Abstract
In angiotensin II (ANG II)-dependent hypertension, the augmented intrarenal ANG II constricts the renal microvasculature and stimulates Rho kinase (ROCK), which modulates vascular contractile responses. Rho may also stimulate angiotensinogen (AGT) expression in preglomerular vascular smooth muscle cells (VSMCs), but this has not been established. Therefore, the aims of this study were to determine the direct interactions between Rho and ANG II in regulating AGT and other renin-angiotensin system (RAS) components and to elucidate the roles of the ROCK/NF-κB axis in the ANG II-induced AGT augmentation in primary cultures of preglomerular VSMCs. We first demonstrated that these preglomerular VSMCs express renin, AGT, angiotensin-converting enzyme, and ANG II type 1 (AT1) receptors. Furthermore, incubation with ANG II (100 pmol/l for 24 h) increased AGT mRNA (1.42 ± 0.03, ratio to control) and protein (1.68 ± 0.05, ratio to control) expression levels, intracellular ANG II levels, and NF-κB activity. In contrast, the ANG II treatment did not alter AT1a and AT1b mRNA levels in the cells. Treatment with H-1152 (ROCK inhibitor, 10 nmol/l) and ROCK1 small interfering (si) RNA suppressed the ANG II-induced AGT augmentation and the upregulation and translocalization of p65 into nuclei. Functional studies showed that ROCK exerted a greater influence on afferent arteriole responses to ANG II in rats subjected to chronic ANG II infusions. These results indicate that ROCK is involved in NF-κB activation and the ROCK/NF-κB axis contributes to ANG II-induced AGT upregulation, leading to intracellular ANG II augmentation.
Keywords: renin-angiotensin system, afferent arteriole, angiotensin II type 1 receptors, renin, parathenolide, H-1152, juxtamedullary nephron preparation
the renal afferent arterioles are primarily responsible for regulating preglomerular resistance, renal blood flow, and glomerular filtration rate (GFR) (3, 34, 42). Elevated renal vascular resistance and preglomerular reactivity are observed in angiotensin II (ANG II)-induced hypertension (16, 36). Although many systemic, neural, paracrine, and autoregulatory mechanisms contribute to afferent arteriolar dynamics (34), ANG II exerts a critical role, in particular in ANG II-dependent hypertension (17). In hypertensive models with increased intrarenal ANG II, afferent arteriolar vasoconstrictor responses to additional ANG II are augmented, indicating an enhanced reactivity (2b, 17, 34). The mechanisms responsible for the enhanced responsiveness remain unclear, but it seems likely that the greater vasoconstriction contributes to impaired renal hemodynamics and the development of hypertension (15, 35).
In addition to the increased preglomerular reactivity to ANG II in ANG II-induced hypertension, there is an augmentation of angiotensinogen (AGT), which is predominantly localized in proximal tubules (23) but is also expressed in other renal cells, including glomerular endothelial cells, mesangial cells, and podocytes (45, 53). Chronic infusions of ANG II significantly increase intrarenal AGT expression accompanied by increased renal ANG II levels. These findings suggest that there is a stimulation of AGT in kidney vascular smooth muscle cells (VSMCs), but this has not been clearly established, and it is unknown whether AGT can be formed in VSMCs or is due to uptake. Although intrarenal AGT mRNA and protein are localized primarily in proximal tubule segments, it is also possible that AGT expression in afferent arterioles can lead to increases in local or intracellular ANG II formation, directly enhancing vascular sensitivity. To address these issues, preglomerular VSMCs were isolated and cultured using special efforts to maintain the normal phenotype.
In other tissues, the Rho (a small GTPase) and Rho kinase (ROCK) pathway is an important factor in signal transduction pathways that mediate cell proliferation, cell migration, apoptosis, smooth muscle contraction, and actin cytoskeleton organization (22, 29). Rho contributes to impairment of renal hemodynamics in some models of hypertension (33, 52). The activation of ROCK alters vascular tone by increasing the cytosolic calcium sensitivity to phosphorylation of myosin light chains (50). Treatment with ROCK inhibitors attenuates the progression of renal vascular injury in ANG II-induced hypertensive rats, suggesting that activation of the ROCK pathway is involved in the development of renal injury (12, 24, 38, 41).
ANG II activates NF-κB, which is involved in immune and inflammatory reactions via induction of cytokines, chemokines, cell adhesion molecules, growth factors, and immunoreceptors (32). NF-κB is a potent regulator of human AGT promoter expression in proximal tubule cells (1). In aortic VSMCs, activated ROCK mediates activation of NF-κB by ANG II (40). However, the role of the ROCK/NF-κB axis in AGT regulation in preglomerular VSMCs has not been delineated. Therefore, the aims of this study were to establish a primary preglomerular VSMC culture system that retains its characteristics to demonstrate the presence of AGT, ANG II, and other components of RAS in the cells and to determine the contribution of ROCK/NF-κB to the ANG II-mediated AGT augmentation. To establish functional relevance, the effects of ROCK inhibition on ANG II stimulates afferent arterioles from control and chronic ANG II-infused rats were determined (18).
MATERIALS AND METHODS
Animals.
The experimental protocol was approved by the Tulane University Institutional Animal Care and Use Committee. In this study, male Sprague-Dawley rats (200–230 g, Charles River Laboratories, Wilmington, MA) were housed in a constant-temperature room with a 12:12-h dark-light cycle with free access to food and water.
For preglomerular VSMC isolation, kidneys were harvested from the rats. The animals were anesthetized with pentobarbital sodium (40 mg/kg, Ovation). The abdominal cavity was exposed by a midline abdominal incision, and the abdominal aorta was cannulated with ligatures above and below the renal arteries. The kidneys were perfused with PBS solution and an iron oxide solution and then were decapsulated.
For immunostaining of AGT and for the experiments evaluating afferent arteriolar responses, rats received ANG II (80 ng/min, 14 days) via implanted osmotic minipumps (Alzet osmotic pump, Alza, Mountain View, CA) or sham operation. On day 14, rats were anesthetized with pentobarbital sodium for left kidney excision after unilateral ligature, and the right kidneys were sequentially perfused with saline solution (0.9% NaCl) and 4% paraformaldehyde. Formalin-fixed kidney sections (4 μm) were used to detect AGT by using an anti-rat AGT antibody. For the juxtamedullary nephron study (8, 9), control and ANG II-infused male Sprague-Dawley rats (350–400 g, Charles River Laboratories) were used.
Antibodies and inhibitors.
Anti-α-smooth muscle actin, anti-tubulin, and anti-desmin antibodies were purchased from Abcam (Cambridge, MA). Anti-CD34, anti-renin, anti-angiotensin-converting enzyme (ACE), anti-ANG II type 1 receptor (AT1R), and anti-β-actin antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Another anti-α-smooth muscle actin antibody was obtained from Dako (Carpinteria, CA) for immunohistochemistry. An anti-AGT antibody was purchased from Immuno-Biological Laboratories (Gunma, Japan). An anti-ANG II antibody was purchased from Phoenix Pharmaceuticals, (Burlingame, CA). IRDye-labeled anti-mouse IgG and anti-rabbit IgG antibodies were obtained from Li-Cor Bioscience (Lincoln, NE) as secondary antibodies for Western blot analyses. Donkey anti-rabbit IgG (Alexa Fluor 488) and donkey anti-mouse IgG (Alexa Fluor 594) antibodies were purchased from Invitrogen (Grand Island, NY). Olmesartan, an AT1R blocker, was provided by Daiichi-Sankyo (Tokyo, Japan). Fasudil, a ROCK inhibitor, was purchased from Asahi Kasei (Tokyo, Japan). H-1152, also a ROCK inhibitor, was obtained from Calbiochem (Darmstadt, Germany), and parthenolide, an NF-κB inhibitor, was purchased from MP Bio Science (Derbyshire, UK).
