Abstract
Restenosis is an adverse outcome of angioplasty, characterized by vascular smooth muscle cell (VSMC) hyperplasia. However, therapies targeting VSMC proliferation delay reendothelialization, increasing the risk of thrombosis. Resveratrol (RESV) inhibits restenosis and promotes reendothelialization after arterial injury, but in vitro studies assessing RESV-mediated effects on endothelial cell growth contradict these findings. We thus hypothesized that fluid shear stress, mimicking physiological blood flow, would recapitulate RESV-dependent endothelial cell wound healing. Since RESV is an estrogen receptor (ER) agonist, we tested whether RESV promotes reendothelialization through an ER-α-dependent mechanism. Mice fed a high-fat diet or a diet supplemented with RESV were subjected to carotid artery injury. At 7 days after injury, RESV significantly accelerated reendothelialization compared with vehicle. In vitro wound healing assays demonstrated that RESV exhibits cell-type selectivity, inhibiting VSMC, but not endothelial cell growth. Under laminar shear stress (LSS), RESV dramatically enhanced endothelial cell wound healing and increased both the activation of extracellular signal-regulated kinase (ERK) and endothelial cell proliferation. Under LSS, small interfering RNA against ER-α, but not endothelial nitric oxide synthase, abolished RESV-induced ERK activation, endothelial cell proliferation, and wound healing. Thus these studies suggest that the EC phenotype induced by LSS better models the prohealing effects of RESV and that RESV and LSS interact to promote an ER-α-dependent mitogenic effect in endothelial cells.
Keywords: endothelial, wound healing, resveratrol, sheer stress, estrogen receptor
balloon angioplasty and stenting are the most common methods of revascularization for coronary artery disease. However, as much as 30% of patients receiving a bare metal stent exhibit in-stent restenosis (37), resulting in decreased blood flow through the stented vessel. Restenosis is primarily due to an exaggerated proliferation and migration of vascular smooth muscle cells (VSMC) following stenting injury. Thus pharmacological targeting of proliferation and migration have been leading approaches for maintaining vessel patency. Drug-eluting stents (DES) provide local delivery of antimitogenic drugs, such as paclitaxel (Taxol) and sirolimus, directly to the vessel wall. These devices have impressively reduced the incidence of restenosis compared with bare metal stents (29), but they are also associated with an increased risk of in-stent thrombosis compared with their bare metal counterparts. Current studies suggest that the potent antimitogenic drugs used in DES may impair vessel healing, leading to delayed reendothelialization, the single best predictor of clinical in-stent thrombosis (41). Therefore, the most effective approach for restoring blood flow through diseased arteries will be therapy that selectively interferes with restenosis, without delaying reendothelialization, thus mitigating thrombotic risk.
Studies on the vascular protective effects of estrogen have revealed paradoxical effects in VSMC vs. endothelial cells (EC). In vitro and in vivo experiments have shown that estrogen stimulates EC growth (20, 28), whereas it inhibits VSMC migration and proliferation (2, 19, 29). However, a complication of the utilization of estradiol itself might be its side effect profile, disruption of the menstrual cycle in women, potentially feminizing effects in men, etc.
Due to the differential effects of estradiol on VSMC and EC growth, we chose to study the vascular cell-type selective activity of the phytoestrogen resveratrol (RESV). Phytoestrogens typically have similar receptor activation profiles of estradiol in tissues such as the vasculature, presumably without some of the unwanted effects, mainly due to their reduced receptor binding affinities. RESV is a potent red wine polyphenol that elicits broad-spectrum cardioprotective effects (44). With respect to restenosis, we and others have shown that oral dosing with RESV blocks vessel stenosis in the rabbit iliac artery (47) and in the mouse carotid injury model, respectively (20). Moreoever, our prior studies showed that RESV inhibits carotid stenosis (19), as well as VSMC proliferation in vitro (7), through an estrogen receptor (ER)-α-dependent increase in nitric oxide (NO) production. In addition, others have reported that RESV blocks VSMC proliferation by inhibiting nuclear factor-κB activation (22). In addition to targeting VSMC proliferation, RESV has been shown to promote EC function (33, 42) and accelerate reendothelialization in a rat model of arterial angioplasty (12). However, the mechanism responsible for a RESV-mediated increase in EC growth remains unclear. This uncertainty has likely been perpetuated by conflicting reports between in vitro and in vivo studies assessing the RESV-mediated effects on EC growth. RESV is commonly presented as an inhibitor of EC wound healing using in vitro methods (16, 24), which is in contrast to reports indicating that RESV accelerates reendothelialization in animal models of arterial injury (12). We postulate that the EC phenotype induced by fluid shear stress, a variable that is missing in static EC culture, will better model the prohealing effects of RESV. Laminar blood flow (high shear stress) typically occurs in linear vascular geometry, whereas disturbed flow (low shear stress) occurs in arches and branches (5). While atherosclerotic lesions requiring angioplasty typically occur in areas of disturbed flow (6), it is the resident EC within the adjacent laminar regions that are primarily responsible for endothelial recovery following stenting injury (8). To increase the face validity of our in vitro wound assay, EC were exposed to laminar shear stress (LSS) using a parallel plate flow chamber to mimic phenotypic changes that occur due to blood flow under physiologic conditions (31).
METHODS
Materials.
RESV (trans-3,4,5-trihydroxystilbene) was obtained from Enzo Life Sciences. Taxol was obtained from EMD Chemicals. Stock solutions were prepared in DMSO and were diluted 1:2,000 in cell culture media. All other reagents were of the highest purity available.
Mice.
Female B6.126 mice, 6–8 wk of age, were obtained from Taconic (Hudson, NY). The mice were acclimated for 1 wk after arrival and were maintained on a 12-h light-dark cycle. Food and water were supplied ad libitum. The mice were provided either a high-fat, “Western” diet (TD88137; Harlan Teklad), or the Western diet powdered together with RESV (500 mg/kg diet). Based on food consumption, the final dose of RESV was estimated at 50 mg/kg body wt. In prior studies, we found that this dose inhibited neointimal hyperplasia 14 days after carotid artery injury (19). Additionally, based on prior reports, this dose of RESV should achieve peak plasma concentrations in the range of 5 μM (1). All animal protocols were approved by the Animal Care and Use Committee at the Louisiana State University Health Sciences Center, Shreveport, in accordance with the policies and guidelines set forth by the Office of Laboratory Animal Welfare at the National Institutes of Health.
Carotid injury model.
On day 14, the mice were anesthetized using pentobarbital (50 mg/kg ip) and were subjected to a mechanically induced carotid artery denudation. Depth of anesthesia was verified by lack of response to toe pinch. The surgical procedure used a modified guidewire to injure the left common carotid artery, as originally described by Lindner et al. (25), and performed as we described previously (19). After surgery, the mice were again administered the Western diet ± RESV. With the use of an overdose of pentobarbital (100 mg/kg ip), the mice were euthanized at 7 days postinjury, a sensitive time point for evaluating endothelialization (36).
Histology and morphometry.
Following euthanasia, the mice were perfused with sterile PBS at physiological pressure, and the carotid arteries were harvested and fixed in 4% buffered formalin for 48 h. The arteries were paraffin embedded and sectioned at 5-μm thickness. Reendothelialization was evaluated using immunohistochemical staining for von Willebrand factor (VWF). An average of four equally spaced cross sections over the lesion area of each animal were immunostained for VWF antibody (1:100 dilution, ab6994; Abcam), followed by biotinylated IgG-streptavidin-HRP (1:20,000) and detection using DAB (Vector Laboratories). Tissues were counterstained with hematoxylin. Percent reendothelialization was calculated by normalizing the positively stained luminal circumference by the total luminal circumference. Measures taken across the lesioned area were averaged together to obtain a within-animal mean, and then the mean measures for each animal were averaged to obtain a mean value within a given treatment group. As such, the coefficient of variation within an animal was up to 45%, but the coefficient of variation between animals within a treatment group was 12.5 and 9% for mice treated with vehicle compared with RESV, respectively.
