Abstract
This study tested the hypothesis that P2Y12 receptor blockade with clopidogrel preserves renal autoregulatory ability during ANG II-induced hypertension. Clopidogrel was administered orally to male Sprague-Dawley rats chronically infused with ANG II. After 14 days of treatment, whole kidney autoregulation of renal blood flow was assessed in vivo in pentobarbital-anesthetized rats using an ultrasonic flow probe placed around the left renal artery. In ANG II-vehicle-treated rats, decreasing arterial pressure over a range from 160 to 100 mmHg resulted in a 25 ± 5% decrease in renal blood flow, demonstrating a significant loss of autoregulation with an autoregulatory index of 0.66 ± 0.15. However, clopidogrel treatment preserved autoregulatory behavior in ANG II-treated rats to levels indistinguishable from normotensive sham-operated (sham) rats (autoregulatory index: 0.04 ± 0.14). Compared with normotensive sham-vehicle-treated rats, ANG II infusion increased renal CD3-positive T cell infiltration by 66 ± 6%, induced significant thickening of the preglomerular vessels and glomerular basement membrane and increased glomerular collagen I deposition, tubulointerstitial fibrosis, damage to the proximal tubular brush border, and protein excretion. Clopidogrel significantly reduced renal infiltration of T cells by 39 ± 9% and prevented interstitial artery thickening, ANG II-induced damage to the glomerular basement membrane, deposition of collagen type I, and tubulointerstitial fibrosis, despite the maintenance of hypertension. These data demonstrate that systemic P2Y12 receptor blockade with clopidogrel protects against impairment of autoregulatory behavior and renal vascular injury in ANG II-induced hypertension, possibly by reducing renal T cell infiltration.
Keywords: autoregulatory response, purinergic P2Y receptors, clopidogrel, thromboxane, lymphocyte, angiotensin II
autoregulation is an important intrinsic mechanism regulating glomerular capillary pressure, primarily by adjusting afferent arteriolar resistance in response to changes in renal perfusion pressure and/or distal tubular fluid flow (52). Buffering glomerular capillary pressure protects against glomerular injury when arterial pressure (AP) is fluctuating (5). Hypertension and end-organ injury often result when ANG II levels are inappropriately elevated. ANG II-induced hypertension leads to impaired renal function and renal injury (20, 31). Rats that receive a chronic low-dose ANG II infusion for 2 wk exhibit a significant elevation in AP, impaired autoregulatory behavior, proteinuria, and renal fibrosis (17).
Recently, chronic elevation of ANG II has been linked to both vascular inflammation and autoregulatory impairment (24, 51). The inflammatory cytokine transforming growth factor (TGF)-β has been implicated in renal injury during hypertension and diabetes (18, 48). Acute administration of TGF-β impairs afferent arteriolar autoregulatory vasoconstriction (53). Chronic use of a general anti-inflammatory agent, pentosan polysulfate, or a lymphocyte antiproliferative drug, mycophenolate mofetil, prevented the increase in plasma TGF-β and preserved autoregulation in ANG II-infused hypertensive rats without lowering AP (24, 25). Monocyte chemoattractant protein-1 receptor blockade in an ANG II + high-salt hypertension model improved autoregulatory reactivity despite persistent hypertension (15). Taken together, these data suggest that anti-inflammatory agents improve autoregulatory capability independent of direct effects on AP.
P2Y12 receptors are one of a family of P2Y purinoceptors (7, 54). P2Y12 receptors are highly expressed by platelets and are involved in platelet activation (16, 58). Clopidogrel (Plavix) is a thienopyridine derivative prodrug that inhibits platelet activation, aggregation, and adhesion by blocking platelet ADP-sensitive P2Y12 receptors (16, 29, 58). Orally administered clopidogrel is catabolized to its active form by cytochrome P-450 3A in the liver (11). The active form of clopidogrel is an irreversible inhibitor of P2Y12 receptors (49, 58). Two recent studies (22, 57) have demonstrated that blockade of ADP sensitive P2Y12 receptors with clopidogrel reduced renal fibrosis. P2Y12 receptor inhibition decreased TGF-β, fibronectin, and plasminogen activator inhibitor-1 in a model of glomerulonephritis (45). In human renal transplant patients, P2Y12 receptor blockade reduced levels of inflammatory biomarkers such as matrix metalloprotease-9 (23). P2Y12 receptor blockers have been useful in reducing myocardial infarction, atherosclerosis, stroke, and the mortality associated with peripheral vascular disease (2, 7, 55, 58). Interactions between inappropriately elevated ANG II levels and P2Y12 receptor activity may have important clinical implications. While these studies have implicated endogenous platelets in the inflammatory cascade that contributes to hypertensive kidney injury, they do not address the potential role that clopidogrel treatment may have on the impaired renal microvascular function in hypertension. The possible protective effect of clopidogrel on autoregulatory behavior in hypertension has not been studied. If clopidogrel preserves renal microvascular autoregulatory function, it may explain the renal protective effects that clopidogrel treatment have during hypertension. Accordingly, the present study tested the hypothesis that P2Y12 receptor blockade with clopidogrel preserves autoregulatory behavior and reduces renal injury in ANG II-induced hypertension.
METHODS
Animals
Experiments were performed in male Sprague-Dawley rats (n = 120, 200–225 g, Charles River Laboratories, Raleigh, NC). Rats had free access to standard rat chow (Harlan Teklad Global Diets no. 8656, Wilmington, DE) and tap water. Rats were treated according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals using procedures approved by the Institutional Animal Care and Use Committee of Georgia Health Sciences University.
Hypertension was induced using low-dose ANG II infusion from osmotic minipumps (model 2002, ALZET, Cupertino, CA) implanted subcutaneously in the dorsum of the neck in anesthetized rats (2–3% isoflurane in oxygen) (30). Rats were divided into five groups. Two sham surgery (sham) groups were treated orally with clopidogrel (10 mg·kg body mass−1·day−1, Sanofi-Aventis, Bridgewater, NJ) or vehicle (fat-free pudding, 1 g) for 14 days. This dose of clopidogrel was selected as it closely matches the commonly prescribed human dose of 75 mg/day. Two groups received ANG II (60 ng/min, Phoenix Pharmaceuticals, Burlingame, CA) while simultaneously receiving either clopidogrel (ANG60-clopidogrel) or vehicle (ANG60-vehicle). The last group received an ANG II infusion at 70 ng/min plus clopidogrel (ANG70-clopidogrel) as a pressure control because clopidogrel treatment slowed the development of hypertension in the ANG60-clopidogrel group despite the fact that the level of hypertension on days 13 and 14 was similar to the 60 ng/min ANG II-alone group. Accordingly, we added the 70 ng/min ANG II infusion group to provide the blood pressure control to obviate the possibility that improved function in the ANG60-clopidogrel group was not due to a lag in the progression of hypertension.
Animal Monitoring
Body weight was measured on day 0 (before minipump implantation) and on days 6 and 13. Activated clopidogrel binds irreversibly to P2Y12 receptors (49, 58), so clopidogrel treatment will irreversibly inhibit P2Y12 receptor-mediated platelet activation. The efficacy of clopidogrel-mediated inhibition of platelet activation was verified by comparing bleeding times in treated and untreated rats. After 14 days of treatment with either clopidogrel or vehicle, rats were placed in individual restrainers, and the tip of the tail (∼3 mm) was cut with a sharp scalpel. Bleeding time was monitored by timed collection of blood droplets. Bleeding that extended beyond a maximum of 1,200 s (common in clopidogrel-treated rats) was stopped using a silver nitrate styptic pencil.
Blood Pressure Monitoring
Tail-cuff plethysmography.
Blood pressure was measured two different ways. The majority of the animals were monitored using tail-cuff plethysmography (IITC Life Science, Woodland Hills, CA) to verify and follow the development of hypertension. This technique allows verification of significant differences in systolic blood pressure (SBP) across treatment groups, and it is the approach that is most suitable for these experiments, where renal tissues need to be perfused in situ and harvested for further study. SBP was measured every 3 days to assure normal functioning of the minipumps and normal hypertension progression.
