Abstract
The external anal sphincter (EAS) may be injured in 25–35% of women during the first and subsequent vaginal childbirths and is likely the most common cause of anal incontinence. Since its first description almost 300 years ago, the EAS was believed to be a circular or a “donut-shaped” structure. Using three-dimensional transperineal ultrasound imaging, MRI, diffusion tensor imaging, and muscle fiber tracking, we delineated various components of the EAS and their muscle fiber directions. These novel imaging techniques suggest “purse-string” morphology, with “EAS muscles” crossing contralaterally in the perineal body to the contralateral transverse perineal (TP) and bulbospongiosus (BS) muscles, thus attaching the EAS to the pubic rami. Spin-tag MRI demonstrated purse-string action of the EAS muscle. Electromyography of TP/BS and EAS muscles revealed their simultaneous contraction and relaxation. Lidocaine injection into the TP/BS muscle significantly reduced anal canal pressure. These studies support purse-string morphology of the EAS to constrict/close the anal canal opening. Our findings have implications for the effect of episiotomy on anal closure function and the currently used surgical technique (overlapping sphincteroplasty) for EAS reconstructive surgery to treat anal incontinence.
Keywords: external anal sphincter muscle architecture, anal incontinence, childbirth-related injury, magnetic resonance diffusion tensor imaging
an understanding of the morphology of the external anal sphincter (EAS), one of the superficial muscles of the pelvic floor, is important, since damage to this muscle may occur in 25–35% of women during vaginal childbirth (34). Injury to the EAS muscle is an important cause of anal incontinence, which has a devastating effect on an individual's quality of life (8). Morphology of the EAS has intrigued many investigators. From the original description by Santorini at the turn of 18th century (27) to the most current texts (2, 19), the EAS is described as a three-component (subcutaneous, superficial, and deep) circular muscle structure (2, 19). Others have argued that the deep part of the EAS is actually the puborectalis muscle (PRM) (7, 14, 23, 25). MRI studies of Hussain et al. (14) and our recent three-dimensional (3-D) ultrasound (US) images, combined with high-definition manometry maps (25), support the idea that the deep part of the EAS is indeed the PRM, because it has a “sling” shape. On the basis of 3-D US images and anal canal pressures obtained by high-definition manometry (HDM), our study shows that the increase in pressure with voluntary contraction of the proximal and distal halves of the anal canal is related to contraction of the PRM and EAS, respectively (25).
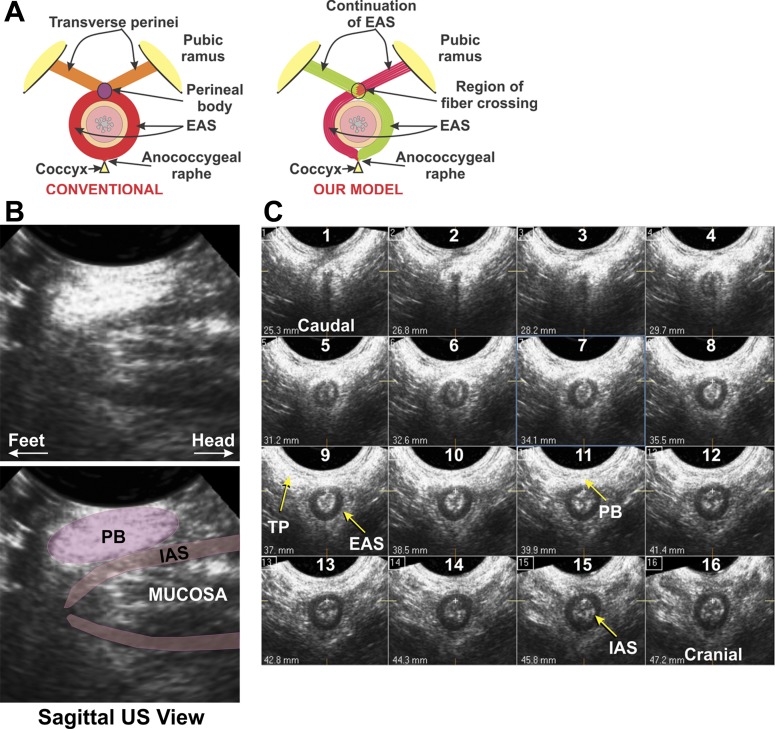
Another aspect of EAS morphology that has never been questioned relates to its ventral attachment. Generally, all skeletal muscles originate from a bone and are inserted into a bone via an aponeurosis or a tendon. Interestingly, that is not the case with the EAS muscle; it has been suggested that the anterior/ventral ends of EAS muscle fibers are inserted into the midline fibroaponeurotic structure of the perineal body (PB) (35). Separate sets of muscles, transverse perineal (TP) and bulbospongiosus [BS, also called bulbocavernosus (17)] that originate from the pubic rami are also inserted into the PB (35) (see supplemental material for this article available online at the Journal website). Crossover of EAS muscle fibers in the midline of the PB, from one side to the other, which has been suggested by several investigators (21, 22, 35), raises the possibility that perhaps the muscle fibers of the EAS cross in the midline structure of the PB to continue as contralateral TP/BS muscles. At the dorsal end, the EAS is attached to the coccyx via the anococcygeal raphe. We tested the hypothesis that the EAS is not a “donut-shaped” muscle but, rather, has “purse-string” morphology (Fig. 1A). We used a combination of “state-of-the-art” imaging techniques, such as 3-D US, proton density (PD) MRI, diffusion tensor (DT) MRI (9), and DT imaging-based muscle fiber tracking (9, 31), to clarify the structural aspects of the EAS muscle complex. Furthermore, we used velocity-encoded dynamic spin-tag (ST) MRI (20), electromyography (EMG), and HDM to investigate the functional aspects of EAS. Thus we correlated the precise morphology with EAS function (comprising EAS, PB, and TP/BS muscles).
Fig. 1.
Schematic of our hypothesis (A) and sagittal and transverse ultrasound (US) images of the anal canal (B and C). A: schematic of conventional external anal sphincter (EAS) muscle architecture compared with our proposed model. B: sagittal US images of the anal canal. Note elliptical shape of the perineal body (PB), which has a well-defined structure. C: 16 serial axial images of the anal canal, spaced 15 mm apart. Note circular configuration of the internal anal sphincter (IAS), while the EAS merges with the transverse perineal (TP)/bulbospongiosus (BS) muscle at the ventral end. Cranial-caudal extent of the EAS and TP/BS is similar, and the EAS and TP/BS are continuous at the ventral end in the PB.
MATERIALS AND METHODS
The University of California San Diego Institutional Human Research Review Board approved the study protocol, and each subject signed an informed consent prior to participation in the study. Thirty-six nulliparous, asymptomatic adult women (mean age 35 yr) were included in the study.
