Abstract
We used the patch-clamp technique to examine the effect of angiotensin II (ANG II) on the basolateral K channels in the thick ascending limb (TAL) of the rat kidney. Application of ANG II increased the channel activity and the current amplitude of the basolateral 50-pS K channel. The stimulatory effect of ANG II on the K channels was completely abolished by losartan, an inhibitor of type 1 angiotensin receptor (AT1R), but not by PD123319, an AT2R antagonist. Moreover, inhibition of phospholipase C (PLC) and protein kinase C (PKC) also abrogated the stimulatory effect of ANG II on the basolateral K channels in the TAL. This suggests that the stimulatory effect of ANG II on the K channels was induced by activating PLC and PKC pathways. Western blotting demonstrated that ANG II increased the phosphorylation of c-Src at tyrosine residue 416, an indication of c-Src activation. This effect was mimicked by PKC stimulator but abolished by calphostin C. Moreover, inhibition of NADPH oxidase (NOX) also blocked the effect of ANG II on c-Src tyrosine phosphorylation. The role of Src-family protein tyrosine kinase (SFK) in mediating the effect of ANG II on the basolateral K channel was further suggested by the experiments in which inhibition of SFK abrogated the stimulatory effect of ANG II on the basolateral 50-pS K channel. We conclude that ANG II increases basolateral 50-pS K channel activity via AT1R and that activation of AT1R stimulates SFK by a PLC-PKC-NOX-dependent mechanism.
Keywords: AT1R, Src-family protein tyrosine kinase, NOX, Kir.4.1
the thick ascending limb (TAL) plays a key role in the regulation of renal salt and water homeostasis. Not only is the TAL responsible for reabsorption of 20–25% filtered NaCl but it also plays a key role in urinary concentrating mechanism by separating water from electrolyte transport thereby generating medullary interstitial hypertonicity (11, 13, 16). While the apical type II Na-K-Cl cotransporter (NKCC2) is responsible for Na/Cl entering the cell (6, 8), the basolateral K channel activity is also involved in sustaining the membrane transport in the TAL (10, 12, 14). First, basolateral K channels participate in generating the cell membrane potential in the TAL thereby providing the driving force for Cl diffusion across the basolateral membrane (34). Second, the basolateral K channel couples to the activity of Na-K-ATPase thereby facilitating the function of the Na pump (29). The patch-clamp experiments have identified a 40- to 50-pS inwardly rectifying K channel as a main type of K channel expressed in the basolateral membrane of the TAL (9, 23). Furthermore, we previously demonstrated that the 50-pS K channel activity was inhibited by high external Ca2+ through activating Ca2+-sensing receptor and by prostaglandin E2 (PGE2) through prostaglandin E (EP) receptor (10, 18). In addition to external Ca2+ and PGE2, ANG II has been shown to be involved in the regulation of Na/Cl transport in the TAL (20, 31). We showed that ANG II activates the basolateral Cl channels in the TAL by stimulating NADPH oxidase (NOX) (35). Since the basolateral K channel activity affects the Cl movement across the basolateral membrane, it is conceivable that ANG II may also regulate the basolateral K channels in the TAL. Therefore, the aim of the present study is to examine whether ANG II also regulates the basolateral 50-pS K channels in the TAL.
METHODS
Preparation of medullary TAL.
Sprague-Dawley (SD) rats (both sex) were used in the experiments. The animals with 60–80 g body wt were purchased from the animal center of the second affiliated hospital of Harbin Medical University (Harbin, China) and they were fed with a normal rat chow before use. Rats were killed by cervical dislocation (<90 g) and the kidneys were removed immediately. The kidneys were cut into several 1-mm-thick slices with a razor blade. The slices were incubated in a buffer solution containing type IA collagenase (1 mg/ml) at 37°C for 50–60 min. After the collagenase treatment, the slices were rinsed with a HEPES-buffered bath solution containing (in mM) 140 NaCl, 5 KCl, 1.8 MgCl2, 1.8 CaCl2, and 10 HEPES (pH 7.4) and kept at 4°C. The TAL was dissected using watch-making forceps in HEPES-buffered bath solution and transferred onto a 5×5-mm cover glass coated with polylysine (Sigma) to immobilize the tubule. The cover glass was placed in a chamber mounted on an inverted microscope (Nikon) and the tubules were superfused with HEPES-buffered bath solution. The animal-using protocol has been approved by an independent animal-using committee in Harbin Medical University.
Patch-clamp technique.
An Axon 200B patch-clamp amplifier was used for the experiments and all experiments were carried at room temperature. The single-channel patches were performed in cell-attached mode and the current was low-pass filtered at 0.2–0.5 kHz. The signals were then digitized by an Axon interface (Digidata 1320). The patch pipettes were pulled from borosilicate glass capillaries (Degan, Minneapolis, MN) using a two-step Narishige PP-830 electrode-puller (Narishige, Tokyo, Japan). The pipettes were then polished (WPI MF-200 electrode-polisher) and filled with the solution containing (in mM) 140 KCl, 1.8 MgCl2, and 10 HEPES (pH 7.4).
The data were analyzed using pClamp software system 9.2 (Axon Instruments, Burlingame, CA). We defined NPo, a product between channel number (N) and channel open probability (Po), as an index of channel activity. The NPo was calculated from data samples of 90-s duration in the steady state as follows: NPo = ∑ (1t1 + 2t2 + . . . iti), where ti is the fractional open time spent at each of the observed current levels.
Western blot.
An equal amount of protein (100 μg) obtained from medullary TAL was separated by electrophoresis using 10% SDS-PAGE and the proteins were transferred to nitrocellulose membranes (Pall Life Sciences). Membranes were blocked with 5% nonfat dry milk in 0.1% Tween-Tris-buffered saline (TTBS) and then washed with 0.1% TTBS. The membrane was incubated with the primary antibody overnight at 4°C. After four time washes (10 min for each) with 0.1% TBS-T, the membranes were incubated with the horseradish peroxidase-conjugated secondary antibody for 1 h. Protein bands were detected by SuperSignal West Pico Chemiluminescent Substrate (Pierce Biotechnology) and quantified by densitometry using Quantity One software (Bio-Rad).