Telemetric measurement of blood pressure.
As noted above, cuff plethysmography is suitable for detecting significant differences in SBP, but it is not sensitive enough to reveal more subtle differences in blood pressure or hypertension development. Accordingly, we also measured AP by telemetry (Data Sciences, St. Paul, MN) in a subset of rats to verify the course of hypertension development and to control for potential blood pressure effects of clopidogrel that might not be resolvable with tail-cuff plethysmography. These animals were used only to follow blood pressure. For these animals, transmitters were implanted under pentobarbital anesthesia (50 mg/kg ip) 10–14 days before the implantation of osmotic minipumps (46). After animals had recovered from surgery, blood pressure was continuously monitored for the 2 wk of ANG II infusion.
In Vivo Autoregulation Experiments
Renal autoregulation was assessed as previously described (44). Rats anesthetized with pentobarbital sodium (50 mg/kg) received 6% BSA fraction V (Calbiochem, Darmstadt, Germany) in saline (100 μl/min iv to 1% of body weight) followed by a constant isotonic saline infusion (25 μl/min). A bladder catheter allowed continuous urine collection. An adjustable occluder around the aorta allowed manual control of left renal artery pressure as measured by a femoral artery catheter. An ultrasonic flow probe (MA1PRB, Transonic Systems, Ithaca, NY) was placed around the left renal artery to measure renal blood flow (RBF). AP and RBF were recorded by an eight-channel Powerlab (model ML870/P, AD Instruments, Colorado Springs, CO). When surgery was completed, the wound was covered in moistened gauze and parafilm.
Autoregulatory curves were generated by decreasing renal perfusion pressure, measured as left femoral artery mean AP (MAP), from baseline to 70 mmHg in 10-mmHg decrements at 2 min/step as previously described (44). Two autoregulatory curves were obtained over 40–60 min and were averaged to determine the overall response. The autoregulatory index (AI) was calculated as follows: AI = [(RBF1 − RBF2)/(RBF1)]/[(AP1 − AP2)/(AP1)] where subscript 1 is the value before pressure was adjusted and subscript 2 is the value after the pressure change. An AI of zero indicates perfect autoregulation, and AIs approaching 1.0 indicate less efficient autoregulation. RBF was normalized to left kidney weight.
Plasma and Urine Collection
Activated platelets can produce significant amounts of thromboxane A2 (TXA2), which has potent vasoconstrictor effects on the renal microcirculation (4, 58, 61). Plasma thromboxane B2 (TXB2) concentrations, a metabolite of TXA2, are increased in ANG II-infused hypertensive rats (13, 35, 41). Accordingly, plasma and urine TXB2 and 2,3-dinor-TXB2 were measured across the treatment groups to establish their thromboxane status and to determine the impact of clopidogrel treatment on endogenous thromboxane levels. In separate rats, aortic blood (5 ml) was collected into a syringe containing 120 μl of 7.5% EDTA (Fisher Scientific, Fairlawn, NJ) and 10 μmol/l indomethacin (Sigma-Aldrich, St. Louis, MO) and centrifuged (1,500 g, 4°C, 10 min). Plasma aliquots were frozen at −80°C until assayed. By preventing Ca2+-mediated platelet activation with EDTA and cyclooxygenase-dependent prostanoid production with indomethacin, the measured levels of plasma thromboxane were likely due to vascular production rather than ex vivo platelet activation (43). Twenty-four-hour urine was collected on days 0, 6, and 14 of treatment. Plasma and urine TXB2 and 2,3-dinor-TXB2 were measured by ELISA (Cayman Chemical, Ann Arbor, MI) according to the manufacturer's instructions. Urinary protein concentration was measured by a modified Bradford method (8) and used to calculate 24-h protein excretion.
Histological and Immunohistochemical Assessment of Renal Tissues
In some rats, kidneys were perfused with physiological salt solution followed by 10% formalin and sectioned longitudinally before being placed in 10% formalin overnight. Fixed kidneys were paraffin embedded, sectioned, and stained with hematoxylin and eosin for the examination of renal structure. The periodic acid-Schiff reaction was used to assess basement membrane thickening. Picrosirius red and Masson's blue trichrome staining were used to determine renal fibrosis. Sections were examined and scored by a trained renal pathologist blinded to the experimental conditions.
For immunohistochemistry, sections were deparaffinized with xylene and ethanol and incubated with proteinase K for antigen retrieval. Endogenous biotin and peroxidase activity were blocked by an incubation with avidin and biotin and H2O2, respectively. Primary antibodies against CD3 (BD Pharmingen, San Diego, CA) and ED-1 (Abcam, Cambridge, MA) were used to detect T lymphocytes and macrophages, respectively. Biotinylated horse anti-mouse secondary antibody was used to develop the avidin-biotinylated horseradish peroxidase complex (Vectastain ABC kits, Vector Laboratory, Burlingame, CA). Slides were counterstained with hematoxylin, and 20 fields/slide were photographed by light microscopy (×20 magnification). CD3- or ED-1-positive cells were counted and averaged over a 0.25-mm2 area of the renal cortex. All sections were scored by a trained observer who was blinded to the treatment protocol.
Statistics
Data are reported as means ± SE. Two-way ANOVA with Holm-Sidak post hoc analysis was used to evaluate statistical significance versus the control group as well as pair-wise differences as appropriate. One-way ANOVA with Bonferroni post hoc analysis was used as appropriate. Histological data were analyzed using one-way ANOVA with a Holm-Sidak post hoc test for multiple comparisons. Statistical analyses were performed using SigmaStat (version 3.5, Systat Software, Point Richmond, CA). P values of <0.05 were considered significantly different.
RESULTS
Physiological Parameters
Baseline SBP, body weight, water intake, and daily urine excretion were similar in all groups (Table 1). Summary data for rats after 14 days of treatment are shown in Table 2. Body weight increased similarly over 14 days of treatment in sham groups, whereas it increased more slowly in ANG II-infused rats. SBP, water intake, and urine excretion increased significantly during ANG II infusion compared with sham groups. Clopidogrel did not affect body weight, SBP, water intake, or urine excretion. Clopidogrel treatment increased bleeding time in sham and ANG II groups by approximately threefold, consistent with its anticoagulant properties conferred through irreversible clopidogrel binding to platelet P2Y12 receptors (49, 58). Statistical analysis was not performed on bleeding time data because the actual variation on the measurement in some groups is not known. Bleeding was terminated deliberately using a silver nitrate styptic pencil in some animals where bleeding time extended beyond a maximum of 1,200 s.
Table 1.
Physiological parameters of experimental groups at baseline
| n | Sham + Vehicle | Sham + Clopidogrel | 60 ng/min ANG II + Vehicle | 60 ng/min ANG II + Clopidogrel | 70 ng/min ANG II + Clopidogrel | |
|---|---|---|---|---|---|---|
| Body weight, g | 24 | 258 ± 3 | 261 ± 4 | 256 ± 5 | 251 ± 6 | 256 ± 4 |
| Systolic blood pressure, mmHg | 24 | 121 ± 3 | 122 ± 3 | 116 ± 2 | 114 ± 2 | 119 ± 2 |
| Water intake, ml/day | 24 | 35.1 ± 3 | 31.8 ± 1 | 33.2 ± 1 | 38.8 ± 5 | 28.1 ± 4 |
| Urine volume, ml/day | 18 | 13.9 ± 1.5 | 13.1 ± 0.9 | 12.8 ± 0.7 | 11.0 ± 0.9 | 11.1 ± 1.1 |
Values are means ± SE; n, number of total rats/group with a subset of the rats undergoing 24-h urine collection.
Table 2.
