Abstract
Ghrelin is a metabolic signal regulating energy homeostasis. Circulating ghrelin levels rise during starvation and fall after a meal, and therefore, ghrelin may function as a signal of negative energy balance. Ghrelin may also act as a modulator of reproductive physiology, as acute ghrelin administration suppresses gonadotropin secretion and inhibits the neuroendocrine reproductive axis. Interestingly, ghrelin's effect in female metabolism varies according to the estrogen milieu predicting an interaction between ghrelin and estrogens, likely at the hypothalamic level. Here, we show that ghrelin receptor (GHSR) and estrogen receptor-α (ERα) are coexpressed in several hypothalamic sites. Higher levels of circulating estradiol increased the expression of GHSR mRNA and the co-xpression of GHSR mRNA and ERα selectively in the arcuate nucleus (ARC). Subsets of preoptic and ARC Kiss1 neurons coexpressed GHSR. Increased colocalization was observed in ARC Kiss1 neurons of ovariectomized estradiol-treated (OVX + E2; 80%) compared with ovariectomized oil-treated (OVX; 25%) mice. Acute actions of ghrelin on ARC Kiss1 neurons were also modulated by estradiol; 75 and 22% of Kiss1 neurons of OVX + E2 and OVX mice, respectively, depolarized in response to ghrelin. Our findings indicate that ghrelin and estradiol may interact in several hypothalamic sites. In the ARC, high levels of E2 increase GHSR mRNA expression, modifying the colocalization rate with ERα and Kiss1 and the proportion of Kiss1 neurons acutely responding to ghrelin. Our findings indicate that E2 alters the responsiveness of kisspeptin neurons to metabolic signals, potentially acting as a critical player in the metabolic control of the reproductive physiology.
Keywords: kisspeptin, hypothalamus, metabolism, growth hormone secretagogue receptor, reproduction
in mammals, the ability to reproduce is gated by the availability of energy stores (25, 49). Reproduction is metabolically expensive, and during starvation fertility is stopped to conserve energy for basic survival. Accumulating evidence has implicated circulating hormones as a means of transmitting information regarding peripheral energy availability to the central circuitry that controls the reproductive system. Among these metabolic signals is the stomach-derived hormone ghrelin (23, 46). Ghrelin was recognized originally for its growth hormone-stimulating action, but extensive research has since demonstrated that ghrelin is also a direct regulator of metabolism and energy balance (22, 28, 55). Ghrelin promotes the storage of lipids as fat, induces glucagon release, and suppresses insulin secretion and sensitivity (4, 5, 47). Interestingly, the effects of ghrelin to increase body weight vary according to the estrogen milieu, as a more pronounced orexigenic effect is observed in females upon ovariectomy (3, 6). In cycling rats, ghrelin stimulation of food intake is observed only in females in diestrus (when estrogen levels are low). Moreover, males treated with estradiol are resistant to the stimulatory effects of ghrelin on food intake (6). Collectively, these findings suggest an interaction between ghrelin and estradiol possibly at the level of the receptors located in the brain.
In addition to its effects on energy balance, accumulating evidence suggests that ghrelin can also influence fertility (34). Studies conducted in several species, including rats, sheep, monkeys, and humans, indicate that ghrelin administration suppresses gonadotropin secretion (19, 27, 30, 48). The exact site of ghrelin action is currently unknown, but several lines of evidence support the idea that ghrelin acts at the hypothalamic level to inhibit gonadotropin secretion. First, the functional ghrelin receptor GHSR (growth hormone secretagogue receptor; GHSR1a) is present in several hypothalamic regions, including those known to participate in the control of the reproductive function, indicating that this hormone can interact directly with hypothalamic neurons (56). Second, the inhibitory action of ghrelin upon gonadotropin secretion is maintained when ghrelin is administered directly to the brain of male and female rats (10, 11, 15). Third, ghrelin inhibits the output of gonadotropin releasing hormone (GnRH) from hypothalamic explants (11). Of note, in rats, fasting conditions (when ghrelin is high) as well as ghrelin administration decrease hypothalamic Kiss1 mRNA expression (13). However, in contrast to mice, the preoptic area of rats is deficient in GHSR (56), suggesting an indirect effect of ghrelin upon Kiss1 gene expression in those neurons.
To gain insights into the brain circuitry mediating ghrelin's effects in the reproductive neuroendocrine axis, we employed a series of neuroanatomic, electrophysiological, and genetic approaches using the mouse as an experimental model. We aimed to identify the hypothalamic sites of ghrelin/estrogen and ghrelin/Kiss1 interaction and the direct effect of ghrelin on Kiss1 cell activity.
MATERIALS AND METHODS
Animals.
Female C57BL/6 and Kiss1-Cre/GFP mice were housed in the University of Texas Southwestern Medical Center Animal Resource Center or in the animal care facility of the Department of Anatomy, Institute of Biomedical Sciences, University of São Paulo, in a light- (12 h on/12 h off) and temperature-controlled (21–23°C) environment. They were fed standard chow diet (Harlan Teklad Global Diet no. 2016; Harlan Laboratories, Madison, WI) and had free access to water. To generate the Kiss1-Cre/GFP mouse model, mice that express Cre recombinase driven by Kiss1 regulatory elements (J2–4 line) (7) were crossed to reporter mice that express eGFP in a Cre-dependent fashion [strain: B6;129-Gt(ROSA)26Sortm2Sho/J; stock no. 004077]. Within the Kiss1-Cre/GFP mice, Cre activity irreversibly excises a transcriptional blocking cassette between two loxP sites (16), thus permitting enhanced green fluorescent protein (eGFP) expression within the entire population of Kiss1-expressing neurons, including those in the preoptic area [anteroventral periventricular nucleus (AVPV) and periventricular nucleus (PeN)] and in the arcuate nucleus (ARC), in varying physiological conditions. All experiments and procedures were carried out in accordance with the guidelines established by the National Institute of Health Guide for the Care and Use of Laboratory Animals and were approved by the University of Texas Institutional Animal Care and Use Committee (AP no. 2008-0150) and by the Committee on Care and Use of Laboratory Animals of the Institute of Biomedical Sciences, University of São Paulo (Protocol CEUA no. 065.129.02).
Ovariectomy and estradiol replacement.
Ovariectomy was performed in mice anesthetized with an intraperitoneal (ip) injection of a ketamine-xylazine cocktail (120 mg/kg ketamine, 16 mg/kg xylazine). A capsule prepared as described previously (8) containing either 1 μg of estradiol (E2; 17β-estradiol 3-benzoate) (Sigma) suspended in sesame oil or oil alone was implanted under the skin at the time of the ovariectomy. Mice were euthanized 7 days, later and brains were collected for histology or quantitative PCR (qPCR). For electrophysiological experiments, mice were ovariectomized (OVX) 7–10 days prior to recording. Ovariectomized E2-primed mice (OVX + E2) were submitted to the surgical procedure 3–4 days prior to recording (14).
Perfusion and histology.
Mice were deeply anesthetized with chloral hydrate and perfused with 10% formalin (pH 7.4). Brains were dissected and cryoprotected overnight at 4°C in diethylpyrocarbonate (DEPC)-treated 0.1 M phosphate-buffered saline (PBS), pH 7.4, containing 20% sucrose. The brains were cut (25-μm sections) in the frontal plane in a freezing microtome. Five series were collected and stored at −20°C in cryoprotectant until they were processed for in situ hybridization and immuhistochemistry.
Single- and double-label in situ hybridization/immunohistochemistry.
