Abstract
Epithelial Na+ channel (ENaC) subunits (α, β, and γ) found in functional complexes are translated from mature mRNAs that are similarly processed by the inclusion of 13 canonical exons. We examined whether individual exons 3–12, encoding the large extracellular domain, are required for functional channel expression. Human ENaCs with an in-frame deletion of a single α-subunit exon were expressed in Xenopus oocytes, and their functional properties were examined by two-electrode voltage clamp. With the exception of exon 11, deletion of an individual exon eliminated channel activity. Channels lacking α-subunit exon 11 were hyperactive. Oocytes expressing this mutant exhibited fourfold greater amiloride-sensitive whole cell currents than cells expressing wild-type channels. A parallel fivefold increase in channel open probability was observed with channels lacking α-subunit exon 11. These mutant channels also exhibited a lost of Na+ self-inhibition, whereas we found similar levels of surface expression of mutant and wild-type channels. In contrast, in-frame deletions of exon 11 from either the β- or γ-subunit led to a significant loss of channel activity, in association with a marked decrease in surface expression. Our results suggest that exon 11 within the three human ENaC genes encodes structurally homologous yet functionally diverse domains and that exon 11 in the α-subunit encodes a module that regulates channel gating.
Keywords: epithelial Na+ channel, exon, domain, open probability, self-inhibition
epithelial na+ channels (ENaCs) mediate Na+ entry into epithelial cells and play a critical role in the regulation of extracellular fluid volume and blood pressure (42). Mutations in human ENaC genes cause Mendelian disorders such as Liddle syndrome and pseudohypoaldosteronism type I (39). Enhanced ENaC activity is thought to increase the risk of developing salt-sensitive disorders, including essential hypertension, and contributes to the pathophysiological changes in cystic fibrosis (7, 10, 34, 38, 50).
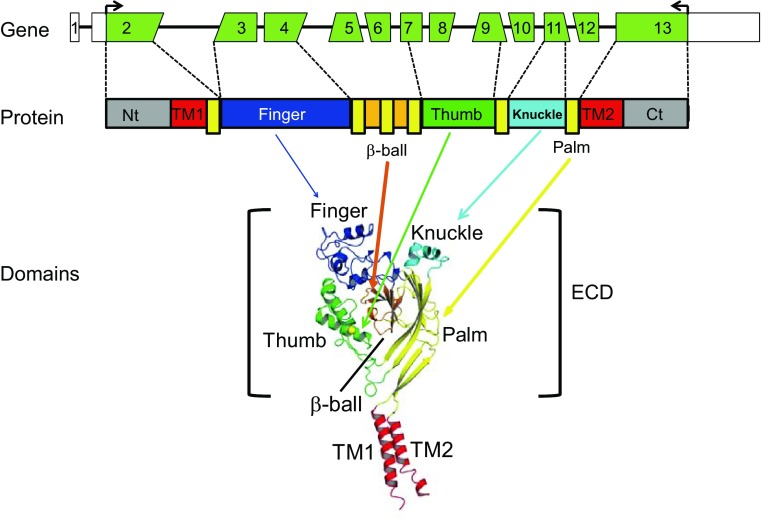
There are four human genes (gene symbols: SCNN1A, SCNN1B, SCNN1D, and SCNN1G) that encode four ENaC subunits (α, β, δ, and γ, respectively). In high-resistance epithelia, functional ENaC complexes contain α-, β-, and γ-subunits. Human SCNN1A is located on chromosome 12, and both SCNN1B and SCNN1G are on chromosome 16. Each gene has a similar architecture with 13 exons and 12 introns, spanning 30.5 kb (SCNN1A), 79.0 kb (SCNN1B), and 34.2 kb (SCNN1G). Common isoforms of all three human ENaC subunits (α, β, and γ) are translated from mature mRNAs that are similarly spliced by inclusion of all 13 constitutive exons. Transmembrane domains 1 and 2 as well as NH2- and COOH-terminal cytoplasmic domains of all three subunits are encoded by exons 2 and 13. Exons 3–12 encode most of the large extracellular domain (ECD; Fig. 1A). It is not known if all of the exons encoding the ECD are required for channel function or whether they impart specific functional roles.
Fig. 1.
Gene structure, protein, and domain model of a human epithelial Na+ channel (ENaC) subunit. Top: exons of a human (h)ENaC gene. Angled edges indicate the codon split between two exons. Middle: linear protein model of an ENaC subunit with colored domains. Elongated yellow boxes show palm domain β-strands interspersed with other domains. Bottom: a structural model of an ENaC-homologous acid-sensing ion channel (ASIC)1 subunit (22), showing the likely domain organization of the extracellular domain (ECD; bracket) and transmembrane domains (TM1 and TM2) of an ENaC subunit. NH2- and COOH-termini (Nt and Ct, respectively) are not shown.
A delineation of exon-specific functions within ENaC genes should aid in the understanding of biological roles of alternatively spliced transcripts of human ENaC genes. Indeed, both human SCNN1A and SCNN1B generate multiple transcripts through alternative splicing or heterogeneous transcription initiation (5, 16, 37, 53, 54). Certain alternative transcripts of SCNN1A and SCNN1B lack one or more coding exons. Like other genes, these alternative transcripts of ENaCs may have important roles in the regulation of transcription, translation, posttranslational modification, trafficking, and channel activity. Furthermore, aberrant splicing induced by mutations in ENaC genes has been associated with pseudohypoaldosteronism type I (1, 52, 55).
