Abstract
Dsk5 mice have a gain of function in the epidermal growth factor receptor (EGFR), caused by a point mutation in the kinase domain. We analyzed the effect of this mutation on liver size, histology, and composition. We found that the livers of 12-wk-old male Dsk5 heterozygotes (+/Dsk5) were 62% heavier compared with those of wild-type controls (+/+). The livers of the +/Dsk5 mice compared with +/+ mice had larger hepatocytes with prominent, polyploid nuclei and showed modestly increased cell proliferation indices in both hepatocytes and nonparenchymal cells. An analysis of total protein, DNA, and RNA (expressed relative to liver weight) revealed no differences between the mutant and wild-type mice. However, the livers of the +/Dsk5 mice had more cholesterol but less phospholipid and fatty acid. Circulating cholesterol levels were twice as high in adult male +/Dsk5 mice but not in postweaned young male or female mice. The elevated total plasma cholesterol resulted mainly from an increase in low-density lipoprotein (LDL). The +/Dsk5 adult mouse liver expressed markedly reduced protein levels of LDL receptor, no change in proprotein convertase subtilisin/kexin type 9, and a markedly increased fatty acid synthase and 3-hydroxy-3-methyl-glutaryl-CoA reductase. Increased expression of transcription factors associated with enhanced cholesterol synthesis was also observed. Together, these findings suggest that the EGFR may play a regulatory role in hepatocyte proliferation and lipid metabolism in adult male mice, explaining why elevated levels of EGF or EGF-like peptides have been positively correlated to increased cholesterol levels in human studies.
Keywords: liver, epidermal growth factor, Dsk5, cholesterol, hepatomegaly, low-density lipoprotein receptor
hepatocytes express an extremely large number of epidermal growth factor receptors (EGFR), which are concentrated on their sinusoidal and laterobasal membranes. EGFR is a 170-kDa glycoprotein that contains a cysteine-rich extracellular ligand-binding domain, a single transmembrane domain, and an intracellular tyrosine kinase domain (44). The intracellular tyrosine kinase domain can be activated immediately after the extracellular domain binds EGF or an EGF-like ligand (44). EGFR, which is also known as ERBB1, belongs to a family that includes three other interacting tyrosine kinases, namely ERBB2, ERBB3, and ERBB4. Upon ligand binding, the constrained extracellular domain of EGFR extends, allowing subsequent dimerization to another EGFR monomer or to an ERBB2, ERBB3, or ERBB4 extended monomer. Dimerization results in activation of the intracellular kinase domains, which leads to transphosphorylation of the dimeric receptors as well as phosphorylation of proximate signaling molecules.
Ligand-mediated ERBB receptor dimerization and signaling has several levels of complexity that can affect cellular response. There is complexity in the ligands themselves. The EGF-like peptide family, including EGF, amphiregulin, transforming growth factor-α (TGF-α), heparin-binding EGF (HBEGF), betacellulin, and epiregulin, can bind EGFR and in some cases ERBB4. In addition, a distinct group of ligands known as the heregulins binds ERBB3 and ERBB4. Moreover, ERBB2 does not bind a known ligand but instead assumes a dimerization-ready extended extracellular confirmation that allows it to heterodimerize with other ligand-bound ERBB molecules. ERBB3, while capable of binding to heregulins, has an inactive kinase domain although it does contain multiple docking sites for phosphatidyl inositol 3-kinase (PI3K). The formation of signaling ERBB dimers bound to distinct EGF or heregulin ligands differentially activates downstream signaling pathways, such as Ras-Raf-MAP-kinase and PI3K-Akt (57). This in turn affects the cellular response to ERBB signaling. The complex interplay between EGF and heregulin ligands, the ERBB receptor tyrosine kinases themselves, and multiple downstream signaling pathways modulates the cellular response, affecting a range of cellular processes, including migration, proliferation, cell viability, differentiation, and metabolism.
The role of the EGF signaling in the hepatocyte has received much attention, partly because of the high density of EGF receptors on these cells. We have studied the ontogeny of ERBB protein expression in the liver of the mouse and rat and found that the hepatocyte expresses EGFR, ERBB2, and ERBB3 during the neonatal phase of development but only EGFR and ERBB3 in adult animals (9). Radioligand binding studies indicate that each hepatocyte of the adult male rodent liver expresses about 600,000 EGF receptors (3) but only 20,000 ERBB3 receptors (8). EGFR has been implicated in the control of liver regeneration and growth; however, the liver of the adult mouse is mitotically inactive under normal circumstances. Few hepatocytes (0.2 to 3.2% dependent on time of day) or sinusoidal nonparenchymal cells (1.1 to 5.7%) traverse the cell cycle each day (50). This implies that EGFR may have other nonproliferative roles.
The physiological function of the extremely large number of EGF receptors in the liver of the normal adult mouse remains unknown. These receptors may play a role in ligand clearance. For example, it has been shown that exogenously administered EGF is efficiently cleared from the portal circulation by hepatocytes and that 19% of EGF undergoes transcytosis and secretion into the bile at the apical canalicular membrane (6, 49). EGFR may also have antiapoptotic roles in the liver (14, 37), protecting cells from apoptotic toxins and xenobiotics derived from the portal blood. EGFR and ERBB3 are also regulated by metabolic events. For example, the expression of EGFR in the liver decreases during fasting (43), hypothyroidism (36), and in type 1 and type 2 diabetes (10), whereas that of ERBB3 increases during fasting and diabetes. These latter increases can be reversed by insulin administration (7). Both EGFR and ERBB3 also show divergent patterns of circadian expression. Peak expression of EGFR occurs late in the dark phase (43), whereas that of ERBB3 occurs at the transition from light to dark (9). Collectively, these results suggest that the two predominant hepatocellular ERBB tyrosine kinases of adult mice may have distinct metabolic roles.
To investigate the physiological role of EGFR in the liver of adult mice, we analyzed several proliferative and metabolic parameters in the Dsk5 mutant mouse model (16). EGFR in this mouse has a Leu863Gln mutation within a region of the kinase domain that stabilizes the receptor activation loop, producing a gain-of-function allele that increases basal EGFR kinase activity. In this study, we describe an unexpected metabolic phenotype in the heterozygote male adult +/Dsk5 mouse on a normal chow diet, namely liver enlargement characterized by increased liver cholesterol as well as increased plasma low-density lipoprotein (LDL) cholesterol and triglyceride. The livers of these mice also exhibit decreased hepatic LDL receptor (LDLR) expression as well as increased hepatic 3-hydroxy-3-methyl-glutaryl (HMG)-CoA-reductase and fatty acid synthase (FAS) expression. Increased expression of transcription factors associated with enhanced cholesterol synthesis was also observed. These results suggest that the ERBB family of receptor tyrosine kinases and their ligands influence hepatic lipid metabolism, raising questions about the mechanisms involved and the significance of this pathway in hypercholesterolemic patients or in patients with cancer treated with EGFR tyrosine kinase inhibitors.
MATERIALS AND METHODS
Mice and genetic crosses.
The EgfrDsk5 allele was initially generated by random mutagenesis with N-ethyl-N-nitrosourea and maintained isogenic on the C3H background (16). 129S1/SvImJ-EgfrDsk5 congenic mice were generated by backcrossing C3H-EgfrDsk5 heterozygous stocks to 129 wild-type mice for more than 10 generations (11). We used heterozygotes primarily because of the difficulty in breeding and maintaining sufficient number of homozygotes. Mice were fed Purina Mills Lab Diet and water ad libitum under specific pathogen-free conditions in an American Association for the Accreditation of Laboratory Animal Care-approved facility. Mice were raised under conditions of regulated lighting (lights on 0600–1800), temperature, and humidity. All experiments were approved by the Institutional Animal Care and Use Committee of Vanderbilt University.
Genotyping.
DNA was extracted from adult ear punches or embryo tail biopsies for genotyping by incubation at 95°C in 100 μl of 25 mM NaOH/0.2 mM EDTA for 20 min and then neutralization with 100 μl of 40 mM Tris·HCl, pH 5.0. For the subsequent genotyping reactions, 1 μl of lysed tissue sample was used per reaction. The Dsk5 allele was amplified by PCR with the following primers: DskF, 5′-AGATGGTTCACTCCCTCACG-3′ and DskR, 5′-ATGCTTCCTGATCTACTCCC-3′ (Qiagen, Valencia, CA). PCR conditions were 40 cycles at 94°C for 20 s, 62°C for 20 s, and 72°C for 60 s. PCR products were digested for 3 h at 37°C with AluI and Restriction Enzyme Buffer 2 and run on a 3% agarose gel to separate a 220-bp product corresponding to wild-type EGFR and a 150- and 70-bp set of products corresponding to the digested EGFR-Dsk5 allele. The heterozygous mice have a wavy fur coat, and it is possible to grossly distinguish them from wild-type mice, confirming the genotyping results.
Collection of livers and other organ samples.
Mice were anesthetized in the middle of the light phase (between 1130 and 1330) with 3% isoflurane. They were subjected to a thoracotomy and cardiac puncture to obtain blood. Organs were rapidly dissected from each animal, and the wet weights of the liver, kidney, spleen, testis, inguinal fat pad, and submandibular gland were recorded. Portions of the liver were either frozen in liquid nitrogen for protein and RNA analyses or fixed in phosphate-buffered 4% paraformaldehyde for subsequent paraffin embedding and various histological analyses.
Histology and morphological analyses.
