Abstract
Dirofilaria immitis (heartworm) infections affect domestic dogs, cats, and various wild mammals with increasing incidence in temperate and tropical areas. More sensitive antibody detection methodologies are required to diagnose asymptomatic dirofilariasis with low worm burdens. Applying current transcriptomic technologies would be useful to discover potential diagnostic markers for D. immitis infection. A filarial homologue of the mammalian translationally controlled tumor protein (TCTP) was initially identified by screening the assembled transcriptome of D. immitis (DiTCTP). A BLAST analysis suggested that the DiTCTP gene shared the highest similarity with TCTP from Loa loa at protein level (97%). A histidine-tagged recombinant DiTCTP protein (rDiTCTP) of 40 kDa expressed in Escherichia coli BL21 (DE3) showed immunoreactivity with serum from a dog experimentally infected with heartworms. Localization studies illustrated the ubiquitous presence of rDiTCTP protein in the lateral hypodermal chords, dorsal hypodermal chord, muscle, intestine, and uterus in female adult worms. Further studies on D. immitis-derived TCTP are warranted to assess whether this filarial protein could be used for a diagnostic purpose.
Keywords: Dirofilaria immitis, heartworm, dirofilariasis, translationally controlled tumor protein, transcriptome
INTRODUCTION
The causative agent of cardiopulmonary dirofilariasis, Dirofilaria immitis (heartworm), affects domestic dogs, cats, and various wild mammals with increasing incidence in temperate and tropical areas [1-5]. As mosquito-borne zoonotic pathogens, heartworms can also be transmitted to humans, where they cause pulmonary dirofilariasis [6]. Adult worms reside in pulmonary arteries and right ventricles, resulting in production of blood-circulating microfilariae in dogs as natural hosts [5]. Because dogs with a low worm burden are usually asymptomatic, primary diagnostic screening by detecting blood microfilariae (Mf) or circulating heartworm antigens are necessary prior to treatment [7]. However, due to unapparent infection without Mf in some cases, antigen testing is considered the most sensitive diagnostic method [7]. Therefore, finding a sensitive diagnostic molecular marker for heartworm infections is crucial to control the disease.
Since the initial description of translationally controlled tumor protein (TCTP) in mouse Ehrlich ascites tumor cells and erythroleukemia cells [8-10], TCTPs have been discovered in a wide variety of organisms, including mammals, plants, lower eukaryotes, and prokaryotes. TCTP genes have also been found in Caenorhabditis elegans and various parasites, such as Plasmodium falciparum, Plasmodium yoelii, Trypanosoma brucei, Brugia malayi, and Wuchereria bancrofti [11-15]. Due to their calcium-binding feature and histamine-releasing function in vitro, filarial TCTPs are thought to be associated with parasite survival in the host and initiation of pathology [13]. Detailed research on the physiological roles of TCTP protein in parasites has been conducted, while the question of whether the filarial homologues have immunological roles in the parasitic life stage remains still unclear. High expression levels of TCTP protein have been detected in microfilarial and adult B. malayi. As secretory proteins, Bm-TCTPs are present abundantly in the excretory-secretory (ES) products of microfilariae [13], which are usually exposed to their host immune systems. Given these observations, it has been supposed that filarial TCTP homologues may have potential for immunodiagnosis of filariasis.
Recently, an adult D. immitis transcriptome dataset containing 20,810 unique expressed genes (unigenes) and 15,698 coding sequences (CDS) has been uncovered by using a next-generation sequencing platform and powerful de novo assembly [16]. Based on the comprehensive annotation information of those unigenes, abundant homologous genes, which have not been described in heartworms, were discovered. Here, we initially screened out a D. immitis unigene that was considered as a TCTP homologue, and we cloned and expressed the filarialderived TCTP molecules for further investigation of their potential value for diagnosis.