3-D US Imaging
For 3-D US imaging, a 3- to 9-MHz transducer (model HD11, Philips Medical Systems, Bothell, WA) was placed on the perineum of subjects in the dorsal lithotomy position. This imaging method captures volume US data, the details of which are described elsewhere (36, 37). The US transducer was directed dorsally to obtain volume data in the axial orientation of the anal canal. Data were stored on a CD-ROM and reviewed later with the QLAB 5.0 3-D view program (Philips Medical Systems). US images along the entire length of the anal canal were displayed at 1.0- to 1.5-mm intervals (Fig. 1B) to visualize various anatomic structures.
MRI
MRI was performed on a 3-T system scanner (GE Medical Systems, Milwaukee, WI) using an eight-channel torso phased-array coil. The final protocols for the high-resolution and DT imaging were used only after extensive optimization of the sequence parameters. All images were read by an imaging physicist (S.S.) with >25 yr of experience and then by a gastroenterologist (R.K.M.) with 30 yr of experience.
High-Resolution MRI
A stack of 28–34 axial slices covering a region from the most distal (inferior) aspect of the anal canal to just proximal to the coccyx were acquired. Slice thickness, spacing, and field of view were optimized for image resolution, both in-plane and along the cranial-caudal direction, as well as for total coverage and signal-to-noise ratio (SNR) within comparable scan times. The type of pulse sequence, namely, T1, T2, and proton-weighted, with or without fat suppression, was examined for best contrast between adjoining tissues. The PD image yielded the best delineation of anatomic structures. PD images were acquired using fast-spin-echo, two-dimensional, fat-saturated PD images, with 11.5-ms echo time, 5,500-ms repetition time, 256 × 192 image matrix, 15-cm field of view, 2.5-mm slice thickness, and 0.4-mm spacing.
DT Imaging
DT imaging was first optimized with respect to several parameters. Parallel imaging, a relatively new option, allows utilization of signals from several orthogonal detection coils, fewer phase-encoding levels, and, hence, proportionately reduced scan time, with equal spatial resolution. This is especially advantageous for DT imaging, since this concomitantly improves image quality and reduces the “point-spread function” and also reduces SNR. We used several parallel-imaging factors and image matrices to optimize in-plane resolution and decided on a factor of 1.6 and a matrix size of 128 × 128. Slice thickness, spacing, and field of view were chosen on a basis similar to that used to choose the same parameters for the previous morphological imaging sequence. The “b value,” a measure of the degree of sensitization to diffusion motion, is an important parameter to consider in DT imaging; higher b values represent greater sensitization. The SNR of particularly DT imaging is much lower in muscle tissue than brain because of much lower spin-spin relaxation times (T2) in muscle than brain and, hence, is much more difficult to acquire. To compensate for this lower SNR, we resorted to much lower b values, which reduces the sensitivity to diffusion motion but yields much higher SNR. On the basis of our extensive experience (30, 31), a b value of 400 s/mm2 is the most optimal in various muscles. The number of diffusion-gradient directions was also optimized with respect to the quality of fiber tracking, and 1 baseline and 32 noncollinear gradient directions were finally used. Our optimal protocol used a spin echo-based echo planar diffusion-weighted sequence with spatial spectral fat saturation, minimum possible echo time of 45–50 ms, repetition time of 4,500–6,500 ms (depending on the number of slices), 20-cm field of view, 3-mm slice thickness, 0.4-mm spacing, and 8 averages. Typically, 24–30 slices were acquired in the axial and perpendicular planes to the anal canal axis.
Data Analysis of DT Images and Fiber Tracking
DtiStudio software (https://www.mristudio.org) was used for image analysis. Detailed descriptions of the pixel-by-pixel analysis of the signal intensities to calculate the DT terms and, subsequently, the eigenvectors, the angle of the major axis of the primary eigenvector relative to three orthogonal axes (x, y, z) of the magnet, and the corresponding three eigenvalues are provided elsewhere (9, 10, 13, 16, 30, 31). These eigenvalues, in turn, yielded fractional anisotropy (FA), a comprehensive intravoxel index equal to 1 for perfectly anisotropic diffusion, such as in a long, infinitely small, thin capillary; 0 for a perfectly symmetrical sphere; and a near-0 low value believed to indicate crossing fibers. Fiber tracking was implemented with the FA threshold set between 0.08 and 0.15, a rather low value, to avoid missing fibers in the low-FA region, and the angular threshold set between 50° and 70°.
Dynamic MRI
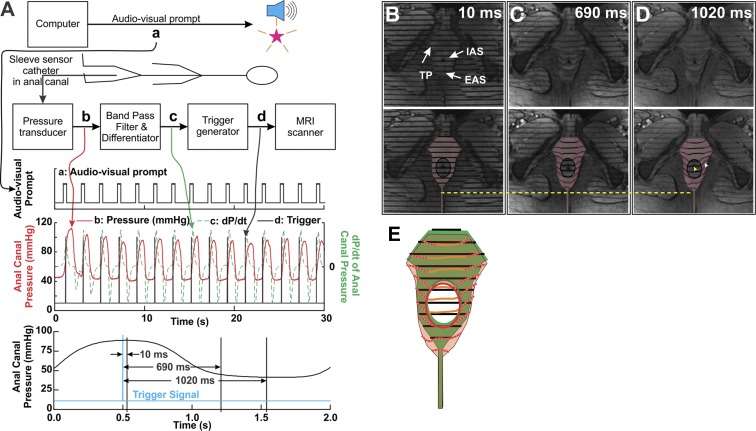
ST MRI allows monitoring of the movement of tissue points in vivo by the extent of distortion of the “tag” lines. Straight tag lines are created by radio-frequency pulses at the initiation of the trigger signal. Distortion of the lines during contraction reveals the trajectory of different tissue points. We trained subjects to contract their anal sphincter muscles at a regular interval of ∼0.5 Hz following a computer-generated audio cue. Anal canal pressure was recorded by a reverse-perfused sleeve sensor (32) placed in the anal canal. Pressure sensed with each “squeeze” was transmitted via a catheter to the electronics outside the scanner room. Visual feedback was provided to the subject by video projection of the sensed pressure to maintain consistent levels of contraction pressure throughout data acquisition. Electronic trigger pulses were produced on the basis of the threshold values of the first derivative of the pressure curve of contraction and used to mimic cardiac R wave to acquire gated scans over ∼80 contractions, yielding 50 phases over a 2-s contraction cycle. A standard fast gradient-recalled echo sequence was used to acquire 5-mm-thick ST images in the axial plane, prescribed on a sagittal image (see Fig. 5), with tag lines prescribed as horizontal stripes with a 5-mm separation.
Fig. 5.