Four equally spaced sections over the lesioned area were also stained with Verhoeff's van Gieson for the assessment of neointimal hyperplasia. Although 7 days was not an ideal time point for assessing neointimal hyperplasia (15), we measured stenosis in alternate sections to determine whether we could confirm our prior findings of RESV-mediated effects on neointimal hyperplasia. Areas encompassed by the lumen and internal elastic lamina were determined using ImageJ software (National Institutes of Health) and, as described above, were averaged across the lesioned artery. Percent stenosis was calculated using the formula: (1-lumen area/area encompassed by the internal elastic lamina) × 100. For this assessment, the resulting coefficient of variation between animals within a treatment group was 40 and 76% for mice treated with vehicle compared with RESV, respectively.
VSMC and human umbilical vein endothelial cell wound healing assay.
Human aortic VSMC (Cell Applications) were cultured using phenol-red free DMEM containing 10% FBS. Passages 1–7 were utilized for experiments. Human umbilical vein endothelial cells (HUVEC; Invitrogen) were cultured in complete phenol-red free endothelial basal medium (EBM-2; Millipore) supplemented with 2% FBS. Only passages 1–3 were used for experiments. Cells were transferred to six-well plates and were synchronized by replacing growth medium with medium containing 0.1% FBS for 24 h (HUVEC; Ref. 46) or 72 h (VSMC; Ref. 7). Uniform wound areas were created in confluent monolayer cell cultures using a sterile 20- to 200-μl pipette tip. The remaining cellular debris was removed with PBS, and the cells were stimulated with 20 ng/ml PDGF-BB (for VSMC) or 25 ng/ml VEGF165 (for HUVEC), followed by the addition of RESV or Taxol. Wound closure was assessed at 24 h (HUVEC) or 48 h (VSMC) using phase contrast microscopy, which captured a ∼50% healing response in stimulated cells at the specified time points. ImageJ was used to calculate the width of the wound at four locations within each well. Each experiment was performed three times using triplicate wells. Wound width was normalized by dividing final wound width by the initial width at time = 0.
Human aortic endothelial cell wound healing assay.
Male human aortic endothelial cells (HAEC; Lonza; passages 8–10) were maintained on 0.2% gelatin-coated 10-cm dishes in complete phenol-red free EBM-2 supplemented with 2% FBS. On the day of the experiment, cells were plated on basement membrane extract (Matrigel; 1:50 dilution)-coated 38 × 75 mm2 glass slides (Corning) in complete EBM-2 containing 0.2% FBS. After 4 h, the cells had formed a confluent monolayer and a uniform wound area was created using a sterile 20- to 200-μl pipette tip. Starting wounds were imaged, and the slides were either placed in a cell culture incubator at 37°C (static) or a parallel plate flow chamber in complete EBM-2 supplemented with 0.2% FBS maintained at 37°C and perfused with 5% CO2. A peristaltic pump induced LSS at 12 dyn/cm2 for 18 h, serving as the stimulus in these experiments, as previously described (31). Wound healing was quantified by dividing final wound width by the initial width at time = 0 and averaging measurements over five fields of view per wound.
Immunocytochemistry and cell alignment.
Fluorescent staining for microtubule organizing center (MTOC) was used to assess HAEC polarity under static or LSS conditions. As the nucleus is reoriented during cell migration, MTOC moves toward the direction of migration, establishing directional polarity (10). HAEC were fixed with PBS containing 2% formaldehyde and were permeabilized with 0.1% Triton X-100. Fixed cells were incubated with PBS containing 10% goat serum to reduce nonspecific antibody binding and were incubated with primary antibodies against Ki67 or pericentrin of the MTOC. Cells were then rinsed in Tris-buffered saline containing 0.1% Tween 20 (TBST) and were incubated for 2 h with Alexa488-conjugated goat anti-rabbit IgG (Invitrogen). Cells were rinsed in TBST, were stained with 4,6-diamidino-2-phenylindole (DAPI), and were mounted on slides with Fluoromount-G (SouthernBiotech). The cells were imaged on a Photometrics Coolsnap120 ES2 camera interfaced with a Nikon Eclipse Ti inverted fluorescent microscope. Images were analyzed using NIS Elements BR 3.00, SP5 imaging software.
NIS Elements software was next used to quantify cell alignment to flow vs. the wound edge. Cells were considered aligned when the angle between the MTOC and flow (or the wound) was <60° off axis. Random positioning results in 33% of the cells presenting the MTOC within the aligned region. At 18 h after treatment, MTOC staining was evaluated in an uninjured control region, as well as on the upstream and downstream boundaries of the wound. Upstream and downstream boundaries were established as within 400 μm of the wound edge. Over 100 cells were evaluated in each region per experiment.
Cell proliferation.
Staining for Ki67 was used to assess HAEC proliferation. 18 h after treatment, Ki67 staining was evaluated in an uninjured control region, as well as on the upstream and downstream boundaries of the wound. Over 100 cells were evaluated in each region. Proliferating cells were assessed by normalizing the Ki67-positive nuclei by the total number of nuclei.
Nuclear pathology.
DAPI nuclear stains were scored for condensed (pyknotic) and fragmented (karyorrhexic) nuclei. Total nuclei were determined using NIS Elements software, and atypical nuclei were expressed as a percentage of the total. Over 100 nuclei were evaluated in each region per experiment.
Transfection.
Transient transfection of small interfering (si)RNA targeting ER-α or endothelial nitric oxide synthase (eNOS; Dharmacon Smartpool ON-TARGETPLUS, cat. nos. I-003401-00 and L-006490-00, respectively), compared with scrambled siRNA (AllStars Negative Control siRNA; Qiagen), was performed using Lipofectamine 2000 (Invitrogen), per the manufacturer's instructions. Briefly, HAEC were transfected with 10 nM of siRNA and were left to recover. Twenty-four hours later, the transfection was repeated to ensure acceptable knockdown. LSS was applied 24 h after the second transfection.
Western blotting.
Lysates were run on Novex 4–20% Tris·HCl gradient gels and were transferred to PVDF membranes. The membranes were blocked and were incubated with primary antibody overnight at 4°C, followed by secondary for 1 h. The ECL Plus system (Amersham) and X-ray film were used for chemiluminescent detection. Densitometry was carried out using ImageJ software, and bands were normalized to GAPDH.
Statistics.
Data are expressed as means ± SD (Fig. 1) or SE (all others). Statistical tests were performed using GraphPad Prism, version 6. A Student's t-test was used to evaluate luminal stenosis and reendothelialization. A one-way ANOVA followed by Tukey's post hoc test was used to assess ER-α expression and the effect of treatment on VSMC and HUVEC wound healing. A two-way ANOVA followed by Student Newman-Keuls post hoc test, conducted as described by Glantz (9), was used to evaluate all other data. P < 0.05 was accepted as the threshold for statistical significance.