The resolved structure of acid-sensing ion channel (ASIC)1, a member of ENaC/degenerin family, revealed a highly organized ECD structure consisting of five distinct domains termed palm, β-ball, finger, thumb, and knuckle (26). Previous studies from our group and others (22, 26, 29, 30, 51) have suggested that the ECDs of ASIC1 and ENaC have similar structural features. The central core of the extracellular region, composed of the palm and β-ball domains, is formed by noncontiguous β-strands that arise from multiple exons (Fig. 1). In contrast, the knuckle, finger, and thumb domains are formed by contiguous α-helices and connecting loops. While the knuckle domain is largely encoded by a single exon (exon 11), the finger (exons 3 and 4) and thumb (exons 7–9) domains are encoded by multiple exons. Previous studies (13–15, 17, 18, 29–31, 44–46, 48) have suggested that the ECDs of ENaC subunits harbor distinct sensors for various extracellular regulators, including ions (Na+, H+, Ni2+, Zn2+, Cu2+, and Cl−) and shear stress. To begin to address the question of whether ENaC ECDs are formed of distinct functional modules encoded by specific exons, we generated and expressed ENaCs lacking individual ECD-coding exons in Xenopus oocytes. Whereas most mutants, when coexpressed with wild-type (WT) β- and γ-subunits, did not result in the expression of functional channels, we found that channels lacking α-subunit exon 11 exhibited increased activity due to an increase in channel open probability (Po).
MATERIALS AND METHODS
Site-directed mutagenesis.
Point mutations were introduced into human α-, β-, and γ-ENaC subunit cDNAs using the QuickChange II XL Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA). Target mutations were verified by DNA sequencing. WT and mutant ENaC cRNAs were synthesized by in vitro transcription using T7 or SP6 RNA polymerase (Ambion), purified by an RNA purification kit (Qiagen), and quantified by spectrophotometry.
ENaC expression and two-electrode voltage clamp.
ENaC expression in Xenopus oocytes and current measurements by two-electrode voltage clamp were performed as previously described (14). Stage V and VI oocytes with the follicle cell layer removed were injected with 3 ng of each subunit (α, β, and γ) cRNA in a volume of 50 nl/cell and incubated at 18°C in modified Barth's solution [MBS; containing 88 mM NaCl, 1 mM KCl, 2.4 mM NaHCO3, 15 mM HEPES, 0.3 mM Ca(NO3)2, 0.41 mM CaCl2, 0.82 mM MgSO4, 10 μg/ml streptomycin sulfate, and 100 μg/ml gentamycin sulfate; pH 7.4]. All experiments were performed at room temperature (20–24°C) 20–52 h after injection. Oocytes were placed in a recording chamber from Warner Instruments (Hamden, CT) and perfused at a constant flow rate of 12–15 ml/min. The perfusion solution contained 110 mM NaCl, 2 mM KCl, 2 mM CaCl2, and 10 mM HEPES and had the pH adjusted at 7.4. Voltage clamp was performed using an Axoclamp 900A Computer-Controlled Microelectrode Amplifier and DigiData 1440A interface controlled by pCLAMP 9.2 (Molecular Devices, Sunnyvale, CA). The protocol for harvesting oocytes from Xenopus laevis was approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh.
Surface expression.
Surface expression of ENaCs in oocytes was examined using human ENaC β- or γ-subunits with an extracellular FLAG epitope tag, as previously described (12). Briefly, oocytes were injected with 3 ng/subunit of three human ENaC cRNAs with either the β- or γ-subunit containing an extracellular FLAG epitope tag (DYKDDDDK). The FLAG tag was placed immediately after residue T137 in the β-subunit, as previously described (20), and residue L128 in the γ-subunit. The γ-subunit epitope tag was placed before the furin cleavage site at R138 to avoid interfering with cleavage at the furin site. Expression of channels with either a FLAG-tagged β- or γ-subunit did not impair channel activity. Two days after cRNA injection, a surface expression assay was performed on ice except for the last step, which was at room temperature (20–24°C). After a 30-min incubation in MBS (without antibiotics) supplemented with 1% BSA (MBS-BSA), oocytes were incubated for 1 h with MBS-BSA supplemented with 1 μg/ml of a mouse monoclonal anti-FLAG antibody (M2, Sigma). Oocytes were then washed in MBS-BSA and incubated with MBS-BSA supplemented with 1 μg/ml of a horseradish peroxidase-coupled secondary antibody [peroxidase-conjugated AffiniPure F(ab′)2 fragment goat anti-mouse IgG, Jackson ImmunoResearch, West Grove, PA] for 1 h. Cells were washed and transferred to MBS without BSA. Individual oocytes were placed in a 96-well plate, and 100 μl of SuperSignal ELISA Femto Maximum Sensitivity Substrates (Thermo Scientific, Rockford, IL) were added to each well. Cells were then incubated at room temperature for 1 min, and chemiluminescence in relative light units was quantified in a GloMax-Multi+ Detection System (Promega, Madison, WI).
Na+ self-inhibition.
Na+ self-inhibition was examined by rapidly replacing low Na+ concentration bath solution (NaCl-1; containing 1 mM NaCl, 109 mM N-methyl-d-glucamine, 2 mM KCl, 2 mM CaCl2, and 10 mM HEPES; pH 7.4) with high Na+ concentration bath solution (NaCl-110; containing 110 mM NaCl, 2 mM KCl, 2 mM CaCl2, and 10 mM HEPES; pH 7.4) while the oocytes were continuously clamped to −100 mV (membrane potential). Bath solution exchange was done with a Teflon valve computer-controlled perfusion system (AutoMate Scientific, Berkeley, CA). Upon completion of the experiment, 10 μM amiloride was added to the bath solution to determine the amiloride-insensitive portion of the whole cell current. Given the variability in the Na+ self-inhibition response of WT ENaCs among different batches of oocytes (43), the response of WT channels was always tested in an alternating manner with mutants in the same batch of oocytes.
Single channel recordings.