Immunostaining of AGT.
To detect AGT protein in afferent arterioles of rat kidneys, paraformaldehyde was perfused into kidneys of chronic ANG II-infused rats (80 ng/min, 14 days). Immunofluorescence staining was performed as previously described using the kidney sections (4 μm) (11). A rabbit anti-AGT antibody and anti-α-smooth muscle actin antibody were used in this experiment. Donkey anti-rabbit IgG (Alexa Fluor 488) and donkey anti-mouse IgG (Alexa Fluor 594) were used as secondary antibodies.
Isolation and culture of preglomerular VSMCs and mesangial cells.
Isolation of preglomerular VSMCs from rat afferent arterioles was performed using the iron oxide perfusion technique with different sieves, as previously described with minor modifications (4, 7). After the kidneys were perfused with 10 ml PBS, the perfusion solution was switched to 5% (wt/vol) suspension of iron oxide particles (Sigma-Aldrich) in culture medium (DMEM supplemented with 1 U/ml penicillin, 1 U/ml streptomycin, Invitrogen Life Technologies). The cortical tissue was carefully dissected from the medulla, minced, and gently pressed through a 150-μm mesh sieve followed by a 100-μm mesh sieve. The microvessels containing iron were separated from major vascular parts using a magnet. The microvessels were placed in 20 ml culture medium containing 0.5 mg/ml type I collagenase and incubated at 37°C for 30 min with shaking. The microvessels were cultured with DMEM containing 15% FBS, 1 U/ml penicillin, and 1 U/ml streptomycin in 75-cm2 tissue culture flasks at 37°C in 5% CO2-95% air for 5 days. Thereafter, the medium was replaced with a fresh one; subsequently, the medium was changed every 2 days until the cells reached confluence. For the experiments, cells were used between passages 3 and 5. The cells at 80% confluence were starved by incubation with serum-free DMEM for 24 h. The serum-starved cells were stimulated with ANG II for 24 h in the presence and absence of inhibitors. Rat mesangial cells were isolated as previously reported (20). In brief, cells were isolated from intact glomeruli of Sprague-Dawley rats by multiple sieving steps and maintained in RPMI 1640 medium supplemented with 15% FBS (Invitrogen).
Rat aortic VSMC culture.
For comparison with the preglomerular VSMCs, aortic VSMCs, which are a representative model of nonrenal VSMCs, were also isolated from the same male Sprague-Dawley rats in accordance with published methods (27). Isolated cells were maintained in DMEM supplemented with 10% FBS (Invitrogen). Aortic VSMCs were used between passages 3 and 9.
Experimental protocols.
For the juxtaglomerular nephron study, a single afferent arteriole was visualized and perfused with each solution. Afferent arteriolar inside diameters were measured during the final 5 min of each treatment period with the following solutions: 1) 1 nmol/l ANG II and 2) 1 nmol/l ANG II plus fasudil from 0.1 to 100 μmol/l.
For cell studies, preglomerular VSMCs and aortic VSMCs were treated with ANG II (from 1 to 100 pmol/l) for 24 h, and cells were harvested for analyses. To determine the effects of each inhibitor on AGT or RelA mRNA expression levels, experiments were repeated during pretreatment with olmesartan, H-1152, and parathenolide 30 min before ANG II administration.
We also performed a transient transfection of preglomerular VSMCs with small interference (si) RNA of ROCK. Silencer-select predesigned siRNA against ROCK1 was designed and synthesized by Ambion (Life Technologies, Carlsbad, CA). The siRNA (100 nM) was transfected to preglomerular VSMCs with Lipofectamine RNAiMAX reagent (Life Technologies) according to the manufacturer's protocol. As a negative control, a nontargeting scrambled negative siRNA (control siRNA) was purchased from Life Technologies. After 24-h transfection, cells were washed and incubated with or without ANG II (100 pmol/l) for 24 h.
RT-PCR and quantitative real-time RT-PCR.
For total RNA isolation, cells were washed with PBS and then total RNA was extracted using a commercially available kit (Qiagen). RT-PCR was performed to detect AGT, AT1a, and AT1b mRNA in both preglomerular and aortic VSMCs. To avoid analyses in plateau conditions, RT-PCR conditions were carefully decided based on results of preliminary tests. RNA amounts in the reverse transcription (400 ng), number of PCR cycles (35 cycles), and loading amount of PCR products (1/5 vol of PCR) were used. The amplified cDNA bands were visualized by ethidium bromide in 1.5% agarose gels. Quantitative real-time RT-PCR (qRT-PCR) was performed to evaluate rat AGT, AT1a, AT1b, and p65 expression using the TaqMan PCR system as previously described (25, 39). All samples were analyzed in triplicate, and the data obtained were normalized to β-actin mRNA expression levels. Primer sequences were as follows: AGT: forward primer, 5′-GAA GAT GAA CTT GCC ACT AGA-3′; reverse primer, 5′-AAG TGA ACG TAG GTG TTG AAA-3′; and probe, 5′/6 FAM/CAG CAC GGA CAG CAC CCT ATT/BHQ1/3′; AT1a: forward primer, 5′-CTT GTT CCC TTT CCT TAT CA-3′; reverse primer, 5′-CGT TTC TTG GTT TGT TCT TT-3′; and probe, 5′/6 FAM/CAC CAG CTA TAC CCT TAT TTG GAA AAG/BHQ/1/3′; AT1b: forward primer, 5′-ATT TCA TCG AGA ACA CCA AT-3′; reverse primer, 5′-TTT GTT AGA CCC AGT CCA AT-3′; and probe, 5′/6 FAM/ATG AAT CTC AGA ACT CAA CAC TCC C/BHQ/1/3′; p65: forward primer, 5′-CAT CAA GAT CAA TGG CTA-3′; reverse primer, 5′-CAC AAG TTC ATG TGG ATG AG-3′; and probe, 5′/6 FAM/AAC AGT TCG AAT CTC CCT GGT BHQ/1/3′; and β-actin: forward primer, 5′-ATC ATG AAG TGT GAC GTT GA-3′; reverse primer, 5′-GAT CTT CAT GGT GCT AGG AGC-3′; and probe, 5′/HEX/TCT ATG CCA ACA CAG TGC TGT CTG GT/BHQ2/3′.
Immunocytochemistry.
To identify the isolated cells, immunocytochemical staining against the cell markers for characterization and major RAS components in preglomerular VSMCs was performed as described previously (4, 7, 21). Preglomerular VSMCs were cultured in four-well chamber slides (Lab-Tek). After fixation with 4% paraformaldehyde for 20 min and incubation with 0.1% Triton X-100 for 3 min, blocking solution (Image-iT FX signal enhancer, Invitrogen) was used. The cells were incubated with primary antibodies overnight at room temperature. After washing of the cells with PBS, the cells were incubated with Alexa Fluor 488- or 595-labeled secondary antibodies. ProLong Gold antifade reagent containing .4,6-diamidino-2-phenylindole (Invitrogen) was used as a nuclear stain. Isolated rat mesangial cells were also characterized by Thy1 staining.
Western blot analysis.
To determine the expression levels of AGT and AT1R proteins in preglomerular VSMCs, Western blot analyses were performed using 60 μg of cell lysate as previously described (25, 37, 39). After harvesting of the preglomerular VSMCs, the cell lysates were prepared and quantified by a bicinchoninic acid protein assay kit (Pierce, Rockford, IL). Proteins were separated using SDS-PAGE and transferred to nitrocellulose membranes. After incubation of the membrane with antibodies, proteins were detected by the Odyssey System (Li-Cor Bioscience) as described previously (37, 43).
Juxtamedullary nephron study.
