Abstract
Intestinal intussusception (ISS) commonly causes intestinal obstruction in children. One mechanism that has been proposed to cause ISS is inflammation-induced alteration of intestinal motility. We investigated whether innate inflammatory factors or altered motility is required for induction of ISS by LPS. We compared rates of ISS among BALB/c and C57BL/6 mice, mice lacking lymphocytes or depleted of phagocytes, or mice with defects in the Toll-like receptor 4 (TLR4) signaling pathway following administration of LPS or the Ca2+ analog MnCl2. At 6 or 2 h after administration of LPS or MnCl2, respectively, mice underwent image analysis to assess intestinal contraction rate or laparotomy to identify ISS. LPS-induced ISS (LPS-ISS) was observed in BALB/c mice, but not in C57BL/6 mice or any BALB/c mice with disruptions of TLR4 signaling. LPS-induced serum TNF-α, IL-6, and nitric oxide (NO) and intestinal NO levels were similar in BALB/c and C57BL/6 mice. The rate of LPS-ISS was significantly reduced in phagocyte-depleted, but not lymphocyte-deficient, mice. Intestinal contraction rates were reduced in LPS-ISS-susceptible BALB/c mice, but not in LPS-ISS-resistant C57BL/6 or TLR4 mutant mice, suggesting a role for reduced intestinal contraction rate in LPS-ISS susceptibility. This was tested with MnCl2, a Ca2+ antagonist that reduced intestinal contraction rates and induced ISS, irrespective of mouse strain. Therefore, LPS-ISS is initiated by innate immune signaling that requires TLR4 and phagocytes but may be independent of TNF-α, IL-6, and NO levels. Furthermore, alteration of intestinal motility, specifically, reduced intestinal contraction rate, is a key factor in the development of ISS.
Keywords: inflammatory response, intestinal disorder, pediatric, mouse model
intestinal intussusception (ISS), the telescoping of one segment of the intestine into an adjacent segment, is the most common cause of potentially life-threatening intestinal obstruction in young pediatric patients. ISS is an important pediatric disease that occurs relatively frequently (0.1–9:1,000 live births) worldwide, although rates vary by country (2). Despite the clinical importance of this disease, the mechanism of ISS is unknown.
ISS is associated with multiple infectious agents in children (16, 26, 42, 53). Whether ISS results from the direct effect of the pathogen on the intestine or indirect effects of infection-induced inflammation is unclear. Inflammation is suspected to play an etiological role in ISS in children (4, 39, 52), because intestinal lymphoid hyperplasia is often observed in children with ISS (3), and compared with children without ISS, IL-6 levels, C-reactive protein levels, and anti-endotoxin antibody titers (52) are elevated in children with ISS.
Data from experimental models also implicate inflammation in ISS. LPS is expressed by gram-negative bacteria, several of which are associated with ISS in children (8, 14, 17, 22, 53), and is a potent stimulator of inflammation mediated by Toll-like receptor 4 (TLR4). Serum LPS is elevated in pigs with ISS (4), and LPS treatment induces ISS in mice and rats (1, 21, 28, 35, 43, 47). Whether TLR4 signaling is required for ISS is unknown. Many leukocytes express TLR4. However, macrophage responses to LPS are pronounced (34, 41), and macrophage depletion protects against other LPS-induced diseases (7, 50). Whether macrophage depletion similarly protects mice from LPS-induced ISS (LPS-ISS) remains unstudied.
LPS treatment rapidly induces a complex cascade of cytokines, including TNF-α, IL-6, and nitric oxide (NO) (5, 41), all indirectly implicated in ISS on the basis of changes in ISS rates following treatment of BALB/c or Swiss albino mice with single agonists/antagonists (1, 28, 35, 43). In addition, NO levels are significantly higher in Swiss albino mice with LPS-ISS than in those without LPS-ISS (43). However, a more critical evaluation of the role of these inflammatory mediators in LPS-ISS is needed, because the agonists and antagonists used lack specificity, a treatment effect on cytokine levels was not confirmed, and comparisons were between treated and untreated mice, not mice with different susceptibilities to developing LPS-ISS.
There is much speculation but little definitive evidence that ISS results from altered intestinal motility (40). Infection, inflammation, and LPS are associated with ISS, and each can alter intestinal motility (1, 43, 50). In vivo assessment of intestinal motility is difficult and for ISS is limited to the observation that LPS inhibits gastrointestinal transit (GIT) in mice (21). However, GIT alterations did not correlate with differences in the rate of ISS between LPS- and rotavirus-LPS-treated mice (48). An alternate in vivo method for assessing intestinal motility would greatly aid in our ability to measure alterations in intestinal motility and to understand how LPS is inducing ISS.
The aims of this study were to use the LPS-ISS mouse model to identify ISS-susceptible and -resistant mouse strains in which to elucidate the mechanism of ISS, evaluate the role of TLR4 signaling and different leukocyte subpopulations in ISS, and determine if alterations in motility are involved in the development of ISS. We identified profound differences in LPS-ISS susceptibility between BALB/c (susceptible) and C57BL/6 (resistant) mice. Neither differences in GIT nor differences in TNF-α, IL-6, or NO levels accounted for the differences in LPS-ISS susceptibility between mouse strains; LPS induced similar alteration of GIT and TNF-α, IL-6, or NO levels in BALB/c and C57BL/6 mice. We identified a requirement for TLR4 signaling and phagocytes in LPS-ISS, a previously unverified role for innate immunity in LPS-ISS. Furthermore, we show that, unlike LPS, MnCl2 induced ISS and reduction in intestinal contraction rate independent of TLR4 signaling and mouse strain. Therefore, induction of ISS by LPS or MnCl2 was predicted by whether the treatment reduced intestinal contraction rate, suggesting that this change in intestinal motility is a key contributor to ISS. Taken together, these results provide new insights into the mechanism of ISS.
MATERIALS AND METHODS
Mice.
Male and female 4- to 10-wk-old BALB/c, C.C3-TlrLps-d/J (TLR4 mutant), C.129P2-Lbptm1Jack/J (LBP−/−), CD14 knockout (CD14−/−) (24), and SCID/NCr mice, all on BALB/c background, and CD-1 and C57BL/6 mice were purchased from the National Cancer Institute (Frederick, MD), Charles River Laboratories (Wilmington, MA), or Jackson Laboratories (Bar Harbor, ME) or bred at Baylor College of Medicine facilities. Mice were housed in microisolator cages under a standard 12:12-h light-dark cycle and fed autoclaved LabDiet 5010 (PMI, Richmond, IN) ad libitum. All studies were approved by the Baylor College of Medicine Institutional Animal Care and Use Committee.
LPS.
Escherichia coli 0111:B4 LPS (Sigma Chemical, St. Louis, MO) or ultra-pure E. coli 0111:B4 LPS (InvivoGen, San Diego, CA) was used in all experiments. LPS was diluted in sterile water in borosilicate glass vials (Wheaton, Millville, NJ) to specified concentrations, mixed at room temperature on a shaker for ≥2 h, and immediately administered to mice.
LPS-ISS studies.
Average mouse weight per cage was determined, and LPS was administered to mice at 6 or 12 mg/kg in a single intraperitoneal injection. Control mice received sterile water but were not included in every experiment, because ISS was never seen in previous studies of >200 water-treated mice (data not shown) (48). At 6 h postinjection, mice were euthanized by cervical dislocation, laparotomy was performed, and ISS was assessed visually. Male and female mice developed LPS-ISS at similar rates following treatment with LPS at 6 mg/kg (data not shown).