Single-label in situ hybridization (ISH) histochemistry (IHC) for GHSR mRNA detection was performed as described previously (5). Briefly, tissue sections from C57BL/6 OVX (n = 4) and OVX + E2 (n = 5) females were mounted onto SuperFrost plus slides (Fisher Scientific), air-dried, and fixed in 4% paraformaldehyde in DEPC-treated PBS for 20 min. Tissue was dehydrated in increasing concentrations of ethanol, cleared in xylenes, rehydrated in decreasing concentrations of ethanol, and placed in prewarmed sodium citrate buffer, pH 6.0. While in buffer, slides were microwaved for 10 min, followed by dehydration in graded ethanol. The 33P-labeled GHSR riboprobe was diluted to 106 counts·min−1·ml−1 in a hybridization solution containing 50% formamide, 10 mM Tris·HCl (pH 8.0), 5 mg of tRNA (Invitrogen), 10 mM dithiotreitol, 10% dextran sulfate, 0.3 M NaCl, 1 mM EDTA, and 1× Denhardt's solution. The GHSR riboprobe was described and validated in previous studies (56). Hybridization solution with probe was applied on each slide and incubated overnight at 57°C. Coverslips were then removed and slides washed in 2× SSC (sodium chloride sodium citrate buffer) and treated with 0.02% RNase A (Roche) in 0.5 M NaCl, 10 mM Tris·HCl, and 1 mM EDTA for 30 min. Sections were then subjected to stringency washes in SSC. Tissue was dehydrated in increasing concentrations of ethanol, and slides were placed in X-ray film cassettes with BMR-2 film (Kodak) for 3 days and then dipped in NTB-2 autoradiographic emulsion (Kodak), dried, and stored in light-protected boxes at 4°C for 3–4 wk. Finally, slides were developed with D-19 developer (Kodak), counterstained with thionin, dehydrated in graded ethanol, cleared in xylenes, and coverslipped with Permaslip.
Double-label ISH and IHC was performed as described previously (7, 56). Briefly, free-floating sections from C57BL/6 OVX (n = 4) and OVX + E2 (n = 4) females or Kiss1-Cre/GFP mice on diestrus (n = 4), OVX (n = 4), and OVX + E2 (n = 4) were rinsed in DEPC-treated PBS and treated with 0.1% sodium borohydride for 15 min. Sections were treated with 0.25% acetic anhydride in 0.1 M triethanolamine (pH 8.0) for 10 min and then washed in 2× SSC. Next, the sections were incubated overnight at 50°C in the above-described hybridization solution containing the 33P-labeled GHSR riboprobe. Subsequently, sections were treated with RNase A and submitted to stringency washes in SSC. Next, the sections from C57BL/6 females were incubated in anti-estrogen receptor-α (ERα; made in rabbit, 1:25,000, C1355; Millipore), and sections from Kiss1-Cre/GFP females were incubated in anti-GFP (made in chicken, 1:5,000; Aves Laboratories) overnight at room temperature. Both antibodies have been validated before (2, 7, 52, 53). The next day, sections were incubated for 1–2 h in secondary antibody [biotin-conjugated donkey anti-rabbit, 1:1,000 (Vector Laboratories) for ERα staining or AlexaFluor 488 conjugated goat anti-chicken, 1:500 (Invitrogen) for GFP labeling]. Sections incubated in Alexa Fluor 488-conjugated secondary antisera (GFP) were mounted onto SuperFrost plus slides and processed for standard autoradiographic procedures, as described above. Sections incubated in biotinylated secondary antisera [ERα were further incubated in avidin-biotin complex (1:500 for 1 h; Vector Laboratories)]. Peroxidase reaction was performed using 3,3′-diaminobenzidine tetrahydrochloride (Sigma) as chromogen and sections were mounted onto SuperFrost plus slides and processed for standard autoradiographic procedures, as described above.
Quantitative PCR.
C57BL/6 female mice (OVX, n = 5; OVX + E2, n = 7) were anesthetized with chloral hydrate and euthanized by decapitation. Brains were immediately dissected, rinsed in ice-cold DEPC-treated PBS, and sectioned with clean razor blades in a metal matrix according to Chuang et al. (5). To quantify relative levels of mRNA, tissue punches from the medial preoptic area [containing the AVPV and the medial preoptic nucleus (MPO)] and from the caudal ARC were collected. Neuroanatomic landmarks were used to identify the AVPV/MPO and ARC regions, and a 14-gauge tissue punch needle attached to a 3-ml syringe was used to bilaterally collect portions of tissue from each region. Histological structures were identified based on The Mouse Brain in Stereotaxic Coordinates (39). To collect punches from the AVPV/MPO, we used sections where the anterior commissure formed an elongated continuum but was not touched by the third ventricle. Tissue was extracted from the region immediately adjacent to the third ventricle. To collect punches from the ARC, tissue was extracted from the region immediately adjacent to the third ventricle at its ventral extreme. RNA was isolated, and qPCR was performed as described by Chuang et al. (5). Total RNA was isolated from each hypothalamic block using Trizol (Qiagen), and RNA concentrations were determined by absorbance at 260 nm with a Nanodrop 100 Spectrophotometer (Thermo Scientific). Total RNA was treated with DNAse (Roche) and reverse-transcribed into cDNA with the High Capacity cDNA Reverse Transcription kit (Applied Biosystems). qPCR analysis was performed using an 7900 HT Fast Real-Time PCR System and TaqMan Gene Expression Assays (Applied Biosystems). Relative RNA level of a target gene in each sample was normalized using 18S values. Primer sequences were as follows: GHSR forward, 5′-ACCGTGATGGTATGGGTGTCG-3′; reverse, 5′-CACAGTGAGGCAGAAGACCG-3′ (product spanning nucleotides 878–937, NM_177330).
Electrophysiological recordings.
To examine the pharmacological effects of ghrelin on the electrophysiological properties of Kiss1 neurons, whole cell patch clamp recordings were performed in Kiss1-Cre/GFP female mice (diestrus, n = 10; OVX, n = 3; OVX + E2, n = 3). Kiss1-Cre-expressing neurons in the preoptic area (AVPV/PeN) and the ARC were recorded. During the recordings, neurons were maintained in hypothalamic slice preparations, and data analyses were performed as described previously (14, 51). Mice were decapitated, and the entire brain was removed. After removal, brains were immediately submerged in ice-cold, carbogen-saturated (95% O2 and 5% CO2) artificial cerebrospinal fluid (aCSF; 126 mM NaCl, 2.8 mM KCl, 1.2 mM MgCl2, 2.5 mM CaCl2, 1.25 mM NaH2PO4, 26 mM NaHCO3, and 5 mM glucose). Coronal sections from a hypothalamic block (250 μM) were cut with a Leica VT1000S Vibratome and then incubated in oxygenated artificial cerebrospinal fluid (aCSF) at room temperature for ≥1 h before recording. Slices were transferred to the recording chamber and allowed to equilibrate for 10–20 min before recording. The slices were bathed in oxygenated aCSF (32–34°C) at a flow rate of ∼2 ml/min. The pipette solution for whole cell recording was modified to include an intracellular dye (Alexa Fluor 594) for whole cell recording: 120 mM K-gluconate, 10 mM KCl, 10 mM HEPES, 5 mM EGTA, 1 mM CaCl2, 1 mM MgCl2, 2 mM Mg-ATP, and 0.03 mM Alexa Fluor 594 hydrazide dye, pH 7.3. Epifluorescence was used briefly to target fluorescent cells, at which time the light source was switched to infrared differential interference contrast imaging to obtain the whole cell recording (Nikon Eclipse FN1 equipped with a fixed stage and a QuantEM:512SC electron-multiplying charge-coupled device camera or a Leica DM6000 FS equipped with a fixed stage and a Leica DFC360 FX high-resolution, high-speed monochrome fluorescence digital camera). Electrophysiological signals were recorded using an Axopatch 700B amplifier (Molecular Devices), low-pass filtered at 2–5 kHz, digitized at 88 kHz (Neuro-corder; Cygnus Technology), and analyzed offline on a PC with pCLAMP programs (Molecular Devices). Recording electrodes had resistances of 2.5–5 MΩ when filled with the K-gluconate internal solution. Input resistance was assessed by measuring voltage deflection at the end of the response to a hyperpolarizing rectangular current pulse (500 ms of −10 to −50 pA). Membrane potential values were compensated to account for junction potential (−8 mV). Solutions containing acylated rat ghrelin (Pi Proteomics) were typically perfused for 5 min. For some experiments, tetrodotoxin (TTX; 1 μM; Tocris) was added to the bath solution to block action potential-dependent presynaptic activity from afferent neurons that might have affected the membrane potential of postsynaptic neurons targeted for recording.
