Abstract
Mutations in the electrogenic Na+/HCO3− cotransporter (NBCe1) that cause proximal renal tubular acidosis (pRTA), glaucoma, and cataracts in patients are recessive. Parents and siblings of these affected individuals seem asymptomatic although their tissues should make some mutant NBCe1 protein. Biochemical studies with AE1 and NBCe1 indicate that both, and probably all, Slc4 members form dimers. However, the physiologic implications of dimerization have not yet been fully explored. Here, human NBCe1A dimerization is demonstrated by biomolecular fluorescence complementation (BiFC). An enhanced yellow fluorescent protein (EYFP) fragment (1–158, EYFPN) or (159–238, EYFPC) was fused to the NH2 or COOH terminus of NBCe1A and mix-and-matched expressed in Xenopus oocyte. The EYFP fluorescent signal was observed only when both EYFP fragments are fused to the NH2 terminus of NBCe1A (EYFPN-N-NBCe1A w/ EYFPC-N-NBCe1A), and the electrophysiology data demonstrated this EYFP-NBCe1A coexpressed pair have wild-type transport function. These data suggest NBCe1A forms dimers and that NH2 termini from the two monomers are in close proximity, likely pair up, to form a functional unit. To explore the physiologic significance of NBCe1 dimerization, we chose two severe NBCe1 mutations (6.6 and 20% wild-type function individually): S427L (naturally occurring) and E91R (for NH2-terminal structure studies). When we coexpressed S427L and E91R, we measured 50% wild-type function, which can only occur if the S427L-E91R heterodimer is the functional unit. We hypothesize that the dominant negative effect of heterozygous NBCe1 carrier should be obvious if the mutated residues are structurally crucial to the dimer formation. The S427L-E91R heterodimer complex allows the monomers to structurally complement each other resulting in a dimer with wild-type like function.
Keywords: Na+/HCO3− cotransporter, protein structure, SLC4, acid base, Xenopus oocyte, electrophysiology, membrane current
a major task of the proximal tubule (PT) is to reabsorb NaHCO3 while maintaining intracellular pH (renal bicarbonate absorption: ∼350 g/day, 1 lb/day). The PT reabsorbs 80–90% of filtered HCO3−, and 70% of the filtered Na+. In PT epithelia, the apical Na+-H+ exchanger (NHE3) and basolateral electrogenic Na+/HCO3− cotransporter (NBCe1A) regulate pH in a coordinated fashion. Both NHE3 and NBCe1A activities increase during chronic metabolic acidosis (1, 31), respiratory acidosis (25), and chronic hyperfiltration (32).
Human missense mutations in the gene encoding NBCe1 (SLC4A4; OMIM No. 603345) were shown to cause permanent proximal renal tubular acidosis (pRTA, type II RTA) and ocular abnormalities including bilateral glaucoma, cataracts (corneal opacity), and band keratopathy without obvious pancreatic defects (19, 34). These data indicate that NBCe1 is the HCO3− and/or Na+ exit transport pathway in the proximal tubule and that NBCe1 plays a key role for maintaining ocular pressure and/or corneal clarity (40).
The crystal structure of band 3 (AE1, SLC4A1) shows a strong preference for dimerization and possibly even tetramerization (with ankyrin binding loop) in its membrane environments (21, 43). Studies with AE1 (3, 22) and NBCe1 (30) biochemically indicate that both, and probably all, Slc4 members form dimers. AE1 monomers can function within a dimer, and both AE1 dominant and recessive mutations causing distal renal tubular acidosis are observed (2). However, the physiologic implications of NBCe1 dimerization have not yet been explored.
We have studied the biophysical and transport properties of human disease caused by NBCe1 mutations (8, 10). NBCe1 mutations found in patients with proximal renal tubular acidosis, glaucoma, and cataracts are only recessive. Although parents and siblings of affected individuals theoretically make some amount of the mutant form of NBCe1 protein, they seem asymptomatic. It is conceivable that NBCe1 forms functional dimers that heterodimer proteins remedy the mutation consequence.
Thus we hypothesize that NBCe1 naturally forms functional dimers and adopted biomolecular fluorescence complementation (BiFC) techniques to determine if membrane NBCe1 dimerization occurs. We mix matched the complementary nonfluorescent fragments linked to NH2 termini or COOH termini of NBCe1 to interpret the dimer folding is tail to tail, head to head, or head to tail fashion. In addition, as an initial study of the physiological significance of NBCe1 dimerization, we coexpressed two mismatched NBCe1 mutations in Xenopus oocytes. We investigated if NBCe1 function can be partially restored in heterodimer mutation.
METHODS
Construction of fluorescent fusion proteins.
The most widely used approach for the visualization of protein complexes in cells is fluorescence resonance energy transfer (FRET) between color variants of the green fluorescent protein (GFP) fused to interacting proteins (12). This approach requires that the donor and acceptor molecules must be in close proximity (typically 10–100 Å) and that there must be overexpression of the fusion proteins to allow quantification of small changes in fluorescence emissions (16). However, there is another elegant and cost-effective way to study protein interaction and/or dimerization (36). We used this newly developed, BiFC technique to visualize NBCe1A dimerization and/or protein-protein interactions. This approach is based on complementation between two nonfluorescent fragments of the yellow fluorescent protein (YFP) when they are brought together by interactions between proteins fused to each fragment (16). This method has been successfully used to visualize dimer assembly of G-protein β- and γ-subunits in vivo (18), as well as visualization of protein interactions in living cells (17, 41).
We employed the BiFC technique to visualize NBCe1A dimer formation. The enhanced YFP (EYFP) containing a Q69M mutation to reduce environmental sensitivity (14) was created from green fluorescent protein plasmid (pEGFP-N1; Clontech) by mutagenesis using QuickChange mutagenesis kit (Stratagene). EYFP fragments were PCR amplified from pEYFP-N1 plasmid, and appropriate complimentary restriction sites were introduced on the 5′- and 3′-end. An amino-terminal EYFP fragment (1–158 amino acids, EYFPN) was ligated to the NH2 terminus of NBCe1A at the multiple cloning site (MCS) of NBCe1A-pTLN2 containing unique restriction sites (Fig. 1A): forward primer: MFR 535 (NruI+ EYFPN-NH2-terminal-fwd) 5′- CCACCGTCGCGAACCATGGTGAGCAAGGG-3′ and reverse primer: MFR-536 (KpnI+ EYFPN-NH2-terminal-rev) 5′-GGGGTACCCTGCTTGTCGGCCATGATATAG-3′ were used to amplify EYFPN fragment flanked by Nrul and KpnI sites to be fused to NH2 terminus of NBCe1A. Likewise, a carboxyl-terminal EYFP fragment (159–238 amino acids, EYFPC) was fused to NH2 terminus of NBCe1A at the MCS of NBCe1A-pTLN2 containing unique restriction sites. To amplify EYFPC fragment and remove the stop codon at the same time, forward primer: MFR 537 (NruI+ EYFPC-NH2-terminal-fwd) 5′-ATATCATCGCGACCATGGGCAAGAACGGCATC-3′ and reverse primer: MFR-538 (KpnI+ EYFPC-NH2-terminal-rev) 5′-CGGGTACCTCCCTTGTACAGCTCGTCCATGC-3′ were used to amplify the fragment flanked by Nrul and KpnI and fused to NH2 terminus of NBCe1A.
