Abstract
Basement membrane abnormalities have often been observed in kidney cysts of polycystic kidney disease (PKD) patients and animal models. There is an abnormal deposition of extracellular matrix molecules, including laminin-α3,β3,γ2 (laminin-332), in human autosomal dominant PKD (ADPKD). Knockdown of PKD1 paralogs in zebrafish leads to dysregulated synthesis of the extracellular matrix, suggesting that altered basement membrane assembly may be a primary defect in ADPKD. In this study, we demonstrate that laminin-332 is aberrantly expressed in cysts and precystic tubules of human autosomal recessive PKD (ARPKD) kidneys as well as in the kidneys of PCK rats, an orthologous ARPKD model. There was aberrant expression of laminin-γ2 as early as postnatal day 2 and elevated laminin-332 protein in postnatal day 30, coinciding with the formation and early growth of renal cysts in PCK rat kidneys. We also show that a kidney cell line derived from Oak Ridge polycystic kidney mice, another model of ARPKD, exhibited abnormal lumen-deficient and multilumen structures in Matrigel culture. These cells had increased proliferation rates and altered expression levels of laminin-332 compared with their rescued counterparts. A function-blocking polyclonal antibody to laminin-332 significantly inhibited their abnormal proliferation rates and rescued their aberrant phenotype in Matrigel culture. Furthermore, abnormal laminin-332 expression in cysts originating from collecting ducts and proximal tubules as well as in precystic tubules was observed in a human end-stage ADPKD kidney. Our results suggest that abnormal expression of laminin-332 contributes to the aberrant proliferation of cyst epithelial cells and cyst growth in genetic forms of PKD.
Keywords: autosomal recessive polycystic kidney disease, laminin-332, polycystic kidney disease rat, cystogenesis, proliferation
polycystic kidney disease (PKD) encompasses a group of monogenic disorders that are a leading cause of end-stage renal disease. The most common form, autosomal dominant PKD (ADPKD), has an estimated prevalence rate of 1:400-1:1,000 worldwide (40) and is caused by mutations in one of two genes, PKD1 and PKD2. The autosomal recessive form of PKD (ARPKD) has an estimated prevalence rate of ∼1:20,000 live births and is associated with significant neonatal mortality and childhood morbidity. ARPKD is caused by mutations in the polycystic kidney and hepatic disease 1 (PKHD1) gene. Despite the observation that cystic epithelia in both ADPKD and ARPKD have defects in terminal differentiation (9, 11), the mechanism of cystogenesis is different in these two diseases. In ADPKD, cysts can form in all nephron segments and fully pinch off to form isolated fluid-filled cysts (32). In contrast, in ARPKD, there is the fusiform dilation of the collecting ducts, which remain contiguous with the nephron (45).
The products of PKD1 (polycystin-1), PKD2 (polycystin-2), and PKHD1 (fibrocystin) are large, multifunctional, membrane-associated proteins. Polycystin-2 is a Ca2+-permeable cation channel also known as the transient receptor potential P2 channel. Polycystin-1 (directly) and fibrocystin (indirectly) interact with polycystin-2 and regulate its channel activity. PKD proteins are localized in the primary cilia, plasma membrane, and endoplasmic reticulum. Mutations to PKD genes disrupt important signaling pathways (e.g., cAMP, MAPK, mammalian target of rapamycin, Wnt-β catenin, etc.), resulting in the loss of planar cell polarity, excessive cell proliferation and fluid secretion, and cystogenesis.
Several studies (4, 13, 37, 38) have shown that basement membrane abnormalities are a feature common to human and several rodent models of PKD. Additionally, human ADPKD epithelial cells in culture exhibit an abnormal basement membrane morphology consisting of banded collagen and numerous unique blebs or spheroids (46). In the Han:SPRD rat model of PKD, cystic transformation starts in the proximal tubule with a sharp transition from a normal to thickened basement membrane and a loss of epithelial cell differentiation restricted to these areas (37). In normal adult kidneys of humans, mice, and rats, laminin-511 (composed of laminin α5-, β1-, and γ1-chains; previously known as laminin-10) is one of the major components of the tubular basement membrane, whereas laminin-332 (composed of laminin α3-, β3-, and γ2-chains; previously known as laminin-5) expression generally is not observed (27). In this context, it is important to note that laminins are multidomain heterotrimers formed by the combination of one α-chain, one β-chain, and one γ-chain. A typical trimeric laminin structure (for example, laminin-111) has a cross structure with one long arm and three short arms, with the latter being formed from the three free NH2-terminal ends of the α-, β-, and γ-chains. The laminin trimer is formed by assembly of the coiled-coil α-helical regions of the three chains, which results in the formation of the long arm structure. Trimeric laminin-332 begins with the stable association of the glycosylated β- and γ-chains to form a heterodimer; the α-chain then combines with this dimer in order for the trimeric molecule to be secreted out of the cell (41).
Recent studies have suggested that abnormalities in basement membrane laminin may be directly involved in PKD cystogenesis. Mice with a hypomorphic mutation in laminin-α5, a major tubular and glomerular basement membrane component, exhibit PKD, proteinuria, and death from renal failure by 4 wk of age. The basement membranes of the cysts are markedly thickened with an aberrant accumulation of laminin-332 (38). In human ADPKD cysts, abnormal expression of laminin-332 has also been observed (14), and an anti-laminin-γ2 function-blocking antibody inhibited ERK activation induced by EGF stimulation in cultured ADPKD cells (13). Furthermore, it was recently observed that the defects that arise from the combined knockdown of the PKD1 paralogs Pkd1a and Pkd1b in zebrafish, such as dorsal axis curvature, were a result of dysregulation of collagen synthesis in these mutants. Based on the results of this study, it was suggested that Pkd1a/b and Pkd2 interact to regulate extracellular matrix (ECM) secretion or assembly and that altered matrix integrity may be a primary defect underlying ADPKD tissue pathologies (5, 26).
While abnormal laminin assembly has been reported in human ADPKD and in a nonorthologous rodent model of PKD, no studies have yet explored whether defects in the tubular basement membrane laminin composition are associated with ARPKD. In this study, we used human ARPKD kidney tissues and primary cells, immortalized cells from Oak Ridge polycystic kidney (orpk) mice (a model of recessive PKD), and the PCK rat (an orthologous model of human ARPKD) to investigate the expression and role of laminin-332 (16, 19, 43).
MATERIALS AND METHODS
Cell culture.
Immortalized orpk mutant cell line 94D-pCDNA (PCDNA) and the rescued cell line 94D-Tg737Bap-2 (BAP2), previously established and characterized by Dr. Bradley Yoder and colleagues, were cultured using a culture medium (48). G418 (200 μg/ml) was routinely added to the culture medium to keep the selection pressure for stable cells. For routine propagation, cells were cultured in a 32°C incubator with 20 U/ml interferon-γ. To promote differentiation and T antigen inactivation, cells were cultured under nonpermissive conditions (40°C in the absence of interferon-γ) until cells became confluent. Cells cultured under nonpermissive conditions were used for the investigation of laminin-332 expression by real-time PCR and ECM extraction.
Human tissues.
We obtained an ADPKD kidney from a deceased 46-yr-old male patient (creatinine: 4.2–4.4 and blood urea nitrogen: 56). The kidney was recovered the same day by the National Disease Research Interchange (Philadelphia, PA; www.ndriresource.org) with appropriate approvals from the University of Rochester Medical Center (URMC) Research Subjects Review Board. A normal kidney was also recovered from a 58-yr-old female subject who had died of a cardiovascular problem. Tissues were frozen in OCT compound and sectioned for immunohistochemistry. Additionally, frozen OCT kidney tissue blocks were obtained from a 5-yr-old male child with ARPKD/congestive heart failure at the time of nephrectomy.
Human ARPKD and normal human kidney cells.
Primary cultures of ARPKD and normal human kidney (NHK) cells were generated by the PKD Research Biomaterials and Cellular Models Core at the University of Kansas Medical Center (35). ARPKD kidneys were obtained at the time of nephrectomy from a 5-wk-old male infant and a 6-yr-old male child. Cystic tissues were digested with DMEM-F-12 containing collagenase type IV to loosen epithelial cells from the stromal tissue (35). The resultant suspension was centrifuged, and the pellet was rinsed and plated in T75 flasks containing DMEM-F-12 supplemented with 5% FBS, penicillin-streptomycin, and 5 μg/ml insulin, 5 μg/ml transferrin, and 5 μg/ml sodium selenite (ITS; Collaborative Biomedical Products, Bedford, MA) until cells were 75% confluent. Normal human kidneys unsuitable for transplantation were obtained from the Midwest Transplant Network (Kansas City, KS). These kidneys were from a 36-yr-old man and 58-yr-old woman. The cortex was minced; cells were released by digestion with collagenase and grown in culture media. The protocol for the collection of surgically discarded kidney tissues complied with federal regulations and was approved by the Institutional Review Board of the University of Kansas Medical Center.
To compare gene expression of the laminin-332 chains, ARPKD and NHK cells were plated on plastic petri dishes (100-mm diameter) and permeable supports (Transwells) and grown in media containing 1% FBS + ITS + penicillin-streptomycin for 3 days. Media were changed to 0.05% FBS for 24 h before RNA extraction. Total RNA was isolated in a buffer containing β-mercaptoethanol and precipitated in 70% ethanol. RNA was loaded onto the column of an RNeasy Mini Kit (Qiagen) and eluted with RNase-free water. The quality of RNA was verified using an Agilent bioanalyzer (Santa Clara, CA).
PCK rats.
PCK rats were originally derived from a spontaneous mutation in a strain of Sprague-Dawley (SD) rats (16, 19). The mutation in the PCK rat has been shown to be an exon deletion in the rat homolog of human PKHD1 (43). Initial colonies of PCK rats were established from rats obtained from Mayo Clinic (Rochester, MN). Subsequently, PCK and SD rats were also purchased from Charles River Laboratories (Wilmington, MA) and maintained in the URMC vivarium. Rats were obtained and maintained as per the regulations approved by the University Committee on Animal Resources under an established and approved animal protocol.
Electron microscopy.
PCK and SD rat kidney sections were immediately immersed in a combination fixative containing 4.0% paraformaldehyde and 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer (pH 7.4). After 24 h of primary fixation, tissue was rinsed in the same buffer and postfixed for 1 h in buffered 1.0% osmium tetroxide, dehydrated in a graded series of ethanol to 100%, transitioned into propylene oxide, infiltrated with EPON/Araldite resin, embedded, and polymerized for 48 h at 60°C. Sections (1 μm) were cut, placed onto glass slides, and then stained with toludine Blue on a hot plate to identify cysts or normal tubules from PCK and SD tissues, respectively. Using an ultramicrotome, thin sections (70 nm) of targeted areas were cut with a diamond knife and collected onto carbon-coated nickel grids. Grids were stained with aqueous uranyl acetate and lead citrate and examined using a Hitachi 7650 transmission electron microscope with an attached Gatan 11 megapixel Erlangshen digital camera and Digital micrograph software.
Real-time PCR.
Total RNA was extracted from whole kidney sections of four different PCK and SD rats using Tripure reagent (Roche), and RNA integrity was analyzed using the Agilent bioanalyzer Nano 6000 kit. Total RNA was then treated with TurboDNAse (Ambion, Grand Island, NY), and first-strand cDNA was synthesized from 1–2 μg of total RNA using the High Capacity Reverse Transcriptase Kit (Applied Biosystems, Carlsbad, CA). Real-time PCR was performed using Taqman method in a 7900HT Sequence Detection System (Applied Biosystems) with Taqman Universal Master Mix. Forward/reverse primers and fluorogenic probes to detect rat laminin-332 and laminin-511 chains and GAPDH were designed using PrimerExpress (shown in Table 1). Real-time PCR results were analyzed in SDS RQ Manager 1.2, and the relative quantity was determined using the ΔΔCt method (where Ct is threshold cycle). GAPDH Ct values were more or less uniform across all samples and were used in the normalization process. A similar protocol was used to perform real-time PCR on kidney samples from postnatal day (PN)2, PN7, PN10, PN21, PN30, PN40, and PN70, with a maximum of 8 samples/condition. An ABI assay for the rat sex-determining region Y (SRY) gene (Rn04224592_u1) was also used in these real-time experiments. Real-time PCR results from male samples (n = 5 samples each except for PN70, where n = 3) were used in the analysis and statistical evaluation. For the orpk experiment, three different confluent monolayers of rescued (BAP2) and mutant (PCDNA) cell lines were used to extract total RNA. Premade primer-probe sets from ABI [Mm01254735_m1 (laminin-α3), Mm0122029_m1 (laminin-α5), Mm00801853_m1 (laminin-β1), Mm00493108_m1 (laminin-β3), Mm00711808_m1 (laminin-γ1), and Mm00500494_m1 (laminin-γ2)] were used for real-time PCR experiments on orpk cell lines. RNA extraction and real-time PCR experiments were performed at the URMC Functional Genomics Center. For human ARPKD cells, real-time PCR was performed as described above except that eukaryotic 18S RNA was used to normalize gene expression levels. The premade Taqman primer-probe assays used for human samples were Hs00165042_m1, Hs00165078_m1, and Hs01043711_m1 for human laminin-α3, laminin-β3, and laminin-γ2, respectively.