Images and data analysis.
All sections used for ISH and ISH/IHC were visualized with a Zeiss M2 microscope. Photomicrographs were produced by capturing images with a Zeiss Axiocam HRc digital camera and AxioVision software. Only the sharpness, contrast, and brightness were adjusted. For single-label ISH, the hybridization signal was estimated by the analysis of the integrated optical density (IOD) using the Image J software (http://rsb.info.nih.gov/ij), and comparison between treatment groups (OVX vs. OVX + E2) was defined. Dark-field photomicrographs were acquired using the same illumination and exposure time for every section, and no image editing was processed. The IOD values for GHSR mRNA were calculated as the total IOD of a constant area subtracting the background. The background was obtained from adjacent nuclei that do not express GHSR. Because of the low expression levels of GHSR and the inaccurate determination of single cells using silver grains, only total IOD was compared, and quantification was further evaluated by qPCR. For double-label ISH/IHC assays, cells were considered double labeled if the density of silver grains overlying the nucleus (ERα-ir) or the cytoplasm (GFP-ir) of the cell was at least three times greater than the background level. Only one representative section and one side of the brain were counted per mouse per group, and therefore, no correction for double counting was used. Background was determined by observing a portion of the tissue where GHSR was expected to be absent based on previous studies (56) and in our own present observations. GHSR-expressing cells were not individually counted because of the diffuse nature of the silver grains.
Data are expressed as means ± SE. Comparison between two groups was carried out using the unpaired two-tailed Student t-test and between three groups using one-way ANOVA. Comparison between the percentage of Kiss1 cells that acutely responded to ghrelin was performed using chi-square two-tailed test. GraphPad Prism software was used for the statistical analysis, and an α-value of 0.05 was considered in all analyses.
RESULTS
GHSR and ERα are coexpressed in defined hypothalamic sites.
GHSR mRNA expression has been described in intact adult male mice (56). Because of the sexually dimorphic responses to exogenous ghrelin described in previous studies (6), we initially evaluated the distribution of GHSR mRNA in the forebrain of the female mice. As described for adult males, females on diestrus showed moderate to high hybridization signals in the medial aspect of the MPO, in the AVPV, in the suprachiasmatic nucleus, in the ventrolateral subdivision of the ventromedial nucleus of the hypothalamus (VMHvl), and in the ARC. Low hybridization signal was also observed in the anterodorsal preoptic, periventricular, paraventricular, dorsomedial, and ventral premammillary nuclei. No clear difference in the distribution pattern of GHSR mRNA was detected comparing males and females, but higher hybridization levels were observed in the VMHvl of females and ventral premammillary nucleus of males (data not shown).
We then performed a double-label assay to determine sites of coexpression of GHSR mRNA and ERα immunoreactivity. Because changing levels of E2 may modify the detection of ERα immunoreactivity in the rodent brain (41, 42, 54), we evaluated the colocalization rate of ERα and GHSR in OVX and OVX + E2 females (Table 1). As a control for E2 levels, uterine weight was assessed at the time of euthanization. OVX females had a reduced uterus size compared with OVX + E2 mice [0.0154 ± 0.001 vs. 0.1736 ± 0.019 g, P < 0.001, t(6) = 8.3]. We observed ERα immunoreactivity (ERα-ir) in cells overlaid with silver grains (representing GHSR mRNA expression) in the AVPV, MPO, VMHvl, and ARC (Fig. 1). In general, the colocalization rate was similar regardless of the steroid hormone milieu [AVPV: P = 0.73, t(4) = 0.36; MPO: P = 0.69, t(7) = 0.40; VMHvl: P = 0.13, t(5) = 1.81]. However, in the ARC, we detected a sharp increase in coexpression of ERα and GHSR in OVX + E2 females compared with the oil-treated group [P < 0.001, t(5) = 7.5; Table 1]. Importantly, ERα-ir in the ARC was not different between OVX vs. OVX + E2 mice.
Table 1.
GHSR and ERα are coexpressed in defined hypothalamic sites
| Region (Groups) | Atlas Level | Total ERα-ir | ERα-ir + GHSR | %Double/ERα-ir |
|---|---|---|---|---|
| AVPV | ||||
| OVX | 28/29 | 103.3 ± 17.1 | 30.3 ± 5.5 | 31.0 ± 6.5 |
| OVX + E2 | 28/29 | 79.6 ± 15.2 | 38.8 ± 9.0 | 47.6 ± 5.5 |
| MPO | ||||
| OVX | 29/30 | 153.0 ± 7.0 | 56.7 ± 2.5 | 37.1 ± 0.4 |
| OVX + E2 | 29/30 | 143.2 ± 11.5 | 57.4 ± 7.2 | 39.2 ± 4.4 |
| VMHvl | ||||
| OVX | 44/45 | 91.0 ± 7.2 | 40.3 ± 5.8 | 44.3 ± 0.7 |
| OVX + E2 | 44/45 | 114.8 ± 9.8 | 45.5 ± 6.1 | 39.6 ± 2.1 |
| ARC | ||||
| OVX | 50/51 | 86.0 ± 11.0 | 15.5 ± 3.5 | 17.8 ± 1.8 |
| OVX + E2 | 50/51 | 79.0 ± 7.9 | 65.8 ± 6.9** | 83.1 ± 1.5*** |
Values represent estimates of mean counts of cells ± SE (n = 4/group). GHSR, ghrelin receptor; ERα, estrogen receptor-α; AVPV, anteroventral periventricular nucleus; OVX, ovariectomized; E2, estradiol; MPO, medial preoptic nucleus; VMHvl, ventrolateral subdivision of the ventromedial nucleus of the hypothalamus; ARC, arcuate nucleus. The atlas level designations correspond to those described in The Mouse Brain in Stereotaxic Coordinates (39).
P < 0.01 and
P < 0.001, Student's t-test comparing ovariectomized oil-treated (OVX) vs. ovariectomized estradiol treated (OVX + E2 mice).
Fig. 1.