Fig. 1.
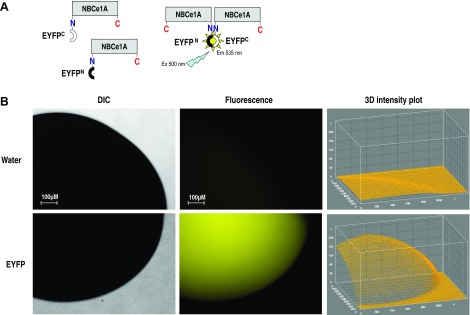
A: schematic illustration of biomolecular fluorescence complementation (BiFC) method used to visualize Na+/HCO3− cotransporter (NBCe1A) dimerization. Two complementary nonfluorescent fragments of the enhanced yellow fluorescent protein (EYFP) were ligated to the NH2 or COOH terminus of NBCe1A. Fluorescent signal reflects the dimer formation when we coexpressing YFPN-NBCe1A and YFPC-NBCe1A in the oocytes. We can mix match the YFPN and YFPC labeling linked to both NH2 termini or COOH termini or on either side to interpret the dimer folding is tail to tail, head to head, or head to tail fashion. B: full-length EYFP-pGEMHE construct expressed in Xenopus oocytes. Strong fluorescent signal from an unaltered EYFP protein in the expressing system verify the integrity of the EYFP protein. Minimum auto fluorescent signal was observed in the water-inject oocytes. The fluorescence intensity measured with ImageJ software was plotted on the Z axis of 3D intensity plot. DIC, differential interference contrast.
All constructs that incorporate YFP fused to the COOH terminus of NBCe1A (NBCe1A-C-EYFPN and NBCe1A-C-EYFPC) used the NBCe1-EGFP-pGH19 constructs (a gift from Drs. W. Boron and M. Parker at Case Western Reserve University as the template) (39). NBCe1A-EGFP-pGH19 was first converted to NBCe1A-EYFP-pGH19 (containing Q69M) using QuickChange mutagenesis kit (Stratagene) as described above, then sequenced and functionally verified (data not shown) before subsequent subcloning. NBCe1A-C-EYFPC-pGH19 was created by adding a stop codon on the NBCe1A-EYFP-pGH19 construct using forward primer: (NBCe1-C-EYFPN-fwd) 5′-GGCCGACAAGCAGAAGTAGGGCATCAAGGTGAACTTCAAGAT-3′ and reverse primer: (NBCe1-C-EYFPC-rev) 5′-CTTCTGCTTGTCGGCCATGATATAGACGTTGTGGCTG-3′. NBCe1A-C-EYFPN-pGH19 was created using a ligation-independent PCR cloning method (27): forward primer (NBCe1-C-EYFPC-fwd) 5′-CGCCACCATGGTGAGCAAGAACGGCATCAAGGTGAACTTCAA-3′ and reverse primer (NBCe1-C -EYFPC-rev) 5′-TGCCGTTCTTGCTCACCATGGTGGCGACCGGT-3′. After ligation, a linker sequence, GTEFAL, was introduced before the start of NBCe1A or a linker sequence, SPVAT, was introduced after the NBCe1A sequence. The same five-amino acid linker “SPVAT” was successfully used between the COOH terminus of NBCe1A and EGFP by other investigators without altering NBCe1A function (39). The integrity of all fusion-protein, plasmid constructs were verified by DNA sequencing.
Xenopus oocyte isolation and injection.
Stage 5/6 oocytes were harvested from female Xenopus laevis purchased from Xenopus Express (Beverly Hills, FL) or Nasco International (Fort Atkinson, WI). The procedure was approved by the Mayo Clinic Institutional Animal Care and Use Committee. An excised piece of ovary containing oocytes was rinsed ∼20 min with 0CaND96 solution (several changes) until the solution was clear. The tissue was agitated in ∼25 ml of sterile-filtered 0CaND96 solution with 1.5 mg/ml collagenase (type IA) for ∼45 min. Isolated oocytes were rinsed several times with sterile ND96 medium, sorted, and stored in OR3 medium.
The pTLN2 or pGH19 vector containing NBCe1A-EYFP fusion protein construct was linearized with MluI or Not I. Capped cRNA was synthesized using a linearized cDNA template and the SP6 or T7 mMessage mMachine kit (Ambion, Austin, TX) as described previously (5, 6). We routinely assessed the concentration and quality of reaction products by ultraviolet absorbance and gel electrophoresis, respectively. Oocytes in OR3 medium were visualized with a dissecting microscope and injected with 46 nl of either cRNA (0.5 μg/μl or 23 ng/oocyte) or water. Injected oocytes were maintained in sterile OR3. The experiments on oocytes were performed 3–7 days following injection.
Oocyte electrophysiology.
The NBCe1A transporter was expressed in oocytes, and transport function was examined using two-electrode voltage clamp. The electrodes were pulled from borosilicate capillary glasses to tip diameters of 1–2 μm, filled with 3 M KCl and had resistance of 0.2–2 MΩ. Oocytes were impaled with microelectrodes for measuring membrane potential (Vm) and passing current. Oocyte membrane potentials were controlled using an OC-725C voltage clamp (Warner Instruments, Hamden, CT), filtered at 2–5 kHz, digitized at 10 kHz, and recorded with Pulse software, and data were analyzed using the PulseFit program (HEKA) as previously (10). Current-voltage (I/V) protocols consisted of 100 ms steps from −160 to +60 mV in 20-mV steps. For periods when voltage-steps protocol was not being run, oocytes were clamped at a holding potential (Vh) of −60 mV, and current was constantly monitored and recorded at 10 Hz.
The oocyte was held on nylon mesh in a chamber through which saline flows continuously (5 ml/min). The standard ND96 saline medium contains the following (in mM) 96 NaCl, 2 KCl, 1 MgCl2, 1.8 CaCl2, and 5 HEPES (pH 7.5, 195–200 mosmol/kgH2O). In 5% CO2 equilibrated HCO3− solutions, 33 mM NaCl was replaced by 33 mM NaHCO3 (pH 7.5). In zero Na+ (0 Na+) solutions, NaCl is iso-osmotic ion substituted with choline chloride and choline-bicarbonate. In each solution manipulation, two I/V protocols were executed. One was at the peak current right after the current start to rectify, and the second one was measured when the current reach the steady sate. The linear conductance is the slope of the transmembrane current (raw current) over the −20- to +20-mV range at the steady state (26). Membrane conductance (G = I/V, units of Siemens, S) was calculated between −20 and +20 mV because extracellular HCO3−-independent currents associated with NBCe1A expression were found minimal in this range (29). The I/V curves in HCO3− solutions were calculated by subtracting the baseline current measured in HCO3− free solution (ND96).
