Abstract
Angiotensin-converting enzyme 2 (ACE2) is located in several tissues and is highly expressed in renal proximal tubules, where it degrades the vasoconstrictor angiotensin II (ANG II) to ANG-(1–7). Accumulating evidence supports protective roles of ACE2 in several disease states, including diabetic nephropathy. A disintegrin and metalloprotease (ADAM) 17 is involved in the shedding of several transmembrane proteins, including ACE2. Our previous studies showed increased renal ACE2, ADAM17 expression, and urinary ACE2 in type 2 diabetic mice (Chodavarapu H, Grobe N, Somineni HK, Salem ES, Madhu M, Elased KM. PLoS One 8: e62833, 2013). The aim of the present study was to determine the effect of insulin on ACE2 shedding and ADAM17 in type 1 diabetic Akita mice. Results demonstrate increased renal ACE2 and ADAM17 expression and increased urinary ACE2 fragments (≈70 kDa) and albumin excretion in diabetic Akita mice. Immunostaining revealed colocalization of ACE2 with ADAM17 in renal tubules. Renal proximal tubular cells treated with ADAM17 inhibitor showed reduced ACE2 shedding into the media, confirming ADAM17-mediated shedding of ACE2. Treatment of Akita mice with insulin implants for 20 wk normalized hyperglycemia and decreased urinary ACE2 and albumin excretion. Insulin also normalized renal ACE2 and ADAM17 but had no effect on tissue inhibitor of metalloproteinase 3 (TIMP3) protein expression. There was a positive linear correlation between urinary ACE2 and albuminuria, blood glucose, plasma creatinine, glucagon, and triglycerides. This is the first report showing an association between hyperglycemia, cardiovascular risk factors, and increased shedding of urinary ACE2 in diabetic Akita mice. Urinary ACE2 could be used as a biomarker for diabetic nephropathy and as an index of intrarenal ACE2 status.
Keywords: diabetic Akita mice, albuminuria, renal and urinary ACE2, ADAM17, TIMP3
the renin-angiotensin system (RAS) is activated and involved in pathogenic mechanisms that result in diabetic nephropathy (DN), partially mediated by angiotensin II (ANG II) (9). Indeed, some reports determined ANG II levels as 1,000 times higher in a diabetic population relative to control patients (25). ANG II, a vasoconstrictor peptide, induces cellular dedifferentiation and proliferation, generates reactive oxygen species, and produces inflammation, apoptosis, and tubuloglomerular fibrosis (53). Consequently, ANG II-mediated effects lead to the development and progress of DN toward end-stage renal disease (ESRD) (21, 55).
Angiotensin converting enzyme 2 (ACE2) is a membrane-bound metallopeptidase with predominant localization to the apical plasma membrane of the proximal tubular epithelium in the kidney (49, 52). ACE2 is one of the major renal peptidases that forms ANG-(1–7); a vasodilator/anti-inflammatory peptide, by cleaving the carboxy-terminal amino acid from ANG II (13, 50). Accordingly, a key renoprotective role has been proposed for ACE2: retarding DN progression (38, 50). Indeed, studies involving diabetic Akita mice (32), streptozotocin (STZ) diabetic mice (8), STZ diabetic rats (29, 49), and mouse models of chronic kidney disease (12) demonstrated decreased renal ACE2 protein expression and activity. In addition, deletion or pharmacological inhibition of ACE2 in diabetic Akita and STZ diabetic mice, respectively, results in increased albuminuria and deteriorated renal function (45, 56). Furthermore, administration of human recombinant ACE2 or mouse recombinant ACE2 to diabetic Akita mice or STZ diabetic rats, respectively, reduced albuminuria and kidney injury (31, 39). Interestingly, a decrease in renal tubular and glomerular ACE2 expression was reported for type 2 diabetic patients with nephropathy (36, 41). Other studies showed evidence for increased renal ACE2 in Akita, STZ, and db/db diabetic mice (10, 39, 56, 58, 59, 62). Taken together, these results suggest ACE2 as a new promising target for preventing the onset and retarding the progression of DN.
At present, the primary biomarker used in the clinical diagnosis of chronic kidney disease (CKD) is urinary albumin excretion (33). However, there is a debate regarding microalbuminuria as an early or specific marker of DN since clinical studies have reported that microalbuminuria subsides in ∼55% of DN patients with significant decline in glomerular filtration rate (GFR) (48), prompting a search for new markers of tubular injury. Components of the RAS, such as ACE and angiotensinogen, have been described as urinary constituents in patients or animal models of CKD (2, 24). Recently, soluble ACE2 protein activity and expression have been detected in human and sheep urine (35, 44), which is most likely due to proteolytic shedding of its ectodomain (10, 22, 27). In clinical studies, urinary levels of ACE2 protein expression and activity were significantly increased in CKD (35) and in diabetic renal transplant patients (60). Furthermore, a strong positive correlation was observed between urinary ACE2 mRNA expression and proteinuria levels in type 2 diabetic patients with nephropathy (51). Consequently, it has been suggested that urinary ACE2 levels can reflect diabetic intrarenal changes and could be used as a potential early biomarker of DN (10, 35).
The shedding of urinary ACE2 has been recently ascribed to actions of a disintegrin and metalloproteinase 17 (ADAM17) in a mouse model of type 2 diabetes (10). In vitro, the catalytically active ectodomain of ACE2 was cleaved by ADAM17 in HEK293, Huh7, and human respiratory epithelial cells (22, 27). ADAM17, also known as tumor necrosis factor-α-converting enzyme (TACE) or CD156q, is a zinc-dependent protease and the most active “sheddase” of the ADAMs family (43). The metalloprotease domain mediates ectodomain cleavage, resulting in the release of several transmembrane proteins, a phenomenon known as ‘shedding.’ The role of ADAM17 in the regulation of the RAS is suggested by a study demonstrating increased ADAM17 levels in mice treated with ANG II (28). Moreover, studies conducted on Chinese hamster ovary cells established that ADAM17 is able to cleave the ectodomain of ACE2 at the peptide sequence between Arg [708] and Ser [709] (26), but not ACE (52). ADAM17 has also been implicated in the pathogenesis of various diseases, including renal inflammatory disease and fibrosis (34, 37). Accumulating evidence suggests that increased ADAM17 activity results in increased insulin resistance and hyperglycemia (15, 17). The tissue inhibitors of metalloproteinases (TIMPs) are endogenous inhibitors of matrix metalloproteinases, including ADAM17 (54). TIMP3 has been shown to play a crucial role in the pathogenesis of various renal diseases, including DN (16), and TIMP3 deficiency resulted in increased ADAM17 activity (15) and exacerbated DN (3). Furthermore, a clinical study conducted in type 2 diabetic patients demonstrated that a decrease in TIMP3 leads to ADAM17 overactivity in the circulation, resulting in increased insulin receptor resistance (5, 6). Additionally, renal TIMP3 is decreased in STZ diabetic mice and in kidney biopsies from type 2 diabetic patients (16).
The current study investigates the effect of hyperglycemia on urinary ACE2 excretion and suggests that renal tubular ACE2 shedding could be mediated via renal ADAM17 in type 1 diabetes-induced nephropathy.
MATERIALS AND METHODS
Study design.
Male (8 wk old) diabetic Akita mice (C57BL/6-Ins2Akita/J) and their age-matched wild-type (WT) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Mice were housed individually in plastic cages at room temperature (22°C) with 12:12 h light-dark cycle for 22 wk. Animals had free access to water and standard 18% protein rodent chow. Mice (10 wk old) were randomly assigned to three groups: 1) control, 2) Akita untreated, and 3) Akita treated with insulin. Blood glucose, body weight, food intake, water intake, and urine output were monitored weekly. All experiments were conducted in accordance to the guidelines of the Wright State University Animal Care and Use Committee.
Insulin treatment.
Insulin treatment was delivered subcutaneously using a sustained-release insulin implant (LinBit for mice, Lin-Shin Canada, Toronto, ONT). The rate of insulin release from LinBit (0.1 U·day−1·implant−1) lasted for at least 30 days. Following brief anesthesia with isoflurane, insulin implants were immersed in betadine solution and implanted subcutaneously with a 12-gauge needle under the middorsal skin. The LinBit insulin implants are made from a mixture of insulin and microrecrystallized palmitic acid.
Body composition measurement.
Body composition was measured using 1H magnetic resonance spectroscopy (EchoMRI-100, Echo Medical System, Houston, TX). After the calibration of the apparatus, mice were introduced into a clean and transparent plastic cylinder and placed into the apparatus to determine fat content, lean mass, and total body water.
Measurement of blood glucose levels.
Blood glucose concentration was determined by using a glucometer (FreeStyle Lite Blood Glucose Monitoring System, Abbott, CA) and FreeStyle Blood Glucose Test Strips. A gentle cut was made at the tip of the mouse's tail to draw venous blood samples for measurement. Values were recorded and expressed in milligrams per deciliter.
Urine collection.
Mice were placed individually in metabolic cages for 24-h urine collection with free access to food and water. Protease inhibitors (Roche Diagnostics, Indianapolis, IN) were added to prevent protein degradation. Urine samples were collected in two steps every 12 h and kept at 4°C until the 24-h collection was completed. Samples were centrifuged at 3,000 g for 5 min at 4°C to remove cellular debris, and supernatants were aliquotted and stored at −80°C until use.