MATERIALS AND METHODS
Parasites and animals
D. immitis parasites used in the present study were originally derived from an adult dog which was experimentally infected with D. immitis, laboratory raised, and known to be free of intestinal helminths. Three 5-month-old naive rabbits (without previous exposure to ectoparasitic drugs), weighing 2.9-3.2 kg, were provided by the Laboratory Animal Center of Sichuan Agricultural University (China). Institutional Ethical and Animal Care Guidelines were adhered to during the sampling exercises, and all procedures were carried out in accordance with the Guide for the Care and Use of Laboratory Animals.
Sera
For immunohistochemistry, the rabbit immune serum was obtained by immunizing them once with 200 µg purified rDiTCTP with complete Freund's adjuvant and twice with incomplete Freund's adjuvant at 14-day intervals. Serum was collected at week 3 after immunization. The animals were bled at week 2 after the last boost. Blood serum including polyclonal anti-DiTCTP antibodies was collected and purified by HiTrap ProteinA (Bio-Rad, Hercules, California, USA).
For immunoblotting analysis, the positive sera were derived from an infected dog provided by the Department of Parasitology, College of Veterinary Medicine, Sichuan Agricultural University, China.
DNA sequence analysis
The full-length cDNA sequence of DiTCTP used for designing primers was derived from unigene 881 of the assembled D. immitis transcriptome dataset (GenBank accession no. JR896809) in the Transcriptome Shotgun Assembly Sequence Database (TSA) at the National Center for Biotechnology Information (NCBI) [16]. An Open Reading Frame Finder (http://www.ncbi.nlm.nih.gov/projects/gorf/) and the BLAST network server of the NCBI (http://blast.ncbi.nlm.nih.gov/Blast.cgi) were used to analyse open reading frames (ORF) of the nucleotide sequence and deduced amino acid sequences to determine similarities with previously reported sequences in GenBank. Signal PV4.0 at the Center of Biological Sequence Analysis (http://www.cbs.dtu.dk/services/SignalP/) was employed to predict the signal sequence. The DNAStar Protean software was used to predict the secondary structure of amino acid sequence encoded by the DiTCTP gene. The presumption of the 3D structure of the DiTCTP protein was performed through the CPHmodels-3.2 Server online program.
Expression and purification of recombinant rDi-TCTP
Extraction of RNA and cDNA synthesis were performed as described previously [17]. DNA encoding the rDiTCTP domain was amplified by PCR using a sense primer, P1 (5'-GGATCCATGCTGATTTTCAAGG-3') containing a BamH1 site (shown in bold) upstream of the start codon and an antisense primer, P2 (5'-CTCGAGTCATTGTTTTTCTTC-3') containing a Xho1 site (shown in bold), just downstream of the terminator codon. The PCR product was digested with BamH1 and Xho1, ligated into the plasmid expression vector Pet32a(+) (TaKaRa, Kyoto, Japan), which had been processed similarly. The ligated product was transformed into Escherichia coli BL21 (DE3) (Novagen, Darmstadt, Germany). Expression of the recombinant protein (rDiTCTP) was induced with 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG). Purification of rDiTCTP was carried out as described previously [18].
Immunoblot analysis
Briefly, rDiTCTP protein was separated by SDS-PAGE and transferred onto PVDF membranes (Millipore, Billerica, Massachusetts, USA) for 1 hr in an electrophoretic transfer cell (Bio-Rad). After blocking with 5% skimmed milk in TBST (40 mM Tris-HCl, 0.5 M NaCl, 0.1 Tween-20, pH 7.4) for 1 hr at room temperature, membranes were then incubated with dog antiserum diluted 1:200 (v/v) in 1% skimmed milk in TBST over night at 4℃. After washing with TBST, the membrane was incubated with horseradish peroxidase (HRP)-conjugated goat anti-dog antibody for 1 hr. After the final washing with TBST, diaminobenzidine (DAB) reagent (Tiangen, Beijing, China) was used for detecting protein signals.