Liposome-mediated cell depletion.
Mice were untreated or injected intraperitoneally with 200 μl of liposomes containing PBS or dichloromethylene bisphosphonate [clodronate (Cl2MDP); a gift from Roche Diagnostics, Mannheim, Germany]. Liposomes were prepared as previously described using phosphatidylcholine (Lipoid, Ludwigshafen, Germany) and cholesterol (Sigma Chemical) (45). At ∼14 h after liposome injection, mice were injected with LPS (6 mg/kg ip) and euthanized or imaged by ultrasound 6 h later to assess ISS or intestinal contraction rate, respectively.
Measurement of TNF-α, IL-6, and NO levels.
Mice were bled 1 or 6 h after LPS treatment for assessment of serum TNF-α or IL-6 levels, respectively, by TNF-α Quantikine ELISA (R & D Systems, Minneapolis, MN) or BD OptEIA mouse IL-6 ELISA (BD Biosciences, San Diego, CA) according to the manufacturers' instructions. NO levels in serum or a 3-cm piece of jejunum collected 6 h after LPS treatment were measured using a nitrate/nitrite colorimetric assay kit (Cayman Chemical, Ann Arbor, MI) according to the manufacturer's instructions.
GIT studies.
GIT was assessed as previously described (25, 48). Briefly, at 6 h after LPS or water injection, BALB/c and C57BL/c mice were gavaged with 100 μl of 5 mM FITC-dextran (Sigma Chemical) in PBS. The stomach and small intestine were removed 15 min later following euthanasia. The intestine was divided into 10 equal segments, each flushed with 1 ml of PBS. FITC-dextran concentration in luminal contents was measured (485-nm excitation, 510-nm emission; VICTOR2 1420 multilabel counter, Perkin Elmer, Waltham, MA). To determine the geometric center of FITC-dextran, FITC-dextran concentrations in the stomach and each intestinal segment were summed. The percentage of total FITC-dextran in the stomach and each segment was determined and multiplied by the segment number of the sample (e.g., 1 for stomach, 2 for 1st intestine segment, 3 for 2nd intestine segment). These values were added to determine the geometric center (25, 48).
MnCl2-induced ISS studies.
MnCl2 (0.4 mmol/kg ip) was administered using a 30 mM MnCl2 solution in 0.9% NaCl. At 2 h postinjection, mice were euthanized and examined for ISS visually following laparotomy.
Ultrasound assessment of intestinal contraction rate.
Depilated mice were lightly anesthetized with isoflurane gas, and ultrasound imaging [Vevo Ultrasound (VisualSonics, Toronto, ON, Canada) using probe 704 at 40 MHz] was performed. Contraction rate was determined 18–22 h before and 2 or 6 h after treatment with MnCl2 (0.4 mmol/kg) or LPS (6 or 12 mg/kg), respectively. The probe was moved along the cephalocaudal axis beginning at the edge of the liver to acquire 6–13 random axial and longitudinal cross sections of bowel, which were assessed for the number of contractions per minute. A contraction in both axial and longitudinal cross sections was defined as a narrowing of the lumen and in the latter case as occurring unidirectionally along the intestinal wall. The contraction rate (average number of contractions per minute) was determined for each mouse pre- and posttreatment, and the percent change in contraction rate induced by treatment was calculated. The mean percent change was calculated for each treatment group.
Intestinal contractile activity ex vivo.
Intestinal contractile activity in intact ileal tissue, in the longitudinal axis, was measured as described previously (44) 6 h after injection of LPS or vehicle. Isometric force was monitored by an external force-displacement transducer (Experimetria, Budapest, Hungary) connected to PowerLab data acquisition hardware (ADInstruments, Colorado Springs, CO). Each strip was stretched to 0.5-g tension and allowed to equilibrate for 30 min, and then 10 min of basal contractile activity were recorded. After recording of contractile activity, the length of each strip was measured. All force development was normalized to tissue length. All contractile activity parameters were calculated over 5 min of recorded data in two separate intestinal sections and averaged. Total contractile activity was calculated as the area under the curve. Basal tone was defined as the average minimum of the contraction cycle. Amplitude was calculated as average cycle height.
Statistical methods.
A χ2 test was used to determine significance in all ISS experiments, and data from multiple experiments were combined on the basis of no significant variation among experiments as determined by χ2 analysis. Differences in TNF-α, IL-6, and NO levels and GIT rates were analyzed using Student's t-test. Contraction rate inhibition differences were analyzed using one- or two-way ANOVA. Data from ex vivo experiments were analyzed using ANOVA with Fisher's least significant difference post hoc test. Spearman rank correlation was calculated on the basis of a permutation test with 10,000 replications. P < 0.05 was considered significant.
RESULTS
LPS-ISS requires TLR4 signaling.
LPS signals through TLR4. We hypothesized that TLR4 signaling was critical to LPS-ISS and tested this idea in multiple strains of mice deficient in TLR4 signaling. TLR4 mutant, LPS-binding protein knockout (LBP−/−), and CD14−/− mice, all on BALB/c background, or BALB/c mice were treated with ultrapure LPS (6 mg/kg), and the incidence of ISS was determined. BALB/c mice developed ISS at a high rate, but all three TLR4-signaling-impaired mouse strains failed to develop ISS (Fig. 1A), demonstrating that TLR4 signaling is essential in LPS-ISS and suggesting that LPS-induced inflammation is required for LPS-ISS.
Fig. 1.
LPS-induced intussusception (LPS-ISS) requires Toll-like receptor 4 (TLR4) and phagocytes. ISS rate was assessed in BALB/c wild-type, TLR4 mutant, LBP−/−, and CD14−/− mice 6 h after injection of 6 mg/kg ultrapure LPS (A) and in BALB/c, BALB/c-SCID, and PBS-liposome- or clodronate-liposome-treated BALB/c mice (B). Similar results were obtained in individual (2–3) experiments, so data were combined to calculate percent ISS. ISS rates were compared between BALB/c and each mutant/knockout strain (A) or genotype or treatment group (B) by χ2 test: *P < 0.001. Numbers above bars represent total number of mice.
Phagocytes are essential for LPS-ISS.
Of TLR4-expressing cells, leukocytes are among the most significantly reactive to LPS (29). We tested the importance of B cells, T cells, and phagocytes, all capable of responding to LPS (20, 32, 41), in ISS by comparing LPS-ISS rates between control mice and B and T cell-deficient SCID mice or between PBS-liposome-treated and clodronate-liposome-treated (phagocyte-depleted) mice. There was no significant difference in LPS-ISS between SCID and BALB/c mice (Fig. 1B). LPS-ISS rates in PBS-liposome-treated mice were not significantly lower than those in BALB/c mice. In contrast, phagocyte-depleted mice showed a near-complete abrogation of ISS following LPS treatment (Fig. 1B). These data demonstrate that phagocytes are essential for the development of LPS-ISS.
Mouse strain determines LPS-ISS susceptibility.