Oocytes were placed in a hypertonic solution (NaCl-110 supplemented with 200 mM sucrose) for 5 min. Vitelline membranes were manually removed, and oocytes were placed in a recording chamber with NaCl-110 at room temperature (22–25°C) for 15 min before recordings were initiated. The pipette solution was the same as bath solution (NaCl-110). Patch pipettes with a tip resistance of 5–10 MΩ were used. Patch clamp in a cell-attached configuration was performed using a PC-One Patch Clamp amplifier (Dagan) and a DigiData 1322A interface connected to a PC. Patches were clamped at a membrane potential (negative value of the pipette potential) of −100 mV. pCLAMP versions 8 and 10 (Molecular Devices) were used for data acquisition and analyses, respectively. Single channel recordings were acquired at 5 kHz, filtered at 1 kHz by a built-in 4-pole low-pass Bessel Filter, and stored on a hard drive. Po was estimated by single channel search function of pCLAMP 10.3 from recordings that contained no more than four current levels (3 channels) and lasted for at least 5 min.
Statistical analyses.
Data are presented as means ± SE. Significance comparisons between groups were performed with a Student's t-test. P values of <0.05 were considered significantly different.
RESULTS
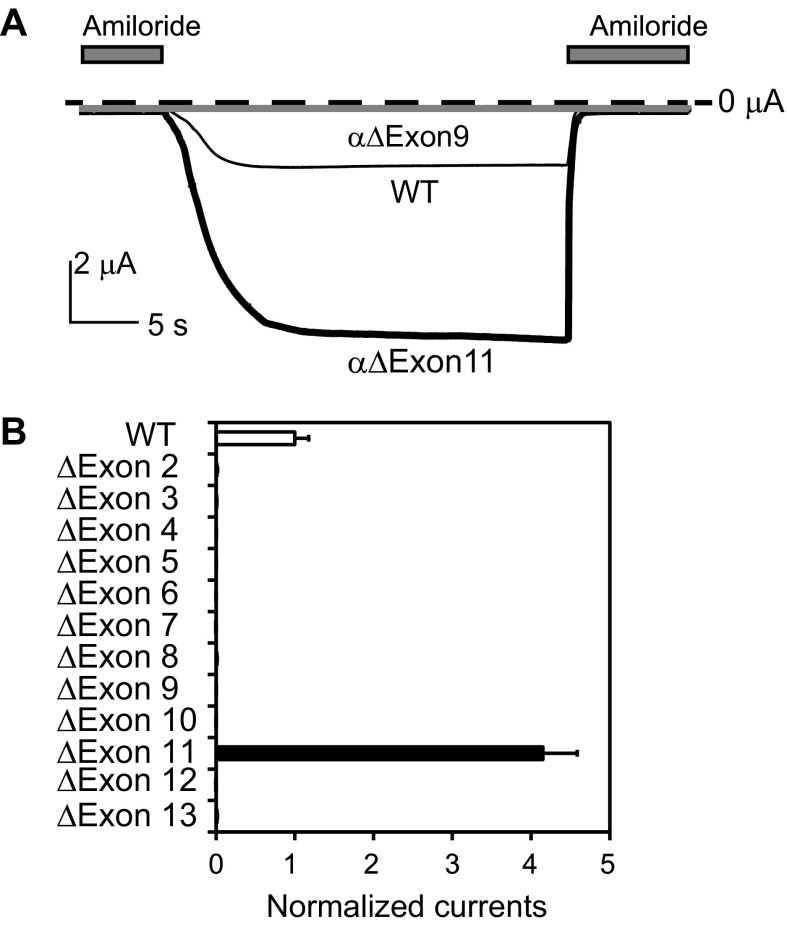
We examined whether all of the coding exons (exons 2–13) in human SCNN1A were required for the expression of function channels. α-Subunit cDNAs were modified with in-frame deletions of individual exons and coexpressed with WT β- and γ-subunits in Xenopus oocytes. In-frame deletions were performed to prevent frame shifts leading to a change in the coding sequence and premature termination. While oocytes expressing WT channels had robust amiloride-sensitive currents (2.1 ± 0.4 μA, n = 23, at −100 mV), we found that oocytes expressing α-subunits that lacked regions encoded by specific exons, with the exception of exon 11, did not have measurable amiloride-sensitive currents. Surprisingly, oocytes expressing channels with an α-subunit exon 11 in-frame deletion (αΔExon11) exhibited whole cell amiloride-sensitive Na+ currents that were fourfold greater than cells expressing WT channels (P < 0.001; Fig. 2). Exon 11 encodes the peripheral knuckle domain of the ECD.
Fig. 2.
Deletion of exon 11-encoded region of the human α-subunit leads to a gain of function. Whole cell currents were measured in oocytes injected with αβγ [wild type (WT)] or αΔExonβγ cRNAs and clamped at −100 mV. A: representative recordings of currents in oocytes expressing αβγ (WT; thin black line), αΔExon9βγ (gray line), or ΔExon11βγ (thick black line) in the absence and presence (gray bars) of 10 μM amiloride. The dashed line indicates the zero current level. All three traces are scaled according to the scale bar. B: normalized currents for WT and mutant channels. Normalized currents represent amiloride-sensitive currents measured at −100 mV from individual oocytes that were normalized to the average of the amiloride-sensitive current of oocytes expressing WT channels from the same batch of oocytes. All mutant values were significantly different from those of WT values (n = 9∼23, P < 0.001). The normalized currents of most mutants were too small to be visible.
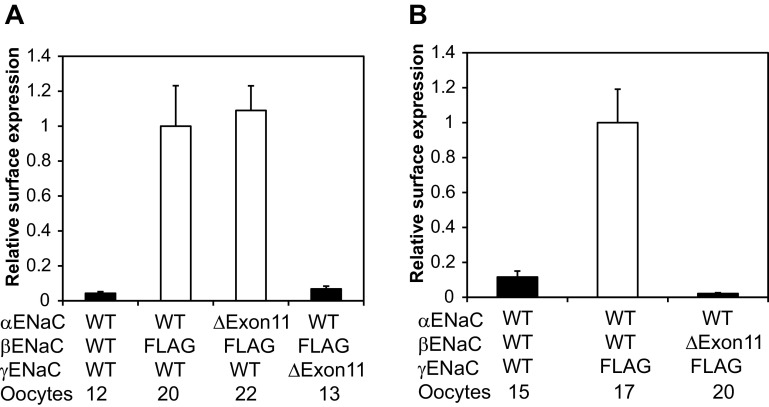
The large increase in amiloride-sensitive currents in oocytes expressing αΔExon11βγ channels suggested that deletion of the α-subunit knuckle domain is associated with an increase in the number of channels at the plasma membrane and/or an increase in channel Po. We performed a chemiluminescence-based surface expression assay in oocytes expressing either WT or αΔExon11βγ channels, with the β-subunit bearing an external epitope FLAG tag. We found similar levels of surface of WT and mutant channels (Fig. 3), suggesting that deletion of the α-subunit knuckle region did not alter channel density at the plasma membrane.