Table 1.
TaqMan real-time PCR primers and probes developed in this study
| Forward Primer | Reverse Primer | Probe | |
|---|---|---|---|
| Rat laminin-α3 | 5′-CAGATGGCCGTTGTCTTTGCTT-3′ | 5′-TCCATTTAGTCCGAACACCACCGT-3′ | 5′-AGCAAGGAGAGATACCATGACGGGAA-3′ |
| Rat laminin-α5 | 5′-ACAACTTTGAGCAGGCCTTCAAGC-3′ | 5′-TCCCATTGTAGGACACAAGACGCA-3′ | 5′-TTTGCCCGCATCACCTTTGAGAAGCA-3′ |
| Rat laminin-γ1 | 5′-CCTGCTTCTGCTTTGGGCATTCTT-3′ | 5′-ATCACAGCAATATCCTGCCGGTCT-3′ | 5′-TGTGTGCACCAATGCTGTTGGCTACA-3′ |
| Rat laminin-γ2 | 5′-TGCTGGATGTGACAACTCTGGACA-3′ | 5′-TCCAGCATCGGTGAGCATATGAA-3′ | 5′-ATGCAGGTGTAAGCCAGGTGTGGTA-3′ |
| Rat laminin-β1 | 5′-ACGCTCCAGCTTGCATGTGTTT-3′ | 5′-ACATTCGGAAGACACGAGCACTGA-3′ | 5′-TGTGTCTGCAATTACCTGGGCACAGT-3′ |
| Rat laminin-β3 | 5′-GCTGTGATCTTTGCAAGCCTGGTT-3′ | 5′-TCACAAGGCATATCTTGCCGGGTA-3′ | 5′-TGCCATCCCTGTGACTGCAGCATCTTA-3′ |
| Rat GAPDH | 5′-GTTACCAGGGCTGCCTTCTC-3′ | 5′-GGGTTTCCCGTTGATGACC-3′ | 5′-AACGGCACAGTCAAGGCTGAGAATG-3′ |
| Mouse GAPDH | 5′-AATGGTGAAGGTCGGTGTGAAC-3′ | 5′-GAAGATGGTGATGGGCTTCC-3′ | 5′-AGGCCGAGAATGGGAAGCTTG-3′ |
Immunoblot analysis.
For the investigation of laminin-γ2 levels in rat kidneys, kidney extracts from 8- to 12-wk-old SD and PCK rats were prepared according to a protocol adapted from Zuk et al. (50). Briefly, 0.15 g of frozen kidney tissue were added to 9 parts of extraction buffer [400 mM Tris (pH 8.9), 10 mM EDTA, and 2% SDS with a Complete Protease Inhibitor cocktail pellet (Roche Diagnostics, Mannheim, Germany)], homogenized (dounce homogenizer) on ice, and then boiled for 8 min at 95°C. Samples were then cooled at room temperature, 40 mM DTT was added, and the suspension was further homogenized and boiled for additional 4 min. Samples were then cooled at room temperature, sonicated (3 times, 15 s), and centrifuged at 16,000 g for 5 min in a microcentrifuge. The BCA protein assay was then performed, 330 μg total protein was loaded onto 3–5% SDS-PAGE gels, and the Western blot membrane was probed with a 1:200 dilution of laminin-γ2 antibody (sc-7652). The same membrane was stripped and reprobed with laminin-γ1 antibody (sc-6019). Equal total protein loading was ascertained by immunoblot analysis with a goat polyclonal actin antibody (sc-1616). Immunoblots were scanned using HP Scanjet 8300, and densitometry was performed using ImageJ software (34). Statistical analysis of these normalized densitometric results was performed using GraphPad Instat3 (La Jolla, CA) software. To detect laminin-332 in the ECM of PCDNA and BAP2 cells, the ECM was extracted directly with SDS extraction buffer [50 mM Tris·HCl (pH 8.8), 2% SDS, and 5 mM EDTA] after cells were removed with Nonidet P-40 lysis buffer (50 mM Tris·HCl, 150 mM NaCl, 1% Nonidet P-40, and 5 mM EDTA) (25). The ECM extract (16 μg) was probed with laminin-γ2 polyclonal antibody (sc-7652). The antibody that detected laminin-γ1 (sc-6019) in rat tissues failed to recognize mouse laminin-γ1 in the ECM of PCDNA/BAP2 cells, and, therefore, a Coomassie gel was used to ascertain equal loading (data not shown). A nonspecific protein band slightly below the 209-kDa marker in the Coomassie stain was used to normalize densitometric values.
Immunohistochemistry.
Frozen sections (8 μm) of adult PCK and SD rats were fixed with ice-cold acetone for 5–10 min, blocked with 0.3% BSA and 1% donkey serum, and incubated with 1:200 rabbit polyclonal antibody (20, 21) and 1:50 goat polyclonal to aquaporin (AQP)2 (sc-9882). After the primary antibodies were washed, slides were incubated with appropriate rhodamine (TRITC)- and fluorescein (FITC)-conjugated secondary antibodies, mounted with Dako mounting solution, and imaged using an FV1000 Olympus laser scanning confocal microscope. For laminin-511 immunostaining, a rabbit antiserum (no. 8948) to mouse laminin-α5 (gift of Dr. Jeffrey H. Miner, Washington University, St. Louis, MO) was used. Samples were processed similarly for immunohistochemistry except that sections were stained with a monoclonal antibody to laminin-332 (clone P3H9-2, MAB2144, R&D Systems, immunogen-human keratinocytes), which has been previously demonstrated to recognize human laminin-332 in several studies (3, 15, 42). Alternate sections were double stained either with a rabbit polyclonal antibody to AQP1 (AQP11-A, Alpha Diagnostics, San Antonio, TX) or with fluorescein-Dolichos biflorus agglutinin (DBA; Vector Laboratories, Burlingame, CA).
3-D Matrigel culture.
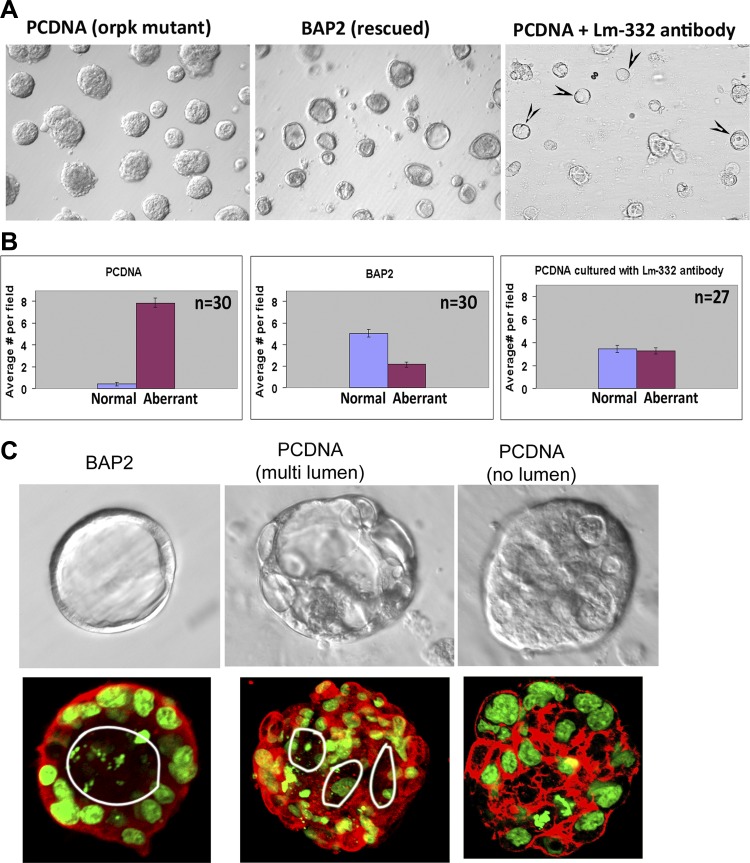
Cilia-negative orpk mutant cells (PCDNA) and rescued BAP2 cells were cultured using a Matrigel culture on-top strategy (22). Briefly, prechilled 12-well plates were coated with 240 μl of growth factor-reduced BD Matrigel matrix (BD Biosciences) and incubated at 37°C for 30 min, and 15,000 cells were then mixed in 400 μl of culture medium without interferon-γ. Cells were allowed to attach for another 30 min at 40°C. An additional 400 μl of the culture containing 10% Matrigel was then added, and cells were cultured in a 40°C incubator with 5% CO2 for up to 5 days and imaged with an Eclipse TE2000-S/4 microscope with Nomarski differential interference microscopy using Spot Advanced software (SPOT Imaging Solutions, Sterling Heights, MI) or TILLvisION (TILL Photonics, Rochester, NY). A rabbit polyclonal antibody to laminin-332 developed by Dr. Lazarova and Dr. Yancey (20), which has been demonstrated to be function blocking in mouse epithelia, was added at a 1:200 dilution in the culture medium. As a control, a 1:200 dilution of rabbit serum (Jackson Immunoresearch, West Grove, PA) was added similarly to the culture medium. Twenty-seven to thirty random fields ×10 magnification were imaged from PCDNA, BAP2, and PCDNA + laminin-332 function-blocking antibody, PCDNA + rabbit serum samples were collected from two to three independent experiments. Cysts with a single lumen (normal) or without any lumen or with multiple lumens (aberrant) were counted from these images and statistically analyzed using a two-tailed Student's t-test in Microsoft Excel.
Proliferation assay.
Cell proliferation assays were performed using Cell Titer 96 Aqueous One Solution Reagent (Promega, Madison, WI). Three sets of 2,000 PCDNA and BAP2 cells were seeded quintuplicate in 96-well plates and allowed to attach for 1 h in a 32°C (5% CO2) incubator. One set of cells was then added to 20 μl of the reagent and incubated for 2 h, and the absorbance at 490 nm was then recorded using a SpectraMax 340 plate reader (Molecular Devices, Sunnyvale, CA). These readings were for day 0. The other two sets of cells were cultured in a 32°C (5% CO2) incubator, and the proliferation assay was repeated at 24 h (day 1) and 48 h (day 2) postplating on these cells. In function-blocking experiments, 1:200 of rabbit polyclonal laminin-332 (as previously described) and 1:200 control rabbit sera were added. Cell numbers were determined using the equation derived from standard curves, and data from 10 data points (from 2 independent experiments) were analyzed using a two-tailed Student's t-test. Standard curves for the proliferation assays were generated as follows: 2,000–14,000 PCDNA and BAP2 cells were plated in triplicate in 96-well plates, allowed to attach for 1 h at 32°C, added to 20 μl of the reagent, and then allowed to incubate for 2 h.
RESULTS
The basement membrane of PCK rat kidneys exhibits structural abnormalities.