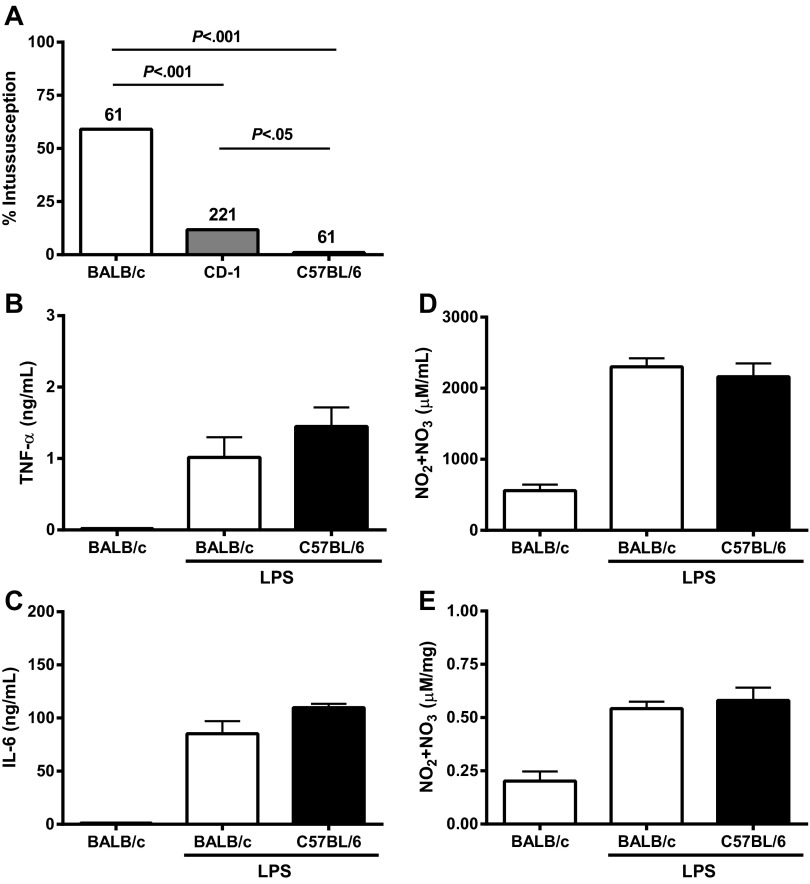
Mouse (21, 28, 35, 48) and various human (2, 18, 23, 30, 31, 38, 49) studies suggest that genetics impact ISS susceptibility. We tested this hypothesis by treating two inbred mouse strains (BALB/c and C57BL/6) and one outbred mouse strain (CD-1) with LPS and comparing ISS rates. BALB/c mice were highly susceptible and C57BL/6 mice were highly resistant to LPS-ISS (Fig. 2A). CD-1 mice were moderately susceptible to LPS-ISS. While the basis of these differences is unknown, the difference between inbred strains provided an important control to pursue identification of the mechanism of ISS.
Fig. 2.
Susceptibility to LPS-ISS differs among mouse strains and is not reflected by differences in levels of proinflammatory mediators. A: LPS-ISS rate in BALB/c, CD-1, and C57BL/6 mice 6 h after administration of 12 mg/kg LPS. LPS-ISS rate was compared among strains using χ2 test. B–E: TNF-α, IL-6, and nitric oxide (NO) levels in serum (D) and intestine (E) in BALB/c and C57BL/6 mice at 1 h (TNF-α) or 6 h (IL-6 and NO) after administration of 12 mg/kg LPS (n = 5–16 mice/group). Mediator levels were similar in water-treated C57BL/6 and BALB/c mice (data not shown). Mediator concentrations in LPS-treated BALB/c mice were compared with those in C57BL/6 mice by Student's t-test.
Inflammatory mediators TNF-α, IL-6, and NO levels are not significantly different in BALB/c and C57BL/6 mice.
Phagocytes produce a well-defined cascade of proinflammatory cytokines following LPS treatment (11). TNF-α, IL-6, and NO are implicated as possible mediators of LPS-ISS (1, 21, 35, 43). We tested whether differential production of TNF-α, IL-6, or NO following LPS treatment explained LPS-ISS susceptibility differences between mouse strains. No significant differences in serum levels of TNF-α, IL-6, and NO were seen between LPS-treated BALB/c and C57BL/6 mice (Fig. 2, B–D). Intestinal NO levels were also not significantly different between BALB/c and C57BL/6 mice (Fig. 2E). Taken together, these results demonstrate that differences in LPS-ISS susceptibility between mouse strains are not accounted for by differences in levels of LPS-induced TNF-α, IL-6, or NO, suggesting that LPS-ISS susceptibility in BALB/c and C57BL/6 mice is defined by another factor(s) or by a differential downstream response to these cytokines between strains. However, treatment of BALB/c mice with inducible NO synthase-specific (1400W, 5 mg/kg) or selective inhibitors (aminoguanidne, 50 mg/kg) immediately before or after LPS administration, respectively, significantly reduced mean serum NO levels compared with LPS treatment alone (1,072 and 1,836 vs. 4263 μmol/ml, respectively, P < 0.0001) without significantly altering LPS-ISS rates. These data strongly suggest that NO itself is not a key mediator of LPS-ISS.
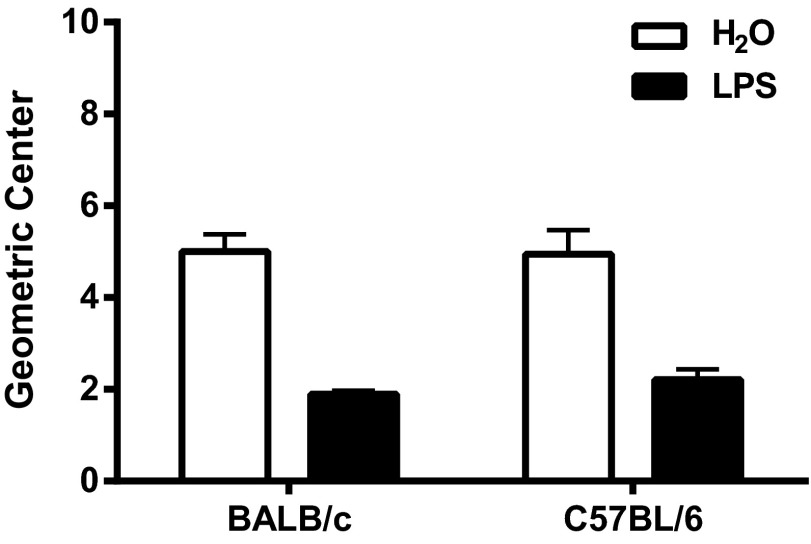
Changes in GIT do not correlate with LPS-ISS rates.
Lin et al. (21) showed that LPS-ISS correlated with reduced GIT. We tested whether GIT rate reflected the susceptibility differences to LPS-ISS in BALB/c and C57BL/6 mice. GIT was determined (25) 6 h after administration of water or LPS (12 mg/kg). GIT was similar in BALB/c and C57BL/6 mice following water treatment (P > 0.05; Fig. 3). Despite large LPS-ISS susceptibility differences, GIT was not significantly different between BALB/c and C57BL/6 mice after LPS treatment (Fig. 3) and was not a predictive correlate of LPS-ISS rate. However, GIT does not always reflect local alterations in intestinal motility (51).
Fig. 3.
Gastrointestinal transit (GIT) is similar in mice with different LPS-ISS susceptibility. GIT was assessed in water- and LPS-treated (12 mg/kg) mice 6 h post treatment (n = 5/treatment group). Student's t-test was used to compare GIT in BALB/c and C57BL/6 mice given the same treatment.