After overnight fixation in 4% buffered paraformaldehyde, liver samples were transferred to 70% ethanol, dehydrated in a graded series of ethanols and xylenes, and embedded in paraffin. Five-micron sections were cut using a Leica Biocut 2030 microtome. Sections were deparaffinized, rehydrated in a graded series of ethanols, and stained with Periodic acid-Schiff (PAS) or Feulgen stain (ScyTek Laboratories, Logan, UT). Stained sections were dehydrated in a series of ethanols. Histological images were photographed on an Olympus Vanex AHBT3 microscope using a NIKON E5000 connected by a PTEM 257009 camera to an objective adaptor. Photomicrographs were taken at a ×40 magnification, and five fields from each liver section were photographed, transferred to a computer, enlarged, and printed. The number of hepatocyte and nonparenchymal nuclei, the average hepatocyte diameter, and the average hepatocellular nuclear diameter were determined. Hepatocyte and nonparenchymal nuclei were marked with different colors, and their nuclei were counted. The longest central axis or diameter of each cell or nucleus was also measured and recorded. The measurements reflected the relative changes in size and nuclear diameter of hepatocytes in the livers of the +/+ and +/Dsk5 mice (not the precise size). The number of binucleated cells was also counted in each field.
Immunohistochemical analysis of Ki67.
Sections were processed by the manufacturer's protocol (Vectastain Elite ABC kit; Vector Laboratories, Burlingame, CA). Endogenous peroxidase activity was quenched by placing the slides in 0.3% H2O2 in methanol for 30 min. After the slides were blocked in a 3.0% goat serum solution for 1 h, the tissue sections were incubated with the Ki67 SP6 rabbit monoclonal (Thermo Scientific, Nashville, TN) in PBS for 90 min in a humidified chamber. The sections were then washed, incubated with biotinylated secondary antibody, rinsed in PBS, and incubated with the Vectastain Elite ABC reagent. Diaminobenzidine was used as the peroxidase substrate.
RNA, DNA, protein, and glycogen analyses.
TRI Reagent was used for the simultaneous isolation of RNA, DNA, and protein (Molecular Research Center, Cincinnati, OH). Glycogen was visualized in liver sections using the PAS stain. It was also biochemically measured by the method of Van Handel (53).
RNA isolation and quantitative real-time RT-PCR analysis of hepatic mRNA levels.
Approximately 100 mg of liver tissue was homogenized in 2 ml of Tri-Reagent/BCP (Molecular Research Center, Cincinnati, OH). Total RNA was isolated according to the manufacturer's instructions, and the purity and integrity were validated. For quantitative real-time RT-PCR assays, reverse transcription was carried out using the RT2 first-strand kit (Qiagen, Hilden, Germany) at 42°C for 15 min. The reaction was then immediately stopped by incubation at 95°C for 5 min in a volume of 20 μl containing 1 μl of total RNA. Real-time PCR was carried out with the 2 μl (15 ng of cDNA) and RT2 SYBY Green Mastermix (Qiagen). To assess HMG-CoA reductase, sterol-regulatory element-binding protein 2 (SREBP2), SREBP1C, FAS, and LDLR mRNA expression levels, PCR was performed in a 25-μl reaction containing 1 μl of cDNA, 12.5 μl of PCR Master Mix, and 2 μl of premixed primers (1). Incubations were at 95°C for 10 min, 95°C for 15 s, and 60°C for 1 min. The samples were amplified for 40 cycles using Bio-Rad CFX95 cycler (Bio-Rad, Hercules, CA). The relative amount of mRNA was related to the GAPDH mRNA by use of the Qiagen RT2 Profiler PCR Data Analysis Program.
Lipid analysis.
Lipids were extracted using the method of Folch et al. (17). The extracts were filtered and lipids recovered in the chloroform phase. Individual lipid classes were separated by thin-layer chromatography using Silica Gel 60 A plates developed in petroleum ether, ethyl ether, acetic acid (80:20:1) and visualized by rhodamine 6G. Phospholipids, triglycerides, and cholesterol esters were scraped from the plates and methylated using BF3/methanol as described by Morrison and Smith (35). The methylated fatty acids were extracted and analyzed by gas chromatography. Gas chromatographic analyses were carried out on an Agilent 7890A gas chromatograph equipped with flame ionization detectors and a capillary column (SP2380, 0.25 mm × 30 m, 0.25-μm film; Supelco, Bellefonte, PA). Helium was used as a carrier gas. The oven temperature was programmed from 160°C to 230°C at 4°C/min. Fatty acid methyl esters were identified by comparing the retention times to those of known standards. Inclusion of lipid standards with odd-chain fatty acids permitted quantitation of the amount of lipid in the sample. Dipentadecanoyl phosphatidylcholine (C15:0), trieicosenoin (C20:1), and cholesteryl eicosenoate (C20:1) were used as standards. Total triglycerides, total cholesterol, and high-density lipoproteins (HDL) were analyzed by commercially available kits adapted to microtiter plates. LDL was calculated from the triglycerides, cholesterol, and HDL.
Western blotting.
Pieces of liver (about 100 mg) were weighed and then homogenized on ice using a 2-ml Wheaton glass tissue homogenizer in TGH buffer (20 weight volumes of 20 mM HEPES, 1% Triton X-100, 10% glycerol, and 50 mM NaCl). This buffer included protease inhibitors (1 mM PMSF, 10 μg/ml aprotinin, and 1 μg/ml leupeptin) as well as phosphatase inhibitors (10 mM sodium molybdate, 1 mM sodium orthovanadate, and 10 mM β-glycerol phosphate). Lysates were immunoblotted as previously described (42). We used the following affinity-purified antibodies from Santa Cruz Biotechnology (Santa Cruz, CA): sc-03 for EGFR; sc-284 for ERBB2; sc-285 for ERBB3; sc-283 for ERBB4; sc-8312 for Akt 1,2,3; sc-33827 for HMG CoA reductase; sc-13 for Erk 1,2; sc-367 for SREBP-1; sc-6556 for hepatocyte nuclear factor (HNF)-4α; and sc-189 for early growth response-1 (Egr-1). Antibodies from Cell Signaling Technology (Beverly, MA) included antiphosphotyrosine [Y845]-EGFR (CS2231), [Y1992]-EGFR (CS2235), [Y1068]-EGFR (CS3777), [Y1289]-ERBB3 (CS4561), phospho-AKT (CS927), and FAS (CS2065). The LDLR antibody (GTX 61553) was from GeneTex (San Antonio, TX). The PCSK9 (C10240) and the SREBP2 antibodies (10007663) were from Cayman Chemicals (Ann Arbor, MI). The pErk 42,44 antibody Anti-ACTIVE MAPK pTEpY (V8031) was from Promega (Madison, WI). The HNF-1α antibody (ARP51375) was from Aviva Systems Biology (San Diego, CA).
The lanes in each immunoblot contained equal amounts of protein, as determined by the Bio-Rad DC Protein Assay. After each transfer, we confirmed equal protein loading and transfer by Ponceau S staining of immunoblots, scanning the image for future reference. All sample cohorts were analyzed on a single blot to ensure reliable comparison. Immunoreactive signal was detected using the enhanced chemiluminescence method using either the SuperSignal West Pico Chemiluminescent Substrate or the SuperSignal West Femto Maximum Sensitivity Substrate (Thermo Scientific, Rockford, IL). The decision to use one or the other depended on the quality of the antibody as well as the abundance of the protein. We performed densitometry using an Epson scanner and the Image J program (45).
Statistical analysis.
Data are expressed as means ± SE. Statistical analysis was performed using an unpaired, two-tailed Student's t-test assuming equal variances between compared groups. A P value of <0.05 was determined to be statistically significant.
RESULTS
EgfrDsk5 mice have enlarged livers.
The gross appearance and lobar structure of the liver in the +/Dsk5 mouse was normal; however, the livers were visibly enlarged. At 3 mo of age, liver weight was increased 62% in +/Dsk5 heterozygotes (P < 0.0002) compared with those from wild-type controls. No difference was seen in the overall body weight (Fig. 1A). We then evaluated the ontogeny of liver weight in male mice at 24 days, 1.5, 3, 5, and 7 mo of age (Fig. 1B). The liver weight in the Dsk5 heterozygote showed only a slight, statistically insignificant increase 3 days after weaning (24 days). The greatest increase occurred between 24 days and 1.5 mo, after which time no further increase relative to the wild-type mice occurred. In contrast to 3-mo-old male +/Dsk5 mice, 3-mo-old female +/Dsk5 mice compared with the wild-type mice showed a smaller but statistically significant increase in liver weight of about 20%. On the basis of reports that other organs, such as the placenta, are enlarged in the +/Dsk5 mouse, we looked at the ontogeny of the weight of the kidney, spleen, fat pad, and testes. As shown in Table 1, +/Dsk5 mice had a consistent but smaller increase in the kidney weight. In contrast, these mice also had a consistent reduction in the size of the inguinal fat pads.
Fig. 1.
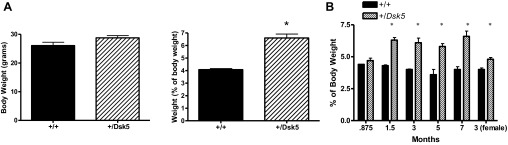
Epidermal growth factor receptor (EGFR) +/Dsk5 mice have enlarged livers. A: body weight (left) and liver weight (right) were determined in 12-wk-old male +/+ and +/Dsk5 mice. The liver weight is expressed as a percentage of total body weight. The +/Dsk5 mouse had a larger average body weight, but they were not significantly different (P < 0.09). In contrast, the liver weight was significantly increased in the +/Dsk5 mouse (P < 0.0002). B: livers of +/+ and +/Dsk5 mice were removed at different ages and expressed as a percentage of total body weight. The livers in the +/Dsk5 mice 3 days after weaning (.875 mo) were larger but not statistically significantly different. The increase in liver weight in all other groups was statistically significantly different *P < 0.05.
Table 1.