Materials and statistics.
ANG II, losartan, PD123319, type 1A collagenase, U73122, 4-amino-5-(4-methyphenyl)-7-(t-buty)-pyrazolo[3,4-d]-pyrimidine (PP1), calphostin C, herbimycin A, phorbol-12-myristate-13-acetate (PMA), sodium 4,5-dihydroxybenzene-1,3-disulfonate (tiron), and diphenyleneiodonium sulfate (DPI) were purchased from Sigma (St. Louis, MO). The antibodies of β-actin, c-Src, and phospho-Tyr416-cSrc (P-Tyr416-cSrc) were obtained from Cell Signaling. U73122, PMA, calphostin C, and DPI were dissolved in DMSO and the final concentrations of DMSO in the bath were <0.1% which had no significant effect on channel activity. Data are shown as means ± SE. We used paired Student's t-tests or one-way ANOVA to determine the significance of the difference between the control and experimental groups. Statistical significance was taken as P < 0.05.
RESULTS
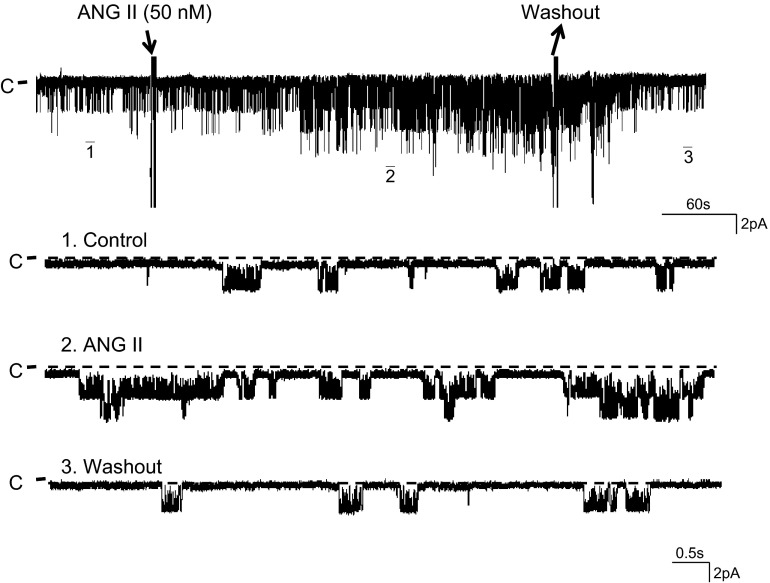
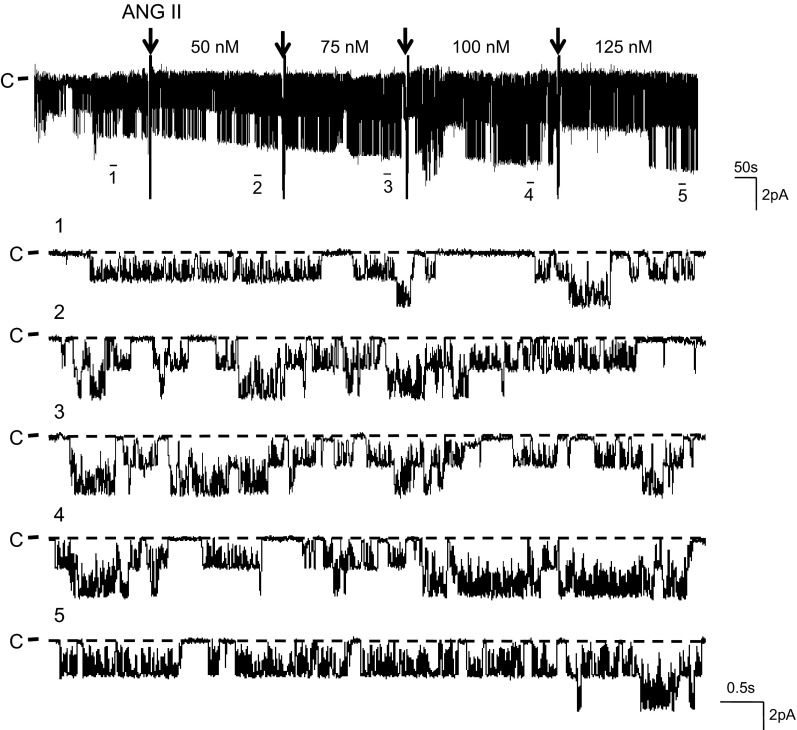
Using the single-channel patch-clamp technique, we confirmed the previous observations that the 50-pS K channel was predominant in the basolateral membrane of the TAL (9). To examine the effect of ANG II on the basolateral 50-pS K channel in the TAL, we added 50 nM ANG II to the bath and monitored the response of the K channel to ANG II. Figure 1 is a typical recording showing the effect of 50 nM ANG II on the 50-pS K channel activity in a cell-attached patch. It is apparent that addition of 50 nM ANG II increased the channel activity defined by NPo from 0.23 ± 0.06 to 0.52 ± 0.07 within 5 min (n = 6). The effect of ANG II was reversible because washout restored the channel activity to the basal level (Fig. 1). Furthermore, an increase in ANG II concentrations enhanced the stimulatory effect of ANG II on the 50-pS K channel in the TAL. Figure 2 is a typical recording demonstrating that increasing ANG II concentrations to 75 and 100 nM not only stimulated the channel activity defined by NPo but also raised the channel current amplitude. This suggests that ANG II-induced stimulation of the 50-pS K channels hyperpolarized the cell membrane potential. Figure 3A is a dose-response curve of ANG II effect on the 50-pS K channel (n = 5–7). Raised ANG II concentrations from 0 to 25, 50, 75, 100, and 125 nM increased NPo from 0.23 ± 0.06 to 0.34 ± 0.07, 0.54 ± 0.08 (P < 0.05), 0.65 ± 0.08 (P < 0.05), 0.74 ± 0.1 (P < 0.05), 0.76 ± 0.12 (P < 0.01), respectively. Since concentrations of ANG II causing the maximal stimulation of the 50-pS K channel were between 50 and 100 nM, we used 50 nM ANG II in the following experiments.