Method of dynamic MRI and results. A: schematic showing signal processing to provide an MRI trigger used for gating the magnetic resonance imager. Audio-video prompt (a) is generated by the computer. In response, the subject generates an anal pressure trace (b, in red). This pressure signal is band-pass-filtered and differentiated (c, in green). Finally, a trigger is generated on the basis of the threshold amplitude of the 1st derivative (black spikes). This signal is used to trigger the magnetic resonance imager. Bottom trace: average anal pressure plot, along with timing (vertical lines) of the 3 magnetic resonance-tagged images in B, C, and D. B–D: 3 different phases (10, 690, and 1,020 ms) of the pressure (contraction/relaxation) cycle, after the start of the spin tag (top). Regions of the EAS, IAS, and anococcygeal raphe with the tag lines are highlighted to show distortions of the tag lines, depicting motion in different regions (bottom). With EAS relaxation, 1) the entire anal complex moves dorsally, 2) tag lines at the inner edge of the EAS, adjacent to the IAS, move dorsally significantly less than tag lines at the outer edge (away from the IAS) of the EAS (25% vs. 6%), and 3) tag lines do not distort within the IAS region. If the muscle fibers of the EAS were circularly oriented and contracted/relaxed isometrically, one would not observe differential movement across its transverse thickness, unlike movement in these images of EAS region. Finally, the direction of distortion of the EAS tag line on either side, when viewed in combination, indicates that the directions of the fibers cross in the PB. E: superimposed tag lines from B and D, bottom. Green region with black lines represents data from B; pink region with red and orange lines represents data from D. Note displacement of the IAS region with minimal distortion of tag lines and distortion of tag lines in the EAS region.
EMG and Lidocaine Injection Into TP Muscle
Studies were conducted in eight asymptomatic nulliparous women. Bipolar needle electrodes were placed in the right or left lateral EAS (at 9 or 3 o'clock, 1 cm lateral to the anal verge) and TP (3 cm ventral to the ventral midline of the anal verge and 2–3 cm lateral to the midline) muscles. A raw EMG signal was recorded during insertion of the electrodes. The EAS and TP were the first muscles encountered during needle insertions. The needle used to record EMG activity of the TP muscle also had a port for injection of lidocaine into the muscle. Two milliliters of 2% lidocaine was injected into the right TP/BS muscle under EMG guidance. Anal canal pressure was compared before and 15 min after the injection with voluntary squeeze.
Anal Canal Pressure Recording
Anal pressures were recorded using a 10-mm-diameter HDM probe [Given Imaging (previously Sierra Scientific), Los Angeles, CA] (4, 5, 24, 25) to determine the effects of lidocaine injection into the TP. The HDM probe consists of 256 pressure transducers (each 4 mm2), located over the 6.4-cm length of the probe, in 16 circumferential rings of transducers, with 16 transducers in each ring. Pressures are displayed as color plots. Anal canal pressures were recorded before and after injection of lidocaine into the TP muscle. Pressure data at the peak of squeezes were exported to an Excel spreadsheet. Average pressure changes from 256 transducers over 3 squeezes were calculated before and 15 min after lidocaine injection.
Statistical Analysis
MR DTI image analysis.
Circular regions of interest (ROIs), four pixels in diameter, were selected in different regions of the left and right EAS, PB, and anococcygeal raphe as identified in the PD and DT images. Three ROIs were selected in each of the right and left EAS (at 8, 9, and 10 o'clock for right and 2, 3, and 4 o'clock for left), three in the PB (at, 11, 12, and 1 o′clock), and one in the anococcygeal raphe (at 6 o'clock). In each subject, ROIs were selected in three slices that were considered to represent the core of the EAS. The mean and SD were calculated over all the slices in each subject. Since the correlation of these parameters in different muscles is not known a priori, two-tailed Student's paired t-test was performed to confirm significant differences between each of the regions (P < 0.05).
Anal canal recordings.
Values are means ± SD. Differences were compared by one-tailed Student's t-tests. Mean pressure changes were calculated from the average of three squeezes before and 15 min after lidocaine injection.