Changes in organ weights at different ages between +/Dsk5 and wild-type mice
| Age, mo | Liver | Kidney | Spleen | Fat Pad | Testes |
|---|---|---|---|---|---|
| 0.875 | 6.82 | 10.47* | 0 | 18.51 | 5.3 |
| 1.5 | 46.51† | 22.55† | 22.08 | 7.21 | −2.11 |
| 3 | 52.5† | 15.6† | 18.28* | −26.7 | −13.1 |
| 3 (F) | 20* | 11.9* | −16.79 | −29.5* | |
| 5 | 61.1* | 8.93 | 42.15 | −42.9 | 0.18.5 |
| 7 | 65* | −0.01 | −51.08 | −56.0* | 31.15 |
The organs from subgroups +/Dsk5 and wild-type mice were weighed. Means and standard errors were calculated. The data show the percent difference in organ weight means for 5 different organs from male mice and 4 organs from female (F) mice. Note that the livers and kidneys tended to be larger in the +/Dsk5 mice compared to their wild-type littermates, whereas the fat pads were smaller.
P < 0.05;
P < 0.001.
EgfrDsk5 mice have a higher rate of cell proliferation, larger nuclei, larger cell volume, and fewer binucleate cells.
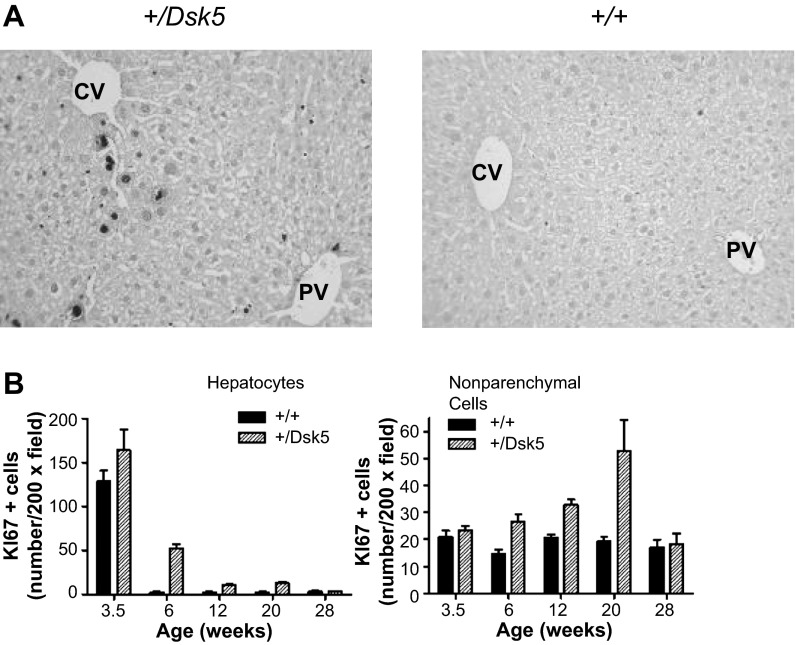
We observed no difference in the overall architecture in histological sections of the liver in adult male +/Dsk5 mice. Because EGF is a known hepatic and stellate cell mitogen, we evaluated hepatocyte and nonparenchymal cell proliferation at various ages for +/Dsk5 mice and their wild-type littermates. Figure 2A shows representative sections from the livers of 6-wk-old male mice immunostained with an antibody against Ki67, a protein that is expressed by cells in all active phases of the cell cycle (G1, S, G2, and M) but not in G0 cells (13). Cells that are positive for Ki67 show intense nuclear labeling. The photomicrograph shows greater Ki67 staining in the livers of +/Dsk5 than wild-type mice. Mitotic figures were seen in microscopic sections in the livers of the +/Dsk5 mice, demonstrating cell cycle progression. The strongest Ki67 immunostaining occurred in cells around the central veins (CV), which synthesize glutamine; however, scattered Ki67-positive cells were identified at other locations as well. We quantified the number of cells that were Ki67 positive (Fig. 2B). Both hepatocytes and nonparenchymal cells in +/Dsk5 mice had more Ki67-positive cells. Although the level of cell proliferation was elevated in +/Dsk5 mice, it was still much lower than one would see in the neonatal liver or in the regenerating liver following 70% resection. The increase was not seen in postweaned (3.5 wk) or older mice (28 wk). Figure 3A shows a Feulgen stain to detect DNA (ScyTek Laboratories). We observed that the nuclei were larger and darker in 3-mo-old +/Dsk5 mice, indicative of increased polyploidization (Fig. 3B, left). We also noted that many of these cells had a larger relative diameter (Fig. 3B, middle). The average hepatocyte in +/Dsk5 mice compared with +/+ mice was 33% larger (P < 0.0001). Finally, livers from wild-type mice had 1.6 times as many binucleate cells compared with livers from +/Dsk5 mice (P < 0.0001; Fig. 3B, right).
Fig. 2.
The hepatocytes and nonparenchymal cells of +/Dsk5 mice have a higher rate of cell proliferation. A: photomicrographs of liver sections from 6-wk-old male mice immunostained for Ki67, a cell proliferation marker. Note the nuclear staining in hepatocytes and the smaller nonparenchymal cells. The staining in hepatocytes tended to be greater around the central vein (CV), as opposed to the portal vein (PV). There were more proliferating cells in the +/Dsk5 mice; however, the number of proliferating cells was still much less than would be seen in early liver development or during liver regeneration following resection. B: quantification of Ki67+ cells obtained by counting the number of positively stained cells per ×20 field. The number of positive cells is shown as a function of age. Note that more nonparenchymal cells than hepatocytes stained for Ki67 after 6 wk of age in both +/+ and +/Dsk5 mice.
Fig. 3.
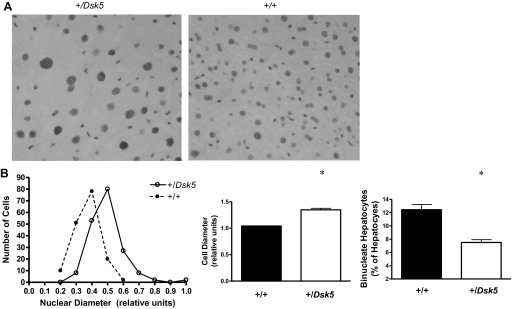
Hepatocytes of +/Dsk5 mice have larger nuclei, larger cell volumes, and fewer binucleate cells. A: photomicrograph of sections of livers from 3-mo-old male mice stained with the Feulgen method to detect DNA. Note that the nuclei of the +/Dsk5 hepatocytes were larger and darker, and their cell diameters were larger than the +/+ hepatocytes. However, the latter had more binucleate cells. B: quantification of the relative nuclear (left) and cellular diameters (middle) and number of binucleate hepatocytes (right). *P < 0.0001.
EGFR signaling is enhanced in EgfrDsk5 livers.
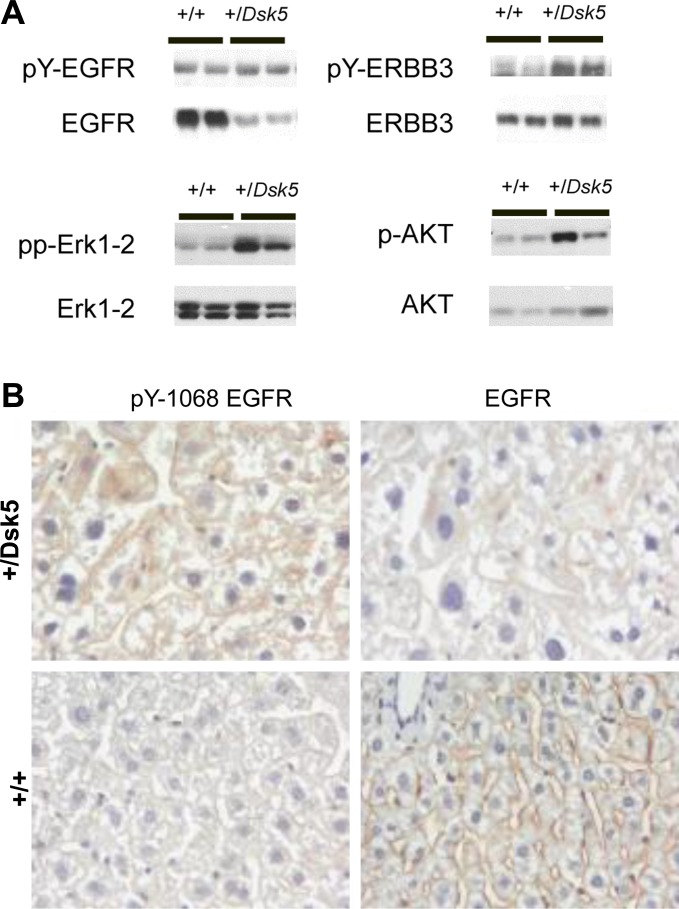
We evaluated the abundance of total and phosphorylated EGFR in the livers from +/Dsk5 heterozygous mice to determine whether there is downregulation and increased tyrosine phosphorylation of the EGFR in the livers, which has been previously reported in +/Dsk5 livers and placentas (11, 16). The EGFR downregulation has been ascribed to enhanced lysosomal degradation of internalized receptor. Figure 4A, top, left, shows EGFR and tyrosine-phosphorylated (pY) EGFR expression in the livers. Each lane contains equally weighted lysate protein derived from two 6-wk-old male mice. In the +/Dsk5 mice, EGFR is downregulated, but pEGFR is increased, consistent with the enhanced tyrosine kinase activity associated with the Dsk5 mutation (16). Furthermore, EGFR levels increase by about fourfold from 24 days to 6 wk in wild-type male mice (data not shown), as previously described (25). We also looked at the levels of ERBB3 and a phosphorylated form of ERBB3 (Fig. 4A, top, right). For ERBB3, we observed a much smaller increase than for EGFR in ERBB3 levels from 24 days to 6 wk and no sex differences in the expression levels in wild-type mice (data not shown). Moreover, in contrast to EGFR, ERBB3 expression in the livers of +/Dsk5 mice was comparable to that in the wild-type mice. Like EGFR, the ERBB3 phosphorylated form was increased, consistent with what one might expect for an EGFR dimeric signaling partner. Both EGFR and ERBB3 showed statistically significant increases in tyrosine phosphorylation when the ratios of pEGFR signal/total EGFR signal were compared between +/Dsk5 and wild-type mice (data not shown). Figure 4B shows immunohistochemical staining for EGFR and pY-1068 EGFR in representative sections of 3-mo-old +/Dsk5 and wild-type mice. These data confirm in hepatocytes that there was diminished expression but greater phosphorylation of EGFR in +/Dsk5 livers compared with wild-type littermates. Nearly all immunostaining was localized to the surface membranes of hepatocytes, and no nuclear staining was observed. Although we did detect a small amount of ERBB2 protein by immunoblot, it was not hyperphosphorylated, suggesting that it might be expressed in EGFR-negative cells (data not shown). Immunoblots revealed no ERBB4 expression (data not shown).