Cell culture.
Human proximal tubular cells (HK-2 cells) were obtained from the American Type Culture Collection (Manassas, VA). Passages 5–8 were used for experiments. Cells were grown for 7–9 days in T25 flasks at 37°C with 5% CO2 in keratinocyte serum-free medium containing 0.05 mg/ml bovine pituitary extract, 5 ng/ml human recombinant epidermal growth factor (Life Technologies, Grand Island, NY), and an antibiotic-antimycotic mixture including 100 IU/ml penicillin, 100 μg/ml streptomycin, and 0.25 μg/ml amphotericin B (Cellgro, Manassas, VA). Media was changed every 2–3 days. At 80% confluence, media was removed and fresh media without or with 10 μM ADAM17 inhibitor TNF-α protease inhibitor-1 (TAPI-1; Enzo Life Sciences, Farmingdale, NY) was added to the cells. After 24, 48, and 72 h of incubation, media was removed and ACE2 expression was analyzed by Western blotting.
Western blot analysis.
Urine samples and kidney protein extracts (lysates) were obtained from nondiabetic WT, diabetic Akita, and diabetic Akita mice treated with insulin. Kidneys were homogenized on ice using Complete Lysis-M EDTA-Free buffer (Roche Applied Science) containing 2.5 mmol/l PMSF and protease inhibitors. Tissue homogenates were centrifuged at 10,000 g for 10 min at 4°C to remove cellular debris. Urine samples (2–10 μl) were normalized to creatinine, determined by using an enzyme-linked immunoassay (see below). Kidney protein lysates (50 μg protein), urine samples (2–10 μl), and HK-2 media (1.2 μg protein) were separated on 10% SDS-PAGE and electroblotted to polyvinylidene fluoride membranes (Millipore, Billerica, MA). Membranes were incubated overnight at 4°C with the following specific primary antibodies: goat anti-ACE2 (cat. no. AF3437, 1:1,000, R&D Systems, Minneapolis, MN), rabbit anti-ADAM17 (cat. no. ADI-905-249-100, 1:500, Enzo Life Sciences), or goat anti-TIMP3 (cat. no. sc-6836, 1:200, Santa Cruz Biotechnology, Dallas, TX) followed by incubation with horseradish peroxidase-conjugated donkey anti-goat (cat. no. HAF017, 1:2,000, R&D Systems) or donkey anti-rabbit (cat. no. 711-035-152, 1:20,000, Jackson ImmunoResearch Laboratories, West Grove, PA) secondary antibodies. The primary antibody for HK-2 media was rabbit anti-ACE2 (cat. no. sc20998, 1:200, Santa Cruz Biotechnology). Signals were detected using supersignal chemiluminescent substrate (Thermo Scientific) and visualized using a Fujifilm image analyzer (LAS 3000, Image Quant).
Kidney histology and immunohistochemistry.
Kidneys were collected from mice anesthetized by ketamine/xylazine (100:8 mg/kg) and perfused transcardially with PBS followed by 4% paraformaldehyde (PFA). Kidneys were fixed in 4% PFA overnight at 4°C and sent to AML Laboratories (Baltimore, MD) for paraffin embedding, sectioning, and periodic acid-Schiff/Masson's trichrome staining. For immunofluorescence experiments, sections were deparaffinized and incubated in 10 mM sodium citrate (pH 8.5) at 95°C for 60 min. Sections were then blocked in 3% normal horse serum (diluted in PBS containing 0.1% Triton X-100) and incubated with goat anti-ACE2 and rabbit anti-ADAM17 primary antibodies diluted in 3% normal horse serum overnight at 4°C. The sections were washed with PBS three times and incubated with donkey anti-goat fluorescein-conjugated and anti-rabbit Cy3-conjugated secondary antibody (1:100, Jackson ImmunoResearch Laboratories), respectively. Slides were allowed to air-dry and coverslipped using Vectashield mounting medium (Vector, Burlingame, CA). Images were obtained using a conventional fluorescence microscope (Optronics, Goleta, CA) or a FV1000 confocal microscope (Olympus). Analysis and computerized quantification of immunostaining were performed using MetaMorph software (Molecular Devices).
ACE2 assay.
ACE2 activity was measured using the fluorogenic substrate 7-Mca-APK(Dnp), purchased from Biomol International, in the presence of the ACE inhibitor lisinopril. Two microliters of kidney lysate (20–30 μg) or 1- to 10-μl urine samples (20–30 μg) were incubated with 100 μl of the reaction buffer (50 mM Tris, pH 7.5, 5 mM ZnCl2, 150 mM NaCl2, and 10 μM lisinopril) and 4 mM Mca-APK(Dnp). After incubation at 37°C for 1–4 h, fluorescence was measured using a Fusion Packard plate reader (Packard BioScience, Meriden, CT) at 328-nm excitation and 393-nm emission wavelength. Specific activity of ACE2 was determined using the specific ACE2 inhibitor MLN-4760 (gift from Millenium Pharmaceuticals, Cambridge, MA). Results were expressed as picomoles per hour per microgram protein for kidney samples and nanomoles per hour per milligram creatinine for the urine samples.
In situ enzyme activity using mass spectrometry imaging.
Consecutive tissue sections (12 μm) were prepared from fresh frozen kidneys and incubated with 0.1 mM ANG II at 37°C for 5 min. A detailed description of the imaging technique has been published previously (19). Briefly, matrix consisting of 10 mg/ml α-cyano-4-hydroxy-cinnamic acid in 60% methanol, 10% acetone, and 0.3% trifluoroacetic acid was spray-coated onto kidney tissue sections using a thin-layer chromatography nebulizer. Mass spectrometry images were obtained using an Autoflex III smartbeam matrix-assisted laser desorption/ionization (MALDI) time-of-flight (TOF)/TOF instrument (Bruker Daltonics). The spectral analysis was performed with proprietary Bruker Imaging software.
Urinary albumin assay.
Urinary albumin excretion was determined using a Mouse Albumin ELISA Quantitation Set (Bethyl Laboratories, Montgomery, TX) following the instructions of the manufacturer. Briefly, a 96-well plate coated with goat anti-mouse albumin antibody was incubated overnight with TBS containing 1% BSA followed by an incubation with diluted albumin standards and urine samples. After addition of horseradish peroxidase-conjugated secondary antibody and the substrate mixture, the reaction was stopped by 2 N H2SO4. Measurements were taken at 450 nm by using a Fusion Packard plate reader.
Urinary and plasma creatinine assay.
Urinary and plasma creatinine levels were carried out using a kit purchased from Quidel (San Diego, CA). Urine, plasma samples, and standards were diluted with distilled water (1:40) and then loaded into a 96-well plate, followed by addition of a color reagent. After a 30-min incubation, measurements of optical density were taken at 450 nm by using a Fusion Packard plate reader.
Plasma hormones and lipid measurements.
Trunk blood was collected in ice-chilled heparinized tubes. Blood was centrifuged at 10,000 g for 10 min at 4°C. Plasma was separated, aliquoted, and stored at −80°C. Plasma samples were analyzed for insulin, glucagon, adiponectin, leptin, and triglyceride levels at the Mouse Metabolic Phenotyping Center (Cincinnati, OH).
Statistics.
Statistical analysis was carried out with Statistica software (version 10) and GraphPad Prism (5.01). All data are presented as means ± SE. An unpaired Student's t-test was used to evaluate the difference between two groups. However, for more than two groups, one-way ANOVA was used. The differences in metabolic parameters (blood glucose, body weight, food intake, water intake, body fat, lean body mass, and total body water) were assessed by repeated measures two-way ANOVA. A probability value of <0.05 was considered significant. If a significant difference was recognized, a Bonferroni multiple comparison test was performed.
RESULTS
Insulin normalizes hyperglycemia in diabetic Akita mice.
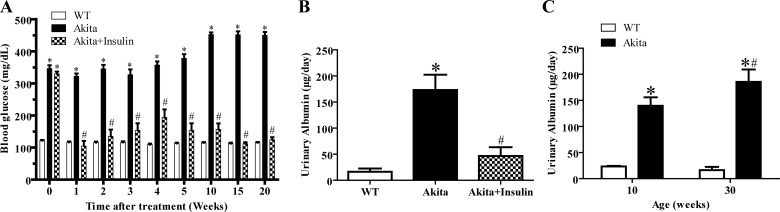
Blood glucose levels were monitored weekly for a period of 20 wk (10–30 wk old). Akita (10 wk old) mice developed significantly higher blood glucose levels compared with WT mice, and the levels consistently increased throughout the study period (Fig. 1A). Chronic treatment of diabetic Akita mice with insulin implants normalized hyperglycemia after 1 wk and throughout the 20-wk duration of treatment (Fig. 1A, P < 0.0001).
Fig. 1.