The juxtamedullary nephron experiments were performed as previously described (8, 9). After anesthesia, blood was collected from the carotid arterial cannula and then centrifuged and filtered to separate the plasma and cellular fractions. The reconstituted blood was pressurized with a 95% O2-5% CO2 gas mixture. The right kidney was perfused with Tyrode solution (pH 7.4) containing 5.1% BSA and a mixture of l-amino acids. The kidney was excised and sectioned longitudinally and prepared as previously described (8, 9). After the dissection was completed, the Tyrode perfusate was replaced with the reconstituted blood. Renal perfusion pressure was set at 100 mmHg. The inner cortical surface of the kidney was continuously superfused with a warmed (37°C) Tyrode solution containing 1% BSA. The tissue was transilluminated, and video images of the microvessels were evaluated with a microscope (Nikon, Melville, NY). Arteriolar inside diameters were measured at 30-s intervals using a calibrated digital image-shearing monitor (Instrumentation for Physiology and Medicine, San Diego, CA). Treatments were administered by superfusing the tissue with a Tyrode solution containing the agent to be tested or the vehicle solution.
Statistical analysis.
Results are expressed as means ± SE. Statistical significance was assessed using one-way ANOVA followed by a post hoc Bonferroni/Dunn multiple comparison test. A value of P <0.05 was considered to be statistically significant.
RESULTS
Expression of AGT in rat afferent arterioles.
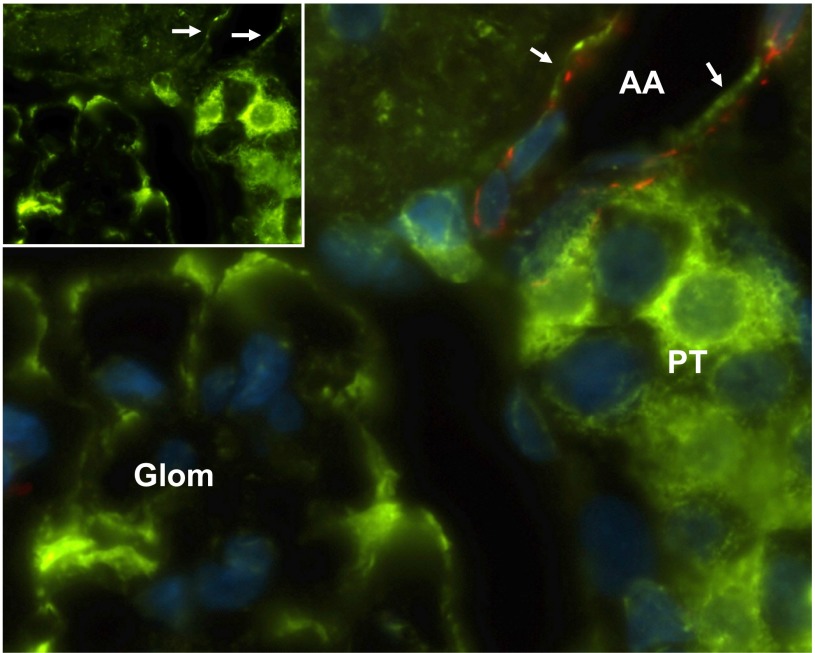
To establish the expression of AGT in afferent arterioles of ANG II-infused hypertensive rats, immnunohistological analysis was performed. Immunoreactivity against AGT protein (green) was observed in renal proximal tubules and glomeruli (Fig. 1). Afferent arterioles were identified by staining of α-smooth muscle actin (red). Importantly, the immunoreactivity of AGT and α-smooth muscle actin was colocalized, indicating that preglomerular VSMCs express AGT protein. AGT was not detected in preglomerular VSMCs from control rat kidneys.
Fig. 1.
Immunofluorescence staining of angiotensinogen (AGT) in afferent arterioles of ANG II-infused rat kidneys. Inset: AGT-positive staining as shown in green is observed in proximal tubular cells, glomerulus, and vascular smooth muscel cells (VSMCs). α-Smooth muscle actin (red) identifies the VSMCs of the afferent arteriole. Nuclei were stained by diamidino-2-phenylindole (DAPI, blue). The larger panel represents an enlarged view of the merged images showing a dual staining for AGT (green) and α-smooth muscle actin, indicating the presence of AGT in the VSMCs of the afferent arteriole. Arrows indicate AGT staining in an afferent arteriole. AA; afferent arteriole, PT; proximal tubular, Glom; glomerulus. Magnification: ×1,000 (oil-emulsion objective).
Establishment of primary culture of preglomerular VSMCs.
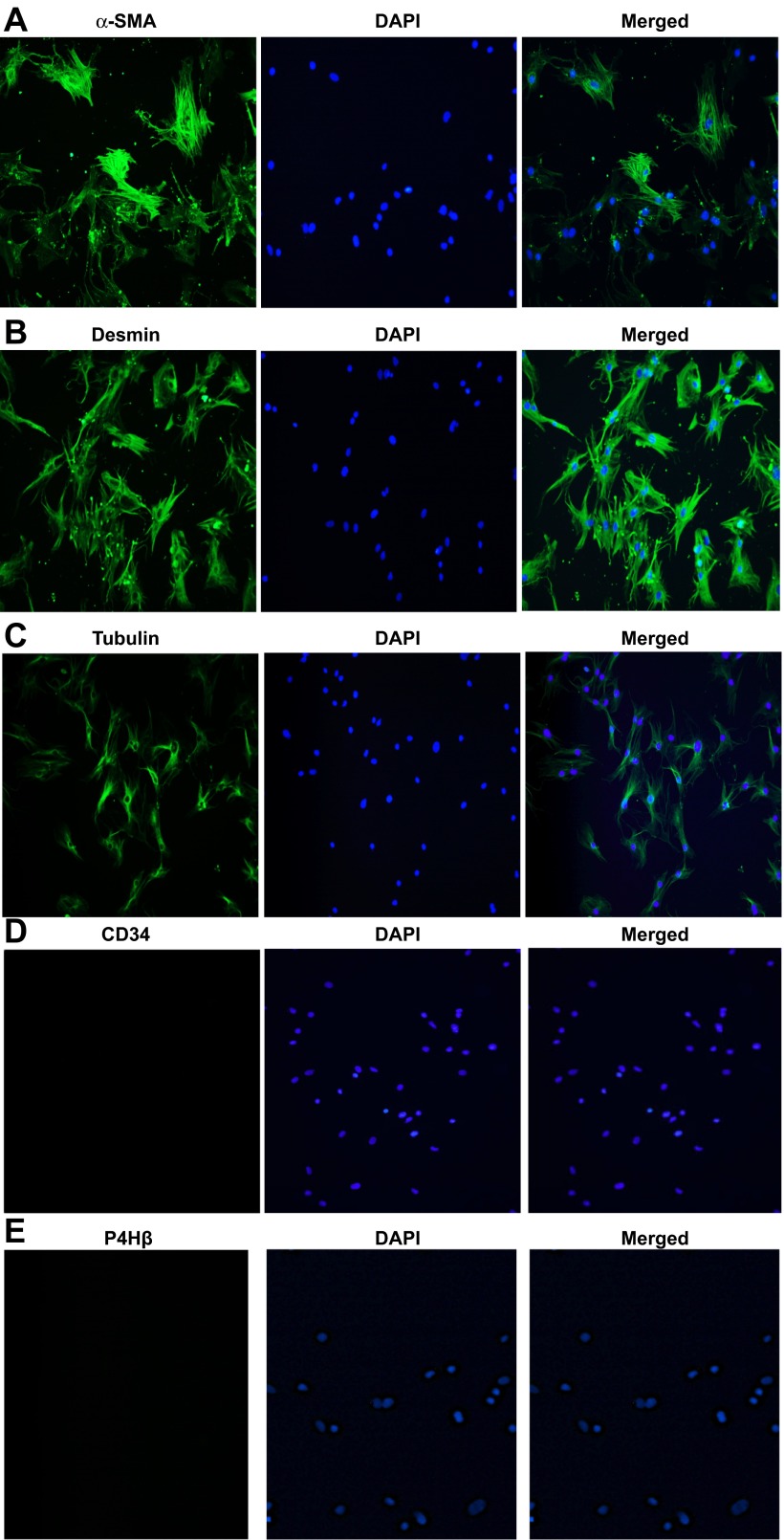
To characterize the purified preglomerular VSMCs, we used cell markers for VSMCs, endothelial cells, and fibroblasts. As shown in Fig. 2, preglomerular VSMCs were identified as being positive for α-smooth muscle actin (Fig. 2A), a marker for microfilaments, desmin (Fig. 2B), a marker for intermediate filaments, and tubulin (Fig. 2C), a marker for microtubules. Staining was negative for CD34, a marker for endothelial cells (Fig. 2D), and prolyl-4-hydroxylase-β, a marker for fibroblasts (Fig. 2E). These data indicate that preglomerular VSMCs were successfully isolated and were not contaminated with other cells. Furthermore, the results suggest that the cultured preglomerular VSMCs retained their phenotypic characteristics.