RESULTS
3-D US Images of the Anal Canal
The midline sagittal image of the anal canal shows a well-defined, elliptical-shaped structure of the PB under the US transducer (Fig. 1A). Figure 1B shows axial images of the anal canal, spaced every 1.5 mm, from the caudal to the cranial end. The internal anal sphincter (IAS) is seen as a hypoechoic (dark) circular ring in these images; it appears 8–10 mm from the caudal edge of anal canal. Surrounded by the IAS is a relatively hyperechoic ring-shaped structure of the EAS. Only a very thin ring of the circular EAS is seen surrounding the anal canal at its ventral end. Lateral edges of the EAS appear to merge into the PB and TP/BS muscles. Ventral to the PB, on the right as well as the left side, TP/BS muscles merge into the midline PB. Cranial-caudal extents of the TP/BS muscles (20.0 ± 3.0 mm) are similar to the cranial-caudal extent of the EAS (20.0 ± 3.5 mm). The length of the PB differed among subjects [20.0 ± 3.3 (SD) mm, n = 12], but in each case, the relationship between the TP/BS and EAS and their cranial-caudal extent are precisely the same. The latter observation raises the possibility that EAS and TP/BS muscles are a continuation of the same muscle, what we refer to as the “EAS muscle complex.”
High-Resolution MRI
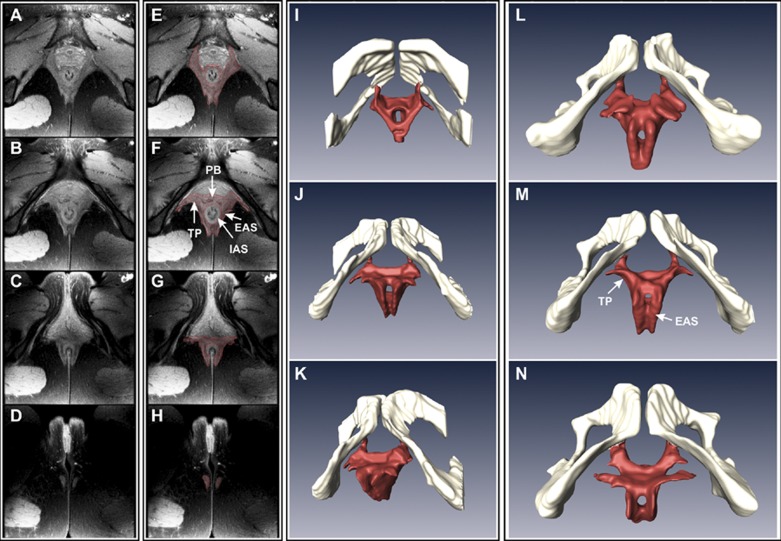
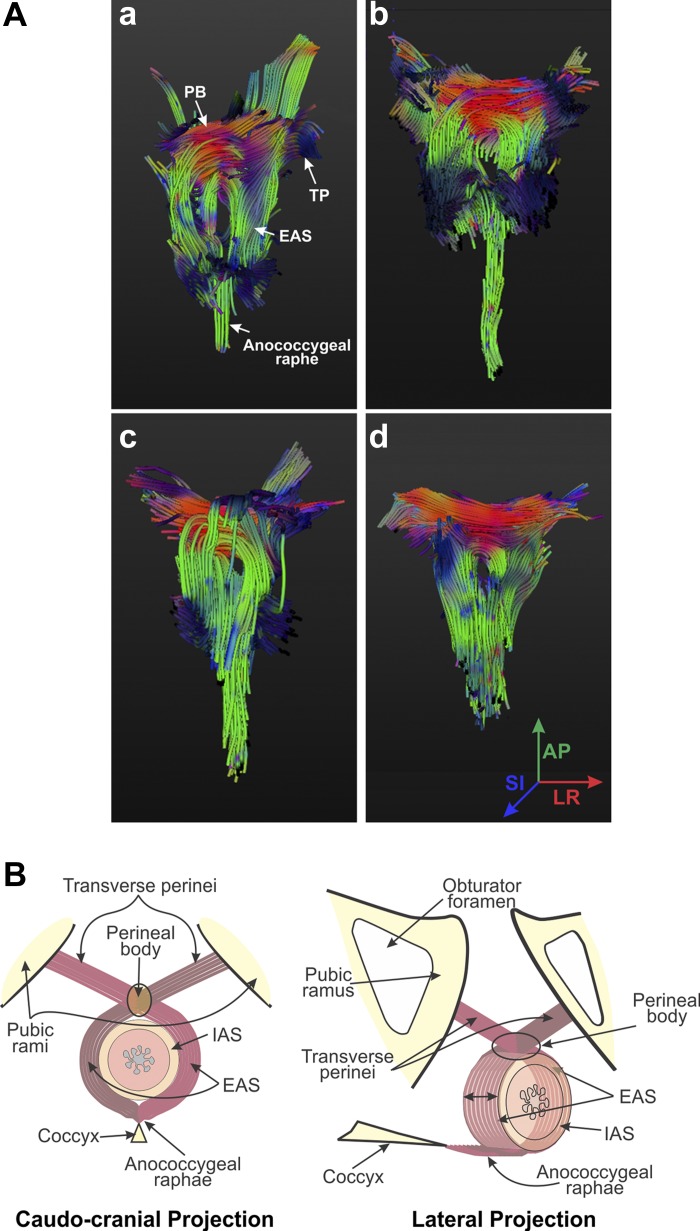
MRIs that include high-resolution morphological imaging and DTI could be carried out successfully in 17 of the 30 subjects initially enrolled in the study. MRIs show a distinct circular IAS. In contrast, a circular shape of the EAS is not clearly appreciated in these MRIs. At the ventral end of the anal canal, TP/BS muscles and the EAS merge into the PB and continue in the posterior direction as right and left sides of the EAS muscles. There is no clear demarcation between the EAS and TP/BS muscle margins in the midline (Fig. 2, A–D). Edges of the EAS and TP/BS muscles were marked manually using Amira software (Visage Imaging, San Diego, CA), and 3-D reconstruction of the EAS and TP/BS muscles is shown in Fig. 2, E–H. These 3-D images raise the possibility of a purse-string shape of the EAS muscle, if one considers TP/BS muscles to be the ventral extension of the EAS with crossing of fibers in the midline. Figure 2, I–N, shows the 3-D shape of the EAS, PB, and TP muscles as one structure. In these images, the cranial edge of the PB was defined as the cranial edge of the EAS.
Fig. 2.
Proton density (PD) MRI of the anal canal (A–H) and 3-dimensional (3-D) reconstruction of the EAS muscle complex (I–N). In A–D, axial images of the anal canal (6 mm apart, from cranial to caudal direction) show various components of the anal sphincter complex. In E–H, muscle margins were marked manually to construct the 3-D anatomy (viewed from caudal to cranial direction, in 4 different subjects, I–N). I–K: images from 1 subject shown in 3 different projections. L–N: anatomy of the EAS muscle in the caudal-cranial projection from 3 additional subjects.
DT Image Analysis of EAS and TP/BS Muscles
Because of typically long times involved in acquiring DT image scans, they are sensitive to subject motion and, hence, were always examined for motion artifacts as soon as they were completed. In few cases, the scans were repeated. All the reported cases were screened for sufficiently good quality, i.e., were devoid of motion artifact. Also, since alignment of the subject with the magnet axis was important, several scout scans were acquired to ascertain that the subjects were in alignment with the magnet axis.
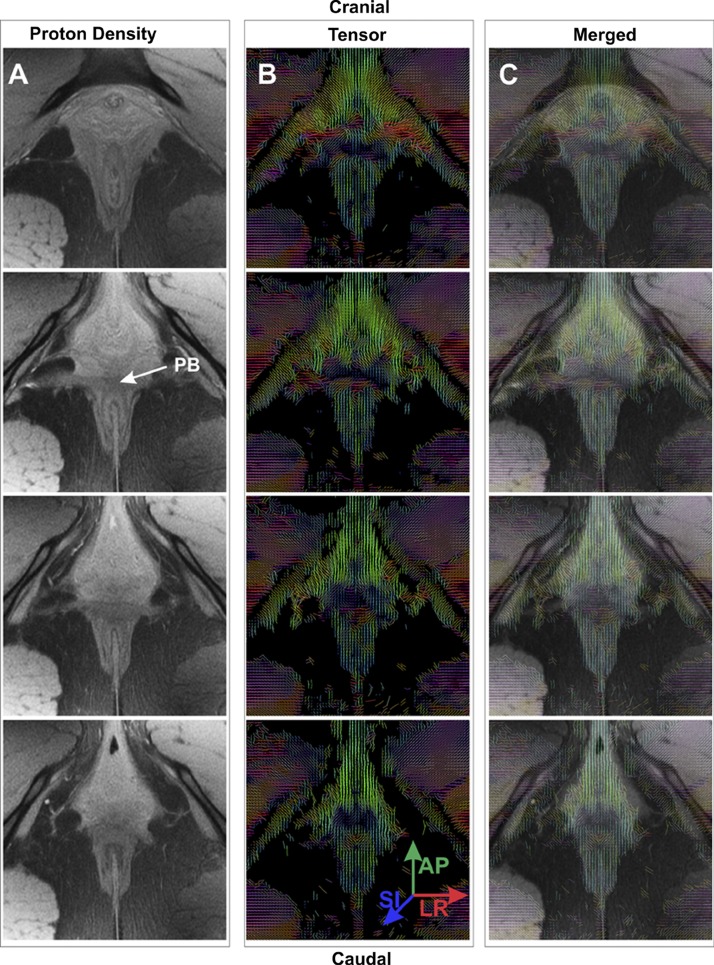
Figure 3 shows the PD, high-resolution images adjacent to the DT image maps, and superimposed tensor images on the corresponding PD images of the anal sphincter in the axial plane of the anal canal (starting with the most cranial end on top). Colors in these maps represent the direction of the primary eigenvector of the DT, which reflects the direction of the muscle fibers. On the basis of the colors in the DT maps, on lateral sides of the EAS, muscle fibers traverse predominantly in the ventral-dorsal direction. In the anococcygeal raphe, the direction is mostly dorsal, but also cranial-caudal. On the other hand, in the ventral midline region (PB), the colors are consistent with the lateral trajectory. Muscle fibers in the TP/BS suggest a ventral, lateral, and cranial course (from the midline), as indicated by the orientation of the measured eigenvectors.
Fig. 3.