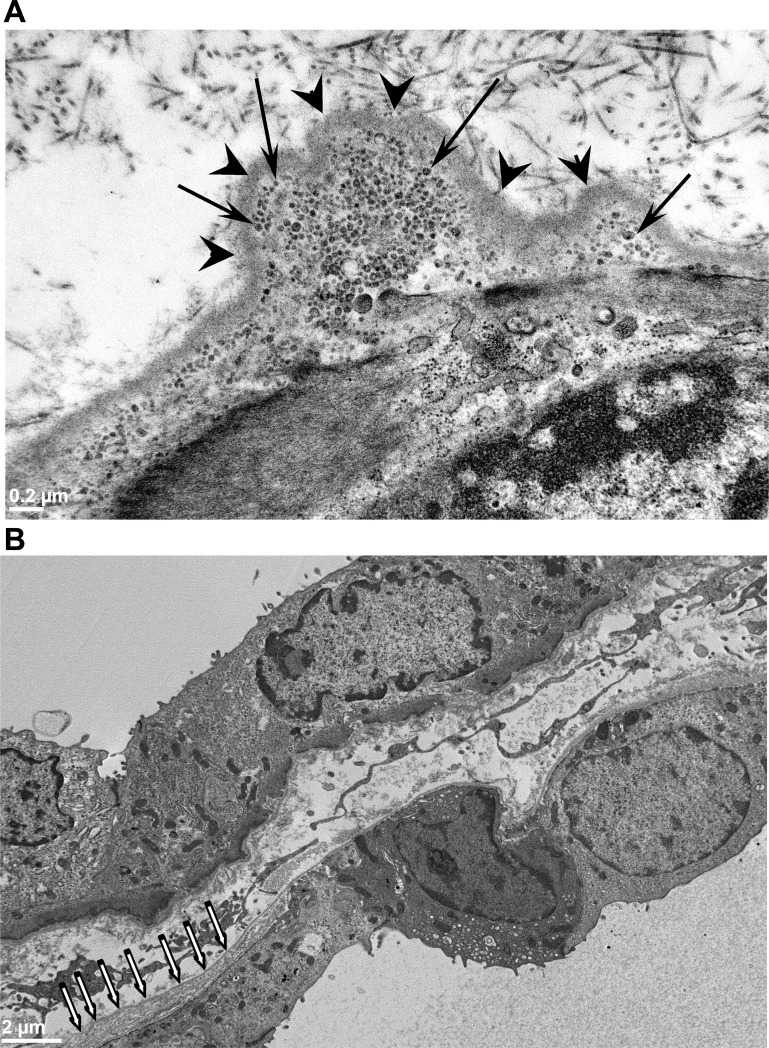
We examined kidney sections from adult (24 wk old) male PCK rats by transmission electron microscopy and observed striking ultrastructural abnormalities of the cyst basement membranes. The basement membranes lining cysts appeared to be focally evaginated into the interstitial compartment (Fig. 1A, arrowheads), and these evaginations were filled with vesicle-like structures (Fig. 1A, arrows). Additionally, we observed thickened multilamellated basement membranes (Fig. 1B). These features were not observed in normal tubular basement membranes of age-matched SD rat kidneys (data not shown). Similar abnormal features in the basement membrane were also observed in 12-wk-old male PCK rat kidney sections (data not shown). However, we did not make any quantitative observations about the extent of these abnormalities.
Fig. 1.
Abnormalities in the basement membranes of adult PCK rat kidney cysts. A: transmission electron microscopy (TEM) images of an adult PCK rat kidney section showing the evaginations of the basement membrane into the interstitium (arrowheads). These evaginations are filled with abnormal 30- to 50-nm vesicle-like structures (arrows). Size bar = 0.2 μm. B: another TEM image of a PCK kidney cyst showing a thickened, multilayered basement membrane (white arrows).
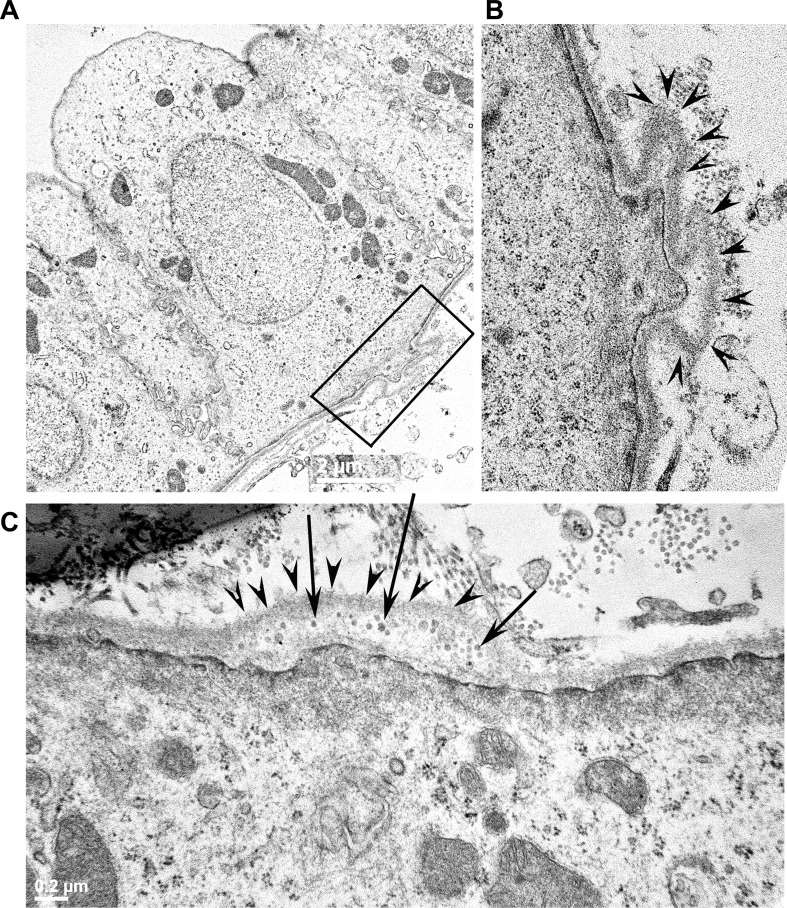
In PCK rats, occasional cysts were found as early as PN7, but these were more distinct by PN21 (Fig. 2A). Remarkably, kidney sections from PN21 PCK rats showed basement abnormalities similar to those of adult PCK rats, including evagination of the cystic basement membrane (Fig. 2B, arrowheads) and abnormal vesicle-like structures (Fig. 2C, arrows). These findings suggest that basement abnormalities begin early in age and coincide with the onset of cystogenesis.
Fig. 2.
Basement membrane abnormalities begin as early as postnatal day (PN)21 in PCK rat kidneys. A: TEM image of pericystic epithelia from a PN21 PCK rat kidney. The region highlighted by the rectangular box shows evaginations of the basement membrane into the interstitial compartment. B: magnified view of the region highlighted by the rectangular box in A. The evaginations of the basement membrane are marked with arrowheads in this image. C: a different image of a PN21 PCK pericystic kidney region, revealing the presence of vesicle-like structures (arrows) in the evaginations of the basement membrane (arrowheads) on the basal surface of the pericystic epithelia.
Laminin-332 is aberrantly expressed in human ARPKD.
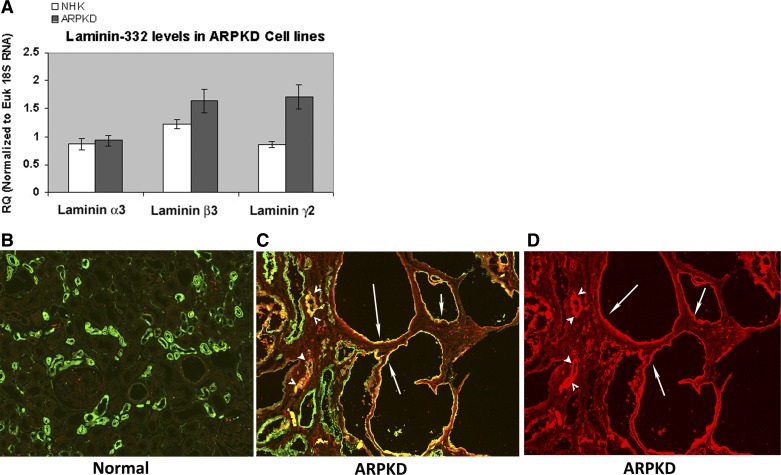
We examined mRNA levels for laminin-332 chains (α3, β3, and γ2) in four human ARPKD kidney and four NHK cell preparations. These samples were obtained by culturing cells from two human ARPKD kidneys and two NHK cells on Transwell filters and cell culture flasks. Ct values were similar for cells cultured on Transwell filters and cell culture flasks. We found that there was a two-fold increase (P < 0.05) in the expression of the laminin γ2-chain, but not α3- or β3-chains, in ARPKD cells compared with NHK cells (Fig. 3A). Fully functional trimeric laminin-332 assembly requires several steps of processing, including glycosylation of the individual laminin chains, β-γ heterodimer formation, association with the α-chain, secretion to the extracellular space, protelolytic processing, and, finally, polymerization. We used a specific antibody that recognized trimeric laminin-332 in immunohistochemistry to investigate whether or not there are differences in the trimeric laminin-332 appearance in ARPKD. Frozen ARPKD tissue embedded in OCT compound was sectioned and stained with a monoclonal antibody that recognizes human laminin-332 [clone P3H9-2, R&D Systems (3, 24, 42)]. Fluorescein-labeled DBA was used in double-labeling experiments to identify collecting ducts and cysts derived from collecting ducts. A representative result showed that there was no laminin-332 (red) staining in the NHK section (Fig. 3B); however, there was significant laminin-332 staining in the cysts of an ARPKD kidney (Fig. 3, C and D). Many of these cystic structures (marked with arrows) were also labeled with DBA (green staining), indicating that these cysts were derived from collecting ducts. Additionally, we also observed that some, but not all, of the precystic collecting ducts stained with the laminin-332 antibody (arrowheads in Fig. 3, C and D).
Fig. 3.
Laminin-332 is aberrantly expressed in human autosomal recessive polycystic kidney disease (ARPKD) kidney epithelia. A: relative expression levels of laminin α3-, β3-, and γ2-chains in human ARPKD cells compared with those of normal human kidney (NHK) cells. There was a significant 2-fold increase in mRNA levels of laminin-γ2 in ARPKD cells compared with NHK cells. B: frozen kidney sections of a human subject with no kidney disease stained with a monoclonal laminin-332 antibody (red) and Dolichos biflorus agglutinin (DBA; green) demonstrated no red (laminin-332) staining in collecting ducts. C: laminin-332 (red) and DBA (green) staining in cystic structures (arrows) and precystic tubules of a typical kidney section procured from an ARPKD/congestive heart failure patient. D: laminin-332 staining alone of the image in C for clarity.
Abnormal expression of laminin-332 in adult PCK rat kidney cysts and precystic tubules.
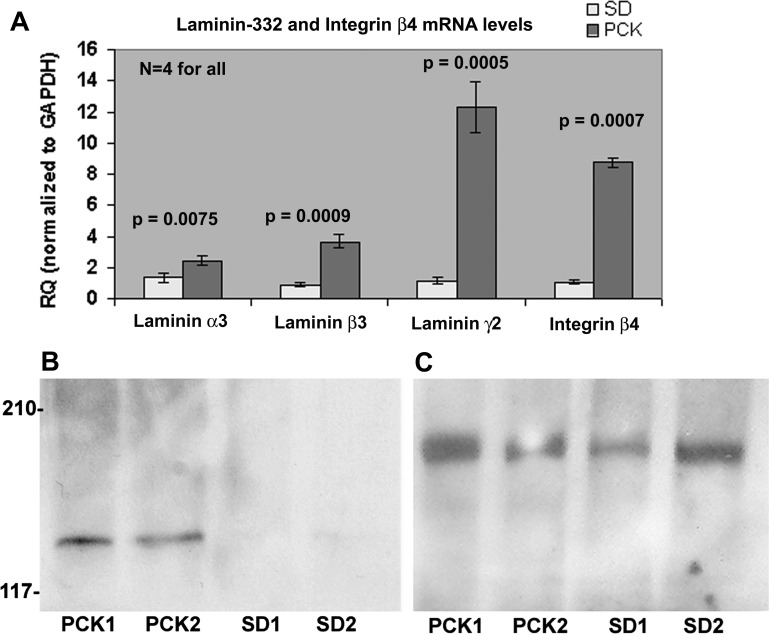
We performed real-time PCR on RNA from four different kidney tissues of adult (12–16 wk of age) PCK and SD rats. GAPDH was used as a normalization control. The results showed a 10-fold increase in the expression of laminin-γ2 in the PCK rat kidneys (Fig. 4A), a 4-fold increase in laminin-β3, a 2-fold increase in laminin-α3, and an 8-fold increase in integrin-β4, a receptor for laminin-332. Increases in mRNA expression levels of laminin-332 chains in PCK rats were highly significant (P < 0.005).