Chronic treatment with insulin decreased hyperglycemia and urinary albumin excretion in Akita mice. A: time-related changes in blood glucose levels in wild-type (WT) mice, diabetic Akita mice, and diabetic Akita mice treated with insulin. Values are means ± SE of group size (n = 8–10). Repeated measurements 2-way ANOVA using a Bonferroni's post hoc test showed that treatment caused a significant decrease in blood glucose levels of Akita+insulin mice {[F (2, 20) = 1076.245], P < 0.0001}. Similarly, duration of treatment showed a significant decrease in blood glucose levels of Akita+insulin mice {[F (8, 80) = 43.172], P < 0.0001}. B: effect of normalizing hyperglycemia in diabetic Akita mice on urinary albumin excretion. One-way ANOVA showed that 20 wk after treatment commenced, there was a significant decrease in urinary albumin excretion in diabetic Akita mice treated with insulin compared with untreated diabetic Akita mice. Values are means ± SE of group size (n = 6–8). #P < 0.001 vs. untreated Akita mice, *P < 0.001 vs. WT. C: age-dependent changes in urinary albumin excretion in WT mice and diabetic Akita mice. There was a significant increase in urinary albumin excretion in 30-wk-old diabetic Akita mice compared with 10-wk-old diabetic Akita mice. Values are means ± SE of group size (n = 6–10). #P < 0.001, *P < 0.001 vs. WT.
Effects of insulin treatment on general metabolic and plasma parameters in diabetic Akita mice.
As shown in Table 1, food intake, water intake, and urine output of diabetic Akita mice consistently increased, whereas body weight and absolute body fat decreased with age compared with WT mice. Plasma insulin, leptin, and adiponectin levels were significantly decreased in diabetic Akita mice compared with WT mice, while plasma glucagon and triglycerides levels were significantly increased (P < 0.05). Chronic treatment of diabetic Akita mice with insulin resulted in a significant increase in absolute body fat, but not body weight, compared with untreated diabetic Akita mice. In addition, insulin treatment of diabetic Akita mice demonstrated a significant decrease in food intake, water intake, and urine output compared with untreated diabetic Akita mice (P < 0.05). Treatment of diabetic Akita mice with insulin significantly increased plasma insulin, leptin, and adiponectin levels, while glucagon and triglycerides levels were significantly decreased to levels observed in WT mice. In addition, plasma creatinine levels were significantly higher in diabetic Akita mice compared with WT mice while insulin treatment significantly reduced plasma creatinine levels in diabetic Akita mice.
Table 1.
Age-dependent changes in metabolic and plasma parameters of wild-type, Akita, and Akita mice treated with insulin
| Parameter | WT | Akita | WT | Akita | Akita+Insulin |
|---|---|---|---|---|---|
| Age, wk | 10 | 10 | 30 | 30 | 30 |
| Duration of treatment, wk | 0 | 0 | 0 | 0 | 20 |
| Group size, n | 10 | 10 | 8 | 8 | 8 |
| Body weight, g | 22 ± 0.5 | 20 ± 0.3* | 30 ± 1 | 26 ± 0.5* | 27 ± 1 |
| Absolute body fat, g | 1.8 ± 0.2 | 1.6 ± 0.2* | 4.9 ± 0.3 | 2.1 ± 0.1* | 4 ± 0.2# |
| Food intake, g/day | 4 ± 0.2 | 6 ± 0.3* | 5 ± 0.1 | 14.7 ± 1.2* | 6.2 ± 0.6# |
| Water intake, ml/day | 5.5 ± 0.3 | 21 ± 1.9* | 7.5 ± 0.2 | 44.7 ± 2.5* | 7 ± 0.5# |
| Urine output, ml/day | 1 ± 0.2 | 12 ± 1.6* | 1.1 ± 0.1 | 33.2 ± 2.4* | 3.2 ± 0.4# |
| Plasma insulin, ng/ml | ND | ND | 2.3 ± 0.2 | 0.2 ± 0.04* | 3.4 ± 0.4# |
| Plasma glucagon, ng/ml | ND | ND | 0.02 ± 0.003 | 0.09 ± 0.011* | 0.04 ± 0.006# |
| Plasma leptin, ng/ml | ND | ND | 2.5 ± 0.2 | 0.4 ± 0.1* | 2.3 ± 0.1# |
| Plasma adiponectin, μg/ml | ND | ND | 12.9 ± 0.8 | 7.5 ± 0.5* | 12.4 ± 0.4# |
| Plasma triglycerides, mg/dl | ND | ND | 98.9 ± 7 | 318.4 ± 17.5* | 89 ± 5.1# |
| Plasma creatinine, mg/dl | ND | ND | 0.47 ± 0.02 | 0.89 ± 0.09* | 0.47 ± 0.08# |
Values are means ± SE WT, wild-type; ND, not determined. Statistically significant:
P < 0.05 vs. age-matched WT mice.
P < 0.05 vs. age-matched Akita mice.
Insulin ameliorates urinary albumin excretion in diabetic Akita mice.
There was a significantly higher urinary albumin excretion in diabetic Akita mice compared with age-matched WT mice (Fig. 1B, *P < 0.001 vs. WT), and chronic treatment with insulin for 20 wk demonstrated a significant decrease in urinary albumin excretion of the treated diabetic Akita mice compared with untreated diabetic Akita mice (Fig. 1B, #P < 0.001 vs. untreated Akita). In addition, progression of the disease manifested by worsening of albuminuria in 10- and 30-wk-old untreated Akita mice (Fig. 1C, #P < 0.001).
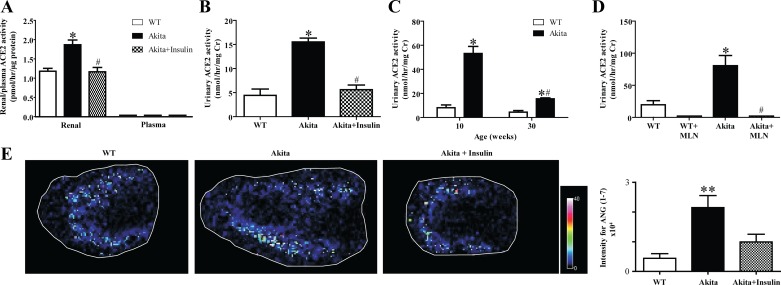
Insulin decreases renal and urinary ACE2 activity and plasma creatinine in diabetic Akita mice.
There was a significant increase in renal and urinary ACE2 activity in diabetic Akita mice compared with WT mice (Fig. 2, A and B, *P < 0.001 vs. WT). Chronic treatment with insulin for 20 wk significantly reduced renal and urinary ACE2 activity in treated diabetic Akita mice compared with untreated diabetic Akita mice (Fig. 2, A and B, #P < 0.001 vs. untreated Akita mice). In addition, there was a significant decrease in urinary ACE2 activity in 30-wk- compared with 10-wk-old diabetic Akita mice (Fig. 2C, #P < 0.001). The ACE2-specific inhibitor MLN-4760 (MLN in Fig. 2), was added to the reaction mixture to confirm the specificity of the assay. There was no detectable urinary ACE2 activity in the WT+MLN or Akita+MLN groups (Fig. 2D, #P < 0.001). In addition, ACE2 activity was not detected in plasma of WT, Akita, or insulin-treated Akita mice (Fig. 2A).
Fig. 2.
Chronic treatment with insulin decreased renal and urinary angiotensin-converting enzyme 2 (ACE2) activity in Akita mice. A: renal and plasma ACE2 activity values determined in samples obtained from WT mice, diabetic Akita, and diabetic Akita mice treated with insulin. While plasma ACE2 activity was not detectable, 1-way ANOVA showed a significant increase in renal ACE2 activity in diabetic Akita mice compared with WT mice and diabetic Akita mice treated with insulin. Values are means ± SE of group size (n = 6–8). #P < 0.001 vs. untreated Akita mice. *P < 0.001 vs. WT. B: urinary ACE2 activity determined in samples obtained from WT mice, diabetic Akita mice, and diabetic Akita mice treated with insulin. One-way ANOVA showed a significant increase in urinary ACE2 activity in diabetic Akita mice compared with WT mice and diabetic Akita mice treated with insulin. Values are means ± SE of group size (n = 6–8). #P < 0.001 vs. untreated Akita mice. *P < 0.001 vs. WT. C: age-dependent changes in urinary ACE2 activity in WT mice and diabetic Akita mice. One-way ANOVA showed a significant decrease in urinary ACE2 activity of 30-wk-old diabetic Akita mice compared with 10-wk-old diabetic Akita mice. Values are means ± SE of group size (n = 6–10). #P < 0.001 vs. untreated Akita mice. *P < 0.001 vs. WT. D: urinary ACE2 activity significantly decreased in the presence of specific ACE2 inhibitor MLN-4760 (MLN). Values are means ± SE of group size (n = 10). #P < 0.001 vs. Akita mice, *P < 0.001 vs. WT. E: MALDI imaging of cortical ANG-(1–7) formation in 30-wk-old WT, Akita, and insulin-treated Akita mice. Quantitation of signal intensities showed a significant increase in cortical ANG-(1–7) formation in Akita mice compared with WT and insulin-treated Akita mice. Values are means ± SE of group size (n = 4). **P < 0.01 vs. WT.
Insulin decreases renal cortical ANG-(1–7) formation in diabetic Akita mice.