Fig. 3.
Effects of exon 11 region deletions on the surface expression of hENaCs. Oocytes were injected with the three hENaC subunit cRNAs shown at the bottom. For α- and γ-subunit mutants, a β-subunit with a FLAG epitope was included in all groups in A except for the negative control (left bar), whereas a γ-subunit with a FLAG epitope was used for the β-mutant in B. Relative light units measured from individual oocytes were normalized to the average relative light units of oocytes expressing the FLAG control (αβFLAGγ in A and αβγFLAG in B). The solid bars indicate that the values were significantly different from the FLAG control group (P < 0.01).
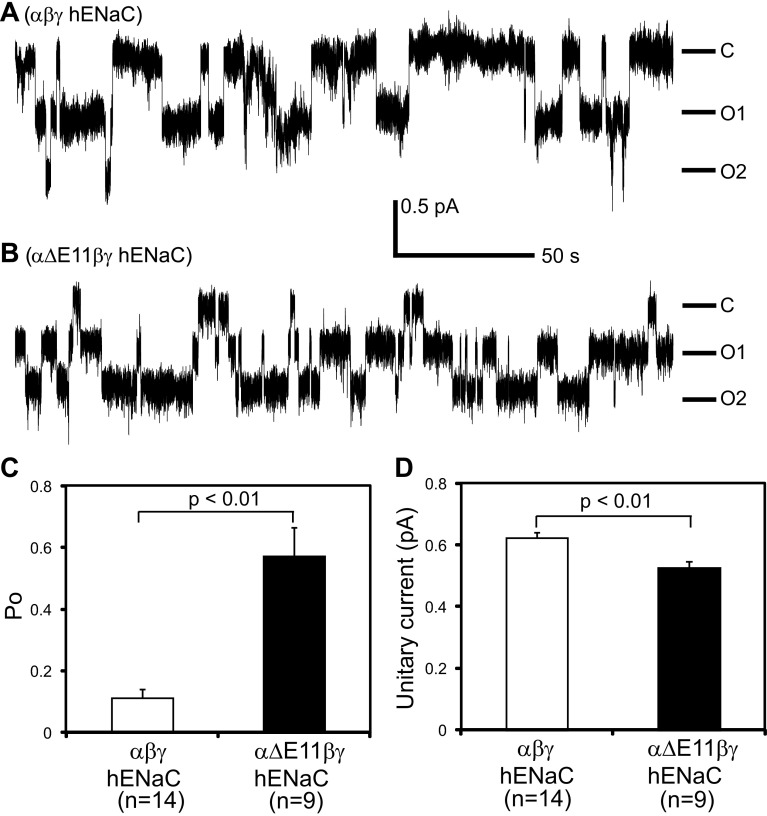
We next performed patch-clamp analyses of oocytes expressing WT or αΔExon11βγ channels. Typical single channel gating transitions are shown in Fig. 4. The Po of mutant channels was 0.57 ± 0.09 (n = 9), significantly greater than that of WT channels (0.11 ± 0.03, n = 14, P < 0.01). In addition, mutant unitary currents (0.52 ± 0.02 pA, n = 9) were slightly less than WT unitary currents (0.62 ± 0.02 pA, n = 14, P < 0.01). These observations suggest that the increase in whole cell Na+ current observed with the deletion of the α-subunit knuckle region reflects a dramatic and significant increase in channel Po.
Fig. 4.
Deletion of exon 11-encoded region of the α-subunit increases channel open probability (Po). Patch-clamp recordings were performed with oocytes expressing either αβγ (A) or αΔExon11βγ (B). Cell-attached patches were clamped at −100 mV (membrane potential). Both bath and pipette solutions contained 110 mM Na+. Downward deflections indicate channel openings. C, closed state; O1 and O2, open states. The scale bar is for both traces. C: averaged Po of WT and mutant channels. Po was estimated using the single channel analysis function of ClampFit 10. D: averaged unitary currents at −100 mV. Unitary currents in each patch were obtained from the event analyses after single channel searching by ClampFit 10.
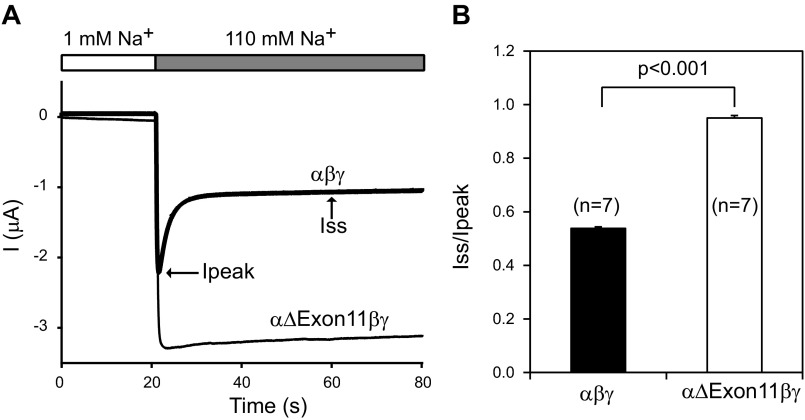
External Na+ inhibits Na+ channels by reducing channel Po, a process referred to as Na+ self-inhibition (30). We examined whether the increased Po observed with the α-subunit exon 11 deletion mutant reflected a suppressed Na+ self-inhibition response. As shown in Fig. 5, when the external Na+ concentration was rapidly increased from 1 to 110 mM, we observed that oocytes expressing WT channels exhibited a rapid increase in Na+ current that was followed by slow fall in current that reflected the Na+ self-inhibition response. In contrast, oocytes expressing αΔExon11βγ channels did not exhibit a fall in Na+ current, suggesting that the increased Po we observed with the mutant channel is due, in part, to a loss of Na+ self-inhibition.