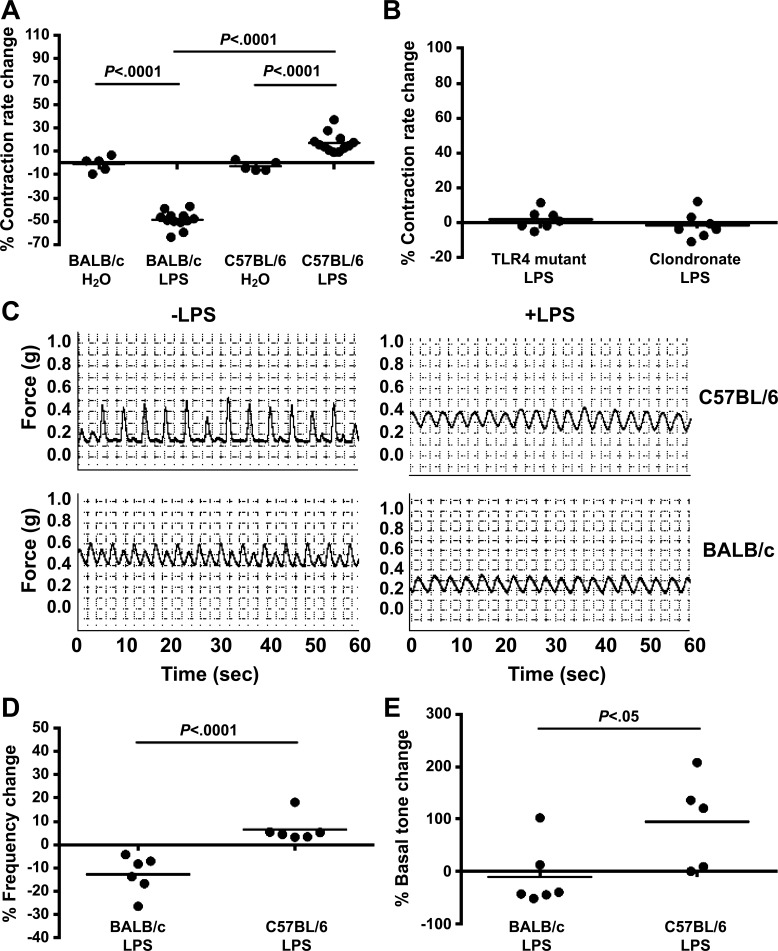
Intestinal contraction rate is inhibited by LPS in mice that are susceptible to ISS.
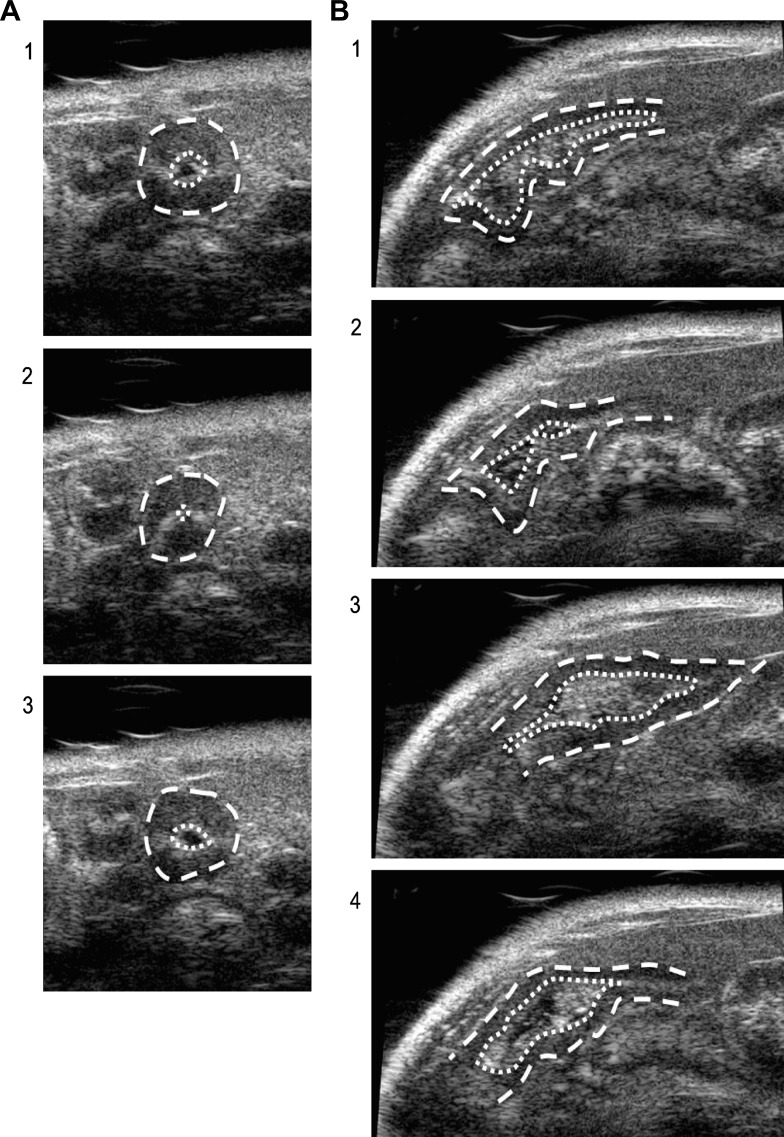
Ultrasound provided an alternative noninvasive in vivo measure of intestinal motility (9, 37) that allowed us to test whether local LPS-induced changes in intestinal contraction rate accounted for BALB/c and C57BL/6 LPS-ISS susceptibility differences. Intestinal contractions were counted at 6–13 random sites throughout the intestine in the axial or longitudinal orientation (Fig. 4). A mean contraction rate was determined for individual mice before and after administration of water or LPS (12 mg/kg), and mean percent contraction rate changes per group were compared. Average basal intestinal contraction rates (20–22 min−1) were not significantly different between the strains of mice (data not shown). However, LPS treatment had different effects on contraction rate in BALB/c and C57BL/6 mice. LPS treatment significantly inhibited contraction rate in LPS-ISS-susceptible BALB/c mice (Fig. 5A), while LPS treatment significantly enhanced contraction rate in LPS-ISS-resistant C57BL/6 mice (Fig. 5A).
Fig. 4.
Ultrasound assessment of intestinal contraction. Sequential frames of a 30-frame/s video loop are shown for an axial (A, 1–3) and a longitudinal (B, 1–4) cross section of the intestine seen by ultrasound. Outlines of outer intestinal wall (dashed) and intestinal lumen (dotted) are indicated. A single contraction is depicted in each orientation from relaxed (1), to contracted with narrowing of the lumen and change of size/shape of intestinal wall (2), to relaxed as intestine reverts to original position (3–4).
Fig. 5.
Change in intestinal contraction rate reflects LPS-ISS susceptibility. A: intestinal contraction rate was determined by ultrasound before and after administration of water or LPS, and percent change was calculated, in BALB/c and C57BL/6 mice. B: intestinal contraction rate was determined by ultrasound before and after LPS administration, and percent change was calculated, in TLR4 mutant and phagocyte-depleted (clondronate-treated) BALB/c mice. C: representative traces show ileal contractile activity, measured ex vivo in an organ bath, in C57BL/6 and BALB/c mice treated with water (−LPS) or LPS (+LPS). D and E: percent change in contractile frequency and basal tone calculated as the difference between the LPS-treated and average control over the average control [100 ∗ (frequency of individual − control average)/control average]. Intestinal contractile activity ex vivo in organ baths on intact ileal tissue was measured in water- and LPS-treated BALB/c and C57BL/6 mice in 2 separate ileal sections and averaged. Each ○ represents 1 mouse (n = 4–6/group); bars represent mean for group. Differences were compared between water- and LPS-treated mice of the same strain by ANOVA (A and B) and between LPS-treated groups of different strains by ANOVA with Fisher's least significant difference post hoc test (D and E).