Fig. 2.
Characterization of primary cultured preglomerular VSMCs. α-Smooth muscle actin (SMA; A), desmin (B), tubulin (C), CD34 (D), and prolyl-4-hydroxylase-β (P4Hβ; E) are shown in green. Nuclei were stained by DAPI and are shown in blue. Merged images are shown on the right. Magnification: ×200.
Expression of RAS components in preglomerular VSMCs.
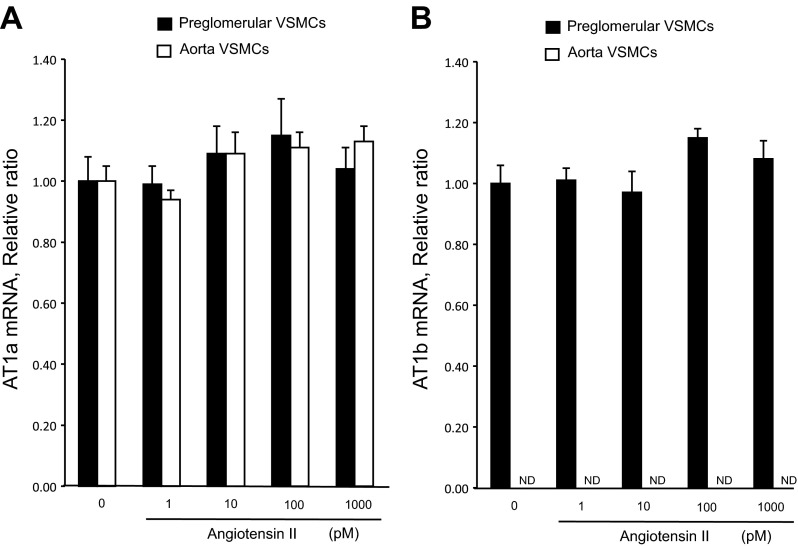
Immunostaining results showed that AGT, renin, ACE, and AT1R proteins are expressed in the isolated preglomerular VSMCs (Fig. 3A). Western blot analyses also demonstrated the presence of AGT protein and AT1R protein in the preglomerular VSMCs (Fig. 3B). AGT expression levels were greater in preglomerular VSMCs than in aortic VSMCs (Fig. 3C). AT1a mRNA was expressed in both preglomerular VSMCs and aortic VSMCs. In contrast, AT1b mRNA was expressed in preglomerular VSMCs but was not detected in aortic VSMCs (Figs. 3C and 4, A and B).
Fig. 3.
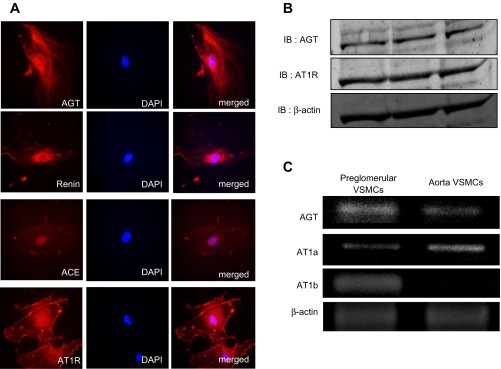
Expression of renin-angiotensin system (RAS) components in preglomerular VSMCs. A: immunostainings of AGT, renin, angiotensin-converting enzyme (ACE), and ANG II receptor (AT1R) in preglomerular VSMCs shown in red. Nuclei were stained by DAPI (middle). Merged images are shown on the right. Magnification: ×600. B: Western blot analyses of AGT, AT1R, and β-actin protein expression in preglomerular VSMCs. C: RT-PCR analyses of AGT, AT1a, AT1b, and β-actin in preglomerular VSMCs (left) and aortic VSMCs (right).
Fig. 4.
Effects of ANG II on AT1a and AT1b expression. Preglomerular VSMCs were incubated with ANG II (1 pM- 1,000 pM) for 24 h, and then, qRT-PCR was performed. A: filled bars, AT1a; open bars, aortic VSMCs. B: filled bars, AT1b. ND, nondetectable levels. Values are means ± SE (n = 4).
Effects of ANG II on AGT and AT1R expression.
AT1a and AT1b mRNA expression levels in preglomerular VSMCs were not changed by ANG II. Similarly, ANG II did not change AT1a expression levels in aortic VSMCs (Fig. 4). Figure 5, A and B, shows effects of ANG II on AGT mRNA and protein augmentation in preglomerular VSMCs but not in aortic VSMCs. AGT mRNA levels were significantly increased by 10 pmol/l, 100 pmol/l, and 1 nmol/l ANG II in preglomerular VSMCs. The maximum augmentation of AGT mRNA and protein by ANG II was observed at 100 pmol/l ANG II. AGT protein levels were also significantly increased by 100 pmol/l ANG II. In aortic VSMCs, ANG II decreased AGT mRNA expression (Fig. 5A). We also tested the effect of ANG II on AGT in mesangial cells. Treatment with 10 pmol/l and 100 nmol/l ANG II did not change AGT expression levels in mesangial cells (data not shown).
Fig. 5.
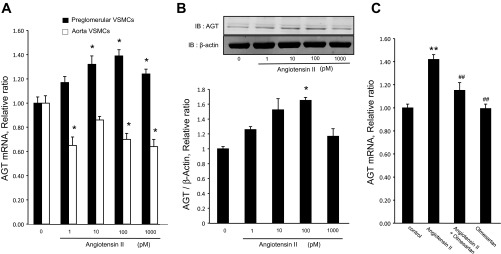
Effects of ANG II on AGT expression. A: preglomerular VSMCs (n = 12) and aortic VSMCs (n = 8) were incubated with ANG II for 24 h, and then, qRT-PCR analysis was performed. B: preglomerular VSMCs were incubated with ANG II for 24 h, and then Western blotting using anti-AGT and anti-β-actin antibodies was performed (n = 3). C: preglomerular VSMCs were incubated with olmesartan (10 nmol/l) for 30 min and then ANG II (100 pmol/l) for 24 h (n = 8–16). Thereafter, AGT expression levels were measured by qRT-PCR. Values are means ± SE. IB, immunoblot. *P < 0.05, **P < 0.01 vs. control. ##P < 0.01 vs. ANG II-treated group.
The role of AT1R activation in mediating AGT augmentation was tested using olmesartan (10 nmol/l). As shown in Fig. 5C, ANG II-induced AGT upregulation was prevented by pretreatment with olmesartan. These data indicate that ANG II-induced AGT augmentation is mediated by activating AT1R.