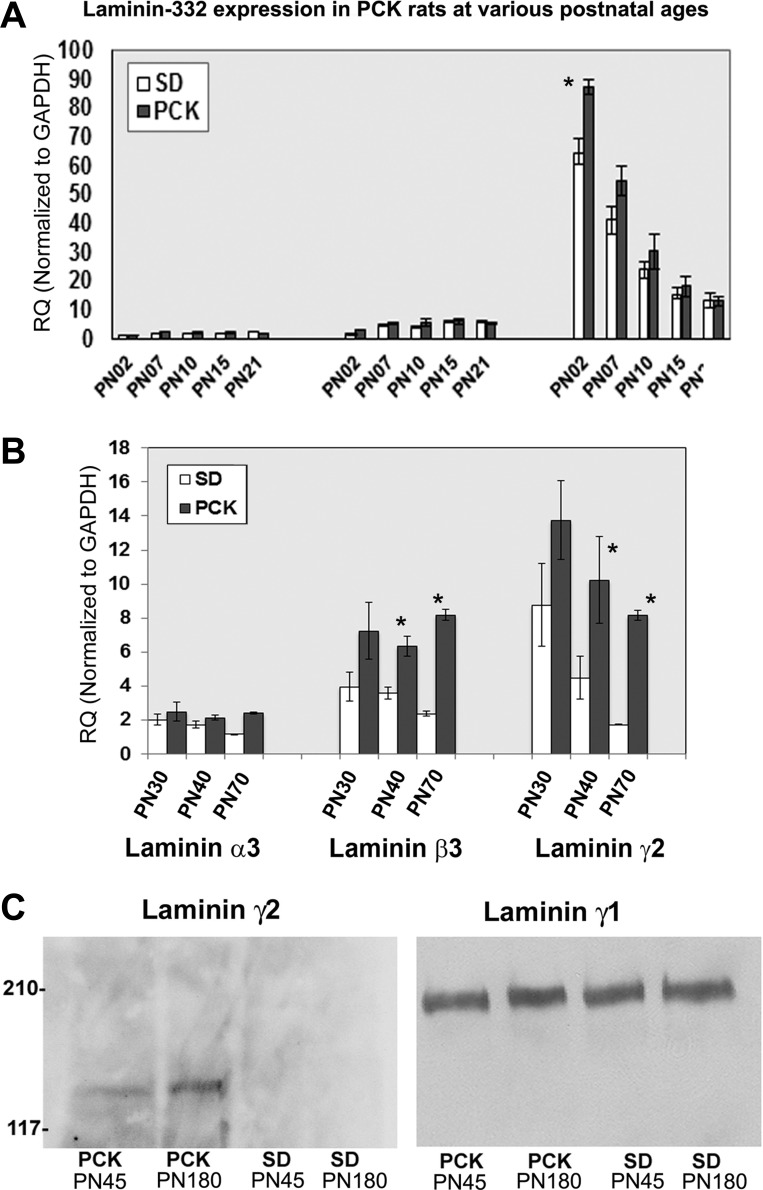
Fig. 4.
Laminin-332 is upregulated in adult PCK kidneys. A: real-time PCR was performed on four independent adult PCK kidney tissues, and the relative quantity (RQ) of gene expression was determined using the ΔΔCt method (where Ct is threshold cycle) with GAPDH as the normalization control. The graph shows the significant increase in the RQ of all laminin-332 chains as well as one of its receptor, integrin-β4, in PCK rat kidneys compared with Sprague-Dawley (SD) kidneys. The RQ of laminin-γ2 in PCK rats was 12.315 ± 1.631 versus 1.215 ± 0.195 in SD rats, a 10-fold increase. The RQ of integrin-β4 was 8.764 ± 0.297 versus 1.087 ± 0.050 in SD, an 8-fold increase. All P values for PCK versus SD relative expression were significant (P < 0.05) and are as shown. B: immunoblot of equal amounts (330μg) of kidney extracts obtained from two 8- to 12-wk old PCK rats (PCK1 and PCK2) and SD rats (SD1 and SD2) probed with a polyclonal antibody to laminin-γ2. C: immunoblot with laminin-γ1 antibody using the same membrane as that in B.
To determine whether there is increased laminin-332 expression at the protein level, we probed equal amounts of kidney extracts from two different adult PCK and SD rat kidneys (8–12 wk of age) using laminin-γ2 antibodies. Typical results are shown in Fig. 4, B and C. We observed laminin-γ2 bands only in the two PCK kidney samples but not in SD kidney samples (Fig. 4B). The same membrane was stripped and reprobed with a laminin-γ1 polyclonal antibody, which showed the presence of laminin-γ1 in both SD and PCK kidney extracts (Fig. 4C) as predicted by the real-time PCR results (see Fig. 7B). Also, immunoblot analysis with goat polyclonal anti-integrin-β4 demonstrated increased integrin-β4 expression in PCK rats (data not shown).
Fig. 7.
Abnormal laminin-332 expression by PN30 in PCK rat kidneys. Total RNA was extracted from whole kidney tissues of PN2, PN7, PN0, PN21, PN30, PN40, and PN70 PCK and SD kidneys, and real-time PCR was performed using Taqman primers and probes for rat laminin-332 chains and the sex-determining region Y (SRY) gene. A: RQ values of laminin α3-, β3-, and γ2-chains in PN2, PN7, PN10, PN15, and PN21 kidneys. There was a significant increase in laminin-γ2 transcript levels in PCK rats as early as PN2. B: RQ values of PCK and SD rat kidney laminin α3-, β3-, and γ2-chains of SRY-positive PN30, PN40, and PN70 SD and PCK rats. Values are means ± SE. RQ values of laminin-332 in PCK rats showed an increasing trend from PN30 onward. Results were analyzed using a two-tailed Student's t-test. For most of the samples n = 5 except for PN70, where n = 4. C: basement membrane fractions were extracted from PN45 and PN180 PCK and SD kidney tissues. Left, Western blot results of 330 μg of basement membrane fractions with a polyclonal antibody to laminin-γ2 (sc-7652). Right, Western blots of the same amounts of basement membrane extract probed with a polyclonal antibody to laminin-γ1 (sc-6019). Similar results were obtained for PN30 PCK rat kidney samples; however, the bands were very faint (data not shown).
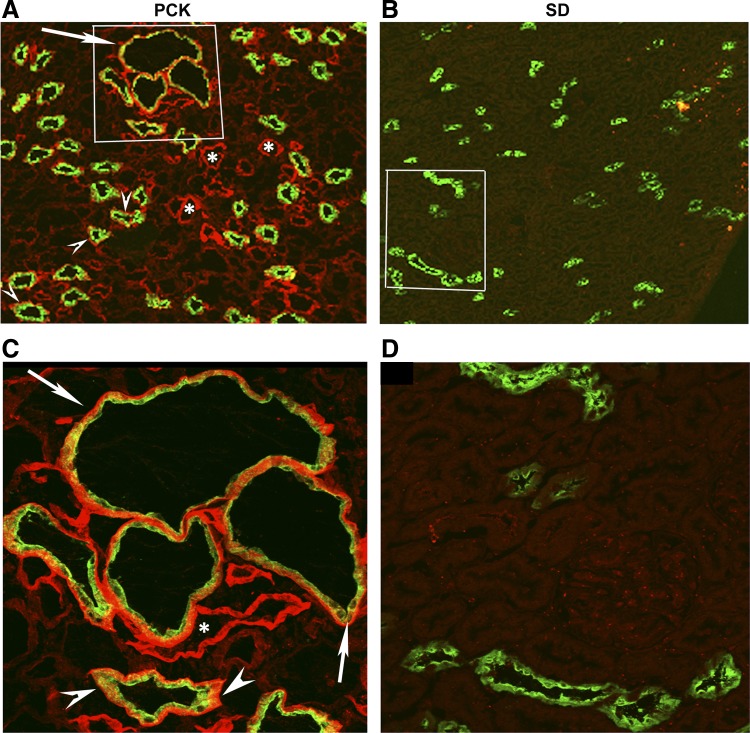
To examine the localization of laminin-332 in PCK kidneys, we double stained frozen sections of adult PCK and SD rat kidneys with a well-characterized rabbit polyclonal antibody to laminin-332 that recognizes all three chains of laminin-332 (20, 21) and a goat polyclonal AQP2 antibody. The AQP2 antibody was used to identify collecting ducts. The immunostaining results demonstrated the pericystic localization of laminin-332 in PCK rat kidney cysts (arrows in Fig. 5, A and C) and basement membrane localization of laminin-332 in precystic collecting ducts (arrowheads in Fig. 5, A and C). Additionally, we observed laminin-332 expression in the basement membrane of kidney tubules that were not stained with AQP2 (marked with asterisks in Fig. 5). However, in PCK kidney sections stained with antibodies to both laminin-332 and Na+/H+ exchanger 3 (NHE3; a proximal tubule marker) we observed that some, but not all, of the proximal tubules were positive for laminin-332 staining (data not shown). Control double immunostaining with antibodies to laminin-332/AQP2 and laminin-332/NHE3 performed on age-matched SD kidney sections did not show any laminin-332 staining either in collecting ducts marked with AQP2 (Fig. 5, B and D) or in proximal tubules stained with NHE3 (data not shown).
Fig. 5.
Laminin-332 expression in PCK rat cysts and precystic tubules but not in SD rat kidneys. Frozen sections of adult PCK rat kidneys and SD rat kidneys were immunostained with a rabbit polyclonal antibody to laminin-332 and a goat polyclonal aquaporin (AQP)2 (collecting duct marker) antibody. A: confocal image of a portion of the PCK kidney section with a few cysts and precystic tubules marked by AQP2 (green). C: magnified image of the area enclosed by the white rectangle in A. Laminin-332 staining (red) can be seen around the cysts (arrow) and precystic collecting ducts (arrowheads) that were stained with AQP2 (green). Laminin-332 staining was also observed in tubular structures that were not stained with AQP2 (*). B and D: laminin-332 (red) and AQP2 (green) double-stained images of SD rat kidneys. No laminin-332 staining was observed in either collecting ducts stained with AQP2 or other tubular structures in adult SD rat kidneys.
Laminin-511 is absent in the basement membrane of PCK cysts and precystic tubules.
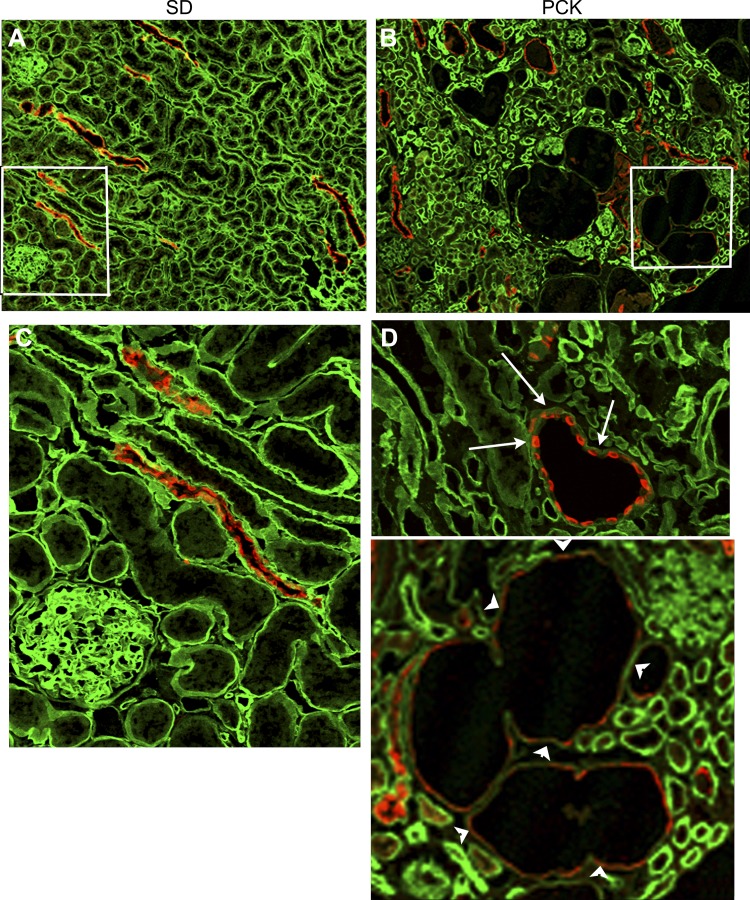
We also investigated the expression of laminin-511 in PCK rat kidney sections using a well-characterized polyclonal antibody that recognizes trimeric laminin-511 (7, 28, 33). Typical immunohistochemistry results are shown in Fig. 6. The results showed that laminin-511 expression (green) could be observed with uniform intensity in the basement membranes of most of the epithelial structures, including proximal and distal tubules and collecting ducts, of control SD rat kidneys (Figs. 6, A and B). However, in PCK rats, the intensity of laminin-511 staining was highly variable. Laminin-511 expression was conspicuously absent in the basement membrane of PCK rat kidney cysts (arrowheads in Fig. 6, C and D) and in most of the precystic collecting ducts that expressed AQP2 (arrows in Fig. 6, C and D), although some tubular segments showed intense laminin-511 staining in their basement membranes.