Bimolecular fluorescence complementation assay.
The full-length EYFP construct was first expressed in Xenopus to verify the integrity of the fluorescent signal from an unaltered EYFP protein in the expressing system (Fig. 1B). The oocytes expressing EYFP or fusion proteins (monoexpressing and coexpressing) were examined using a Zeiss Observer Z1 inverted fluorescent microscope (Carl Zeiss, Germany). The excitation and emission filter set 46HE (500/25, 535/30) were used for observing EYFP fluorescent signal. Epifluorescent images were acquired with an AxioCam digital camera and AxioVision software (Carl Zeiss, Germany) (10, 35). Exposure times and gain for images were set to be constant within the same batch of oocyte. These settings were determined empirically for auto fluorescence correction depending on the background fluorescent signal intensity from water-injected oocytes. The image fluorescence intensity was measured with ImageJ software (version 1.47; National Institutes of Health) and plotted on the Z axis of a 3D intensity plot.
Coexpressing NBCe1A mutations.
We selected two severe NBCe1A mutations (i.e., S427L and E91R) to use in dimer complementation experiments. S427L is a naturally occurred recessive mutation found in a patient (10) and E91R is an artificial mutation created for NH2-terminal structure studies (5). These two mutations were individually expressed or coexpressed in oocytes, and electrophysiology assays were performed in the three groups to compare the transporter function to that of wild-type NBCe1A and water-injected oocytes.
Statistical analysis.
Currents and linear conductances are represented as the means ± SE. Statistical analyses were performed using a one-tailed Student's t-test to have a significant difference at P < 0.05 or less.
RESULTS
Bimolecular fluorescence complementation.
The full-length EYFP-pGEMHE construct were injected into Xenopus oocytes. Three days after injection, Xenopus oocytes showed obvious fluorescent signals (Fig. 1B) confirming the integrity of the EYFP protein in the expressing system. Minimum autofluorescence was found in the water-injected oocytes, but this small value was used for background signal correction.
Monoexpressing fluorescent fusion proteins.
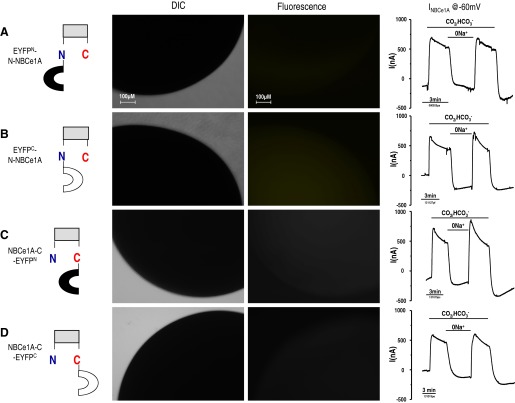
The amino-terminal EYFP fragment (1–158 amino acids, EYFPN) fused to the NH2 or COOH terminus of NBCe1A (EYFPN-N-NBCe1A or NBCe1A-C-EYFPN) was expressed in Xenopus oocytes. Likewise, the carboxyl-terminal EYFP fragment (159–238 amino acids, EYFPC) fused to NH2 or COOH terminus of NBCe1A (EYFPC-N-NBCe1A or NBCe1A-C-EYFPC) was expressed in Xenopus oocytes. Three days after injection, using fluorescent microscope, Xenopus oocyte showed no fluorescent signal in any of the monoexpressed, EYFP fragment-NBCe1A, fusion proteins (Fig. 2). These results verified the integrity of the EYFP-NBCe1A fusion protein that will be used for the BiFC analyses.
Fig. 2.
Monoexpressing of EYFP-NBCe1A fusion protein. The amino-terminal EYFPN fragment (1–158 amino acids) or the carboxyl-terminal EYFPC fragment (159–238 amino acids) was fused to the NH2 or COOH terminus of NBCe1A and expressed in Xenopus oocytes individually. No fluorescent signal was observed from any of the monoexpressed EYFP fragment-NBCe1A fusion protein. Transmembrane current traces (Vh = −60 mV) show no difference between these 4 (A–D) EYFP-NBCe1A fusion transporters.
Electrophysiology of monoexpressed NBCe1A-EYFP fusion transporter.
NBCe1A-EYFP fusion transporters were individually expressed in oocytes, and their transport function was examined using two-electrode voltage clamp (Fig. 2, right). Transmembrane current traces of these four EYFP-NBCe1A fusion transporters (at Vh= −60 mV) are almost identical in response to HCO3− addition or Na+ removal. These fusion transporters behaved as one would expect a wild-type NBCe1A to behave (see Fig. 6). That is, the EYFP fragments do not interfere with normal NBCe1A transport function regardless of whether the EYFP tag is fused to NH2 or COOH terminus of NBCe1A.
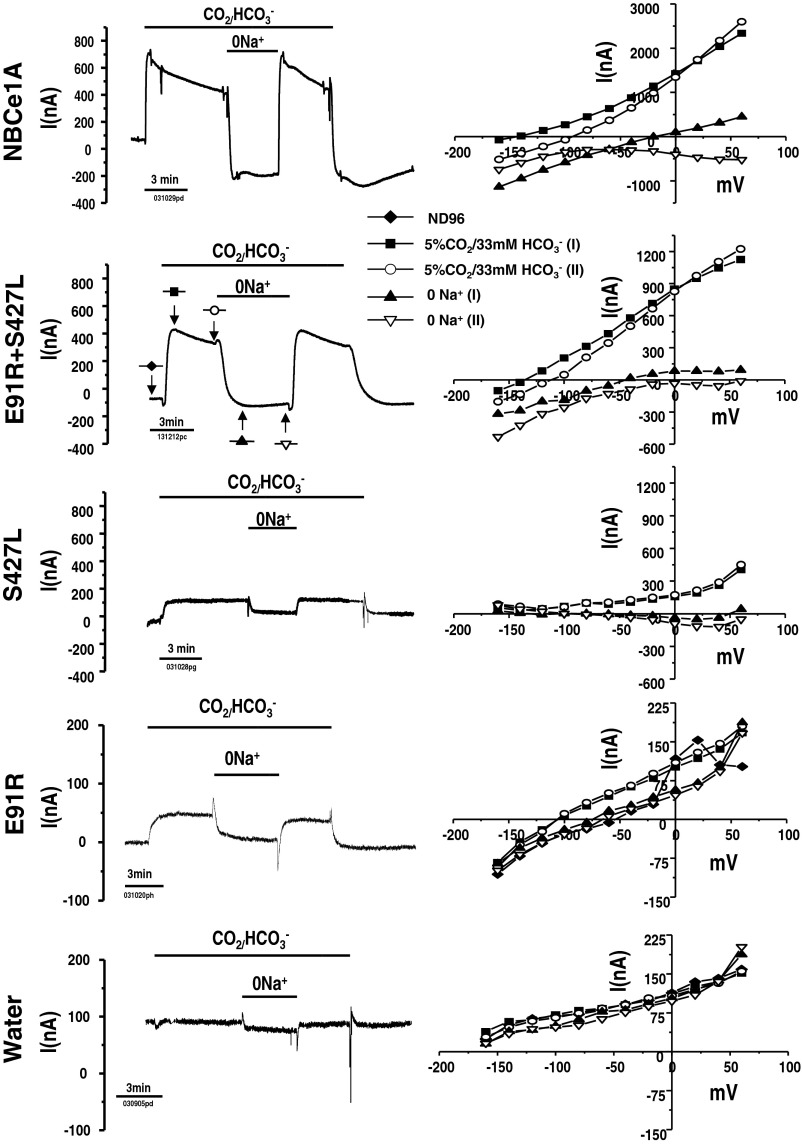
Fig. 6.