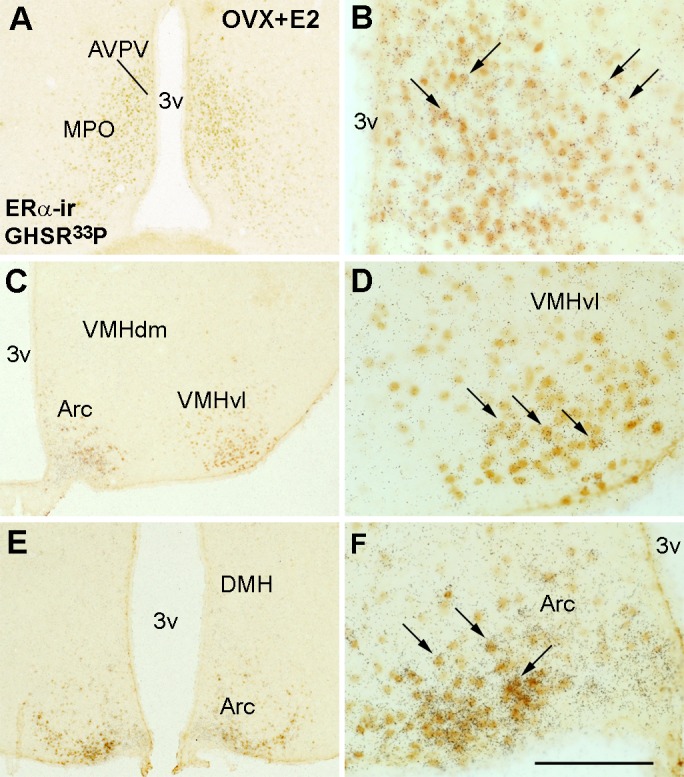
Ghrelin receptor (GHSR) and estrogen receptor-α (ERα) are coexpressed in defined hypothalamic sites. A–F: bright-field illumination showing distribution of ERα immunoreactivity and GHSR mRNA (hybridization signal, silver grains) in the medial aspect of the medial preoptic nucleus (MPO) and in the anteroventral periventricular nucleus (AVPV) (A and B; B is higher magnification of A) in the ventrolateral subdivision of the ventromedial nucleus of the hypothalamus/ventromedial nucleus of the hypothalamus (VMHvl) (C and D; D is higher magnification of C) and in the arcuate nucleus (ARC) (E and F; F is higher magnification of E). Images illustrate data from an ovariectomized estradiol-primed mouse (OVX + E2). Arrows indicate neurons expressing both receptors. 3v, 3rd ventricle; VMHdm, dorsomedial subdivision of the ventromedial nucleus of the hypothalamus. Scale bar: A, C, and E = 400 μm; B, D, and F = 200 μm.
GHSR mRNA expression is altered by E2 treatment.
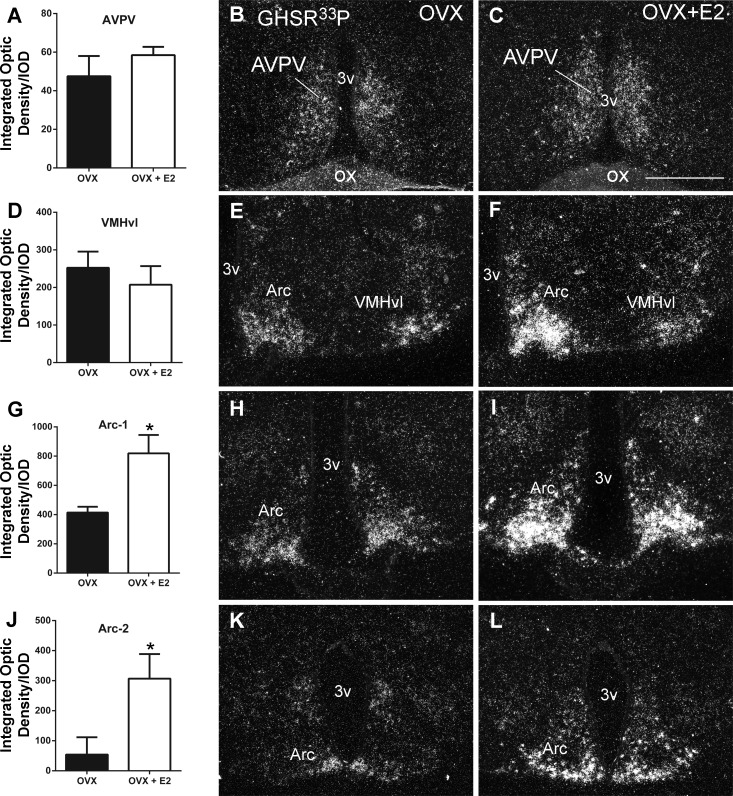
Because of the unexpected observation of an increased colocalization rate of GHSR mRNA and ERα-ir upon E2 exposure, we performed a systematic evaluation of GHSR expression in the hypothalamus of oil-treated and E2-treated OVX mice. GHSR mRNA expression was not altered in the AVPV, MPO, and VMHvl [AVPV/MPO: P = 0.3919, t(3.9) = 0.96; VMHvl: P = 0.26, t(4.8) = 1.29]. In contrast, the GHSR signal was very robust and widespread in the ARC of OVX + E2 compared with OVX mice [ARC1: P < 0.01, t(3.7) = 6.1; ARC2: P < 0.05; t(3.6) = 4.4; Fig. 2].
Fig. 2.
Estradiol (E2) increases GHSR mRNA expression in neurons of the ARC. A, D, G, and J: bar graphs showing quantification of GHSR mRNA expression [hybridization signal by integrated optic density (IOD)] in the AVPV (A), in the VMHvl (D), and in 2 rostro-to-caudal levels of the ARC (G and J) of OVX oil-treated (OVX) and E2-treated (OVX + E2) mice. B, C, E, F, H, I, K, and L: dark-field illumination showing GHSR mRNA (hybridization signal, silver grains) in the AVPV (B and C), in the VMHvl (E and F), and in 2 rostro-to-caudal levels of the ARC (H, I, and K, and L) of OVX and OVX + E2 mice. Note the higher density of silver grains in the ARC of OVX + E2 compared with OVX mice. Data are expressed as means ± SE. *P < 0.01. OX, optic chiasm. Scale bar, 400 μm.
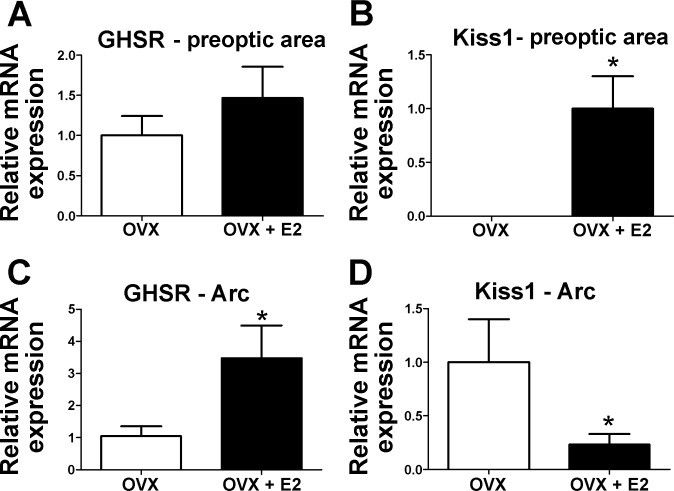
To further quantify the effects of E2 on GHSR mRNA expression, we isolated tissue from the preoptic area (AVPV and MPO) and caudal ARC of oil-treated and E2-treated OVX mice. Kiss1 mRNA expression was used as control (43). Similar to the above histochemistry findings, qPCR analysis revealed that GHSR mRNA expression was unchanged in the preoptic area of OVX vs. OVX + E2 mice (Fig. 3, A and B), whereas in the ARC GHSR mRNA expression increased threefold in E2-treated mice (Fig. 3, C and D).
Fig. 3.