Fig. 1.
ANG II stimulates the 50-pS K channel. A channel recording showing the effect of ANG II (50 nM) on the 50-pS K channel activity in the basolateral membrane of the thick ascending limb (TAL). The experiment was performed in a cell-attached patch with 140 mM KCl in the pipette and 140 mM NaCl/5 mM KCl in the bath. Top trace shows the time course of the experiment. Three parts of the trace, indicated by numbers, are extended to show the fast time resolution. The holding potential was 0 mV, and the channel closed current is indicated by “C.”
Fig. 2.
Effect of ANG II on the 50-pS K channel is dose-dependent. A channel recording demonstrating the effect of ANG II (50 to 125 nM) on the 50-pS K channels in the basolateral membrane of the TAL. The arrow indicates the addition of ANG II and the total ANG II concentration is indicated on the top of the corresponding trace. The experiment was performed in a cell-attached patch with 140 mM K in the pipette and the Ringer solution in the bath. Top trace shows the experimental time course. Five parts of the trace, indicated by numbers, are extended to show the fast time resolution. The holding potential was 0 mV, and the channel closed current is indicated by “C” and a dotted line.
Fig. 3.
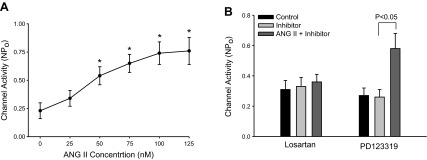
Effect of ANG II is mediated by type 1 angiotensin receptor (AT1R). A: dose-response curve of ANG II effect on the 50-pS K channels in cell-attached patches. *Difference is significant compared with the control value. B: bar graph summarizes the experiments in which the effect of ANG II (50 nM) on the 50-pS K channels was tested in the presence of 10 μM losartan or PD123319.
We next examined whether the stimulatory effect of ANG II was mediated by AT1R or AT2R because they are expressed in the TAL (21). We examined the effect of ANG II on the 50-pS K channels in the presence of either losartan or PD123319. Results are summarized in Fig. 3B showing that neither losartan (10 μM) nor PD123319 (10 μM) had a significant effect on the channel activity (n = 4). However, in the presence of losartan, application ANG II (50 nM) failed to increase 50-pS K channel activity (control 0.31 ± 0.06; losartan 0.33 ± 0.03; ANG II+losartan 0.36 ± 0.05). In contrast, inhibition of AT2R with PD123319 did not abolish the stimulatory effect of ANG II on the 50-pS K channel (control 0.27 ± 0.03; PD123319 0.26 ± 0.04; ANG II+PD123319 0.58 ± 0.11). This suggests that ANG II stimulates the basolateral 50-pS K channels in the TAL through the activation of AT1R.
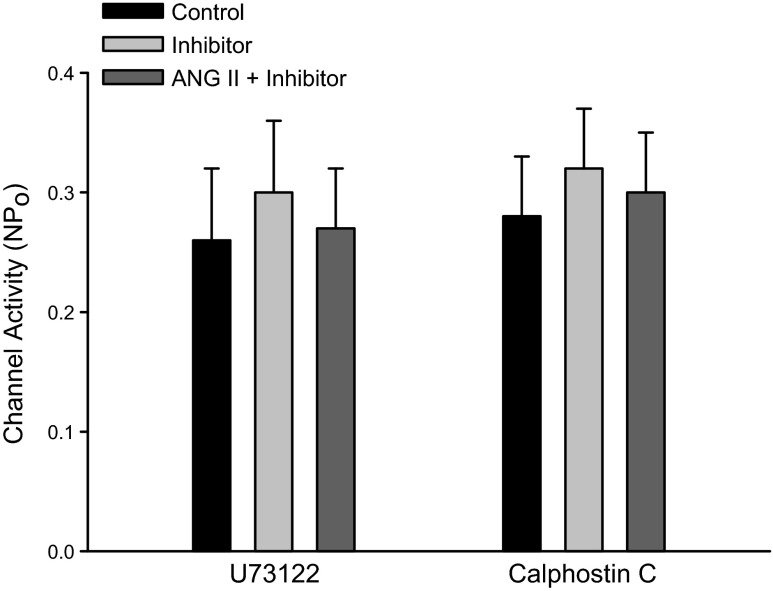
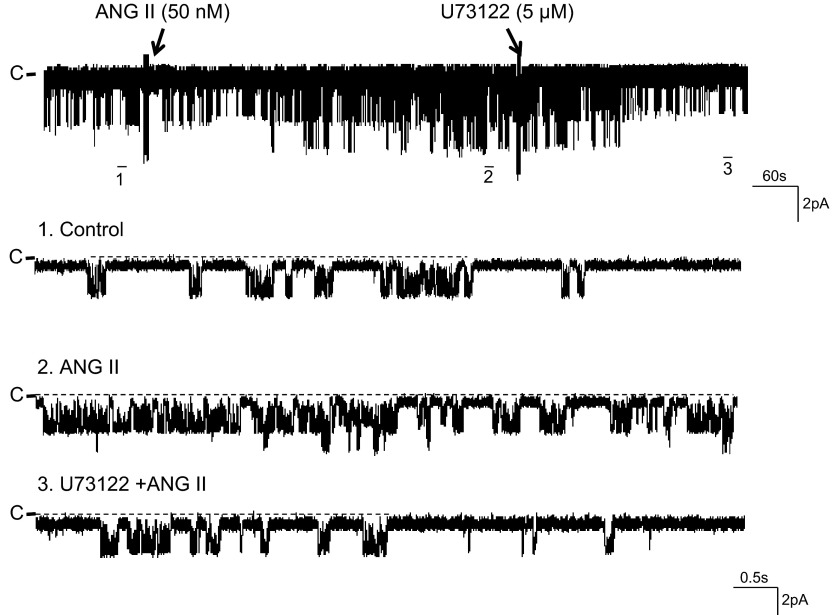
The stimulation of AT1R has been shown to augment NKCC2 activity by phospholipase C (PLC)- and protein kinase C (PKC)-dependent pathways in the TAL (1). Hence, we examined the effect of ANG II (50 nM) on the basolateral 50-pS K channel in the TAL pretreated with U73122 (5 μM), an inhibitor of PLC. Data summarized in Fig. 4 show that U73122 had no significant effect on the K channel activity (control 0.26 ± 0.04 and U73122 0.3 ± 0.06, n = 5). However, the inhibition of PLC abolished the effect of ANG II on the 50-pS K channel (NPo 0.27 ± 0.03, n = 5). In addition, inhibition of PLC also reversed the ANG II-induced stimulation of the 50-pS K channel. Figure 5 is a representative recording showing that adding 50 nM ANG II increased channel activity from 0.23 ± 0.05 to 0.56 ± 0.09 (n = 6). But, in the presence of ANG II, adding U73122 reversed the stimulatory effect of ANG II and reduced NPo to 0.2 ± 0.05 (n = 6). We next examined the effect of ANG II on the K channel in the TAL treated with calphostin C, an inhibitor of PKC (Fig. 4). Although calphostin C (100 nM) had no significant effect on the 50-pS K channel activity, it abrogated ANG II-induced stimulation of K channels (control 0.28 ± 0.05; calphostin C 0.32 ± 0.03; ANG II+calphostin C 0.3 ± 0.05; n = 7). Thus, our data strongly suggest that the effect of ANG II on 50-pS K channels was mediated by PLC- and PKC-dependent mechanisms.
Fig. 4.
