Abstract
We have previously reported that doublecortin-like kinase 1 (Dclk1) is a putative intestinal stem cell (ISC) marker. In this report, we evaluated the use of Dclk1 as a marker of surviving ISCs in response to treatment with high-dose total body irradiation (TBI). Both apoptotic and mitotic Dclk1+ cells were observed 24 h post-TBI associated with a corresponding loss of intestinal crypts observed at 84 h post-TBI. Although the Notch signaling pathway plays an important role in regulating proliferation and lineage commitment within the intestine, its role in ISC function in response to severe genotoxic injury is not yet fully understood. We employed the microcolony assay to functionally assess the effects of Notch inhibition with difluorophenacetyl-l-alanyl-S-phenylglycine t-butyl ester (DAPT) on intestinal crypt stem cell survival following severe (>8 Gy) radiation injury. Following treatment with DAPT, we observed a nearly 50% reduction in the number of surviving Dclk1+ crypt epithelial cells at 24 h after TBI and similar reduction in the number of surviving small intestinal crypts at 84 h. These data indicate that inhibition of Notch signaling decreases ISC survival following radiation injury, suggesting that the Notch signaling pathway plays an important role in ISC-mediated crypt regeneration. These results also suggest that crypt epithelial cell Dclk1 expression can be used as one potential marker to evaluate the early survival of ISCs following severe radiation injury.
Keywords: doublecortin-like kinase 1, radiation, crypt survival
the adult intestinal epithelium is continuously and rapidly replaced by cell replication within the crypts of Lieberkühn and subsequent migration of their progeny on the villus epithelium in the small intestine or on the surface epithelium in the colon (9). Intestinal epithelial cells are ultimately derived from multipotent stem cell(s) located near the base of each intestinal crypt (3, 4, 32, 37). In the adult mouse small intestine, these crypt stem cells divide to produce a daughter stem cell (self-renewal) as well as a more rapidly replicating transit cell. Their progeny subsequently differentiate into the mature epithelial cell types found in the small intestine as the epithelial cells migrate away from the proliferative zone in each intestinal crypt (3, 26, 27).
Crypt stem cells also play a central role in mucosal regeneration following injury (22). Intestinal injury induced by genotoxic/cytotoxic agents can disrupt the epithelial barrier, resulting in the loss of crypts. Restoration of normal epithelial architecture and barrier function is a multistep process: 1) stem cells proliferate to increase their numbers and to give rise to the more rapidly proliferating transit cell population; 2) the transit cell population expands rapidly to form a regenerative crypt; and 3) normal patterns of epithelial differentiation are reestablished by migration and differentiation of cells produced in these regenerative crypts (9). Moreover, if the injury has completely destroyed some crypts, the surviving crypt stem cells can divide to replete the number of viable crypts (22).
A functional assay for quantifying stem cell survival following acute radiation injury to the replicating cell population has been developed based on the capacity of the surviving stem cells to regenerate crypt-like foci of cells (termed microcolonies) (22, 38). The actively proliferating transit cells are the most sensitive to ionizing radiation-induced injury; the slowly proliferating stem cells are less sensitive to radiation. In this assay, 3 or 4 days after irradiation (IR), the number of crypt-like foci of surviving epithelial cells is scored on histological sections of intestine. Each epithelial foci is thought to represent the survival of one or more clonogenic stem cells able to give rise to the regenerative crypt. This assay allows for quantitative assessment of the role of exogenous agents and/or molecular signal transduction pathways on crypt survival and indirectly stem cell survival following lethal dose total body irradiation (TBI).
We have reported that doublecortin-like kinase 1 (Dclk1), previously known as DCAMKL-1, is a putative intestinal stem cell (ISC) marker (14, 15). Dclk1+ cells were primarily found at the +4 position of the normal uninjured mouse intestinal crypt. Dclk1+ cells were also found in other positions along the crypt villus axis, including crypt-based columnar cells (15). Some Dclk1+ cells, particularly on the villus, appear to be cells with the morphological appearance of Tuft/brush cells (8). The precise function of this morphologically distinct cell remains unknown. Yet, in the crypt, Dclk1 also appears to mark a subpopulation of non-Tuft cells and occasionally Lgr5-expressing cells (15). In a modified label retention (following 10 Gy IR) assay, Dclk1 label-retaining cells were functionally quiescent and appeared to be functionally “anchored” at or near the +4 position (15). These data suggest that postradiation injury, all of the label retaining Dclk1+ cells are not sloughed off at the villus tip. Furthermore, Dclk1+ cells sorted from the mouse small intestine using anti-Dclk1 antibody form spheroids in suspension culture and develop into nodules when injected in the flanks of athymic nude mice. These nodules contain cells demonstrating markers of early intestinal epithelial lineage commitment (15). These studies demonstrate that Dclk1 cells isolated from intestinal crypts have self-renewal capacity.
The Notch signaling pathway plays an important role in the regulation of critical biological processes, including cellular proliferation and lineage commitment within the intestine. Inhibition of Notch signaling accelerates epithelial differentiation, whereas overactivation results in inhibition of all secretory lineage commitment and amplification of the intestinal progenitor pool (6). The Notch signaling pathway is considered as an intestinal stem and progenitor cell gatekeeper (13). Whether the Notch signaling pathway plays a role in ISC functions in response to radiation is not clear.
In this report we investigated the effects of inhibiting the Notch signaling pathway using DAPT on the number of surviving crypts 3.5 days after IR. We also evaluated the effects of Notch inhibition on the number of Dclk1+ crypt epithelial cells at 24 h after IR. Our results demonstrate that the effect of Notch inhibitor on Dclk1+ crypt epithelial cells was proportional to the effect on surviving crypts, which suggests that Dclk1+ cells may provide a useful surrogate for evaluating the survival of ISCs following radiation injury. This study also demonstrates that inhibition of Notch signaling decreases ISC survival with an associated loss of surviving Dclk1+ stem cells following radiation injury, suggesting that the Notch signaling pathway plays an important role in ISC-mediated crypt regeneration.
MATERIALS AND METHODS
Experimental animals.
Six- to eight-week-old female C57Bl/6 mice (The Jackson laboratory, Bar Harbor, ME) were used in the experiments. Mice were housed under controlled conditions, including a 12:12-h light-dark cycle, with ad libitum access to food and water. All animal experiments were performed with the approval and authorization from the Institutional Review Board and the Institutional Animal Care and Use Committee, University of Oklahoma Health Sciences Center.