GHSR mRNA expression is increased in the ARC of E2-treated mice. A and D: bar graphs showing the relative GHSR and Kiss1 mRNA expression in the preoptic area (including the AVPV; A and B) and in the ARC (C and D) of OVX and OVX + E2 mice. Kiss1 mRNA levels were used as a control for the treatment groups. Data are presented as means ± SE; *P < 0.05.
E2 induces GHSR expression in subsets of Kiss1 neurons of the ARC.
ERα in the AVPV and ARC colocalizes with kisspeptin (45), and previous studies have indicated that ghrelin may alter Kiss1 gene expression (13). As such, we assessed whether Kiss1 neurons coexpress GHSR in both sites. To do this, we used double-label ISH/IHC in hypothalamic sections of previously described Kiss1 reporter mice (Kiss1-Cre/eGFP) (7, 14). We observed that 10–20% of Kiss1 cells in the AVPV of female mice coexpress GHSR regardless of sex steroid status (Table 2). We further observed that 25% of Kiss1 cells in the ARC of OVX oil-treated mice express GHSR mRNA (Fig. 4A). In contrast, treatment with E2 had a profound effect on these parameters. In particular, in the ARC of OVX + E2 mice, much more GHSR signal overall was detected, and ∼80% of Kiss1 cells were overlaid with silver grains (Fig. 4B). No effect of E2 was noticed in Kiss1 cells of the AVPV. The exact counts of Kiss1- and double-labeled cells are shown in Table 2. As expected for this transgenic Cre-dependent eGFP reporter mouse line, the number of eGFP-expressing Kiss1 cells was unaltered by the presence or absence of E2 (7).
Table 2.
GHSR mRNA is coexpressed in subsets of Kiss1 neurons
| Region (Groups) | Atlas Level | Total Kiss1 | Kiss1+ GHSR | %Double/Kiss1 |
|---|---|---|---|---|
| AVPV | ||||
| Diestrus | 29 | 32.2 ± 2.5 | 3.2 ± 0.4 | 9.34 ± 0.7 |
| OVX | 29 | 36.7 ± 6.6 | 6.7 ± 2.0 | 17.9 ± 4.6 |
| OVX + E | 29 | 21.0 ± 2.5 | 3.0 ± 0.9 | 22.7 ± 3.1 |
| ARC1 | ||||
| Diestrus | 44/45 | 46.33 ± 4.8 | 17.67 ± 3.2 | 37.6 ± 3.5% |
| OVX | 44/45 | 35.3 ± 17.9 | 7.7 ± 0.7 | 25.6 ± 7.2 |
| OVX + E | 44/45 | 33.0 ± 3.4 | 26.5 ± 3.0* | 80.6 ± 4.3** |
| ARC2 | ||||
| Diestrus | 50/51 | 42.33 ± 2.8 | 14.33 ± 2.4 | 33.4 ± 3.3% |
| OVX | 50/51 | 43.0 ± 4.7 | 9.7 ± 2.8 | 22.0 ± 6.0 |
| OVX + E | 50/51 | 47.7 ± 7.1 | 39.3 ± 9.0* | 80.6 ± 9.2** |
Values represent estimates of mean counts of cells ± SE (n = 4/group). ARC1 and -2, the 2 rostro-to-caudal levels of the arcuate nucleus. The atlas level designations correspond to those described in The Mouse Brain in Stereotaxic Coordinates (39).
P < 0.05 and
P < 0.01 (2-way ANOVA comparing the 3 groups).
Fig. 4.
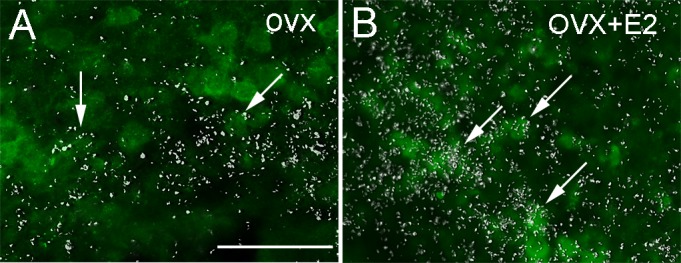
E2 increases GHSR mRNA expression in Kiss1 neurons of the ARC. A and B: fluorescent images showing expression of GHSR mRNA (hybridization signal, silver grains) in Kiss1 neurons [green fluorescent protein (GFP) immunoreactivity] of the ARC of OVX (A) and OVX + E2 mice (B). Arrows indicate dual-labeled neurons. Note the higher expression of GHSR mRNA (silver grains) and higher no. of dual-labeled neurons (GHSR + Kiss1) in the ARC of OVX + E2 mice. Scale bar, 100 μm.
Ghrelin depolarizes subsets of Kiss1 neurons of the ARC.
Our observation that GHSR is expressed in a subset of Kiss1 neurons suggested that ghrelin indeed can signal directly to these neurons. Thus, we used acute hypothalamic slice preparations from Kiss1-Cre/GFP transgenic mice to examine the effects of ghrelin (100 nM) on the membrane potential of Kiss1 neurons. Kiss1 neurons were identified by GFP signals under a fluorescent microscope (Fig. 5, A and B). Alexa Fluor 594 was added to the intracellular pipette solutions (Fig. 5C) for real-time confirmation that GFP-positive neurons were targeted for recording (Fig. 5D). We recorded from 46 Kiss1-Cre/GFP neurons in aCSF bath solutions. Similar to previous observations (14, 17), ARC Kiss1 neurons had a resting membrane potential (RMP) of −51.0 ± 2.0 mV in current clamp mode (n = 21 cells from 8 mice on diestrus). Application of ghrelin depolarized six out of 13 ARC Kiss1 neurons from females on diestrus (46%, 4.5 ± 0.6 mV; Fig. 5E). These data are in agreement with the colocalization rate of Kiss1-Cre/GFP and GHSR mRNA observed in the ARC of diestrous females (Table 2). In the presence of TTX (1 μM), application of ghrelin (100 nM) resulted in a depolarization from rest in four out of eight Kiss1 neurons (50%, 5.8 ± 0.8 mV; Fig. 5F), which is indicative of a direct membrane depolarization independent of action potential-mediated synaptic transmission.
Fig. 5.
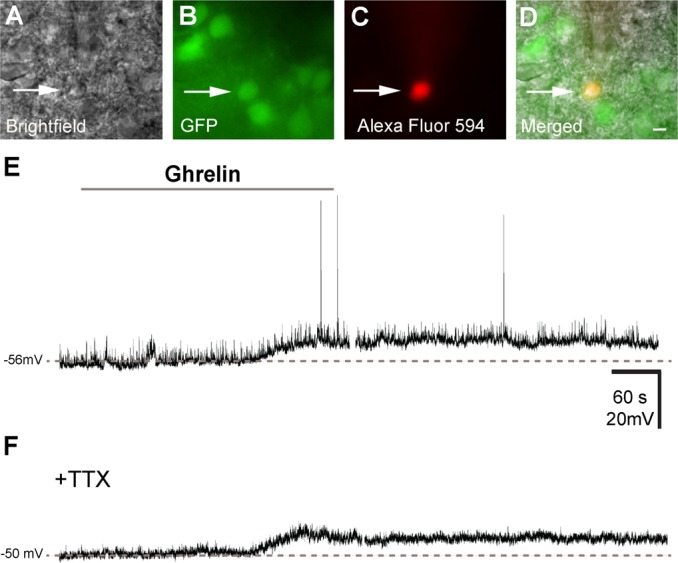
Ghrelin depolarizes a subset of Kiss1 neurons of the ARC. A: bright-field illumination of Kiss1-Cre/GFP neuron during acquisition of a whole recording (arrow). B: the same neuron under fluorescent (FITC) illumination to identify Kiss1-Cre/GFP signal. C: complete dialysis of Alexa Fluor 594 from the intracellular pipette at the end of the recording. D: colocalization of Alexa Fluor 594 and GFP in the same neuron. E: current clamp recording demonstrates that ghrelin (100 nM) depolarizes some Kiss1-Cre/GFP neurons. Dashed line indicates the resting membrane potential. F: current clamp recording demonstrates that ghrelin depolarizes Kiss1-Cre/GFP neurons in the presence of tetrodotoxin (TTX; 1 mM). Scale bar, 10 μm.