Inhibition of phospholipase C (PLC) and protein kinase C (PKC) abrogates the effect of ANG II. A bar graph summarizes the experiments in which the effect of ANG II (50 nM) on the 50-pS K channels in the TAL was examined in the presence or in the absence of 5 μM U73122 or 100 nM calphostin C. All experiments were performed in cell-attached patches at 0 mV and the channel activity was determined at the steady state of each treatment.
Fig. 5.
Inhibition of PLC reversed the stimulatory effect of ANG II on the K channels. A channel recording showing that adding U73122 reversed ANG II (50 nM)-induced stimulation of the basolateral 50-pS K channel activity in the TAL. The experiment was performed in a cell-attached patch with 140 mM KCl in the pipette and 140 mM NaCl/5 mM KCl in the bath. Top trace shows the time course of the experiment. Three parts of the trace, indicated by numbers, are extended to show the fast time resolution. The holding potential was 0 mV, and the channel closed current is indicated by “C.”
The effect of PKC on the basolateral 50-pS K channel in the TAL was complex. A previous study demonstrated that PGE2 inhibited the 50-pS K channel by activating PKC which further stimulated mitogen-activated protein kinase (10). The present study demonstrated that inhibition of PKC also abrogated the stimulatory effect of ANG II on the 50-pS K channel in the TAL. Therefore, we speculate that ANG II and PGE2 may activate two different PKC isoforms that might mediate the stimulatory effect and the inhibitory effect on the 50-pS K channel, respectively. Alternatively, PGE2-PKC and ANG II-PKC pathways may be in two separate compartments such that PKC mediates both effects. Activation of PKC has been shown to stimulate SFK (3, 4). Our recent study demonstrated that SFK phosphorylated Kir.4.1 and activated the Kir.4.1 activity (36). Since Kir.4.1 is also expressed in the basolateral membrane of the TAL and is likely an important component of the basolateral K channel in the TAL (19), it is conceivable that ANG II might activate the 50-pS K channel in the TAL by a PKC-SFK-dependent mechanism. Thus, we examined the effect of ANG II on tyrosine phosphorylation of c-Src at Tyr416, which is known as an indication of c-Src activation (17). We isolated medullary TALs and the tubules were treated with ANG II (50 nM) for 5, 15, and 30 min, respectively. Figure 6A is a typical Western blot showing that incubation of the medullary TAL with 50 nM ANG II for 5, 15, and 30 min augmented tyrosine phosphorylation of c-Src by 90 ± 20, 60 ± 15, and 40 ± 10%, respectively (n = 3). We next examined the dose-response curve of ANG II effect on c-Src phosphorylation at Tyr416 by treating the TAL with 50, 75, and 100 nM ANG II for 5 min. As shown in Fig. 6B, ANG II treatment significantly increased the tyrosine phosphorylation by 90 ± 20% (50 nM), 115 ± 25% (75 nM), and 120 ± 25% (100 nM), respectively (n = 3).
Fig. 6.
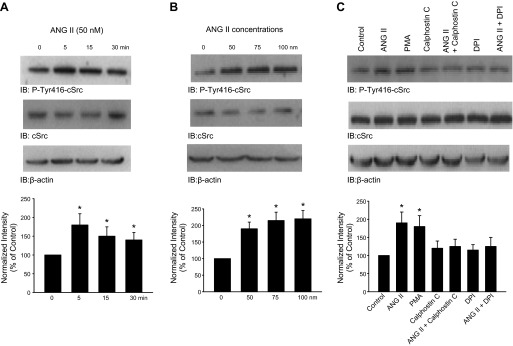
ANG II stimulates c-Src phosphorylation at Tyr416. A: Western blotting shows the effect of ANG II on the phosphorylation of c-Src at Tyr416 (detected with P-Tyr416-cSrc antibody). The isolated medullary TALs were incubated with 50 nM ANG II for 5, 15, and 30 min, respectively. The tubules were then lysed for harvesting proteins after ANG II treatment. Results were summarized in a bar graph (bottom). *Significant difference compared with the control (untreated group). B: Western blot showing the effect of ANG II treatment (5 min) on Tyr416 phosphorylation at 50, 75, and 100 nM, respectively. A bar graph (bottom) summarizes. C: Western blot demonstrating the effect of ANG II on Tyr416 phosphorylation in the presence of 10 μM phorbol-12-myristate-13-acetate (PMA), 100 nM calphostin C, or 10 μM diphenyleneiodonium sulfate (DPI). The expression of total c-Src and actin (load control) is shown in the bottom 2 lanes. The isolated TALs were treated with 50 nM ANG II and corresponding inhibitors for 5 min. A bar graph summarizes the results from 3 similar experiments (bottom). *Significant difference compared with the control value (no ANG II). We normalized the band intensity by comparing the total c-Src expression with actin level and then calculated the ratio between the phosphorylated c-Src and total c-Src.