Renal ACE2 activity in WT, Akita, and insulin-treated Akita mice was further investigated by MALDI imaging. This mass spectrometry-based in situ enzyme assay allows for the localization of regional ANG-(1–7) formation within a tissue section using the endogenous substrate ANG II. An elevated ANG-(1–7) formation was detected in diabetic Akita mice, confirming the results obtained with the fluorogenic enzyme assay (Fig. 2E). In addition, the increased ANG-(1–7) formation was predominantly located in the renal cortex of diabetic Akita mice.
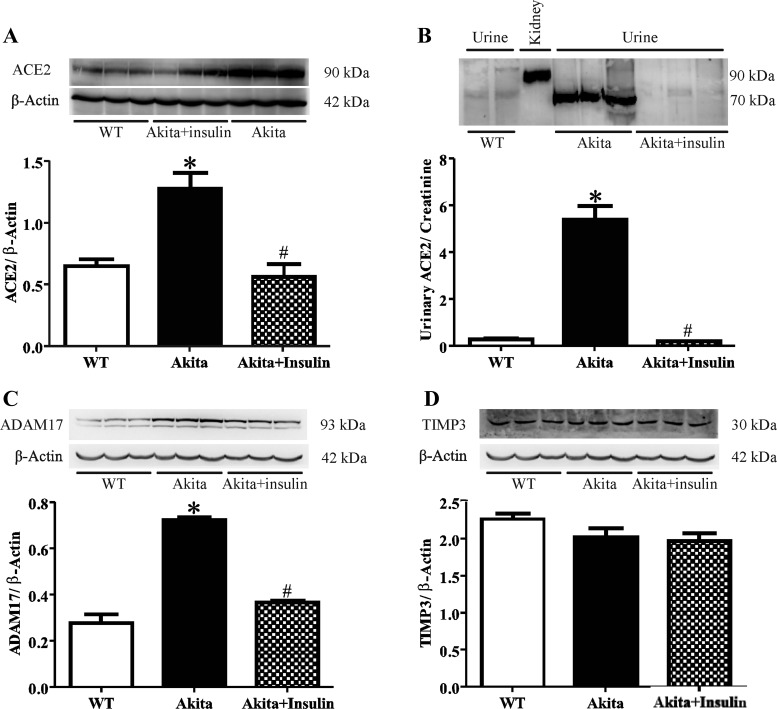
Insulin attenuates expression of renal and urinary ACE2, renal ADAM17, but not renal TIMP3 in diabetic Akita mice.
Western blotting revealed that renal ACE2 protein expression, represented by immunoreactive bands at ∼90 kDa, was increased in diabetic Akita mice compared with WT (Fig. 3A, *P < 0.001 vs. WT). Renal ACE2 significantly decreased in insulin-treated Akita compared with untreated diabetic Akita mice (Fig. 3A, #P < 0.001 vs. untreated Akita mice). In addition, Western blotting detected immunoreactive bands of urinary ACE2 at ∼70 kDa (Fig. 3B). Urinary ACE2 protein expression was increased in diabetic Akita mice compared with WT mice (Fig. 3B, *P < 0.001 vs. WT) and significantly reduced in insulin-treated diabetic Akita mice (Fig. 3B, #P < 0.001 vs. untreated Akita mice). The protein expression profile of ADAM17 was identical to renal and urinary ACE2 expression showing an increase in diabetic Akita mice compared with WT mice (Fig. 3C, *P < 0.001 vs. WT), which was reversed by insulin treatment (Fig. 3C, #P < 0.001 vs. untreated Akita mice). In contrast, renal TIMP3 protein expression was not significantly different in diabetic Akita mice compared with WT mice and was also not changed by insulin treatment in diabetic Akita mice compared with untreated diabetic Akita mice (Fig. 3D).
Fig. 3.
Western blot analysis for renal and urinary ACE2 and renal a disintegrin and metalloprotease (ADAM) 17 and tissue inhibitor of metalloproteinase 3 (TIMP3) protein expression in WT, diabetic Akita, and Akita mice treated with insulin. A: renal ACE2 protein expression values determined in samples obtained from WT mice, diabetic Akita mice, and diabetic Akita mice treated with insulin. One-way ANOVA showed a significant increase in renal ACE2 protein expression of diabetic Akita mice compared with WT mice and diabetic Akita mice treated with insulin. Values are means ± SE of group size (n = 6–8). #P < 0.001 vs. untreated Akita mice. *P < 0.001 vs. WT. B: urinary ACE2 protein expression values determined in samples obtained from WT mice, diabetic Akita mice, and diabetic Akita mice treated with insulin. One-way ANOVA showed a significant increase in urinary ACE2 protein expression of diabetic Akita mice compared with WT mice and diabetic Akita mice treated with insulin. Values are means ± SE of group size (n = 6–10). #P < 0.001 vs. untreated diabetic Akita mice. *P < 0.001 vs. WT. C: renal ADAM17 protein expression values determined in samples obtained from WT mice, diabetic Akita mice, and diabetic Akita mice treated with insulin. One-way ANOVA showed a significant increase in renal ADAM17 protein expression of diabetic Akita mice compared with WT mice and diabetic Akita mice treated with insulin. #P < 0.001 vs. untreated Akita mice. Values are means ± SE of group size (n = 6–8). *P < 0.001 vs. WT. D: renal TIMP3 protein expression values determined in samples obtained from WT mice, diabetic Akita mice, and diabetic Akita mice treated with insulin. One-way ANOVA showed that there was no significant change in renal TIMP3 protein expression of diabetic Akita mice compared with WT mice or diabetic Akita mice treated with insulin. Values are means ± SE of group size (n = 6–8).
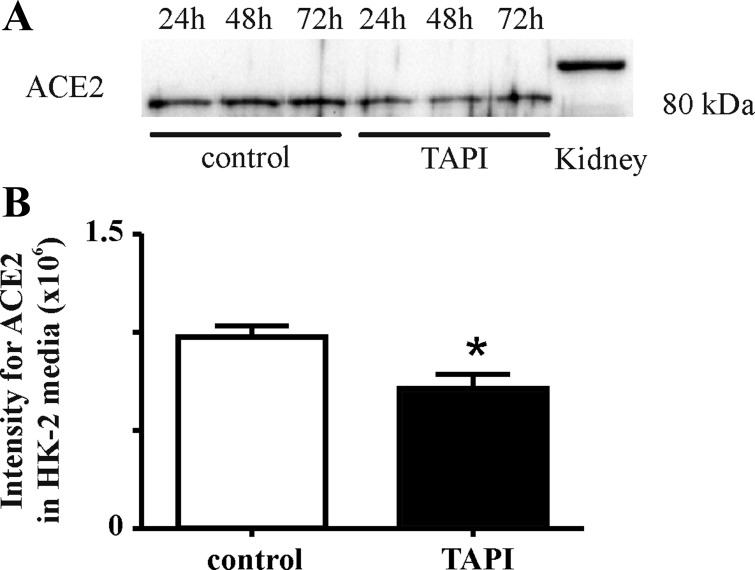
Inhibition of ADAM17 decreases renal ACE2 shedding in human renal proximal tubular HK-2 cells.
To confirm ADAM17-mediated ACE2 shedding, ACE2 expression was analyzed in cell media obtained from human renal proximal tubular HK-2 cells in the absence or presence of 10 μM ADAM17 inhibitor TAPI-1. Results show that ACE2 shedding into the cell media was significantly decreased by treatment of the cells with the ADAM17 inhibitor (Fig. 4, *P < 0.05).
Fig. 4.
Inhibition of ADAM17 reduced ACE2 shedding in human renal proximal tubular HK-2 cells. A: Western blot of ACE2 protein expression in cell media obtained after 24, 48, and 72 h from control HK-2 cells or from HK-2 cells after treatment with the ADAM17 inhibitor TNF-α protease inhibitor-1 (TAPI-1). B: ACE2 levels in cell media after treatment with ADAM17 inhibitor, TAPI-1. An unpaired Student's t-test showed significantly decreased ACE2 protein expression in media obtained from treated HK-2 cells. Values are means ± SE of group size (n = 3). *P < 0.05 vs. control.
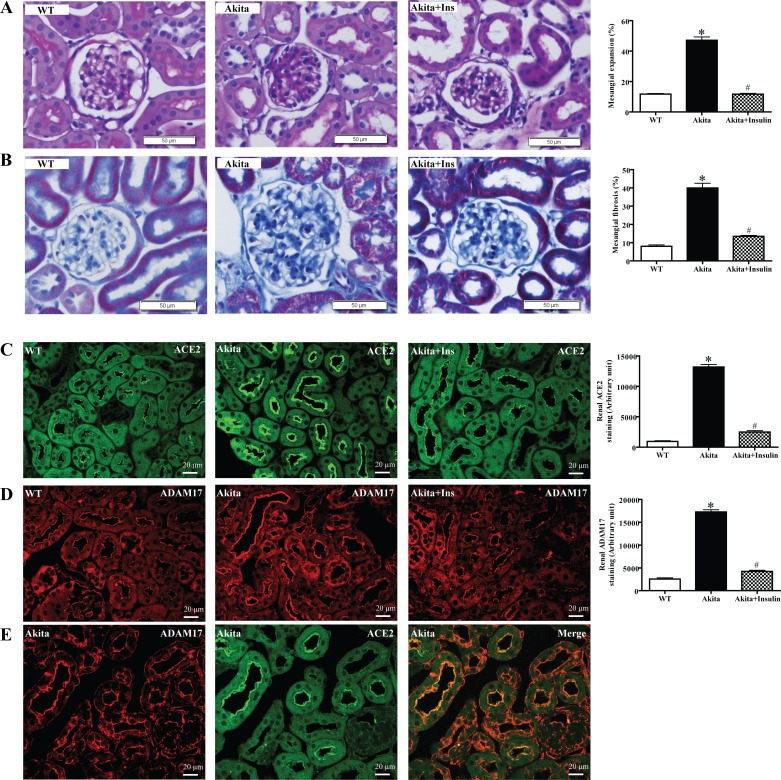
Insulin abolishes renal histopathological changes in diabetic Akita mice.