IR procedure.
Adult mice were exposed to TBI with air being pumped in the chamber during exposure. A Gamma-cell 40 137Cs gamma irradiator was used with a dose rate of 1 Gy IR/min. For DAPT (Sigma Aldrich, St. Louis, MO) treatment, mice (n = 5) were injected with DAPT (100 mg/kg in corn oil ip) 24 h before IR exposure. Mice in the control group (TBI without DAPT, n = 5) were injected with corn oil only. Two hours before the 84-h time interval, each mouse was injected with 5-bromo-2′-deoxyuridine (BrdU, 200 μl of 5 mg/ml BrdU solution in PBS; Sigma Aldrich). Mice were killed at 6, 24, or 84 h post-IR exposure.
Immunohistochemistry.
Heat-induced epitope retrieval was performed on 4-μm formalin-fixed paraffin-embedded sections by using a pressurized Decloaking Chamber (Biocare Medical, Concord, CA) in citrate buffer (pH 6.0) at 99°C for 18 min. For brightfield microscopy, slides were exposed to peroxidase blocking solution before the addition of primary antibodies [anti-Dclk1 ab31704, 1:8,000 dilution (Abcam, Cambridge, MA), anti-phosphorylated β-catenin-Ser552 (p-β-cat-Ser552), 1:500 dilution, anti-Ki67, 1:1,000]. After incubation with primary antibody overnight at 4°C, the slides were incubated in peroxidase-conjugated polymer (Promark Series-Biocare Medical). Slides were developed with either Betazoid DAB or Bajoran Purple HRP chromogens (Biocare Medical). To detect apoptotic cells, the ApopTag Peroxidase in Situ Apoptosis Detection Kit was used following the manufacturer's instructions (Millipore, Billerica, MA). The apoptotic cells were detected with anti-digoxigenin conjugated with FITC. To detect Dclk1+ apoptotic cells, following the incubation with anti-Dclk1 polyclonal antibody, anti-rabbit secondary antibody conjugated with Alexa 547 was used.
Microscopic examination.
Slides were examined with a Nikon 80i microscope and DXM1200C camera for brightfield microscopy. Fluorescent images were taken with PlanFluoro objectives, using a CoolSnap ES2 camera (Photometrics, Tucson, AZ). Images were processed using NIS-Elements software (Nikon Instruments, Melville, NY).
Crypt survival study.
The number of surviving crypts was scored across each intestinal cross section circumference [a surviving crypt was defined as containing five or more adjacent BrdU-positive nuclei (7)]. Twenty cross sections were measured for each mouse and four mice per experimental group.
Real-time RT-PCR analyses.
Total RNA isolated from small intestine was subjected to reverse transcription using Superscript II RNase H-Reverse Transcriptase and random hexanucleotide primers (Invitrogen, Carlsbad, CA). The cDNA was subsequently used to perform real-time PCR by SYBR chemistry (SYBR Green I; Molecular Probes, Eugene, OR) for specific transcripts using gene-specific primers and JumpStart Taq DNA polymerase (Sigma-Aldrich). The crossing threshold value assessed by real-time PCR was noted for the transcripts and normalized with β-actin mRNA. The quantitative changes in mRNA were expressed as fold change relative to control ± SE values.
The following primers were used: β-actin: forward 5′-GGTGATCCACATCTGCTGGAA-3′, reverse 5′-ATCATTGCTCCTCCTCAGGG-3′; Dclk1: forward 5′-CAGCAACCAGGAATGTATTGGA-3′, reverse 5′- ctcaactcggaatcggaagact-3′; Notch1: forward 5′-CGGGTCCACCAGTTTGAATG-3′, reverse 5′-GTTGTATTGGTTCGGCACCAT-3′; Hes1: forward 5′-TCTGACCACAGAAAGTCATCA-3′, reverse 5′-AGCTATCTTTCTTAAGTGCATC-3′.
Statistical analysis.
All experiments were performed in triplicate. Results were reported as averages ± SE. Data were analyzed using the Student's t-test for comparison of mean values between groups. A P value <0.05 was considered statistically significant.
RESULTS
Intestinal crypt response to TBI.
Adult C57Bl/6 mice were subjected to 12 Gy TBI to study the response of the small intestinal crypt to genotoxic injury. The small intestines were isolated at 6, 24, 84, and 168 h post-TBI, fixed, and stained with hematoxylin and eosin (Fig. 1). Morphologically apoptotic cells appeared at 6 h postradiation, suggesting the initiation of cell death induced by radiation damage. Morphologically mitotic cells appeared at 24 h postradiation, suggesting the release of stem/progenitor cells from radiation-induced cell cycle arrest, allowing surviving stem/progenitor cells to divide following radiation injury. Regenerative crypts appeared at 3.5 days postradiation, and the return of normal crypt/villus axis architecture appeared at 7 days postradiation even though the crypt was still hyperplastic.
Fig. 1.
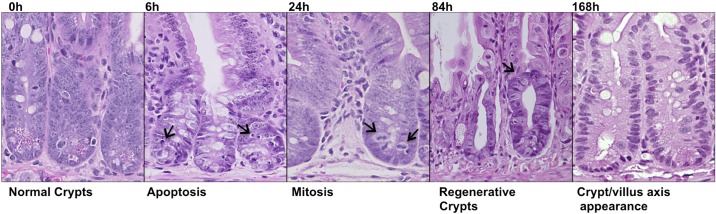
The intestinal crypts response to radiation injury. Wild-type C57Bl/6 mice were subjected to 12 Gy IR. The small intestinal crypts isolated 6, 24, 84, and 168 h post-IR were stained with hematoxylin and eosin and presented. The magnification is ×400.
Response of Dclk1+ crypt cells and small intestinal crypts to TBI.