Fig. 4.
EGFR signaling is enhanced in EGFR +/Dsk5 livers. A: changes in the phosphorylation (top) and total expression (bottom) of EGFR (left) and ERBB3 (right) in livers of 6-wk-old male mice. Note that there is decreased expression of EGFR but increased phosphorylation of this protein in the +/Dsk5 mice compared with +/+ littermates. In contrast, ERBB3 levels are similar if not increased in the +/Dsk5 mice but also show increased phosphorylation. Bottom: phosphorylation and total expression of 2 proteins involved in EGFR signaling, namely Erk 1–2 and Akt. Note the enhanced phosphorylation of Erk 1–2 and Akt in the +/Dsk5 mice. B: representative immunohistochemical sections from the livers of +/Dsk5 and +/+ mice that have been stained with antibodies against pY-1068 EGFR or EGFR.
To examine whether +/Dsk5 mice showed increased activation of classic signaling pathways downstream from receptor activation, we evaluated the total and phosphorylated forms of serine kinases involved in Ras and PI3K signaling, extracellular-related kinase (Erk) 1 and 2, and AKT (Fig. 4A, bottom, left and bottom, right, respectively). There is increased phosphorylation of these proteins in the livers of the +/Dsk5 mice. We observed pronounced increases in pAkt, but not in ppErk 1–2, in +/Dsk5 relative to wild-type mice even at 5 mo of age (data not shown). We did not see such differences in phosphorylation for either of these proteins at 3 or 28 wk of age (data not shown).
EgfrDsk5 mice show alterations in glycogen and cholesterol.
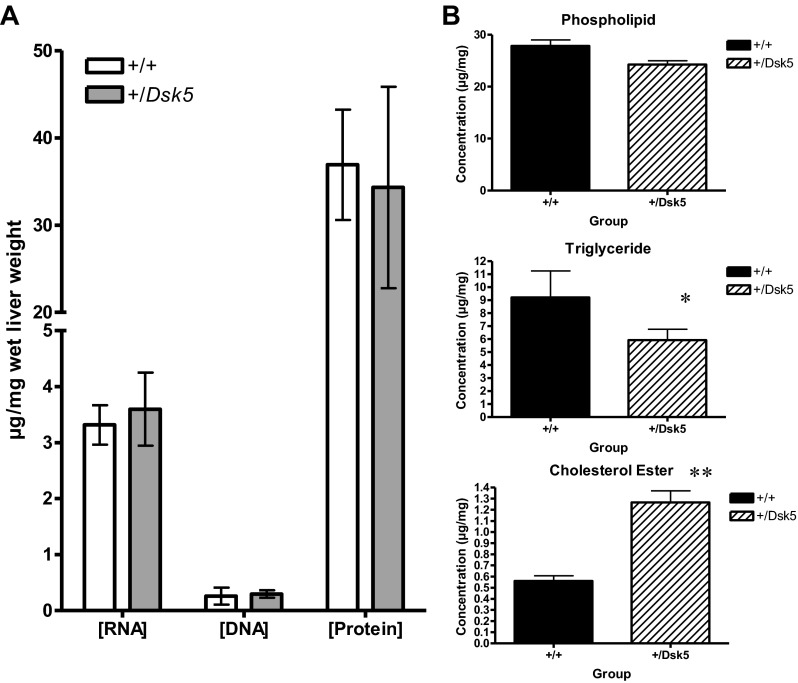
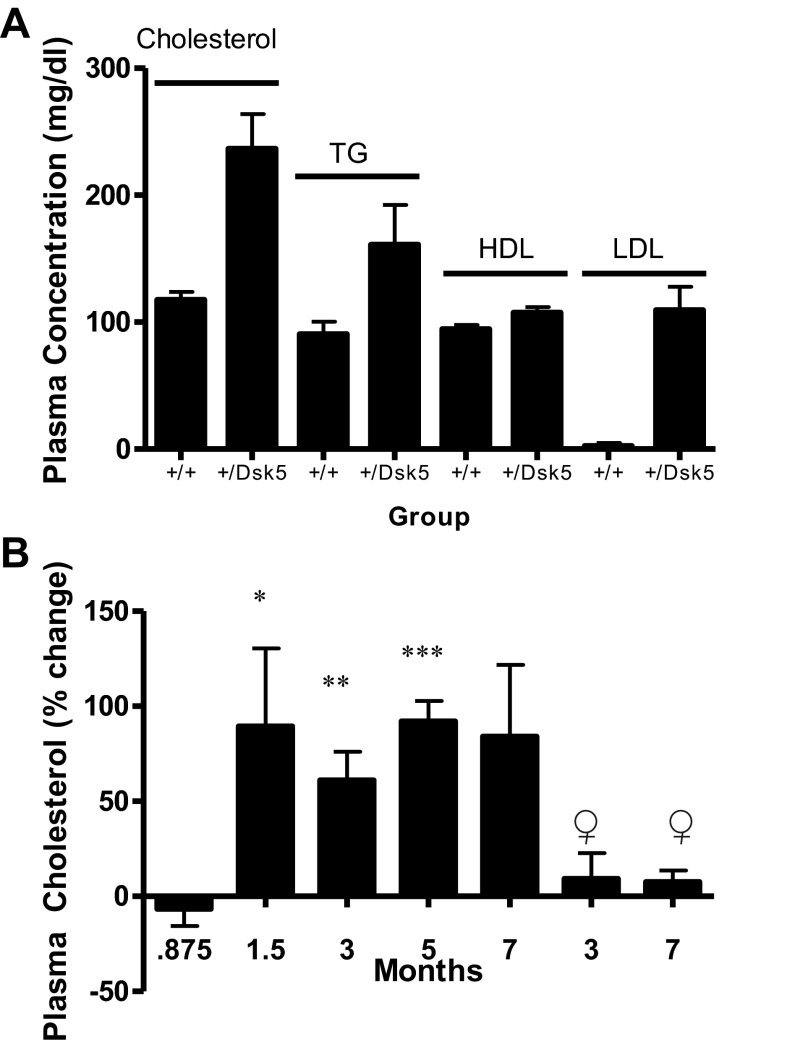
We found no differences in the concentration of RNA, DNA, and protein (expressed per unit wet weight) between +/Dsk5 mice and their wild-type littermates (Fig. 5A). The number of glycogen-positive cells is greater in the placenta of +/Dsk5 mice compared with wild-type mice (11). Because the liver plays an important role in glycogen metabolism, we examined the hepatic glycogen content using the PAS stain. Upon histological examination, the glycogen in +/Dsk5 hepatocytes appeared to be more uniformly distributed and present in higher quantity than in the wild-type mice. Therefore, we quantified the glycogen between the groups biochemically. The glycogen was 39% greater in +/Dsk5 compared with wild-type mice (P < 0.05; data not shown). These data reflect the total hepatic glycogen content in the middle of the resting phase of the light-dark cycle, when glycogen levels decline. We then evaluated the phospholipid, triglyceride, and cholesterol ester content of the livers (Fig. 5B). There was an increase in cholesterol esters and corresponding decreases in triglycerides and phospholipids +/Dsk5 livers. Cholesterol esters doubled in +/Dsk5 livers even though these mice consumed a standard chow diet. The plasma lipid profile (Fig. 6A) of 3-mo-old male mice showed a doubling of total cholesterol (P < 0.0001) between +/Dsk5 and wild-type mice. Triglyceride concentrations were also increased. However, HDL concentrations showed little or no change, whereas calculated LDL concentrations increased by 20-fold. We then examined the ontogeny of plasma cholesterol levels (Fig. 6B). We observed that the plasma cholesterol concentrations increased slightly with age and were slightly lower in 3- and 7-mo-old female mice (data not shown). The most significant change was a near doubling of the plasma cholesterol concentrations in male +/Dsk5 mice by 6 wk of age. Postweaned male and 3- and 7-mo-old female mice did not show significant increases in plasma cholesterol.
Fig. 5.
The livers of +/Dsk5 mice have elevated cholesterol esters but decreased phospholipids and triglycerides. A: RNA, DNA, and protein were measured in the +/+ and +/Dsk5 mice using the TRI Reagent. The values were referenced to the wet weight of the tissue. No significant differences were found for these molecules. B: Phospholipid (PL), triglyceride (TG), and cholesterol ester (CE) concentrations were measured in pieces of liver obtained from the +/+ or +/Dsk5 mice and expressed relative to the wet weight. There is increased CE in the liver of the +/Dsk5 mice compared with the +/+ mice but decreased PL and TG. *P < 0.02; **P < 0.001.
Fig. 6.
Male but not female or postweaned male mice show elevated levels of plasma cholesterol associated with increased low-density lipoprotein (LDL) levels. A: we measured plasma cholesterol, triglycerides, and high-density lipoproteins (HDL) and calculated LDL in the plasma in +/+ and +/Dsk5 3-mo-old male mice. The total cholesterol was nearly doubled in +/Dsk5 mice on a normal chow diet. P < 0.01. This was associated with an increase in the circulating triglyceride level and a 20-fold increase in the calculated LDL level. P < 0.005. B: we measured the changes in the circulating levels in male mice at different stages of development. We also measured the levels of cholesterol in 3- and 7-mo-old female mice. Compared with +/+ mice, we found a consistent, statistically significant increase in plasma cholesterol in male +/Dsk5 mice between 1.5 and 5 mo (the level was higher in the 7-mo-old male but did not attain statistical significance due to a smaller sample size) but not in postweaned male mice or adult female mice. *P < 0.0009; **P < 0.005; ***P < 0.02.