Glomerular mesangial expansion in diabetic Akita mice was significantly increased compared with WT mice (Fig. 5A, *P < 0.001 vs. WT). Quantitative analysis of Masson's trichrome staining of individual glomerular tufts revealed a significant increase in renal fibrosis in diabetic Akita mice compared with WT mice (Fig. 5B, *P < 0.001 vs. WT). Mesangial expansion and renal fibrosis were significantly decreased upon treatment with insulin (Fig. 5, A and B, #P < 0.001 vs. untreated Akita mice). Immunofluorescence analysis confirmed increased renal protein expression of ACE2 and ADAM17 in diabetic Akita mice, which was reversed by insulin treatment (Fig. 5, C and D). Furthermore, immunofluorescence detected strong colocalization of renal ACE2 and ADAM17 to the apical side of the proximal tubule brush-border membrane in diabetic Akita mice (Fig. 5E).
Fig. 5.
Histological analysis and immunofluorescence staining of renal ACE2 and ADAM17 after 12 wk of insulin treatment. A: representative periodic acid-Schiff (PAS) staining of 22-wk-old kidney sections obtained from WT mice, diabetic Akita mice, and diabetic Akita mice treated with insulin. The y-axis (%) is defined as the area of PAS staining in the glomerulus divided by the total area of the glomerulus multiplied by 100. There was a significant increase in glomerular mesangial expansion in diabetic Akita mice compared with WT mice and diabetic Akita mice treated with insulin. Values are means ± SE of group size (n = 25 glomeruli/group). #P < 0.001 vs. untreated Akita. *P < 0.001 vs. WT. B: representative Masson's trichrome staining of 22-wk-old kidney sections in WT mice, diabetic Akita mice, and diabetic Akita mice treated with insulin. The y-axis (%) is defined as the area of Masson's trichrome staining in the glomerulus divided by the total area of the glomerulus multiplied by 100. There was a significant increase in glomerular mesangial fibrosis in diabetic Akita mice compared with WT mice and diabetic Akita mice treated with insulin. Values are means ± SE of group size (n = 25 glomeruli/group). #P < 0.001 vs. untreated Akita. *P < 0.001 vs. WT. C: immunofluorescence staining with ACE2 in the kidney sections from WT mice, diabetic Akita mice, and diabetic Akita mice treated with insulin. Strong ACE2 staining is observed in the cortical tubules in diabetic Akita mice compared with WT mice and insulin-treated diabetic Akita mice. #P < 0.001 vs. untreated Akita. *P < 0.001 vs. WT. D: immunofluorescence staining for ADAM17 in the kidney sections from WT mice, diabetic Akita mice, and diabetic Akita mice treated with insulin. Strong ADAM17 staining is observed in the cortical tubules in diabetic Akita mice compared with WT mice and insulin-treated diabetic Akita mice. #P < 0.001 vs. untreated Akita. *P < 0.001 vs. WT. E: immunofluorescence staining of ACE2 and ADAM17 in a kidney obtained from an Akita mouse. Merging of both images shows colocalization of ACE2 and ADAM17 in cortical tubules.
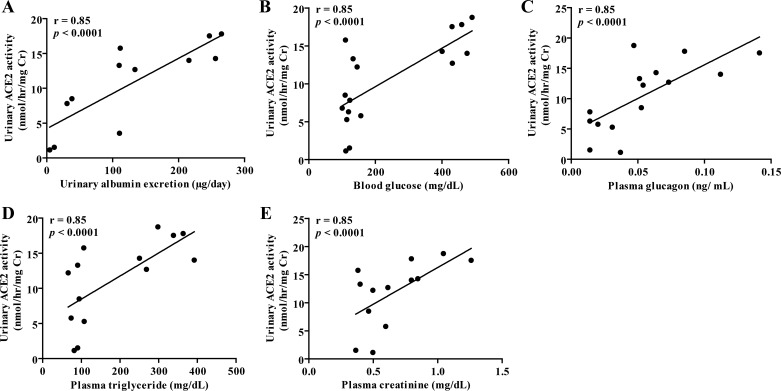
Regression analysis between urinary ACE2 activity and albuminuria, blood glucose, and plasma levels of glucagon, triglycerides, and creatinine.
Urinary ACE2 activity correlated positively with urinary albumin, blood glucose, plasma glucagon, triglycerides, and creatinine levels (Fig. 6, P < 0.0001).
Fig. 6.
Regression analysis between urinary ACE2 activity and albuminuria, blood glucose, and plasma levels of glucagon, triglycerides, and creatinine. A: regression analysis showed a significant positive correlation between urinary ACE2 activity and urinary albumin excretion. B: regression analysis showed a significant positive correlation between urinary ACE2 activity and blood glucose. C: regression analysis showed a significant positive correlation between urinary ACE2 activity and plasma glucagon. D: regression analysis showed a significant positive correlation between urinary ACE2 activity and plasma triglyceride. E: regression analysis showed a significant positive correlation between urinary ACE2 activity and plasma creatinine.
DISCUSSION
DN is still the leading cause of end-stage renal disease and is diagnosed by the presence of albuminuria within the range of microalbuminuria (23). In the present study, Akita mice developed significant, progressive, and durable hyperglycemia through the 20-wk study period, which was associated with albuminuria and renal functional and structural abnormalities. By 30 wk of age, the level of hyperglycemia and albuminuria in Akita mice matched other studies conducted in a model with the same (C57BL/6) genetic background (3, 32). Monitoring of the metabolic parameters demonstrated that Akita mice had a higher daily water intake (polydipsia), food intake (polyphagia), and urine output (polyuria), whereas body weight and fat composition were significantly less than in WT mice. Additionally, as expected, analysis of plasma hormones and lipid profiles revealed that Akita mice had severe insulin deficiency and reduced adiponectin and leptin concentrations. Conversely, there was an increase in plasma glucagon, triglycerides, and creatinine in Akita mice compared with WT mice. Numerous studies have suggested the association between hyperglycemia and albuminuria in various genetic backgrounds of Akita mice (7). Our results are in agreement with those studies and demonstrate that as the disease progresses, hyperglycemia increases, which results in a significant increase in albuminuria in 30-wk- compared with 10-wk-old Akita mice. Albumin excretion levels observed in our study were similar to the ones reported by others using Akita with a C57BL/6 background (3, 32). Interestingly, albumin excretion in (C57BL/6) Akita mice is less robust compared with 20-wk-old (FVB/NJ) Akita mice (7). Based on quantitative histomorphometric assessments, Akita kidneys developed a moderate increase in glomerular mesangial matrix expansion and glomerular fibrosis, which resembles the early histological lesions seen in humans with type 1 diabetes-induced nephropathy (7, 32). The renal histopathological findings of the present study were similar with other Akita genetic strains (3, 7, 32, 39).
As recent studies suggested the presence of urinary ACE2 in type 2 diabetic patients (35), we investigated in a similar manner whether type 1 diabetic Akita mice excrete ACE2. Indeed, the present study is the first to show increased urinary ACE2 in a genetic mouse model of type 1 diabetes. The higher urinary ACE2 levels were associated with increased renal ACE2 and ADAM17 protein expression. Since ADAM17 has been shown to increase the ectodomain shedding of ACE2 (27), we propose that elevated levels of urinary ACE2 may be due to a rise in ectodomain shedding of renal ACE2 mediated by ADAM17 in the tubular membrane facing the luminal side instead of increases in plasma ACE2 and glomerular filtration rate. Indeed, authors of a recent clinical study speculated that in patients with chronic kidney disease, ACE2 sheds directly from the proximal renal tubules into the urine via ADAM17, although at the time this notion was not confirmed experimentally (35). This conclusion is supported by our findings showing the colocalization of renal ACE2 and ADAM17 in cortical tubules. Additionally, the molecular mass of urinary ACE2 was ∼20 kDa lower than the molecular mass of kidney ACE2. Furthermore, plasma ACE2 activity was not detectable in untreated or treated Akita mice or WT mice. Work in our laboratory and others have confirmed the lack of ACE2 in plasma of db/db diabetic mice or lean control mice (10), or in CKD patients or healthy subjects (35). The absence of detectable ACE2 activity in plasma has been attributed to the presence of endogenous ACE2 inhibitor (30); however, other studies have shown an elevation of serum ACE2 activity in type 1 diabetic patients (46), in serum of sheep (44), in plasma of STZ diabetic mice (38), and in rats (61). The discrepancy between these results might be explained by differences between plasma or serum preparations, species, incubation time, type of buffer, or substrate used in the ACE2 enzyme activity assays.