To further delineate whether acute actions of ghrelin on ARC Kiss1 neurons are modulated by E2, neurons from OVX and OVX + E2 mice were recorded. E2 levels did not influence RMP of ARC Kiss1 neurons [OVX: −59.6 ± 3.4 mV, n = 9 cells from 3 mice; OVX + E2: −55.7 ± 0.9 mV, n = 8 cells from 3 mice, t(15) = 1.0; P > 0.05], although steady-state capacitance was influenced by E2 [OVX: 17.2 ± 2.0 pF, n = 9 cells from 3 mice; OVX + E2: 8.9 ± 1.1 pF, n = 8 cells from 3 mice, t(15) = 3.3, P < 0.05], as reported previously (14). Interestingly, the number of cells that responded to acute ghrelin action varied depending on E2 levels. Ghrelin depolarized two out of nine ARC Kiss1 neurons in OVX mice (22%, 7.0 ± 3.0 mV; from 3 mice), whereas in OVX + E2 mice, six out of eight cells were depolarized by ghrelin application (75%, 6.8 ± 1.1 mV, from 3 mice). The difference in percentage of Kiss1 cells responding to ghrelin comparing both groups (P < 0.05, df = 1) is in agreement with the colocalization rate of Kiss1-Cre/GFP and GHSR mRNA observed in the ARC of OVX and OVX + E2 females (Table 2). In current clamp mode, ghrelin did not affect the RMP of recorded AVPV Kiss1 neurons [RMP before ghrelin's application: −63 ± 3.9 mV; RMP after ghrelin: −63 ± 4.0 mV; t(14) = 0.1, P > 0.05; n = 8 cells from 6 mice].
DISCUSSION
In the present study, we have shown that GHSR and ERα are coexpressed in several hypothalamic sites, including the AVPV, the MPO, the VMHvl, and the ARC. Notably, high levels of E2 induced GHSR mRNA expression selectively in the ARC. The E2 induction of GHSR gene expression was detected in ARC neurons expressing ERα and Kiss1. Ghrelin acutely depolarizes ARC Kiss1 neurons, and this effect is modulated by changing levels of E2.
The potential relevance of these findings is broad. A series of studies in different species and physiological conditions have demonstrated that ghrelin administration decreases the pulsatile release of LH (10, 11, 15, 27, 30, 34, 48). For example, in rats, acute ghrelin administration decreased LH secretion in intact and orchidectomized prepubertal males, in ovariectomized prepubertal females, and in cycling and aged females (10, 11, 13, 15, 30). This effect seems to be exerted primarily through the brain since in vitro approaches have demonstrated that ghrelin may act in an opposite manner at the pituitary level; i.e., it stimulates gonadotropin release directly from gonadotropes (11, 40). However, little is known about the sites of action of ghrelin to suppress LH pulsatility in the brain. One potential candidate is kisspeptin neurons, as ghrelin administration decreases Kiss1 mRNA expression in the preoptic area of rats (13). The data here support the model that ghrelin acts in kisspeptin neurons of the ARC to modulate the neuroendocrine reproductive axis. They further indicate that this effect is dependent on circulating E2 levels. The physiology behind these findings is not currently clear, but ghrelin actions in Kiss1 neurons may contribute to the negative feedback actions of E2. Although it seems a logical prediction, further studies are necessary to assess the role of direct ghrelin signaling in Kiss1 neurons.
Previous studies have shown that an interaction between ghrelin and estrogens in metabolic control may exist (3, 6). With this in mind, and because of the crucial role of estrogen in LH secretion, we first determined the potential hypothalamic sites involved in ghrelin/estrogen interaction. Notably, we found coexpression of ERα and GHSR in areas highly implicated in reproductive control. In the preoptic area, the AVPV is critical for the positive feedback actions of estrogen (20, 50), and the MPO, a sexually dimorphic site, has a central integrative role in reproductive-related behaviors (e.g., male sexual behavior and maternal behavior) in sensing environmental cues and in neuroendocrine regulation (1, 29, 37, 38). The VMHvl has a pivotal role in female sexual behavior (12, 35), and the ARC is thought to be essential in the negative feedback actions of estrogen (31, 44, 45). Importantly, ARC neurons are prime sensors of the internal environment integrating metabolic and reproductive functions (9, 23). Therefore, ghrelin may act directly in several nodes of the hypothalamic circuitry to inhibit reproduction.
Of special interest for our study is that two sites of GHSR expression and colocalization with ERα are those expressing Kiss1. Thus, ghrelin could potentially act through Kiss1 neurons to regulate the hypothalamus-pituitary-gonads axis (13). Because studies thus far have yielded little evidence for direct signaling of ghrelin to Kiss1 neurons, we sought to determine whether indeed Kiss1 neurons express GHSR and to more closely examine the effects of ghrelin signaling upon these cells. We observed that GHSR mRNA is present in both the AVPV and ARC regions of the female mouse hypothalamus (where Kiss1 expressing neurons reside), but only in the ARC do a substantial number of Kiss1 cells contain GHSR mRNA.
Electrophysiological recordings revealed that roughly half of ARC Kiss1 neurons of females on diestrus responded to ghrelin. If ghrelin does interact directly with Kiss1 cells to diminish their output, we would expect that ghrelin treatment would suppress neuronal activity and perhaps hyperpolarize the cell, making it more difficult to fire an action potential. Surprisingly, we observed that when ghrelin evoked a response, it was always depolarizing. These data suggest that ghrelin enhances the electrical activity of Kiss1 neurons in the ARC.
At first glance, this finding of a depolarizing effect of ghrelin on Arc Kiss1 neurons seems counterintuitive, but another unexpected observation changes the perspective. In particular, our analysis of Kiss1 and Ghsr expression not only revealed that these two genes are coexpressed in subsets of the same neuronal populations but also showed that Ghsr expression patterns and activity are influenced by E2. Whether this is a direct effect of ERα upon the transcription of Ghsr gene is unknown, but one could argue that if that was the case we would expect to also see changes in other brain sites that colocalize both receptors. However, the mechanisms by which changing levels of E2 modulate Ghsr expression are undefined, and whether specific intracellular pathways or neuromediators are required for this effect to take place in subsets of neurons is yet to be determined. Of note is that data obtained from OVX and OVX + E2 approaches, although widely used by multiple laboratories in studies assessing E2 actions, should be interpreted as indicative of a particular phenomenon. Additional studies using distinct methodologies are necessary to test the model.