Fig. 6.
Laminin-511 is absent in the basement membranes of PCK cysts. Frozen sections of adult PCK rat kidneys and SD rat kidneys were double stained with a rabbit antiserum to laminin-α5 and a goat polyclonal antibody to AQP2 (red). The green color shows laminin-α5 staining. A: confocal image of a SD rat kidney section immunostained with laminin-α5 andAQP2 antibodies. There was uniform intensity of the green laminin-α5 staining in all tubular segments in this image. B: portion of a PCK kidney section with few cysts and precystic collecting ducts marked by AQP2 (red). Note that the green laminin-α5 staining was uneven, comparatively more in some tubular regions than in others, in the PCK kidney. C: magnified image of the region of SD rat kidney section enclosed by the white rectangle in A demonstrating the uniform distribution of laminin-511 in the collecting ducts (marked by red AQP2 staining) and other tubular regions not marked by AQP2 staining. D: two magnified images of PCK rat kidney sections showing the absence of laminin-511 (green) in the pericystic basement membrane (arrowheads) and in the basement membrane of precystic tubule (arrows).
Despite this considerable variation in the expression pattern, laminin-511 expression levels were not significantly different. Specifically, Western blot analysis of equal amounts of tissue lysates from PCK and SD kidneys revealed that the amount of laminin-γ1 was not significantly different (Figs. 4C and 7C). Although real-time PCR showed a 1.5- to 1.8-fold increase in the mRNA levels of laminin-α5, the expression levels of β1- and γ1-chains were not statistically different (data not shown).
Laminin-γ2 is abnormally expressed as early as PN2 and the appearance of aberrant laminin-332 protein coincides with the onset of cystogenesis in PCK rats.
Hematoxylin and eosin staining of frozen sections of PCK kidneys from various postnatal ages from PN2 to PN70 confirmed the earlier observation that although occasional small cysts were observed in PN7-PN10 kidneys, distinct moderately sized cysts were visible only by PN21- PN30 (data not shown) (19). We then examined PCK and SD kidneys from various postnatal ages, namely, PN2, PN7, PN10, PN15, PN21, PN30, PN40, and PN70, using real-time PCR. Since male PCK rats develop a more severe phenotype, using the SRY gene expression as a marker for males, we selected male PCK rat kidneys for gene expression analysis. As shown in Fig. 7A, the results demonstrated expression levels of laminin-α3, -β3, and -γ2 up to PN21. We observed that the expression of laminin-γ2 was significantly higher than of the other two components. Moreover, at PN2, the expression of laminin was significantly higher in PCK rats compared with SD rats (87.43 ± 2.39 in PCK rats vs. 64.826 ± 4.44 in SD rats, P = 0.001184). The increased expression of laminin-α3, -β3, and -γ2 in other time points up to PN21 was not statistically significant. As shown in Fig. 7B, the expression levels of laminin-α3, -β3, and -γ2 were consistently greater in PCK kidneys compared with SD kidneys beginning at PN30. There was a significant 1.8-fold increase in the expression of laminin-β3 by PN40 (6.340 ± 0.56 in PCK rats vs. 3.589 ± 0.37 in SD rats, P = 0.011) and a 3.5-fold increase by PN70 (8.179 ± 0.34 in PCK rats vs. 2.372 ± 0.13 in SD rats, P = 0.008). Also, there was a significant 4.7-fold increase in laminin-γ2 by PN70 (8.168 ± 0.29 in PCK rats vs. 1.740 ± 0.008 in SD rats, P = 0.004). In addition, in PN30 samples, we observed a 2-fold increase in the expression levels of laminin-β3 (7.237 ± 1.68 in PCK rats vs 3.961 ± 0.87 in SD rats) and a 1.5-fold increase in the levels of laminin-γ2 (13.764 ± 2.29 in PCK rats vs. 8.768 ± 2.45 in SD rats), although our results for PN30 were not statistically significant. It is also interesting to note that, whereas the expression levels of laminin-α3, -β3, and -γ2 generally showed a decreasing trend from PN30 to PN70 in SD rats, in PCK rats the laminin-332 expression remained elevated.
Western blot analysis of equal amounts of kidney extracts of PN21 to PN80 kidney tissues with laminin-γ2 polyclonal antibody demonstrated the presence of laminin-γ2 in PCK kidneys as early as PN45 but not in age-matched SD kidneys (Fig. 7C, left). However, laminin-γ1 was observed in equal amounts in all four samples (Fig. 7C, right). A faint laminin-γ2 band was observed in PN30 samples (data not shown). Equal amounts of loading were ascertained by Western blot analysis with a polyclonal antibody to actin (data not shown). In summary, these experiments demonstrate that although there was a statistically significant expression of laminin-γ2 as early as PN2, the aberrant expression of trimeric laminin-332 in PCK rat kidneys began as early as PN30.
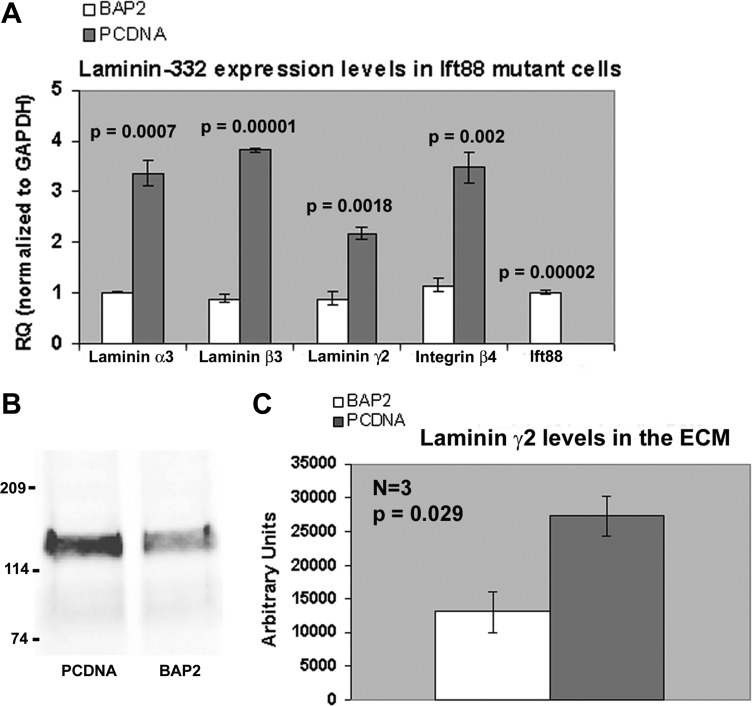
Upregulation of laminin-332 expression in the Ift88 mutant cell line.
The orpk mouse has a mutation in the Ift88 gene that disrupts the expression of polaris (IFT88), an intraflagellar transport protein required for ciliogenesis (30, 47, 48). This defect causes complete loss or severe stunting of the apical primary cilia, and orpk mice exhibit an ARPKD phenotype with cysts arising in the proximal tubule and collecting duct segments of the kidney. In the absence of an established cell line from PCK rat kidneys, we investigated confluent monolayers of an immortalized cell line (PCDNA) derived from collecting duct principal cells of orpk mice transfected with the empty vector pCDNA3.1 and a rescued cell line (BAP2), which expresses endogenous levels of Ift88 using real-time PCR. As shown in Fig. 8A, the results demonstrated a substantial (2.5- to 4.3-fold) and significant (P < 0.002) upregulation of all three laminin-332 chains as well as integrin-β4 in cilia mutant PCDNA cells compared with rescued cells (P < 0.05). Additionally, Western blot analysis of equal amounts (16 μg) of the ECM fraction of confluent monolayers of PCDNA and BAP2 cells revealed that PCDNA (mutant) cells had increased laminin-332 in their ECM compared with their rescued counterparts (Fig. 8B). Densitometric analysis of three independent Western blots showed that the average densitometric value of the PCDNA bands was 27,246 ± 2,891, which was significantly higher (2-fold increase, P = 0.027) than the average BAP2 value of 13,092 ± 2,982. These results indicate that aberrant laminin-332 expression is a hallmark of ARPKD and that one should be able to obtain valuable insights on the mechanism by which laminin-332 regulates cystogenesis using the orpk cell line.
Fig. 8.
Ift88 mutant cell lines derived from the Oak Ridge polycystic kidney (orpk) mouse also show increased expression of laminin-332. Immortalized orpk mutant cell line 94D-pCDNA (PCDNA) and the rescued cell line 94D-Tg737Bap-2 (BAP2) were cultured. A: real-time PCR experiments were performed on RNA extracted from three independent monolayer cultures of PCDNA and BAP2 cells with mouse-specific probes and primers to all three laminin-332 chains, integrin-β4, and Ift88. The RQ was determined using the ΔΔCt method with GAPDH as the normalization control. The graph shows the significant increase (P < 0.005) for all laminin-332 chains and integrin-β4 in PCDNA compared with BAP2 cells. Note the minimal expression of Ift88 in PCDNA cells, confirming that PCDNA cells do not express Ift88. B: 16 μg of extracellular matrix (ECM) extracts from PCDNA and BAP2 cells were probed with a laminin-γ2 polyclonal antibody (sc-7652). The results indicate that laminin-γ2 expression was more intense in PCDNA cells than in BAP2 cells (left). A Coomassie-stained gel loaded with 16 μg of ECM extracts of PCDNA and BAP2 cells was used to normalize the densitometric values of the laminin-γ2 bands (data not shown). Densitometric analysis of three independent Western blots showed that there was a significant 2-fold increase (P < 0.05) of laminin-γ2 in the ECM of PCDNA cells compared with that of BAP2 cells (right).
Laminin-332 function-blocking antibodies inhibit the formation of the abnormal cyst phenotype in Matrigel culture of Ift88 mutant cells.
When we cultured cilia mutant PCDNA and rescued BAP2 cells in Matrigel, we observed a dramatic difference in the morphology of the cyst-like structures that developed after 4–5 days in culture. The representative light microscopic images (at ×10 magnification) shown in Fig. 9A, left and middle, demonstrated that, whereas most of the rescued BAP2 cysts had a clear and well defined lumen (normal), most of the PCDNA cysts lacked a distinct lumen or had multiple lumens (aberrant). This observation is consistent with a loss of planar cell polarity and disorganized proliferation of cells lacking primary cilia (12, 31). Laser scanning confocal microscopy analysis of phalloidin (F-actin)-stained images of these structures confirmed a single lumen in most of the BAP2 cysts, whereas multiple lumens or no lumen were observed in mutant PCDNA cysts (Fig. 9C). The results of the statistical analysis of normal and aberrant structures from 30 random fields are shown in Fig. 9B. The results show that whereas only ∼5% of the cysts in PCDNA cells had normal cyst-like structures with distinct lumens, nearly 70% of the BAP2 cyst-like structures had a distinct single lumen.
Fig. 9.