In the present study, Western blot analysis of urinary ACE2 detected immunoreactive bands at ∼70 kDa in Akita mice, suggesting that the soluble form of ACE2 (∼70 kDa, urinary ACE2) is the shed fragment of membrane-bound ACE2 (∼90 kDa, renal ACE2). This finding is in agreement with two recent clinical studies showing ACE2 expression as three bands in immunoblots of urine samples from patients with CKD and diabetic renal transplant recipients, the glycosylated form of ACE2 at ∼120 kDa, membrane-bound ACE2 at ∼90 kDa, and a cleaved fragment of ACE2 at ∼75 kDa (35, 60). To confirm whether an increase in urinary ACE2 expression is directly associated with an increase in urinary ACE2 activity, we quantified ACE2 enzymatic activity. In line with the Western blot results, there was a significant increase in urinary ACE2 activity in Akita mice compared with wild type mice. These results confirm a recent study in type 2 db/db diabetic mice (10). In the present study, addition of ACE inhibitor lisinopril to the buffer of ACE2 enzymatic activity assay did not affect urinary ACE2 levels. This finding supports a clinical study that used ACE inhibitors (ACEi) or angiotensin receptor blockers (ARB) in patients with DN, in which urinary ACE2 levels were not altered (35). In contrast to urinary ACE2, microalbuminuria was decreased by ACEi in type 2 diabetic patients but did not prevent the progression toward end-stage renal disease as shown in the ADVANCE trial (11). Similarly, treatment with ACEi or ARB diminished urinary albumin excretion but did not affect urinary ACE2 in type 2 diabetic db/db mice (58). In the current study, MLN-4760, the pharmacological inhibitor of ACE2, significantly reduced urinary ACE2 activity in both Akita and WT mice, which confirms the specificity of the conducted ACE2 activity assay.
To investigate the effect of aging on urinary ACE2 levels, urine specimens from 10-wk-old Akita mice were compared with 30-wk-old Akita mice. It was found that younger animals had higher urinary ACE2 activity than older animals, indicating that with the progression of disease, the kidney is unable to maintain the same levels of renal ACE2, most likely due to depletion into the urine. It also reflects the possibility of using urinary ACE2 as an early biomarker of diabetic kidney disease. This notion is supported by several clinical studies that reported increased urinary ACE2 levels in patients with CKD and diabetic renal transplant recipients (35, 60). There was also a strong correlation between urinary ACE2 mRNA and the degree of proteinuria in type 2 diabetic patients (51). The present study showed that urinary ACE2 levels are positively correlated with albuminuria, blood glucose, plasma glucagon, triglycerides, and creatinine. This supports our previous results conducted in type 2 db/db diabetic mice (10) and a recent clinical study (40). The relationship between urinary ACE2 levels and other risk factors of metabolic and kidney dysfunction implies that urinary ACE2 levels may be considered as an additional and independent risk factor of diabetic kidney disease.
It is well known that overactivation of the RAS in both types of diabetes with subsequent abundant generation of ANG II plays an important role in the progression of DN (20). Therefore, it is assumed that ACE2 has an endogenous renoprotective function due to its ability to degrade ANG II, thereby reducing the deleterious ANG II-mediated effects in diabetes (47). The present study showed a significant increase in protein expression and activity of renal ACE2 in Akita mice compared with WT mice. Western blotting, immunostaining, and fluorogenic enzymatic activity assay have confirmed this finding. Moreover, MALDI imaging analysis confirmed an increase in ANG-(1–7) formation in diabetic Akita mice compared with WT mice. Mass spectrometry is superior to the current existing detection methods in terms of specificity, accuracy, selectivity, and the ability to simultaneously detect multiple analytes. Our new in situ method has the added advantage of allowing for the regional quantification of enzymatic activity within tissue sections (19). Using this powerful technique, we demonstrated that the increase in ANG-(1–7) formation was predominantly localized in the cortex, the key site of ANG-(1–7) formation in the kidney. This finding is also in agreement with previous studies, which demonstrated an increase in renal tubular ACE2 expression and activity in type 2 db/db diabetic mice compared with lean control mice (10, 58). Increased renal ACE2 protein expression or activity was also observed in various other studies using Akita, db/db, and STZ diabetic mice (39, 56, 58, 59, 62). Interestingly, renal ACE2 mRNA levels were elevated in diabetic Akita animals (39, 56) but not in STZ and db/db mice (59, 62), suggesting that other mechanisms may be responsible for upregulation of renal ACE2 in addition to transcriptional regulators. These findings are in contrast to other studies reporting on decreased renal ACE2 expression and activity in experimental models of diabetes including Akita mice (32), STZ mice (8), STZ rats (29, 49), and mouse models of chronic kidney disease (12). Despite the controversy, we postulate that an increase in renal ACE2 in cortical tubules could be the earliest positive feedback response to hyperglycemia and a possible compensatory protective mechanism opposing the toxic effects of sustained hyperglycemia, elevated levels of ANG II, and increased ADAM17-mediated ACE2 shedding during the initial stages of kidney damage.
This study is the first report that shows an increase in renal ADAM17 protein expression in type 1 diabetic Akita mice as shown by Western blotting and immunostaining, confirming our previous study conducted in type 2 diabetic db/db mice (10). In addition, a significant increase in renal ADAM17 activity was found in STZ and OVE26 diabetic mice (16, 18). However, there was no significant difference in renal ADAM17 mRNA expression between Akita and WT mice, suggesting that increased ADAM17 protein expression in diabetes may not be regulated at the mRNA level (3). A 2005 study reported that ADAM17 mediates active ectodomain shedding of ACE2, but not ACE, in vitro (52). In addition, several studies conducted in different cell lines reported that overexpression of ADAM17 increases shedding of active extracellular domain of ACE2 in HEK293 cells and in Huh7 cells (27). The present study shows that pharmacological inhibition of ADAM17 reduced shedding of ACE2 in human renal proximal tubular HK-2 cells, which have been shown to provide a reliable model for the study of proximal tubular cells (42).
Clinical studies conducted in patients with DN proposed that TIMP3, a physiological inhibitor of ADAM17, could contribute to the development and progression of DN (14, 57). We speculated that hyperglycemia decreases renal TIMP3 protein expression, resulting in increased ADAM17. Our speculation was supported by recent studies that reported a significant decrease in renal TIMP3 in STZ diabetic mice and in type 2 diabetic patients (16), leading to elevated circulating levels of ADAM17 (5, 6). Interestingly, Western blotting performed in the present study revealed no significant difference in renal TIMP3 protein expression in Akita mice compared with WT mice, indicating other indirect, TIMP3-independent pathways.
Finally, the present study investigated the effect of normalizing hyperglycemia on cardiovascular risk factors, renal ADAM17, ACE2, TIMP3 expression, urinary ACE2, and albumin excretion in Akita mice. Insulin treatment normalized hyperglycemia and other metabolic and plasma parameters. The results shown above were confirmed by a previous study conducted in STZ diabetic mice treated with insulin implants (8). Moreover, there was a significant attenuation in urinary albumin excretion and renal histopathological lesions of treated Akita mice. As expected, previous findings in STZ diabetic mice, nonobese diabetic mice, and type 1 diabetic patients treated with subcutaneous insulin showed the significant role of glycemic control in the delayed onset or reduced progression of diabetic nephropathy (1, 4, 8). Interestingly, treatment with insulin decreased both urinary and renal ACE2 protein expression and activity in treated Akita mice. This contrasts previous results using type 2 diabetic mice where normalizing hyperglycemia with the insulin sensitizer rosiglitazone had no effect on renal ACE2 expression, highlighting the difference in both diabetic models (10). Accordingly, we also tested the effect of normalizing hyperglycemia on renal ADAM17 and TIMP3 protein expression. Treatment with insulin decreased renal ADAM17 protein expression in Akita mice. As expected, treatment with insulin had no effect on renal TIMP3 protein expression in Akita mice, suggesting that hyperglycemia has no immediate impact on ADAM17 via TIMP3.
In conclusion, our results suggest that hyperglycemia increases renal ACE2 and ADAM17 expression in coalition with a rise in urinary ACE2 excretion most likely due to increased shedding of renal ACE2 mediated by ADAM17. The euglycemic effect of insulin decreased urinary ACE2 excretion, restored renal ACE2 and ADAM17 expression back to physiological levels, and normalized the rate of shedding. A strong positive correlation of urinary ACE2 with other independent risk factors for diabetic kidney disease suggests the important clinical relevance of urinary ACE2 as a marker for diabetic renal impairment and an indicator for medical therapy intervention.
GRANTS
This work was supported by American Heart Association Grant SDG 0735112N (K. M. Elased), a National Institutes of Health fellowship (F32DK093226 to N. Grobe), a Wright State University BSOM emerging science seed grant (K. M. Elased and N. Grobe), and financial support from the Libyan government (E. S. B. Salem).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: E.S.S., N.G., and K.M.E. provided conception and design of research; E.S.S. and N.G. performed experiments; E.S.S., N.G., and K.M.E. analyzed data; E.S.S., N.G., and K.M.E. interpreted results of experiments; E.S.S. and N.G. prepared figures; E.S.S., N.G., and K.M.E. drafted manuscript; E.S.S., N.G., and K.M.E. edited and revised manuscript; E.S.S., N.G., and K.M.E. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Harshita Chodavarapu, Hari K. Somineni, Lesan K. Mattis, and Teresa L. Garrett for excellent technical assistance.