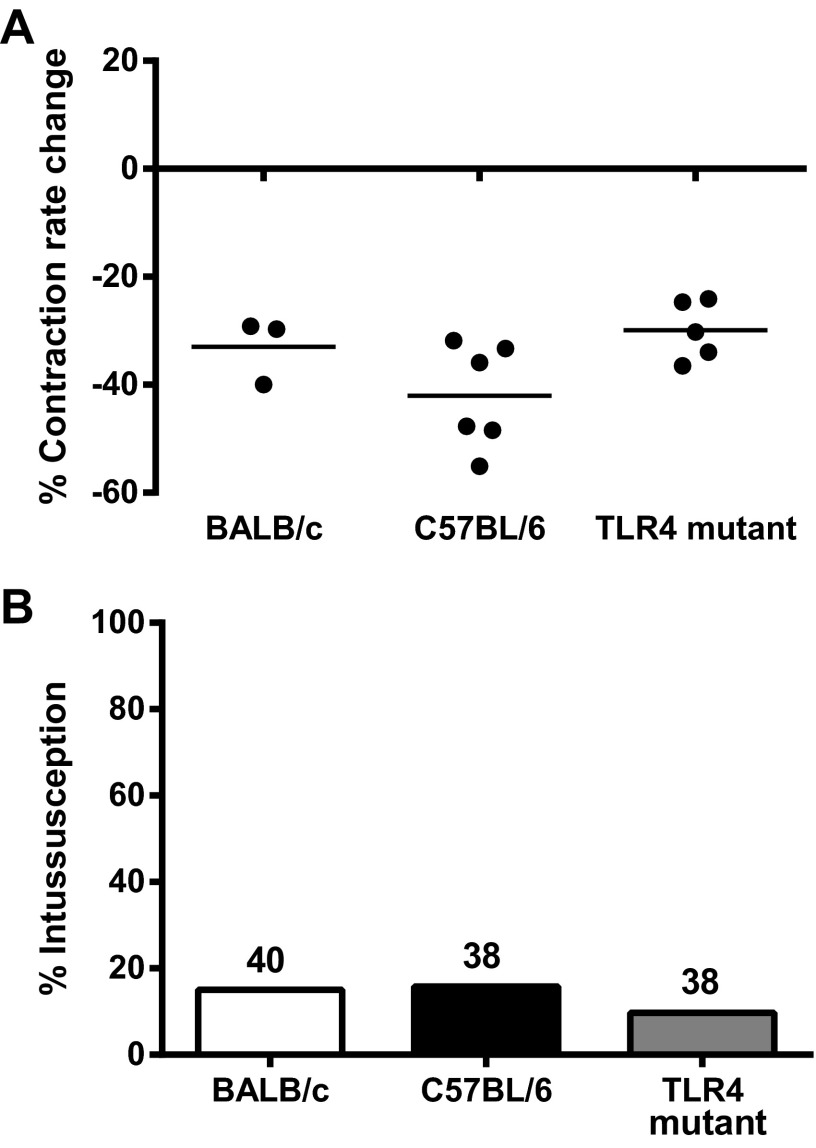
A lack of contraction rate reduction in C57BL/6 mice suggested that other mice resistant to LPS-ISS would also be resistant to LPS-induced reduction of contraction rate. To test this, we examined the effect of LPS on intestinal contraction rate in LPS-ISS-resistant TLR4 mutant and phagocyte-depleted BALB/c mice. LPS treatment did not significantly alter intestinal contraction rate in TLR4 mutant or phagocyte-depleted BALB/c mice (Fig. 5B).
To validate our ultrasound results, we determined whether ex vivo spontaneous contractile activity (integral, frequency, contraction minimum, and amplitude) was altered in the ileum of water- and LPS-treated BALB/c and C57BL/6 mice (Fig. 5C). Percent contractile frequency and basal tone change were significantly decreased in BALB/c and increased in C57BL/6 ileum (Fig. 5, D and E). These data corroborate our ultrasound findings and indicate that LPS differentially alters small intestinal spontaneous contractile frequency and basal tone between BALB/c and C57BL/6 mice. Together, the in vivo and ex vivo data strongly support our hypothesis that reduced intestinal contraction rate is required for ISS susceptibility.
MnCl2 induces similar changes in intestinal contraction rate and ISS rates in BALB/c and C57BL/6 mice.
We further reasoned that chemical treatments that reduced intestinal contraction rate would cause ISS, thereby demonstrating that reduced intestinal contraction rate is a key component of the mechanism of ISS. MnCl2 is a Ca2+ analog that inhibits Ca2+ uptake, thereby reducing intestinal smooth muscle contraction in vivo (15). To test our hypothesis, BALB/c, C57BL/6, and TLR4 mutant mice were treated with MnCl2 (0.4 mmol/kg ip) and examined 2 h later by ultrasound to determine intestinal contraction rate or following laparotomy to determine ISS rate. As expected, MnCl2 treatment significantly reduced intestinal contraction rate to similar levels in all three strains of mice (Fig. 6A). Consistent with our prediction, MnCl2 also induced equivalent rates of ISS in all three strains of mice (Fig. 6B). These data suggest that MnCl2 induced ISS by altering motility, similar to LPS, but bypassing the factor(s) mediating susceptibility differences in LPS-ISS and strongly support a mechanism of ISS that requires a reduction of intestinal contraction rate.
Fig. 6.
MnCl2 inhibits intestinal contraction rate and induces ISS irrespective of LPS-ISS susceptibility. A: inhibitory effect of MnCl2 on intestinal contraction rate was determined by comparing contraction rate before and after administration of 0.4 mmol/kg MnCl2 to BALB/c, C57BL/6, and TLR4 mutant mice (n = 3–6 mice/group). B: ISS rate in BALB/c, C57BL/6, and TLR4 mutant mice following administration of 0.4 mmol/kg MnCl2. Numbers above each bar represent total number of mice.
Contraction rate changes negatively correlate with intussusception rate.
If changes in contraction rate predict susceptibility to ISS, then these parameters should be highly correlative. When examined across all strains and treatment groups (Figs. 5 and 6), contraction rate and ISS rate negatively correlated (r = −0.95, P < 0.001), providing further experimental evidence to support altered intestinal contraction as key in ISS.