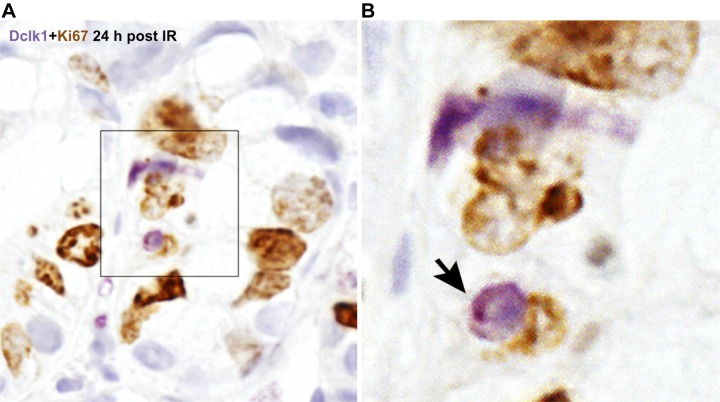
To investigate the response of Dclk1+ crypt epithelial cells and intestinal crypts to genotoxic injury, C57Bl/6 mice were subjected to 12 Gy TBI. Apoptotic cells were identified by TUNEL assay at both 6 and 24 h post-TBI (Fig. 2, center). Dclk1 in the crypt cells was identified by specific antibody immunostaining (Fig. 2, left). Dclk1 staining was observed occasionally in apoptotic cells at both 6 and 24 h post-TBI (Fig. 2, right for overlay), indicating apoptosis of potential resident stem cells after high-dose radiation injury. Approximately 15% of Dclk1-positive cells was detected undergoing apoptosis at both 6 and 24 h post-TBI. In our previous report, apoptotic Dclk1-positive cells were only found at 24 h after low-dose radiation (6 Gy) but not found at 6 h post 6 Gy radiation (14). These data suggested that Dclk1 cells were resistant to low-dose IR at 6 h. Moreover, these studies suggested that the 24-h mitotic activity of Dclk1+ cells could compensate for any stem cell loss, since there is essentially no substantial crypt loss at doses below 8 Gy. He et al. have reported that ISCs that undergo crypt fission and crypt budding contain nuclear p-β-cat-Ser552 (10). To assess this following TBI, crypt cells were immunostained with anti-p-β-cat-Ser552 antibody 24 h post-TBI. We found nuclear p-β-cat-Ser552 staining in the crypts, indicating the presence of mitotic stem cells (Fig. 3). Dclk1 in the crypt cells was identified by specific antibody immunostaining (Fig. 3). Moreover, Dclk1-positive cells were found adjacent to or colocalized with p-β-cat-Ser552-positive cells with ∼40% incidence (Fig. 3). Furthermore, Dclk1 staining was also found in some Ki67-positive cells 24 h post 12 Gy IR (Fig. 4). These data suggest that the potential descendants of Dclk1+ cells are able to divide and proliferate 24 h after high-dose TBI. We also observed the loss of intestinal crypts 84 h post 12 Gy, again demonstrating the high-dose requirement for crypt loss following TBI (see Fig. 6A). These results provide additional support for the long-standing Potten hypothesis that lethal dose (12 Gy) IR induces crypt stem cell sterilization in a majority of intestinal crypts, and the descendants of the few surviving cells are able to divide and ultimately repopulate the entire intestinal epithelium (25, 28, 30).
Fig. 2.
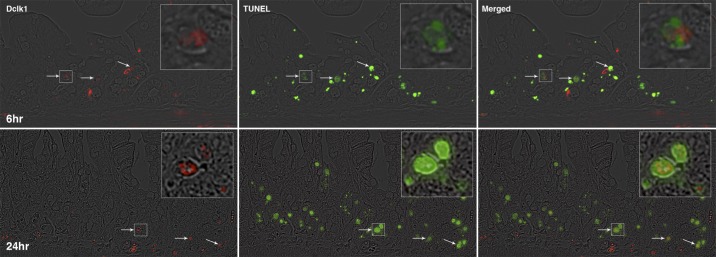
Identification of doublecortin-like kinase 1 (Dclk1)-positive apoptotic cells in small intestinal crypts after total body irradiation (TBI). The small intestine isolated 6 and 24 h post-TBI were fixed with formalin and embedded in paraffin. Paraffin-embedded sections were immunostained with anti-Dclk1 antibody (red on left) and ApopTag Peroxidase for apoptotic cells (green in middle). Colocalization of Dclk1 (red) in apoptotic cells (green) is indicated by arrows in the overlay pictures (right). Panels on top are 6 h post-TBI and bottom are 24 h post-TBI. The magnification is ×200, and the magnification for the inset is approximately ×600.
Fig. 3.
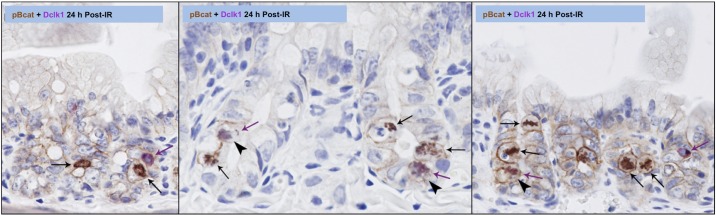
Identification of Dclk1-positive mitotic cells in small intestinal crypts after TBI. The small intestine isolated 24 h post-TBI was fixed with formalin and embedded in paraffin. Paraffin-embedded sections were immunostained with anti-Dclk1 antibody (purple, purple arrow) and anti-phospho β-catenin Ser552 antibody (brown, black arrow). Colocalization of Dclk1 with phospho β-catenin was indicated by arrowhead. The magnification is ×400.
Fig. 4.
Identification of Dclk1-positive proliferative cells in small intestinal crypts after TBI. The small intestine isolated 24 h post-TBI was fixed with formalin and embedded in paraffin. Paraffin-embedded sections were immunostained with anti-Dclk1 antibody (purple) and anti-Ki67 antibody (brown) (A). Colocalization of Dclk1 with Ki67 was indicated by arrow. The magnification for the inset is approximately ×600 (B).
Fig. 6.