Physiological parameters of experimental groups after 14 days of treatment
| n | Sham + Vehicle | Sham + Clopidogrel | 60 ng/min ANG II + Vehicle | 60 ng/min ANG II + Clopidogrel | 70 ng/min ANG II + Clopidogrel | |
|---|---|---|---|---|---|---|
| Body weight, g | 24 | 354 ± 6 | 369 ± 7 | 326 ± 6* | 320 ± 6 | 336 ± 8* |
| Systolic blood pressure, mmHg | 24 | 118 ± 3 | 120 ± 3 | 198 ± 5* | 195 ± 4 | 195 ± 4* |
| Water intake, ml/day | 24 | 37.5 ± 4 | 38.3 ± 3 | 55.7 ± 4* | 55.3 ± 3* | 55.0 ± 5* |
| Urine volume, ml/day | 18 | 20.0 ± 1.9 | 20.1 ± 1.8 | 36.7 ± 3.0* | 38.9 ± 3 | 36.9 ± 3.9* |
| Bleeding time, s | 24 | 407 ± 24 | 1200 ± 0 | 304 ± 17 | 1120 ± 17 | 987 ± 81 |
| Anesthetized mean arterial pressure, mmHg | 6 | 131 ± 5 | 126 ± 3 | 162 ± 12* | 169 ± 3 | 165 ± 16* |
| Anesthetized renal blood flow, ml·min−1·g kidney mass−1 | 6 | 10.7 ± 0.9 | 9.1 ± 1.1 | 11.0 ± 1.0 | 9.7 ± 0.8 | 8.6 ± 1.6 |
Values are mean ± SE; n, number of total rats/group with a subset of the rats undergoing 24-h urine collection. Statistical analysis was not performed on bleeding time data because the variation on the measurement is not known. Bleeding was terminated deliberately using a silver nitrate styptic pencil in cases where bleeding time extended beyond a maximum of 1,200 s.
P < 0.05 vs. the respective sham group.
Telemetric Blood Pressure Measurements
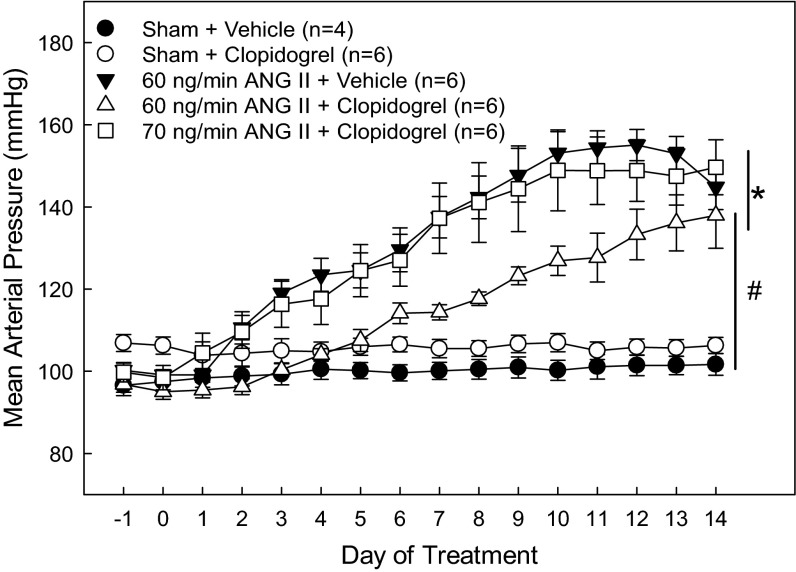
MAP was similar between sham rats irrespective of clopidogrel treatment (Fig. 1). ANG II infusion significantly increased MAP. By day 4, ANG60-vehicle rats had significantly elevated MAP compared with sham-vehicle rats. Elevation of MAP plateaued around day 9 and remained in the hypertensive range through day 14. However, in ANG60-clopidogrel rats, hypertension development was delayed. While MAP increased significantly by day 9 compared with sham-vehicle rats, it took longer to reach the same level of hypertension compared with sham-vehicle rats. To control for the discrepancy in hypertension progression due to clopidogrel treatment, we added a separate group of rats that received an ANG II infusion (70 ng/min ANG II with clopidogrel) titrated to match the MAP profile exhibited by ANG60-vehicle rats. As shown in Fig. 1, the development of hypertension in rats that received 70 ng/min ANG II plus clopidogrel almost exactly mirrored the hypertension progression observed in ANG60-vehicle rats. This group with a similar hypertension profile served as the primary focus group for the autoregulatory experiments.
Fig. 1.
Mean arterial pressure (MAP) in rats treated with ANG II and clopidogrel. Twenty-four-hour MAP was measured by telemetry in sham-operated (sham) rats (circles) and rats that received either 60 ng/min ANG II (ANG60; triangles) or 70 ng/min ANG II (ANG70; squares) via osmotic minipump. Solid symbols represent rats that received vehicle; open symbols represent rats treated with clopidogrel (10 mg·kg−1·day−1). *P < 0.05 vs. sham + vehicle-treated rats; #P < 0.05 vs. 60 ng/min ANG II + vehicle-treated rats.
Autoregulatory Behavior in ANG II- and Clopidogrel-Treated Rats
Anesthetized MAP and RBF data are shown in Table 2. There were no statistical differences in MAP or RBF between anesthetized sham-vehicle and sham-clopidogrel rats. MAP and RBF were also similar between ANG60-vehicle and ANG70-clopidogrel groups, and MAP in both groups was significantly increased compared with the sham + clopidogrel group.
Figure 2 shows the effect of clopidogrel treatment on actual RBF (A) and normalized RBF (B). In both sham groups, RBF remained stable as renal perfusion pressure was reduced from 120 to 100 mmHg (Fig. 2, A and B), consistent with normal autoregulatory behavior. However, in ANG60-vehicle rats, RBF decreased with each reduction of renal perfusion pressure across a range of 160 to 100 mmHg. Clopidogrel preserved autoregulatory ability in both ANG60-clopidogrel and ANG70-clopidogrel hypertensive rats, resulting in stable RBF over a pressure range from 160 to 100 mmHg.
Fig. 2.
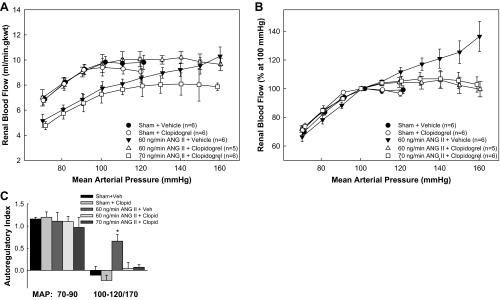
Autoregulation of renal blood flow (RBF) in vivo. Autoregulation of RBF in anesthetized rats from sham, sham + clopidogrel, ANG60 + vehicle, ANG60 + clopidogrel, and ANG70 + clopidogrel. A: actual RBF against MAP. B: RBF expressed as a percentage of the control blood flow at 100 mmHg. C: autoregulatory index over the range from initial arterial pressure to 100 and 70–90 mmHg, respectively. *P < 0.05 vs. sham rats.
As shown in Fig. 2C, the AI calculated over the 120- to 100-mmHg pressure range in the sham-vehicle and sham-clopidogrel groups averaged −0.11 ± 0.20 and −0.23 ± 0.12, respectively, consistent with efficient RBF autoregulation. In contrast, the AI for ANG60-vehicle rats averaged 0.66 ± 0.15 from 160 to 100 mmHg, indicating significant autoregulatory impairment. The AI for ANG60-clopidogrel and ANG70-clopidogrel rats averaged 0.04 ± 0.14 and 0.07 ± 0.06, respectively, demonstrating intact autoregulatory behavior. Retention of the autoregulatory capability in the ANG60-clopidogrel group occurred in the face of a slower developing hypertension (Fig. 1), making interpretation difficult. Accordingly, it is important to note that clopidogrel treatment also prevented the loss of autoregulatory control of RBF in the ANG70-clopidogrel group, despite nearly identical hypertension profiles (Fig. 1) with the ANG60-vehicle group.