EgfrDsk5 mice show altered hepatic expression of enzymes involved in cholesterol and fatty acid metabolism.
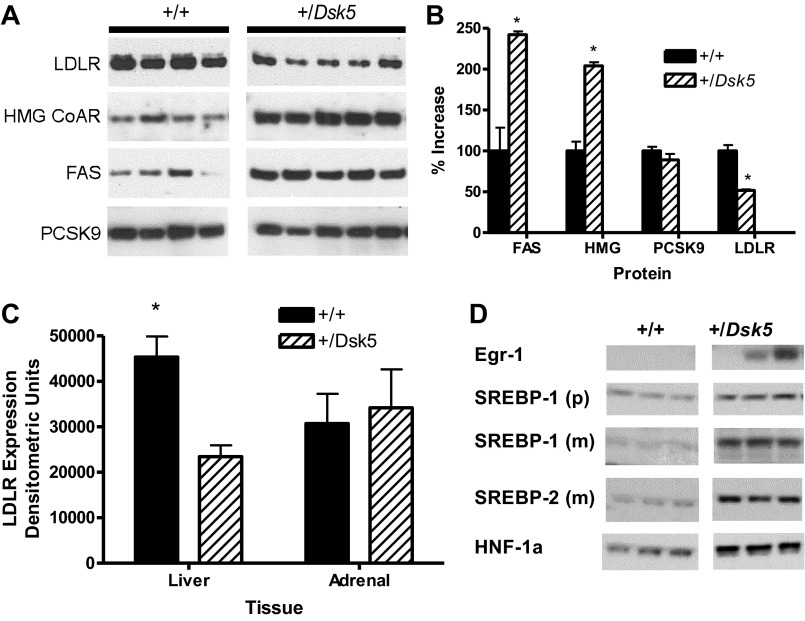
To determine whether the changes in hepatic lipid composition were related to changes in the expression of hepatic proteins known to be important in the regulation of cholesterol and fatty acid concentrations, we evaluated the hepatic protein expression of LDLR, HMG CoA reductase, FAS, and PCSK9. Figure 7A shows an immunoblot of +/Dsk5 mice and their controls. The relative expression obtained by ImageJ analysis is shown in Fig. 7B. We observed a twofold increase in the expression of HMG CoA reductase and FAS (P < 0.0001). The former is the rate-limiting enzyme for cholesterol synthesis in the liver and in other tissues. The latter catalyzes the synthesis of fatty acids but is also involved in fatty acid oxidative catabolism through peroxisome proliferator-activated receptor-α, which mediates the hepatic response to fasting (27). In contrast, LDLR levels fell by half (P < 0.0001), providing an additional mechanism for the elevated plasma LDL total cholesterol concentrations in the blood (Fig. 7, A and C). We did not see a reduction in the LDLR in the adrenal gland (Fig. 7C). This organ expressed comparable LDLR levels to the liver in +/+ mice (Fig. 7C) but less than 1% as much EGFR compared with the liver (data not shown). We found no changes in the liver PCSK9, an enzyme that binds the LDLR and targets it for lysosomal degradation (Fig. 7, A, bottom, and B) (15). This suggests that decreased LDLR expression in +/Dsk5 mice is not caused by increased PCSK9 expression. Finally, to provide an initial evaluation as to the mechanism for these differences, we evaluated changes in the mRNA (Table 2) and protein (Fig. 7D) for several of these enzymes as well as for transcription factors associated with hepatic lipid synthesis. Real-time PCR revealed statistically significant two- to threefold increases in mRNA levels for SREBP2, SREBP1c, HMG-CoA reductase, and FAS. Immunoblot analysis also showed increases of several times in the protein expression for various transcription factors involved in the positive control of hepatic cholesterol levels, including Egr-1, SREBP-1, SREBP-2, and HNF-1α (Fig. 7D).
Fig. 7.
Adult male +/Dsk5 mice show decreased hepatic LDLR expression and increased hepatic 3-hydroxy-3-methyl-glutaryl (HMG) CoA reductase and fatty acid synthase (FAS) expression. A: an immunoblot analysis was carried out for 4 proteins that play a role in the lipid profile of the liver, including LDLR, HMG CoA reductase, FAS, and PCSK9. Each lane includes protein from the liver of a single animal. B: we quantified the results obtained in the immunoblot from A and found a significant increase in expression for FAS and HMG CoA reductase, as well as a significant decrease for the LDLR in the +/Dsk5 mice compared with the +/+ littermates. No differences were observed for PCSK9 *P < 0.05. C: we analyzed by immunoblot the expression of LDLR in several different tissues. The signal intensity for LDLR expression was comparable in the liver and adrenal gland. There is a significant difference (*P < 0.015) in LDLR expression between the +/+ and +/Dsk5 liver but not for the adrenal. D: we evaluated the hepatic expression levels of several transcription factors by immunoblot that regulate cholesterol and fatty acid levels including early growth response-1 (Egr-1), sterol-regulatory element-binding protein (SREBP)-1, SREBP-2, and hepatocyte nuclear factor (HNF)-1α. The antibodies used recognize the SREBP1 precursor (p) as well as the smaller, cleaved “mature” form (m) but only the mature SREBP2 form (m). The expression levels were increased by several times for each protein in the +/Dsk5 compared with +/+, with P < 0.05 or lower.
Table 2.
Changes in hepatic mRNA expression of key genes involved in lipid metabolism
| Gene Symbol | +/Dsk5 Relative Expression | +/+ Relative Expression | Fold Change | P Value |
|---|---|---|---|---|
| HMG-CoA Reductase | 52.08 | 18.87 | 2.76 | 0.019 |
| SREBP2 | 39.22 | 17.28 | 2.27 | 0.042 |
| SREBP1c | 22.73 | 7.83 | 2.90 | 0.036 |
| LDLR | 13.86 | 5.85 | 2.37 | 0.097 |
| FAS | 3.44 | 1.00 | 3.44 | 0.020 |
The relative amounts of mRNA for 3-hydroxy-3-methyl-glutaryl (HMG)-CoA reductase, sterol-regulatory element-binding protein (SREBP)2, SREBP1c, low-density lipoprotein receptor (LDLR), and fatty acid synthase (FAS) were measured by quantitative RT-PCR assay. The amount was calculated relative to the mRNA for GAPDH. The analysis is derived from 3-mo-old +/+ (n = 4) and +/Dsk5 (n = 5) male mice.
The ontogeny of LDLR, HMG CoA reductase, FAS, and PCSK9 expression in the livers of +/Dsk5 mice and wild-type controls.
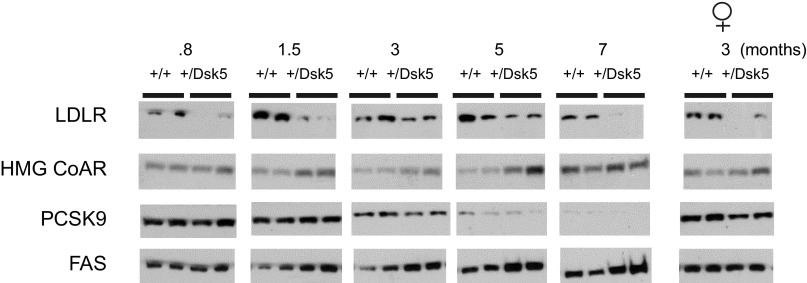
We examined the ontogeny of hepatic expression of LDLR, HMG CoA reductase, PCSK9, and FAS. Because of the large number of samples (n = 60 mice), we prepared liver homogenates from five individual mice, measured the protein in individual samples, and then created subpools. This allowed loading of all the samples onto a single SDS electrophoretic gel. Figure 8 shows the immunoblots for these molecules. For each wild-type and +/Dsk5 age, we show two pools from liver homogenates prepared from two to three different mice. The LDLR blots indicate that the levels of this protein are notably lower in +/Dsk5 mice as early as three days after weaning, even when there is no change in the circulating cholesterol concentrations or in the liver weight. The LDLR levels are lower in the +/Dsk5 mice at all ages. The levels are lower even in 3-mo-old female mice, even though female mice did not show a significant change in circulating cholesterol levels. Increased HMG CoA reductase did not manifest until the 1.5-mo time point. However, this enzyme shows circadian variation, peaking in the dark phase. We evaluated expression only in the middle of the light phase and do not know whether increased nocturnal expression occurred earlier in development in the livers of +/Dsk5 mice. A similar pattern of increased FAS expression was observed in male but not in female mice. Finally, we did not observe consistent differences in PCSK9 expression between wild-type and +/Dsk5 mice; however, we did see an interesting marked decline in older mice beginning as early as 3 mo of age.
Fig. 8.
The ontogeny of expression of LDLR, HMG CoA reductase, PCSK9, and FAS in male +/+ and +/Dsk5 mice. We examined the ontogeny of expression of LDLR, HMG CoA reductase, PCSK9, and FAS in male mice of different ages as well as in young adult female mice. Compared with +/+ mice, we observed a consistent reduction in hepatic LDLR protein expression in all ages and sexes of +/Dsk5 mice. HMG CoA reductase and FAS were increased beginning at 1.5 mo but showed no significant change in the livers of postweaned male mice. HMG CoA reductase but not FAS was increased in the livers of the 3-mo-old female mice. Each point represents a homogenate obtained by combining equal amounts of protein from homogenates obtained from 2 or 3 mice. No consistent differences were seen in PCSK9 for the male mice although the expression of this protein was high in age-matched female mice and appeared to be slightly higher in the +/+ compared with +/Dsk5 mice.
