: 175–183, 2000 [DOI] [PubMed] [Google Scholar]
- 25.Haffner S, Taegtmeyer H. Epidemic obesity and the metabolic syndrome. Circulation 108: 1541–1545, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Harper ME, Dent RM, Bezaire V, Antoniou A, Gauthier A, Monemdjou S, McPherson R. UCP3 and its putative function: consistencies and controversies. Biochem Soc Trans 29: 768–773, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Harper ME, Green K, Brand MD. The efficiency of cellular energy transduction and its implications for obesity. Annu Rev Nutr 28: 13–33, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Heo M, Allison DB, Faith MS, Zhu S, Fontaine KR. Obesity and quality of life: mediating effects of pain and comorbidities. Obes Res 11: 209–216, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Iossa S, Lionetti L, Mollica MP, Barletta A, Liverini G. Thermic effect of food in hypothyroid rats. J Endocrinol 148: 167–174, 1996 [DOI] [PubMed] [Google Scholar]
- 30.Johannsen DL, Welk GJ, Sharp RL, Flakoll PJ. Differences in daily energy expenditure in lean and obese women: the role of posture allocation. Obesity (Silver Spring) 16: 34–39, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Joosen AM, Gielen M, Vlietinck R, Westerterp KR. Genetic analysis of physical activity in twins. Am J Clin Nutr 82: 1253–1259, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Katzmarzyk PT. Physical activity, sedentary behavior, and health: paradigm paralysis or paradigm shift? Diabetes 59: 2717–2725, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klein S. The national obesity crisis: a call for action. Gastroenterology 126: 6, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Koch LG, Britton SL, Wisloff U. A rat model system to study complex disease risks, fitness, aging, and longevity. Trends Cardiovasc Med 22: 29–34, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koch LG, Britton SL. Artificial selection for intrinsic aerobic endurance running capacity in rats. Physiol Genomics 5: 45–52, 2001 [DOI] [PubMed] [Google Scholar]
- 36.Koch LG, Kemi OJ, Qi N, Leng SX, Bijma P, Gilligan LJ, Wilkinson JE, Wisløff H, Høydal MA, Rolim N, Abadir PM, van Grevenhof EM, Smith GL, Burant CF, Ellingsen O, Britton SL, Wisløff U. Intrinsic aerobic capacity sets a divide for aging and longevity. Circ Res 109: 1162–1172, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kodama S, Saito K, Tanaka S, Maki M, Yachi Y, Asumi M, Sugawara A, Totsuka K, Shimano H, Ohashi Y, Yamada N, Sone H. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. JAMA 301: 2024–2035, 2009 [DOI] [PubMed] [Google Scholar]
- 38.Kodera Y, Takeyama K, Murayama A, Suzawa M, Masuhiro Y, Kato S. Ligand type-specific interactions of peroxisome proliferator-activated receptor γ with transcriptional coactivators. J Biol Chem 275: 33201–33204, 2000 [DOI] [PubMed] [Google Scholar]
- 39.Kokkinos P, Myers J, Kokkinos JP, Pittaras A, Narayan P, Manolis A, Karasik P, Greenberg M, Papademetriou V, Singh S. Exercise capacity and mortality in black and white men. Circulation 117: 614–622, 2008 [DOI] [PubMed] [Google Scholar]
- 40.Kotz CM, Teske JA, Billington CJ. Neuroregulation of nonexercise activity thermogenesis and obesity resistance. Am J Physiol Regul Integr Comp Physiol 294: R699–R710, 2008 [DOI] [PubMed] [Google Scholar]
- 41.Kotz CM, Wang C, Levine AS, Billington CJ. Urocortin in the hypothalamic PVN increases leptin and affects uncoupling proteins-1 and -3 in rats. Am J Physiol Regul Integr Comp Physiol 282: R546–R551, 2002 [DOI] [PubMed] [Google Scholar]