Immunohistochemistry
For immunohistochemistry, adult female worms were fixed overnight in 4% buffered paraformaldehyde solution and embedded in paraffin. The tissue sections were deparaffinized in xylene, rehydrated through an ethanol to water gradient, and treated with 0.01 M citrate buffer (citric acid), pH 6.0, in a microwave oven at 700 W for 5 min. Before immunostaining, the tissue sections with anti-DiTCTP antibody (diluted to 1:2,000, at 4℃overnight) or pre-immune rabbit sera, non-specific binding was blocked with 0.1% (w/v) glycine in 0.01 M PBS and followed by 4% (w/v) bovine serum albumin (BSA) in 0.01 M PBS for 30 min each. The sections were washed and then incubated with horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG (Life Technologies, Carlsbad, California, USA), diluted to 1:1,000 at room temperature, for 1 hr in darkness. Sections were rinsed with TBS, and then immunoreactivity was detected using the EnvisionTM+ System, HRP (DAB) (DAKO, Glostrup, Denmark). The sections were counterstained with hematoxylin, dehydrated in graded series of alcohol, cleared in xylene, and then observed under a light microscope.
RESULTS
Sequence analysis of DiTCTP
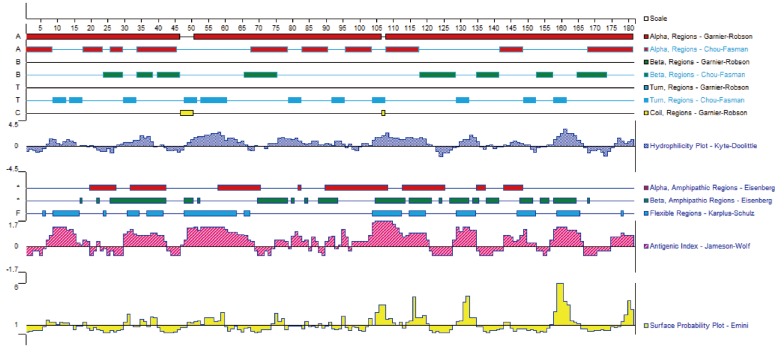
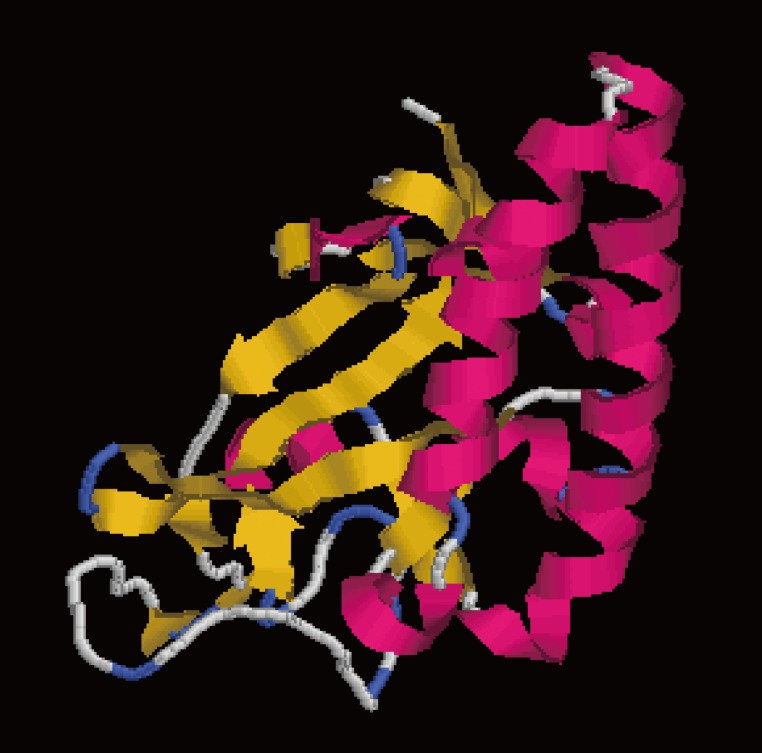
DiTCTP was identified initially while screening the assembled D. immitis transcriptome dataset. According to the annotation information of the unigenes, one of them (unigene 881) showed significant homology with TCTP from Loa loa. ORF analysis suggested that unigene 881 contains a single ORF of 546 base pairs encoding a putative polypeptide of 181 amino acids, with a putative molecular mass of 20.7396 kDa and a pl of 4.62. The sequence produced in this study has been deposited in the GenBank (accession no. JX469415). GenBank analysis showed that DiTCTP