PD MRIs and diffusion tensor (DT) images of the EAS complex. A and B: PD images (3-mm thick, 0.4 mm apart) and corresponding DT images of 4 axial slices of the anal canal from cranial to caudal direction in 1 subject. C: DT images superimposed on PD images. DT images were calculated from diffusion-weighted images and placed closest to the location of PD images (since DT image slices were 5-mm thick and separated by 0.4 mm). PB identified in A (arrow) corresponds to that in Fig. 2B. Tensor images show direction of the principal eigenvector, which corresponds to direction of the muscle fiber in each voxel. Colors represent direction of the eigenvector: blue, cranial-caudal; red, left-right (LR); green, ventral-dorsal [anterior-posterior (AP)]. In 2nd and 3rd panels in B and C, mainly red color of the PB indicates left-to-right-oriented muscle fibers. In 1st and 3rd panels of B, mixed colors in PB suggest crossing of muscle fibers. All DT images show ventral-dorsal orientation of the EAS muscle fibers in lateral positions and no crossing of muscle fibers in the dorsal region. AP, anterior-posterior; LR, left-right; SI, superior-inferior or craniocaudal.
Table 1 lists the FA, the eigenvalues (λ1, λ2, and λ3), the angle of orientation with the z- and y-axes of the PB, the right and left EAS, and the anococcygeal raphe. Calculation of angles from the eigenvector values yields the following angles of orientation with respect to the z-axis (cranial-caudal) and y-axis (anterior-posterior) of the magnet: 88 ± 0° and 88 ± 0° for the PB, 52 ± 0° and 55 ± 1° (z-axis) and 8 ± 0° and 12 ± 0° (y-axis) for the left and right EAS, 81 ± 0° and 62 ± 0° for the anococcygeal raphe, and 85 ± 0° and 86 ± 0° (z-axis) and 77 ± 0° and 62 ± 0° (y-axis) for the left and right TP/BS, respectively. The angle with respect to the x-axis (left-right) is not given, since the x-axis angle is complementary (i.e., 90° angle with y-axis) to the y-axis angle. The FA values in the left and right EAS are 0.28 ± 0.03 and 0.31 ± 0.07, respectively, which are consistent with each other as well as with the established values in the literature (10, 40). On the other hand, the FA value in the PB (0.21 ± 0.04) is significantly lower than that in the right and left EAS, a finding consistent with crossing of muscle fibers in this region (33). The FA value in the anococcygeal raphe is 0.20 ± 0.09. Comparison of FA values using a two-tailed test between PB and EAS (both right and left) revealed significant differences (P < 0.05), with no significant difference between the right and left EAS. The primary eigenvalue λ1 was consistently larger than λ2 and λ3, validating the fibrous character of these tissues, and the eigenvalues for the PB were significantly different (P < 0.05) from those of the EAS.
Table 1.
FA, eigenvalues, and fiber orientations of components of the EAS complex
| Eigenvalues, ×10−3 mm2/s |
Fiber Orientation, degrees |
|||||
|---|---|---|---|---|---|---|
| Muscle Component | FA | λ1 | λ2 | λ3 | Angle With z-Axis | Angle With y-Axis |
| Perineal body | 0.21 ± 0.04* | 2.01 ± 0.04* | 1.67 ± 0.03* | 1.32 ± 0.03* | 88 ± 0 | 88 ± 0 |
| EAS | ||||||
| Right | 0.31 ± 0.04 | 1.70 ± 0.02 | 1.24 ± 0.02 | 0.99 ± 0.01 | 55 ± 1 | 12 ± 0 |
| Left | 0.28 ± 0.04 | 1.65.±0.02 | 1.20 ± 0.02 | 0.91 ± 0.02 | 52 ± 0 | 8 ± 0 |
| Annococcygeal raphe | 0.20 ± 0.09* | 1.37 ± 0.01 | 1.08 ± 0.01 | 0.88 ± 0.01 | 80.68 ± 0.07 | 61.48 ± 0.3 |
Values are means ± SD. FA, fractional anisotropy; EAS, external anal sphincter.
P < 0.05 (by 2-tailed t-test).
DT-Based Fiber Tracking in the EAS, PB, and TP/BS
Individual fibers were tracked using the DT imaging maps by drawing ROIs that encompass the PB: the right and the left lateral arms of the EAS. In each subject, the majority of fibers exhibited a trajectory that started from the left or the right side of the EAS, traversed through the central structure of the PB, and then crossed over to the opposite side to continue as TP/BS muscles (Fig. 4A). This type of analysis further suggests crossing of muscle fibers in the PB and purse-string morphology of the EAS complex consisting of TP/BS, right and left EAS, and anococcygeal raphe (Fig. 4B).
Fig. 4.
A: fiber tracking of the EAS muscle complex. Fiber tracking, utilizing the tensor images in Fig. 3, illustrates results obtained in 4 different subjects (a–d). Colors of the fibers at different points are indicative of their directions, as shown in d. As shown in a–d, EAS muscle fibers cross over from one side to the other in the PB to continue as the TP/BS muscles. Dorsally and caudally, muscle fibers continue as the anococcygeal raphe. Regions of the IAS and anal canal are depicted as a signal void in a–d. B: schematic of the EAS muscle complex. On the basis of US images, PD MRIs, DT images, and magnetic resonance fiber tracking, we propose “purse-string” morphology of the EAS complex.
Dynamic Motion of the EAS, PB, and TP/BS
Because of the complicated nature of dynamic MRI studies, in which subjects exerted >70 consistent sphincter muscle contractions, and the duration of these studies, only 7 subjects successfully completed the studies. The images in Fig. 5 show that the anal sphincter complex moves in the ventral direction with contraction and in the dorsal direction with relaxation (average displacement 8–10 mm in 7 subjects). In Fig. 5A, the first phase is acquired at the peak of anal contraction when tag lines are observed to be straight. As the subject relaxes the muscles, these lines become angulated in the EAS region (Fig. 5, E–G), which suggests tissue displacement. If the “circular EAS” muscle were to contract or relax isometrically, one would expect uniform displacement with no distortion of the horizontal tag lines around the whole circumference of the anal canal, which is not the case. There is greater ventral (anterior) movement of EAS outer edge than its inner edge (∼25% vs. ∼7%). On the other hand, there is no distortion of outer and inner edges of the IAS during contraction, suggesting that IAS contraction is isometric (Fig. 5H).