- 42.Kotz CM, Wang CF, Briggs JE, Levine AS, Billington CJ. Effect of NPY in the hypothalamic paraventricular nucleus on uncoupling proteins 1, 2, and 3 in the rat. Am J Physiol Regul Integr Comp Physiol 278: R494–R498, 2000 [DOI] [PubMed] [Google Scholar]
- 43.Kozak LP. Brown fat and the myth of diet-induced thermogenesis. Cell Metab 11: 263–267, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lessard SJ, Rivas DA, Chen ZP, van Denderen BJ, Watt MJ, Koch LG, Britton SL, Kemp BE, Hawley JA. Impaired skeletal muscle beta-adrenergic activation and lipolysis are associated with whole-body insulin resistance in rats bred for low intrinsic exercise capacity. Endocrinology 150: 4883–4891, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lessard SJ, Rivas DA, Stephenson EJ, Yaspelkis BB, 3rd, Koch LG, Britton SL, Hawley JA. Exercise training reverses impaired skeletal muscle metabolism induced by artificial selection for low aerobic capacity. Am J Physiol Regul Integr Comp Physiol 300: R175–R182, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Levine JA, Eberhardt NL, Jensen MD. Role of nonexercise activity thermogenesis in resistance to fat gain in humans. Science 283: 212–214, 1999 [DOI] [PubMed] [Google Scholar]
- 47.Levine JA, Kotz CM. NEAT—non-exercise activity thermogenesis—egocentric & geocentric environmental factors vs. biological regulation. Acta Physiol Scand 184: 309–318, 2005 [DOI] [PubMed] [Google Scholar]
- 48.Levine JA, Lanningham-Foster LM, McCrady SK, Krizan AC, Olson LR, Kane PH, Jensen MD, Clark MM. Interindividual variation in posture allocation: possible role in human obesity. Science 307: 584–586, 2005 [DOI] [PubMed] [Google Scholar]
- 49.Levine JA. Nonexercise activity thermogenesis (NEAT): environment and biology. Am J Physiol Endocrinol Metab 286: E675–E685, 2004 [DOI] [PubMed] [Google Scholar]
- 50.Lucía A, Hoyos J, Pérez M, Santalla A, Chicharro JL. Inverse relationship between VO2max and economy/efficiency in world-class cyclists. Med Sci Sports Exerc 34: 2079–2084, 2002 [DOI] [PubMed] [Google Scholar]
- 51.MacLean PS, Higgins JA, Johnson GC, Fleming-Elder BK, Donahoo WT, Melanson EL, Hill JO. Enhanced metabolic efficiency contributes to weight regain after weight loss in obesity-prone rats. Am J Physiol Regul Integr Comp Physiol 287: R1306–R1315, 2004 [DOI] [PubMed] [Google Scholar]
- 52.MacLean PS, Higgins JA, Johnson GC, Fleming-Elder BK, Peters JC, Hill JO. Metabolic adjustments with the development, treatment, and recurrence of obesity in obesity-prone rats. Am J Physiol Regul Integr Comp Physiol 287: R288–R297, 2004 [DOI] [PubMed] [Google Scholar]
- 53.Miller GA, Chapman JP. Misunderstanding analysis of covariance. J Abnorm Psychol 110: 40–48, 2001 [DOI] [PubMed] [Google Scholar]
- 54.Miyaki T, Fujikawa T, Kitaoka R, Hirano N, Matsumura S, Fushiki T, Inoue K. Noradrenergic projections to the ventromedial hypothalamus regulate fat metabolism during endurance exercise. Neuroscience 190: 239–250, 2011 [DOI] [PubMed] [Google Scholar]
- 55.Mountjoy KG. Distribution and function of melanocortin receptors within the brain. Adv Exp Med Biol 681: 29–48, 2010.s56 [DOI] [PubMed] [Google Scholar]
- 56.Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med 346: 793–801, 2002 [DOI] [PubMed] [Google Scholar]