REFERENCES
- 1.Anonymous The effect of intensive treatment of diabetes on the development, and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med 329: 977–986, 1993 [DOI] [PubMed] [Google Scholar]
- 2.Arita DY, Cunha TS, Perez JD, Colucci JA, Ronchi FA, Nogueira MD, Arita LS, Aragao DS, Teixeira VP, Casarini DE. Overexpression of urinary N-domain ACE in chronic kidney dysfunction in Wistar rats. Clin Exp Hypertens 34: 389–396, 2012 [DOI] [PubMed] [Google Scholar]
- 3.Basu R, Lee J, Wang Z, Patel VB, Fan D, Das SK, Liu GC, John R, Scholey JW, Oudit GY, Kassiri Z. Loss of TIMP3 selectively exacerbates diabetic nephropathy. Am J Physiol Renal Physiol 303: F1341–F1352, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brezar V, Culina S, Gagnerault MC, Mallone R. Short-term subcutaneous insulin treatment delays but does not prevent diabetes in NOD mice. Eur J Immunol 42: 1553–1561, 2012 [DOI] [PubMed] [Google Scholar]
- 5.Cardellini M, Menghini R, Luzi A, Davato F, Cardolini I, D'Alfonso R, Gentileschi P, Rizza S, Marini MA, Porzio O, Lauro D, Sbraccia P, Lauro R, Federici M. Decreased IRS2 and TIMP3 expression in monocytes from offspring of type 2 diabetic patients is correlated with insulin resistance and increased intima-media thickness. Diabetes 60: 3265–3270, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cardellini M, Menghini R, Martelli E, Casagrande V, Marino A, Rizza S, Porzio O, Mauriello A, Solini A, Ippoliti A, Lauro R, Folli F, Federici M. TIMP3 is reduced in atherosclerotic plaques from subjects with type 2 diabetes and increased by SirT1. Diabetes 58: 2396–2401, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang JH, Paik SY, Mao L, Eisner W, Flannery PJ, Wang L, Tang Y, Mattocks N, Hadjadj S, Goujon JM, Ruiz P, Gurley SB, Spurney RF. Diabetic kidney disease in FVB/NJ Akita mice: temporal pattern of kidney injury and urinary nephrin excretion. PLoS One 7: e33942, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang SY, Chen YW, Chenier I, Tran SM, Zhang SL. Angiotensin II type II receptor deficiency accelerates the development of nephropathy in type I diabetes via oxidative stress and ACE2. Exp Diabetes Res 2011: 521076, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chawla T, Sharma D, Singh A. Role of the renin angiotensin system in diabetic nephropathy. World J Diabetes 1: 141–145, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chodavarapu H, Grobe N, Somineni HK, Salem ES, Madhu M, Elased KM. Rosiglitazone treatment of type 2 diabetic db/db mice attenuates urinary albumin and angiotensin converting enzyme 2 excretion. PLoS One 8: e62833, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Galan BE, Perkovic V, Ninomiya T, Pillai A, Patel A, Cass A, Neal B, Poulter N, Harrap S, Mogensen CE, Cooper M, Marre M, Williams B, Hamet P, Mancia G, Woodward M, Glasziou P, Grobbee DE, MacMahon S, Chalmers J. Lowering blood pressure reduces renal events in type 2 diabetes. J Am Soc Nephrol 20: 883–892, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dilauro M, Zimpelmann J, Robertson SJ, Genest D, Burns KD. Effect of ACE2 and angiotensin-(1–7) in a mouse model of early chronic kidney disease. Am J Physiol Renal Physiol 298: F1523–F1532, 2010 [DOI] [PubMed] [Google Scholar]
- 13.Donoghue M, Hsieh F, Baronas E, Godbout K, Gosselin M, Stagliano N, Donovan M, Woolf B, Robison K, Jeyaseelan R, Breitbart RE, Acton S. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1–9. Circ Res 87: E1–E9, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Ewens KG, George RA, Sharma K, Ziyadeh FN, Spielman RS. Assessment of 115 candidate genes for diabetic nephropathy by transmission/disequilibrium test. Diabetes 54: 3305–3318, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Federici M, Hribal ML, Menghini R, Kanno H, Marchetti V, Porzio O, Sunnarborg SW, Rizza S, Serino M, Cunsolo V, Lauro D, Mauriello A, Smookler DS, Sbraccia P, Sesti G, Lee DC, Khokha R, Accili D, Lauro R. Timp3 deficiency in insulin receptor-haploinsufficient mice promotes diabetes and vascular inflammation via increased TNF-alpha. J Clin Invest 115: 3494–3505, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fiorentino L, Cavalera M, Menini S, Marchetti V, Mavilio M, Fabrizi M, Conserva F, Casagrande V, Menghini R, Pontrelli P, Arisi I, D'Onofrio M, Lauro D, Khokha R, Accili D, Pugliese G, Gesualdo L, Lauro R, Federici M. Loss of TIMP3 underlies diabetic nephropathy via FoxO1/STAT1 interplay. EMBO Mol Med 5: 441–455, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fiorentino L, Vivanti A, Cavalera M, Marzano V, Ronci M, Fabrizi M, Menini S, Pugliese G, Menghini R, Khokha R, Lauro R, Urbani A, Federici M. Increased tumor necrosis factor alpha-converting enzyme activity induces insulin resistance and hepatosteatosis in mice. Hepatology 51: 103–110, 2010 [DOI] [PubMed] [Google Scholar]
- 18.Ford BM, Eid AA, Gooz M, Barnes JL, Gorin YC, Abboud HE. ADAM17 mediates Nox4 expression and NADPH oxidase activity in the kidney cortex of OVE26 mice. Am J Physiol Renal Physiol 305: F323–F332, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grobe N, Elased KM, Cool DR, Morris M. Mass spectrometry for the molecular imaging of angiotensin metabolism in kidney. Am J Physiol Endocrinol Metab 302: E1016–E1024, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gurley SB, Coffman TM. The renin-angiotensin system and diabetic nephropathy. Semin Nephrol 27: 144–152, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Hoffmann S, Podlich D, Hahnel B, Kriz W, Gretz N. Angiotensin II type 1 receptor overexpression in podocytes induces glomerulosclerosis in transgenic rats. J Am Soc Nephrol 15: 1475–1487, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Jia HP, Look DC, Tan P, Shi L, Hickey M, Gakhar L, Chappell MC, Wohlford-Lenane C, McCray PB., Jr Ectodomain shedding of angiotensin converting enzyme 2 in human airway epithelia. Am J Physiol Lung Cell Mol Physiol 297: L84–L96, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jim B, Santos J, Spath F, Cijiang HJ. Biomarkers of diabetic nephropathy, the present and the future. Curr Diabetes Rev 8: 317–328, 2012 [DOI] [PubMed] [Google Scholar]
- 24.Kobori H, Urushihara M. Augmented intrarenal and urinary angiotensinogen in hypertension and chronic kidney disease. Pflügers Arch 465: 3–12, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koitka A, Cooper ME, Thomas MC, Tikellis C. Angiotensin converting enzyme 2 in the kidney. Clin Exp Pharmacol Physiol 35: 420–425, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lai ZW, Hanchapola I, Steer DL, Smith AI. Angiotensin-converting enzyme 2 ectodomain shedding cleavage-site identification: determinants and constraints. Biochemistry 50: 5182–5194, 2011 [DOI] [PubMed] [Google Scholar]
- 27.Lambert DW, Yarski M, Warner FJ, Thornhill P, Parkin ET, Smith AI, Hooper NM, Turner AJ. Tumor necrosis factor-alpha convertase (ADAM17) mediates regulated ectodomain shedding of the severe-acute respiratory syndrome-coronavirus (SARS-CoV) receptor, angiotensin-converting enzyme-2 (ACE2). J Biol Chem 280: 30113–30119, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lautrette A, Li S, Alili R, Sunnarborg SW, Burtin M, Lee DC, Friedlander G, Terzi F. Angiotensin II and EGF receptor cross-talk in chronic kidney diseases: a new therapeutic approach. Nat Med 11: 867–874, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Leehey DJ, Singh AK, Bast JP, Sethupathi P, Singh R. Glomerular renin angiotensin system in streptozotocin diabetic and Zucker diabetic fatty rats. Transl Res 151: 208–216, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Lew RA, Warner FJ, Hanchapola I, Yarski MA, Manohar J, Burrell LM, Smith AI. Angiotensin-converting enzyme 2 catalytic activity in human plasma is masked by an endogenous inhibitor. Exp Physiol 93: 685–693, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu CX, Hu Q, Wang Y, Zhang W, Ma ZY, Feng JB, Wang R, Wang XP, Dong B, Gao F, Zhang MX, Zhang Y. Angiotensin-converting enzyme (ACE) 2 overexpression ameliorates glomerular injury in a rat model of diabetic nephropathy: a comparison with ACE inhibition. Mol Med 17: 59–69, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lo CS, Liu F, Shi Y, Maachi H, Chenier I, Godin N, Filep JG, Ingelfinger JR, Zhang SL, Chan JS. Dual RAS blockade normalizes angiotensin-converting enzyme-2 expression and prevents hypertension and tubular apoptosis in Akita angiotensinogen-transgenic mice. Am J Physiol Renal Physiol 302: F840–F852, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lv J, Perkovic V, Foote CV, Craig ME, Craig JC, Strippoli GF. Antihypertensive agents for preventing diabetic kidney disease. Cochrane Database Syst Rev 12: CD004136, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Melenhorst WB, Visser L, Timmer A, van den Heuvel MC, Stegeman CA, van GH. ADAM17 upregulation in human renal disease: a role in modulating TGF-α availability? Am J Physiol Renal Physiol 297: F781–F790, 2009 [DOI] [PubMed] [Google Scholar]