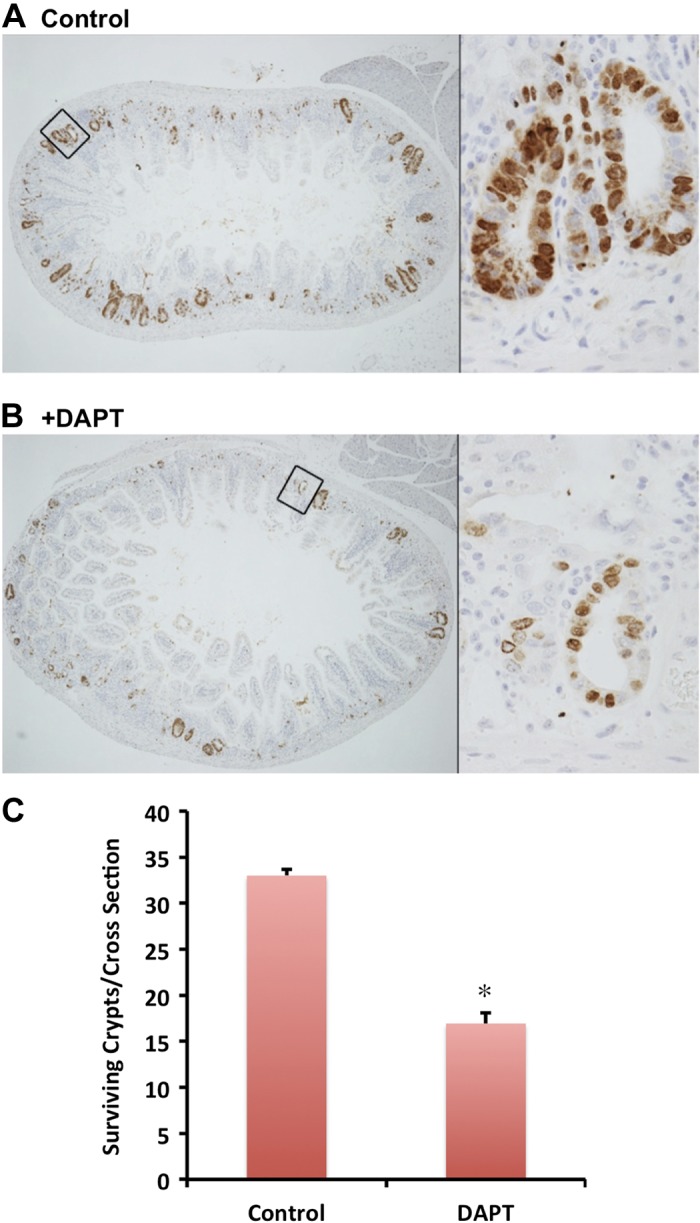
Inhibition of Notch1 signaling pathway reduced the number of surviving crypts after radiation injury. C57Bl/6 mice in the control group (A) or DAPT-treated group (B) were subjected to TBI. These mice were injected with 5-bromo-2′-deoxyuridine (BrdU) solution 82 h post-IR and killed 84 h post-IR. The small intestines were isolated, fixed, and processed. BrdU-positive cells are the indication for surviving crypts in A and B. The magnification is ×40 for the cross section pictures and approximately ×600 for the inset. The average number of surviving crypts per cross section was counted for total of 80 cross sections and presented in C. Values in the bar graph are given as averages ± SE. *P < 0.01, statistically significant difference compared with control.
Inhibition of the Notch signaling pathway reduces surviving Dclk1+ cells in response to TBI.
In uninjured adult mice, there are ∼20–30 Dclk1+ cells/cross section (∼150 crypts) (data not shown). Post-TBI (24 h), there are ∼11 Dclk1+ cells/cross section counted (Fig. 5A), a >50% reduction. This reduction is presumably the result of apoptotic cell death induced by radiation injury. Apoptotic Dclk1+ cells were detected post-TBI (Fig. 2). To investigate the role of the Notch signaling pathway on crypt epithelial Dclk1+ cell fate after TBI, mice were treated with the γ-secretase inhibitor DAPT 24 h before IR exposure. DAPT treatment alone for 48 h did not affect the number of Dclk1+ cells per cross section (data not shown). Following DAPT pretreatment and 24 h post-IR exposure, there were ∼6 Dclk1+ cells/cross section (Fig. 5, B and C), an ∼50% reduction in the number of Dclk1+ crypt epithelial cells after DAPT treatment. These data suggest that Notch signaling pathway plays an important role in the survival of Dclk1-expressing cells measured in situ 24 h after TBI, and inhibition of Notch pathway may sensitize Dclk1 cells to the effects of high-dose TBI.
Fig. 5.
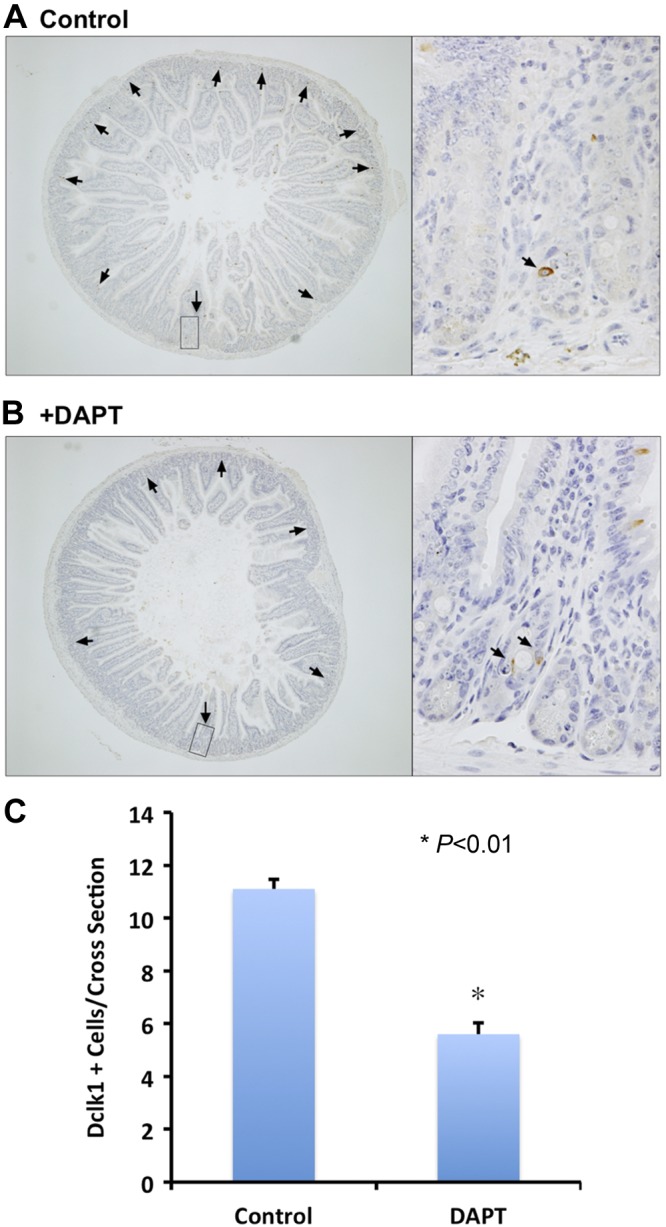
Inhibition of Notch1 signaling pathway reduced surviving Dclk1-positive cells after radiation injury. C57Bl/6 mice in control group (A) or difluorophenacetyl-l-alanyl-S-phenylglycine t-butyl ester (DAPT)-treated group (B) are subjected to TBI. Post-IR (24 h), the small intestines were isolated, fixed, processed, and stained with anti-Dclk1 antibody. Dclk1-positive cells in one cross section were indicated by arrows in A and B. The magnification is ×40 for the cross section pictures, and approximately ×600 for the inset. The average number of Dclk1 positive cells per cross section was counted for total of 80 cross sections and presented in C. Values in the bar graph are given as averages ± SE. *P < 0.01, statistically significant difference compared with control.