Effects of inhibition of ROCK and NF-κB on ANG II-induced AGT augmentation and intracellular ANG II formation.
To investigate the role of ROCK and NF-κB in ANG II-induced AGT augmentation in preglomerular VSMCs, the effects of a pharmacological ROCK inhibitor; H-1152 (10 nmol/l), and ROCK1 siRNA on AGT mRNA expression levels were examined. As shown in Fig. 6A, ANG II-induced AGT mRNA augmentation was completely prevented by H-1152. Moreover, the transfection of siRNA targeting ROCK1 attenuated AGT augmentation, while scrambled negative siRNA did not affect AGT mRNA augmenation (Fig. 6B).
Fig. 6.
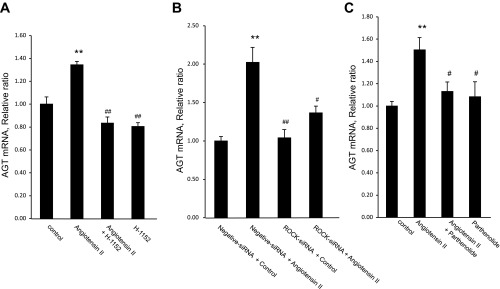
Effect of Rho kinase (ROCK) inhibition and NF-κB inhibition on ANG II-induced AGT mRNA augmentation in preglomerular VSMCs. A: preglomerular VSMCs were preincubated with H-1,152 (10 μmol/l) for 30 min and then ANG II (100 pmol/l) for 24 h (n = 8). B: preglomerular VSMCs were treated with Lipofectamine RNAiMAX reagent and 100 pmol ROCK1 small interfering (si) RNA. After 24-h transfection, cells were incubated with or without ANG II (100 pmol/l) for 24 h (n = 3). C: preglomerular VSMCs were incubated with parthenolide (10 μmol/l) for 30 min and then ANG II (100 pmol/l) for 24 h (n = 8–12). Values are means ± SE. A and C: *P < 0.05, **P < 0.01 vs. control. #P < 0.05, ##P < 0.01 vs. ANG II-treated group. B: **P < 0.01 vs. negative siRNA without ANG II. #P < 0.05, ##P < 0.01 vs. negative siRNA with ANG II-treated group.
Pretreatment with NF-κB inhibitor parthenolide (10 nmol/l) inhibited ANG II-induced AGT augmentation (Fig. 6C). These results suggest that AGT augmentation is mediated by the activity of ROCK and NF-κB in preglomerular VSMCs.
To test the role of ROCK activation in intracellular ANG II formation in preglomerular VSMCs, intracellular ANG II was stained after treatment with ANG II at 100 pmol/l for 24 h. Intracellular ANG II staining showed weak signals in nonstimulated preglomerular VSMCs. Intracellular ANG II levels were increased in ANG II-stimulated preglomerular VSMCs. The increase in intracellular ANG II was prevented by H-1152 (Fig. 7).
Fig. 7.
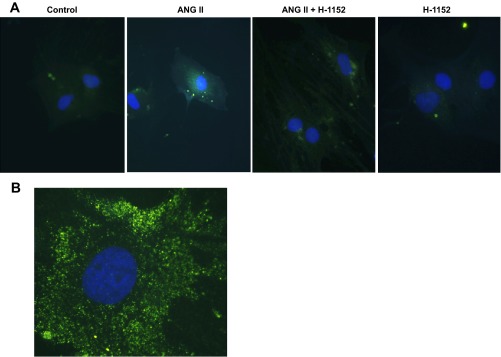
ANG II increases intracellular ANG II formation in preglomerular VSMCs. A: preglomerular VSMCs were incubated with ANG II (100 pmol/l) for 24 h with or without pretreatment with H-1152 (10 μmol/l) for 30 min. Intracellular ANG II was stained with anti-ANG II antibody (green). Nuclei were stained with DAPI (blue). Magnification: ×400. B: preglomerular VSMCs were incubated with ANG II (100 pmol/l) for 24 h. Magnification: ×1,000 (oil-emulsion objective).
Effects of ROCK inhibition on ANG II-induced NF-κB activation.
We further tested the sequential order of ROCK and NF-κB in the pathway underlying the ANG II-induced AGT augmentation. As shown in Fig. 8A, 100 pmol/l ANG II induced an increase in p65 (a major component of NF-κB) mRNA expression in preglomerular VSMCs. The upregulation of p65 mRNA expression by ANG II was inhibited by pretreatment with H-1152. Furthermore, ANG II induced translocation of p65 into nuclei in preglomerular VSMCs (Fig. 8B). The translocation of p65 was prevented by H-1152.
Fig. 8.
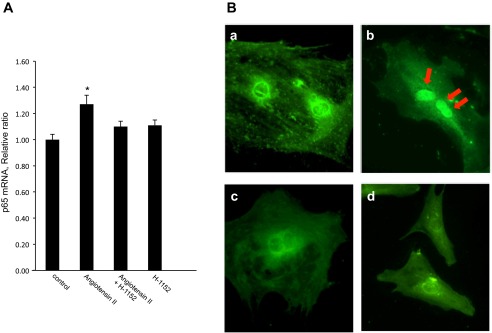
Effects of ROCK inhibition on ANG II-induced NF-κB activation. Preglomerular VSMCs were pretreated with H-1152 (10 μmol/l) for 30 min and then with ANG II (100 pmol/l) for 24 h. A: p65 (an NF-κB subunit) mRNA expression in preglomerular VSMCs was determined by qRT-PCR (n = 8). Values are means ± SE. *P < 0.05 vs. control. B: effects of H-1152 on ANG II-induced translocation of p65 into nuclei. Preglomerular VSMCs were treated with ANG II (100 pmol/l) for 15 min. p65 was stained with anti-p65 antibody (green). a: control. b: ANG II-treated cell. c: ANG II with ROCK inhibitor-treated cell. d: ROCK inhibitor-treated cell. Nuclei were stained with DAPI (blue). Magnification: ×600.
Effects of Rho-kinase inhibition on afferent arteriolar diameters in ANG II-infused rats.
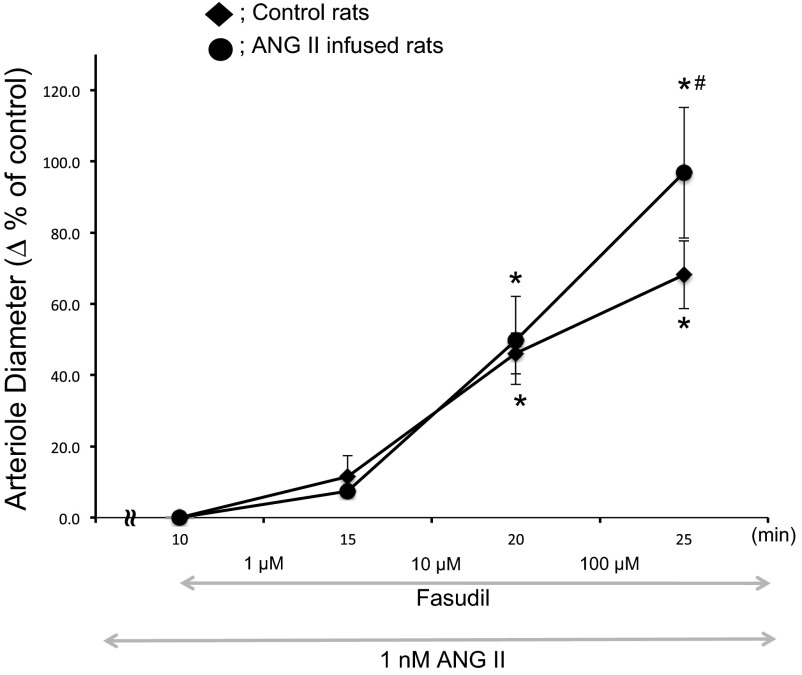
The ability of Rho-kinase inhibition to dilate afferent arterioles was tested by treating with fasudil in the presence of ANG II at 1 nmol/l concentration. Ten and 100 μmol/l fasudil significantly dilated afferent arterioles from both control and ANG II-infused rats. However, the relative increases in afferent arteriolar diameters with 10 and 100 μmol/l fasudil were significantly greater in ANG II-infused rats than in control rats, suggesting a greater Rho-kinase-dependent afferent arteriolar tone following chronic treatment with ANG II (Fig. 9).