EMG of TP/BS and EAS Muscles
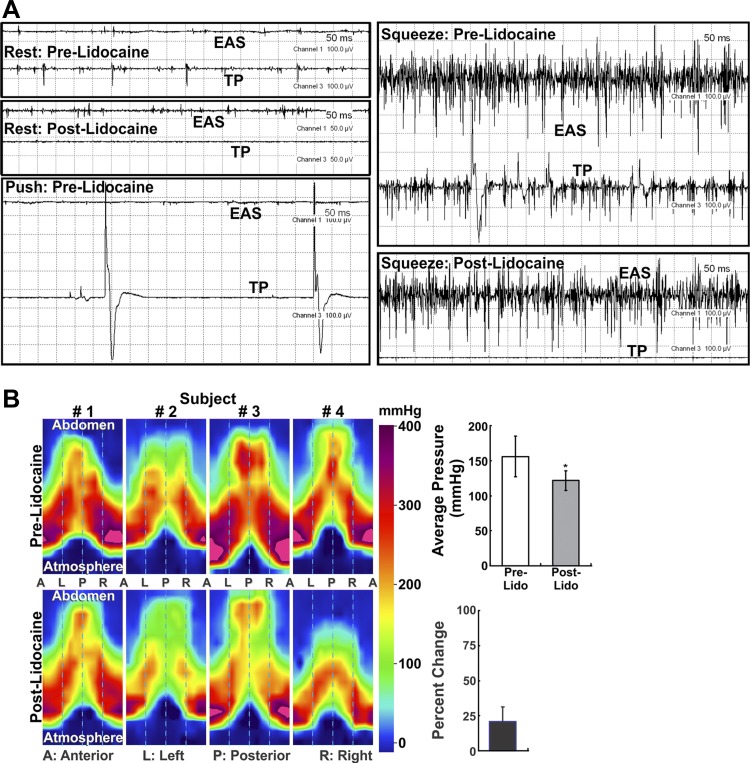
EMG studies were conducted in five nulliparous women. Bipolar needles/wires were placed in the right EAS (at 9 o′clock, 1 cm from the anal verge) and TP/BS (right side, 3 cm ventral and 2 cm lateral to the midline of the anal verge). Recordings demonstrated tonic EMG activity in TP/BS and EAS muscles. With voluntary squeeze, electrical firing increased in both muscles, suggesting synchronous activation. On the other hand, with voluntary push (defecatory maneuver), the two muscles are inhibited at the same time (Fig. 6A).
Fig. 6.
A: electromyographic (EMG) recording of the TP/BS muscle. Note tonic EMG activity (spikes) in EAS and TP/BS muscles at rest. Before lidocaine injection, push (defecatory) maneuver resulted in a decrease and voluntary contraction (squeeze) resulted in an increase in EMG activity in both muscles. Lidocaine injection into the TP/BS muscle abolishes EMG activity in the TP/BS muscle without affecting the EAS muscle, at rest and during the squeeze maneuver. B: anal canal pressures recorded by high-definition anal canal manometry before and after lidocaine injection into the TP/BS muscle. Anal canal pressures were recorded during voluntary squeeze before and 15 min after lidocaine injection into the TP/BS muscle. Subjects were instructed to make a similar voluntary effort (confirmed by amplitude of the EMG signal) before and after injection. Reduction of anal canal pressure after lidocaine injection was observed in the lower half of the anal canal. In subject 4, the cranial-caudal extent of the pressure profile decreased significantly because of marked reduction of EAS contraction-related pressure. Bar graphs show effect of lidocaine on anal canal pressure.
Effect of Lidocaine Injection Into the TP/BS Muscle on Anal Canal Pressure
The anal canal pressure profile is split in the ventral midline to display a surface plot of the “cylindrical” anal canal anatomy. With voluntary squeeze, anal canal pressure increases in all regions. EMG traces revealed that, soon after injection, the electrical signal disappeared in the TP/BS muscle. On the other hand, there was no effect on the EMG pattern of the EAS muscle at rest and with voluntary contraction, arguing against the possibility that lidocaine affected the pudendal nerve or its branches that innervate the EAS muscle. Additional EMG recordings were obtained in three subjects in whom lidocaine was injected into the right TP/BS and EMG activity was recorded from the left EAS muscle (at 3 o'clock). There was no difference in the amplitude of EMG spikes before and after lidocaine injection in these three subjects (120 vs. 116, 139 vs. 137, and 50 vs. 51 mV). These findings prove that lidocaine did not diffuse from the right TP/BS muscle into the left EAS. Figure 6B shows a significant decrease (21 ± 5%) in anal canal squeeze pressures after lidocaine injection. Furthermore, anal canal pressure decreased in the caudal half of the anal canal region, which is under the influence of EAS muscle (5).
US image analysis of the pelvic floor in five subjects shows that lidocaine injection had no effect on the pelvic floor hiatus length at rest (6.1 ± 0.6 vs. 5.8 ± 0.8 cm) and during squeeze (4.8 ± 0.4 vs. 5.0 ± 0.5 cm, n = 5). Since pelvic floor hiatus is a measure of PRM length (11, 36), we conclude that the injected lidocaine did not diffuse into the PRM.
DISCUSSION
Controversy regarding EAS morphology, based on gross anatomic dissection, has centered on whether the EAS is a two- or three-part structure. Frustrated by innumerable descriptions and convinced that the EAS is a two-part structure, Daley stated, “three-part external anal sphincter be removed from the gross anatomy texts, and be relegated to the junkyard of anatomic trivia where it may languish for the sake of the historical anatomist or the rare individual who spends time carving out the most meticulous of dissection” (6). MRIs of Hussain et al. (14) and our 3-D US images (25) present strong evidence that the EAS is indeed a two-part structure; the deep EAS is most likely the PRM. The part of the EAS located caudal to the level of the IAS is probably referred to as the subcutaneous portion (based on anatomic dissection), and the cranial part of the EAS that surrounds the IAS is referred to as the superficial component of the EAS. On the basis of MRI and US images, it is clear that the proximal half of the anal canal is surrounded by the “U”-shaped PRM, and closure of the proximal half of the anal canal is related to contraction of the IAS and PRM (18). In fact, the PRM forms the inferior margin of the pelvic floor hiatus, and its contraction causes closure of not only the anal canal (18), but the vagina (24) and urethra as well (26).
Our 3-D US images, high-resolution MRIs, and DT images strongly suggest a purse-string morphology of the EAS. DT imaging and fiber tracking have been used extensively to visualize white matter tracts in the brain in vivo and muscle fiber direction in vitro (28, 29, 33, 38, 39). However, its application to muscle fiber tracking in vivo has been limited, primarily because of lower SNR related to the motion in muscle. We compensated for the lower SNR by using a relatively low b value of 400 s/mm2, which allows less echo time and, consequently, increases the SNR. The SNR was further enhanced by use of a large number (8–10) of averages. Our use of parallel imaging limited the extent of blurring by decreasing the point-spread function. Using this strategy, we succeeded in producing some of the earliest work in vivo in humans in diffusion-weighted imaging of breast (28), prostate (29), and lower leg muscle (30). Similar success in recording DT images of muscles has also been reported by other groups (13, 16).