Inhibition of Notch signaling pathway reduces the number of regenerative crypts in response to TBI.
Lethal TBI injury induces crypt stem cell sterilization in a majority of intestinal crypts (11). The appearance of regenerative crypts 84 h postradiation injury is thought to represent the survival of at least one progenitor/stem cell per crypt (22). There were several BrdU-incorporated crypts (regenerative crypts) detected 84 h post-TBI (Fig. 6A). After DAPT pretreatment and 12 Gy IR exposure, the number of BrdU-incorporated crypts was dramatically decreased (Fig. 6B). The number of BrdU+ cells per cross section was quantified, and an ∼50% reduction in crypt survival was observed (Fig. 6C). These data taken together suggest that the Notch signaling pathway plays an important role in crypt stem cell survival and/or crypt regeneration following severe radiation injury.
Inhibition of Notch signaling pathway reduces Dclk1 expression levels in the small intestine in response to high-dose radiation injury.
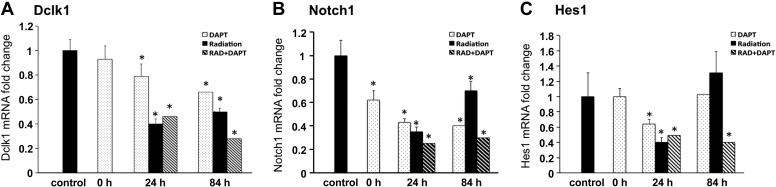
In our previous report, immunoreactive Dclk1 staining was not observed in regenerative crypt epithelial cells 84 h post-TBI (14). Restoration of Dclk1 expression within the crypts was only observed 7 days postradiation injury when the morphological features of the intestine are returning to normal. In this study, we measured Dclk1 mRNA levels by quantitative real-time RT-PCR 24 and 84 h post-IR with or without DAPT pretreatment. In the DAPT alone groups, Dclk1 mRNA levels were decreased in intestine ∼20 and 30% 48 and 108 h post-DAPT pretreatment, respectively (Fig. 7A, Note: 24 and 84 h in Fig. 7A indicate post-TBI time, DAPT pretreatment started 24 h before TBI). This result suggests that the Notch signaling pathway regulates Dclk1 expression in the small intestine. In the 24-h post-TBI groups, Dclk1 mRNA levels decreased by 60% in the absence of DAPT and ∼50% in the presence of DAPT (Fig. 7A). Thus there was no further reduction of Dclk1 mRNA levels in the presence of DAPT. In the 84-h post-TBI groups, Dclk1 mRNA levels decreased about 45% in the absence of DAPT and ∼70% in the presence of DAPT. Because regenerative crypts at 84 h post-IR were reduced 50% in the presence of DAPT (Fig. 6), these data taken together support the hypothesis that inhibition of Notch signaling results in a reduction in the number of Dclk1-expressing cells.
Fig. 7.
Inhibition of Notch1 signaling pathway decreased Dclk1 mRNA expression after TBI. Total RNAs were isolated from the small intestine after each treatment. mRNA expression levels were measured by quantitative real-time RT-PCR and normalized against β-actin level. A: Dclk1 mRNA after DAPT and radiation injury. B: Notch1 mRNA after DAPT and radiation injury. C: Hes1 mRNA after DAPT and radiation injury. Values in the bar graphs are given as averages ± SE. *P < 0.05, statistically significant difference compared with control.
We also measured mRNA levels of Notch1, and Hes1, one of the downstream effectors of Notch signaling pathway, in the small intestine after DAPT and TBI treatment. In the DAPT alone groups, Notch1 mRNA levels decreased ∼40% 24 h after treatment, and >50% 48 and 108 h after treatment (Fig. 7B, Note: 24 and 84 h indicate for post-TBI time, DAPT pretreatment started 24 h before TBI). In the 24 h post-TBI groups, Notch1 mRNA levels decreased dramatically in the absence of DAPT, and DAPT treatment furthered the reduction (Fig. 7B). In the 84-h post-TBI groups, Notch1 mRNA levels recovered >30% in the absence of DAPT but remained decreased (70%) in the presence of DAPT. In the DAPT alone groups, Hes1 mRNA levels remained unchanged 24 h after DAPT treatment, but decreased significantly (30%) after 48 h, and recovered back to baseline levels 108 h after DAPT (Fig. 7C). In the 24-h post-TBI groups, Hes1 mRNA levels decreased 60% in the absence of DAPT and 50% in the presence of DAPT (Fig. 7C). In the 84-h post-TBI groups, Hes1 mRNA levels increased about 30% in the absence of DAPT and remained decreased (60% reduction) in the presence of DAPT. These results suggest that the Notch signaling pathway is very active during crypt regeneration, supporting its importance in crypt stem epithelial cell dynamics after radiation injury.
DISCUSSION
In this study we detected apoptotic and mitotic Dclk1+ cells at 24 h and loss of crypts at 84 h post 12 Gy TBI. Using Notch pathway inhibitor DAPT, we demonstrated the effect of Notch inhibitor on Dclk1+ crypt epithelial cells was proportional to the effect on surviving crypts, suggesting that Dclk1+ cells may provide a useful surrogate for evaluating the survival of ISCs following radiation injury. A recent report by Hua et al. has shown that, following 12 Gy TBI, the Lgr5-labeled crypt-based columnar stem cells undergo apoptosis starting at 6 h and peaking at 24 h, followed by mitotic death at 24–48 h post-TBI (12). Their results suggest that examining at the 24-h time point may overestimate the number of surviving stem cells, and the surviving stem cells at 48 h may better predict crypt regeneration at 3.5 days post-TBI (12).