Fig. 9.
Effects of ROCK inhibition on afferent arteriolar diameters in ANG II-infused rats. After establishing steady-state diameters, ANG II (1 nmol/l) was superfused on the blood vessels. Afferent arteriolar responses to fasudil superfusion from 1 to 100 μmol/l were evaluated in control rats (⧫; n = 6) and chronic ANG II infused rats (●; n = 5). Values are means ± SE. *P < 0.05 vs. Δ% at 10 min. #P < 0.05 vs. Δ% of control.
DISCUSSION
In many types of hypertension, renal vascular resistance is increased. Structurally narrowed renal afferent arterioles were observed in spontaneously hypertensive rats (SHR) (46), which increases preglomerular resistance and reduces renal blood flow and GFR (3, 34, 42). ANG II is a key factor mediating afferent arteriolar vasoconstriction, particularly in ANG II-dependent hypertension (17). Elevated renal microvascular resistance and preglomerular overreactivity that is specific to ANG II-induced blood pressure elevation after infusion of ANG II has been demonstrated in chronic ANG II-infused animals (17). ANG II impairs autoregulation, which may contribute to hypertensive injury (18, 19). In vivo studies also showed impaired renal blood flow and GFR autoregulation in ANG II-infused rats (5). Chronic infusion of ANG II caused marked impairment of sodium excretion, suppression of the pressure-natriuresis relationship, and reduced renal blood flow and GFR (51). Although several factors contribute to the enhanced vascular reactivity in ANG II-dependent hypertension, the molecular mechanisms have not been completely delineated.
Preglomerular VSMCs isolated from SHR showed greater expression levels of receptor for activated C kinase 1 than preglomerular VSMCs isolated from normotensive rats (5). In addition, renal vascular resistance remained elevated even after withdrawal of RAS blockade (2). These findings provide evidence that preglomerular VSMCs establish and sustain unique molecular mechanisms during hypertension, which contribute to sustained increases in afferent arteriolar resistance. In ANG II-infused hypertensive models, the augmented afferent arteriolar vasoconstrictor responses to additional ANG II indicate that preglomerular VSMCs undergo intracellular changes underlying the increase in sensitivity to ANG II that occurs (2b, 17, 34). In this study, we show that the acute administration of ANG II induced vasoconstriction of the afferent arterioles in control rats and chronic ANG II-infused rats. Moreover, during ANG II superfusion, the Rho-kinase inhibition in ANG II-infused rats led to greater vasodilation compared with control rats. These results present functional relevance to this study and indicate that ANG-II mediated activation of the Rho-kinase pathway contributes to the increased afferent arteriolar vascular sensitivity.
Immunohistological analysis demonstrated immunoreactivity against AGT in afferent arterioles of ANG II-infused rats whereas this could not be detected in control rats. In addition, preglomerular VSMCs isolated from rats in this study express the major RAS components, which are required to produce ANG II locally. The AGT expression levels in preglomerular VSMCs were higher than in aortic VSMCs. Importantly, ANG II stimulated both AGT mRNA and protein expression in the preglomerular VSMCs but not aortic VSMCs and mesangial cells. In mesangial cells, other groups have reported that AGT is expressed in rat glomeruli and mesangial cells, which is upregulated in glomerular disease (47–49). It has been previously indicated that elevation of reactive oxygen species induces AGT augmentation in mesangial cells (37). However, there is no report regarding the effect of ANG II on AGT expression in rat mesangial cells. Therefore, we tested the effect of ANG II on AGT expression in mesangial cells and demonstrated that ANG II does not alter AGT expression levels in the cells. These results suggest that mechanisms underlying AGT regulation in preglomerular VSMCs differ from those found in aortic VSMCs and mesangial cells.
Increases in intracellular ANG II levels were also demonstrated in ANG II-treated preglomerular VSMCs. Since ROCK inhibition attenuated the increases in intracellular ANG II, ANG II production rather than ANG II internalization seems to contribute to the intracellular ANG II augmentation in preglomerular VSMCs. This elevated intracellular ANG II can explain the increase in afferent arteriolar reactivity to ANG II that occurs in ANG II-dependent hypertension. Indeed, intracellular ANG II has been shown to induce intracellular calcium elevation in VSMCs (13). Moreover, AT1R expression levels in preglomerular VSMCs were not increased by ANG II. Thus the enhanced reactivity to ANG II in afferent arterioles in ANG II-dependent hypertension is not dependent on increased AT1R levels. In the present study, the maximum induction of AGT augmentation was observed with 100 pM ANG II. In normal rats, plasma ANG II levels are <50 pM (28, 30). Chronic ANG II infusion at 80 ng/min has been shown to increase plasma ANG II levels in the range of 150–400 pM (10, 26, 28, 44). These concentrations are similar to those used in our vitro studies, suggesting that the AGT augmentation in preglomerular VSMCs occurs in the range of plasma ANG II concentrations achieved in ANG II-dependent hypertension. In contrast to the AGT augmentation in preglomerular VSMCs, ANG II reduced AGT expression in aortic VSMCs. It has been established that glomeruli and the efferent arterioles express only AT1b receptors whereas the afferent arterioles express both types of the AT1R gene (14). Our study also shows that AT1b mRNA was not detected in aortic VSMCs. Thus VSMCs in the aorta and afferent arterioles may have different molecular systems in the regulation of RAS.
The present study demonstrates that ROCK and NF-κB mediate the ANG II-induced increase in AGT expression in preglomerular VSMCs. These results are in agreement with previous findings that NF-κB inhibition suppresses basal AGT mRNA expression in renal proximal tubular cells (43). In vivo studies have demonstrated that renal injury is associated with the activation of the ROCK/NF-κB pathway in chronic ANG II-infused rats (24). In the present study, we showed that AGT mRNA augmentation was blocked by knockdown of ROCK1 using siRNA as well as a pharmacological inhibition. These data indicate that ROCK contributes to ANG II-induced AGT augmentation. We also show that an NF-κB inhibitor attenuates ANG II-induced increases in AGT mRNA expression in preglomerular VSMCs, indicating that NF-κB is directly involved in the AGT augmentation in preglomerular VSMCs as well as in renal proximal tubular cells. In aortic VSMCs, activation of ROCK by ANG II leads to activation of NF-κB (15) but does not stimulate AGT expression. It has also been reported that activated NF-κB augments ROCK expression, resulting in increases in ROCK activity in VSMCs (6), which is an inverse pathway to the ROCK/NF-κB axis. Therefore, the sequential order of ROCK and NF-κB in the ANG II-induced AGT augmentation was examined in preglomerular VSMCs. The results showing that ROCK inhibition prevents NF-κB activation indicate that ROCK activation precedes and mediates NF-κB activation, leading to AGT augmentation in preglomerular VSMCs.
Since ROCK is an important signaling factor in the increased afferent arteriolar wall thickness and arterial blood pressure in ANG II-dependent hypertension (38), the ROCK/NF-κB/AGT in preglomerular VSMCs can enhance the development of ANG II-associated renal arterial hypertrophy.