Functional complementation data illustrating NBCe1A forms dimers. E91R and S427L are 2 severe NBCe1A missense mutations when expressed individually results in 6.6 and 20% wild-type NBCe1A transport function. Coexpression of these 2 mutations (E91R + S427L; with the same amount of total cRNA injected) in Xenopus oocytes is shown, and the net transporter activity is ∼50% wild-type function rather than some average activity of the parts (i.e., <13%). Left: transmembrane current traces recorded when membrane potential was held at −60 mV. Right: I/V curves at various points of solution protocol. One was at peak current and other one was measured when the current reach the steady sate. The I/V curves shown were corrected current [subtracting the baseline current measured in HCO3−-free solution (ND96)] except for E91R and water-injected control oocyte, the I/V curve shown were raw currents (unsubtracted).
Coexpressing fluorescent fusion proteins.
Pairs of YFPN-NBCe1A and YFPC-NBCe1A fusion transporter were coexpressed in Xenopus oocytes. Three days after injection, oocytes were examined for EYFP fluorescent signals using a fluorescent microscope (Fig. 3). One coexpressed pair, where both EYFP fragments were fused to the NH2 terminus of NBCe1A (EYFPN-N-NBCe1A w/ EYFPC-N-NBCe1A), showed a strong EYFP fluorescent signal. Nevertheless, when we coexpressed the pair in which EYFP fragments were both fused to the COOH terminus of NBCe1A (NBCe1A-C-EYFPN w/ NBCe1A-C-EYFPC), oocytes showed only slight fluorescent signals as quantified by 3D intensity plots (Fig. 3). Similarly, very weak fluorescent signals were detected when we coexpressed a mix-and-matched pair where EYFP fragments are fused to either NH2 terminus or COOH terminus (EYFPN-N-NBCe1A w/ NBCe1A-C-EYFPC or NBCe1A-C-EYFPN w/ EYFPC-N-NBCe1A). The 3D intensity plots showed only marginal fluorescent signal at the oocyte edge. This result could be an optical artifact due to oocyte spherical shape. Alternatively, there is a low, but discrete probability that some EYFPN and EYFPC fragments are distributed in membrane proximity of the EYFP-NBCe1A fusion proteins. Similar results were observed in three separate oocyte batches expressing pairs of EYFP-NBCe1A fusion proteins.
Fig. 3.
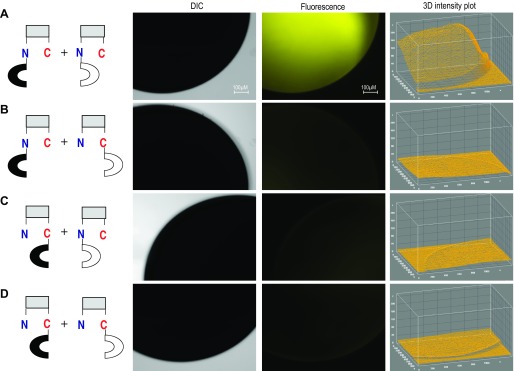
Coexpressing of EYFP-NBCe1A fusion protein. Pairs of YFPN-NBCe1A and YFPC-NBCe1A fusion transporter were coexpressed in Xenopus oocytes (A–D, see models at left). Fluorescent signals reflect dimer formation when we coexpressing mix matched YFPN-NBCe1A and YFPC-NBCe1A pair in the oocytes (A). The Z axis of 3D intensity plot depicts the fluorescence intensity. Fluorescent signal was observed only when we coexpressed the pair where both EYFP fragments are fused to the NH2 terminus of NBCe1A while other pairs show minimum fluorescent signals.
Electrophysiology of coexpressed NBCe1A-EYFP fusion transporter.
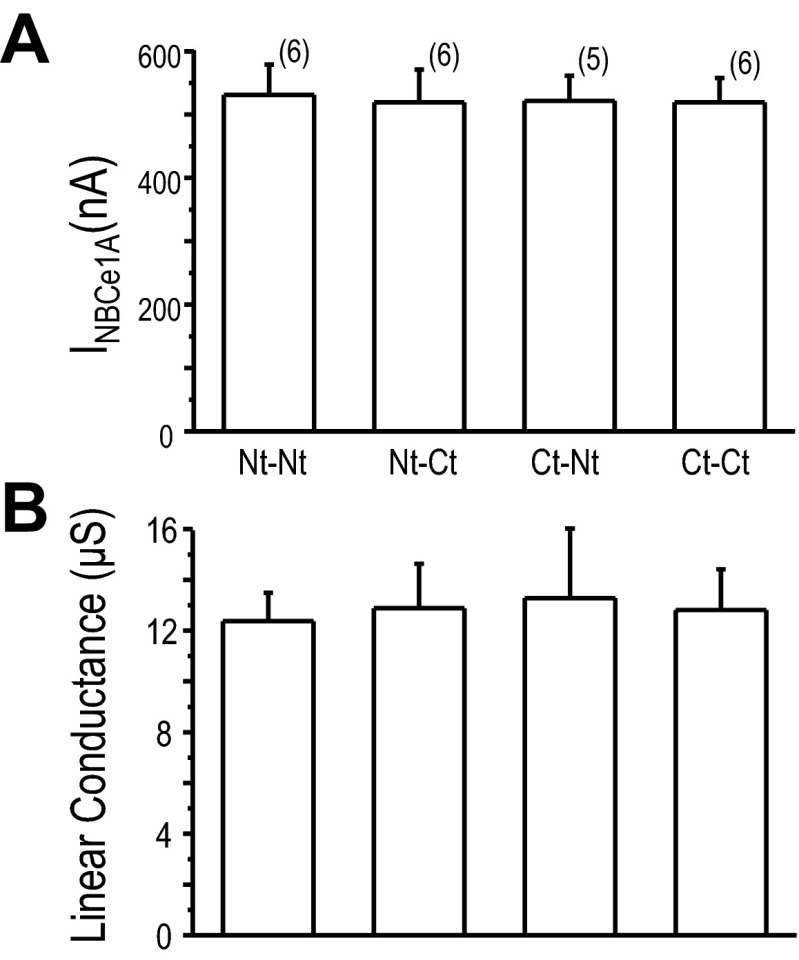
Pairs of NBCe1A-EYFP fusion transporters were coexpressed in oocytes, and their transport function was measured by electrophysiology (Fig. 4). When EYFPN-N-NBCe1A was coexpressed with EYFPC-N-NBCe1A, oocytes showed wild-type NBCe1A function (Fig. 4A). This result complements the BiFC finding described in the previous section (Fig. 3A). Interesting, when either NBCe1A-C-EYFPN was coexpressed with EYFPC-N-NBCe1A (Fig. 4B), or when EYFPN-N-NBCe1A was paired with NBCe1A-C-EYFPC (Fig. 4C), oocytes showed wild-type NBCe1A like transport function. Similar results were elicited by the NBCe1A-C-EYFPN and NBCe1A-C-eYFPC pair (Fig. 4D). There was no statistical difference when we compared the HCO3−-dependent current (measured at −60 mV) among various pairs of NBCe1A-EYFP expressed in oocytes (Fig. 5). The linear conductance also showed similar transport characteristics among them. These results were somewhat expected as monoexpress NBCe1A-EYFP fusion transporters yielded similar transport function as wild-type NBCe1A (Fig. 2).