Notch signaling has been implicated in stem cell maintenance in many systems through its downstream target Hes1 (5). Notch signaling drives progenitor cells toward an enterocyte lineage, whereas inhibition of Notch signaling drives progenitor cells toward a secretory lineage resulting in an abundance of Goblet cells (17). Despite this seemingly protective phenotype, Notch inhibitors were not protective against colitis induced by the chemical irritant Dextran sodium sulfate. Moreover, Notch inhibition appears to exacerbate colitis in other models of inflammatory injury (20). In addition, gut toxicity has been a major limiting factor in many clinical trials of Notch inhibitors for a variety of liquid tumors. This toxicity can be attenuated by limiting the dosing schedule to every 4 days, allowing for recovery of the intestinal epithelium, which turns over every 3–4 days. This may represent a plausible explanation for the improved tolerability of this regimen. These data led us to investigate whether stem cells were actually deleted or at least functionally inhibited by Notch inhibition. Our results demonstrate that systemic Notch inhibition beginning 24 h before IR results in a 50% reduction in crypt survival after IR compared with vehicle-treated controls, suggesting that inhibition of Notch can functionally reduce ISC's activity. This result seems to confirm the well-known role of the Notch pathway in stem cell biology, and these studies demonstrate a direct effect of Notch inhibition on ISCs in vivo.
To further analyze this result mechanistically, we sought to determine the effect of Notch inhibition on the expression of the putative ISC marker Dclk1 in response to radiation injury. In response to DAPT treatment alone without radiation injury, we found an ∼20% decrease in Dclk1 mRNA levels but no change in the number of Dclk1+ cells per cross section. Because the Dclk1+ cells were detected by immunostaining, it may not be sensitive enough to detect small changes in Dclk1 protein levels. We have reported that treating HCT116 colon cancer xenografts with DAPT results in both Dclk1 mRNA and protein downregulation in the tumor, suggesting a Notch regulatory mechanism (33). We evaluated the effects of radiation injury on Dclk1 expression 24 h post-IR, the postulated time point at which p53 independent stem cell apoptosis occurs following lethal dose IR (14, 16). We chose Dclk1 for several reasons: 1) its limited expression pattern within the crypt allowed for quantitative assessment; 2) its distinct subcellular expression pattern in conjunction with labeling with nuclear markers (such as p-β-cat-Ser552) within a particular crypt could be used to determine the identity of DNA-damaged cells; and 3) other putative stem cell markers are expressed in crypt-based columnar and progenitor populations, making it impossible to quantitatively assess the effects of Notch inhibition on crypt dynamics in two-dimensional histological sections (24). Although skepticism still exists regarding the identity of Dclk1-expressing cells (8), there is increasing data in other models suggesting that Dclk1 plays a functional role in many cancers and is unlikely to be a marker of wholly differentiated cells. Studies are currently underway to determine the function of Dclk1 in the gut in normal homeostasis and in cancer. The possibility that Dclk1 and other markers that are observed at the +4 position (i.e., Msi1, Bmi1, Lrig1, and mTert) (18, 23, 29, 31, 34, 39) may serve to mark “rescue stem cells” in response to severe genotoxic/cytotoxic injury must also be considered.
The studies presented here attempt to assess the role of Notch signaling on epithelial crypt stem cell fate in response to genotoxic/cytotoxic injury using Dclk1 as an in situ marker of reserve or rescue stem cells. Notch signaling may play a key role as a regulatory mechanism for protection of the stem cell under severe DNA damage. Recent studies using Notch-deficient mice have demonstrated that Notch deletion results in a reduction in Wnt target genes, including the novel stem cell marker Lgr5 (21). These findings suggest in a correlative manner that Notch may be upstream of Wnt with respect to stem cell dynamics and that Notch inhibition may indeed result in reduced Wnt signaling. This combined reduction in stem cell pathway signaling may be a welcome approach in attenuating the stem cell phenotype in cancer by reducing the cell of origin as well as the proliferative potential of the stem cell offspring. This hypothesis would certainly be in play if a hierarchical or interconversion model of the stem cell niche were the reality. Because the identity and validation of novel stem cell markers continues to be debated, functional assays of stem cell survival such as clonogenic microcolony assays remain necessary. Evaluation of Dclk1 expression in radiation and related injury models may serve as an effective model to test the effects of therapeutic agents on gut stem cells directly. This use of Dclk1 is currently feasible, since commercial antibodies that are easy to use and yield reproducible results in many tissues are available. Furthermore, the expression in a minority of cells allows for quantitative assessment of the response to genotoxic/cytotoxic injury, particularly 24 h after radiation injury. We are aware of reports that Dclk1 represents a fully differentiated Tuft cell or enteroendocrine cell based on immunohistochemistry (8). We contend the functional properties of Dclk1, and previous reported sorting studies identify a subset of Dclk1 cells that are not fully differentiated. In fact, a recent study by Bjerknes et al. confirmed the presence of an early Tuft cell in the crypt with a different marker expression profile (1). Finally, there is a possibility that a subset of tuft cells has stem cell-like properties. In fact, several recent reports suggest that other cell types can revert to stem cells upon crypt damage (2, 35, 36). Using a transgenic mouse model (Cyp1A1-H2B-YFP), Buczacki et al. reported that label-retaining cells (Histone 2B positive) at the crypt base of the small intestine have a combined secretory and stem-cell signature and demonstrate clonogenicity after injury (2). Additionally, van Es et al. showed that Dll1+ secretory progenitor cells can revert to stem cells during intestinal crypt regeneration following injury (35). Using the Sox9-enhanced green fluorescent protein (EGFP) transgenic mouse model, Van Landeghem et al. found that Sox9-EGFP high cells have both an enteroendocrine and a +4 ISC signature and express Dclk1 protein. Moreover, isolated Sox9-EGFP high cells form organoids in vitro after radiation injury, suggesting that Sox9-EGFP high cells contain radiation-activated cells with ISC characteristics that likely participate in crypt regeneration postinjury (36). A recent report by Nakanishi et al. showed that there was no detectable BrdU incorporation in Dclk1+ cells in the normal intestine, and extremely small number of blue stripes comprised LacZ-labeled Dclk1+ cell lineages 14 days after 8 Gy IR in the Dclk1-Cre-ERT2 lineage tracing mouse (19). It is hypothesized that the +4 position quiescent stem cell is a potential rescue stem cell and may play a minor role in normal intestinal homeostasis where Lgr5+ rapidly cycling stem/progenitor cells may have a predominant role. In this report, they used 8 Gy IR to activate lineage tracing. However, it may be necessary to use a >8 Gy high-dose IR to functionally achieve lineage tracing from a “rescue stem cell.”