In summary, this study demonstrates that ANG II can directly induce upregulation of AGT, leading to increases in intracellular ANG II formation in preglomerular VSMCs. In vivo, the increased ANG II levels can elicit afferent arteriolar vasoconstriction and contribute to an enhanced reactivity to ANG II which is mediated by Rho-kinases in ANG II-dependent hypertension. In addition, this study revealed that the ROCK/NF-κB pathway is implicated in the ANG II-induced AGT augmentation in preglomerular VSMCs. These findings suggest that new approaches to suppress ROCK and NF-κB activities would be useful adjunct therapy in controlling ANG II-dependent renal vascular constriction and hypertension. Furthermore, primary cultured cells are useful tools for future studies that relate to the mechanisms of renal and vascular injury in various pathological conditions.
GRANTS
This study was supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (R01DK072408), the National Heart, Lung, and Blood Institute (R01-26371), the National Institute of General Medical Sciences IDeA Program (COBRE, P30GM103337), and the American Heart Association, Greater Southeast Affiliate (10GRT3020018).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: K.M., M.U., and H.K. provided conception and design of research; K.M., R.S., W.S., and M.C.P. performed experiments; K.M., R.S., and W.S. analyzed data; K.M. and R.S. interpreted results of experiments; K.M., R.S., and M.C.P. prepared figures; K.M. and R.S. drafted manuscript; K.M., R.S., M.C.P., M.U., H.K., and L.G.N. edited and revised manuscript; K.M., R.S., M.C.P., M.U., H.K., and L.G.N. approved final version of manuscript.
ACKNOWLEDGMENTS
Present address of M. Urushihara: Dept. of Pediatrics, Institute of Health Bioscience, University of Tokushima Graduate School, Kuramoto, Tokushima, Japan.
Present address of H. Kobori: Dept. of Pharmacology, Kagawa Medical University, Kagawa, Japan.
The authors acknowledge the valuable comments and excellent technical assistance of Akemi Katsurada, Nina A. Perrault, and Salem I. Elkhayat (Tulane University).
REFERENCES
- 1.Acres OW, Satou R, Navar LG, Kobori H. Contribution of a nuclear factor-kappaB binding site to human angiotensinogen promoter activity in renal proximal tubular cells. Hypertension 57: 608–613, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arendshorst WJ, Brannstrm K, Ruan X. Actions of angiotensin II on the renal microvasculature. J Am Soc Nephrol 10: 149–161, 1999 [PubMed] [Google Scholar]
- 2b.Brands MW, Banes-Berceli AK, Inscho EW, Al-Azawi H, Allen AJ, Labazi H. Role of renal vasoconstriction and janus kinase 2/signal transducer and activator of transcription 3 activation. Hypertension 56: 1–6, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Casellas D, Bouriquet N, Artuso A, Walcott B, Moore LC. New method for imaging innervation of the renal preglomerular vasculature. Alterations in hypertensive rats. Microcirculation 7: 429–437, 2000 [PubMed] [Google Scholar]
- 4.Che Q, Carmines P. Src family kinase involvement in rat preglomerular microvascular contractile and [Ca2+]i responses to ANG II. Am J Physiol Renal Physiol 288: F658–F664, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng D, Zhu X, Gillespie DG, Jackson EK. Role of RACK1 in the differential proliferative effects of neuropeptide Y(1–36) and peptide YY(1–36) in SHR vs. WKY preglomerular vascular smooth muscle cells. Am J Physiol Renal Physiol 304: F770–F780, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chun-Yu W, Kuo-Chin H, Yin-Hsiang C, Pe-Fang H, Kuei-Hui L, Wan-Wan L. The role of Rho-associated kinase in differential regulation by statins of interleukin-1β- and lipopolysaccharide-medoated nuclear factor kappa B activation and inducible nitric-oxide synthase gene expression in vascular smooth muscle cells. Mol Pharmacol 69: 960–967, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Dubey RK, Roy A, Overbeck HW. Culture of renal arteriolar smooth muscle cells. Mitogenic responses to angiotensin II. Circ Res 71: 1143–1152, 1992 [DOI] [PubMed] [Google Scholar]
- 8.Feng MG, Navar LG. Afferent arteriolar vasodilator effect of adenosine predominantly involves adenosine A2B receptor activation. Am J Physiol Renal Physiol 299: F310–F315, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feng MG, Prieto -M, Navar LG. Nebivolol-induced vasodilation of renal afferent arterioles involves β3-adrenergic receptor and nitric oxide synthase activation. Am J Physiol Renal Physiol 303: F775–F782, 2012 [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez AA, Lara LS, Luffman C, Seth DM, Prieto MC. Soluble form of the (pro)renin receptor is augmented in the collecting duct and urine of chronic angiotensin II-dependent hypertensive rats. Hypertension 57: 859–864, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gonzalez AA, Luffman C, Bourgeois CR, Vio CP, Prieto MC. Angiotensin II-independent upregulation of cyclooxygenase-2 by activation of the (Pro)renin receptor in rat renal inner medullary cells. Hypertension 61: 443–449, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guilluy C, Bregeon J, Toumaniantz G, Rolli-Derkinderen M, Retailleau K, Loufrani L, Henrion D, Scalbert E, Bril A, Torres RM, Offermanns S, Pacaud P, Loirand G. The Rho exchange factor Arhgef1 mediates the effects of angiotensin II on vascular tone and blood pressure. Nat Med 16: 183–190, 2010 [DOI] [PubMed] [Google Scholar]
- 13.Haller H, Lindscau C, Quass P, Luft FC. Intracellular actions of angiotensin II in vascular smooth muscle cells. J Am Soc Nephrol 10: s75–83, 1999 [PubMed] [Google Scholar]
- 14.Harrison-Bernard LM, Cook A, Oliverio MI, Coffman TM. Renal segmental microvascular responses to ANG II in AT1A receptor null mice. Am J Physiol Renal Physiol 284: F538–F545, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Higuchi S, Ohtsu H, Suzuki H, Shirai H, Frank GD, Eguchi S. Angiotensin II signal transduction through the AT1 receptor: novel insights into mechanisms and pathophysiology. Clin Sci (Lond) 112: 417–428, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Ichihara A, Imig J, Inscho EW, Navar LG. Interactive nitric oxide-angiotensin II influences on renal microcirculation in angiotensin II-induced hypertension. Hypertension 31: 1255–1260, 1998 [DOI] [PubMed] [Google Scholar]
- 17.Imig JD. Afferent arteriolar reactivity to angiotensin II is enhanced during the early phase of angiotensin II hypertension. Am J Hypertens 13: 810–818, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Inscho EW, Cook A, Murzynowski JB, Imig JD. Elevated arterial pressure impairs autoregulation independently of AT1 receptor activation. J Hypertens 22: 811–818, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Inscho EW, Cook A, Webb RC, Jin LM. Rho-kinase inhibition reduces pressure-mediated autoregulatory adjustments in afferent arteriolar diameter. Am J Physiol Renal Physiol 296: F590–F597, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kagami S, Urushihara M, Kitamura A, Kondo S, Hisayama T, Kitamura M, Löster K, Reutter W, Kuroda Y. PDGF-BB enhances alpha1beta1 integrin-mediated activation of the ERK/AP-1 pathway involved in collagen matrix remodeling by rat mesangial cells. J Cell Physiol 198: 470–478, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Kamiyama M, Garner M, Farragut KM, Kobori H. The establishment of a primary culture system of proximal tubule segments using specific markers