shared the highest similarity with a TCTP (GenBank: EFO28099.1) (97%) from L. loa at the protein level, followed by 96% similarity with TCTP from W. bancrofti (GenBank no. AAK71499.1) and 95% similarity with TCTP from B. malayi (GenBank no. EDP33421.1). A Signal PV4.0 analysis suggested that no signal peptide exists in DiTCTP. The protein encoded by the DiTCTP cDNA sequence was predominantly hydrophilic (hydropathy analysis, data not shown). Fig. 1 illustrates that the secondary structures of the DiTCTP protein predicted by different methods showed significant diversity. Three alpha-helices and 2 random coils with no extended strand were predicted by the Garnier-Robson method, while under Chou-Fasman modern, 10 α-helices, 8 extended strand, and 11 random coils were predicted from the amino acid sequence deduced from the DiTCTP gene. Thirteen flexible regions from DiTCTP protein were predicted by the Karplus-Schulz method. The tertiary structure prediction of the DiTCTP protein is shown in Fig. 2.
Fig. 1.
Secondary structures of amino acid sequence encoded by DiTCTP gene predicted by different methods.
Fig. 2.
Tertiary structure prediction of DiTCTP protein. Red parts represent α-helix; the extended strand is coloured in yellow; the random coil is coloured in blue and grey.
Characterization and immunoreactivity of rDiTCTP
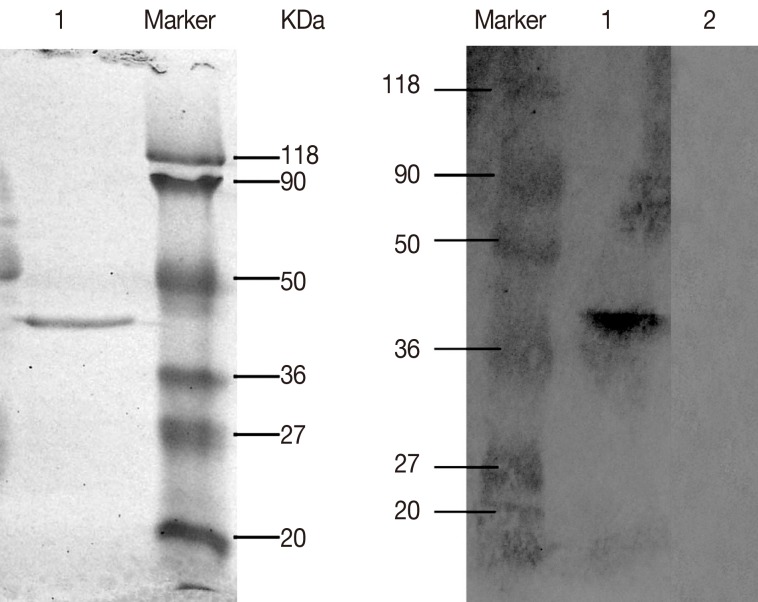
The ORF of DiTCTP cloned into Pet32a (+) was expressed as a His-tagged proteins in E. coli BL21 (DE3). A 10 ml HisBind resin column was used for single step purification of His-tagged DiTCTP protein. The purified histidine-tagged rDiTCTP had an apparent molecular mass of approximately 40 kDa by SDS-PAGE (Fig. 3A). Immunoblot using the serum from dogs with heartworm infections (Fig. 3B), suggested the rDiTCTP protein to be immunoreactive.
Fig. 3.