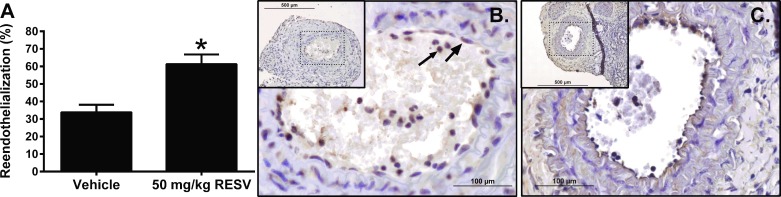
Fig. 1.
Resveratrol (RESV) accelerates carotid artery reendothelialization. A: reendothelialization quantified as the percent of the lumen exhibiting positive staining for von Willebrand factor (VWF). Representative ×40 images VWF staining in mice receiving Western diet (B) or Western diet containing RESV (C, inset: ×10). Large arrow: indicates VWF staining. Small arrow indicates nonspecific VWF staining that was excluded from the analysis. Data are means ± SD. *P < 0.05, compared with vehicle-treated mice; n = 3.
RESULTS
RESV accelerates arterial reendothelialization.
Mice receiving RESV showed a nearly twofold increase in reendothelialization compared with mice on the Western diet alone (33.8 vs. 61.3%; P < 0.05; Fig. 1). Additionally, a Student's t-test revealed a RESV-mediated trend toward a decrease in luminal stenosis (39.4 ± 9.2 vs. 12.1 ± 5.3%; P = 0.06; not shown). We have previously shown that a robust inhibition in mice is observed at 14 days postinjury; moreover, in these earlier studies, RESV inhibited neointimal hyperplasia in wild-type but not ER-α-deficient animals (19). Thus our findings here are consistent with our prior observations.
Taxol and RESV exert opposite selectivity in VSMC and EC.
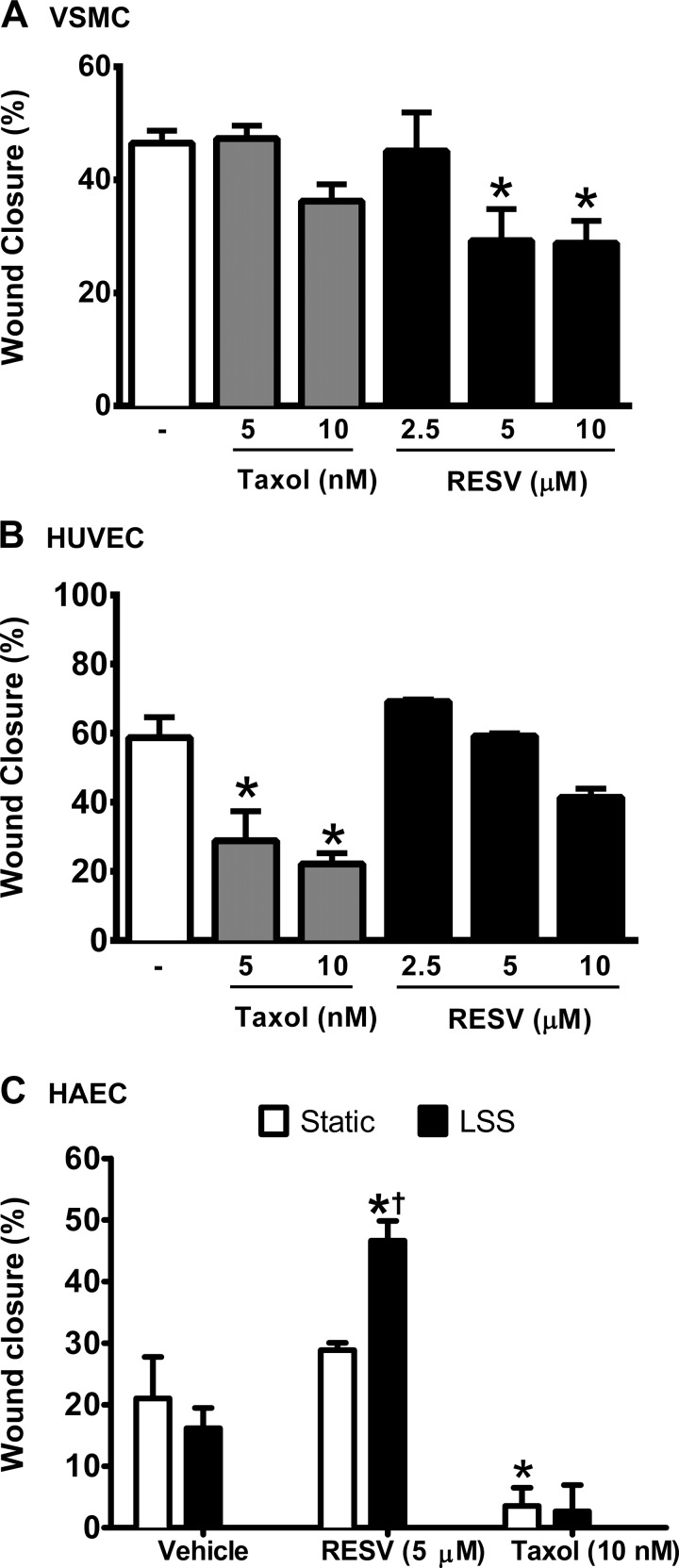
We next sought to model these effects using in vitro wound healing assays. At 48 h after incubation, Taxol (up to 10 nM) did not significantly inhibit wound closure in PDGF-stimulated VSMC (Fig. 2A). On the other hand, RESV at 5 and 10 μM reduced VSMC wound closure by 37.2 and 38.1%, respectively (P < 0.05). VEGF165-stimulated HUVEC were sensitive to Taxol, with 5 nM and 10 nM causing a significant reduction in wound healing (Fig. 2B). RESV did not significantly affect wound closure in HUVEC.
Fig. 2.
Taxol and RESV exert opposite selectivity in the vascular smooth muscle cell (VSMC) and endothelial cell (EC) wound healing assay. Wound closure was quantified in VSMC (A) compared with HUVEC (B), expressed as a percentage of the initial wound width that has healed. C: RESV and laminar sheer stress (LSS) interact to increase human aortic endothelial cell (HAEC) wound closure, quantified as the distance between the wound edges at 5 locations before and after injury. Data are means ± SE. *P < 0.05, compared with the appropriate vehicle control. †P < 0.05, compared with RESV-treated cells under static culture; n = 3–4.
RESV accelerates wound closure under LSS.
Due to our negative findings for RESV-mediated effects on wound healing in HUVEC, despite our findings of increased levels of reendothelialization in mice, we next set out to determine whether RESV promotes endothelial wound healing in a more relevant cell type and in cells exposed to physiologically relevant conditions. EC in stented arteries are exposed to a number of environmental cues to direct wound healing, including shear stress. To replicate the shear force that EC are exposed to under flow within the vessel wall, LSS (12 dyn/cm2) was applied to HAEC in a parallel plate flow apparatus for 18 h. Results showed that LSS alone did not alter wound closure compared with HAEC under static conditions (Fig. 2C). Compared with vehicle, RESV accelerated wound closure under LSS (16.2 ± 3.3 vs. 46.6 ± 3.2%, respectively; P < 0.05). RESV treatment under LSS also enhanced wound closure compared with static HAEC treated with RESV. Consistent with data from HUVEC, there was no effect of RESV under static conditions, and 10 nM Taxol significantly attenuated HAEC wound healing compared with vehicle treated cells. A two-way ANOVA revealed a significant main effect of drug treatment and a significant interaction between treatment and shear stress (P < 0.05). Given that arterial levels of Taxol within 30 days of stenting are reportedly in the range of 3.6 to 10 nM, the concentration of Taxol used in this study is representative of arterial levels after placement of a Taxus stent (27).