Fig. 5.
Exon 11 region deletion from the α-subunit eliminates Na+ self-inhibition. A: representative traces for Na+ self-inhibition responses. Oocytes were clamped at −100 mV, and whole cell currents were continuously recorded while bath Na+ concentration was rapidly increased from 1 mM (open bar) to 110 mM (gray bar). Inward currents are shown as negative values by convention. The current (I) decline after the increase in bath Na+ concentration from a peak current (Ipeak) to a steady-state current (Iss) represents Na+ self-inhibition. The current traces for αβγ (WT; thick line) and αΔExon11βγ (thin line) are superimposed on the same scales. B: magnitude of Na+ self-inhibition responses of WT and mutant channels. Iss/Ipeak values were obtained as described in materials and methods.
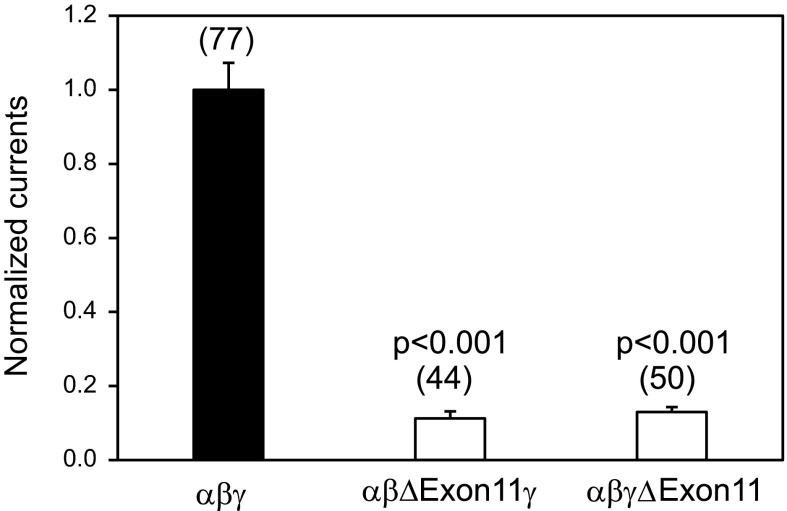
All three ENaC subunits (α, β, and γ) contribute to the conducting pore in the heteromultimeric channel complexes, although they have distinct functional roles (30, 32, 40, 42, 49). We examined whether deletions of the residues encoded by exon 11 in the β- or γ-subunit altered the properties of channels similar to those observed with the α-subunit mutant. We found that expression of a mutant β- or γ-subunit in an αβγ-channel complex in oocytes resulted in very small amiloride-sensitive currents (Fig. 6). Furthermore, levels of surface expression levels in oocytes expressing either mutant β- or γ-subunits were markedly reduced (Fig. 3).
Fig. 6.
Deletion of exon 11-encoded region of β- or γ-hENaC subunit results in a loss of function. Whole cell currents were measured in oocytes injected with αβγ (WT), αβΔExon11γ, or αβγΔExon11 cRNAs and clamped at −100 mV. Normalized currents for WT and mutant channels were obtained in the same way as in Fig. 2B. Both mutant values were significantly different from the WT value (P < 0.001). Data were collected in four batches of oocytes, and the numbers of cells for each group are shown in parentheses.
DISCUSSION
We observed that most coding exons in the α-subunit are required for the generation of functional channels in Xenopus oocytes. While we did not assess the underlining reasons for the loss of functional expression of these mutant channels, it is likely that the deletion of specific regions resulted in misfolded subunits or impaired subunit assembly in the endoplasmic reticulum (ER), which results in their targeting for ER-associated degradation. In this regard, it was surprising that deletion of the knuckle region of the α-subunit, encoded by exon 11, did not appear to impair channel exit from the ER and expression at the plasma membrane. In contrast, deletion of this region from either the β- or γ-subunit resulted in a dramatic reduction in both whole cell currents and surface expression of channels, suggesting an impairment of protein folding and ER exit. At present, it is unclear why channels lacking the α-subunit knuckle domain do not have the same fate as channels lacking the β- or γ-subunit knuckle domain. This observation is in accordance with numerous previous studies (18, 33, 40–42, 48, 49) demonstrating subunit asymmetry with regard to the functional effects of mutations. We speculate that the strikingly distinct consequences of homologous exon 11 deletions among the three ENaC subunits may reflect, in part, how the different subunits interact with factors that influence ENaC biogenesis. For example, in yeast, the ER lumen-resident heat shock protein (Hsp)70-related Lhs1 selectively targets the α-subunit for ER-associated degradation but does not affect the degradation of the β- or γ-subunit (9). Its mammalian homolog, Grp170, also enhances α-subunit turnover in human embryonic kidney cells (9). In contrast, luminal Hsp40s enhance the degradation of all three subunits in yeast (8). It is notable that an ER exit motif has been identified in the COOH-terminal cytoplasmic domain of the α-subunit, whereas β- and γ-subunits appear to lack ER exit motifs (35). Furthermore, posttranslational modifications, such as furin-dependent proteolysis, palmitoylation, and phosphorylation by specific kinases, occur in a subunit-specific manner (3, 25, 35, 36).