Our results also alter our take on the effects of ghrelin on kisspeptin neurons of the ARC, because when E2 levels are high, Ghsr expression is increased, but Kiss1 expression is diminished (44, 45). Thus, if ghrelin stimulates the activity of Kiss1 neurons, as our electrophysiological data suggest, it seems likely that these neurons would be releasing less kisspeptin and more of another factor. Two known factors are dynorphin and neurokinin B, both neuropeptides that are present in Kiss1 neurons, and dynorphin has inhibitory effects on GnRH output (18, 26, 36). However, evidence suggests that, similar to Kiss1, expression of these neuropeptides is downregulated by E2 in the ARC (36). Kiss1 neurons of the ARC also contain the neurotransmitters GABA and glutamate, which can modulate the activity of GnRH neurons (7, 21, 24, 32, 33). Thus, any effects of ghrelin upon kisspeptin-containing neurons may in fact be mediated by the release of another neurotransmitter rather than the stimulatory kisspeptin signal. Together, these results suggest that E2 sensitizes Kiss1 neurons to ghrelin and that ghrelin in turn increases the activity of Kiss1 neurons. It remains to be seen what neurotransmitters are released by Kiss1 neurons in the ARC under these conditions and whether the net effect would be stimulatory or inhibitory to GnRH neurons.
Overall, our study suggests a model in which ghrelin interacts directly with a subpopulation of Kiss1-expressing neurons in the ARC to modulate their activity and that exposure to E2 increases the sensitivity of these neurons to ghrelin signals. In this context, our findings reinforce those from other groups indicating that the estrogen milieu functions as a critical player in the metabolic control of the reproductive physiology.
GRANTS
This work was supported by the Foundation for Prader-Willi Research (to C. F. Elias), by the National Institutes of Health (Grants R01-HD-061539 and R01-HD-69702 to C. F. Elias, R01-DA-024680 and R01-MH-085298 to J. M. Zigman, K01-DK-087780 to K. W. Williams, and F32-DK-085834 to H. M. Dungan Lemko), and by the São Paulo Research Foundation [State of Sao Paulo Research Foundation (FAPESP) Grant nos. 2012/12202-4 and 2012/12554-8 to R. Frazao and 2013/00801-3 to R. P. da Silva].
AUTHOR CONTRIBUTIONS
R.F., H.M.D.L., R.P.d.S., D.V.r., and C.E.L. performed experiments; R.F., H.M.D.L., R.P.d.S., and K.W.W. analyzed data; R.F., H.M.D.L., K.W.W., J.M.Z., and C.F.E. interpreted results of experiments; R.F. and H.M.D.L. prepared figures; R.F., H.M.D.L., and C.F.E. drafted manuscript; R.F., H.M.D.L., and C.F.E. edited and revised manuscript; J.M.Z. and C.F.E. conception and design of research; J.M.Z. and C.F.E. approved final version of manuscript.
REFERENCES
- 1.Balthazart J, Ball GF. Topography in the preoptic region: differential regulation of appetitive and consummatory male sexual behaviors. Front Neuroendocrinol 28: 161–178, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burke MC, Letts PA, Krajewski SJ, Rance NE. Coexpression of dynorphin and neurokinin B immunoreactivity in the rat hypothalamus: Morphologic evidence of interrelated function within the arcuate nucleus. J Comp Neurol 498: 712–726, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Butera PC, Wojcik DM, Clough SJ. Effects of estradiol on food intake and meal patterns for diets that differ in flavor and fat content. Physiol Behav 99: 142–145, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castaneda TR, Tong J, Datta R, Culler M, Tschop MH. Ghrelin in the regulation of body weight and metabolism. Front Neuroendocrinol 31: 44–60, 2010 [DOI] [PubMed] [Google Scholar]
- 5.Chuang JC, Sakata I, Kohno D, Perello M, Osborne-Lawrence S, Repa JJ, Zigman JM. Ghrelin directly stimulates glucagon secretion from pancreatic alpha-cells. Mol Endocrinol 25: 1600–1611, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clegg DJ, Brown LM, Zigman JM, Kemp CJ, Strader AD, Benoit SC, Woods SC, Mangiaracina M, Geary N. Estradiol-dependent decrease in the orexigenic potency of ghrelin in female rats. Diabetes 56: 1051–1058, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Cravo RM, Margatho LO, Osborne-Lawrence S, Donato J, Jr, Atkin S, Bookout AL, Rovinsky S, Frazao R, Lee CE, Gautron L, Zigman JM, Elias CF. Characterization of Kiss1 neurons using transgenic mouse models. Neuroscience 173: 37–56, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dungan HM, Gottsch ML, Zeng H, Gragerov A, Bergmann JE, Vassilatis DK, Clifton DK, Steiner RA. The role of kisspeptin-GPR54 signaling in the tonic regulation and surge release of gonadotropin-releasing hormone/luteinizing hormone. J Neurosci 27: 12088–12095, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elias CF, Purohit D. Leptin signaling and circuits in puberty and fertility. Cell Mol life Sci 70: 841–862, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fernández-Fernández R, Tena-Sempere M, Aguilar E, Pinilla L. Ghrelin effects on gonadotropin secretion in male and female rats. Neurosci Lett 362: 103–107, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Fernández-Fernández R, Tena-Sempere M, Navarro VM, Barreiro ML, Castellano JM, Aguilar E, Pinilla L. Effects of ghrelin upon gonadotropin-releasing hormone and gonadotropin secretion in adult female rats: in vivo and in vitro studies. Neuroendocrinology 82: 245–255, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Flanagan-Cato LM. Sex differences in the neural circuit that mediates female sexual receptivity. Front Neuroendocrinol 32: 124–136, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Forbes S, Li XF, Kinsey-Jones J, O'Byrne K. Effects of ghrelin on Kisspeptin mRNA expression in the hypothalamic medial preoptic area and pulsatile luteinising hormone secretion in the female rat. Neurosci Lett 460: 143–147, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Frazao R, Cravo RM, Donato J, Jr, Ratra DV, Clegg DJ, Elmquist JK, Zigman JM, Williams KW, Elias CF. Shift in Kiss1 cell activity requires estrogen receptor alpha. J Neurosci 33: 2807–2820, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Furuta M, Funabashi T, Kimura F. Intracerebroventricular administration of ghrelin rapidly suppresses pulsatile luteinizing hormone secretion in ovariectomized rats. Biochem Biophys Res Commun 288: 780–785, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Gavériaux-Ruff C, Kieffer BL. Conditional gene targeting in the mouse nervous system: Insights into brain function and diseases. Pharmacol Ther 113: 619–634, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Gottsch ML, Popa SM, Lawhorn JK, Qiu J, Tonsfeldt KJ, Bosch MA, Kelly MJ, Ronnekleiv OK, Sanz E, McKnight GS, Clifton DK, Palmiter RD, Steiner RA. Molecular properties of Kiss1 neurons in the arcuate nucleus of the mouse. Endocrinology 152: 4298–4309, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grachev P, Li XF, Kinsey-Jones JS, di Domenico AL, Millar RP, Lightman SL, O'Byrne KT. Suppression of the GnRH pulse generator by neurokinin B involves a kappa-opioid receptor-dependent mechanism. Endocrinology 153: 4894–4904, 2012 [DOI] [PubMed] [Google Scholar]