DISCUSSION
In this study, we characterized a mutant mouse model with enhanced EGFR activity and then analyzed several aspects of liver physiology that influence growth and metabolism. We used the heterozygous Dsk5 mouse (+/Dsk5), which has a hypermorphic Egfr allele. EGFR in this mouse has a Leu863Gln mutation within the kinase domain (16). This mutation stabilizes the receptor activation loop, producing a gain-of-function allele and resulting in increased EGFR kinase activity. This strain derives the designation Dsk from its dark skin, with melanocytosis thought to be secondary to increased keratinocyte proliferation and epidermal thickening, rather than a direct response of altered EGFR signaling. Others have created mice that overexpress specific EGF ligands, such as TGF-α. These mice also have evidence of increased EGFR signaling and epithelial hyperplasia in many organs, including the liver, which doubled in weight and DNA (41). The increases in weight in some organs were offset by losses in others, such as bone, muscle, and fat. In the +/Dsk5 adult male mice, we found that the liver weights relative to wild-type littermates also nearly doubled (Fig. 1). We found smaller weight increases in the kidney and spleen even though the kidney did not become enlarged in the TGF-α transgenic mouse (41). The inguinal fat pad weight decreased in +/Dsk5 mice although the overall body weight was not different (Table 1, Fig. 1). The liver weight in postweaned 24-day-old male +/Dsk5 mice did not differ from that of wild-type mice, and we documented an acceleration in hepatic weight gain of the male +/Dsk5 mice between 3 and 6 wk of age, roughly during the onset of puberty (Fig. 1B). The changes in adult female +/Dsk5 liver weight were smaller than those of the male mice but statistically significant. The effect of the Dsk5 mutation on skin color and toenail appearance varies somewhat with the strain, but we have not yet examined whether the phenotype described in this paper is strain dependent. Nevertheless, these results suggest that, although the EGFR may not play a major role in liver development, there is a sexual dimorphism in EGFR regulation of liver size. Along this line, the increased expression of EGFR in the liver (25) and of EGF in the submandibular gland (18) does not occur until after weaning when the livers in +/Dsk5 mice began to enlarge. Because there is a fourfold increase in EGFR expression after weaning and because adult male mice have seven times as many EGF receptors compared with their female counterparts (3), changes in liver size or metabolism might not occur until there is a threshold in the number of mutant EGFR required to override homeostatic mechanisms that counterbalance enhanced EGF signaling, such as receptor-associated late transducer or tyrosine phosphatases.
Because EGF stimulates the proliferation of hepatocytes as well as nonparenchymal cells, we expected that +/Dsk5 mice would show an increase in basal hepatocellular proliferation. We demonstrated this by performing immunohistochemical analysis for the cell proliferation marker Ki67 (Fig. 2). Many but not all of the proliferating cells in +/Dsk5 livers were located adjacent to the central vein. These cells produce glutamine synthetase and are responsible for ammonia metabolism and glutamine synthesis (54). The reasons for the increased proliferation of these cells are not clear, but our data show increased immunostaining for active caspase 3, a marker of apoptosis, at this site in +/Dsk5 livers. In addition, there is increased staining for betacellulin, an EGF-like ligand (data not shown). This suggests that cells in this region had accelerated cell turnover secondary to increased apoptosis. Although we did not observe differences in the cell density or overall architecture of the liver, the morphology of the hepatocytes differed between +/Dsk5 and wild-type mice (Fig. 3). For example, the average nuclear size increased in +/Dsk5 mice, whereas the number of binucleate cells decreased. Increases in nuclear size and Feulgen staining intensity correlate with increased hepatocellular DNA ploidy, which normally increases during aging. Polyploidy in the liver represents terminal differentiation and senescence, resulting in decreased cell replication ability. It increases in situations that stress the liver, such as drug and toxin exposure and surgical resection, and it has been proposed as a mechanism to regulate organ size (19). Thus some hepatocytes in +/Dsk5 livers may respond to the proliferative stress of sustained and enhanced EGFR activation by losing the ability to divide after DNA synthesis. In future studies, it will be of interest to assess whether liver regeneration following partial hepatectomy is accelerated in this mouse as a result of increased EGFR tyrosine kinase activity or retarded due to increased hepatocellular polyploidization.
Because the adult mouse liver has a low basal rate of cell proliferation, the high density of EGFR (600,000 per hepatocyte) may serve other functions. By analyzing the phosphorylation of molecules associated with EGFR signal transduction, including EGFR tyrosine phosphorylation itself, we found evidence for ongoing EGF signaling in the +/Dsk5 and wild-type livers, even in the absence of significant cell proliferation (Fig. 4). In +/Dsk5 mice, decreased EGFR expression, presumably due to ligand-mediated internalization, was evident as early as 3.5 wk and persisted at 28 wk. Despite reductions in the number of EGF receptors, increased EGFR cell signaling was reflected by increased levels of the tyrosine-phosphorylated forms of ERBB3 and EGFR in both male and female mice irrespective of age (data not shown). The enhanced tyrosine phosphorylation of ERBB3 in +/Dsk5 mice supports the idea that the EGFR hypermorph and ERBB3 interact in vivo.
Although a functional, nonproliferative role for EGFR conceivably may involve EGF clearance or hepatocyte survival, considerable evidence suggests that EGF and EGFR have metabolic roles in this organ. In neonatal mice, the injection of EGF induces fatty liver due to triglyceride accumulation (23) and reduces the size of the inguinal fat pads (47). This latter result is partly due to a failure of precursor cells to differentiate into mature adipocytes. In adult mice, EGF has been shown to increase hepatic glycogenolysis (21) and gluconeogenesis (48). Although it stimulates glycogenolysis, it can also partially inhibit catecholamine-induced glycogenolysis by dampening the cyclic AMP signal (but not the cytosolic Ca++ rise) associated with adrenaline (21). Along this line, we observed a 39% increase in midday glycogen +/Dsk5 livers (data not shown) compared with wild-type littermates. Transport and handling of mice on the day of the experiment activate the sympathetic nervous system, including the release of adrenalin and glycogenolysis. Conceivably, increased activation of EGFR in +/Dsk5 livers may impede adrenalin-mediated glycogen breakdown, accounting for the higher levels of glycogen in +/Dsk5 mice compared with their wild-type littermates.
Additional support for a metabolic role for hepatic EGFR emerges when the hormonal, developmental, and metabolic regulation of EGFR and EGF are considered. EGFR expression in the liver and EGF in the submandibular gland, the main source of circulating EGF, are positively regulated by several hormones with important metabolic roles, including insulin, growth hormone, corticosterone, androgens, and thyroxine (36, 56). Moreover, various metabolic diseases alter the expression of ERBB receptor tyrosine kinases and EGF. Hepatic EGFR expression and submandibular gland EGF decrease in diabetic (38, 46) or obese mice (5). Fasting causes a reduction in hepatic EGFR expression (43). In contrast, ERBB3 expression levels increase in diabetic or fasted mice (7). Collectively, these findings imply that EGFR and ERBB3 are regulated by metabolic events and may have metabolic roles.
We initially analyzed whether the composition of the liver changed in +/Dsk5 mice by measuring the expression of RNA, DNA, and protein as a function of organ wet weight (Fig. 5A). We found no significant differences between +/Dsk5 and control mice. This prompted us to measure other biomolecules, including phospholipids, triglycerides, and cholesterol esters (Fig. 5B). We found marked differences in the hepatic levels of these lipids in +/Dsk5 and control mice fed a standard chow diet, with increases in cholesterol ester and reductions in phospholipid and triglyceride content. Moreover, we observed that the hepatic cholesterol content correlated with increases in the level of plasma cholesterol. Plasma triglycerides increased by 30%. There was also a doubling of total cholesterol that was accounted for by circulating LDL levels (Fig. 6). Interestingly, the increase was age- and sex-dependent and correlated with increased EGFR and EGF expression in the liver and submandibular gland. We saw a persistent increase in cholesterol between weaning and 6 wk of age in male but not female mice, reminiscent of the developmental and sexual differences observed for EGFR and EGF themselves. There are no reports as to whether similar changes were ever found in transgenic mice overexpressing TGF-α.
The expression of a number of hepatic molecules that are associated with the synthesis or catabolism of lipids was altered in +/Dsk5 mice (Figs. 7 and 8). We found that the hepatic LDLR levels decreased, whereas HMG CoA reductase levels increased. Both of these changes would contribute to increases in hepatic and circulating cholesterol levels. LDLR is involved in LDL and cholesterol catabolism, whereas HMG CoA reductase is the rate-limiting enzyme in cholesterol synthesis. Targeted disruption of LDLR (Ldlrtm/Her) in the mouse on a standard chow diet leads to increases in circulating LDL levels comparable to those reported here (26). Mice homozygous for Ldlrtm/Her have an elevated plasma cholesterol level of 200–400 mg/dl when fed a standard chow diet that can increase to levels above 2,000 mg/dl on a high-fat diet. The reduction in LDLR in the +/Dsk5 livers manifested shortly after weaning before increases in liver weight, cholesterol levels, HMG CoA reductase, or FAS expression. The reduction in LDLR was not associated with changes in PCSK9, an enzyme that decreases LDLR by binding and targeting it for lysosomal degradation.