(A) Purification of recombinant DiTCTP protein, which was resolved on a 12% SDS-PAGE under non-reducing conditions. Lane 1 shows purified recombinant DiTCTP by a single step with 10 ml HisBind resin column. (B) Western blot demonstrating that approximately 2 mg of rDiTCTP reacts to the D. Immitis-infected dog antiserum in 1:200 dilution. Lane 1, western blot detection with D. Immitis-infected dog antisera; Lane 2, western blot detection with naive serum.
rDiTCTP immunolocalization
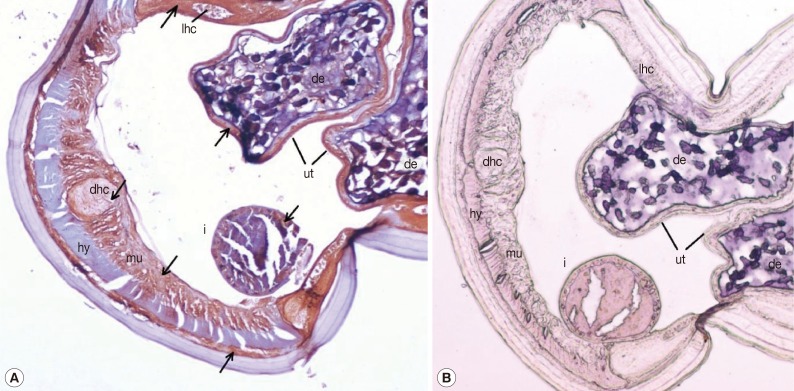
Sections of adult female worms probed with anti-rDiTCTP antibody and preimmune serum are shown in Fig. 4A and 4B, respectively. The purified anti-rDiTCTP antibody stained discrete portions of female adult worms, while pre-immune serum showed minimal or no staining. Intensely stained anatomic features in the cross-sections of female worms were the lateral hypodermal chords, dorsal hypodermal chord, muscle cells, and parenchyma and epithelia of the intestine and uterus. Developing embryos also showed clear staining. The hypodermis was remarkable by its lack of staining.
Fig. 4.
Tissue localization of rDiTCTP protein in transverse sections of female adult worms. Worms were fixed in paraformaldehyde and embedded in paraffin. Sections were immunostained with either purified rabbit anti-rDiTCTP-Ig (1:2,000) (A) or stained with pre-immune rabbit sera (1:2,000) (B). Magnification, × 100. Arrows indicate antibody-labelled regions. i, intestine; ut, uterus; de, developing embryos; hy, hypodermis; mu, muscle; dhc, dorsal hypodermal chord; lhc, lateral hypodermal chord.
DISCUSSION
Here, we cloned and expressed for the first time the TCTP homologue from D. immitis. The assembled heartworm transcriptome provided a more precise full-length reference sequence to design primers for the expression of DiTCTP. The sequencing result showed a 100% similarity between DiTCTP and the putative ORF of unigene 881, which also experimentally verified that the assembled heartworm transcriptome dataset is a creditable and powerful tool to find new genes of D. immitis.
According to the heartworm transcriptome research [16], unigene 881 was included in the heartworm intestinal-expressed transcripts. This could be verified by the significant staining of rDiTCTP in the parenchyma and epithelia of the intestine. However, the dark staining in other organs of heartworms indicated that DiTCTP might not be an intestine-specifically expressed gene, but be a widely expressed, muti-functional gene in D. immitis. Developing embryos also showed clear staining, while non-immunostained cells present in the uterus were probably infertile eggs, which had been passed along with developing microfilariae. However, this wide distribution in tissues seems to be in sharp contrast to the situation with female Ostertagia ostertagi [18], for which TCTP has been reported to be dominant only in eggs, but not in other organs. The study of O. ostertagi-derived TCTP indicated its potentially pivotal role in egg production and development. In contrast to O. ostertagi-derived TCTP, the protein has been identified as an abundant ES product from the microfilarial stage of B. malayi [13], suggesting that the filarial TCTP-induced histamine release will play a major role in the pathogenesis of filariasis. The different distribution of TCTPs indicates that the O. ostertagi-derived TCTP may play a different role in the survival of parasites within the host than the filarial-derived TCTPs. Given the wide distribution of D. immitis-derived TCTP of females and embryos, it is supposed that this protein may be a multistage expressed protein. Previous studies have reported TCTP to be secreted by parasites to modulate the host immune responses, and itself has immunogenicity [13,15,19,20], which coincides with our immunoblot results. Further studies on D. immitis-derived TCTP are warranted to assess whether this filarial protein could be used for a diagnostic purpose.