One of the key functions of the ECDs of ENaC subunits is to confer a downregulation of channel activity by extracellular Na+ (i.e., Na+ self-inhibition). The location of the Na+-binding sites and the structural transitions that occur after Na+ binding that results in a reduction in channel Po remain elusive. It is interesting that the absence of the exon 11-encoded knuckle domain from the α-subunit resulted in a loss of Na+ self-inhibition, in parallel with an increase in channel Po. Based on recently resolved structures of ASIC1 at high and low pH, the knuckle domain together with the upper portion of the palm domain functions as a scaffold, in contrast to a more mobile low portion of the palm domain, where large conformational movements were observed in channels in different conformational states (i.e., conducting vs. nonconducting) (2). The knuckle domain of the α-subunit borders the finger domain of the γ-subunit (14, 18, 22, 26, 29, 30). There are a number of sites in the γ-subunit finger domain that have roles in modulating the channel's response to extracellular factors, including Na+, Zn2+, and proteases (6, 15, 24, 41, 47, 56). Based on these findings, we speculate that interactions at the interface between the α-subunit knuckle domain and γ-subunit finger domain may have an important role in the allosteric regulation of ENaC Po by external factors.
There are a growing number of single nucleotide polymorphisms, small insertion/deletion variants, and alternatively spliced mRNA variants that are present in the databases of sequenced genes and RNAs (19, 21). In addition to the well-described Liddle syndrome mutations, a number of ENaC single-nucleotide polymorphisms have been associated with hypertension or salt sensitivity (4, 11, 23, 27, 28, 57). To date, we have not found any human ENaC transcript lacking exon 11. Future studies using RNA sequencing and other techniques will reveal if such transcripts indeed exist in vivo.
In summary, by analyzing channels with exon level deletions, we found that the exon 11-encoded knuckle domain in the α-subunit is not required for the functional expression of heteromeric channels. Furthermore, our results suggest that the α-subunit knuckle domain has a role in facilitating Na+ self-inhibition and dampening ENaC Po in the presence of external Na+. Delineation of exon-encoded functions within ENaC subunits should enhance our understanding of the biological roles of alternatively spliced transcripts of human ENaC genes, which are largely unknown.
GRANTS
This work was supported by National Institutes of Health Grants R01-ES-014701, R01-DK-051391, R01-DK-065161, and P30-DK-079307.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.C., T.R.K., and S.S. conception and design of research; J.C. and S.S. performed experiments; J.C., T.R.K., and S.S. analyzed data; J.C., T.R.K., and S.S. interpreted results of experiments; J.C., T.R.K., and S.S. edited and revised manuscript; J.C., T.R.K., and S.S. approved final version of manuscript; T.R.K. and S.S. drafted manuscript; S.S. prepared figures.
REFERENCES
- 1.Adachi M, Tachibana K, Asakura Y, Abe S, Nakae J, Tajima T, Fujieda K. Compound heterozygous mutations in the gamma subunit gene of ENaC (1627delG and 1570-1G→A) in one sporadic Japanese patient with a systemic form of pseudohypoaldosteronism type 1. J Clin Endocrinol Metab 86: 9–12, 2001 [DOI] [PubMed] [Google Scholar]
- 2.Baconguis I, Gouaux E. Structural plasticity and dynamic selectivity of acid-sensing ion channel-spider toxin complexes. Nature 489: 400–405, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baines D. Kinases as targets for ENaC regulation. Curr Mol Pharmacol 6: 50–64, 2013 [DOI] [PubMed] [Google Scholar]
- 4.Baker EH, Duggal A, Dong Y, Ireson NJ, Wood M, Markandu ND, MacGregor GA. Amiloride, a specific drug for hypertension in black people with T594M variant? Hypertension 40: 13–17, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Banasikowska K, Post M, Cutz E, O'Brodovich H, Otulakowski G. Expression of epithelial sodium channel α-subunit mRNAs with alternative 5′-untranslated regions in the developing human lung. Am J Physiol Lung Cell Mol Physiol 287: L608–L615, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Bruns JB, Carattino MD, Sheng S, Maarouf AB, Weisz OA, Pilewski JM, Hughey RP, Kleyman TR. Epithelial Na+ channels are fully activated by furin- and prostasin-dependent release of an inhibitory peptide from the γ-subunit. J Biol Chem 282: 6153–6160, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Bubien JK. Epithelial Na+ channel (ENaC), hormones, and hypertension. J Biol Chem 285: 23527–23531, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buck TM, Kolb AR, Boyd CR, Kleyman TR, Brodsky JL. The endoplasmic reticulum-associated degradation of the epithelial sodium channel requires a unique complement of molecular chaperones. Mol Biol Cell 21: 1047–1058, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buck TM, Plavchak L, Roy A, Donnelly BF, Kashlan OB, Kleyman TR, Subramanya AR, Brodsky JL. The Lhs1/GRP170 chaperones facilitate the endoplasmic reticulum associated degradation of the epithelial sodium channel. J Biol Chem 288: 18366–18380, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Busst CJ. Blood pressure regulation via the epithelial sodium channel: from gene to kidney and beyond. Clin Exp Pharmacol Physiol 40: 495–503, 2013 [DOI] [PubMed] [Google Scholar]