While the DT maps give a qualitative description of fiber directions, the directional vectors of the primary eigenvector (Table 1) are able to give quantitative values of the angle of orientation of the fiber in reference to the three orthogonal magnet axes. Note that these angles are relative to the magnet axis, not the subject axis. However, in most cases, these two coincide, since care was taken to align the subject with the axis of the magnet, including use of the initial prescription. In some cases, the subject may be slightly misaligned in any of the three directions. The angles yielded by the primary eigenvector for the different muscles of the EAS complex represent a very elegant validation of this novel noninvasive, nonionizing, high-resolution DT method of visualizing muscle fibers within the human body. The fibers in the ventral midline, i.e., in the PB, are expected to traverse left to right, which is implied by an angle of ∼90° with the y-axis. Similarly, an angle of 87° with the z-axis implies that the fibers lie in the transaxial plane, roughly perpendicular to the cranial-caudal direction. Similar validation is seen for the left and right EAS with the angle of ∼8° and 12° with the y- or anterior-posterior axis. On the basis of the physics, while the eigenvector directions in the PB cannot prove crossover of muscle fibers, i.e., cannot distinguish between left-to-right and right-to-left traversal, as noted above, a lower FA value possibly does imply such crossing (33). Lower FA values are attributed to crossing fibers in the white matter tracts of the brain (33). Hence, we suspect that lower FA values in the PB region likely represent crossing of muscle fibers from one side to the other. FA values in the anococcygeal raphe are similar to those in the right and left EAS, implying the existence of muscle fibers (tensor behavior of water diffusion) in the anococcygeal raphe, a finding similar to a recent histological study (15) and contrary to the conventional thinking that the anococcygeal raphe is a fibroaponeurotic structure.
Fiber tracking based on DT imaging and dynamic MRI also confirms our proposed purse-string configuration of the EAS. Interestingly, Oh and Kark (21) performed a histological study and described “decussation” of EAS muscle fibers at the ventral end, i.e., in the PB. In fact, a schematic by Thompson (35) and his description of the EAS published in 1899 clearly demonstrate crossing of muscle fibers in the PB to continue as TP and BS muscles (see figures in supplemental material). Dynamic MRI shows that the EAS muscle does not contract in an isometric fashion, which also argues against the circumferential nature of EAS contraction. Therefore, we believe that, on the basis of even earlier dissection studies, there is strong support for our hypothesis that the EAS has a purse-string morphology, rather than a donut shape. A recent developmental study concluded that the BS is an important part of the EAS muscle (1). Our functional data that paralysis of TP/BS muscles by lidocaine injection decreases anal canal pressure provide convincing evidence that the TP/BS muscles are part of the EAS muscle. Hence, we propose that the EAS, TP, and BS muscles should be referred to as the “EAS complex muscles.”
Why does it matter whether EAS has a “circular” or a purse-string configuration, since closure of the anal canal can be achieved by both configurations? We believe that the purse-string configuration is mechanically more advantageous than the circular morphology for several reasons: 1) if the TP/BS muscles are actually part of the EAS muscle, the effective length of the EAS muscle is greater, which can generate greater muscle shortening and a stronger anal canal closure; and 2) on the basis of the circular configuration, EAS muscle contraction is suspected to be isometric, in which case no increase in muscle thickness may occur with contraction. The latter would result in an increase in muscle stress to a much greater degree than in a muscle that thickens with contraction. In fact, with contraction, the coccyx moves in the ventral and cranial direction (3), thus decreasing the length and increasing the thickness of the EAS muscle. In addition to the above-mentioned biomechanical advantages, there are important clinical considerations relevant to the EAS morphology. 1) Lateral episiotomy performed at the time of childbirth transects the TP/BS muscle. It is considered to be a “sphincter-sparing” operation, in contrast to the midline dorsal episiotomy. If the TP/BS muscle is actually a part of the EAS, lateral episiotomy is not a sphincter-sparing operation. 2) A commonly performed surgical procedure to treat anal incontinence, i.e., “overlapping sphincteroplasty,” restores the circular configuration of the EAS muscle. If the EAS is not a circular muscle to begin with, restoration of circular anatomy cannot restore normal EAS muscle function. Perhaps overlapping sphincteroplasty is not an effective surgical procedure to treat anal incontinence (12). 3) Finally, the possibility that the other sphincters in the body do not have a circular/donut configuration requires careful exploration.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant R01-DK-091348.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.K.M. is responsible for conception and design of the research; R.K.M. drafted the manuscript; R.K.M., V.B., and S.S. edited and revised the manuscript; R.K.M. approved the final version of the manuscript; R.K.M., V.B., G.S., M.L., and S.S. performed the experiments; R.K.M., V.B., M.L., and S.S. analyzed the data; V.B. prepared the figures.
Supplementary Material
REFERENCES
- 1.Arakawa T, Hayashi S, Kinugasa Y, Murakami G, Fujimiya M. Development of the external anal sphincter with special reference to intergender difference: observations of mid-term fetuses (15–30 wk of gestation). Okajimas Folia Anat Jpn 87: 49–58, 2010. [DOI] [PubMed] [Google Scholar]
- 2.Bharucha AE. Pelvic floor: anatomy and function. Neurogastroenterol Motil 18: 507–519, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Bo K, Lilleas F, Talseth T, Hedland H. Dynamic MRI of the pelvic floor muscles in an upright sitting position. Neurourol Urodyn 20: 167–174, 2001. [DOI] [PubMed] [Google Scholar]
- 4.Cheeney G, Nguyen M, Valestin J, Rao SS. Topographic and manometric characterization of the recto-anal inhibitory reflex. Neurogastroenterol Motil 24: e147–154, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheeney G, Remes-Troche JM, Attaluri A, Rao SS. Investigation of anal motor characteristics of the sensorimotor response (SMR) using 3-D anorectal pressure topography. Am J Physiol Gastrointest Liver Physiol 300: G236–G240, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dalley AF., 2nd The riddle of the sphincters. The morphophysiology of the anorectal mechanism reviewed. Am Surg 53: 298–306, 1987. [PubMed] [Google Scholar]