The results reported here lend additional support to the hypothesis that the Notch signaling pathway is a valid target for anti-stem cell-based therapies. Furthermore, these studies provide a reasonable explanation for the side effect profile observed with gamma secretase inhibitors and establish Dclk1 expression as an effective marker for testing the effects of therapeutic agents in radiation and related injury models. Additional approaches to the inhibition of the Notch signaling pathway may be beneficial for anti-cancer drug development.
GRANTS
This research was performed as a project of the Intestinal Stem Cell Consortium, a collaborative research project funded by the National Institute of Diabetes and Digestive and Kidney Diseases and the National Institute of Allergy and Infectious Diseases (NIH U01 DK-085508 to C. W. Houchen), and also funded by a Veterans Affairs Merit Award to C. W. Houchen.
DISCLOSURES
C.W. Houchen is a founder of COARE Biotechnology, Inc. The other authors have declared that no conflict of interest exists.
AUTHOR CONTRIBUTIONS
Author contributions: D.Q., R.J.M., S.M.S., N.A., L.L., T.A.B., and C.W.H. conception and design of research; D.Q., R.J.M., S.M.S., N.W., and P.C. performed experiments; D.Q., R.J.M., S.M.S., N.W., and P.C. analyzed data; D.Q., R.J.M., S.M.S., and C.W.H. interpreted results of experiments; D.Q., R.J.M., and S.M.S. prepared figures; D.Q. drafted manuscript; D.Q., R.J.M., S.M.S., and C.W.H. edited and revised manuscript; D.Q. and C.W.H. approved final version of manuscript.
REFERENCES
- 1.Bjerknes M, Khandanpour C, Moroy T, Fujiyama T, Hoshino M, Klisch TJ, Ding Q, Gan L, Wang J, Martin MG, Cheng H. Origin of the brush cell lineage in the mouse intestinal epithelium. Dev Biol 362: 194–218, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buczacki SJ, Zecchini HI, Nicholson AM, Russell R, Vermeulen L, Kemp R, Winton DJ. Intestinal label-retaining cells are secretory precursors expressing Lgr5. Nature 495: 65–69, 2013 [DOI] [PubMed] [Google Scholar]
- 3.Cheng H, Leblond CP. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. V. Unitarian Theory of the origin of the four epithelial cell types. Am J Anat 141: 537–561, 1974 [DOI] [PubMed] [Google Scholar]
- 4.Cohn SM, Simon TC, Roth KA, Birkenmeier EH, Gordon JI. Use of transgenic mice to map cis-acting elements in the intestinal fatty acid binding protein gene (Fabpi) that control its cell lineage-specific and regional patterns of expression along the duodenal-colonic and crypt-villus axes of the gut epithelium. J Cell Biol 119: 27–44, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fre S, Hannezo E, Sale S, Huyghe M, Lafkas D, Kissel H, Louvi A, Greve J, Louvard D, Artavanis-Tsakonas S. Notch lineages and activity in intestinal stem cells determined by a new set of knock-in mice. PLoS One 6: e25785, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fre S, Huyghe M, Mourikis P, Robine S, Louvard D, Artavanis-Tsakonas S. Notch signals control the fate of immature progenitor cells in the intestine. Nature 435: 964–968, 2005 [DOI] [PubMed] [Google Scholar]
- 7.George RJ, Sturmoski MA, May R, Sureban SM, Dieckgraefe BK, Anant S, Houchen CW. Loss of p21Waf1/Cip1/Sdi1 enhances intestinal stem cell survival following radiation injury. Am J Physiol Gastrointest Liver Physiol 296: G245–G254, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gerbe F, van Es JH, Makrini L, Brulin B, Mellitzer G, Robine S, Romagnolo B, Shroyer NF, Bourgaux JF, Pignodel C, Clevers H, Jay P. Distinct ATOH1 and Neurog3 requirements define tuft cells as a new secretory cell type in the intestinal epithelium. J Cell Biol 192: 767–780, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gordon JI, Hermiston ML. Differentiation and self-renewal in the mouse gastrointestinal epithelium. Curr Opin Cell Biol 6: 795–803, 1994 [DOI] [PubMed] [Google Scholar]
- 10.He XC, Yin T, Grindley JC, Tian Q, Sato T, Tao WA, Dirisina R, Porter-Westpfahl KS, Hembree M, Johnson T, Wiedemann LM, Barrett TA, Hood L, Wu H, Li L. PTEN-deficient intestinal stem cells initiate intestinal polyposis. Nat Genet 39: 189–198, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Houchen CW, George RJ, Sturmoski MA, Cohn SM. FGF-2 enhances intestinal stem cell survival and its expression is induced after radiation injury. Am J Physiol Gastrointest Liver Physiol 276: G249–G258, 1999 [DOI] [PubMed] [Google Scholar]
- 12.Hua G, Thin TH, Feldman R, Haimovitz-Friedman A, Clevers H, Fuks Z, Kolesnick R. Crypt base columnar stem cells in small intestines of mice are radioresistant. Gastroenterology 143: 1266–1276, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koch U, Lehal R, Radtke F. Stem cells living with a Notch. Development 140: 689–704, 2013 [DOI] [PubMed] [Google Scholar]
- 14.May R, Riehl TE, Hunt C, Sureban SM, Anant S, Houchen CW. Identification of a novel putative gastrointestinal stem cell and adenoma stem cell marker, doublecortin and CaM kinase-like-1, following radiation injury and in adenomatous polyposis coli/multiple intestinal neoplasia mice. Stem Cells 26: 630–637, 2008 [DOI] [PubMed] [Google Scholar]
- 15.May R, Sureban SM, Hoang N, Riehl TE, Lightfoot SA, Ramanujam R, Wyche JH, Anant S, Houchen CW. Doublecortin and CaM kinase-like-1 and leucine-rich-repeat-containing G-protein-coupled receptor mark quiescent and cycling intestinal stem cells, respectively. Stem Cells 27: 2571–2579, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Merritt AJ, Allen TD, Potten CS, Hickman JA. Apoptosis in small intestinal epithelial from p53-null mice: evidence for a delayed, p53-independent G2/M-associated cell death after gamma-irradiation. Oncogene 14: 2759–2766, 1997 [DOI] [PubMed] [Google Scholar]