RESV allows, while Taxol blocks, EC alignment to LSS.
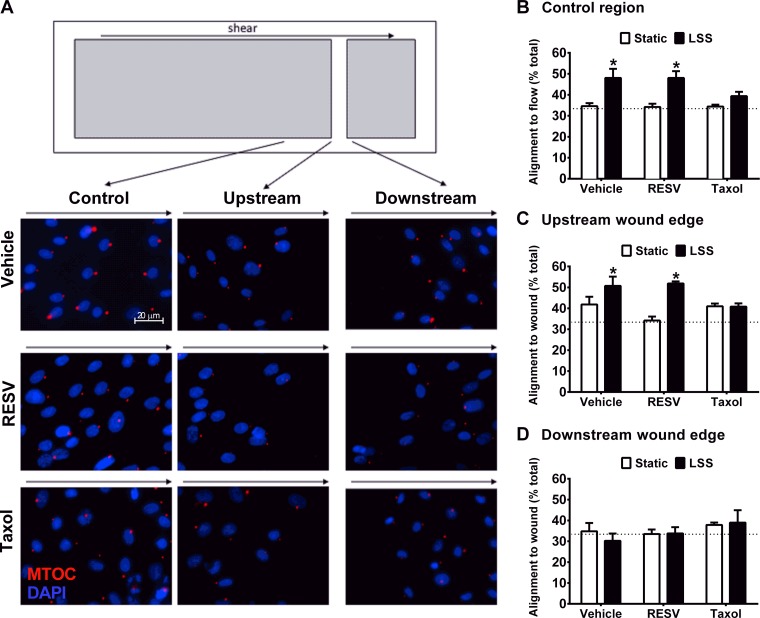
Both shear stress and monolayer wounding affect polarized EC migration. To determine if RESV treatment promoted wound healing by altering EC polarity, fluorescent imaging of MTOC polarity was assessed 18 h after wounding the EC monolayer (Fig. 3A). Polarity was assessed in three regions: the upstream wound edge, the downstream wound edge, and an uninjured portion of the monolayer. Within the uninjured control region, LSS induced cell alignment to flow in the presence of vehicle or RESV (Fig. 3B). However, Taxol significantly blocked LSS-induced EC alignment to fluid flow. Similarly, endothelial alignment to shear stress and into the wound in the upstream wound edge was enhanced by LSS in the vehicle and RESV treated groups but not with Taxol. On the downstream wound boundary, EC did not show significant alignment either into the wound or in the direction of flow in any of the experimental groups (Fig. 3D).
Fig. 3.
Taxol inhibits HAEC alignment under LSS. A: schematic of a glass slide, highlighting the uninjured region, upstream wound boundary, and downstream wound boundary. Representative images from each region after 18-h LSS and RESV. Nuclei were identified using DAPI staining (blue) and microtubule organizing center (MTOC; red) was used to determine polarity. B: control region was used to quantify cell alignment to flow. Alignment into the wound was assessed with respect to the upstream (C) and downstream (D) boundaries. Cells were considered aligned when the angle to flow (or wound) was <60° off axis. Random positioning results in 33% of the cells presenting the MTOC showing alignment to the target. *P < 0.05, compared with the matched static condition; n = 3–6.
RESV promotes EC proliferation under LSS.
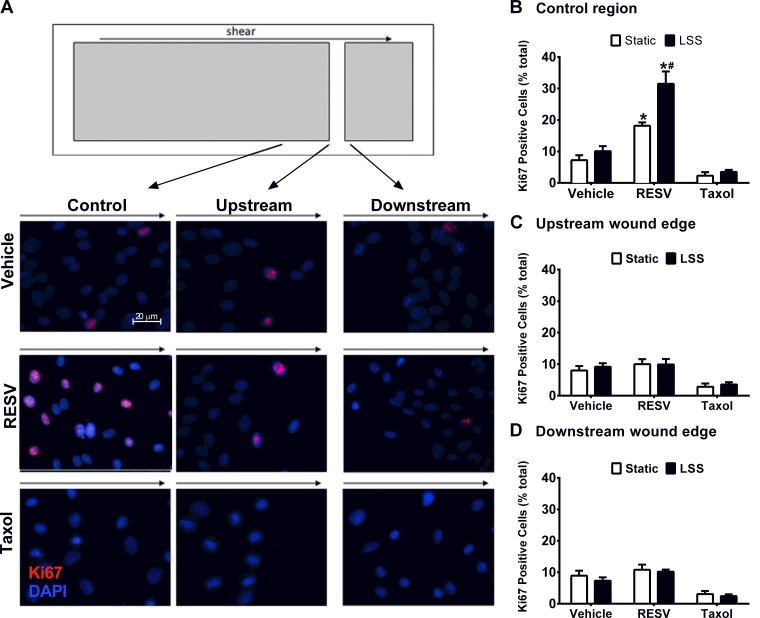
Fluorescent imaging for Ki67 was used to assess HAEC proliferation in the regions of interest (Fig. 4A). Within the uninjured control region, RESV enhanced cell proliferation in static cultures by 1.5-fold compared with vehicle treated cells (Fig. 4B). In addition, RESV treatment promoted even higher numbers of Ki67-positive proliferating cells under LSS compared with RESV-exposed static cultures (73% increase; P < 0.05) and increased proliferation compared with vehicle treated cells exposed to LSS (P < 0.05). Compared with vehicle, Taxol did not alter cell proliferation rates under either static or LSS conditions. Two-way ANOVA revealed a significant interaction between drug treatment and shear stress, which were both found to be significant main effects. Quantification of Ki67 staining immediately adjacent to the wound edge revealed no effect of treatment or shear stress (Fig. 4, C and D).
Fig. 4.
RESV promotes HAEC proliferation. A: schematic of a glass slide, highlighting the uninjured region, upstream wound boundary, and downstream wound boundary. Representative images from each region after 18-h LSS and RESV. Nuclei were identified using DAPI staining (blue) and Ki67 (red) was used to determine cell proliferation. B: control region was used to quantify cell proliferation. Ki67 staining was assessed with respect to both the upstream (C) and downstream (D) boundaries. *P < 0.05, compared with the appropriate vehicle control. #P < 0.05, compared with RESV-treated cells under static conditions; n = 3–8.
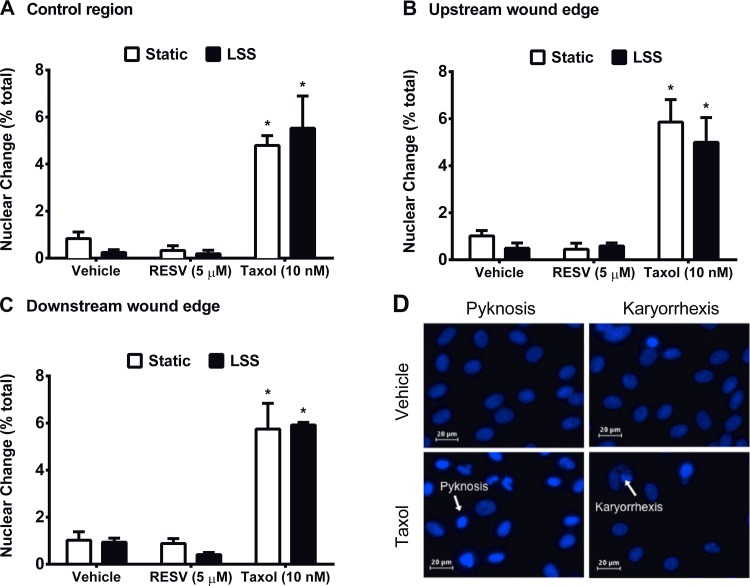
Taxol induces nuclear pathology indicative of cell death.