- 7.Fritsch H, Brenner E, Lienemann A, Ludwikowski B. Anal sphincter complex: reinterpreted morphology and its clinical relevance. Dis Colon Rectum 45: 188–194, 2002. [DOI] [PubMed] [Google Scholar]
- 8.Gaige TA, Kwon HS, Dai G, Cabral VC, Wang R, Nam YS, Engelward BP, Wedeen VJ, So PT, Gilbert RJ. Multiscale structural analysis of mouse lingual myoarchitecture employing diffusion spectrum magnetic resonance imaging and multiphoton microscopy. J Biomed Opt 13: 064005, 2008. [DOI] [PubMed] [Google Scholar]
- 9.Galban CJ, Maderwald S, Uffmann K, Ladd ME. A diffusion tensor imaging analysis of gender differences in water diffusivity within human skeletal muscle. NMR Biomed 18: 489–498, 2005. [DOI] [PubMed] [Google Scholar]
- 10.Goh V, Tam E, Taylor NJ, Stirling JJ, Simcock IC, Jones RG, Padhani AR. Diffusion tensor imaging of the anal canal at 3 tesla: feasibility and reproducibility of anisotropy measures. J Magn Reson Imaging 35: 820–826, 2012. [DOI] [PubMed] [Google Scholar]
- 11.Guaderrama NM, Liu J, Nager CW, Pretorius DH, Sheean G, Kassab G, Mittal RK. Evidence for the innervation of pelvic floor muscles by the pudendal nerve. Obstet Gynecol 106: 774–781, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Halverson AL, Hull TL. Long-term outcome of overlapping anal sphincter repair. Dis Colon Rectum 45: 345–348, 2002. [DOI] [PubMed] [Google Scholar]
- 13.Heemskerk AM, Strijkers GJ, Vilanova A, Drost MR, Nicolay K. Determination of mouse skeletal muscle architecture using three-dimensional diffusion tensor imaging. Magn Reson Med 53: 1333–1340, 2005. [DOI] [PubMed] [Google Scholar]
- 14.Hussain SM, Stoker J, Lameris JS. Anal sphincter complex: endoanal MR imaging of normal anatomy. Radiology 197: 671–677, 1995. [DOI] [PubMed] [Google Scholar]
- 15.Kinugasa Y, Arakawa T, Abe H, Abe S, Cho BH, Murakami G, Sugihara K. Anococcygeal raphe revisited: a histological study using mid-term human fetuses and elderly cadavers. Yonsei Med J 53: 849–855, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lansdown DA, Ding Z, Wadington M, Hornberger JL, Damon BM. Quantitative diffusion tensor MRI-based fiber tracking of human skeletal muscle. J Appl Physiol 103: 673–681, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Larson KA, Yousuf A, Lewicky-Gaupp C, Fenner DE, DeLancey JO. Perineal body anatomy in living women: 3-dimensional analysis using thin-slice magnetic resonance imaging. Am J Obstet Gynecol 203: 494.e15–21, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu J, Guaderrama N, Nager CW, Pretorius DH, Master S, Mittal RK. Functional correlates of anal canal anatomy: puborectalis muscle and anal canal pressure. Am J Gastroenterol 101: 1092–1097, 2006. [DOI] [PubMed] [Google Scholar]
- 19.Netter F. (Editor) Atlas of Human Anatomy. Teteboro, NJ: Icon Learning System, 2003, plates 361 and 364. [Google Scholar]
- 20.Niitsu M, Campeau NG, Holsinger-Bampton AE, Riederer SJ, Ehman RL. Tracking motion with tagged rapid gradient-echo magnetization-prepared MR imaging. J Magn Reson Imaging 2: 155–163, 1992. [DOI] [PubMed] [Google Scholar]
- 21.Oh C, Kark AE. Anatomy of the external anal sphincter. Br J Surg 59: 717–723, 1972. [DOI] [PubMed] [Google Scholar]
- 22.Oh C, Kark AE. Anatomy of the perineal body. Dis Colon Rectum 16: 444–454, 1973. [DOI] [PubMed] [Google Scholar]
- 23.Peschers UM, DeLancey JO, Fritsch H, Quint LE, Prince MR. Cross-sectional imaging anatomy of the anal sphincters. Obstet Gynecol 90: 839–844, 1997. [DOI] [PubMed] [Google Scholar]
- 24.Raizada V, Bhargava V, Jung SA, Karstens A, Pretorius D, Krysl P, Mittal RK. Dynamic assessment of the vaginal high-pressure zone using high-definition manometery, 3-dimensional ultrasound, and magnetic resonance imaging of the pelvic floor muscles. Am J Obstet Gynecol 203: 172.e1–8, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raizada V, Bhargava V, Karsten A, Mittal RK. Functional morphology of anal sphincter complex unveiled by high definition anal manometery and three dimensional ultrasound imaging. Neurogastroenterol Motil 23: 1013–1019, e1460, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rajasekaran MR, Dongwan S, Salehi M, Bhargava V, Fritsch H, Mittal RK. Role of puborectalis muscle in the gnesis of urethral pressure. J Urol 188: 1382–1388, 2012. [DOI] [PubMed] [Google Scholar]
- 27.Santorini J. Septemdecim tabulae. 1715. [Google Scholar]
- 28.Sinha S, Lucas-Quesada FA, Sinha U, DeBruhl N, Bassett LW. In vivo diffusion-weighted MRI of the breast: potential for lesion characterization. J Magn Reson Imaging 15: 693–704, 2002. [DOI] [PubMed] [Google Scholar]
- 29.Sinha S, Sinha U. In vivo diffusion tensor imaging of the human prostate. Magn Reson Med 52: 530–537, 2004. [DOI] [PubMed] [Google Scholar]
- 30.Sinha S, Sinha U, Edgerton VR. In vivo diffusion tensor imaging of the human calf muscle J. Magn Reson Imaging 24: 182–190, 2006. [DOI] [PubMed] [Google Scholar]
- 31.Sinha U, Sinha S, Hodgson JA, Edgerton RV. Human soleus muscle architecture at different ankle joint angles from magnetic resonance diffusion tensor imaging. J Appl Physiol 110: 807–819, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sivri B, Mittal RK. Reverse-perfused sleeve: an improved device for measurement of sphincteric function of the crural diaphragm. Gastroenterology 101: 962–969, 1991. [DOI] [PubMed] [Google Scholar]
- 33.Sotiropoulos SN, Bai L, Morgan PS, Auer DP, Constantinescu CS, Tench CR. A regularized two-tensor model fit to low angular resolution diffusion images using basis directions. J Magn Reson Imaging 28: 199–209, 2008. [DOI] [PubMed] [Google Scholar]
- 34.Sultan AH, Kamm MA, Hudson CN, Thomas JM, Bartram CI. Anal-sphincter disruption during vaginal delivery. N Engl J Med 329: 1905–1911, 1993. [DOI] [PubMed] [Google Scholar]
- 35.Thompson P. The Myology of the Pelvic Floor. Newton: McCorquoddale, 1899. [Google Scholar]
- 36.Weinstein MM, Jung SA, Pretorius DH, Nager CW, den Boer DJ, Mittal RK. The reliability of puborectalis muscle measurements with 3-dimensional ultrasound imaging. Am J Obstet Gynecol 197: 68.e1–6, ]?2007. [DOI] [PubMed] [Google Scholar]
- 37.Weinstein MM, Pretorius DH, Jung SA, Nager CW, Mittal RK. Transperineal three-dimensional ultrasound imaging for detection of anatomic defects in the anal sphincter complex muscles. Clin Gastroenterol Hepatol 7: 205–211, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Whitehead WE. Control groups appropriate for behavioral interventions. Gastroenterology 126: S159–S163, 2004. [DOI] [PubMed] [Google Scholar]
- 39.Whitehead WE, Norton NJ, Wald A. Advancing the treatment of fecal and urinary incontinence through research. Gastroenterology 126: S1–S2, 2004. [DOI] [PubMed] [Google Scholar]
- 40.Zijta FM, Froeling M, van der Paardt MP, Lakeman MM, Bipat S, van Swijndregt AD, Strijkers GJ, Nederveen AJ, Stoker J. Feasibility of diffusion tensor imaging (DTI) with fibre tractography of the normal female pelvic floor. Eur Radiol 21: 1243–1249, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.