Fig. 4.
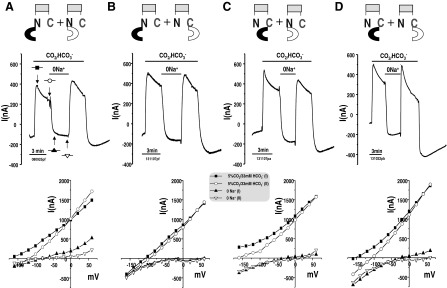
Current responses of NBCe1A-EYFP fusion transporters. Mix-matched pairs of NBCe1A-EYFP fusion transporters were coexpressed in oocytes and their transport function were examined using electrophysiology assay. A–D are analogous to the BiFC fluorescence pairs in Fig. 3, with explicit models at the top of each column. Current traces shown resulted from holding the membrane potential at −60 mV. Bottom: current-voltage (I/V) curves from different pairs of fusion transporters at various points during solution changes. The I/V curves displayed are subtracted currents [minus the current in HCO3−-free solution (ND96)], and 2 I/V curves at peak current and at the steady state are shown.
Fig. 5.
Functional comparison of NBCe1A-EYFP fusion transporter. A: HCO3−-dependent current measured at −60 mV. B: linear conductance (μS) is the slope of the transmembrane current (raw current) over the −20- to +20-mV range. Values shown are means ± SE and sample sizes are in parentheses. The data from any of the clone were collected from at least 4 different batches of oocytes.
Coexpressing NBCe1A mutations.
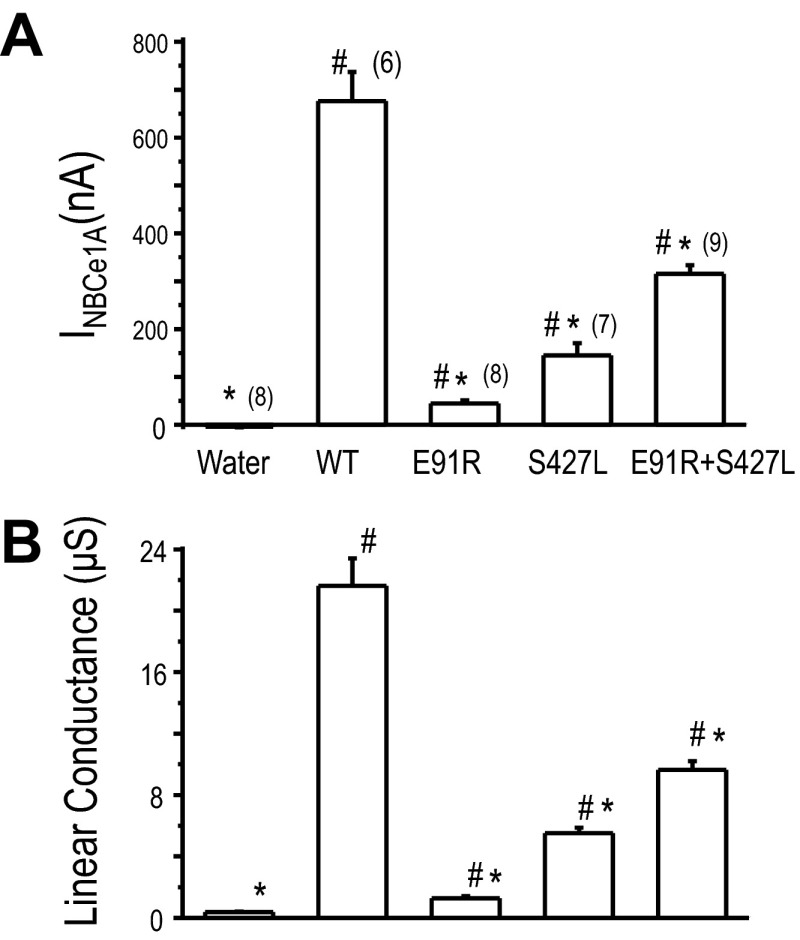
We expressed NBCe1A mutations, namely S427L and E91R, in Xenopus oocytes. Expression of each of these mutations individually results in deficient NBCe1A transport activity (Fig. 6). The HCO3−-dependent currents at −60 mV of the S427L and E91R mutation were significantly lower than those of wild-type NBCe1A. Their currents are 20 and 6.6% of wild-type NBCe1A expressing oocytes, respectively (Fig. 7A). Although the E91R mutation has low transport activity, the HCO3−-elicited current (44.6 ± 6.1 nA) at −60 mV was significantly higher compared to that of water-injected control oocyte (−3.7 ± 1.5 nA). The linear conductance of E91R (1.28 ± 0.12 μS) was also significantly higher than that of water-injected control oocyte (0.37 ± 0.03 μS).
Fig. 7.
Functional comparison of single or coexpressing of NBCe1A mutations in Xenopus oocytes. A: HCO3−-dependent transmembrane current measured at −60 mV. B: linear conductance (μS) is the slope of the transmembrane current over the −20- to +20-mV range. Value shown are means ± SE and sample sizes are in parentheses. *P < 0.05, significantly different from wild-type NBCe1A. #P < 0.05, significantly different from water-injected control oocytes. The data from any of the clone were collected from at least 4 different batches of oocytes.
The linear conductance is the slope of the HCO3− elicited current over the −20 to +20 mV range (Fig. 7B). The linear conductance of the S427L mutation (5.52 ± 0.35 μS) is significantly lower than that of the wild-type NBCe1A(21.61 ± 1.79 μS). The linear HCO3− conductance of the E91R mutation (1.28 ± 0.12 μS) is significantly lower than that of the wild-type NBCe1A but significantly higher than water-injected control oocyte (0.37.7 ± 0.04 μS). However, when we coexpressed S427L and E91R, we observe ∼50% weight function (Figs. 6 and 7). The HCO3−-elicited current at −60 mV of the S427L/E91R coexpression was 315.2 ± 18.2 nA while wild-type NBCe1A has 676.0 ± 60.6 nA. The linear HCO3− conductance of S427L/E91R coexpression oocytes was (9.64 ± 0.56 μS) significantly higher than expressing S427L (5.52 μS) or E91R (1.28 μS) alone.