In addition to hindering EC proliferation and polarity, Taxol significantly increased the percentage of HAEC with pyknotic or karyorrhexic nuclei under both static and LSS compared with vehicle-treated cells (Fig. 5). These results are consistent with an ability of Taxol to promote HAEC apoptosis or necrosis. With respect to nuclear pathology, results from a two-way ANOVA revealed a significant main effect of drug treatment (P < 0.05); but no effect of shear stress. Quantification of nuclear pathology at the upstream and downstream wound boundaries showed similar results.
Fig. 5.
Taxol induces nuclear pathology consistent with EC death. HAEC were stained with DAPI 18 h after treatment with vehicle, RESV, or Taxol under static or LSS conditions. Quantification of condensed (pyknotic) and fragmented (karyorrhexic) nuclei expressed as a percent of the total cells within the control region (A), upstream wound edge (B), and downstream wound edge (C). D: representative images from vehicle treated (top) and Taxol treated (bottom) cells exposed to static or LSS conditions. Statistical analysis was performed using two-way ANOVA followed by Student-Newman-Keuls post hoc test. *P < 0.05, significant difference compared with the appropriate vehicle control. Data are means ± SE; n = 3–6.
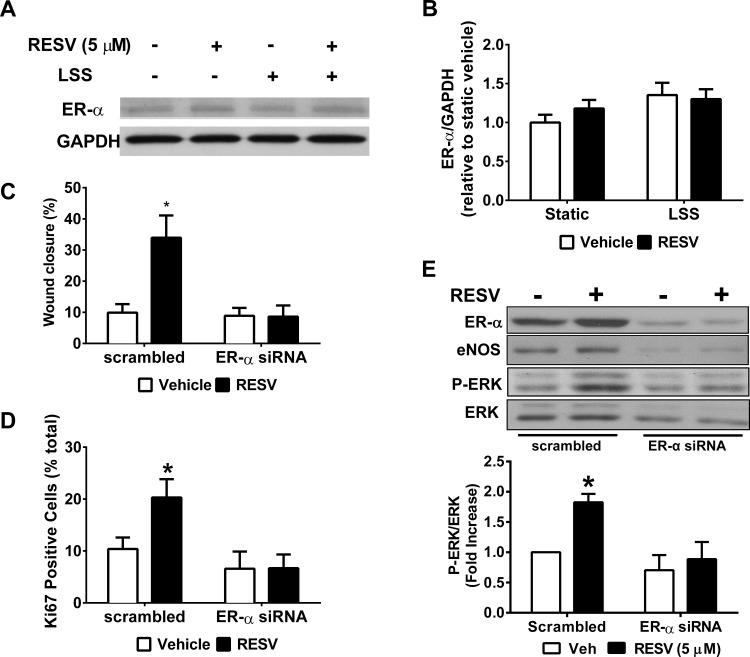
RESV-mediated acceleration in wound healing under LSS depends on ER-α.
Western blot was used to confirm ER-α expression in HAEC under static or LSS culture (Fig. 6, A and B). At 18 h, there was no significant effect of RESV or LSS on the expression of ER-α, although in some experiments, RESV exhibited a trend toward an increased level. siRNA knockdown of ER-α (70.1% reduction; Fig. 6C) was used to determine the role of ER-α in mediating the prohealing effect of RESV under LSS. In cells transfected with scrambled siRNA, RESV maintained its ability to promote wound closure compared with vehicle (Fig. 6C; P < 0.05). However, ER-α siRNA abolished the RESV-mediated increase in wound healing and Ki67 expression (Fig. 6), indicating that EC proliferation is a critical component in the RESV-dependent prohealing response. Moreover, in cells under LSS (Fig. 6E), RESV increased ERK activation, expressed as the ratio of phospho-ERK (P-ERK)-to-ERK, an effect that was blocked by ER-α siRNA.
Fig. 6.
RESV-mediated wound healing in HAEC under LSS is dependent on signaling though estrogen receptor (ER)-α. Representative Western blot (A) and its quantitation (B) showing expression of ER-α 18 h after treatment with 5 μM RESV and/or LSS. C: quantitation of HAEC wound healing under LSS in cells transfected with scrambled or small interfering (si)RNA against ER-α. D: nuclei were identified using DAPI staining and Ki67 was used to determine cell proliferation within the control region. E: representative Western blot showing levels of ER-α, endothelial nitric oxide synthase (eNOS), phospho-ERK (P-ERK), and ERK proteins in HAEC under LSS and transfected with scrambled or siRNA against ER-α. P-ERK levels were quantified as a fold increase in P-ERK/ERK compared with vehicle-treated cells transfected with scrambled siRNA. *P < 0.05, compared with the appropriate vehicle control; n = 3.
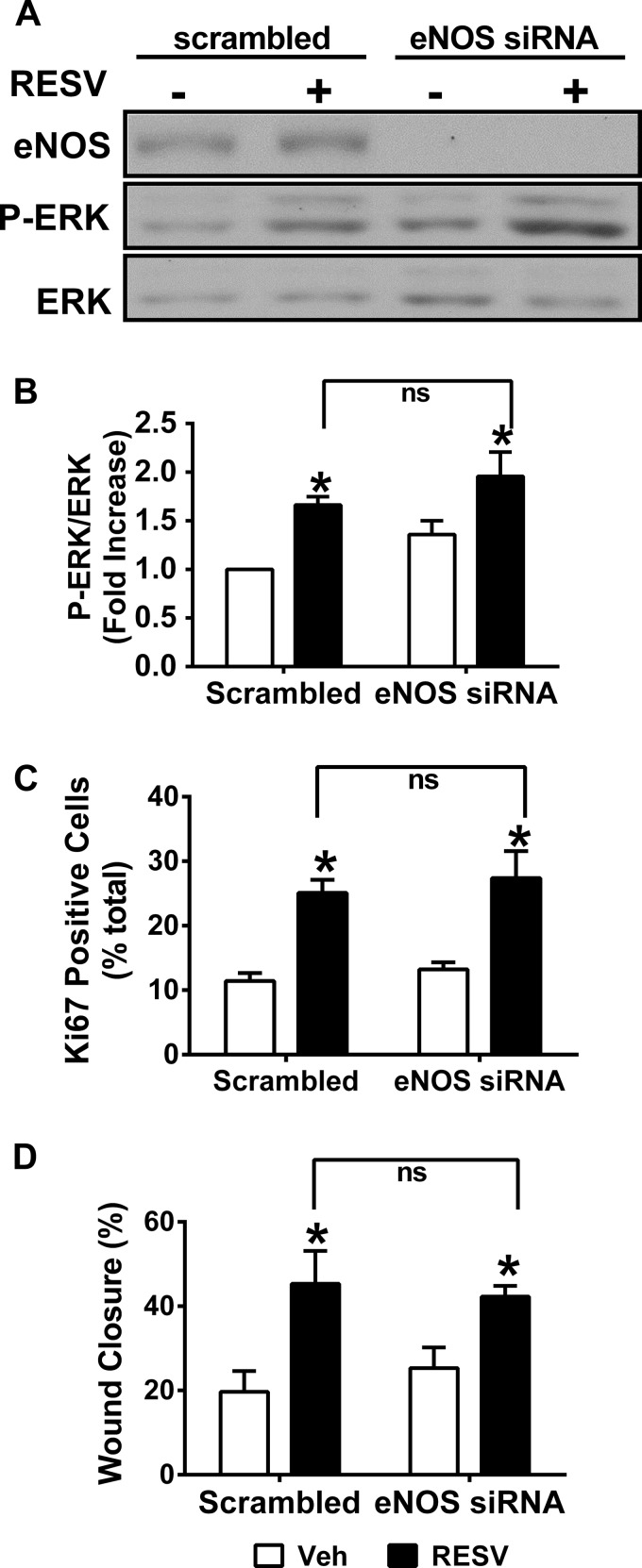
Finally, although neither LSS nor RESV treatment affected eNOS expression in HAEC, siRNA against ER-α reduced eNOS protein levels by ∼70% (Fig. 6E). Therefore, to test for a link between ER-α and eNOS in RESV-induced acceleration of EC proliferation under LSS, we utilized siRNA against eNOS. Although RESV significantly increased EC proliferation (i.e., Ki67-positive staining), wound closure, and ERK activation, eNOS siRNA did not alter any of these parameters (Fig. 7).