Our previous study demonstrated that ANG II stimulated the NOX activity by a PKC-dependent mechanism that increased the phosphorylation of P47phox (34). To examine the role of superoxide anions in mediating the effect of ANG II on the 50-pS K channel in the TAL, we tested the effect of ANG II on the 50-pS K channel in the TAL treated with 10 μM tiron, a free radicle scavenger. As shown in Fig. 7, A and B, tiron treatment had no significant effect on the K channel activity under control conditions but it abolished the effect of ANG II on the 50-pS K channel in the TAL (control NPo 0.27 ± 0.06; tiron 0.26 ± 0.06; and ANG II+tiron 0.34 ± 0.07; n = 7). Because increasing superoxide anions may enhance SFK activity through inhibiting protein tyrosine phosphatase (2), we next examined whether ANG II-induced increase in c-Src phosphorylation at Tyr416 was due to stimulation of PKC and NOX. Thus, we examined the effect of ANG II on c-Src phosphorylation in the TAL cotreated with PMA, calphostin C, or DPI, an inhibitor of NOX. Figure 6C is a typical Western blot demonstrating that the inhibition of PKC or NOX abolished the effect of ANG II, on c-Src tyrosine phosphorylation at Tyr416, while PMA mimicked the effect of ANG II. The results from three experiments are summarized in Fig. 6C, bottom showing that 50 nM ANG II stimulated c-Src phosphorylation at Tyr416 by 90 ± 18% but failed to increase the phosphorylation of c-Src in the TAL treated with 100 nM calphostin C (108 ± 15% of the control) or with 10 μM DPI (115 ± 20%) of the control. Moreover, application of PMA (10 μM) to stimulate PKC also increased c-Src phosphorylation by 75 ± 20% (n = 3).
Fig. 7.
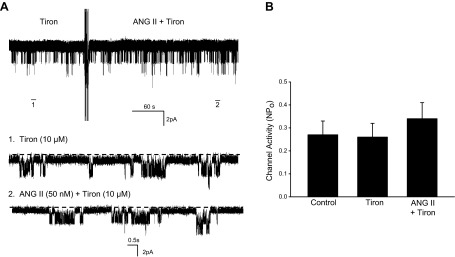
Superoxide anions are required for the effect of ANG II on the K channel. A: channel recording showing the effect of 50 nM ANG II on the 50-pS K channels in the TAL treated with 10 μM sodium 4,5-dihydroxybenzene-1,3-disulfonate (tiron). The experiments were performed in a cell-attached patch and the holding potential was 0 mV. Top trace is a time course of the experiment. Two parts of the trace, indicated by numbers, are extended to show the fast time resolution. B: bar graph summarizes the results from 6 experiments.
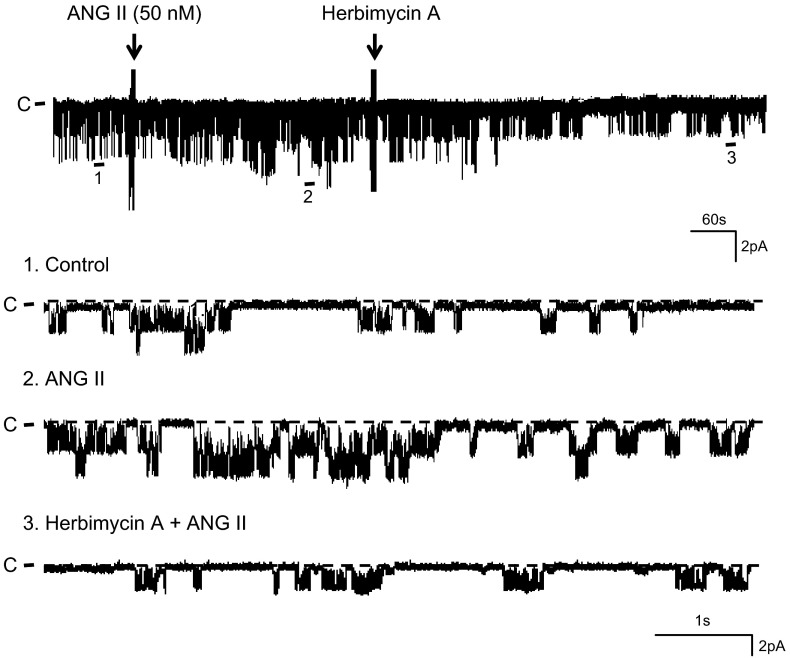
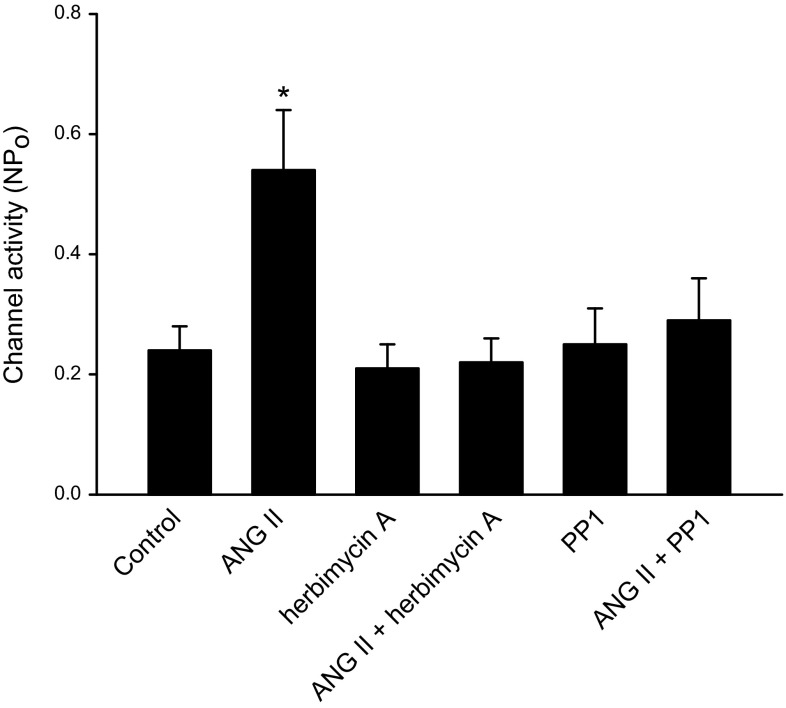
After demonstrating that ANG II activated SFK, we examined the role of SFK in mediating the effect of ANG II on the 50-pS K channel in the TAL. Figure 8 is a representative recording showing that addition of 1 μM herbimycin A abolished the stimulatory effect of ANG II. From inspection of Fig. 8, it is apparent that ANG II increased the basolateral 50-pS K channel activity. However, application of herbimycin A reversed the stimulatory effect of ANG II and reduced the channel activity (control 0.24 ± 0.05; ANG II 0.51 ± 0.09; ANG II+herbimycin A 0.24 ± 0.07, n = 4; Fig. 9). Since herbimycin A has been reported to have effects other than inhibiting SFK (27), we repeated the experiments with PP1, a specific SFK inhibitor that also has a different chemical structure from herbimycin A to avoid the off-target effect of herbimycin A. With PP1, we observed the same results in the experiments in which the effect of ANG II on the 50-pS K channel was examined in the TAL treated with PP1 (1 μM), a SFK inhibitor. Results are summarized in Fig. 9 showing that although PP1 had no significant effect on the channel activity (PP1 0.26 ± 0.1), inhibition of SFK abolished the effect of ANG II on the K channel activity in the TAL (ANG II+PP1 0.32 ± 0.12, n = 6). This strongly suggested that the stimulatory effect of ANG II was mediated by a SFK-dependent mechanism.
Fig. 8.