- 11.Busst CJ, Bloomer LD, Scurrah KJ, Ellis JA, Barnes TA, Charchar FJ, Braund P, Hopkins PN, Samani NJ, Hunt SC, Tomaszewski M, Harrap SB. The epithelial sodium channel gamma-subunit gene and blood pressure: family based association, renal gene expression, and physiological analyses. Hypertension 58: 1073–1078, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carattino MD, Hill WG, Kleyman TR. Arachidonic acid regulates surface expression of epithelial sodium channels. J Biol Chem 278: 36202–36213, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Chen J, Kleyman TR, Sheng S. Gain-of-function variant of the human epithelial sodium channel. Am J Physiol Renal Physiol 304: F207–F213, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen J, Myerburg MM, Passero CJ, Winarski KL, Sheng S. External Cu2+ inhibits human epithelial Na+ channels by binding at a subunit interface of extracellular domains. J Biol Chem 286: 27436–27446, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen J, Winarski KL, Myerburg MM, Pitt BR, Sheng S. Probing the structural basis of Zn2+ regulation of the epithelial Na+ channel. J Biol Chem 287: 35589–35598, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chow YH, Wang Y, Plumb J, O'Brodovich H, Hu J. Hormonal regulation and genomic organization of the human amiloride-sensitive epithelial sodium channel alpha subunit gene. Pediatr Res 46: 208–214, 1999 [DOI] [PubMed] [Google Scholar]
- 17.Collier DM, Snyder PM. Extracellular protons regulate human ENaC by modulating Na+ self-inhibition. J Biol Chem 284: 792–798, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Collier DM, Snyder PM. Identification of epithelial Na+ channel (ENaC) intersubunit Cl− inhibitory residues suggests a trimeric αγβ channel architecture. J Biol Chem 286: 6027–6032, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Djebali S, Davis CA, Merkel A, Dobin A, Lassmann T, Mortazavi A, Tanzer A, Lagarde J, Lin W, Schlesinger F, Xue C, Marinov GK, Khatun J, Williams BA, Zaleski C, Rozowsky J, Roder M, Kokocinski F, Abdelhamid RF, Alioto T, Antoshechkin I, Baer MT, Bar NS, Batut P, Bell K, Bell I, Chakrabortty S, Chen X, Chrast J, Curado J, Derrien T, Drenkow J, Dumais E, Dumais J, Duttagupta R, Falconnet E, Fastuca M, Fejes-Toth K, Ferreira P, Foissac S, Fullwood MJ, Gao H, Gonzalez D, Gordon A, Gunawardena H, Howald C, Jha S, Johnson R, Kapranov P, King B, Kingswood C, Luo OJ, Park E, Persaud K, Preall JB, Ribeca P, Risk B, Robyr D, Sammeth M, Schaffer L, See LH, Shahab A, Skancke J, Suzuki AM, Takahashi H, Tilgner H, Trout D, Walters N, Wang H, Wrobel J, Yu Y, Ruan X, Hayashizaki Y, Harrow J, Gerstein M, Hubbard T, Reymond A, Antonarakis SE, Hannon G, Giddings MC, Ruan Y, Wold B, Carninci P, Guigo R, Gingeras TR. Landscape of transcription in human cells. Nature 489: 101–108, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Firsov D, Schild L, Gautschi I, Merillat AM, Schneeberger E, Rossier BC. Cell surface expression of the epithelial Na channel and a mutant causing Liddle syndrome: a quantitative approach. Proc Natl Acad Sci USA 93: 15370–15375, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Genomes Project C, Abecasis GR, Auton A, Brooks LD, DePristo MA, Durbin RM, Handsaker RE, Kang HM, Marth GT, McVean GA. An integrated map of genetic variation from 1,092 human genomes. Nature 491: 56–65, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gonzales EB, Kawate T, Gouaux E. Pore architecture and ion sites in acid-sensing ion channels and P2X receptors. Nature 460: 599–604, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hannila-Handelberg T, Kontula K, Tikkanen I, Tikkanen T, Fyhrquist F, Helin K, Fodstad H, Piippo K, Miettinen HE, Virtamo J, Krusius T, Sarna S, Gautschi I, Schild L, Hiltunen TP. Common variants of the beta and gamma subunits of the epithelial sodium channel and their relation to plasma renin and aldosterone levels in essential hypertension. BMC Med Genet 6: 4, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hughey RP, Bruns JB, Kinlough CL, Harkleroad KL, Tong Q, Carattino MD, Johnson JP, Stockand JD, Kleyman TR. Epithelial sodium channels are activated by furin-dependent proteolysis. J Biol Chem 279: 18111–18114, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Hughey RP, Bruns JB, Kinlough CL, Kleyman TR. Distinct pools of epithelial sodium channels are expressed at the plasma membrane. J Biol Chem 279: 48491–48494, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Jasti J, Furukawa H, Gonzales EB, Gouaux E. Structure of acid-sensing ion channel 1 at 1.9 A resolution and low pH. Nature 449: 316–323, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Jones ES, Owen EP, Davidson JS, Van Der Merwe L, Rayner BL. The R563Q mutation of the epithelial sodium channel β-subunit is associated with hypertension. Cardiovasc J Africa 22: 241–244, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jones ES, Owen EP, Rayner BL. The association of the R563Q genotype of the ENaC with phenotypic variation in Southern Africa. Am J Hypertens 25: 1286–1291, 2012 [DOI] [PubMed] [Google Scholar]
- 29.Kashlan OB, Adelman JL, Okumura S, Blobner BM, Zuzek Z, Hughey RP, Kleyman TR, Grabe M. Constraint-based, homology model of the extracellular domain of the epithelial Na+ channel α subunit reveals a mechanism of channel activation by proteases. J Biol Chem 286: 649–660, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kashlan OB, Kleyman TR. ENaC structure and function in the wake of a resolved structure of a family member. Am J Physiol Renal Physiol 301: F684–F696, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kashlan OB, Kleyman TR. Epithelial Na+ channel regulation by cytoplasmic and extracellular factors. Exp Cell Res 318: 1011–1019, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kellenberger S, Schild L. Epithelial sodium channel/degenerin family of ion channels: a variety of functions for a shared structure. Physiol Rev 82: 735–767, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Li J, Sheng S, Perry CJ, Kleyman TR. Asymmetric organization of the pore region of the epithelial sodium channel. J Biol Chem 278: 13867–13874, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Mall MA. Role of the amiloride-sensitive epithelial Na+ channel in the pathogenesis and as a therapeutic target for cystic fibrosis lung disease. Exp Physiol 94: 171–174, 2009 [DOI] [PubMed] [Google Scholar]