Measurements of Plasma Thromboxane
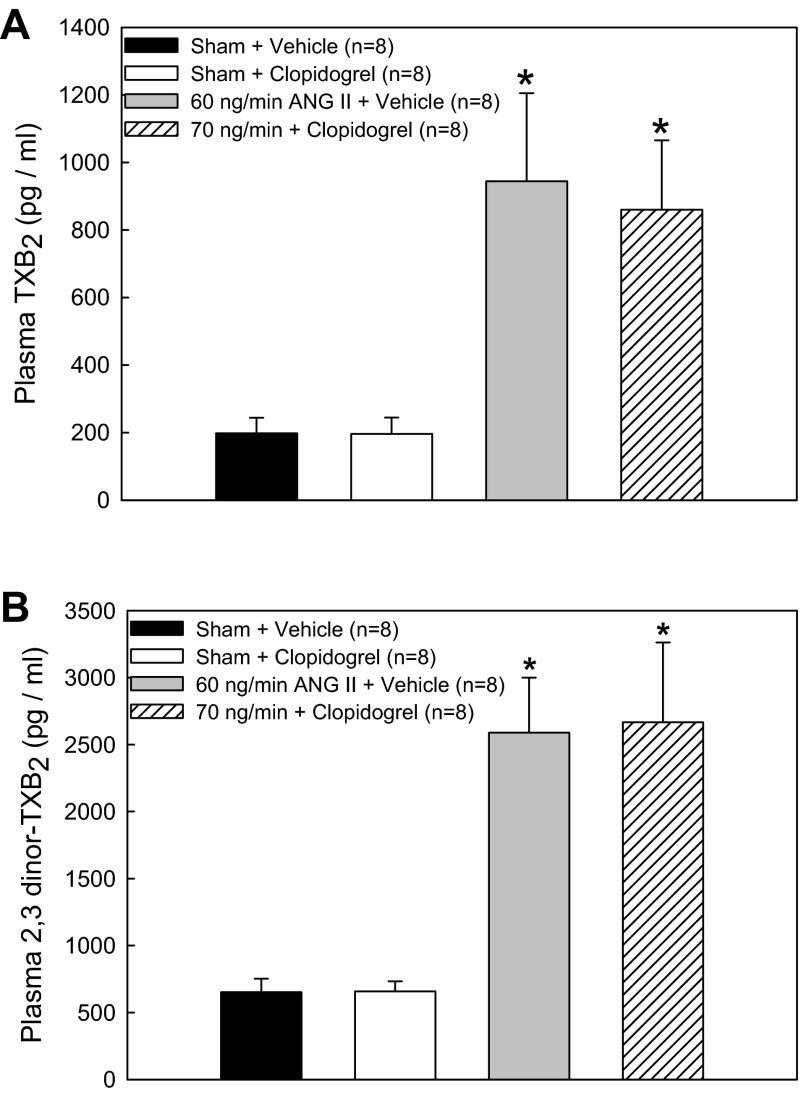
The plasma TXB2 concentration, an indicator of vascular TXA2 production, was significantly increased in ANG60-vehicle rats (944 ± 261 pg/ml) compared with sham-vehicle rats (198 ± 46 pg/ml; Fig. 3A). P2Y12 receptor blockade had no effect on plasma TXB2 concentration in either sham-clopidogrel (196 ± 49 pg/ml) or ANG70-clopidogrel (860 ± 205 pg/ml) groups. Similar results were found for the plasma 2,3-dinor-TXB2 concentration. As shown in Fig. 3B, plasma 2,3-dinor-TXB2 was increased in ANG60-vehicle rats (2,590 ± 410 pg/ml) compared with sham-vehicle rats (652 ± 100 pg/ml). P2Y12 receptor blockade had no effect on 2,3-dinor-TXB2 concentration in either sham-clopidogrel (657 ± 76 pg/ml) or ANG70-clopidogrel (2,667 ± 596 pg/ml) groups. No differences were detected between sham rats that received clopidogrel or vehicle, suggesting that the preservation of RBF autoregulation with clopidogrel cannot be attributed to changes in vascular TXA2 levels.
Fig. 3.
Plasma thromboxane B2 (TXB2; A) and the thromboxane A2 metabolite 2,3-dinor-TXB2 (B) from rats treated with ANG II and clopidogrel. Average plasma concentrations of TXB2 or 2,3-dinor-TXB2 (in pg/ml) are shown for sham rats that received dietary vehicle, sham rats that received clopidogrel, 60 ng/min Ang II-infused rats that received vehicle, and 70 ng/min ANG II-infused rats that received clopidogrel. *P < 0.05 vs. respective sham rats.
Renal Cortical Inflammatory Cell Infiltration Measurements
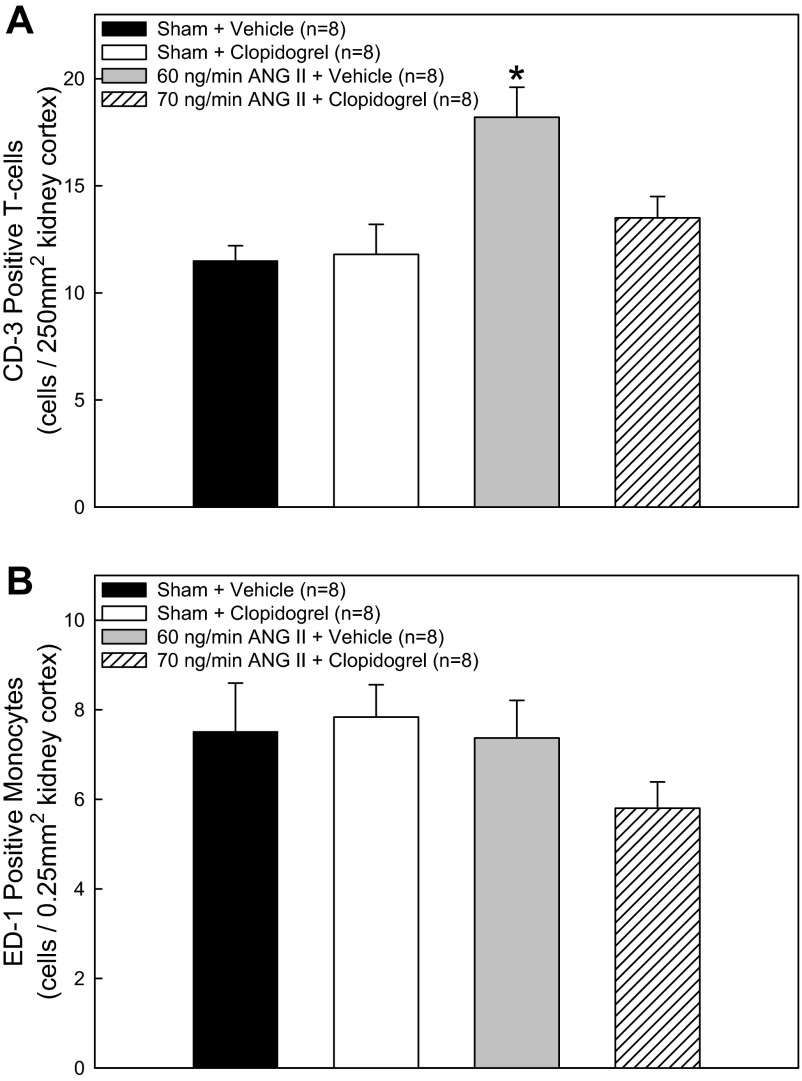
Immunolocalization of inflammatory cells in renal cortex from sham and ANG II-treated rats revealed that T cells (CD3 marker) and monocytes/macrophages (ED-1 marker) were present in both normotensive and hypertensive rats. CD3-positive T cell counts in sham-vehicle and sham-clopidogrel rats (Fig. 4A) were similar and averaged 11.5 ± 0.7 and 11.8 ± 1.4 cells/field, respectively. ANG II infusion significantly increased CD3-positive cells to 18.2 ± 1.4 cells/field. Clopidogrel treatment of ANG II-infused rats prevented the increase in CD3-positive cells seen with ANG II alone (13.5 ± 1.0 cells/field), suggesting a role for P2Y12 receptors in lymphocyte infiltration during hypertension. Overall ED1-positive monocyte/macrophage cell counts averaged 6.8 ± 0.4 cells/field and were similar across groups. ED-1-positive monocyte/macrophage cell counts in sham-vehicle, sham-clopidogrel, ANG60-clopidogrel, and ANG70-clopidogrel rats (Fig. 4B) were similar and averaged 7.5 ± 1.1, 7.8 ± 0.7, 7.4 ± 0.8, and 5.8 ± 0.6 cells/field, respectively.