Fig. 7.
RESV-induced wound healing in HAEC under LSS is not dependent on signaling though eNOS. A: representative Western blot showing levels of eNOS, P-ERK, and ERK in HAEC transfected with scrambled or eNOS siRNA 18 h after treatment with LSS and 5 μM RESV. B: P-ERK was quantified as a fold increase in P-ERK/ERK compared with vehicle-treated cells transfected with scrambled siRNA. C: nuclei were identified using DAPI staining and Ki67 was used to assess cell proliferation within the control region. Data were quantified as a percentage of cells positive for Ki67. D: quantitation of HAEC wound healing under LSS in cells transfected with scrambled or eNOS siRNA and treated with RESV. *P < 0.05, compared with the appropriate vehicle control; n = 3.
DISCUSSION
Epidemiological studies suggest that moderate red wine consumption is associated with significant cardioprotection (38). Among the components of red wine, RESV has been most strongly implicated in its vascular-protective effects (35). Consistent with prior reports, RESV was found to significantly accelerate reendothelialization 7 days after mechanical denudation (12). To examine the mechanisms responsible for the RESV-mediated increase in reendothelialization, we used an in vitro wound healing paradigm. Using this model we found that RESV more potently inhibits wound healing in VSMC than in HUVEC (Fig. 2). Our finding that Taxol more potently interfered with HUVEC wound healing compared with VSMC agrees with prior reports showing that Taxol impairs EC proliferation and migration to a greater extent compared with inhibitory effects on VSMC (43). It has been demonstrated here and in prior studies that RESV promotes reendothelialization in vivo (12). However, the current study shows that RESV did not promote wound healing under static culture in either HAEC or HUVEC and may even reduce wound healing in HUVEC. However, under physiological conditions, EC are constantly exposed to shear stress. Our finding that RESV significantly promoted wound closure under LSS suggests that EC exposed to fluid flow are more sensitive to the mitogenic effects of RESV.
It should be noted here that a caveat of our in vivo study was that we did not stagger our animal studies such that each animal commenced and ended the study at precisely the same point in the female animals' estrous cycles. However, given that the mice were exposed to RESV for 21 days, each should have experienced approximately four cycles. Moreover, our endpoint was reendothelialization, assessed as a positive staining for VWF, i.e., a quantal measure of staining or no staining, rather than a degree of expression of a protein. Thus our findings are likely valid, albeit plagued with a bit more variability.
We assessed MTOC polarization under static and LSS conditions to evaluate the contribution of endothelial migration to the wound healing response. These results suggest that the RESV-mediated acceleration in wound closure is not due to effects on endothelial migration polarity. Certainly, time-lapse video would likely have provided a more conclusive readout for other migration parameters (distance migrated, migration speed, and persistence), but such an approach is beyond our current technical capabilities.
On the other hand, Taxol, a known microtubule-stabilizing agent, inhibits cell migration and proliferation by preventing microtubule disassembly. Because microtubule dynamics play a critical role in cell motility, the inhibitory effects of Taxol on cell polarity likely contribute to delayed endothelial wound healing (39). Interestingly, we found that shear stress induces EC migration polarity specifically in the upstream side of the wound. These data are consistent with prior reports indicating that laminar flow stimulates EC migration into the wound primarily from the upstream side (14).
Ki67 staining was used to evaluate the contribution of proliferation to endothelial wound healing. Notably, RESV only increased endothelial proliferation in uninjured cells within the control region. This observation corresponds to reports (8, 34) describing a “regenerative area” located upstream of endothelial injury, contributing to endothelial healing after denudation. Heterogeneity in cell proliferation based on proximity to the wound edge may reflect a time-dependent healing process involving both migration and proliferation. In vivo studies have shown that endothelial migration at the wound edge precedes proliferation (8). Thus it is likely that at 18 h the onset of proliferation has not reached cells immediately adjacent to the wound edge. However, further studies are needed to investigate the spatial and temporal changes that are implicated in the endothelial wound healing response.
Nuclear pathology showed that 10 nM Taxol induced pyknosis (nuclear condensation) and karyorrhexis (nuclear fragmentation) under both static and LSS conditions. Vacca et al. (40) observed similar morphologic changes in HUVEC treated with 5 nM Taxol for 6 days. Additionally, a 12-h exposure to 10 nM Taxol has been shown to induce apoptosis (17). Taken together, these results indicate that Taxol-induced cell death may be a contributing factor in delaying reendothelialization after placement of a DES during angioplasty (13).
Our initial interest in RESV was its ability to alter estrogen signaling pathways and its ability to stimulate the receptor in vascular tissue. RESV has structural similarity to 17-β-estradiol, resulting in binding affinity for the ER (7). However, given its lower binding affinity compared with estradiol (3), it might be expected to have fewer adverse effects. It has been well established that estradiol exerts differential modulation of vascular cell proliferation and migration via ER signaling (4, 20). ER-α and -β subtypes are both expressed in VSMC and EC, and depending on the presence of coactivators and corepressors (genomic pathway) or second messengers (nongenomic pathway), ER activation can lead to distinct cell-specific outcomes (11, 23). Studies using ER-α or ER-β null mice have shown that the protective effects of estrogen in the cardiovascular system are attributed primarily to ER-α (4). The dichotomous effects of RESV on VSMC wound healing vs. EC wound healing observed in the current study are reminiscent of the differential cell-type specific effects of ER-α activation. Several experiments have shown that estrogen stimulates EC growth (20, 28), whereas it inhibits VSMC migration and proliferation (2, 20, 30). Further, RESV binds to ER-α with an IC50 of 9 μM (7), which is similar to the concentrations of RESV used in this study.
Based on evidence showing that estradiol accelerates reendothelialization through ER-α (4), we tested whether RESV promotes EC wound healing via an ER-α-dependent pathway. As such, siRNA knockdown of ER-α in HAEC abrogated RESV-mediated acceleration of wound healing under LSS (Fig. 6, C and D), likely due to a loss in RESV-induced HAEC proliferation. Since we observed no effect of RESV on ER-α expression (Fig. 6, A and B), these findings likely reflect an ability of RESV to activate the receptor.