In other cells types, EGF has been shown to increase the expression of HMG CoA reductase (29) and FAS (22, 51). A recent gene-profiling study revealed that heregulin-β1 induced the expression of HMG CoA reductase in two mammary luminal epithelial cell lines (58). In ovarian cancer cells, EGFR and ERBB2 have been shown to upregulate FAS through AKT activation (22). In cancer cells, FAS generates lipids for membrane rafts, which accommodate ERBB and other receptor tyrosine kinases, and is now regarded as a potential target for chemotherapy in breast, prostate, and colonic cancer (15, 27, 33, 34). Acute or repeated injections of EGF have not been reported as yet to alter cholesterol or fatty acid synthesis in the liver of a normal mouse. However, the nocturnal increases in the expression of EGF, ERBB3 (9), and EGFR (43) do precede or parallel the nocturnal increases in the synthesis of cholesterol (12) and fatty acid synthesis (24), as well as in the expression of HMG CoA reductase and FAS. Others have previously proposed a role for a nocturnally secreted, PV-delivered, food-regulated gastrointestinal factor(s) in the regulation of cholesterol synthesis and associated circadian rhythms in the liver. The proposed GI factor(s), conceivably EGF, could regulate not only hepatic cholesterol synthesis, but also cholesterol elimination by its conversion to bile acids essential for intestinal fat absorption (28).
We hypothesize that EGF or HRG bind EGFR and ERBB3 in hepatocytes of +/Dsk5 livers, creating EGFR homodimers or EGFR-ERBB3 heterodimers. One of these signaling dimers conceivably activates yet to be defined signaling pathways that eventually stimulate the synthesis or activation of key transcription factors involved in cholesterol and fatty acid synthesis. We evaluated the expression of the various SREBP as well as Egr-1 in these mice and their wild-type littermates and found increases in mRNA (Table 2) and protein (Fig. 7D). Others have shown that EGF increases the expression level of early Egr-1 in hepatocytes of wild-type mice (30, 52). Interestingly, insulin also increases Egr-1, and it appears to increase cholesterol synthesis in the liver mainly through this transcription factor as opposed to STEBP (20). One must also consider the possibility that EGF may increase cholesterogenesis by decreasing hepatocellular bile acid production because removal of bile acids from the intestine by any means increases cholesterol synthesis (28). The neutral and alternative pathways of bile acid synthesis are regulated by cholesterol 7α-hydroxylase (CYP7A1) and mitochondrial sterol 27-hydroxylase (CYP27A1), and it will be important to determine whether the effects of EGF signaling in +/Dsk5 mice downregulate the expression or activity of these enzymes or other ones involved in bile acid production. Finally, it is possible that the phenotype is not mediated by the Dsk5 mutation in the liver and in hepatocytes themselves. Because this mutation affects EGFR in all cells in the body, the liver phenotype may be generated by neural or hormonal signals emanating from nonhepatic tissue (s). Could the heightened cell proliferation in epithelial tissues, such as the gastrointestinal tract or skin, trigger increased cholesterol production in the liver independent of hepatic EGFR itself? This possibility seems unlikely because the sexual and developmental differences in hepatic EGFR parallel the phenotype; however, creation of a hepatocyte-specific EGFR hypermorph or hepatocyte-specific EGFR or ERBB3 knockout mice could be used to address this issue. Furthermore, we observed a reduction in LDLR in the liver but not in the adrenal of the +/Dsk5 mice (Fig. 7C), which both expressed LDLR at comparable levels in wild-type mice by immunoblot analysis.
EGFR and its ligands have been heavily studied because EGF was one of the first growth factors discovered and because EGFR is the prototypical tyrosine kinase receptor. Isolated clinical studies over 40 yr have measured the levels of EGF-like ligands in patients with assorted diseases. Several studies have reported a positive correlation between EGF ligands and serum cholesterol levels in humans. For example, Berrahmoune et al. (4) reported a positive correlation between circulating EGF levels and LDL levels. Sanchez-Vizcaino et al. (40) reported a similar correlation with HBEGF. Moreover, patients with psoriasis, who show a two- to tenfold increase in circulating EGF (2, 32), are known to have increased LDL levels (31) and are at risk for atherosclerosis and associated diseases, including stroke and myocardial infarction (39). Thus EGFR activation or ligand overproduction may be relevant to cholesterol levels in humans, but further study is required. The current study raises a number of new questions. Is there a subset of hypercholesterolemic patients who have a hyperactive EGFR kinase or who express high levels of EGF ligands? Do EGFR tyrosine kinase inhibitors, which are used in cancer therapy, downregulate cholesterol levels by interfering with EGFR signaling in the liver? Do differences in circulating EGF levels account for the increase in circulating LDL levels in men and postmenopausal women? This latter question is relevant because the traditional view that the difference is due to the direct effects of sex hormones themselves has been challenged recently (55).
GRANTS
We acknowledge the generous support of a student research stipend from the Vanderbilt Diabetes Center to O. Garcia. This work was supported by R01DK53804 and R21CA149708 (to W. Russell) and R01CA092479 (to D. Threadgill). ARRA support (R56 DK053804) provided funding for R. Wang. Lipid determinations were carried out with the assistance and support of the MMPC/DRTC Lipid Lab (R56 DK053804).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: L.A.S., D.W.T., and W.E.R. conception and design of research; L.A.S., X.Z., O.A.G., R.F.W., and M.C.S. analyzed data; L.A.S., O.A.G., and R.F.W. interpreted results of experiments; L.A.S., O.A.G., and M.C.S. prepared figures; L.A.S. drafted manuscript; L.A.S., D.W.T., and W.E.R. edited and revised manuscript; L.A.S., D.W.T., and W.E.R. approved final version of manuscript; X.Z., O.A.G., R.F.W., and M.C.S. performed experiments.
REFERENCES
- 1.Ai D, Chen C, Han S, Ganda A, Murphy AJ, Haeusler R, Thorp E, Accili D, Horton JD, Tall AR. Regulation of hepatic LDL receptors by mTORC1 and PCSK9 in mice. J Clin Invest 122: 1262–1270, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson KS, Petersson S, Wong J, Shubbar E, Lokko NN, Carlstrom M, Enerback C. Elevation of serum epidermal growth factor and interleukin 1 receptor antagonist in active psoriasis vulgaris. Br J Dermatol 163: 1085–1089, 2010 [DOI] [PubMed] [Google Scholar]
- 3.Benveniste R, Danoff TM, Ilekis J, Craig HR. Epidermal growth factor receptor numbers in male and female mouse primary hepatocyte cultures. Cell Biochem Funct 6: 231–235, 1988 [DOI] [PubMed] [Google Scholar]
- 4.Berrahmoune H, Lamont JV, Herbeth B, Lambert D, Masson C, McPhillips M, Fitzgerald PS, Visvikis-Siest S. Association between EGF and lipid concentrations: a benefit role in the atherosclerotic process? Clin Chim Acta 402: 196–198, 2009 [DOI] [PubMed] [Google Scholar]
- 5.Blackshear PJ, Stumpo DJ, Kennington EA, Tuttle JS, Orth DN, Thompson KL, Hung MC, Rosner MR. Decreased levels of hepatic epidermal growth factor receptors in obese hyperglycemic rodents. J Biol Chem 262: 12356–12364, 1987 [PubMed] [Google Scholar]
- 6.Burwen SJ, Barker ME, Goldman IS, Hradek GT, Raper SE, Jones AL. Transport of epidermal growth factor by rat liver: evidence for a nonlysosomal pathway. J Cell Biol 99: 1259–1265, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carver RS, Mathew PM, Russell WE. Hepatic expression of ErbB3 is repressed by insulin in a pathway sensitive to PI-3 kinase inhibitors. Endocrinology 138: 5195–5201, 1997 [DOI] [PubMed] [Google Scholar]
- 8.Carver RS, Sliwkowski MX, Sitaric S, Russell WE. Insulin regulates heregulin binding and ErbB3 expression in rat hepatocytes. J Biol Chem 271: 13491–13496, 1996 [DOI] [PubMed] [Google Scholar]
- 9.Carver RS, Stevenson MC, Scheving LA, Russell WE. Diverse expression of ErbB receptor proteins during rat liver development and regeneration. Gastroenterology 123: 2017–2027, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Chua BH, Chua CC, Zhao ZY, Krebs CJ. Estrone modulates the EGF receptor in the liver of db/db mouse. J Recept Res 11: 941–957, 1991 [DOI] [PubMed] [Google Scholar]
- 11.Dackor J, Li MY, Threadgill DW. Placental overgrowth and fertility defects in mice with a hypermorphic allele of epidermal growth factor receptor. Mamm Genome 20: 339–349, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edwards PA, Muroya H, Gould RG. In vivo demonstration of the circadian thythm of cholesterol biosynthesis in the liver and intestine of the rat. J Lipid Res 13: 396–401, 1972 [PubMed] [Google Scholar]
- 13.Endl E, Gerdes J. The Ki-67 protein: fascinating forms and an unknown function. Exp Cell Res 257: 231–237, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Ethier C, Raymond VA, Musallam L, Houle R, Bilodeau M. Antiapoptotic effect of EGF on mouse hepatocytes associated with downregulation of proapoptotic Bid protein. Am J Physiol Gastrointest Liver Physiol 285: G298–G308, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Ferri N, Tibolla G, Pirillo A, Cipollone F, Mezzetti A, Pacia S, Corsini A, Catapano AL. Proprotein convertase subtilisin kexin type 9 (PCSK9) secreted by cultured smooth muscle cells reduces macrophages LDLR levels. Atherosclerosis 220: 381–386, 2012 [DOI] [PubMed] [Google Scholar]