Function-blocking antibodies to laminin-332 inhibit the formation of an aberrant phenotype in Matrigel cultures of Ift88 mutant PCDNA cells. A: PCDNA and BAP2 cells were cultured in 12-well plates containing Matrigel for 4 days and imaged with Nomarski differential interference light microscopy. Typical ×10 fields of BAP2 cells (middle) showed distinct single lumens, which were absent in PCDNA cells (left). Culture of PCDNA cells in the presence of a 1:200 dilution of function-blocking polyclonal antibody to laminin-332 resulted in the appearance of cyst-like structures with lumen (arrowheads; right). B: cyst-like structures with a distinct single lumen were termed as “normal” and cyst-like structures that lacked a distinct single lumen or had multiple lumens were termed as “aberrant.” Normal and aberrant structures were quantified from 30 different random fields from PCDNA and BAP2 Matrigel cultures and evaluated for statistical significance using a two-tailed Student's t-test. The results from PCDNA (left) and BAP2 (middle) cells revealed that only 5% of PCDNA cysts had well-defined lumens, whereas 70% of BAP2 cysts had distinct lumens (lilac bars). In the presence of laminin-332 function-blocking antibody, 51% of PCDNA cysts had well-defined lumens (right). C, top: Nomarski differential interference contrast images of a typical BAP2 cyst-like structure with a single lumen (left), a PCDNA cyst-like structure with multiple lumens (middle), and a PCDNA cyst-like structure with poorly defined lumens. Bottom, confocal slices of phalloidin (F-actin, red)- and Sytox green (nuclear stain)-stained cyst-like structures derived from BAP2 (left) and PCDNA (middle and right) in Matrigel culture. The single lumen in the BAP2 cyst-like structure and multiple lumens in the PCDNA cyst-like structure are indicated by the white circles.
We then examined the effect of a laminin-332 function-blocking antibody on the phenotypes of PCDNA cysts. For this purpose, we used a laminin-332 function-blocking antibody that has been previously demonstrated to induce subepidermal blisters in mice (21). We also confirmed that this antibody recognized laminin-332 expressed by PCDNA cells using Western blot analysis (data not shown). The light microscopic image of a typical field (×10 magnification) of PCDNA cells cultured with this laminin-332 function-blocking antibody is shown in Fig. 9A, right, and demonstrated that at least 50% of the cysts had a well-defined single lumen. Quantification of images from 27 fields obtained from 3 independent experiments confirmed that 51% of the cyst-like structures formed in PCDNA cells had single lumens in the presence of laminin-332 function-blocking antibody as opposed to a mere 5% in the absence of this antibody (Fig. 9B). No significant difference was observed in PCDNA cells cultured in the presence of nonspecific rabbit IgG (data not shown).
Laminin-332 function-blocking antibodies inhibit the proliferation rates of Ift88 mutant cells.
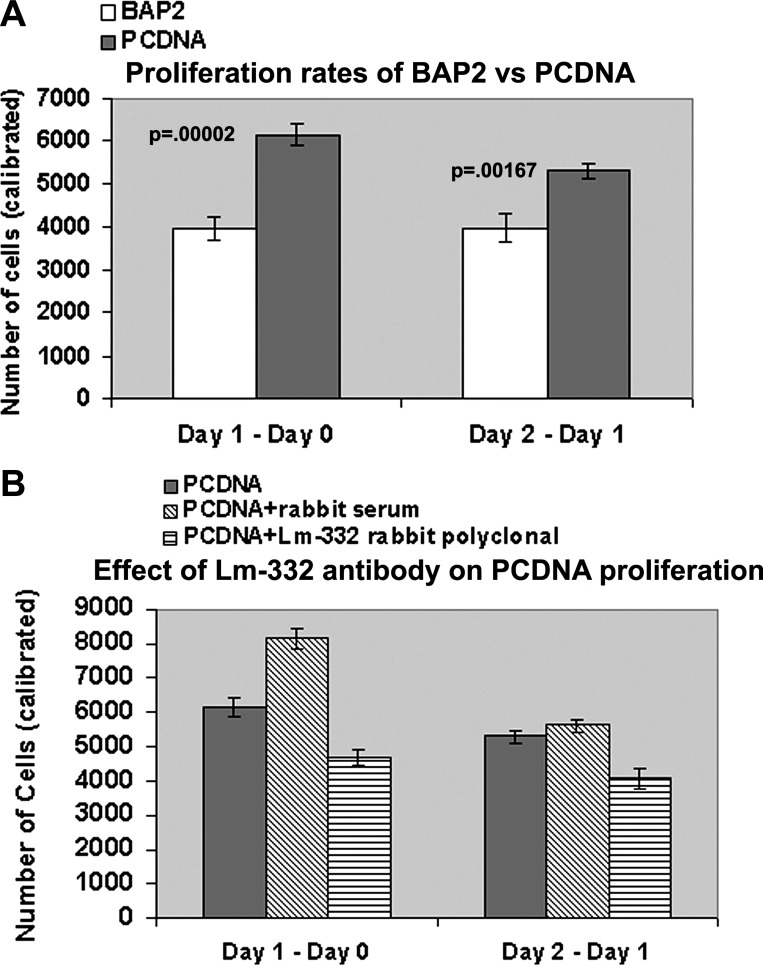
We have consistently observed that cilia mutant PCDNA cells proliferated at a faster rate than rescued BAP2 cells. To ascertain this and to quantify the proliferation rates, we performed a CellTiter 96 Aqueous One Solution Cell Proliferation Assay, in which the absorbance at 490 nm is directly proportional to the number of living cells in culture. After the standard curve was determined (using cell titration performed using various numbers of cells, 2,000–14,000), PCDNA and BAP2 cells were plated at 2,000 cells/well (in quintuplicate), and the proliferation assay was performed after 1 h, 1 day, and 2 days of plating. Proliferation rates were determined as described in materials and methods. The results shown in Fig. 10A demonstrated that PCDNA cells quadrupled their number by day 1 (∼6,100 cells increased; day 1 − day 0) and added another 5,300 cells on day 2 (day 2 − day 1). This was significantly higher than the increase in the number of BAP2 cells, which was ∼3,900 cells.
Fig. 10.
Laminin-332 function-blocking antibody inhibits the aberrant proliferation of Ift88 mutant PCDNA cells. A: CellTiter 96 Aqueous One Solution cell proliferation assays were performed on day 0 (1 h after plating), day 1 (24 h after plating), and day 2 (48 h after plating) on 2,000 PCDNA and BAP2 cells seeded on 12-well plates. Left, increase in the number of cells on day 1 (number of cells on day 1 − number cells on day 0); right, increase in the number of cells on day 2 compared with day 1 (number of cells on day 2 − number cells on day 1). There was a significant increase (P < 0.002) in the number of PCDNA cells compared with BAP2 cells on both days. B: proliferation assays were performed on PCDNA cells that were either cultured in the presence of a 1:200 dilution of polyclonal antibody to laminin-332 or in the presence of a 1:200 dilution of control rabbit serum. There was a significant reduction in the proliferation rates of PCDNA cells cultured in the presence of laminin-332 function-blocking antibody for both day 1 − day 0 and for day 2 − day 1 compared with either PCDNA cells alone (P < 0.0002) or PCDNA cells cultured in the presence of control serum (P < 0.0002).
To determine if blockade of laminin-332 signaling inhibits the increased proliferation of PCDNA cells, we performed proliferation assays on PCDNA cells cultured in the presence of the function-blocking polyclonal antibody. Similar amounts of preimmune rabbit serum were used in control experiments. The addition of laminin-332 polyclonal antibody significantly decreased the proliferation of PCDNA cells significantly to 4,687 ± 192 cells for day 1 − day 0 and to 4,086 ± 279 cells for day 2 − day 1 (Fig. 10B). The P value was <0.002 for PCDNA + laminin-332 compared with PCDNA alone (n = 10). This inhibition in the proliferation rates in the presence of laminin-332 antibody was more pronounced and more significant compared with the proliferation rates of PCDNA cells cultured in the presence of serum, which showed increased proliferation compared with PCDNA cells alone (to 8,146 ± 289 vs. 6,151 ± 249 for day 1 − day 0). In summary, the addition of laminin-332 polyclonal antibody significantly decreased the proliferation rates of Ift88 mutant (cilia defective) cells.
Laminin-332 is aberrantly expressed in cysts and precystic tubules of human ADPKD tissues.
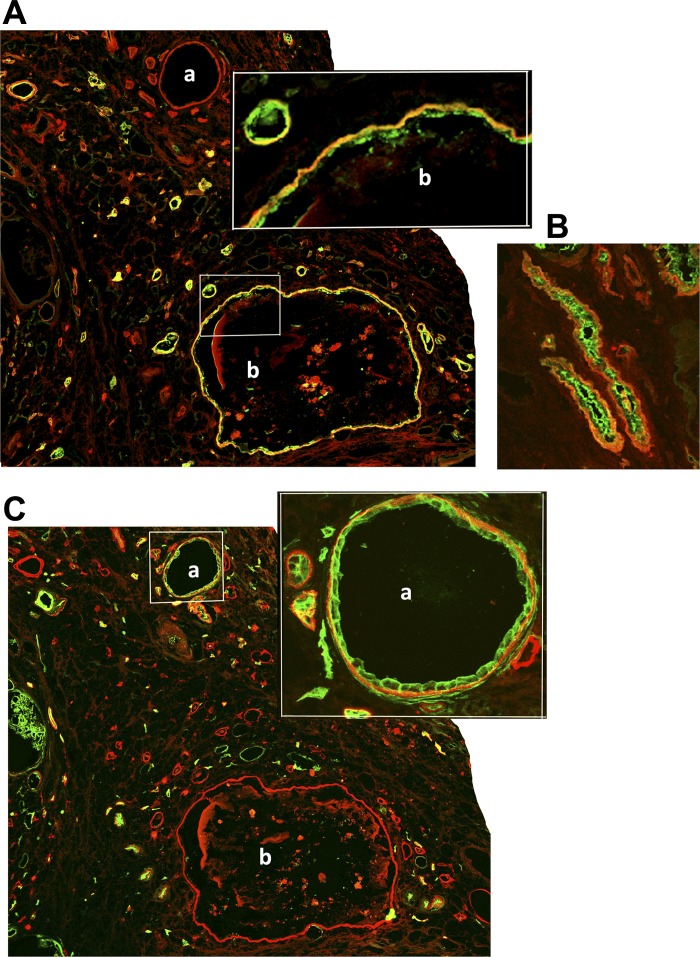
We investigated snap-frozen human end-stage ADPKD and control age-matched NHK samples by immunostaining with the clone P3H9-2 monoclonal antibody to laminin-332. Alternate frozen sections were counterstained with DBA to identify collecting ducts and with a polyclonal antibody to AQP1, a proximal tubule marker. As shown in Fig. 11A, the cyst (labeled “b”) stained with DBA (green) exhibited laminin-332 staining in its basement membrane (red), indicating that there was elevated expression of laminin-332 in cysts that originated from the collecting duct. We also observed laminin-332 in the basement membranes of DBA-stained collecting ducts that were not yet cystic (Fig. 11B), indicating laminin-332 expression is an early event in ADPKD. In a parallel section of the same tissue, a cyst that was positive for AQP1 staining (labeled “a”), a marker for proximal tubules, also expressed laminin-332 in the basement membrane (Fig. 11C). Control experiments performed in a similar manner on NHK samples did not show any laminin-332 staining either in AQP1-stained proximal tubules (data not shown) or in DBA-stained collecting ducts (see Fig. 3B). Our results show that human ADPKD cysts exhibited aberrant basement membrane laminin-332 irrespective of whether they originated from collecting ducts or proximal tubules. In addition, these results also show that not only cysts but also precystic collecting ducts of human ADPKD patients express laminin-332 abnormally.
Fig. 11.
Laminin-332 is aberrantly expressed in the cysts and precystic tubules of human autosomal dominant polycystic kidney disease (ADPKD) kidneys. A: frozen sections of a cystic kidney from a 46-yr-old male ADPKD patient (from the National Disease Research Interchange) were stained with a monoclonal antibody to laminin-332 (clone P3H9-2, red) and with DBA (collecting duct marker, green). A magnified image of the region marked by the rectangular box is shown as an inset. The cyst labeled “b” in A is of collecting duct origin because it is stained with DBA. B: an adjacent section of the same human ADPKD kidney tissue showing that precystic collecting ducts stained with DBA (green) exhibited laminin-332 in their basement membrane (red). C: double staining of a parallel section to the one shown in A with the same monoclonal antibody to laminin-332 and with a polyclonal antibody to AQP1 (proximal tubule marker) showing that the cyst labeled “a” stained with both AQP1 (green) and laminin-332 (red). A magnified image of the region marked with a square box is shown as an inset for clarity.