Fig. 4.
CD3-positive T cell counts (A) and ED-1-positive monocyte/macrophage cell counts (B) in the renal cortex from rats treated with ANG II and clopidogrel. Average numbers of CD3-positive T cells or ED-1-positive monocytes/macrophages were calculated from 0.25-mm2 fields (×20 magnification) of the renal cortex in sham rats that received dietary vehicle, sham rats that received clopidogrel, 60 ng/min ANG II-infused rats that received vehicle, and 70 ng/min ANG II-infused rats that received clopidogrel. *P < 0.05 vs. respective sham rats.
Analysis of Renal Injury
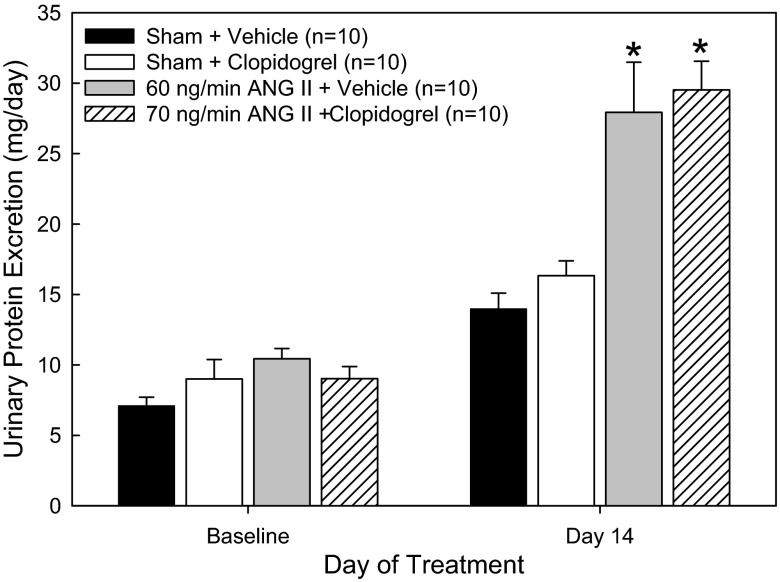
Indices of renal injury were determined by urinary protein excretion as well as by histological assessments. Proteinuria was significantly increased in ANG60-vehicle rats compared with sham-vehicle control rats after 14 days (Fig. 5). Daily protein excretion in ANG70-clopidogrel rats was similar to that observed in ANG60-vehicle rats. Clopidogrel treatment did not decrease ANG II-mediated proteinuria. While the reasons for this are unclear, the data suggest that impairment of glomerular filtration barrier selectivity is not directly influenced by P2Y12 receptors.
Fig. 5.
Urinary protein excretion (in mg/day) in rats treated with dietary vehicle, sham rats that received clopidogrel, 60 ng/min ANG II-infused rats that received vehicle, and 70 ng/min ANG II-infused rats that received clopidogrel. Urine was collected over a 24-h period. *P < 0.05 vs. respective sham rats.
Rats treated with ANG II + vehicle presented significant thickening of the glomerular basement membrane (GBM), glomerular collagen type I deposition, tubulointerstitial fibrosis, and damage to the proximal tubular brush border (Figs. 6 and 7). Treatment with clopidogrel effectively prevented the ANG II-induced damage to the GBM, deposition of collagen type I, and tubulointerstitial fibrosis (Figs. 6 and 7). These results suggest the involvement of PY2Y12 receptors in ANG II-induced renal injury. Significant preglomerular arterial thickening as well as a tendency to develop preglomerular arterial hyalinosis and necrosis were also observed in rats treated with ANG II + vehicle. No significant difference in protein cast formation or thickening of the tubular basement membrane were observed among the groups.
Fig. 6.
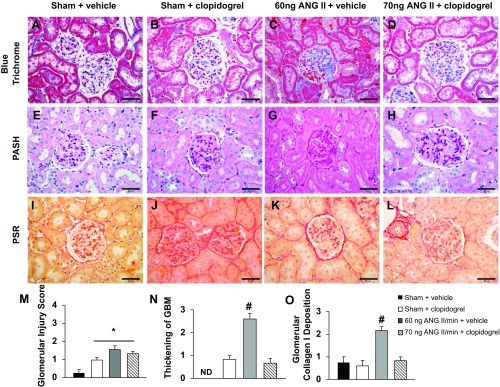
A–L: representative images of glomeruli from the four experimental groups stained with Masson's blue trichrome (A–D), periodic acid-Schiff-hematoxylin (PASH; E–H), or picrosirius red (PSR; I–L). Bars = 50 μm. M–O: quantification of the glomerular injury score (M), degree of glomerular basement membrane (GBM) thickening (N), and degree of glomerular collagen type I deposition (O). ND, not detectable. *P < 0.05 vs. the sham + vehicle-treated group; #P < 0.05 vs. all other groups.
Fig. 7.
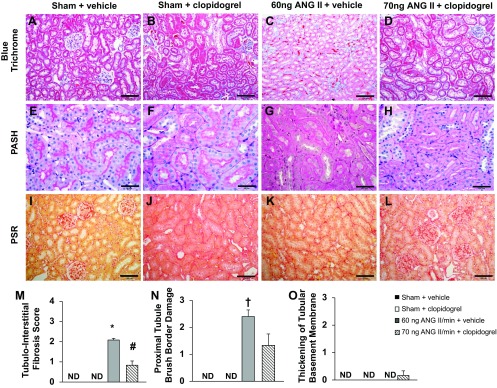
A–L: representative images of tubular structure in the four experimental groups stained with Masson's blue trichrome (A–D), PASH (E–H), or PSR (I–L). Bars = 100 μm in A–D and I–L and 50 μm in E–H. M–O: quantification of tubule-interstitial fibrosis (M), degree of damage to the proximal tubule brush border (N), and degree of tubular basement membrane thickening (O). * P < 0.05 vs. all other groups; #P < 0.05 vs. the 60 ng ANG II/min + vehicle group; †P < 0.05 vs. the sham + vehicle and sham + clopidogrel groups.
DISCUSSION
This study was designed to determine the effect of P2Y12 receptor blockade on ANG II-mediated impairment of whole kidney autoregulation of RBF. The data reveal that ANG II-induced hypertension impairs whole kidney autoregulatory behavior and stimulates the infiltration of CD3-positive lymphocytes into the renal cortex. The major and novel findings of this study demonstrate that chronic treatment with clopidogrel preserves autoregulatory responses despite persistent hypertension and reduces or prevents T lymphocyte infiltration. P2Y12 receptor activation is most likely involved in impaired autoregulatory function in ANG II-induced hypertension.
ANG II-hypertensive rats gained weight more slowly than normotensive control rats, and SBP was found to be similar between ANG II-infused rats and clopidogrel-treated ANG II-infused rats. Urine flow increased in ANG II-infused rats consistent with the increase in water consumption in those animals. An important finding of the present study is that 24-h measurements of MAP with telemetry reveals an attenuation in the development and magnitude of the hypertensive state in 60 ng/min ANG II-hypertensive rats treated with clopidogrel that was not easily detected by tail-cuff plethesmography. Consequently, to control for the delayed onset of hypertension, we added an additional clopidogrel group where we titrated the ANG II infusion rate to obtain a hypertensive pressure profile that mirrored that observed in the ANG II-alone group. In our laboratory, this was achieved using a 70 ng/min ANG II infusion rate, which was slightly higher than our ANG II-alone group and slightly lower than the rate used by Graciano et al. (22). In this setting, the specific effects of clopidogrel treatment could be examined without the confounding variable of differences in hypertensive status, underscoring the need for using the most appropriate measurement techniques for subtle pressure changes that may be missed by less sensitive methods (39). The ability of clopidogrel to delay the development of ANG II-induced hypertension suggests a link between the progression of ANG II-induced hypertension and P2Y12 receptor activity.