- 57.Nabben M, Hoeks J. Mitochondrial uncoupling protein 3 and its role in cardiac- and skeletal muscle metabolism. Physiol Behav 94: 259–269, 2008 [DOI] [PubMed] [Google Scholar]
- 58.Naples SP, Borengasser SJ, Rector RS, Uptergrove GM, Morris EM, Mikus CR, Koch LG, Britton SL, Ibdah JA, Thyfault JP. Skeletal muscle mitochondrial and metabolic responses to a high-fat diet in female rats bred for high and low aerobic capacity. Appl Physiol Nutr Metab 35: 151–162, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nichols CG. KATP channels as molecular sensors of cellular metabolism. Nature 440: 470–476, 2006 [DOI] [PubMed] [Google Scholar]
- 60.Nogueiras R, Wiedmer P, Perez-Tilve D, Veyrat-Durebex C, Keogh JM, Sutton GM, Pfluger PT, Castaneda TR, Neschen S, Hofmann SM, Howles PN, Morgan DA, Benoit SC, Szanto I, Schrott B, Schürmann A, Joost HG, Hammond C, Hui DY, Woods SC, Rahmouni K, Butler AA, Farooqi IS, O'Rahilly S, Rohner-Jeanrenaud F, Tschöp MH. The central melanocortin system directly controls peripheral lipid metabolism. J Clin Invest 117: 3475–3488, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Novak CM, Burghardt PR, Levine JA. The use of a running wheel to measure activity in rodents: relationship to energy balance, general activity, and reward. Neurosci Biobehav Rev 36: 1001–1014, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Novak CM, Escande C, Burghardt PR, Zhang M, Barbosa MT, Chini EN, Britton SL, Koch LG, Akil H, Levine JA. Spontaneous activity, economy of activity, and resistance to diet-induced obesity in rats bred for high intrinsic aerobic capacity. Horm Behav 58: 355–367, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Novak CM, Escande C, Gerber SM, Chini EN, Zhang M, Britton SL, Koch LG, Levine JA. Endurance capacity, not body size, determines physical activity levels: role of skeletal muscle PEPCK. PLoS One 4: e5869, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Novak CM, Levine JA. Central neural and endocrine mechanisms of non-exercise activity thermogenesis and their potential impact on obesity. J Neuroendocrinol 19: 923–940, 2007 [DOI] [PubMed] [Google Scholar]
- 64a.Otter T, King SM, Witman GB. A two-step procedure for efficient electrotransfer of both high-molecular-weight (>400,000) and low-molecular-weight (<20,000) proteins. Anal Biochem 162: 370–377, 1987 [DOI] [PubMed] [Google Scholar]
- 65.Packard GC, Boardman TJ. The misuse of ratios, indices, and percentages in ecophysiological research. Physiol Zool 61: 1–9, 1988 [Google Scholar]
- 66.Piers LS, Soares MJ, McCormack MJ, O'Dea K. Is there evidence for an age related reduction in metabolic rate? J Appl Physiol 85: 2196–2204, 1998 [DOI] [PubMed] [Google Scholar]
- 67.Ravussin E, Lillioja S, Knowler WC, Christin L, Freymond D, Abbott WG, Boyce V, Howard BV, Bogardus C. Reduced rate of energy expenditure as a risk factor for bodyweight gain. N Engl J Med 318: 467–472, 1988 [DOI] [PubMed] [Google Scholar]
- 68.Rivas DA, Lessard SJ, Saito M, Friedhuber AM, Koch LG, Britton SL, Yaspelkis BB, 3rd, Hawley JA. Low intrinsic running capacity is associated with reduced skeletal muscle substrate oxidation and lower mitochondrial content in white skeletal muscle. Am J Physiol Regul Integr Comp Physiol 300: R835–R843, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Roberts SB, McCrory MA, Saltzman E. The influence of dietary composition on energy intake and body weight. J Am Coll Nutr 21: 140S–145S, 2002 [DOI] [PubMed] [Google Scholar]