DISCUSSION
Before the physiological implications of NBCe1 dimerization can be assessed, it must first be demonstrated that NBCe1 forms dimers naturally.
In this study, we used the BiFC technique, which relies on the formation of a fluorescent complex by two nonfluorescent fragments of YFP brought together by association of interacting proteins fused to these fragments (16). The EYFP fluorescent signal was observed only when two specific NBCe1A proteins, each with an EYFP fragment fused to the NH2 terminus (EYFPN-N-NBCe1A w/ EYFPC-N-NBCe1A), were coexpressed in oocytes (Fig. 3). Oocytes mono- or coexpressing NBCe1A-YFP fusion proteins have NBCe1A wild-type function (Figs. 2, 4, and 5), which means that the EYFP fragments (EYFPN or EYFPC) do not disrupt the NBCe1A transport function. Together, these data suggest that NBCe1A form dimers and that the NH2 termini from the two monomers are in close proximity, and likely pair up, to form a functional unit. A similar result was reported in another study measuring FRET when NBCe1A was tagged on the NH2 terminus with EYFP or Cerulean and expressed in HEK293 cells (23). However, the fluorescent tags were not fused onto the COOH terminus of NBCe1A in this study.
Interestingly, no EYFP fluorescent signal was observed in oocytes coexpressing mix-and-matched NBCe1A-YFP fusion proteins when EYFP fragments are fused to either the NH2 terminus or COOH terminus (EYFPN-N-NBCe1A with NBCe1A-C-EYFPC or NBCe1A-C-EYFPN with EYFPC-N-NBCe1A). However, the coexpressed NBCe1A-EYFP fusion proteins remain functional like wild-type NBCe1A. These data suggest that the EYFP fragments (EYFPN or EYFPC) do not disrupt the NBCe1A transport function. Additionally, when a NBCe1-dimer forms, the NH2 terminus and COOH terminus are not in close proximity. Furthermore, the two COOH termini do not associate in NBCe1A-dimer formation as no EYFP fluorescence was observed with coexpression of both EYFP fragments fused to the NBCe1A COOH terminus (i.e., NBCe1A-C-EYFPN w/ NBCe1A-C-EYFPC).
Immunoblot analysis of NBCe1 from membrane fractions from rat kidney cortex shows a band of ∼280 kDa with nonreducing conditions (30). This strong band disappears, but a band of ∼140 kDa appears with extreme reducing conditions. Together these data suggest that the NBCe1 exists in a dimeric form. Similar findings were reported where membrane fractions were isolated from mouse renal cortex or HEK293 cells expressing NBCe1A and analyzed by PAGE using mild solubilization in the nondissociative detergent perfluoro-octanoic acid (PFO) (23).This study found that the dimeric form was the most dominant oligomer, but a minor tetramer coexisted with nondenaturing conditions (i.e., no DTT). Biochemical studies of the AE1 (SLC4A1) oligomeric structure have indicated that the AE1 protein forms dimers and tetramers with similar nondenaturing conditions (3, 4, 22). The dimeric nature of the AE1 membrane domain was shown in the low resolution structure obtained by electron microscopy of 2D crystals (42), and a high-resolution (at 0.26 nm) crystal structure of AE1 cytosolic domain shows a strong preference for dimerization (43). Likewise, preliminary X-ray diffraction analysis of the NH2-terminal cytoplasmic domain of NBCe1A, have indicated that NBCe1A is likely in dimeric form (13).
Heterozygous mutations in the AE1 Cl−/HCO3− exchanger cause autosomal dominant distal renal tubular acidosis (dRTA) (24). It was reported that the dominant dRTA may be caused by mistargeting of mutant AE1 to the apical membrane (9) or the cytosolic retention of hetero-oligomer complexes formation between wild-type and mutant AE1 membrane protein (33). Interestingly, kidney AE1 isoform (kAE1) showed a “dominant-positive effect” by partially rescuing the recessive mutant kAE1 (S773P or G701D) trafficking to the plasma membrane of MDCK cells (7). In contrast, the dominant mutant kAE1 (R589H) resulted in a “dominant-negative effect” when heterodimerized with the wild-type kAE1. Since NBCe1A forms dimers and tetramers like AE1, one should expect to find attenuated NBCe1 transport function through a dominant negative effect due to heterodimer complexes formation of wild-type and mutant protein. Nevertheless, all reported NBCe1 mutations found in patients with pRTA, glaucoma, and cataracts are exclusively recessive (8, 10, 15, 19, 20). Although pRTA has not been found in the human heterozygous kindreds, it is plausible that heterozygous NBCe1 mutation carrier may exhibit pathological phenotypes in tissues with insufficient compensatory capacity for the loss of NBCe1 function (37, 38) or as a result of natural decreased function, as in aging.
A homozygous 65-bp deletion (Δ65bp) in the C terminus of NBCe1 was recently reported in a patient with pRTA, ocular abnormalities, and hemiplegic migraine (37). Intriguingly, several heterozygous members of this family also presented with glaucoma and migraines. A dominant negative effect of heterodimer complexes formation of wild-type and the Δ65bp NBCe1 mutation was speculated to be responsible for the occurrence of glaucoma and migraine in the heterozygous carriers (37). Moreover, in a mouse nbce1-mutation model, the heterozygous NBCe1 mutations were not asymptomatic as observed in human mutation data. Both of the heterozygous NBCe1-deficient mice (NBCe1+/− and NBCe1+/W516X) showed mild acidemia and aciduria (11, 28). The intracellular pH and HCO3− absorption measurements in the renal proximal tubules from the NBCe1+/W516X mice were significantly lower compared to that of NBCe1+/+ mice.
Why are the heterozygous NBCe1 mutation carriers less likely to develop a pathological phenotype? Heterozygous parents and siblings of affected individuals (e.g., the S427L patient) should make some mutant NBCe1 protein as both our data and those of others suggest that NBCe1 naturally forms dimers in native membrane environments. In the human heterozygous kindreds, there should be at least 50% chance to form heterodimer complexes (wt-S427L) in the whole dimer population, as well as a 25% probability to form wt-wt and S427L-S427L homodimers. We previously showed that the S427L mutation is a functional defected of the NBCe1 transporter (10). The plasma membrane abundance of E91R and S427L was verified by either immunohistochemistry or chemiluminescence using an HA-tagged NBCe1A (5, 10). This meant that both mutations are properly trafficked to the plasma membrane but are functionally deficient. It is only logical to assume that wt-S427L heterodimer complexes would be functionally similar to wt-wt homodimer complexes.