DISCUSSION
The results of the study demonstrate the following: 1) there are early ultrastructural abnormalities in the basement membranes of cystic epithelial cells of PCK rats; 2) the laminin γ2-chain is elevated in primary cultures of ARPKD cells and there is aberrant expression of laminin-332 in human ARPKD kidneys; 3) in PCK rats, laminin-γ2 expression is elevated as early as PN2 and laminin-332 protein is increased by PN30, demonstrating that laminin-332 expression is an early feature of cyst formation in ARPKD; 4) elevated laminin-332 expression coincides with a marked decrease in laminin-511; 5) the loss of primary cilia formation in collecting duct orpk cells leads to increased cell proliferation and an abnormal epithelial phenotype, including multilumen cyst formation, characteristics that are corrected by treatment of the cells with a laminin-332-blocking antibody; and 6) laminin-332 is aberrantly expressed in cysts and precystic tubules of human ADPKD kidneys, indicating that abnormal laminin-332 expression is a common feature of renal cystic disease regardless of the gene mutation.
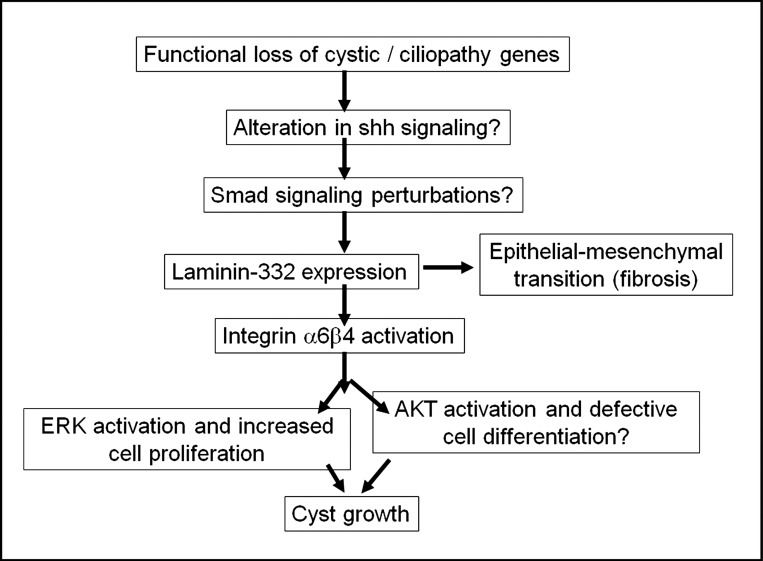
Abnormal matrix turnover and matrix deposition in PKD is well established; however, it unclear if these changes occur early in cyst formation or are secondary to tissue damage due to the expansion of cysts. Here, we show elevated expression of laminin-γ2 transcripts in collecting ducts before notable cyst formation in PCK rats and abnormal trimeric laminin-332 expression as early as PN30 in PCK rats, suggesting that laminin-332 expression may be an important factor in the initial phase of renal cystogenesis. It should be noted that while our results showed a significant increase in laminin-γ2 expression, we were unable to detect trimeric laminin-332 in PCK kidneys at PN2 or PN10. Additionally, laminin-γ2 expression was significantly higher than that of the other two components (laminin-α3 and -β3), making it unclear if laminin-γ2 alone may have a unique function. Previously, laminin-γ2 expression has been observed in the ureteric bud and has been suggested to play a role in kidney development (1). Laminin-γ2, but not trimeric laminin-332, is highly expressed in anaplastic thyroid carcinoma, suggesting that laminin-γ2 itself promotes cell proliferation by modulating signaling of the EGF receptor (6). It is also possible that while transient expression of laminin-γ2 during kidney development is required, failure to downregulate its expression as the kidney develops could result in the activation of pathways involved in cyst growth (Fig. 12). Further experiments are necessary to confirm this possibility.
Fig. 12.
Proposed mechanisms for aberrant laminin-332 signaling in the progression in polycystic kidney disease. shh, sonic hedgehog.
The mechanism for increased cell proliferation induced by laminin-332 remains undefined. We found that the expression levels of integrin-β4, an essential component of one of the two main receptors of laminin-332, namely, integrin-α6β4, is also upregulated in PCK rat kidneys (Fig. 4). This is consistent with previous observations suggesting that laminin-332 promotes aberrant cell proliferation via integrin-α6β4-ERK activation in ADPKD cells. Subconfluent cultures of Madin-Darby canine kidney cells also express laminin-332, possibly mediated by transforming growth factor (TGF)-β-Smad2/4 activation, and the assembly of laminin-332 in the ECM promotes proliferation (25, 29). In this study, we found that a function-blocking antibody to laminin-332 significantly inhibits the proliferation of orpk cells, providing further support for the hypothesis that laminin-332 expression contributes to tubule dilation in the early stages of cystogenesis.
Aberrant laminin-332 expression and the upregulation of integrin-β4 were accompanied by the downregulation of laminin-511 assembly in collecting ducts and cysts of PCK rats (Fig. 6). Previously, mice with a hypomorphic mutation in the laminin α5-chain were shown to develop renal cysts, and many of the cysts had an aberrant expression of laminin-332 (38). Laminin-332 is thought to promote epithelial cell proliferation, whereas laminin-511 has been implicated in the terminal differentiation of various epithelia, including the intestine and hair follicles (7, 23). Laminin-332 mediates the formation of hemidesmosomes in keratinocytes in association with collagen type XVII and integrin-α6β4, and it has been postulated that hemidesmosomes may critically regulate the organization of the cytoskeleton, polarization, and proliferation of the cells (2, 44). Defects in planar cell polarity are thought to contribute to cyst growth in ARPKD (12, 31), and a recent study by Greciano et al. (10) showed that both laminin-332 and laminin-511 regulate polarity in Madin-Darby canine kidney cells (10). In Matrigel cultures of orpk cells, the abnormal epithelial cyst structures with defective lumen formation strongly suggest that orpk mutant cells have a defect in planar cell polarity that could be at least partially rescued by the addition of laminin-332 function-blocking antibody. It is conceivable that a reduction in differentiation-promoting laminin-511 and expression of laminin-332 triggers abnormalities in ciliopathic or cystic genes contributing to the cystic phenotype of cells within entire regions of the nephron.
Additionally, our results confirmed that there is aberrant expression of laminin-332 in human ADPKD (13, 14) and that there is increased expression of this laminin in precystic tubules. Aberrant expression of laminin-332 in the ADPKD kidney is not restricted to collecting duct cysts but is also expressed by cysts derived from proximal tubules. The demonstration of aberrant laminin-332 expression in ARPKD and ADPKD suggests that it plays a role in cyst growth irrespective of the primary mutation. How defects in these diverse genes responsible for cystic disease lead to abnormal expression of laminin-332 remains unclear. There is increasing evidence showing that primary cilia regulate sonic hedgehog signaling (17), and results of a recent study (49) suggest that hedgehog signaling regulates the Smad signaling pathway. As mentioned above, the TGF-β-Smad2/4 pathway regulates the assembly of laminin-332. Hence, it is conceivable that defects in cystic genes (PKD1, PKD2, and PKHD1) or ciliopathy genes (Ift88 and Kif1) could upregulate laminin-332 synthesis via the sonic hedgehog-TGF-β-Smad pathway. A previous study (18) has shown that laminin-332 overexpression in stromal fibroblasts alters their phenotype via an Akt pathway. A model illustrating possible mechanisms by which aberrant laminin-332 contributes to cyst growth in early cystogenesis and disease progression in PKD is shown in Fig. 12.
Our results do not rule out the possibility that laminin-332 could contribute to the progression of PKD by inducing renal fibrosis. In hepatocellular carcinoma and in oral squamous cell carcinoma cells, laminin-332, in conjunction with TGF-β, promotes the epithelial-mesenchymal transition (8, 36), and such mechanisms could be involved in cystogenesis. Consistent with this idea, renal cysts from PN1, PN3, and PN10 as well as 14-wk-old and 4-mo-old PCK rats express mesenchymal markers such as vimentin and α-smooth muscle actin (39).
Therapeutic interventions aimed at interfering with cell proliferation mechanisms have been considered in the treatment of ADPKD. For example, drugs developed to suppress cell proliferation, such as inhibitors of ErbB1, ErbB2, Src kinase, mammalian target of rapamycin, and cyclin-dependent kinases, have been tested in animal models of PKD as potential therapeutic agents (40). However, targeting these signaling pathways in neonates and infants will pose serious side effects and may not be suitable for the treatment of ARPKD. If aberrant laminin-332 contributes to or causes renal pathology in ARPKD by enhancing tubular cell proliferation or alternate mechanisms, the development of single-chain antibodies directed against the appropriate laminin-332 chains could be envisioned as a therapy for ARPKD. Since laminin composition in the basement membranes of different tissues is quite distinct, inhibition of aberrant laminin-332 assembly with the help of biologics such as single-chain antibodies might be able to interfere only with the proliferation of selected cells, such as cystic epithelial cells.
GRANTS
S. Vijayakumar was supported by National Institutes of Health (NIH) Grant UL1-RR-024160-05 (Clinical and Translational Support Institute, Principal Investigator: Thomas A. Pearson) and by the Department of Pediatrics, University of Rochestoer Medical Center. M. P. Marinkovich acknowledges financial support from NIH Grants AR-44012 and AR-47223 (Office of Research and Development, Palo Alto Veterans Affairs Health Care System). B. Yoder acknowledges support from NIH Grants P30-DK-074038 and R01-DK-065655 (University of Alabama at Birmingham Hepatorenal Fibrocystic Kidney Disease Core Center). Z. Lazarova acknowledges financial support from a Froedtert Hospital Foundation Skin Cancer research grant. V. E. Torres acknowledges support of NIH Grant P30-DK-09072 (Mayo Translational PKD Center). D. Wallace is supported by NIH Grant R01-DK-081579. Some human tissues were procured by the National Disease Research Interchange with support from NIH Grant 5-U42-RR-006042. The generation of primary cell cultures by the PKD Biomaterials and Cellular Models Core was funded in part by a grant from the PKD Foundation (to D. P. Wallace).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.V. and V.E.T. conception and design of research; S.V., S.D., and D.P.W. performed experiments; S.V. and S.D. analyzed data; S.V., M.P.M., Z.L., B.K.Y., V.E.T., and D.P.W. interpreted results of experiments; S.V. and S.D. prepared figures; S.V. drafted manuscript; S.V., M.P.M., Z.L., B.K.Y., V.E.T., and D.P.W. edited and revised manuscript; S.V. and D.P.W. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Tricia Nienaber, Jessica Stolar, and Kanchan Parkhi for technical help. The authors appreciate Dr. Vincent Gattone's help in the interpretation of electron microscopy (EM) results and Dr. Jeffrey H. Miner for the laminin-α5 antibodies. Undergraduate students Carolyn Thornton and Alisa Johnson aided in carrying out some aspects of this study. The authors acknowledge the assistance of Dr. Stephen Welle and Michelle Zanche (Functional Genomics Center, University of Rochester Medical Center), Dr. Linda Callahan (Confocal and Conventional Microscopy Core, University of Rochester Medical Center), Karen Bentley (EM Core), and Karen Vanderbilt (Histology).
Some aspects of this study were presented as a poster at the American Society of Nephology annual meeting in 2012.