Our prior studies have shown that RESV dramatically reduces neointimal hyperplasia in an ER-α-dependent manner (19). Further, these effects were dependent at least in part on nitric oxide production, as cotreatment with nitro-l-arginine methyl ester attenuated the observed RESV-mediated protection. In addition, our colleagues have shown that sheer stress (LSS) activates eNOS and its concomitant NO production (31). Thus, given that efficient reendothelialization may be a factor contributing to reduced neointimal hyperplasia, and given the findings our colleagues of LSS-induced NO signaling, we hypothesized that RESV acted via an ER-α-dependent NO production. Although estradiol, through nongenomic pathways, can activate ER-α to promote both eNOS activity and ERK activation (45), our findings support that for RESV-mediated effects on EC exposed to LSS, these pathways are segregated and increased cell proliferation mainly involves an ER-dependent induction in ERK activation. With respect to the specific contribution of LSS, we have not yet identified the particular contribution of LSS in and of itself. However, we hypothesize that LSS induces some modification of signaling and that a given pathway or pathways converge(s) with signaling pathways targeted by RESV to promote a dramatic increase in EC proliferation. Although we were not able to identify the particular contribution of LSS, our studies suggest a mechanism of action for RESV and likely also suggest that cell studies better predict in vivo outcomes if and when we utilize cells in physiologically relevant conditions.
It is important to note that vascular cells in both females and males express a significant amount of ER-α protein (26). The fact that siRNA targeting of ER-α in male HAEC blocked the wound healing effect of RESV suggests this pathway may be conserved in females due to slightly higher expression of ER-α. Collectively, our findings highlight the importance of ER-α in mediating the prohealing effects of RESV on EC under physiological LSS.
By utilizing shear stress in cell culture we were able to recapitulate the RESV-mediated acceleration of wound repair that is observed in vivo. Using this novel approach to the traditional wound healing assay may facilitate understanding of the mechanisms involved in reendothelialization after arterial injury. Considering that RESV shows cell type selectivity, inhibiting VSMC growth while promoting EC growth, these results may have clinical implications in the context of stenting angioplasty. However, while preclinical studies utilizing small animal models and, in some cases, even pigs, have failed to predict efficacy in clinical trials (32), much further study will be required to determine if local delivery of RESV can reduce the risk of late stent thrombosis associated with current-generation DES.
GRANTS
This work was supported by the National Heart, Lung, and Blood Institute Grant R01-HL-098435 (to A. W. Orr) and the Superior Toxicology Fellowship from the Louisiana Board of Regents Grant LEQSF(2008-13)-GF-20 (to A. Yurdagul, Jr.)
DISCLOSURES
J.J.K., A.R.K., and T.R.D have a patent pending that is peripherally related to this work. T.R.D. is a cofounder of ReQuisite Biomedical.
AUTHOR CONTRIBUTIONS
Author contributions: A.Y., J.K., A.R.K., A.W.O., and T.R.D. conception and design of research; A.Y., J.K., M.C.M., A.R.K., and A.S. performed experiments; A.Y., J.K., M.C.M., A.R.K., A.S., A.W.O., and T.R.D. analyzed data; A.Y., J.K., A.W.O., and T.R.D. prepared figures; A.Y., J.K., A.W.O., and T.R.D. edited and revised manuscript; A.Y., J.K., A.W.O., and T.R.D. approved final version of manuscript; J.K., A.W.O., and T.R.D. interpreted results of experiments; J.K. drafted manuscript.
ACKNOWLEDGMENTS
We thank Valeria Hebert for technical contributions.
REFERENCES
- 1.Asensi M, Medina I, Ortega A, Carretero J, Bano MC, Obrador E, Estrela JM. Inhibition of cancer growth by resveratrol is related to its low bioavailability. Free Radic Biol Med 33: 387–398, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Bhalla RC, Toth KF, Bhatty RA, Thompson LP, Sharma RV. Estrogen reduces proliferation and agonist-induced calcium increase in coronary artery smooth muscle cells. Am J Physiol Heart Circ Physiol 272: H1996–H2003, 1997 [DOI] [PubMed] [Google Scholar]
- 3.Bowers JL, Tylmenkov VV, Jernigan SC, Klinge CM. Resveratrol acts as a mixed agonist/antagonist for estrogen receptors alpha and beta. Endocrinology 141: 3657–3667, 2000 [DOI] [PubMed] [Google Scholar]
- 4.Brouchet L, Krust A, Dupont S, Chambon P, Bayard F, Arnal JF. Estradiol accelerates reendothelialization in mouse carotid artery through estrogen receptor-alpha but not estrogen receptor-beta. Circulation 103: 423–428, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Chiu JJ, Chien S. Effects of disturbed flow on vascular endothelium: pathophysiological basis and clinical perspectives. Physiol Rev 91: 327–387, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cunningham KS, Gotlieb AI. The role of shear stress in the pathogenesis of atherosclerosis. Lab Invest 85: 9–23, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Ekshyyan VP, Hebert VY, Khandelwal A, Dugas TR. Resveratrol inhibits rat aortic vascular smooth muscle cell proliferation via estrogen receptor dependent nitric oxide production. J Cardiovasc Pharmacol 50: 83–93, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Filipe C, Lam Shang Leen L, Brouchet L, Billon A, Benouaich V, Fontaine V, Gourdy P, Lenfant F, Arnal JF, Gadeau AP, Laurell H. Estradiol accelerates endothelial healing through the retrograde commitment of uninjured endothelium. Am J Physiol Heart Circ Physiol 294: H2822–H2830, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Glantz SA. Primer of Applied Regression and Analysis of Variance. New York: McGraw-Hill, 1990 [Google Scholar]
- 10.Gotlieb AI, May LM, Subrahmanyan L, Kalnins VI. Distribution of microtubule organizing centers in migrating sheets of endothelial cells. J Cell Biol 91: 589–594, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grandas OH, Mountain DH, Kirkpatrick SS, Cassada DC, Stevens SL, Freeman MB, Goldman MH. Regulation of vascular smooth muscle cell expression and function of matrix metalloproteinases is mediated by estrogen and progesterone exposure. J Vasc Surg 49: 185–191, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Gu J, Wang CQ, Fan HH, Ding HY, Xie XL, Xu YM, Wang BY, Huang DJ. Effects of resveratrol on endothelial progenitor cells and their contributions to reendothelialization in intima-injured rats. J Cardiovasc Pharmacol 47: 711–721, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Hayashi S, Yamamoto A, You F, Yamashita K, Ikegame Y, Tawada M, Yoshimori T, Shimizu S, Nakashima S. The stent-eluting drugs sirolimus and paclitaxel suppress healing of the endothelium by induction of autophagy. Am J Pathol 175: 2226–2234, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsu PP, Li S, Li YS, Usami S, Ratcliffe A, Wang X, Chien S. Effects of flow patterns on endothelial cell migration into a zone of mechanical denudation. Biochem Biophys Res Commun 285: 751–759, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Hui DY. Intimal hyperplasia in murine models. Curr Drug Targets 9: 251–260, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.In K, Park J, Park H. Resveratrol at high doses acts as an apoptotic inducer in endothelial cells. Cancer Res Treat 38: 6, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jordan MA, Wendell K, Gardiner S, Derry WB, Copp H, Wilson L. Mitotic block induced in HeLa cells by low concentrations of paclitaxel (Taxol) results in abnormal mitotic exit and apoptotic cell death. Cancer Res 56: 816–825, 1996 [PubMed] [Google Scholar]
- 18.Khalil RA. Estrogen, vascular estrogen receptor and hormone therapy in postmenopausal vascular disease. Biochem Pharmaol 86: 1627–1642, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khandelwal AR, Hebert VY, Dugas TR. Essential role of ER-α-dependent NO production in resveratrol-mediated inhibition of restenosis. Am J Physiol Heart Circ Physiol 299: H1451–H1458, 2010 [DOI] [PubMed] [Google Scholar]
- 20.Kolodgie FD, Jacob A, Wilson PS, Carlson GC, Farb A, Verma A, Virmani R. Estradiol attenuates directed migration of vascular smooth muscle cells in vitro. Am J Pathol 148: 969–976, 1996 [PMC free article] [PubMed] [Google Scholar]
- 21.Krasinski K, Spyridopoulos I, Asahara T, van der Zee R, Isner JM, Losordo DW. Estradiol accelerates functional endothelial recovery after arterial injury. Circulation 95: 1768–1772, 1997 [DOI] [PubMed] [Google Scholar]
- 22.Lee B, Moon SK. Resveratrol inhibits TNF-alpha-induced proliferation and matrix metalloproteinase expression in human vascular smooth muscle cells. J Nutr 135: 2767–2773, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Li G, Chen YF, Greene GL, Oparil S, Thompson JA. Estrogen inhibits vascular smooth muscle cell-dependent adventitial fibroblast migration in vitro. Circulation 100: 1639–1645, 1999 [DOI] [PubMed] [Google Scholar]
- 24.Lin MT, Yen ML, Lin CY, Kuo ML. Inhibition of vascular endothelial growth factor-induced angiogenesis by resveratrol through interruption of Src-dependent vascular endothelial cadherin tyrosine phosphorylation. Mol Pharmacol 64: 1029–1036, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Lindner V, Fingerle J, Reidy MA. Mouse model of arterial injury. Circ Res 73: 792–796, 1993 [DOI] [PubMed] [Google Scholar]
- 26.Ma Y, Qiao X, Falone AE, Reslan OM, Sheppard SJ, Khalil RA. Gender-specific reduction in contraction is associated with increased estrogen receptor expression in single vascular smooth muscle cells of female rat. Cell Physiol Biochem 26: 457–470, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller KM. A Drug-Eluting Stent Case Study: Taxus Express 2–from Development to Approval. New England Chapter Parenteral Drug Association Workshop (Online). http://www.pda.org/Chapters/North-America-cont/New-England/Presentations/Development-and-Commercialization-of-Combination-Products-A-Drug-Eluting-Stent-Case-Study-TAXUS-E.aspx [2004].