In the present study, a TCTP homologue protein of D. immitis was initially screened out from our heartworm transcriptome dataset and was subsequently cloned and expressed. The immunogenicity of this protein and its wide distribution in tissues was demonstrated. Further studies on D. immitis-derived TCTP are warranted to assess whether this filarial protein could be used for a diagnostic purpose.
ACKNOWLEDGMENTS
We thank Jun Wang and Chengwei Li for their technical assistance and constructive suggestions. This work was supported by grants from the Research Fund for the Chengdu Giant Panda Breeding Research Foundation (project no. CPF-08005) and Changjiang Scholars and Innovative Research Team in University (PCSIRT) (project no. IRT0848).
Footnotes
We have no conflict of interest related to this work.
References
- 1.Abraham D. Biology of Dirofilaria immitis. In: Boreham PFL, Atwell RB, editors. Dirofilariasis. Boca Raton: CRC Press; 1988. pp. 29–46. [Google Scholar]
- 2.Neiffer DL, Klein EC, Calle PP, Linn M, Terrell SP, Walker RL, Todd D, Vice CC, Marks SK. Mortality associated with melarsomine dihydrochloride administration in two North American river otters (Lontra canadensis) and a red panda (Ailurus fulgens fulgens) J Zoo Wildl Med. 2002;33:242–248. doi: 10.1638/1042-7260(2002)033[0242:MAWMDA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 3.Riley SP, Foley J, Chomel B. Exposure to feline and canine pathogens in bobcats and gray foxes in urban and rural zones of a national park in California. J Wildl Dis. 2004;40:11–22. doi: 10.7589/0090-3558-40.1.11. [DOI] [PubMed] [Google Scholar]
- 4.Ruiz de Ybáñez MR, Martinez-Carrasco C, Martínez JJ, Ortiz JM, Attout T, Bain O. Dirofilaria immitis in an African lion (Panthera leo) Vet Rec. 2006;158:240–242. doi: 10.1136/vr.158.7.240. [DOI] [PubMed] [Google Scholar]
- 5.Simón F, Morchón R, González-Miguel J, Marcos-Atxutegi C, Siles-Lucas M. What is new about animal and human dirofilariosis? Trends Parasitol. 2009;25:404–409. doi: 10.1016/j.pt.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 6.McCall JW, Genchi C, Kramer LH, Guerrero J, Venco L. Heartworm disease in animals and humans. Adv Parasitol. 2008;66:193–285. doi: 10.1016/S0065-308X(08)00204-2. [DOI] [PubMed] [Google Scholar]
- 7.Nelson CT, McCall JW, Rubin SB, Buzhardt LF, Dorion DW, Graham W, Longhofer SL, Guerrero J, Robertson-Plouch C, Paul A. 2005 Guidelines for the diagnosis, prevention and management of heartworm (Dirofilaria immitis) infection in dogs. Vet Parasitol. 2005;133:255–266. doi: 10.1016/j.vetpar.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 8.Böhm H, Benndorf R, Gaestel M, Gross B, Nürnberg P, Kraft R, Otto A, Bielka H. The growth-related protein P23 of the Ehrlich ascites tumor: translational control, cloning and primary structure. Biochem Int. 1989;19:277–286. [PubMed] [Google Scholar]