- 70.Rothwell NJ, Stock MJ. A role for brown adipose tissue in diet-induced thermogenesis. Nature 281: 31–35, 1979 [DOI] [PubMed] [Google Scholar]
- 71.Rousset S, Alves-Guerra MC, Mozo J, Miroux B, Cassard-Doulcier AM, Bouillaud F, Ricquier D. The biology of mitochondrial uncoupling proteins. Diabetes 53: S130–S135, 2004 [DOI] [PubMed] [Google Scholar]
- 72.Samec S, Seydoux J, Dulloo AG. Interorgan signaling between adipose tissue metabolism and skeletal muscle uncoupling protein homologs: is there a role for circulating free fatty acids? Diabetes 47: 1693–1698, 1998 [DOI] [PubMed] [Google Scholar]
- 73.Sawyer BJ, Blessinger JR, Irving BA, Weltman A, Patrie JT, Gaesser GA. Walking and running economy: inverse association with peak oxygen uptake. Med Sci Sports Exerc 42: 2122–2127, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schoeller DA, Jefford G. Determinants of the energy costs of light activities: inferences for interpreting doubly labeled water data. Int J Obes Relat Metab Disord 26: 97–101, 2002 [DOI] [PubMed] [Google Scholar]
- 75.Sheehan MT, Jensen MD. Metabolic complications of obesity. Pathophysiologic considerations. Med Clin North Am 84: 363–385, vi, 2000 [DOI] [PubMed] [Google Scholar]
- 76.Shi H, Bowers RR, Bartness TJ. Norepinephrine turnover in brown and white adipose tissue after partial lipectomy. Physiol Behav 81: 535–542, 2004 [DOI] [PubMed] [Google Scholar]
- 77.Shieh CC, Coghlan M, Sullivan JP, Gopalakrishnan M. Potassium channels: molecular defects, diseases, and therapeutic opportunities. Pharmacol Rev 52: 557–594, 2000 [PubMed] [Google Scholar]
- 78.Shiuchi T, Haque MS, Okamoto S, Inoue T, Kageyama H, Lee S, Toda C, Suzuki A, Bachman ES, Kim YB, Sakurai T, Yanagisawa M, Shioda S, Imoto K, Minokoshi Y. Hypothalamic orexin stimulates feeding-associated glucose utilization in skeletal muscle via sympathetic nervous system. Cell Metab 10: 466–480, 2009 [DOI] [PubMed] [Google Scholar]
- 79.Shukla C, Britton SL, Koch LG, Novak CM. Region-specific differences in brain melanocortin receptors in rats of the lean phenotype. Neuroreport 23: 596–600, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Simmons RK, Griffin SJ, Steele R, Wareham NJ, Ekelund U; ProActive Research Team Increasing overall physical activity and aerobic fitness is associated with improvements in metabolic risk: cohort analysis of the ProActive trial. Diabetologia 51: 787–794, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sohn JW, Harris LE, Berglund ED, Liu T, Vong L, Lowell BB, Balthasar N, Williams KW, Elmquist JK. Melanocortin 4 receptors reciprocally regulate sympathetic and parasympathetic preganglionic neurons. Cell 152: 3612–3619, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stephenson EJ, Stepto NK, Koch LG, Britton SL, Hawley JA. Divergent skeletal muscle respiratory capacities in rats artificially selected for high and low running ability: a role for Nor1? J Appl Physiol 113: 1403–1412, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stock MJ, Rothwell NJ. Role of brown adipose tissue thermogenesis in overfeeding: a review. J R Soc Med 76: 71–73, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Swanson DL, Thomas NE, Liknes ET, Cooper SJ. Intraspecific correlations of basal and maximal metabolic rates in birds and the aerobic capacity model for the evolution of endothermy. PLoS One 7: e34271, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tanaka T, Masuzaki H, Yasue S, Ebihara K, Shiuchi T, Ishii T, Arai N, Hirata M, Yamamoto H, Hayashi T, Hosoda K, Minokoshi Y, Nakao K. Central melanocortin signaling restores skeletal muscle AMP-activated protein kinase phosphorylation in mice fed a high-fat diet. Cell Metab 5: 395–402, 2007 [DOI] [PubMed] [Google Scholar]