In our NBCe1A mutation coexpressing experiments, two severe mutations, i.e., S427L and E91R, both result in only minor (20 and 6.6%) wt NBCe1A transport activity. However, when these two mutations are coexpressed in oocytes, we observe ∼50% wt NBCe1A function (Figs. 6 and 7). According to probability, there should be 25% for each of the homodimers (S427L-S427L and E91R-E91R), and 50% E91R-S427L heterodimer in the NBCe1A dimer assembly population. Only if the S427L monomer can complement the E91R monomer in this heterodimer complex would S427L-E91R be functionally similar to wt-wt homodimer. This complementation model seems the most likely explanation for our results.
Similar to AE1 mutations, we believe that the dominant negative effect of the heterodimer will be obvious if the mutated residues are structurally crucial to the dimer formation. The S427L-NBCe1A (TM1) and E91R-NBCe1A (NH2 terminus) mutations are located in different parts of the NBCe1A molecule. The S427L-E91R heterodimer complex allows the monomers to structurally complement each other resulting in a dimer with wild-type-like function. This BiFC study provides visual confirmation that NBCe1A forms dimers in oocytes membranes and that functional complementation in the NBCe1A dimer may explain why patients heterozygous for NBCe1A-mutations are not clinically symptomatic.
GRANTS
This work was supported by American Heart Association Grant 10SDG2640146 (to M.-H. Chang), National Eye Institute Grant EY-017732 (to M. F. Romero), the Robert and Arlene Kogod Aging Center (to A. P. Chen), and the Mayo Foundation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.-H.C. conception and design of research; M.-H.C. and A.-P.C. performed experiments; M.-H.C. and M.F.R. analyzed data; M.-H.C. and M.F.R. interpreted results of experiments; M.-H.C. prepared figures; M.-H.C. drafted manuscript; M.-H.C. and M.F.R. edited and revised manuscript; M.-H.C. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Heather Holmes and Elyse Scileppi for excellent technical support.
REFERENCES
- 1.Akiba T, Rocco VK, Warnock DG. Parallel adaptation of the rabbit renal cortical sodium/proton antiporter and sodium/bicarbonate cotransporter in metabolic acidosis and alkalosis. J Clin Invest 80: 308–315, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alper SL. Genetic diseases of acid-base transporters. Annu Rev Physiol 64: 899–923, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Boodhoo A, Reithmeier RA. Characterization of matrix-bound Band 3, the anion transport protein from human erythrocyte membranes. J Biol Chem 259: 785–790, 1984 [PubMed] [Google Scholar]
- 4.Casey JR, Reithmeier RA. Analysis of the oligomeric state of Band 3, the anion transport protein of the human erythrocyte membrane, by size exclusion high performance liquid chromatography. Oligomeric stability and origin of heterogeneity. J Biol Chem 266: 15726–15737, 1991 [PubMed] [Google Scholar]
- 5.Chang MH, DiPiero JM, Sönnichsen FD, Romero MF. Entry to “HCO3 tunnel” revealed by human mutation and structural model. J Biol Chem 283: 18402–18410, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang MH, Plata C, Kurita Y, Kato A, Hirose S, Romero MF. Euryhaline pufferfish NBCe1 differs from nonmarine species NBCe1 physiology. Am J Physiol Cell Physiol 302: C1083–C1095, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cordat E, Kittanakom S, Yenchitsomanus PT, Li J, Du K, Lukacs GL, Reithmeier RA. Dominant and recessive distal renal tubular acidosis mutations of kidney anion exchanger 1 induce distinct trafficking defects in MDCK cells. Traffic 7: 117–128, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Demirci FY, Chang MH, Mah TS, Romero MF, Gorin MB. Proximal renal tubular acidosis and ocular pathology: L522P, a novel missense mutation in the gene (SLC4A4) for sodium bicarbonate cotransporter protein (NBCe1). Mol Vis 12: 324–330, 2006 [PubMed] [Google Scholar]
- 9.Devonald MA, Smith AN, Poon JP, Ihrke G, Karet FE. Non-polarized targeting of AE1 causes autosomal dominant distal renal tubular acidosis. Nat Genet 33: 125–127, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Dinour D, Chang MH, Satoh JI, Smith BL, Angle N, Knecht A, Serban I, Holtzman EJ, Romero MF. A novel missense mutation in the sodium bicarbonate cotransporter (NBCe1/SLC4A4) causes proximal tubular acidosis and glaucoma through ion transport defects. J Biol Chem 279: 52238–52246, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Gawenis LR, Bradford EM, Prasad V, Lorenz JN, Simpson JE, Clarke LL, Woo AL, Grisham C, Sanford LP, Doetschman T, Miller ML, Shull GE. Colonic anion secretory defects and metabolic acidosis in mice lacking the NBC1 Na+/HCO3− cotransporter. J Biol Chem 282: 9042–9052, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Giese B, Roderburg C, Sommerauer M, Wortmann SB, Metz S, Heinrich PC, Muller-Newen G. Dimerization of the cytokine receptors gp130 and LIFR analysed in single cells. J Cell Sci 118: 5129–5140, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Gill HS, Boron WF. Preliminary X-ray diffraction analysis of the cytoplasmic N-terminal domain of the Na/HCO3 cotransporter NBCe1-A. Acta Crystallograph Sect F Struct Biol Cryst Commun 62: 534–537, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Griesbeck O, Baird GS, Campbell RE, Zacharias DA, Tsien RY. Reducing the environmental sensitivity of yellow fluorescent protein. Mechanism and applications. J Biol Chem 276: 29188–29194, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Horita S, Yamada H, Inatomi J, Moriyama N, Sekine T, Igarashi T, Endo Y, Dasouki M, Ekim M, Al-Gazali L, Shimadzu M, Seki G, Fujita T. Functional analysis of NBC1 mutants associated with proximal renal tubular acidosis and ocular abnormalities. J Am Soc Nephrol 16: 2270–2278, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Hu CD, Chinenov Y, Kerppola TK. Visualization of interactions among bZIP and Rel family proteins in living cells using bimolecular fluorescence complementation. Mol Cell 9: 789–798, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Hu CD, Kerppola TK. Simultaneous visualization of multiple protein interactions in living cells using multicolor fluorescence complementation analysis. Nat Biotechnol 21: 539–545, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hynes TR, Tang L, Mervine SM, Sabo JL, Yost EA, Devreotes PN, Berlot CH. Visualization of G protein betagamma dimers using bimolecular fluorescence complementation demonstrates roles for both beta and gamma in subcellular targeting. J Biol Chem 279: 30279–30286, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Igarashi T, Inatomi J, Sekine T, Cha SH, Kanai Y, Kunimi M, Tsukamoto K, Satoh H, Shimadzu M, Tozawa F, Mori T, Shiobara M, Seki G, Endou H. Mutations in SLC4A4 cause permanent isolated proximal renal tubular acidosis with ocular abnormalities. Nat Genet 23: 264–266, 1999 [DOI] [PubMed] [Google Scholar]