- 19.Harrison JL, Miller DW, Findlay PA, Adam CL. Photoperiod influences the central effects of ghrelin on food intake, GH and LH secretion in sheep. Neuroendocrinology 87: 182–192, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Herbison AE. Estrogen positive feedback to gonadotropin-releasing hormone (GnRH) neurons in the rodent: The case for the rostral periventricular area of the third ventricle (RP3V). Brain Res Rev 57: 277–287, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herbison AE, Moenter SM. Depolarising and hyperpolarising actions of GABA(A) receptor activation on gonadotrophin-releasing hormone neurones: towards an emerging consensus. J Neuroendocrinol 23: 557–569, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Higgins SC, Gueorguiev M, Korbonits M. Ghrelin, the peripheral hunger hormone. Ann Med 39: 116–136, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Hill JW, Elmquist JK, Elias CF. Hypothalamic pathways linking energy balance and reproduction. Am J Physiol Endocrinol Metab 294: E827–E832, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iremonger KJ, Constantin S, Liu X, Herbison AE. Glutamate regulation of GnRH neuron excitability. Brain Res 1364: 35–43, 2010 [DOI] [PubMed] [Google Scholar]
- 25.Kennedy GC, Mitra J. Body weight and food intake as initiating factors for puberty in the rat. J Physiol 166: 408–418, 1963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kinsey-Jones JS, Grachev P, Li XF, Lin YS, Milligan SR, Lightman SL, O'Byrne KT. The inhibitory effects of neurokinin B on GnRH pulse generator frequency in the female rat. Endocrinology 153: 307–315, 2012 [DOI] [PubMed] [Google Scholar]
- 27.Kluge M, Schussler P, Schmidt D, Uhr M, Steiger A. Ghrelin suppresses secretion of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) in women. J Clin Endocrinol Metab 97: E448–E451, 2012 [DOI] [PubMed] [Google Scholar]
- 28.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 402: 656–660, 1999 [DOI] [PubMed] [Google Scholar]
- 29.Kollack-Walker S, Newman SW. Mating and agonistic behavior produce different patterns of Fos immunolabeling in the male Syrian hamster brain. Neuroscience 66: 721–736, 1995 [DOI] [PubMed] [Google Scholar]
- 30.Martini AC, Fernández-Fernández R, Tovar S, Navarro VM, Vigo E, Vazquez MJ, Davies JS, Thompson NM, Aguilar E, Pinilla L, Wells T, Dieguez C, Tena-Sempere M. Comparative analysis of the effects of ghrelin and unacylated ghrelin on luteinizing hormone secretion in male rats. Endocrinology 147: 2374–2382, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Mayer C, Acosta-Martinez M, Dubois SL, Wolfe A, Radovick S, Boehm U, Levine JE. Timing and completion of puberty in female mice depend on estrogen receptor alpha-signaling in kisspeptin neurons. Proc Natl Acad Sci USA 107: 22693–22698, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moenter SM, Chu Z, Christian CA. Neurobiological mechanisms underlying oestradiol negative and positive feedback regulation of gonadotrophin-releasing hormone neurones. J Neuroendocrinol 21: 327–333, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moenter SM, DeFazio RA. Endogenous gamma-aminobutyric acid can excite gonadotropin-releasing hormone neurons. Endocrinology 146: 5374–5379, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Muccioli G, Lorenzi T, Lorenzi M, Ghe C, Arnoletti E, Raso GM, Castellucci M, Gualillo O, Meli R. Beyond the metabolic role of ghrelin: a new player in the regulation of reproductive function. Peptides 32: 2514–2521, 2011 [DOI] [PubMed] [Google Scholar]
- 35.Musatov S, Chen W, Pfaff DW, Kaplitt MG, Ogawa S. RNAi-mediated silencing of estrogen receptor {alpha} in the ventromedial nucleus of hypothalamus abolishes female sexual behaviors. Proc Natl Acad Sci USA 103: 10456–10460, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J Neurosci 29: 11859–11866, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Numan M, Woodside B. Maternity: neural mechanisms, motivational processes, and physiological adaptations. Behav Neurosci 124: 715–741, 2010 [DOI] [PubMed] [Google Scholar]
- 38.Paredes RG, Baum MJ. Role of the medial preoptic area/anterior hypothalamus in the control of masculine sexual behavior. Annu Rev Sex Res 8: 68–101, 1997 [PubMed] [Google Scholar]
- 39.Paxinos G, Franklin C. The Mouse Brain in Stereotaxic Coordinates. Sydney, Australia: Academic, 2001 [Google Scholar]
- 40.Sakata I, Park WM, Walker AK, Piper PK, Chuang JC, Osborne-Lawrence S, Zigman JM. Glucose-mediated control of ghrelin release from primary cultures of gastric mucosal cells. Am J Physiol Endocrinol Metab 302: E1300–E1310, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Simerly RB, Carr AM, Zee MC, Lorang D. Ovarian steroid regulation of estrogen and progesterone receptor messenger ribonucleic acid in the anteroventral periventricular nucleus of the rat. J Neuroendocrinol 8: 45–56, 1996 [DOI] [PubMed] [Google Scholar]
- 42.Simerly RB, Young BJ. Regulation of estrogen receptor messenger ribonucleic acid in rat hypothalamus by sex steroid hormones. Mol Endocrinol 5: 424–432, 1991 [DOI] [PubMed] [Google Scholar]
- 43.Smith HR, Pang KC. Orexin-saporin lesions of the medial septum impair spatial memory. Neuroscience 132: 261–271, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Smith JT, Clay CM, Caraty A, Clarke IJ. KiSS-1 messenger ribonucleic acid expression in the hypothalamus of the ewe is regulated by sex steroids and season. Endocrinology 148: 1150–1157, 2007 [DOI] [PubMed] [Google Scholar]
- 45.Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology 146: 3686–3692, 2005 [DOI] [PubMed] [Google Scholar]
- 46.Tena-Sempere M. Ghrelin and reproduction: ghrelin as novel regulator of the gonadotropic axis. Vitam Horm 77: 285–300, 2008 [DOI] [PubMed] [Google Scholar]
- 47.Tschop M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature 407: 908–913, 2000 [DOI] [PubMed] [Google Scholar]
- 48.Vulliemoz NR, Xiao E, Xia-Zhang L, Germond M, Rivier J, Ferin M. Decrease in luteinizing hormone pulse frequency during a five-hour peripheral ghrelin infusion in the ovariectomized rhesus monkey. J Clin Endocrinol Metab 89: 5718–5723, 2004 [DOI] [PubMed] [Google Scholar]
- 49.Wade GN, Schneider JE, Li HY. Control of fertility by metabolic cues. Am J Physiol Endocrinol Metab 270: E1–E19, 1996 [DOI] [PubMed] [Google Scholar]
- 50.Wiegand SJ, Terasawa E. Discrete lesions reveal functional heterogeneity of suprachiasmatic structures in regulation of gonadotropin secretion in the female rat. Neuroendocrinology 34: 395–404, 1982 [DOI] [PubMed] [Google Scholar]
- 51.Williams KW, Margatho LO, Lee CE, Choi M, Lee S, Scott MM, Elias CF, Elmquist JK. Segregation of acute leptin and insulin effects in distinct populations of arcuate proopiomelanocortin neurons. J Neurosci 30: 2472–2479, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu D, Lin G, Gore AC. Age-related changes in hypothalamic androgen receptor and estrogen receptor alpha in male rats. J Comp Neurol 512: 688–701, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xu X, Roby KD, Callaway EM. Mouse cortical inhibitory neuron type that coexpresses somatostatin and calretinin. J Comp Neurol 499: 144–160, 2006 [DOI] [PubMed] [Google Scholar]
- 54.Yamada S, Noguchi D, Ito H, Yamanouchi K. Sex and regional differences in decrease of estrogen receptor alpha-immunoreactive cells by estrogen in rat hypothalamus and midbrain. Neurosci Lett 463: 135–139, 2009 [DOI] [PubMed] [Google Scholar]
- 55.Zigman JM, Elmquist JK. Minireview: From anorexia to obesity—the yin and yang of body weight control. Endocrinology 144: 3749–3756, 2003 [DOI] [PubMed] [Google Scholar]
- 56.Zigman JM, Jones JE, Lee CE, Saper CB, Elmquist JK. Expression of ghrelin receptor mRNA in the rat and the mouse brain. J Comp Neurol 494: 528–548, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]