DISCUSSION
The complexity of ISS induction is underscored by lack of clearly identified key mediators of disease. We found that TLR4 signaling and phagocytes are required in LPS-ISS, demonstrating a role for innate immunity. However, TNF-α, IL-6, or NO responses to LPS were similar between mouse strains and did not reflect LPS-ISS susceptibility differences between BALB/c and C57BL/6 mice. Similarly, motility alterations measured by GIT did not predict LPS-ISS susceptibility. In contrast, a reduction of intestinal contraction rate measured by ultrasound and confirmed in vitro predicted differences in LPS-ISS susceptibility between BALB/c and C57BL/6 mice, leading us to hypothesize that reduction of intestinal contraction rate is part of the mechanism of ISS. Consistent with this hypothesis, LPS-ISS-refractory TLR4 mutant and phagocyte-depleted BALB/c mice showed no reduction of contraction rate in response to LPS. To further test this hypothesis, mice were treated with MnCl2, a Ca2+ analog that inhibits intestinal smooth muscle contractions. MnCl2 treatment reduced intestinal contraction rate similarly in BALB/c, C57BL/6, and TLR4 mutant mice, and all three strains developed MnCl2-induced ISS at equal rates. Together, our data indicate that LPS-ISS is triggered by inflammatory signaling through TLR4 and requires phagocytes. Furthermore, alteration of intestinal motility, specifically, reduced intestinal contraction rate, induced by LPS innate signaling or by MnCl2-induced modification of contraction, is a key factor in the development of ISS.
The requirement for phagocytes and TLR4 in LPS-ISS indicates that innate immunity and inflammatory signaling are important mediators of LPS-ISS. On the basis of previous studies, this inflammatory signaling is not, however, accompanied by a discernable influx of leukocytes into or surrounding the site of ISS. We and others previously observed that LPS-ISS does not display discernable gross or histological evidence of acute inflammation at 6 h (21, 48), which is when ISS rates were assessed in the current study. These data, together with our finding that only depletion of phagocytic cells reduced ISS rates, suggest that a large influx of immunological cells is not required to initiate ISS but, instead, is dependent on inflammatory signaling by resident TLR4+ and phagocytic cells. Whether there is an increase in other inflammatory markers such as myeloperoxidase awaits further testing. Although LPS-ISS is a TLR4/phagocyte-dependent inflammatory process, TLR4 and phagocytes are unlikely to mediate all ISS. Certainly, TLR4 was not required for MnCl2-induced ISS. Gram-negative bacteria associated with ISS (8, 12, 14) may signal via LPS through TLR4 to wholly or partially induce ISS. However, gram-positive bacteria, viruses, and parasites that lack TLR4 ligands are also associated with ISS (27, 36, 42), suggesting that non-TLR4-signaling pathways also induce ISS. We propose that changes in intestinal smooth muscle contraction required for ISS are an indirect downstream effect of infection-induced inflammation. This idea is supported by the 1- or 6-day lag in ISS development in mice infected with Salmonella or Yersinia, respectively (13, 46). Pathogen-specific virulence factors likely differentially affect the kinetics of key inflammatory mediator(s) involved in ISS that must reach a threshold before ISS formation.
TNF-α, IL-6, and NO, part of the complex inflammatory cascade induced by LPS, have been previously proposed as possible mediators of LPS-ISS (1, 28, 35, 43). In our studies, serum TNF-α, IL-6, and NO and intestinal NO levels were not different between LPS-ISS-susceptible BALB/c and LPS-ISS-resistant C57BL/6 mice. These data suggest that differential susceptibility is determined by 1) another mediator altogether, 2) differential downstream responses to TNF-α, IL-6, or NO between these strains, or 3) differences in intestinal levels of TNF-α and IL-6 (not examined in our studies). We could not use a knockout approach to test whether these molecules were required, because knockouts were not available on BALB/c background. However, NO is unlikely to mediate LPS-ISS, because reduction of NO levels with inducible NO synthase-specific inhibitors did not reduce LPS-ISS rates. Identification of key inflammatory mediators in LPS-ISS awaits further studies.
Mouse strain profoundly impacted LPS-ISS susceptibility. Discrepancies in immunological, neuronal, and/or hormonal signaling may exist between BALB/c and C57BL/6 mice that trigger reduced contraction rate in one strain and increased contraction rate in the other in response to LPS. The identity of specific genetic differences and the key signaling event responsible for LPS-ISS susceptibility determinants remain to be identified. However, inheritance is unlikely due to a single dominant/recessively inherited gene, because BALB/c × C57BL/6 F1 mice developed LPS-ISS at intermediate rates compared with parental strains (data not shown). Heritability of idiopathic ISS in children is not considered typical, although reports of familial (18, 30, 31, 38) and population (2, 23, 49) predispositions to ISS support a role for genetics in ISS. Together, these data are highly suggestive of a genetic predisposition to ISS.
Altered intestinal motility is thought to underlie ISS (3), although definitive evidence is lacking. One reason is that assessment of intestinal motility in vivo is challenging without use of invasive procedures that may, themselves, impact intestinal motility (51). To avoid potential confounding factors of invasive procedures, we utilized two noninvasive modalities to measure motility, GIT (25, 48) and ultrasound (9, 37). Measurement of GIT was uninformative; it did not reflect LPS-ISS susceptibility. In contrast, ultrasound demonstrated that reduced contraction rate was induced by LPS only in LPS-ISS-susceptible mouse strains and by MnCl2, irrespective of mouse strain, and that this reduction was predictive of ISS susceptibility. Bowel wall movement measured by ultrasound may not always reflect a smooth muscle contraction. However, use of in vitro organ baths to detect differential responses of spontaneous contractile activity in whole-mount small intestine from LPS-treated BALB/c and C57BL/6 mice corroborated the ultrasound results, supporting the value of ultrasound in identifying a component of altered intestinal motility. To our knowledge, this is the first report of differential responses of intestinal contraction rate to LPS between mouse strains in vivo or in vitro. Together, our in vivo and in vitro data provide strong evidence for reduction of intestinal contraction rate in the etiology of ISS.
Our data with LPS suggest that reduced intestinal contraction rate results in ISS. We confirmed that an unrelated drug, MnCl2, that reduced intestinal contraction rate induced ISS in BALB/c, C57BL/6, and TLR4 mutant mice (Fig. 6), irrespective of their susceptibility to LPS-ISS. However, MnCl2 antagonizes multiple types of Ca2+ channels and affects cell types other than smooth muscle cells, thereby raising the possibility that the increased ISS rate in MnCl2-treated mice was due to effects other than the reduction of intestinal contraction rate (Fig. 6A). We think this unlikely, as there was a strong negative correlation between intestinal contraction rate and ISS when all treatment groups were combined. However, we further verified our conclusion by testing whether nifedipine would also increase ISS rate in C57BL/6 mice that were not susceptible to LPS-ISS. Nifedipine, an L-type Ca2+ channel antagonist, more specifically targets smooth muscle cells than does MnCl2 and is known to reduce intestinal contraction rate (6). As our data with LPS and MnCl2 predicted, C57BL/6 mice treated with nifedipine developed ISS (5%, 4 of 72 mice), whereas mice treated with vehicle (DMSO) did not (0%, 0 of 49 mice). Together, LPS, MnCl2, and nifedipine data indicate that altered contraction rate is key to ISS.
Rodents generate three types of contractions, rhythmic phasic contractions, giant migrating contractions, and tonic contractions (33). It was not possible by ultrasound to determine which type of contraction pattern is altered in LPS-ISS. Giant migrating contractions are strong contractions that occlude the lumen and propagate distally in small intestine and colon with concomitant reduction of rhythmic phasic contractions and tone in the distal segment and, thus, are intriguing candidates as initiators of ISS. Consistent with this possibility, LPS caused a decrease in percent basal tone in BALB/c, but not C57BL/6, mouse intestine in vitro. However, LPS-ISS occurs in both directions, sometimes within the same mouse (data not shown) (21, 48), not just distally, suggesting that retrograde migrating contractions also may be involved. Identification of the contraction pattern(s) disrupted in ISS will require use of noninvasive imaging modalities that allow pattern identification or use of invasive in vivo methodologies, such as myoelectrical or manometric monitoring (10, 19) after validation, to ensure that the invasive monitoring does not disrupt LPS-ISS development.