from normal mouse kidneys. Int J Mol Sci 13: 5098–5111, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kataoka C, Egashira K, Inoue S, Takemoto M, Ni W, Koyanagi M, Kitamoto S, Usui M, Kaibuchi K, Shimokawa H, Takeshita A. Important role of Rho-kinase in the pathogenesis of cardiovascular inflammation and remodeling induced by long-term blockade of nitric oxide synthesis in rats. Hypertension 39: 245–250, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Kobori H, Harrison-Bernard L, Navar LG. Enhancement of angiotensinogen expression in angiotensin II-dependent hypertension. Hypertension 37: 1329–1335, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kobori H, Ozawa Y, Acres OW, Miyata K, Satou R. Rho-kinase/nuclear factor-κβ/angiotensinogen axis in angiotensin II-induced renal injury. Hypertens Res 34: 976–979, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kobori H, Ozawa Y, Suzaki Y, Nishiyama A. Enhanced intrarenal angiotensinogen contributes to early renal injury in spontaneously hypertensive rats. J Am Soc Nephrol 16: 2073–2080, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kobori H, Prieto-Carrasquero M, Ozawa Y, Navar LG. AT1 receptor mediated augmentation of intrarenal angiotensinogen in angiotensin II-dependent hypertension. Hypertension 43: 1126–1132, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kyaw M, Yoshizumi M, Tsuchiya K, Kagami S, Izawa Y, Fujita Y, Ali N, Kanematsu Y, Toida K, Ishimura K, Tamaki T. Src and Cas are essentially but differentially involved in angiotensin II-stimulated migration of vascular smooth muscle cells via extracellular signal-regulated kinase 1/2 and c-Jun NH2-terminal kinase activation. Mol Pharmacol 65: 832–841, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Liu L, Gonzales A, McCormack M, Seth DM, Kobori H, Navar LG, Prieto MC. Increased renin excretion is associated with augmented urinary angiotensin II levels in chronic angiotensin II-infused hypertensive rats. Am J Physiol Renal Physiol 301: F1195–F1201, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Loirand G, Guerin P, Pacaud P. Rho kinases in cardiovascular physiology and pathophysiology. Circ Res 17: 322–334, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Mendes AC, Ferreira A, Pinheiro SV, Santos RA. Chronic infusion of angiotensin-(1–7) reduces heart angiotensin II levels in rats. Regul Pept 15: 29–34, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Muller DN, Dechend R, Mervaala EM, Park JK, Schmidt F, Fiebeler A, Theuer J, Breu V, Ganten D, Haller H, Luft FC. NF-kappaB inhibition ameliorates angiotensin II-induced inflammatory damage in rats.. Hypertension 35: 193–201, 2000 [DOI] [PubMed] [Google Scholar]
- 33.Nakamura A, Hayashi K, Ozawa Y, Fujiwara K, Okubo K, Kanda T, Wakino S, Saruta T. Vessel- and vasoconstrictor-dependent role of rho/rho-kinase in renal microvascular tone. J Vasc Res 40: 244–251, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Navar LG, Arendshorst W, Pallone TL, Inscho EW, Imig JD, Bell PD. The renal microcirculation. In: Handbook of Physiology. Microcirculation, edited by Tuma RF, Duran WN, Ley K. San Diego, CA: Elsevier, 2008, p. 550–683 [Google Scholar]
- 35.Navar LG, Harrison-Bernard L, Imig JD, Cervenka L, Mitchell KD. Renal responses to AT1 receptor blockade. Am J Hypertens 13: 45S-54S, 2000 [DOI] [PubMed] [Google Scholar]
- 36.Navar LG, Harrison-Bernard L, Nishiyama A, Kobori H. Regulation of intrarenal angiotensin II in hypertension. Hypertension 33: 316–322, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ohashi N, Urushihara M, Satou R, Kobori H. Glomerular angiotensinogen is induced in mesangial cells in diabetic rats via reactive oxygen species–ERK/JNK pathways. Hypertens Res 293: 1174–1181, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ozawa Y, Kobori H. Crucial role of Rho-nuclear factor-κB axis in angiotensin II-induced renal injury. Am J Physiol Renal Physiol 292: F100–F109, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ozawa Y, Kobori H, Suzaki Y, Navar LG. Sustained renal interstitial macrophage infiltration following chronic angiotensin II infusions. Am J Physiol Renal Physiol 292: F330–F339, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ruiz-Ortega M, Lorenzo O, Rupérez M, König S, Wittig B, Egido J. Angiotensin II activates nuclear transcription factor kappaB through AT1 and AT2 in vascular smooth muscle cells: molecular mechanisms. Circ Res 23: 1266–1272, 2000 [DOI] [PubMed] [Google Scholar]
- 41.Rupérez M, Sanchez-Lorez E, Blanco-Colio LM, Esteban V, Rodríguez-Vita J, Plaza JJ, Egido J, Ruiz-Ortega M. The Rho-kinase pathway regulates angiotensin II-induced renal damage. Kidney Int Suppl 99: S39–S45, 2005 [DOI] [PubMed] [Google Scholar]
- 42.Salomonsson M, Sorensen C, Arendshorst WJ, Steendahl J, Holstein-Rathlou NH. Calcium handling in afferent arterioles. Acta Physiol Scand 181: 421–429, 2004 [DOI] [PubMed] [Google Scholar]
- 43.Satou R, Miyata K, Katsurada A, Navar LG, Kobori H. Tumor necrosis factor-α suppresses angiotensinogen expression through formation of a p50/p50 homodimer in human renal proximal tubular cells. Am J Physiol Cell Physiol 299: C750–C759, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shao W, Seth D, Navar LG. Angiotensin II type 1 receptor-mediated augmentation of urinary excretion of endogenous angiotensin II in Val5-angiotensin II-infused rats. Hypertension 56: 378–383, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Singh R, Singh A, Alavi N, Leehey DJ. Mechanism of increased angiotensin II levels in glomerular mesangial cells cultured in high glucose. J Am Soc Nephrol 14: 873–880, 2003 [DOI] [PubMed] [Google Scholar]
- 46.Skov K, Mulvamy M. Structure of renal afferent arterioles in the pathogenesis of hypertension. Acta Physiol Scand 181: 397–405, 2004 [DOI] [PubMed] [Google Scholar]
- 47.Takamatsu M, Urushihara M, Kondo S, Shimizu M, Morioka T, Oite T, Kobori H, Kagami S. Glomerular angiotensinogen protein is enhanced in pediatric IgA nephropathy. Pediatr Nephrol 23: 1257–1267, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Urushihara M, Kobori H. Angiotensinogen expression is enhanced in the progression of glomerular disease. Int J Clin Med 1: 378–387, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Urushihara M, Ohashi N, Miyata K, Satou R, Acres OW, Kobori H. Addition of angiotensin II type 1 receptor blocker to CCR2 antagonist markedly attenuates crescentic glomerulonephritis. Hypertension 57: 586–593, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van Nieuw Amerongen GP, van Hinsbergh V. Cytoskeletal effects of rho-like small guanine nucleotide-binding proteins in the vascular system. Arterioscler Thromb Vasc Biol 21: 300–311, 2001 [DOI] [PubMed] [Google Scholar]
- 51.Wang C, Chin SY, Navar LG. Impairment of pressure-natriuresis and renal autoregulation in ANG II-induced hypertensive rats. Am J Renal Physiol 279: F319–F325, 2000 [DOI] [PubMed] [Google Scholar]
- 52.Yamakawa T, Tanaka S, Numaguchi K, Yamakawa Y, Motley ED, Ichihara S, Inagami T. Involvement of Rho-kinase in angiotensin II-induced hypertrophy of rat vascular smooth muscle cells. Hypertension 35: 313–318, 2000 [DOI] [PubMed] [Google Scholar]
- 53.Yoo TH, Li JJ, Kim JJ, Jung DS, Kwak SJ, Ryu DR, Choi HY, Kim JS, Kim HJ, Han SH, Lee JE, Han DS, Kang SW. Activation of the renin-angiotensin system within podocytes in diabetes. Kidney Int 71: 1019–1027, 2007 [DOI] [PubMed] [Google Scholar]