- 20.Igarashi T, Inatomi J, Sekine T, Seki G, Shimadzu M, Tozawa F, Takeshima Y, Takumi T, Takahashi T, Yoshikawa N, Nakamura H, Endou H. Novel nonsense mutation in the Na+/HCO3− cotransporter gene (SLC4A4) in a patient with permanent isolated proximal renal tubular acidosis and bilateral glaucoma. J Am Soc Nephrol 12: 713–718, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Jennings ML. Oligomeric structure and the anion transport function of human erythrocyte band 3 protein. J Membr Biol 80: 105–117, 1984 [DOI] [PubMed] [Google Scholar]
- 22.Jennings ML, Nicknish JS. Localization of a site of intermolecular cross-linking in human red blood cell band 3 protein. J Biol Chem 260: 5472–5479, 1985 [PubMed] [Google Scholar]
- 23.Kao L, Sassani P, Azimov R, Pushkin A, Abuladze N, Peti-Peterdi J, Liu W, Newman D, Kurtz I. Oligomeric structure and minimal functional unit of the electrogenic sodium bicarbonate cotransporter NBCe1-A. J Biol Chem 283: 26782–26794, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karet FE, Gainza FJ, Gyory AZ, Unwin RJ, Wrong O, Tanner MJ, Nayir A, Alpay H, Santos F, Hulton SA, Bakkaloglu A, Ozen S, Cunningham MJ, di Pietro A, Walker WG, Lifton RP. Mutations in the chloride-bicarbonate exchanger gene AE1 cause autosomal dominant but not autosomal recessive distal renal tubular acidosis. Proc Natl Acad Sci USA 95: 6337–6342, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krapf R. Mechanisms of adaptation to chronic respiratory acidosis in the rabbit proximal tubule. J Clin Invest 83: 890–896, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee SK, Boron WF, Parker MD. Relief of autoinhibition of the electrogenic Na-HCO3− cotransporter NBCe1-B: role of IRBIT vs. amino-terminal truncation. Am J Physiol Cell Physiol 302: C518–C526, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li C, Wen A, Shen B, Lu J, Huang Y, Chang Y. FastCloning: a highly simplified, purification-free, sequence- and ligation-independent PCR cloning method. BMC Biotechnol 11: 92, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lo YF, Yang SS, Seki G, Yamada H, Horita S, Yamazaki O, Fujita T, Usui T, Tsai JD, Yu IS, Lin SW, Lin SH. Severe metabolic acidosis causes early lethality in NBC1 W516X knock-in mice as a model of human isolated proximal renal tubular acidosis. Kidney Int 79: 730–741, 2011 [DOI] [PubMed] [Google Scholar]
- 29.Lu J, Boron WF. Reversible and irreversible interactions of DIDS with the human electrogenic Na/HCO3− cotransporter NBCe1-A: role of lysines in the KKMIK motif of TM5. Am J Physiol Cell Physiol 292: C1787–C1798, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Maunsbach AB, Vorum H, Kwon TH, Nielsen S, Simonsen B, Choi I, Schmitt BM, Boron WF, Aalkjaer C. Immunoelectron microscopic localization of the electrogenic Na/HCO(3) cotransporter in rat and ambystoma kidney. J Am Soc Nephrol 11: 2179–2189., 2000 [DOI] [PubMed] [Google Scholar]
- 31.Preisig PA, Alpern RJ. Chronic metabolic acidosis causes an adaptation in the apical membrane Na/H antiporter and basolateral membrane Na(HCO3)3 symporter in the rat proximal convoluted tubule. J Clin Invest 82: 1445–1453, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Preisig PA, Alpern RJ. Increased Na/H antiporter and Na/3HCO3 symporter activities in chronic hyperfiltration. A model of cell hypertrophy. J Gen Physiol 97: 195–217, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Quilty JA, Cordat E, Reithmeier RA. Impaired trafficking of human kidney anion exchanger (kAE1) caused by hetero-oligomer formation with a truncated mutant associated with distal renal tubular acidosis. Biochem J 368: 895–903, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Romero MF. Defects of the electrogenic Na+/HCO3− cotransporter, NBC1/SLC4a4. In: Membrane Transporter Diseases, edited by Bröer S, Wagner C. New York: Kluwer Academic/Plenum, 2004, p. 65–79 [Google Scholar]
- 35.Sciortino CM, Fletcher BR, Shrode LD, Harte PJ, Romero MF. Localization of endogenous and recombinant Na+-driven anion exchanger protein, NDAE1, from Drosophila melanogaster. Am J Physiol Cell Physiol 281: C449–C463, 2001 [DOI] [PubMed] [Google Scholar]
- 36.Shyu YJ, Liu H, Deng X, Hu CD. Identification of new fluorescent protein fragments for bimolecular fluorescence complementation analysis under physiological conditions. Biotechniques 40: 61–66, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Suzuki M, Van Paesschen W, Stalmans I, Horita S, Yamada H, Bergmans BA, Legius E, Riant F, De Jonghe P, Li Y, Sekine T, Igarashi T, Fujimoto I, Mikoshiba K, Shimadzu M, Shiohara M, Braverman N, Al-Gazali L, Fujita T, Seki G. Defective membrane expression of the Na(+)-HCO(3)(-) cotransporter NBCe1 is associated with familial migraine. Proc Natl Acad Sci USA 107: 15963–15968, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Svichar N, Esquenazi S, Chen HY, Chesler M. Preemptive regulation of intracellular pH in hippocampal neurons by a dual mechanism of depolarization-induced alkalinization. J Neurosci 31: 6997–7004, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Toye AM, Parker MD, Daly CM, Lu J, Virkki LV, Pelletier MF, Boron WF. The human NBCe1-A mutant R881C, associated with proximal renal tubular acidosis, retains function but is mistargeted in polarized renal epithelia. Am J Physiol Cell Physiol 291: C788–C801, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Usui T, Hara M, Satoh H, Moriyama N, Kagaya H, Amano S, Oshika T, Ishii Y, Ibaraki N, Hara C, Kunimi M, Noiri E, Tsukamoto K, Inatomi J, Kawakami H, Endou H, Igarashi T, Goto A, Fujita T, Araie M, Seki G. Molecular basis of ocular abnormalities associated with proximal renal tubular acidosis. J Clin Invest 108: 107–115, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Walter M, Chaban C, Schutze K, Batistic O, Weckermann K, Nake C, Blazevic D, Grefen C, Schumacher K, Oecking C, Harter K, Kudla J. Visualization of protein interactions in living plant cells using bimolecular fluorescence complementation. Plant J 40: 428–438, 2004 [DOI] [PubMed] [Google Scholar]
- 42.Wang DN, Sarabia VE, Reithmeier RA, Kuhlbrandt W. Three-dimensional map of the dimeric membrane domain of the human erythrocyte anion exchanger, Band 3. EMBO J 13: 3230–3235, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang D, Kiyatkin A, Bolin JT, Low PS. Crystallographic structure and functional interpretation of the cytoplasmic domain of erythrocyte membrane band 3. Blood 96: 2925–2933, 2000 [PubMed] [Google Scholar]