- 87.Thompson LV, Brown M. Age-related changes in contractile properties of single skeletal fibers from the soleus muscle. J Appl Physiol 86: 881–886, 1999 [DOI] [PubMed] [Google Scholar]
- 88.Toda C, Shiuchi T, Kageyama H, Okamoto S, Coutinho EA, Sato T, Okamatsu-Ogura Y, Yokota S, Takagi K, Tang L, Saito K, Shioda S, Minokoshi Y. Extracellular signal-regulated kinase in the ventromedial hypothalamus mediates leptin-induced glucose uptake in red-type skeletal muscle. Diabetes 62: 2295–2307, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Toda C, Shiuchi T, Lee S, Yamato-Esaki M, Fujino Y, Suzuki A, Okamoto S, Minokoshi Y. Distinct effects of leptin and a melanocortin receptor agonist injected into medial hypothalamic nuclei on glucose uptake in peripheral tissues. Diabetes 58: 2757–2765, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tschöp MH, Speakman JR, Arch JR, Auwerx J, Brüning JC, Chan L, Eckel RH, Farese RV, Jr, Galgani JE, Hambly C, Herman MA, Horvath TL, Kahn BB, Kozma SC, Maratos-Flier E, Müller TD, Münzberg H, Pfluger PT, Plum L, Reitman ML, Rahmouni K, Shulman GI, Thomas G, Kahn CR, Ravussin E. A guide to analysis of mouse energy metabolism. Nat Methods 9: 57–63, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tweedie C, Romestaing C, Burelle Y, Safdar A, Tarnopolsky MA, Seadon S, Britton SL, Koch LG, Hepple RT. Lower oxidative DNA damage despite greater ROS production in muscles from rats selectively bred for high running capacity. Am J Physiol Regul Integr Comp Physiol 300: R544–R553, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang C, Billington CJ, Levine AS, Kotz CM. Effect of CART in the hypothalamic paraventricular nucleus on feeding and uncoupling protein gene expression. Neuroreport 11: 3251–3255, 2000 [DOI] [PubMed] [Google Scholar]
- 93.Webber J. Energy balance in obesity. Proc Nutr Soc 62: 539–543, 2003 [DOI] [PubMed] [Google Scholar]
- 94.Wisløff U, Najjar SM, Ellingsen O, Haram PM, Swoap S, Al-Share Q, Fernström M, Rezaei K, Lee SJ, Koch LG, Britton SL. Cardiovascular risk factors emerge after artificial selection for low aerobic capacity. Science 307: 418–420, 2005 [DOI] [PubMed] [Google Scholar]
- 95.Yuan CX, Ito M, Fondell JD, Fu ZY, Roeder RG. The TRAP220 component of a thyroid hormone receptor- associated protein (TRAP) coactivator complex interacts directly with nuclear receptors in a ligand-dependent fashion. Proc Natl Acad Sci USA 95: 7939–7944, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhan WZ, Swallow JG, Garland T, Jr, Proctor DN, Carter PA, Sieck GC. Effects of genetic selection and voluntary activity on the medial gastrocnemius muscle in house mice. J Appl Physiol 87: 2326–2333, 1999 [DOI] [PubMed] [Google Scholar]
- 97.Zinbarg RE, Suzuki S, Uliaszek AA, Lewis AR. Biased parameter estimates and inflated Type I error rates in analysis of covariance (and analysis of partial variance) arising from unreliability: alternatives and remedial strategies. J Abnorm Psychol 119: 307–319, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zurlo F, Larson K, Bogardus C, Ravussin E. Skeletal muscle metabolism is a major determinant of resting energy expenditure. J Clin Invest 86: 1423–1427, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]