In addition to its anticoagulation properties, clopidogrel is also reported to have other, less well-defined anti-inflammatory properties (2, 23, 42, 57) that may provide some antihypertensive and/or tissue protective benefit. Prevention of renal injury with clopidogrel has been reported and suggests unique mechanisms for the elevation of MAP and renal injury (22, 23, 57). The function of P2Y12 receptors in influencing MAP in ANG II-hypertensive rats may be more related to the coincident hypertensive state than to a direct modulatory effect on blood pressure per se, because clopidogrel treatment did not influence MAP in sham rats. The findings of the present study extend and strengthen previous findings of Graciano et al. (22) and Tu et al. (57), who reported that clopidogrel treatment reduces renal injury independent of reductions in SBP measured by tail-cuff plethysmography. In those reports, there could have been a muted hypertensive state that was not resolved using tail cuff that may have contributed to the reduction in renal injury that could have arisen from improved autoregulatory efficiency. The present study demonstrates that with nearly identical hypertensive states, autoregulatory behavior is improved and renal injury is reduced, suggesting a pressure-independent role for clopidogrel in mitigating renal injury in this setting and consistent with previous reports (22, 57).
An important objective of this study was to determine the effect of P2Y12 receptor blockade on RBF autoregulation in ANG II-induced hypertension. Whole kidney autoregulatory efficiency was examined in vivo using vehicle- and clopidogrel-treated rats with similar blood pressure profiles. Chronic P2Y12 receptor blockade preserved normal autoregulatory behavior despite persistent hypertension. Evidence supports the preservation of vascular function by anti-inflammatory interventions in settings of persistent hypertension (6, 27, 40, 50). For example, a nonspecific anti-inflammatory agent, pentosan polysulfate, preserved afferent arteriolar autoregulation without affecting AP (24). Also, ANG II-treated rats fed a high-salt diet developed marked afferent arteriolar vasoconstriction, hypertension, decreased glomerular filtration rate, and increased tubulointerstitial injury, all of which were decreased by immune suppression with mycophenolate mofetil (17). In the DOCA-salt model of hypertension, TNF-α inhibition with etanercept also reduced renal injury without affecting blood pressure (14). These data suggest that inflammation contributes importantly to autoregulatory impairment in models of hypertension-mediated renal injury.
Vascular smooth muscle, the endothelium, platelets, lymphocytes, and sympathetic neurons all express P2Y12 receptors (7, 38, 47, 59, 60). Expression of P2Y12 receptors in nonrenal vascular tissue has been reported in both humans and rats (21, 60). In a similar model of ANG II-induced hypertension, clopidogrel treatment improved vasodilation to acetylcholine and normalized vasoconstriction to phenylephrine in second-order mesenteric arteries (21). However, direct involvement of clopidogrel-inhibitable P2Y12 receptors in vasoconstriction was not found. Therefore, the beneficial effects of clopidogrel most likely reflect the prevention of vascular or leukocyte-derived inflammatory processes associated with hypertension. Clopidogrel is a potent inhibitor of P2Y12 receptor activation. Clopidogrel binding to the receptor is irreversible, so in the presence of clopidogrel, the only way to produce active P2Y12 receptors is to synthesize new receptors or traffic new receptors to the membrane surface. We verified effective blockade of P2Y12 receptors in our study by assessing bleeding time in all groups. As noted, effective P2Y12 receptor blockade was confirmed by the dramatically increased bleeding time exhibited by rats treated with clopidogrel compared with the untreated sham control or ANG II-alone groups. Platelets express high levels of P2Y12 receptors and are a rich source of thromboxanes, which are potent vasoconstrictors of the renal microcirculation, and TXB2 levels are markedly increased in ANG II-hypertensive rats (19, 35, 41). Recently, clopidogrel has been reported to reduce platelet-derived thromboxanes in healthy normotensive male volunteers both in vivo and ex vivo (3); however, in our study, clopidogrel treatment did not reduce the plasma thromboxane concentration, possibly because of chronic overstimulation of vascular thromboxane production. The inability of P2Y12 receptor inhibition to reduce TXB2 levels is consistent with previous work demonstrating separate mechanisms for thromboxane synthesis and P2Y12 receptor-mediated platelet aggregation (9, 10). Therefore, the beneficial effects of P2Y12 receptor inhibition on renal autoregulation, renal injury, and hypertension appear to be independent of TXB2 production.
The mechanisms responsible for autoregulatory adjustments have been extensively studied. Both ATP and adenosine have been postulated to serve as chemical mediators of renal autoregulation. A previous study (56) has reported that genetic removal of adenosine A1 receptors in knockout mice inhibits the tubuloglomerular feedback component of autoregulatory afferent arteriolar vasoconstriction. In a rat model, pharmacological blockade of P2X1 receptors inhibits afferent arteriolar autoregulatory responses in vitro and whole kidney autoregulation of renal blood flow in vivo. Mice lacking ATP-sensitive P2X1 receptors exhibit impaired autoregulatory responses (32–34, 44). In addition, afferent arteriolar reactivity to P2X1 receptor activation is markedly attenuated in ANG-II hypertensive rats, and this blunted reactivity can be preserved by prevention of lymphocyte activation (25). Whether or not P2X1 and P2Y 12 receptors interact is unclear, but these data, combined with the data provided in the present report, suggest that broader consideration should be given to the role chronic inflammation plays in altering renal autoregulatory efficiency in hypertension and how that might impact hypertension-induced renal injury.
P2Y12 receptors have the highest mRNA expression in lymphocytes isolated from humans, and blockade of P2Y12 receptors significantly reduces platelet-leukocyte aggregation (36, 37, 59). A recent report (1) has indicated that P2Y12 receptors play an important role in the activation of dendritic cells with subsequent activation of T cells. Clopidogrel treatment also exerted an anti-inflammatory effect on ischemic coronary arteries, and cessation of clopidogrel treatment was associated with proinflammatory and prothrombotic effects in patients with diabetes or coronary artery disease (2, 42). Notably, several studies (12, 26, 28) have implicated T lymphocytes in the renal and vascular injury associated with hypertension. ANG II-hypertensive rats exhibit increased cortical infiltration of CD3-positive T cells, which was prevented by clopidogrel treatment. These data agree with the findings that T lymphocytes contribute significantly to renal injury in hypertension (12). However, the finding that hypertension can be maintained while T cell infiltration is reduced suggests that the deleterious effects of local T cell infiltration may be independent from induction of hypertension (25).
In summary, the present study shows that P2Y12 receptor inhibition prevents ANG II-induced impairment of renal autoregulation. This potentially beneficial effect of clopidogrel may involve a reduction of local intrarenal T cell infiltration, inflammatory events, and renal injury. A possible link between platelet-leukocyte interactions during hypertensive injury and P2Y12 receptor activation suggests multifaceted vascular anti-inflammatory effects of clopidogrel. These data support the growing contention that hypertension is a strong initiator of inflammation. P2Y12 receptor inhibition with clopidogrel could provide a useful benefit for individuals at risk of progression to chronic renal disease.
GRANTS
D. A. Osmond was supported by Southeast Affiliate of the American Heart Association Predoctoral Fellowship Award 0815156E. E.W. Inscho is supported by National Institutes of Health (NIH) Grants DK-044628, HL-098135, and HL-095499, and J. S. Pollock is supported by NIH Grant HL-095499.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: D.A.O. and E.W.I. conception and design of research; D.A.O. and S.Z. performed experiments; D.A.O., T.Y., and C.D.M. analyzed data; D.A.O., J.S.P., C.D.M., and E.W.I. interpreted results of experiments; D.A.O., C.D.M., and E.W.I. prepared figures; D.A.O. drafted manuscript; D.A.O., S.Z., J.S.P., T.Y., C.D.M., and E.W.I. edited and revised manuscript; D.A.O., S.Z., J.S.P., T.Y., C.D.M., and E.W.I. approved final version of manuscript.