In summary, ISS is a complex process. LPS-ISS is dependent on TLR4 signaling and phagocyte functions and has an intestinal motility component. The interaction of these components results in ISS only in mice with all the susceptibility factors, including genetics, among those yet unidentified. However, we demonstrate here that intestinal motility is a critical mediator of ISS, regardless of the inducing agent, and that reduced intestinal contraction rate is a key factor in the intestinal motility component of ISS. Our data support the hypothesis that multiple pathways converge to effect intestinal contraction and cause ISS. The complex cascade of inflammatory mediators induced by LPS and the effects of MnCl2 may act indirectly or directly to alter intestinal contractions in such a way that invagination and ISS occur.
GRANTS
This work was supported by National Institutes of Health Grants R01 AI-24998 (M. E. Conner), R21 AI-064468 (M. E. Conner), AI-023859 (S. M. Goyert), R01 AG-029977 (R. G. Pautler), K01 DK-070758 (K. S. Uray), P30 DK-079638 (R. G. Pautler), and P30 DK-56338, which funds the Texas Medical Center Digestive Diseases Center, and a Merit Review Grant from the Office of Research and Development, Medical Research Service, Department of Veterans Affairs (M. E. Conner).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.E.K., K.S.U., N.W.W., R.G.P., and M.E.C. are responsible for conception and design of the research; K.E.K., A.D.M., and K.S.U. performed the experiments; K.E.K., A.D.M., K.S.U., and M.E.C. analyzed the data; K.E.K., A.D.M., K.S.U., and M.E.C. interpreted the results of the experiments; K.E.K. and K.S.U. prepared the figures; K.E.K. drafted the manuscript; K.E.K., A.D.M., K.S.U., N.W.W., R.G.P., S.M.G., and M.E.C. edited and revised the manuscript; K.E.K., A.D.M., K.S.U., N.W.W., R.G.P., S.M.G., N.v.R., and M.E.C. approved the final version of the manuscript.
ACKNOWLEDGMENTS
The authors thank the Mouse Phenotyping Core at the Baylor College of Medicine for assistance with ultrasound studies, Cara Olsen (Uniformed Services University of the Health Sciences) for assistance with statistical analysis, and Drs. Sarah Blutt, Mary K. Estes, Robert Schulman, Douglas Burrin, and Mark Rhodes for critical reading of the manuscript.
Present address of K. E. Killoran: Department of Microbiology and Immunology, Uniformed Services University of the Health Sciences, Bethesda, MD 20814.
REFERENCES
- 1.Badriyyah M, Mazeh H, Brocke S, Osmanova V, Freund HR, Hanani M. Prevention of lipopolysaccharide-induced intussusception in mice by the COX2 inhibitor rofecoxib. Pediatr Surg Int 24: 333–336, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Bines JE, Ivanoff B. Acute intussusception in infants and children. Incidence, clinical presentation and management: a global perspective. In: Vaccines and Biologicals. Geneva, Switzerland: World Health Organization, 2002 [Google Scholar]
- 3.Brandt ML. Intussusception. In: Oski's Pediatrics: Principles and Practice, edited by McMillan DJ, Deangelis CS, Feigin RD, Warshaw JB. Philadelphia, PA: Lippincott/Williams & Wilkins, 1999, p. 1652–1655 [Google Scholar]
- 4.Chan KL, Chan JK, Peh WC, Chan KW, Tam PK. Endotoxemia associated with intussusception and its diagnostic and surgical interventions. Pediatr Surg Int 18: 685–688, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Cohen J. The immunopathogenesis of sepsis. Nature 420: 885–891, 2002 [DOI] [PubMed] [Google Scholar]
- 6.De Ponti F, D'Angelo L, Frigo GM, Crema A. Inhibitory effects of calcium channel blockers on intestinal motility in the dog. Eur J Pharmacol 168: 133–144, 1989 [DOI] [PubMed] [Google Scholar]
- 7.Emoto M, Emoto Y, Brinkmann V, Miyamoto M, Yoshizawa I, Staber M, van Rooijen N, Hamann A, Kaufmann SH. Increased resistance of LFA-1-deficient mice to lipopolysaccharide-induced shock/liver injury in the presence of TNF-α and IL-12 is mediated by IL-10: a novel role for LFA-1 in the regulation of the proinflammatory and anti-inflammatory cytokine balance. J Immunol 171: 584–593, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Georgoula C, Ward HC. Recurrent intussusception associated with Escherichia coli 0157 infection. Dig Liver Dis 36: 557, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Gimondo P, Mirk P. A new method for evaluating small intestinal motility using duplex Doppler sonography. Am J Roentgenol 168: 187–192, 1997 [DOI] [PubMed] [Google Scholar]
- 10.Gourcerol G, Wang L, Adelson DW, Larauche M, Tache Y, Million M. Cholinergic giant migrating contractions in conscious mouse colon assessed by using a novel noninvasive solid-state manometry method: modulation by stressors. Am J Physiol Gastrointest Liver Physiol 296: G992–G1002, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guha M, Mackman N. LPS induction of gene expression in human monocytes. Cell Signal 13: 85–94, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Hamlyn AN, James OF, Douglas AP, Lavelle MI, Venables CW. Caroli's disease with intrahepatic gall-stones and Salmonella infection. Postgrad Med J 52: 656–659, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Handley SA, Dube PH, Miller VL. Histamine signaling through the H2 receptor in the Peyer's patch is important for controlling Yersinia enterocolitica infection. Proc Natl Acad Sci USA 103: 9268–9273, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hansen MG, Pearl G, Levy M. Intussusception due to Yersinia enterocolitica enterocolitis in a patient with β-thalassemia. Arch Pathol Lab Med 125: 1486–1488, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Itoh T, Kuriyama H, Nanjo T. Effects of calcium and manganese ions on mechanical properties of intact and skinned muscles from the guinea-pig stomach. J Physiol 333: 555–576, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jakab F, Peterfai J, Verebely T, Meleg E, Banyai K, Mitchell DK, Szucs G. Human astrovirus infection associated with childhood intussusception. Pediatr Int 49: 103–105, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Koo JW, Cho CR, Cha SJ, Chung CY. Intussusception associated with Yersinia pseudotuberculosis infection. Acta Paediatr 85: 1253–1255, 1996 [DOI] [PubMed] [Google Scholar]
- 18.Kurzbart E, Cohen Z, Yerushalmi B, Yulevich A, Newman-Heiman N, Mares AJ. Familial idiopathic intussusception: a report of two families. J Pediatr Surg 34: 493–494, 1999 [DOI] [PubMed] [Google Scholar]
- 19.Li M, Johnson CP, Adams MB, Sarna SK. Cholinergic and nitrergic regulation of in vivo giant migrating contractions in rat colon. Am J Physiol Gastrointest Liver Physiol 283: G544–G552, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Liew FY, Komai-Koma M, Xu D. A toll for T cell costimulation. Ann Rheum Dis 63 Suppl 2: ii76–ii78, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin Z, Cohen P, Nissan A, Allweis TM, Freund HR, Hanani M. Bacterial wall lipopolysaccharide as a cause of intussusception in mice. J Pediatr Gastroenterol Nutr 27: 301–305, 1998 [DOI] [PubMed] [Google Scholar]