Herbimycin A reversed the stimulatory effect of ANG II on the K channels. A channel recording showing the effect of 1 μM herbimycin A on the 50-pS K channels in the TAL in the presence ANG II (50 nM). The experiments were performed in a cell-attached patch and the holding potential was 0 mV. Top trace is a time course of the experiment. Three parts of the trace, indicated by numbers, are extended to show the fast time resolution.
Fig. 9.
Inhibition of Src-family protein tyrosine kinase (SFK) abolished the effect of ANG II on the 50-pS K channel. A bar graph summarizes the experiments in which the effect of ANG II (50 nM) on the 50-pS K channels in the TAL was examined in the presence or in the absence of 1 μM herbimycin A or 4-amino-5-(4-methyphenyl)-7-(t-buty)-pyrazolo[3,4-d]-pyrimidine (PP1). All experiments were performed in cell-attached patches. *Significant difference from the rest of the groups.
DISCUSSION
The main finding of the present study is that ANG II stimulates the basolateral 50-pS K channel in the TAL. Moreover, the observation that the stimulatory effect of ANG II on the K channels was absent in the TAL treated with losartan indicated that the effect of ANG II was mediated by stimulation of AT1R. A large body of evidence suggests that ANG II plays an important role in the regulation of electrolyte transport in the kidney. It has been reported that ANG II increases Na and HCO3− reabsorption in both proximal and distal tubules (7, 33). Also, ANG II stimulated NKCC2 activity in the TAL (31) and NCC function in the distal convoluted tubule (5, 26). We and others found that ANG II stimulated the epithelial sodium channel in the cortical collecting duct via an aldosterone-independent mechanism (24, 32). Recently, we demonstrated that ANG II stimulates the 10-pS Cl channels in the basolateral membrane of the TAL (35). Since stimulation of the basolateral K channel by ANG II is expected to increase the driving force of Cl exit across the basolateral membrane, the results strongly suggest that ANG II should enhance Cl absorption in the TAL.
Two lines of evidence suggest that the stimulatory effect of ANG II on the 50-pS K channels is due to the activation of SFK: 1) inhibition of SFK abolished the stimulatory effect of ANG II; 2) the incubation of the TAL with ANG II increased the phosphorylation of c-Src at Tyr416. ANG II has been shown to increase SFK activity by stimulating NOX activity and increasing superoxide anions generation in vascular tissue (22, 30). A recent study indicates that ANG II stimulated superoxide production via PKC-α-dependent pathway that phosphorylated NOX (20). The observation that inhibition of PKC also abrogated the ANG II-induced stimulation of the basolateral 50-pS K channels supports the role of PKC in mediating the effect of ANG II. Because PKC was involved in mediating the inhibitory effect of PGE2 on the 50-pS K channel (10) and the stimulatory effect of ANG II, we speculate that either two types of PKC isoforms or the PKC in two distinctive compartments are involved in regulating the effect of PGE2 and ANG II. Moreover, the stimulatory effect of PKC on the basolateral K channel following ANG II application was the result of the activation of SFK because inhibition of PKC abolished the stimulatory effect of ANG II on the K channel and on the c-Src tyrosine phosphorylation. The mechanism by which PKC or ANG II activates SFK is not clear. However, it is possible that ANG II-induced stimulation of SFK was the result of PKC-induced stimulation of NOX. First, inhibition of PKC and NOX abolished the effect of ANG II on the c-Src phosphorylation. Second, a previous study showed that stimulation of PKC mimicked the effect of ANG II and increased NOX phosphorylation in the TAL (35). Our finding that ANG II stimulates basolateral K and Cl channels through stimulating NOX activity is consistent with the report that superoxide stimulates NaCl absorption in the TAL (15).