ACKNOWLEDGMENTS
Present address of D. A. Osmond: 9423 Cydni Ct. NE, Albuquerque, NM 87112.
REFERENCES
- 1.Addi AB, Cammarata D, Conley PB, Boeynaems JM, Robaye B. Role of the P2Y12 receptor in the modulation of murine dendritic cell function by ADP. J Immunol 185: 5900–5906, 2010 [DOI] [PubMed] [Google Scholar]
- 2.Angiolillo DJ, Fernandez-Ortiz A, Bernardo E, Ramirez C, Sabate M, Jimenez-Quevedo P, Hernandez R, Moreno R, Escaned J, Alfonso F, Banuelos C, Costa MA, Bass TA, Macaya C. Clopidogrel withdrawal is associated with proinflammatory and prothrombotic effects in patients with diabetes and coronary artery disease. Diabetes 55: 780–784, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Armstrong PCJ, Dhanji ARA, Tucker AT, Mitchell JA, Warner TD. Reduction of platelet thromboxane A2 production ex vivo and in vivo by clopidogrel therapy. J Thromb Haemost 8: 613–615, 2010 [DOI] [PubMed] [Google Scholar]
- 4.Baylis C. Effects of administered thromboxanes on the intact, normal rat kidney. Renal Physiol 10: 110–121, 1987 [DOI] [PubMed] [Google Scholar]
- 5.Bidani AK, Griffin KA, Williamson G, Wang X, Loutzenhiser R. Protective importance of the myogenic response in the renal circulation. Hypertension 54: 393–398, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bobadilla NA, Tack I, Tapia E, Sanchez-Lozada LG, Santamaria J, Jimenez F, Striker LJ, Striker GE, Herrera-Acosta J. Pentosan polysulfate prevents glomerular hypertension and structural injury despite persisting hypertension in 5/6 nephrectomy rats. J Am Soc Nephrol 12: 2080–2087, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Boeynaems JM, Communi D, Robaye B. Overview of the pharmacology and physiological roles of P2Y receptors. WIREs Membr Transport Signal 1: 581–588, 2012 [Google Scholar]
- 8.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254, 1976 [DOI] [PubMed] [Google Scholar]
- 9.Cattaneo M, Lecchi A, Lombardi R, Gachet C, Zighetti ML. Platelets from a patient heterozygous for the defect of P2CYC receptors for ADP have a secretion defect despite normal thromboxane A2 production and normal granule stores: Further evidence that some cases of platelet “primary secretion defect” are heterozygous for a defect of P2CYC receptors. Arterioscler Thromb Vasc Biol 20: e101-e106, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Cattaneo M, Lombardi R, Zighetti ML, Gachet C, Ohlmann P, Cazenave JP, Mannucci PM. Deficiency of (33P)2MeS-ADP binding sites on platelets with secretion defect, normal granule stores and normal thromboxane A2 production. Evidence that ADP potentiates platelet secretion independently of the formation of large platelet aggregates and thromboxane A2 production. Thromb Haemost 77: 986–990, 1997 [PubMed] [Google Scholar]
- 11.Clarke TA, Waskell LA. The metabolism of clopidogrel is catalyzed by human cytochrome P450 3A and is inhibited by atorvastatin. Drug Metab Dispos 31: 53–59, 2003 [DOI] [PubMed] [Google Scholar]
- 12.De Miguel C, Das S, Lund H, Mattson DL. T lymphocytes mediate hypertension and kidney damage in Dahl salt-sensitive rats. Am J Physiol Regul Integr Comp Physiol 298: R1136–R1142, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dey A, Williams RS, Pollock DM, Stepp DW, Newman JW, Hammock BD, Imig JD. Altered kidney CYP2C and cyclooxygenase-2 levels are associated with obesity-related albuminuria. Obes Res 12: 1278–1289, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Elmarakby AA, Quigley JE, Imig JD, Pollock JS, Pollock DM. TNF-α inhibition reduces renal injury in DOCA-salt hypertensive rats. Am J Physiol Regul Integr Comp Physiol 294: R76–R83, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elmarakby AA, Quigley JE, Olearczyk JJ, Sridhar A, Cook AK, Inscho EW, Pollock DM, Imig JD. Chemokine receptor 2b inhibition provides renal protection in Angiotensin II salt hypertension. Hypertension 50: 1069–1076, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foster CJ, Prosser DM, Agans JM, Zhai Y, Smith MD, Lachowicz JE, Zhang FL, Gustafson E, Monsma FJ, Wiekowski MT, Abbondanzo SJ, Cook DN, Bayne ML, Lira SA, Chintala MS. Molecular identification and characterization of the platelet ADP receptor targeted by thienopyridine antithrombotic drugs. J Clin Invest 107: 1591–1598, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Franco M, Tapia E, Santamaria J, Zafra I, Garcia-Torres R, Gordon KL, Pons H, Rodriguez-Iturbe B, Johnson RJ, Herrera-Acosta J. Renal cortical vasoconstriction contributes to development of salt-sensitive hypertension after angiotensin II exposure. J Am Soc Nephrol 12: 2263–2271, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Fukuda N, Tahira Y, Matsuda H, Matsumoto K. Transforming growth factor-β as a treatment target in renal diseases. J Nephrol 22: 708–715, 2009 [PubMed] [Google Scholar]
- 19.Gachet C. The platelet P2 receptors as molecular targets for old and new antiplatelet drugs. Pharmacol Ther 108: 180–192, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Garovic V, Textor SC. Renovascular hypertension: current concepts. Semin Nephrol 25: 261–271, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Giachini FRC, Osmond DA, Zhang S, Carneiro FS, Lima VV, Inscho EW, Webb RC, Tostes RC. Clopidogrel, independent of the vascular P2Y12 receptor, improves arterial function in small mesenteric arteries from AngII-hypertensive rats. Clin Sci 118: 463–471, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Graciano ML, Nishiyama A, Jackson K, Seth DM, Ortiz RM, Prieto-Carrasquero MC, Kobori H, Navar LG. Purinergic receptors contribute to early mesangial cell transformation and renal vessel hypertrophy during angiotensin II-induced hypertension. Am J Physiol Renal Physiol 294: F161–F169, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Graff J, Harder S, Wahl O, Scheuermann EH, Gossmann J. Anti-inflammatory effects of clopidogrel intake in renal transplant patients: effects on platelet-leukocyte interactions, platelet CD40 ligand expression, and proinflammatory biomarkers. Clin Pharmacol Ther 78: 468–476, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Guan Z, Fuller BS, Yamamoto T, Cook AK, Pollock JS, Inscho EW. Pentosan polysulfate treatment preserves renal autoregulation in Ang II-infused hypertensive rats via normalization of P2X1 receptor activation. Am J Physiol Renal Physiol 298: F1276–F1284, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guan Z, Giddens MI, Osmond DA, Cook AK, Hobbs JL, Zhang S, Yamamoto T, Pollock JS, Pollock DM, Inscho EW. Immunosuppression preserves renal autoregulatory function and microvascular P2X1 receptor reactivity in ANG II hypertensive rats. Am J Physiol Renal Physiol 304: F801–F807, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med 204: 2449–2460, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herrera-Acosta J, Tapia E, Sanchez-Lozada LG, Franco M, Striker LJ, Striker GE, Rodriguez-Iturbe B. Restoration of glomerular haemodynamics and renal injury independent of arterial hypertension in rats with subtotal renal ablation. J Hypertens 20: S29–S35, 2002 [PubMed] [Google Scholar]