- 22.Matsushita M, Suzaki T, Hajiro K. Intussusception associated with Salmonella typhimurium enterocolitis. Am J Gastroenterol 89: 1246–1248, 1994 [PubMed] [Google Scholar]
- 23.Mayell MJ. Intussusception in infancy and childhood in Southern Africa. A review of 223 cases. Arch Dis Child 47: 20–25, 1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Metkar S, Awasthi S, Denamur E, Kim KS, Gangloff SC, Teichberg S, Haziot A, Silver J, Goyert SM. Role of CD14 in responses to clinical isolates of Escherichia coli: effects of K1 capsule expression. Infect Immun 75: 5415–5424, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller MS, Galligan JJ, Burks TF. Accurate measurement of intestinal transit in the rat. J Pharmacol Methods 6: 211–217, 1981 [DOI] [PubMed] [Google Scholar]
- 26.Montgomery EA, Popek EJ. Intussusception, adenovirus, and children: a brief reaffirmation. Hum Pathol 25: 169–174, 1994 [DOI] [PubMed] [Google Scholar]
- 27.Murphy TV, Gargiullo PM, Massoudi MS, Nelson DB, Jumaan AO, Okoro CA, Zanardi LR, Setia S, Fair E, LeBaron CW, Wharton M, Livengood JR. Intussusception among infants given an oral rotavirus vaccine. N Engl J Med 344: 564–572, 2001 [DOI] [PubMed] [Google Scholar]
- 28.Nissan A, Zhang JM, Lin Z, Haskel Y, Freund HR, Hanani M. The contribution of inflammatory mediators and nitric oxide to lipopolysaccharide-induced intussusception in mice. J Surg Res 69: 205–207, 1997 [DOI] [PubMed] [Google Scholar]
- 29.O'Mahony DS, Pham U, Iyer R, Hawn TR, Liles WC. Differential constitutive and cytokine-modulated expression of human Toll-like receptors in primary neutrophils, monocytes, and macrophages. Int J Med Sci 5: 1–8, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oshio T, Ogata H, Takano S, Ishibashi H. Familial intussusception. J Pediatr Surg 42: 1509–1514, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Papadopoulou F, Efremidis SC. Familial intussusception. J Pediatr Surg 37: 1549–1551, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Peng SL. Signaling in B cells via Toll-like receptors. Curr Opin Immunol 17: 230–236, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Sarna SK. Molecular, functional, and pharmacological targets for the development of gut promotility drugs. Am J Physiol Gastrointest Liver Physiol 291: G545–G555, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Sasmono RT, David A. The biology of macrophages. In: The Innate Immune Response to Infection, edited by Kaufmann SH, Medzhitov R, Gordon S. Washington, DC: Am. Soc. Microbiol., 2004, p. 71–94 [Google Scholar]
- 35.Sonmez K, Karabulut R, Turkyilmaz Z, Demirogullari B, Ozen IO, Gulen S, Basaklar AC, Kale N. Association of tumor necrosis factor, interleukin-6 and cyclooxygenase pathway with lipopolysaccharide-induced intussusception. Eur J Pediatr Surg 18: 103–106, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Staatz G, Alzen G, Heimann G. [Intestinal infection, the most frequent cause of invagination in childhood: results of a 10-year clinical study]. Klin Padiatr 210: 61–64, 1998 [DOI] [PubMed] [Google Scholar]
- 37.Stidham RW, Xu J, Johnson LA, Kim K, Moons DS, McKenna BJ, Rubin JM, Higgins PD. Ultrasound elasticity imaging for detecting intestinal fibrosis and inflammation in rats and humans with Crohn's disease. Gastroenterology 141: 819–826, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stringer MD, Holmes SJ. Familial intussusception. J Pediatr Surg 27: 1436–1437, 1992 [DOI] [PubMed] [Google Scholar]
- 39.Stringer MD, Pledger G, Drake DP. Childhood deaths from intussusception in England and Wales, 1984–9. BMJ 304: 737–739, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stringer MD, Willetts IE. John Hunter, Frederick Treves and intussusception. Ann R Coll Surg Engl 82: 18–23, 2000 [PMC free article] [PubMed] [Google Scholar]
- 41.Sweet MJ, Hume DA. Endotoxin signal transduction in macrophages. J Leukoc Biol 60: 8–26, 1996 [DOI] [PubMed] [Google Scholar]
- 42.To KK, Cheng VC, Hung IF, Tang BS, Wong SS, Yuen KY. Caecal-caecal intussusception caused by Trichuris trichiura in a young healthy adult. Scand J Infect Dis 38: 813–815, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Turkyilmaz Z, Karabulut R, Gulen S, Demirogullari B, Ozen IO, Sonmez K, Basaklar AC, Kale N. Role of nitric oxide and cyclooxygenase pathway in lipopolysaccharide-induced intussusception. Pediatr Surg Int 20: 598–601, 2004 [DOI] [PubMed] [Google Scholar]
- 44.Uray KS, Laine GA, Xue H, Allen SJ, Cox CS., Jr Intestinal edema decreases intestinal contractile activity via decreased myosin light chain phosphorylation. Crit Care Med 34: 2630–2637, 2006 [DOI] [PubMed] [Google Scholar]
- 45.Van Rooijen N, Sanders A. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J Immunol Methods 174: 83–93, 1994 [DOI] [PubMed] [Google Scholar]
- 46.Vijay-Kumar M, Wu H, Jones R, Grant G, Babbin B, King TP, Kelly D, Gewirtz AT, Neish AS. Flagellin suppresses epithelial apoptosis and limits disease during enteric infection. Am J Pathol 169: 1686–1700, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang P, Liu B, Ou H, Tong L, Yang J, Tang C. Nitric oxide synthase/nitric oxide pathway mediates intussusception pathogenesis in rats. Chin Med J (Engl) 112: 1016–1019, 1999 [PubMed] [Google Scholar]
- 48.Warfield KL, Blutt SE, Crawford SE, Kang G, Conner ME. Rotavirus infection enhances lipopolysaccharide-induced intussusception in a mouse model. J Virol 80: 12377–12386, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Webby RJ, Bines JE, Barnes GL, Tindall H, Krause V, Patel M. Intussusception in the Northern Territory: the incidence is low in Aboriginal and Torres Strait Islander children. J Paediatr Child Health 42: 235–239, 2006 [DOI] [PubMed] [Google Scholar]
- 50.Wehner S, Behrendt FF, Lyutenski BN, Lysson M, Bauer AJ, Hirner A, Kalff JC. Inhibition of macrophage function prevents intestinal inflammation and postoperative ileus in rodents. Gut 56: 176–185, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weisbrodt NW, Bass P. In vivo motility techniques. In: Handbook of Methods in Gastrointestinal Pharmacology, edited by Gaginella TS. Boca Raton, FL: CRC, 1996, p. 163–187 [Google Scholar]
- 52.Willetts IE, Kite P, Barclay GR, Banks RE, Rumley A, Allgar V, Stringer MD. Endotoxin, cytokines and lipid peroxides in children with intussusception. Br J Surg 88: 878–883, 2001 [DOI] [PubMed] [Google Scholar]
- 53.Winesett MP, Pietsch JB, Barnard JA. Yersinia enterocolitica in a child with intussusception. J Pediatr Gastroenterol Nutr 23: 77–80, 1996 [DOI] [PubMed] [Google Scholar]