ANG II-induced stimulation of the basolateral 50-pS K channel activity in the TAL is expected to play an important role in mediating electrolyte transport in the distal nephrons. Basolateral K channels are responsible for generating the cell membrane potential, which is essential for Cl diffusion across the basolateral membrane in the TAL. Hence, stimulation of the basolateral K channels increases while inhibition of the basolateral K channels decreases the electrochemical gradient for Cl exit. The inhibition of Cl exit across the basolateral membrane is expected to raise the intracellular Cl concentration which in turn inhibits the apical NKCC2 in the TAL (26). Hence, not only affecting Cl transport in the TAL, the inhibition of basolateral K channels should also increase Na delivery to the distal nephron. This stimulates Na reabsorption in the expanse of K in the connecting tubule thereby causing K wasting. The role of basolateral K channel in regulating renal epithelial transport is demonstrated by the observation that loss-of-function mutation of KCNJ10, a main component of the basolateral K channel in the TAL and distal nephrons, caused an autosomal recessive syndrome featuring seizures, sensorineural deafness, ataxia, mental retardation, and electrolyte imbalance (SeSAME) (25, 28). Thus, our finding supports the role of ANG II in regulating the membrane transport in the TAL and the distal nephrons via an aldosterone-independent mechanism. We conclude that ANG II stimulates the basolateral 50-pS K channels by activating AT1R and that stimulation of AT1R augments SFK activity by a PKC- and NOX-dependent mechanism.
GRANTS
The work is supported by Chinese National Natural Science Foundation no. 31171110 (to R. M. Gu), no. 31071017 (to R. M. Gu), Guizhou Science and Technology Joint Fund LKZ[2013]06 (to M. Wang), and National Institutes of Health Grants HL34100 (to W. H. Wang) and DK 54983 (to W. H. Wang).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.W., H.L., P.W., L.F., L.W., X.D., and D.Z. performed experiments; M.W., H.L., P.W., L.W., and R.G. analyzed data; M.W., P.W., W.-H.W., and R.G. prepared figures; M.W., W.-H.W., and R.G. drafted manuscript; M.W., H.L., P.W., L.F., L.W., X.D., D.Z., W.-H.W., and R.G. approved final version of manuscript; W.-H.W. and R.G. conception and design of research; W.-H.W. and R.G. interpreted results of experiments; W.-H.W. and R.G. edited and revised manuscript.
REFERENCES
- 1.Amlal H, LeGoff C, Vernimmen C, Soleimani M, Paillard M, Bichara M. ANG II controls Na+-K+(NH4+)-2Cl− cotransport via 20-HETE and PKC in medullary thick ascending limb. Am J Physiol Cell Physiol 274: C1047–C1056, 1998 [DOI] [PubMed] [Google Scholar]
- 2.Bedard K, Krause KH. The Nox family of ROS-generating NADPH oxidase: physiology and pathophysiology. Physiol Rev 87: 245–313, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Brandt DT, Goerke A, Heuer M, Gimona M, Leitges M, Kremmer E, Lammers R, Hallaer H, Mischak H. Protein kinase Cd induces Src kinase activatity via activation of the protein tyrosine phosphatase PTPa. J Biol Chem 278: 34073–34078, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Brandt D, Gimona M, Hillmann M, Haller H, Mischak H. Protein kinase C induces actin reorganization via a Src- and Rho-dependent pathway. J Biol Chem 277: 20903–20910, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Castaeda-Bueno MA, Cervantes-Prez LG, Vízquez N, Uribe N, Kantesaria S, Morla L, Bobadilla NA, Doucet A, Alessi DR, Gamba G. Activation of the renal Na+:Cl cotransporter by angiotensin II is a WNK4-dependent process. Proc Natl Acad Sci USA 109: 7929–7934, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gamba G. Molecular physiology and pathophysiology of electroneutral cation-chloride cotransporters. Physiol Rev 85: 423–493, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Geibel J, Giebisch G, Boron WF. Angiotensin II stimulates both Na+-H+ exchange and Na+/HCO3− cotransport in the rabbit proximal tubule. Proc Natl Acad Sci USA 87: 7917–7920, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gimenez I, Isenring P, Forbush B. Spatially distributed alternative splice variants of the renal Na-K-Cl cotransporter exhibit dramatically different affinities for the transported ions. J Biol Chem 277: 8767–8770, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Gu RM, Wang WH. Arachidonic acid inhibits K channels in basolateral membrane of the thick ascending limb. Am J Physiol Renal Physiol 283: F407–F414, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Gu R, Jin Y, Zhai Y, Yang L, Zhang C, Li W, Wang L, Kong S, Zhang Y, Yang B, Wang WH. PGE2 inhibits basolateral 50 pS potassium channels in the thick ascending limb of the rat kidney. Kidney Int 74: 478–485, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hebert SC, Andreoli TE. Control of NaCl transport in the thick ascending limb. Am J Physiol Renal Fluid Electrolyte Physiol 246: F745–F756, 1984 [DOI] [PubMed] [Google Scholar]
- 12.Hebert SC, Desir G, Giebisch G, Wang W. Molecular diversity and regulation of renal potassium channels. Physiol Rev 85: 319–371, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hebert SC, Friedman PA, Culpepper RM, Andreoli TE. Salt absorption in the thick ascending limb of Henle's loop: NaCl cotransport mechanism. Semin Nephrol 2: 316–327, 1982 [Google Scholar]
- 14.Hurst AM, Duplain M, Lapointe JY. Basolateral membrane potassium channels in rabbit cortical thick ascending limb. Am J Physiol Renal Fluid Electrolyte Physiol 263: F262–F267, 1992 [DOI] [PubMed] [Google Scholar]