- 35.Mizuiri S, Aoki T, Hemmi H, Arita M, Sakai K, Aikawa A. Urinary angiotensin-converting enzyme 2 in patients with CKD. Nephrology (Carlton) 16: 567–572, 2011 [DOI] [PubMed] [Google Scholar]
- 36.Mizuiri S, Hemmi H, Arita M, Ohashi Y, Tanaka Y, Miyagi M, Sakai K, Ishikawa Y, Shibuya K, Hase H, Aikawa A. Expression of ACE and ACE2 in individuals with diabetic kidney disease and healthy controls. Am J Kidney Dis 51: 613–623, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Mulder GM, Melenhorst WB, Celie JW, Kloosterhuis NJ, Hillebrands JL, Ploeg RJ, Seelen MA, Visser L, van Dijk MC, van GH. ADAM17 up-regulation in renal transplant dysfunction and non-transplant-related renal fibrosis. Nephrol Dial Transplant 27: 2114–2122, 2012 [DOI] [PubMed] [Google Scholar]
- 38.Nadarajah R, Milagres R, Dilauro M, Gutsol A, Xiao F, Zimpelmann J, Kennedy C, Wysocki J, Batlle D, Burns KD. Podocyte-specific overexpression of human angiotensin-converting enzyme 2 attenuates diabetic nephropathy in mice. Kidney Int 82: 292–303, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oudit GY, Liu GC, Zhong J, Basu R, Chow FL, Zhou J, Loibner H, Janzek E, Schuster M, Penninger JM, Herzenberg AM, Kassiri Z, Scholey JW. Human recombinant ACE2 reduces the progression of diabetic nephropathy. Diabetes 59: 529–538, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Park SE, Kim WJ, Park SW, Park JW, Lee N, Park C, Youn BS. High urinary ACE2 concentrations are associated with severity of glucose intolerance and microalbuminuria. Eur J Endocrinol 168: 203–210, 2013 [DOI] [PubMed] [Google Scholar]
- 41.Reich HN, Oudit GY, Penninger JM, Scholey JW, Herzenberg AM. Decreased glomerular and tubular expression of ACE2 in patients with type 2 diabetes and kidney disease. Kidney Int 74: 1610–1616, 2008 [DOI] [PubMed] [Google Scholar]
- 42.Ryan MJ, Johnson G, Kirk J, Fuerstenberg SM, Zager RA, Torok-Storb B. HK-2: an immortalized proximal tubule epithelial cell line from normal adult human kidney. Kidney Int 45: 48–57, 1994 [DOI] [PubMed] [Google Scholar]
- 43.Scheller J, Chalaris A, Garbers C, Rose-John S. ADAM17: a molecular switch to control inflammation and tissue regeneration. Trends Immunol 32: 380–387, 2011 [DOI] [PubMed] [Google Scholar]
- 44.Shaltout HA, Westwood BM, Averill DB, Ferrario CM, Figueroa JP, Diz DI, Rose JC, Chappell MC. Angiotensin metabolism in renal proximal tubules, urine, and serum of sheep: evidence for ACE2-dependent processing of angiotensin II. Am J Physiol Renal Physiol 292: F82–F91, 2007 [DOI] [PubMed] [Google Scholar]
- 45.Soler MJ, Wysocki J, Ye M, Lloveras J, Kanwar Y, Batlle D. ACE2 inhibition worsens glomerular injury in association with increased ACE expression in streptozotocin-induced diabetic mice. Kidney Int 72: 614–623, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Soro-Paavonen A, Gordin D, Forsblom C, Rosengard-Barlund M, Waden J, Thorn L, Sandholm N, Thomas MC, Groop PH. Circulating ACE2 activity is increased in patients with type 1 diabetes and vascular complications. J Hypertens 30: 375–383, 2012 [DOI] [PubMed] [Google Scholar]
- 47.Su Z, Zimpelmann J, Burns KD. Angiotensin-(1–7) inhibits angiotensin II-stimulated phosphorylation of MAP kinases in proximal tubular cells. Kidney Int 69: 2212–2218, 2006 [DOI] [PubMed] [Google Scholar]
- 48.Thomas MC, MacIsaac RJ, Jerums G, Weekes A, Moran J, Shaw JE, Atkins RC. Nonalbuminuric renal impairment in type 2 diabetic patients and in the general population (national evaluation of the frequency of renal impairment cO-existing with NIDDM [NEFRON] 11). Diabetes Care 32: 1497–1502, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tikellis C, Johnston CI, Forbes JM, Burns WC, Burrell LM, Risvanis J, Cooper ME. Characterization of renal angiotensin-converting enzyme 2 in diabetic nephropathy. Hypertension 41: 392–397, 2003 [DOI] [PubMed] [Google Scholar]
- 50.Tipnis SR, Hooper NM, Hyde R, Karran E, Christie G, Turner AJ. A human homolog of angiotensin-converting enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidase. J Biol Chem 275: 33238–33243, 2000 [DOI] [PubMed] [Google Scholar]
- 51.Wang G, Lai FM, Lai KB, Chow KM, Kwan CH, Li KT, Szeto CC. Urinary mRNA expression of ACE and ACE2 in human type 2 diabetic nephropathy. Diabetologia 51: 1062–1067, 2008 [DOI] [PubMed] [Google Scholar]
- 52.Warner FJ, Lew RA, Smith AI, Lambert DW, Hooper NM, Turner AJ. Angiotensin-converting enzyme 2 (ACE2), but not ACE, is preferentially localized to the apical surface of polarized kidney cells. J Biol Chem 280: 39353–39362, 2005 [DOI] [PubMed] [Google Scholar]
- 53.Watanabe T, Barker TA, Berk BC. Angiotensin II and the endothelium: diverse signals and effects. Hypertension 45: 163–169, 2005 [DOI] [PubMed] [Google Scholar]
- 54.Wei S, Xie Z, Filenova E, Brew K. Drosophila TIMP is a potent inhibitor of MMPs and TACE: similarities in structure and function to TIMP-3. Biochemistry 42: 12200–12207, 2003 [DOI] [PubMed] [Google Scholar]
- 55.Whaley-Connell AT, Chowdhury NA, Hayden MR, Stump CS, Habibi J, Wiedmeyer CE, Gallagher PE, Tallant EA, Cooper SA, Link CD, Ferrario C, Sowers JR. Oxidative stress and glomerular filtration barrier injury: role of the renin-angiotensin system in the Ren2 transgenic rat. Am J Physiol Renal Physiol 291: F1308–F1314, 2006 [DOI] [PubMed] [Google Scholar]
- 56.Wong DW, Oudit GY, Reich H, Kassiri Z, Zhou J, Liu QC, Backx PH, Penninger JM, Herzenberg AM, Scholey JW. Loss of angiotensin-converting enzyme-2 (Ace2) accelerates diabetic kidney injury. Am J Pathol 171: 438–451, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Woroniecka KI, Park AS, Mohtat D, Thomas DB, Pullman JM, Susztak K. Transcriptome analysis of human diabetic kidney disease. Diabetes 60: 2354–2369, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wysocki J, Garcia-Halpin L, Ye M, Maier C, Sowers K, Burns KD, Batlle D. Regulation of urinary ACE2 in diabetic mice. Am J Physiol Renal Physiol 305: F600–F611, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wysocki J, Ye M, Soler MJ, Gurley SB, Xiao HD, Bernstein KE, Coffman TM, Chen S, Batlle D. ACE and ACE2 activity in diabetic mice. Diabetes 55: 2132–2139, 2006 [DOI] [PubMed] [Google Scholar]
- 60.Xiao F, Hiremath S, Knoll G, Zimpelmann J, Srivaratharajah K, Jadhav D, Fergusson D, Kennedy CR, Burns KD. Increased urinary Angiotensin-converting enzyme 2 in renal transplant patients with diabetes. PLoS One 7: e37649, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yamaleyeva LM, Gilliam-Davis S, Almeida I, Brosnihan KB, Lindsey SH, Chappell MC. Differential regulation of circulating and renal ACE2 and ACE in hypertensive mRen2.Lewis rats with early-onset diabetes. Am J Physiol Renal Physiol 302: F1374–F1384, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ye M, Wysocki J, Naaz P, Salabat MR, LaPointe MS, Batlle D. Increased ACE 2 and decreased ACE protein in renal tubules from diabetic mice: a renoprotective combination? Hypertension 43: 1120–1125, 2004 [DOI] [PubMed] [Google Scholar]