- 35.Mueller GM, Kashlan OB, Bruns JB, Maarouf AB, Aridor M, Kleyman TR, Hughey RP. Epithelial sodium channel exit from the endoplasmic reticulum is regulated by a signal within the carboxyl cytoplasmic domain of the α subunit. J Biol Chem 282: 33475–33483, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Mueller GM, Maarouf AB, Kinlough CL, Sheng N, Kashlan OB, Okumura S, Luthy S, Kleyman TR, Hughey RP. Cys palmitoylation of the β subunit modulates gating of the epithelial sodium channel. J Biol Chem 285: 30453–30462, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oh YS, Lee S, Won C, Warnock DG. An Alu cassette in the human epithelial sodium channel. Biochim Biophys Acta 1520: 94–98, 2001 [DOI] [PubMed] [Google Scholar]
- 38.Pratt JH. Central role for ENaC in development of hypertension. J Am Soc Nephrol 16: 3154–3159, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Rossier BC, Pradervand S, Schild L, Hummler E. Epithelial sodium channel and the control of sodium balance: interaction between genetic and environmental factors. Annu Rev Physiol 64: 877–897, 2002 [DOI] [PubMed] [Google Scholar]
- 40.Schild L, Schneeberger E, Gautschi I, Firsov D. Identification of amino acid residues in the α, β, and γ subunits of the epithelial sodium channel (ENaC) involved in amiloride block and ion permeation. J Gen Physiol 109: 15–26, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sheng S, Bruns JB, Kleyman TR. Extracellular histidine residues crucial for Na+ self-inhibition of epithelial Na+ channels. J Biol Chem 279: 9743–9749, 2004 [DOI] [PubMed] [Google Scholar]
- 42.Sheng S, Hallows KR, Kleyman TR. Epithelial Na+ channels. In: Seldin and Giebisch's The Kidney: Physiology and Pathophysiology, edited by Alpern RJ, Caplan MJ, Moe OW. New York: Academic, 2012, p. 983–1017 [Google Scholar]
- 43.Sheng S, Maarouf AB, Bruns JB, Hughey RP, Kleyman TR. Functional role of extracellular loop cysteine residues of the epithelial Na+ channel in Na+ self-inhibition. J Biol Chem 282: 20180–20190, 2007 [DOI] [PubMed] [Google Scholar]
- 44.Sheng S, Perry CJ, Kleyman TR. External nickel inhibits epithelial sodium channel by binding to histidine residues within the extracellular domains of α and γ subunits and reducing channel open probability. J Biol Chem 277: 50098–50111, 2002 [DOI] [PubMed] [Google Scholar]
- 45.Sheng S, Perry CJ, Kleyman TR. Extracellular Zn2+ activates epithelial Na+ channels by eliminating Na+ self-inhibition. J Biol Chem 279: 31687–31696, 2004 [DOI] [PubMed] [Google Scholar]
- 46.Shi S, Blobner BM, Kashlan OB, Kleyman TR. Extracellular finger domain modulates the response of the epithelial sodium channel to shear stress. J Biol Chem 287: 15439–15444, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shi S, Carattino MD, Hughey RP, Kleyman TR. ENaC regulation by proteases and shear stress. Curr Mol Pharmacol 6: 28–34, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shi S, Ghosh DD, Okumura S, Carattino MD, Kashlan OB, Sheng S, Kleyman TR. Base of the thumb domain modulates epithelial sodium channel gating. J Biol Chem 286: 14753–14761, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shi S, Kleyman TR. Gamma subunit second transmembrane domain contributes to epithelial sodium channel gating and amiloride block. Am J Physiol Renal Physiol 305: F1585–F1592, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Soundararajan R, Pearce D, Hughey RP, Kleyman TR. Role of epithelial sodium channels and their regulators in hypertension. J Biol Chem 285: 30363–30369, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stockand JD, Staruschenko A, Pochynyuk O, Booth RE, Silverthorn DU. Insight toward epithelial Na+ channel mechanism revealed by the acid-sensing ion channel 1 structure. IUBMB Life 60: 620–628, 2008 [DOI] [PubMed] [Google Scholar]
- 52.Strautnieks SS, Thompson RJ, Gardiner RM, Chung E. A novel splice-site mutation in the gamma subunit of the epithelial sodium channel gene in three pseudohypoaldosteronism type 1 families. Nat Genet 13: 248–250, 1996 [DOI] [PubMed] [Google Scholar]
- 53.Thomas CP, Auerbach S, Stokes JB, Volk KA. 5′ heterogeneity in epithelial sodium channel α-subunit mRNA leads to distinct NH2-terminal variant proteins. Am J Physiol Cell Physiol 274: C1312–C1323, 1998 [DOI] [PubMed] [Google Scholar]
- 54.Tucker JK, Tamba K, Lee YJ, Shen LL, Warnock DG, Oh Y. Cloning and functional studies of splice variants of the α-subunit of the amiloride-sensitive Na+ channel. Am J Physiol Cell Physiol 274: C1081–C1089, 1998 [DOI] [PubMed] [Google Scholar]
- 55.Welzel M, Akin L, Buscher A, Guran T, BPH, Hogler W, Leonards J, Karges B, Kentrup H, Kirel B, Senses EE, Tekin N, Holterhus PM, Riepe FG. Five novel mutations in the SCNN1A gene causing autosomal recessive pseudohypoaldosteronism type 1. Eur J Endocrinol 168: 707–715, 2013 [DOI] [PubMed] [Google Scholar]
- 56.Winarski KL, Sheng N, Chen J, Kleyman TR, Sheng S. Extracellular allosteric regulatory subdomain within the γ subunit of the epithelial Na+ channel. J Biol Chem 285: 26088–26096, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhao Q, Gu D, Hixson JE, Liu DP, Rao DC, Jaquish CE, Kelly TN, Lu F, Ma J, Mu J, Shimmin LC, Chen J, Mei H, Hamm LL, He J. Common variants in epithelial sodium channel genes contribute to salt sensitivity of blood pressure: the GenSalt study. Circ Cardiovasc Genet 4: 375–380, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]