- 15.Juncos R, Garvin JL. Superoxide enhances Na-K-2Cl cotransporter activity in the thick ascending limb. Am J Physiol Renal Physiol 288: F982–F987, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Jung J, Basile DP, Pratt JH. Sodium reabsorption in the thick ascending limb in relation to blood pressure: a clinical perspective. Hypertension 57: 873–879, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kmiecik TE, Shalloway D. Activation and suppression of pp60c-src transforming ability by mutation of its primary sites of tyrosine phosphorylation. Cell 49: 65–73, 1987 [DOI] [PubMed] [Google Scholar]
- 18.Kong S, Zhang C, Li W, Wang L, Luan H, Wang WH, Gu R. Stimulation of Ca2+-sensing receptor inhibits the basolateral 50-pS K channels in the thick ascending limb of rat kidney. Biochim Biophys Acta 1823: 273–281, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lachheb S, Cluzeaud F, Bens M, Genete M, Hibino H, Lourdel S, Kurachi Y, Vandewalle A, Teulon J, Paulais M. Kir4.1/Kir5.1 channel forms the major K+ channel in the basolateral membrane of mouse renal collecting duct principal cells. Am J Physiol Renal Physiol 294: F1398–F1407, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Massey KJ, Hong NJ, Garvin JL. Angiotensin II stimulates superoxide production in the thick ascending limb by activating NOX4. Am J Physiol Cell Physiol 303: C781–C789, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mujais SK, Kauffman S, Katz AI. Angiotensin II binding sites in individual segments of the rat nephron. J Clin Invest 77: 315–318, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ohtsu H, Suzuki H, Nakashima H, Dhobale S, Frank GD, Motley ED, Eguchi S. Angiotensin II signal transduction through small GTP-binding proteins: mechanism and significance in vascular smooth muscle cells. Hypertension 48: 534–540, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Paulais M, Lourdel S, Teulon J. Properties of an inwardly rectifying K+ channel in the basolateral membrane of mouse TAL. Am J Physiol Renal Physiol 282: F866–F876, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Peti-Peterdi J, Warnock DG, Bell PD. Angiotensin II directly stimulates ENaC activity in the cortical collecting duct via AT1 receptors. J Am Soc Nephrol 13: 1131–1135, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Reichold M, Zdebik AA, Lieberer E, Rapedius M, Schmidt K, Bandulik S, Sterner C, Tegtmeier I, Penton D, Baukrowitz T, Hulton SA, Witzgall R, Ben Zeev B, Howie AJ, Kleta R, Bockenhauer D, Warth R. KCNJ10 gene mutations causing EAST syndrome (epilepsy, ataxia, sensorineural deafness, and tubulopathy) disrupt channel function. Proc Natl Acad Sci USA 107: 14490–14495, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.San Cristobal P, Pacheco-Alvarez D, Richardson C, Ring AM, Vazquez N, Rafiqi FH, Chari D, Kahle KT, Leng Q, Bobadilla NA, Hebert SC, Alessi DR, Lifton RP, Gamba G. Angiotensin II signaling increases activity of the renal Na-Cl cotransporter through a WNK4-SPAK-dependent pathway. Proc Natl Acad Sci USA 106: 4384–4389, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Satoh T, Uehara Y, Kaziro Y. Inhibition of interleukin 3 and granulocytes-macrophage colony-stimulating factor stimulated increase of active Ras-GTP by herbimycin A, a specific inhibitor of tyrosine kinases. J Biol Chem 267: 2537–2541, 1992 [PubMed] [Google Scholar]
- 28.Scholl UI, Choi M, Liu T, Ramaekers VT, Hausler MG, Grimmer J, Tobe SW, Farhi A, Nelson-Williams C, Lifton RP. Seizures, sensorineural deafness, ataxia, mental retardation, and electrolyte imbalance (SeSAME syndrome) caused by mutations in KCNJ10. Proc Natl Acad Sci USA 106: 5842–5847, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schultz SG. Homocellular regulatory mechanisms in sodium-transporting epithelia: avoidance of extinction by “flush-through”. Am J Physiol Renal Fluid Electrolyte Physiol 241: F579–F590, 1981 [DOI] [PubMed] [Google Scholar]
- 30.Seshiah PN, Weber DS, Rocic P, Valppu L, Taniyama Y, Griendling KK. Angiotensin II stimulation of NAD(P)H oxidase activity: upstream mediators. Circ Res 91: 406–413, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Silva GB, Garvin JL. Angiotensin II dependent hypertension increases Na transport-related oxygen consumption by the thick ascending limb. Hypertension 52: 1091–1098, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun P, Yue P, Wang WH. Angiotensin II stimulates epithelial sodium channels in the cortical collecting duct of the rat kidney. Am J Physiol Renal Physiol 302: F679–F687, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang T, Giebisch G. Effects of angiotensin II on electrolyte transport in the early and late distal tubule in rat kidney. Am J Physiol Renal Fluid Electrolyte Physiol 271: F143–F149, 1996 [DOI] [PubMed] [Google Scholar]
- 34.Wang W, Hebert SC, Giebisch G. Renal K+ channels: structure and function. Annu Rev Physiol 59: 413–436, 1997 [DOI] [PubMed] [Google Scholar]
- 35.Wu P, Wang M, Luan H, Li L, Wang L, Wang WH, Gu R. Angiotensin II stimulates basolateral 10-pS Cl channels in the thick ascending limb. Hypertension 61: 1211–1217, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang C, Wang L, Thomas S, Wang K, Lin DH, Rinehart J, Wang WH. Src-family protein tyrosine kinase regulates the basolateral K channel in the distal convoluted tubule (DCT) by phosphorylation of KCNJ10. J Biol Chem 288: 26135–26146, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]