- 28.Morales DE, McGowan KA, Grant DS, Maheshwari S, Bhartiya D, Cid MC, Kleinman HK, Schnaper HW. Estrogen promotes angiogenic activity in human umbilical vein endothelial cells in vitro and in a murine model. Circulation 91: 755–763, 1995 [DOI] [PubMed] [Google Scholar]
- 29.Morice MC, Serruys PW, Sousa JE, Fajadet J, Ban Hayashi E, Perin M, Colombo A, Schuler G, Barragan P, Guagliumi G, Molnàr F, Falotico R; RAVEL Study Group A randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization. N Engl J Med 346: 1773–1780, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Nakamura Y, Suzuki T, Miki Y, Tazawa C, Senzaki K, Moriya T, Saito H, Ishibashi T, Takahashi S, Yamada S, Sasano H. Estrogen receptors in atherosclerotic human aorta: inhibition of human vascular smooth muscle cell proliferation by estrogens. Mol Cell Endocrinol 219: 17–26, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Yurdagul A, Jr, Chen J, Funk SD, Albert P, Kevil CG, Orr AW. Altered nitric oxide production mediates matrix-specific PAK2 and NF-κB activation by flow. Mol Biol Cell 24: 398–408, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pires NM, Jukema JW, Daemen MJ, Quax PH. Drug-eluting stents studies in mice: do we need atherosclerosis to study restenosis? Vascul Pharmacol 44: 257–264, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Rush JW, Quadrilatero J, Levy AS, Ford RJ. Chronic resveratrol enhances endothelium-dependent relaxation but does not alter eNOS levels in aorta of spontaneously hypertensive rats. Exp Biol Med (Maywood) 232: 814–822, 2007 [PubMed] [Google Scholar]
- 34.Schwartz SM, Haudenschild CC, Eddy Endothelial regeneration EM. I Quantitative analysis of initial stages of endothelial regeneration in rat aortic intima. Lab Invest 38: 568–580, 1978 [PubMed] [Google Scholar]
- 35.Siemann EH, Creasy LL. Concentration of the phytoalexin resveratrol in wine. Am J Enol Vitic 43: 4, 1992 [Google Scholar]
- 36.Tan H, Jiang X, Yang F, Li Z, Liao D, Trial J, Magera MJ, Durante W, Yang X, Wang H. Hyperhomocysteinemia inhibits post-injury reendothelialization in mice. Cardiovasc Res 69: 253–262, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trabattoni D, Bartorelli AL. Late occlusive in-stent restenosis of a bare-metal stent presenting with ST-elevation anterior MI: is restenosis better than a late stent thrombosis? Int J Cardiol 135: e65-e67, 2009 [DOI] [PubMed] [Google Scholar]
- 38.Truelsen T, Gronbaek M, Schnohr P, Boysen G. Intake of beer, wine, and spirits and risk of stroke: the Copenhagen City heart study. Stroke 29: 2467–2472, 1998 [DOI] [PubMed] [Google Scholar]
- 39.Ueda M, Graf R, MacWilliams HK, Schliwa M, Euteneuer U. Centrosome positioning and directionality of cell movements. Proc Natl Acad Sci USA 94: 9674–9678, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vacca A, Ribatti D, Iurlaro M, Merchionne F, Nico B, Ria R, Dammacco F. Docetaxel versus paclitaxel for antiangiogenesis. J Hematother Stem Cell Res 11: 103–118, 2002 [DOI] [PubMed] [Google Scholar]
- 41.Vorpahl M, Yazdani SK, Nakano M, Ladich E, Kolodgie FD, Finn AV, Virmani R. Pathobiology of stent thrombosis after drug-eluting stent implantation. Curr Pharm Des 16: 4064–4071, 2010 [DOI] [PubMed] [Google Scholar]
- 42.Wallerath T, Deckert G, Ternes T, Anderson H, Li H, Witte K, Forstermann U. Resveratrol, a polyphenolic phytoalexin present in red wine, enhances expression and activity of endothelial nitric oxide synthase. Circulation 106: 1652–1658, 2002 [DOI] [PubMed] [Google Scholar]
- 43.Wessely R, Blaich B, Belaiba RS, Merl S, Gorlach A, Kastrati A, Schömig A. Comparative characterization of cellular and molecular anti-restenotic profiles of paclitaxel and sirolimus. Implications for local drug delivery. Thromb Haemost 97: 1003–1012, 2007 [PubMed] [Google Scholar]
- 44.Wu JM, Wang ZR, Hsieh TC, Bruder JL, Zou JG, Huang YZ. Mechanism of cardioprotection by resveratrol, a phenolic antioxidant present in red wine (Review). Int J Mol Med 8: 3–17, 2001 [DOI] [PubMed] [Google Scholar]
- 45.Wu Q, Chambliss K, Lee WR, Yuhanna IS, Mineo C, Shaul PW. Point mutations in the ERα Gαi binding domain segregate nonnuclear from nuclear receptor function. Mol Endocrinol 27: 2–11, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu XM, Sansores-Garcia L, Chen XM, Matijevic-Aleksic N, Du M, Wu KK. Suppression of inducible cyclooxygenase 2 gene transcription by aspirin and sodium salicylate. Proc Natl Acad Sci USA 96: 5292–5297, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zou J, Huang Y, Cao K, Yang G, Yin H, Len J, Hsieh TC, Wu JM. Effect of resveratrol on intimal hyperplasia after endothelial denudation in an experimental rabbit model. Life Sci 68: 153–163, 2000 [DOI] [PubMed] [Google Scholar]