- 17.Milano J, McKay J, Dagenais C, Foster-Brown L, Pognan F, Gadient R, Jacobs RT, Zacco A, Greenberg B, Ciaccio PJ. Modulation of notch processing by gamma-secretase inhibitors causes intestinal goblet cell metaplasia and induction of genes known to specify gut secretory lineage differentiation. Toxicol Sci 82: 341–358, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Montgomery RK, Carlone DL, Richmond CA, Farilla L, Kranendonk ME, Henderson DE, Baffour-Awuah NY, Ambruzs DM, Fogli LK, Algra S, Breault DT. Mouse telomerase reverse transcriptase (mTert) expression marks slowly cycling intestinal stem cells. Proc Natl Acad Sci USA 108: 179–184, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakanishi Y, Seno H, Fukuoka A, Ueo T, Yamaga Y, Maruno T, Nakanishi N, Kanda K, Komekado H, Kawada M, Isomura A, Kawada K, Sakai Y, Yanagita M, Kageyama R, Kawaguchi Y, Taketo MM, Yonehara S, Chiba T. Dclk1 distinguishes between tumor and normal stem cells in the intestine. Nat Genet 45: 98–103, 2013 [DOI] [PubMed] [Google Scholar]
- 20.Okamoto R, Tsuchiya K, Nemoto Y, Akiyama J, Nakamura T, Kanai T, Watanabe M. Requirement of Notch activation during regeneration of the intestinal epithelia. Am J Physiol Gastrointest Liver Physiol 296: G23–G35, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Pellegrinet L, Rodilla V, Liu Z, Chen S, Koch U, Espinosa L, Kaestner KH, Kopan R, Lewis J, Radtke F. Dll1- and dll4-mediated notch signaling are required for homeostasis of intestinal stem cells. Gastroenterology 140: 1230–1240, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Potten CS. A comprehensive study of the radiobiological response of the murine (BDF1) small intestine. Int J Radiation Biol 58: 925–973, 1990 [DOI] [PubMed] [Google Scholar]
- 23.Potten CS, Booth C, Hargreaves D. The small intestine as a model for evaluating adult tissue stem cell drug targets. Cell Prolif 36: 115–129, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Potten CS, Gandara R, Mahida YR, Loeffler M, Wright NA. The stem cells of small intestinal crypts: where are they? Cell Prolif 42: 731–750, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Potten CS, Grant HK. The relationship between ionizing radiation-induced apoptosis and stem cells in the small and large intestine. Br J Cancer 78: 993–1003, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Potten CS, Loeffler M. A comprehensive model of the crypts of the small intestine of the mouse provides insight into the mechanisms of cell migration and the proliferation hierarchy. J Theor Biol 127: 381–391, 1987 [DOI] [PubMed] [Google Scholar]
- 27.Potten CS, Loeffler M. Stem cells: attributes, cycles, spirals, pitfalls and uncertainties. Lessons for and from the crypt. Development 110: 1001–1020, 1990 [DOI] [PubMed] [Google Scholar]
- 28.Potten CS, Merritt A, Hickman J, Hall P, Faranda A. Characterization of radiation-induced apoptosis in the small intestine and its biological implications. Int J Radiation Biol 65: 71–78, 1994 [DOI] [PubMed] [Google Scholar]
- 29.Potten CS, Owen G, Booth D. Intestinal stem cells protect their genome by selective segregation of template DNA strands. J Cell Sci 115: 2381–2388, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Riehl TE, George RJ, Sturmoski MA, May R, Dieckgraefe B, Anant S, Houchen CW. Azoxymethane protects intestinal stem cells and reduces crypt epithelial mitosis through a COX-1-dependent mechanism. Am J Physiol Gastrointest Liver Physiol 291: G1062–G1070, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Sangiorgi E, Capecchi MR. Bmi1 is expressed in vivo in intestinal stem cells. Nat Genet 40: 915–920, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmidt GH, Wilkinson MM, Ponder BA. Cell migration pathway in the intestinal epithelium: an in situ marker system using mouse aggregation chimeras. Cell 40: 425–429, 1985 [DOI] [PubMed] [Google Scholar]
- 33.Sureban SM, May R, Mondalek FG, Qu D, Ponnurangam S, Pantazis P, Anant S, Ramanujam RP, Houchen CW. Nanoparticle-based delivery of siDCAMKL-1 increases microRNA-144 and inhibits colorectal cancer tumor growth via a Notch-1 dependent mechanism (Abstract). J Nanobiotechnol 9: 40, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tian H, Biehs B, Warming S, Leong KG, Rangell L, Klein OD, de Sauvage FJ. A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature 478: 255–259, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Es JH, Sato T, van de Wetering M, Lyubimova A, Nee AN, Gregorieff A, Sasaki N, Zeinstra L, van den Born M, Korving J, Martens AC, Barker N, van Oudenaarden A, Clevers H. Dll1+ secretory progenitor cells revert to stem cells upon crypt damage. Nature Cell Biol 14: 1099–1104, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van Landeghem L, Santoro MA, Krebs AE, Mah AT, Dehmer JJ, Gracz AD, Scull BP, McNaughton K, Magness ST, Lund PK. Activation of two distinct Sox9-EGFP-expressing intestinal stem cell populations during crypt regeneration after irradiation. Am J Physiol Gastrointest Liver Physiol 302: G1111–G1132, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Winton DJ, Ponder BA. Stem-cell organization in mouse small intestine. Proc Biol Sci 241: 13–18, 1990 [DOI] [PubMed] [Google Scholar]
- 38.Withers HR, Elkind MM. Microcolony survival assay for cells of mouse intestinal mucosa exposed to radiation. Int J Radiation Biol 17: 261–267, 1970 [DOI] [PubMed] [Google Scholar]
- 39.Wong VW, Stange DE, Page ME, Buczacki S, Wabik A, Itami S, van de Wetering M, Poulsom R, Wright NA, Trotter MW, Watt FM, Winton DJ, Clevers H, Jensen KB. Lrig1 controls intestinal stem-cell homeostasis by negative regulation of ErbB signalling. Nat Cell Biol 14: 401–408, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]