- 16.Fitch KR, McGowan KA, van Raamsdonk CD, Fuchs H, Lee D, Puech A, Herault Y, Threadgill DW, de Angelis MH, Barsh GS. Genetics of dark skin in mice. Gene Dev 17: 214–228, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226: 497–509, 1957 [PubMed] [Google Scholar]
- 18.Gattone VH, Sherman DA, Hinton DA, Niu FW, Topham RT, Klein RM. Epidermal growth-factor in the neonatal mouse salivary-gland and kidney. Biol Neonate 61: 54–67, 1992 [DOI] [PubMed] [Google Scholar]
- 19.Gentric G, Celton-Morizur S, Desdouets C. Polyploidy and liver proliferation. Clin Res Hepatol Gastroenterol 36: 29–34, 2012 [DOI] [PubMed] [Google Scholar]
- 20.Gokey NG, Lopez-Anido C, Gillian-Daniel AL, Svaren J. Early growth response 1 (Egr1) regulates cholesterol biosynthetic gene expression. J Biol Chem 286: 29501–29510, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grau M, Soley M, Ramirez I. Interaction between adrenaline and epidermal growth factor in the control of liver glycogenolysis in mouse. Endocrinology 138: 2601–2609, 1997 [DOI] [PubMed] [Google Scholar]
- 22.Grunt TW, Wagner R, Grusch M, Berger W, Singer CF, Marian B, Zielinski CC, Lupu R. Interaction between fatty acid synthase- and ErbB-systems in ovarian cancer cells. Biochem Biophys Res Commun 385: 454–459, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Heimberg M, Weinstein I, LeQuire VS, Cohen S. The induction of fatty liver in neonatal animals by a purified protein (EGF) from mouse submaxillary gland. Life Sci 4: 1625–1633, 1965 [DOI] [PubMed] [Google Scholar]
- 24.Hems DA, Rath EA, Verrinder TR. Fatty acid synthesis in liver and adipose tissue of normal and genetically obese (ob/ob) mice during the 24-hour cycle. Biochem J 150: 167–173, 1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ichii S, Satoh Y, Hoshikawa Y, Yoshida A. Reinvestigation of ontogeny of epidermal growth-factor receptor messenger-RNA in the liver of rats—quantitative-evaluation of Northern blot analysis. Endocrinol Jpn 38: 511–516, 1991 [DOI] [PubMed] [Google Scholar]
- 26.Ishibashi S, Brown MS, Goldstein JL, Gerard RD, Hammer RE, Herz J. Hypercholesterolemia in low density lipoprotein receptor knockout mice and its reversal by adenovirus-mediated gene delivery. J Clin Invest 92: 883–893, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jensen-Urstad AP, Semenkovich CF. Fatty acid synthase and liver triglyceride metabolism: housekeeper or messenger? Biochim Biophys Acta 1821: 747–753, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krumdieck CL, Ho KJ. Intestinal regulation of hepatic cholesterol synthesis: an hypothesis. Am J Clin Nutr 30: 255–261, 1977 [DOI] [PubMed] [Google Scholar]
- 29.Larsson O. The role of HMG CoA reductase and dolichol synthesis in the control of 3T6 cell proliferation: effects of cell crowding, serum depletion and addition of epidermal growth factor. J Cell Sci 90: 613–620, 1988 [DOI] [PubMed] [Google Scholar]
- 30.Liu L, Tsai JC, Aird WC. Egr-1 gene is induced by the systemic administration of the vascular endothelial growth factor and the epidermal growth factor. Blood 96: 1772–1781, 2000 [PubMed] [Google Scholar]
- 31.Ma C, Harskamp CT, Armstrong EJ, Armstrong AW. The association between psoriasis and dyslipidaemia: a systematic review. Br J Dermatol 168: 486–495, 2013 [DOI] [PubMed] [Google Scholar]
- 32.Ma LL, Chen PF, Luo F. Impact of serum epidermal growth factor on progressive psoriasis vulgaris and regulation of Chinese herbal medicine for cleaving heat, cooling blood, and detoxicating on it. Zhongguo Zhong Xi Yi Jie He Za Zhi 28: 733–735, 2008 [PubMed] [Google Scholar]
- 33.Menendez JA, Lupu R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer 7: 763–777, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Menendez JA, Vellon L, Lupu R. Targeting fatty acid synthase-driven lipid rafts: a novel strategy to overcome trastuzumab resistance in breast cancer cells. Med Hypotheses 64: 997–1001, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Morrison WR, Smith LM. Fatty acid composition of milk phospholipids. II. Sheep, Indian buffalo and human milks. Lipids 2: 178–182, 1967 [DOI] [PubMed] [Google Scholar]
- 36.Mukku VR. Regulation of epidermal growth factor receptor levels by thyroid hormone. J Biol Chem 259: 6543–6547, 1984 [PubMed] [Google Scholar]
- 37.Murthy A, Defamie V, Smookler DS, Di Grappa MA, Horiuchi K, Federici M, Sibilia M, Blobel CP, Khokha R. Ectodomain shedding of EGFR ligands and TNFR1 dictates hepatocyte apoptosis during fulminant hepatitis in mice. J Clin Invest 120: 2731–2744, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nagy A, Nagashima H, Cha SH, Oxford GE, Zelles T, Peck AB, Humphreys-Beher MG. Reduced oral wound healing in the NOD mouse model for type 1 autoimmune diabetes and its reversal by epidermal growth factor supplementation. Diabetes 50: 2100–2104, 2001 [DOI] [PubMed] [Google Scholar]
- 39.Prodanovich S, Kirsner RS, Kravetz JD, Ma F, Martinez L, Federman DG. Association of psoriasis with coronary artery, cerebrovascular, and peripheral vascular diseases and mortality. Arch Dermatol 145: 700–703, 2009 [DOI] [PubMed] [Google Scholar]
- 40.Sanchez-Vizcaino E, Vehi C, Camprecios G, Morcillo C, Soley M, Ramirez I. Heparin-binding EGF-like growth factor in human serum. Association with high blood cholesterol and heart hypertrophy. Growth Factors 28: 98–103, 2010 [DOI] [PubMed] [Google Scholar]
- 41.Sandgren EP, Luetteke NC, Palmiter RD, Brinster RL, Lee DC. Overexpression of TGF alpha in transgenic mice: induction of epithelial hyperplasia, pancreatic metaplasia, and carcinoma of the breast. Cell 61: 1121–1135, 1990 [DOI] [PubMed] [Google Scholar]
- 42.Scheving LA, Stevenson MC, Zhang X, Russell WE. Cultured rat hepatocytes upregulate Akt and ERK in an ErbB-2-dependent manner. Am J Physiol Gastrointest Liver Physiol 295: G322–G331, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scheving LA, Tsai TH, Cornett LE, Feuers RJ, Scheving LE. Circadian variation of epidermal growth factor receptor in mouse liver. Anat Rec 224: 459–465, 1989 [DOI] [PubMed] [Google Scholar]
- 44.Schlessinger J. Ligand-induced, receptor-mediated dimerization and activation of EGF receptor. Cell 110: 669–672, 2002 [DOI] [PubMed] [Google Scholar]
- 45.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9: 671–675, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Serrero G, Lepak NM, Hayashi J, Goodrich SP. Impaired epidermal growth factor production in genetically obese ob/ob mice. Am J Physiol Endocrinol Metab 264: E800–E803, 1993 [DOI] [PubMed] [Google Scholar]
- 47.Serrero G, Mills D. Physiological role of epidermal growth factor on adipose tissue development in vivo. Proc Natl Acad Sci USA 88: 3912–3916, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Soley M, Hollenberg MD. Epidermal Growth-Factor (Urogastrone)-Stimulated Gluconeogenesis in Isolated Mouse Hepatocytes. Arch Biochem Biophys 255: 136–146, 1987 [DOI] [PubMed] [Google Scholar]
- 49.St Hilaire RJ, Hradek GT, Jones AL. Hepatic sequestration and biliary secretion of epidermal growth factor: evidence for a high-capacity uptake system. Proc Natl Acad Sci USA 80: 3797–3801, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Surur JM, Moreno FR, Badran AF, Llanos JM. Variations in DNA synthesis and mitotic indices in hepatocytes and sinusoid litoral cells of adult intact male mouse along a circadian time span. Chronobiol Int 2: 161–168, 1985 [DOI] [PubMed] [Google Scholar]
- 51.Swinnen JV, Heemers H, Deboel L, Foufelle F, Heyns W, Verhoeven G. Stimulation of tumor-associated fatty acid synthase expression by growth factor activation of the sterol regulatory element-binding protein pathway. Oncogene 19: 5173–5181, 2000 [DOI] [PubMed] [Google Scholar]
- 52.Tsai JC, Liu L, Zhang J, Spokes KC, Topper JN, Aird WC. Epidermal growth factor induces Egr-1 promoter activity in hepatocytes in vitro and in vivo. Am J Physiol Gastrointest Liver Physiol 281: G1271–G1278, 2001 [DOI] [PubMed] [Google Scholar]
- 53.Van Handel E. Estimation of glycogen in small amounts of tissue. Anal Biochem 11: 256–265, 1965 [DOI] [PubMed] [Google Scholar]
- 54.Wagenaar GTM, Moorman AFM, Chamuleau RAFM, Deutz NEP, Degier C, Deboer PAJ, Verbeek FJ, Lamers WH. Vascular branching pattern and zonation of gene-expression in the mammalian liver. Anat Rec 239: 441–452, 1994 [DOI] [PubMed] [Google Scholar]
- 55.Wang X, Magkos F, Mittendorfer B. Sex differences in lipid and lipoprotein metabolism: it's not just about sex hormones. J Clin Endocrinol Metab 96: 885–893, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wilson CM, Griffin JE, Reynolds RC, Wilson JD. The interaction of androgen and thyroid hormones in the submandibular gland of the genetically hypothyroid (hyt/hyt) mouse. Endocrinology 116: 2568–2577, 1985 [DOI] [PubMed] [Google Scholar]
- 57.Wilson KJ, Mill C, Lambert S, Buchman J, Wilson TR, Hernandez-Gordillo V, Gallo RM, Ades LM, Settleman J, Riese DJ., 2nd EGFR ligands exhibit functional differences in models of paracrine and autocrine signaling. Growth Factors 30: 107–116, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Worthington J, Bertani M, Chan HL, Gerrits B, Timms JF. Transcriptional profiling of ErbB signalling in mammary luminal epithelial cells—interplay of ErbB and IGF1 signalling through IGFBP3 regulation. BMC Cancer 10: 490, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]