- 28.Hoch NE, Guzik TJ, Chen W, Deans T, Maalouf SA, Gratze P, Weyand C, Harrison DG. Regulation of T cell function by endogenously produced angiotensin II. Am J Physiol Regul Integr Comp Physiol 296: R208–R216, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hollopeter G, Jantzen HM, Vincent D, Li G, England L, Ramakrishnan V, Yang RB, Nurden P, Nurden A, Julius D, Comley PB. Identification of the platelet ADP receptor targeted by antithrombotic drugs. Nature 409: 202–207, 2001 [DOI] [PubMed] [Google Scholar]
- 30.Ichihara A, Imig JD, Inscho EW, Navar LG. Interactive nitric oxide angiotensin II influences on renal microcirculation in angiotensin II induced hypertension. Hypertension 31: 1255–1260, 1998 [DOI] [PubMed] [Google Scholar]
- 31.Imig JD, Inscho EW. Adaptations of the renal microcirculation to hypertension. Microcirculation 9: 315–328, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Inscho EW, Cook AK, Imig JD, Vial C, Evans RJ. Physiological role for P2X1 receptors in renal microvascular autoregulatory behavior. J Clin Invest 112: 1895–1905, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Inscho EW, Cook AK, Imig JD, Vial C, Evans RJ. Renal autoregulation in P2X1 knockout mice. Acta Physiol Scand 181: 445–453, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Inscho EW, Cook AK, Navar LG. Pressure-mediated vasoconstriction of juxtamedullary afferent arterioles involves P2-purinoceptor activation. Am J Physiol Renal Fluid Electrolyte Physiol 271: F1077–F1085, 1996 [DOI] [PubMed] [Google Scholar]
- 35.Keen HL, Brands MW, Smith MJ, Shek EW, Hall JE. Thromboxane is required for full expression of angiotensin hypertension in rats. Hypertension 29: 310–314, 1997 [DOI] [PubMed] [Google Scholar]
- 36.Klinkhardt U, Bauersachs R, Adams J, Graff J, Lindhoff-Last E, Harder S. Clopidogrel but not aspirin reduces P-selectin expression and formation of platelet-leukocyte aggregates in patients with atherosclerotic vascular disease. Clin Pharmacol Ther 73: 232–241, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Klinkhardt U, Kuczka K, Harder S. Effects of the NHE-1 inhibitor cariporide alone or together with the P2Y12 antagonist AR-C 69331 MX on CD62p expression and formation of platelet-leukocyte aggregates. Thromb Res 111: 251–257, 2003 [DOI] [PubMed] [Google Scholar]
- 38.Kunapuli SP. Functional characterization of platelet ADP receptors. Platelets 9: 343–351, 1998 [DOI] [PubMed] [Google Scholar]
- 39.Kurtz TW, Griffin KA, Bidani AK, Davisson RL, Hall JE. Recommendations for blood pressure measurement in humans and experimental animals: part 2: blood pressure measurement in experimental animals: a statement for professionals from the subcommittee of professional and public education of the American Heart Association. Hypertension 45: 299–310, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Luft FC, Dechend R, Müller DN. Immune mechanisms in angiotensin II-induced target-organ damage. Ann Med 44: S49–S54, 2012 [DOI] [PubMed] [Google Scholar]
- 41.Mistry M, Nasjletti A. Role of pressor prostanoids in rats with angiotensin II-salt-induced hypertension. Hypertension 11: 758–762, 1988 [DOI] [PubMed] [Google Scholar]
- 42.Molero L, Lopez-Farre A, Mateos-Caceres PJ, Fernandez-Sanchez R, Maestro ML, Silva J, Rodriguez E, Macaya C. Effect of clopidogrel on the expression of inflammatory markers in rabbit ischemic coronary artery. Br J Pharmacol 146: 419–424, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murphy RC, FitzGerald GA. Current approaches to estimation of eicosanoid formation in vivo. Adv Prostaglandin Thromboxane Leukot Res 22: 341–348, 1994 [PubMed] [Google Scholar]
- 44.Osmond DA, Inscho EW. P2X1 receptor blockade inhibits whole kidney autoregulation of renal blood flow in vivo. Am J Physiol Renal Physiol 298: F1360–F1368, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peters H, Eisenberg R, Daig U, Liefeldt L, Westenfeld R, Gaedeke J, Kramer S, Neumayer HH. Platelet inhibition limits TGF-beta overexpression and matrix expansion after induction of anti-thy1 glomerulonephritis. Kidney Int 65: 2238–2248, 2004 [DOI] [PubMed] [Google Scholar]
- 46.Pollock DM, Pollock JS. Evidence for endothelin involvement in the response to high salt. Am J Physiol Renal Physiol 281: F144–F150, 2001 [DOI] [PubMed] [Google Scholar]
- 47.Quintas C, Fraga S, Goncalves J, Queiroz G. The P2Y1 and P2Y12 receptors mediate autoinhibition of transmitter release in sympathetic innervated tissues. Neurochem Int 55: 505–513, 2009 [DOI] [PubMed] [Google Scholar]
- 48.Roson MI, Cao G, Silvana DP, Gorzalczany S, Pandolfo M, Toblli JE, Fernandez BE. Angiotensin II increases intrarenal transforming growth factor-β1 in rats submitted to sodium overload independently of blood pressure. Hypertens Res 31: 707–715, 2008 [DOI] [PubMed] [Google Scholar]
- 49.Savi P, Nurden AT, Nurden S, Levy-Toledano JM, Herbert P. Clopidogrel: a review of its mechanism of action. Platelets 9: 251–255, 1998 [DOI] [PubMed] [Google Scholar]
- 50.Savoia C, Schiffrin EL. Vascular inflammation in hypertension and diabetes: molecular mechanisms and therapeutic interventions. Clin Sci 112: 375–384, 2007 [DOI] [PubMed] [Google Scholar]
- 51.Schiffrin EL. T lymphocytes: a role in hypertension? Curr Opin Nephrol Hypertens 19: 181–186, 2010 [DOI] [PubMed] [Google Scholar]
- 52.Schnermann J, Levine DZ. Paracrine factors in tubuloglomerular feedback: adenosine, ATP and nitric oxide. Ann Rev Physiol 65: 501–529, 2003 [DOI] [PubMed] [Google Scholar]
- 53.Sharma K, Cook A, Smith M, Valancius C, Inscho EW. TGF-β impairs renal autoregulation via generation of ROS. Am J Physiol Renal Physiol 288: F1069–F1077, 2005 [DOI] [PubMed] [Google Scholar]
- 54.Storey RF. Biology and pharamcology of the platelet P2Y12 receptors. Curr Pharm Des 12: 1255–1259, 2006 [DOI] [PubMed] [Google Scholar]
- 55.Storey RF. New P2Y12 inhibitors. Heart 97: 1262–1267, 2011 [DOI] [PubMed] [Google Scholar]
- 56.Sun D, Samuelson LC, Yang T, Huang Y, Paliege A, Saunders T, Briggs J, Schnermann J. Mediation of tubuloglomerular feedback by adenosine: evidence from mice lacking adenosine A1 receptors. Proc Natl Acad Sci USA 98: 9983–9988, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tu X, Chen X, Xie Y, Shi S, Wang J, Chen Y, Li J. Anti-inflammatory renoprotective effect of clopidogrel and irbesartan in chronic renal injury. J Am Soc Nephrol 19: 77–83, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ueno M, Kodali M, Tello-Monoliu A, Angiolillo DJ. Role of platelets and antiplatelet therapy in cardiovascular disease. J Atheroscler Thromb 18: 431–442, 2011 [DOI] [PubMed] [Google Scholar]
- 59.Wang L, Jacobson SEW, Bengtsson A, Erlinge D. P2 receptor mRNA expression profiles in human lymphocytes, monocytes and CD34+ stem and progenitor cells. BMC Immunol 5: 16, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wihlborg AK, Wang L, Braun OO, Eyjolfsson A, Gustafsson R, Gudbjartsson T, Erlinge D. ADP receptor P2Y12 is expressed in vascular smooth muscle cells and stimulates contraction in human blood vessels. Arterioscler Thromb Vasc Biol 24: 1810–1815, 2004 [DOI] [PubMed] [Google Scholar]
- 61.Wilcox CS, Welch WJ, Snellen H. Thromboxane mediates renal hemodynamic response to infused angiotensin II. Kidney Int 40: 1090–1097, 1991 [DOI] [PubMed] [Google Scholar]