- 9.Chitpatima ST, Makrides S, Bandyopadhyay R, Brawerman G. Nucleotide sequence of a major messenger RNA for a 21 kilodalton polypeptide that is under translational control in mouse tumor cells. Nucleic Acids Res. 1988;16:2350. doi: 10.1093/nar/16.5.2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yenofsky R, Cereghini S, Krowczynska A, Brawerman G. Regulation of mRNA utilization in mouse erythroleukemia cells induced to differentiate by exposure to dimethyl sulfoxide. Mol Cell Biol. 1983;3:1197–1203. doi: 10.1128/mcb.3.7.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhisutthibhan J, Pan XQ, Hossler PA, Walker DJ, Yowell CA, Carlton J, Dame JB, Meshnick SR. The Plasmodium falciparum translationally controlled tumor protein homolog and its reaction with the antimalarial drug artemisinin. J Biol Chem. 1998;273:16192–16198. doi: 10.1074/jbc.273.26.16192. [DOI] [PubMed] [Google Scholar]
- 12.Bini L, Heid H, Liberatori S, Geier G, Pallini V, Zwilling R. Two-dimensional gel electrophoresis of Caenorhabditis elegans homogenates and identification of protein spots by microsequencing. Electrophoresis. 1997;18:557–562. doi: 10.1002/elps.1150180337. [DOI] [PubMed] [Google Scholar]
- 13.Gnanasekar M, Rao KV, Chen L, Narayanan RB, Geetha M, Scott AL, Ramaswamy K, Kaliraj P. Molecular characterization of a calcium binding translationally controlled tumor protein homologue from the filarial parasites Brugia malayi and Wuchereria bancrofti. Mol Biochem Parasitol. 2002;121:107–118. doi: 10.1016/s0166-6851(02)00027-0. [DOI] [PubMed] [Google Scholar]
- 14.Haghighat NG, Ruben L. Purification of novel calcium binding proteins from Trypanosoma brucei: properties of 22-, 24- and 38-kilodalton proteins. Mol Biochem Parasitol. 1992;51:99–110. doi: 10.1016/0166-6851(92)90205-x. [DOI] [PubMed] [Google Scholar]
- 15.Walker DJ, Pitsch JL, Peng MM, Robinson BL, Peters W, Bhisutthibhan J, Meshnick SR. Mechanisms of artemisinin resistance in the rodent malaria pathogen Plasmodium yoelii. Antimicrob Agents Chemother. 2000;44:344–347. doi: 10.1128/aac.44.2.344-347.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fu Y, Lan J, Zhang Z, Hou R, Wu X, Yang D. Novel Insights into the Transcriptome of Dirofilaria immitis. PLoS One. 2012;7:e41639. doi: 10.1371/journal.pone.0041639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen WJ, Niu DS, Zhang XY, Chen ML, Cui H, Wei WJ, Wen BH, Chen XR. Recombinant 56-kilodalton major outer membrane protein antigen of Orientia tsutsugamushi Shanxi and its antigenicity. Infect Immun. 2003;71:4772–4779. doi: 10.1128/IAI.71.8.4772-4779.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meyvis Y, Houthoofd W, Visser A, Borgonie G, Gevaert K, Vercruysse J, Claerebout E, Geldhof P. Analysis of the translationally controlled tumour protein in the nematodes Ostertagia ostertagi and Caenorhabditis elegans suggests a pivotal role in egg production. Int J Parasitol. 2009;39:1205–1213. doi: 10.1016/j.ijpara.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 19.MacDonald SM, Bhisutthibhan J, Shapiro TA, Rogerson SJ, Taylor TE, Tembo M, Langdon JM, Meshnick SR. Immune mimicry in malaria: Plasmodium falciparum secretes a functional histamine-releasing factor homolog in vitro and in vivo. Proc Natl Acad Sci U S A. 2001;98:10829–10832. doi: 10.1073/pnas.201191498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rao KV, Chen L, Gnanasekar M, Ramaswamy K. Cloning and characterization of a calcium-binding, histamine-releasing protein from Schistosoma mansoni. J Biol Chem. 2002;277:31207–31213. doi: 10.1074/jbc.M204114200. [DOI] [PMC free article] [PubMed] [Google Scholar]