REFERENCES
- 1.Aberdam D, Aguzzi A, Baudoin C, Galliano MF, Ortonne JP, Meneguzzi G. Developmental expression of nicein adhesion protein (laminin-5) subunits suggests multiple morphogenic roles. Cell Adhes Commun 2: 115–129, 1994 [DOI] [PubMed] [Google Scholar]
- 2.Borradori L, Sonnenberg A. Structure and function of hemidesmosomes: more than simple adhesion complexes. J Invest Dermatol 112: 411–418, 1999 [DOI] [PubMed] [Google Scholar]
- 3.Carpenter PM, Dao AV, Arain ZS, Chang MK, Nguyen HP, Arain S, Wang-Rodriguez J, Kwon SY, Wilczynski SP. Motility induction in breast carcinoma by mammary epithelial laminin 332 (laminin 5). Mol Cancer Res 7: 462–475, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Cuppage FE, Huseman RA, Chapman A, Grantham JJ. Ultrastructure and function of cysts from human adult polycystic kidneys. Kidney Int 17: 373–381, 1980 [DOI] [PubMed] [Google Scholar]
- 5.Drummond IA. Polycystine, focal adhesions and extracellular matrix interactions. Biochim Biophys Acta 1812: 1322–1326, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garg M, Kanojia D, Okamoto R, Jain S, Madan V, Chien W, Sampath A, Ding LW, Xuan M, Said JW, Doan NB, Liu LZ, Yang H, Gery S, Braunstein GD, Koeffler HP. Laminin-5γ-2 (LAMC2) is highly expressed in anaplastic thyroid carcinoma and is associated with tumor progression, migration and invasion by modulating signaling of EGFR. J Clin Endocrinol Metab 99: E62–E72, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gao J, DeRouen MC, Chen CH, Nguyen M, Nguyen NT, Ido H, Harada K, Sekiguchi K, Morgan BA, Miner JH, Oro AE, Marinkovich MP. Laminin-511 is an epithelial message promoting dermal papilla development and function during early hair morphogenesis. Genes Dev 22: 2111–2124, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giannelli G, Bergamini C, Fransvea E, Sgarra C, Antonaci S. Laminin-5 with transforming growth factor-β1 induces epithelial to mesenchymal transition in hepatocellular carcinoma. Gastroenterology 129: 1375–1383, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Grantham JJ, Mulamalla S, Swenson-Fields SL. Why kidneys fail in autosomal dominant polycystic kidney disease. Nat Rev Nephrol 7: 556–566, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Greciano PG, Moyano JV, Buschmann MM, Tang J, Lu Y, Rudnicki J, Manninen A, Matlin KS. Laminin 511 partners with laminin 332 to mediate directional migration of Madin-Darby canine kidney epithelial cells. Mol Biol Cell 23: 121–136, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guay-Woodford LM. Murine models of polycystic kidney disease: molecular and therapeutic insights. Am J Physiol Renal Physiol 285: F1034–F1049, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Fischer E, Legue E, Doyen A, Nato F, Nicolas JF, Torres V, Yaniv M, Pontoglio M. Defective planar cell polarity in polycystic kidney disease. Nat Genet 38: 21–23, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Joly D, Berissi S, Bertrand A, Strehl L, Patey N, Knebelmann B. Laminin 5 regulates polycystic kidney cell proliferation and cyst formation. J Biol Chem 281: 29181–29189, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Joly D, Morel V, Hummel A, Ruello A, Nusbaum P, Patey N, Noel LH, Rousselle P, Knebelmann B. β4 Integrin and laminin 5 are aberrantly expressed in polycystic kidney disease: role in increased cell adhesion and migration. Am J Pathol 163: 1791–1800, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kariya Y, Sato H, Katou N, Kariya Y, Miyazaki K. Polymerized laminin-332 matrix supports rapid and tight adhesion of keratinocytes, suppressing cell migration. PLos One 7: e35546, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Katsuyama M, Masuyama T, Komura I, Hibino T, Takahashi H. Characterization of a novel polycystic kidney rat model with accompanying polycystic liver. Exp Anim 49: 51–55, 2000 [DOI] [PubMed] [Google Scholar]
- 17.Kiefer J. Diverse connections between primary cilia and hedgehog signaling. Dev Dyn 239: 1255–1262, 2010 [DOI] [PubMed] [Google Scholar]
- 18.Kim BG, Gao MQ, Choi YP, Kang S, Park HR, Kang KS, Cho NH. Invasive breast cancer induces laminin-332 upregulation and integrin β4 neoexpression in myofibroblasts to confer an anoikis-resistant phenotype during tissue remodeling. Breast Cancer Res 14: R88, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lager DJ, Qian Q, Bengal RJ, Ishibashi M, Torres VE. The pck rat: a new model that resembles human autosomal dominant polycystic kidney and liver disease. Kidney Int 59: 126–136, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Lazarova Z, Sitaru C, Zillikens D, Yancey KB. Comparative analysis of methods for detection of anti-laminin 5 autoantibodies in patients with anti-epiligrin cicatricial pemphigoid. J Am Acad Dermatol 51: 886–892, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Lazarova Z, Yee C, Darling T, Briggaman RA, Yancey KB. Passive transfer of anti-laminin 5 antibodies induces subepidermal blisters in neonatal mice. J Clin Invest 98: 1509–1518, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee GY, Kenny PA, Lee EH, Bissell MJ. Three-dimensional culture models of normal and malignant breast epithelial cells. Nat Methods 4: 359–365, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lepage M, Beaulieu J. Important role of laminin-511 in differentiation of the intestinal epithelium. Can J Gastroenterol 25A: 98A–99A, 2011 [Google Scholar]
- 24.Liu Y, Petreaca M, Yao M, Martins-Green M. Cell and molecular mechanisms of keratinocyte function stimulated by insulin during wound healing BMC. Cell Biol 10: 1–15, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mak GZ, Kavanaugh GM, Buschmann MM, Stickley SM, Koch M, Goss KH, Waechter H, Zuk A, Matlin KS. Regulated synthesis and functions of laminin 5 in polarized Madin-Darby canine kidney epithelial cells. Mol Biol Cell 17: 3664–3677, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mangos S, Lam PY, Zhao A, Liu Y, Mudumana S, Vasilyev A, Liu A, Drummond IA. The ADPKD genes pkd1a/b and pkd2 regulate extracellular matrix formation. Dis Model Mech 3: 354–365, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miner JH. Renal basement membrane components. Kidney Int 56: 2016–2024, 1999 [DOI] [PubMed] [Google Scholar]
- 28.Miner JH, Patton BL, Lentz SI, Gilbert OJ, Snider WD, Jenkins NA, Copeland NG, Sanes JR. The laminin α chains: expression, developmental transitions, and chromosomal locations of a 1–5, identification of heterotrimeric laminins 8–11, and cloning of a novel α3 isoform. J Cell Biol 137: 685–701, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moyano JV, Greciano PG, Buschmann MM, Koch M, Matlin KS. Autocrine transforming growth factor-β1 activation mediated by integrin αVβ3 regulates transcriptional expression of laminin-332 in Madin-Darby canine kidney epithelial cells. Mol Biol Cell 21: 3654–3668, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moyer JH, Lee-Tischler MJ, Kwon HK, Schrick JJ, Avner ED, Sweeney WE, Godfrey VL, Cacheiro NLA, Wilkinson JE, Woychik RP. Candidate gene associated with a mutation causing recessive polycystic kidney disease in mice. Science 264: 1329–1332, 1994 [DOI] [PubMed] [Google Scholar]
- 31.Nishio S, Tian X, Gallagher AR, Yu Z, Patel V, Igarashi P, Somlo S. Loss of oriented cell division does not initiate cyst formation. J Am Soc Nephrol 21: 295–302, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Olteanu D, Yoder BK, Liu W, Croyle MJ, Welty EA, Rosborough K, Wyss JM, Bell PD, Guay-Woodford LM, Bevensee MO, Satlin LM, Schwiebert EM. Heightened epithelial Na+ channel-mediated Na+ absorption in a murine polycystic kidney disease model epithelium lacking apical monocilia. Am J Physiol Cell Physiol 290: C952–C963, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Patton BL, Connoll AM, Martin PT, Cunningham JM, Mehta S, Pestronk A, Miner JH, Sanes JR. Distribution of ten laminin chains in dystrophic and regenerating muscles. Neuromuscul Disord 9: 423–433, 1999 [DOI] [PubMed] [Google Scholar]
- 34.Rasband WS, National Institutes of Health ImageJ (online) http://imagej.nih.gov/ij/ [6 January 2014]. [Google Scholar]
- 35.Reif GA, Yamaguchi T, Nivens E, Fujiki H, Pinto CS, Wallace DP. Tolvaptan inhibits ERK-dependent cell proliferation Cl− secretion and in vitro cyst growth of human ADPKD cells stimulated by vasopressin. Am J Physiol Renal Physiol 301: F1005–F1113, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Richter P, Umbreit C, Franz M, Berndt A, Grimm S, Uecker A, Böhmer FD, Kosmehl H, Berndt A. EGF/TGFβ1 co-estimulation of oral squamous cell carcinoma cells causes an epithelial-mesenchymal transition cell phenotype expressing laminin 332. J Oral Pathol Med 40: 46–54, 2011 [DOI] [PubMed] [Google Scholar]
- 37.Schäfer K, Gretz N, Bader M, Obermüller I, Eckardt KU, Kriz W, Bachmann S. Characterization of the Han:SPRD rat model for hereditary polycystic kidney disease. Kidney Int 46: 134–152, 1994 [DOI] [PubMed] [Google Scholar]
- 38.Shannon MB, Patton BL, Harvey SJ, Miner JH. A hypomorphic mutation in the mouse laminin α5 gene causes polycystic kidney disease. J Am Soc Nephrol 17: 1913–1922, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Togawa H, Nakanishi K, Mukaiyama H, Hama T, Shima Y, Sako M, Miyajima M, Nozu K, Nishii K, Nagao S, Takahashi H, Iijima K, Yoshikawa N. Epithelial-to-mesenchymal transition in cyst lining epithelial cells in an orthologous PCK rat model of autosomal-recessive polycystic kidney disease. Am J Physiol Renal Physiol 300: F511–F520, 2011 [DOI] [PubMed] [Google Scholar]
- 40.Torres VE, Harris PC. Autosomal dominant polycystic kidney disease: the last 3 years. Kidney Int 76: 149–168, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tzu J, Marinkovich MP. Bridging structure with function: structural, regulatory, and developmental role of laminins. Int J Biochem Cell Biol 40: 199–214, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Van den Bergh F, Eliason SL, Giudice GJ. Type XVII collagen (BP180) can function as a cell-matrix adhesion molecule via binding to laminin 332. Matrix Biol 30: 100–108, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ward CJ, Hogan MC, Rossetti S, Walker D, Sneddon T, Wang X, Kubly V, Cunningham JM, Bacallao R, Ishibashi M, Milliner DS, Torres VE, Harris PC. The gene mutated in autosomal recessive polycystic kidney disease encodes a large, receptor-like protein. Nat Genet 30: 259–269, 2002 [DOI] [PubMed] [Google Scholar]
- 44.Wilhelmsen K, Litjens SH, Sonnenberg A. Multiple functions of the integrin α6β4 in epidermal homeostasis and tumorigenesis. Mol Cell Biol 26: 2877–2886, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wilson PD. Polycystic kidney disease. N Engl J Med 350: 151–164, 2004 [DOI] [PubMed] [Google Scholar]
- 46.Wilson PD, Schrier RW, Breckon RD, Gabow PA. A new method for studying human polycystic kidney disease epithelia in culture. Kidney Int 30: 371–378, 1986 [DOI] [PubMed] [Google Scholar]
- 47.Yoder BK, Richards WG, Sommardahl C, Sweeney WE, Michaud EJ, Wilkinson JE, Avner ED, Woychik RP. Functional correction of renal defects in a mouse model for ARPKD through expression of the cloned wild-type Tg737 cDNA. Kidney Int 50: 1240–1248, 1996 [DOI] [PubMed] [Google Scholar]
- 48.Yoder B, Tousson A, Millican L, Wu J, Bugg C, Schafer J, Balkovetz D. Polaris, a protein disrupted in orpk mice, is required for assembly of renal cilium. Am J Physiol Renal Physiol 282: F541–F552, 2002 [DOI] [PubMed] [Google Scholar]
- 49.Yoo YA, Kang MH, Kim JS, Oh SC. Sonic hedgehog signaling promotes motility and invasiveness of gastric cancer cells through TGF-β-mediated activation of the ALK5-Smad 3 pathway. Carcinogenesis 29: 480–490, 2008 [DOI] [PubMed] [Google Scholar]
- 50.Zuk A, Bonventre JV, Matlin KS. Expression of fibronectin splice variants in the postischemic rat kidney. Am J Physiol Renal Physiol 280: F1037–F1053, 2001 [DOI] [PubMed] [Google Scholar]