Abstract
Urocortin II (UcnII), a cardioactive peptide with beneficial effects in normal and failing hearts, is also arrhythmogenic and prohypertrophic. We demonstrated that cardiac effects are mediated by a phosphatidylinositol-3 kinase (PI3K)/Akt kinase (Akt)/endothelial nitric oxide synthase (eNOS)/nitric oxide (NO) signaling pathways. Nuclear factor of activated T-cells (NFAT) transcription factors play a key role in the regulation of gene expression in cardiac development, maintenance of an adult differentiated cardiac phenotype, and remodeling processes in cardiac hypertrophy and heart failure (HF). We tested the hypothesis that UcnII differentially regulates NFAT activity in cardiac myocytes from both normal and failing hearts through the PI3K/Akt/eNOS/NO pathway. Isoforms NFATc1 and NFATc3 revealed different basal subcellular distribution in normal and HF rabbit ventricular myocytes with a nuclear NFATc1 and a cytosolic localization of NFATc3. However, in HF, the nuclear localization of NFATc1 was less pronounced, whereas the nuclear occupancy of NFATc3 was increased. In normal myocytes, UcnII induced nuclear export of NFATc1 and attenuated NFAT-dependent transcriptional activity but did not affect the distribution of NFATc3. In HF UcnII facilitated nuclear export of both isoforms and reduced transcriptional activity. NFAT regulation was mediated by a PI3K/Akt/eNOS/NO signaling cascade that converged on the activation of several kinases, including glycogen synthase kinase-3β (GSK3β), c-Jun NH2-terminal kinase (JNK), p38 mitogen-activated kinase (p38), and PKG, resulting in phosphorylation, deactivation, and nuclear export of NFAT. In conclusion, while NFATc1 and NFATc3 reveal distinct subcellular distribution patterns, both are regulated by the UcnII-PI3K/Akt/eNOS/NO pathway that converges on the activation of NFAT kinases and NFAT inactivation. The data reconcile cardioprotective and prohypertrophic UcnII effects mediated by different NFAT isoforms.
Keywords: urocortin, cardiac myocytes, nuclear factor of activated T-cells, NFAT, hypertrophy, heart failure
urocortins (Ucn: isoforms I, II, and III) are peptides of the corticotropin-releasing factor (CRF) family. The cellular effects of urocortin are mediated by G-protein coupled CRF receptors (CRFR1 and CRFR2 subtypes). UcnI can activate both CRF receptor subtypes, whereas UcnII and UcnIII are selective agonists of CRFR2. CRFR2s are expressed throughout the cardiovascular system (27, 30, 48, 57), and urocortins have cardiovascular effects including an increase in heart rate, cardiac contractility, and left ventricular ejection fraction, as well as vasodilation (8, 12, 26, 38, 57). Several studies have shown that in cardiovascular diseases CRFR2s are upregulated, and the plasma concentration of urocortins is increased (8, 26, 36, 37). Urocortins have been shown to exert beneficial effects in heart failure (HF), including a reduction in total peripheral resistance and left ventricular filling pressure by decreasing cardiac afterload and increasing cardiac output (13, 14, 43–45). On the other hand, urocortins have also been demonstrated to potentially facilitate cardiac hypertrophy (7, 22).
Despite the demonstration of a myriad of cardiovascular effects, the underlying cellular mechanisms and signaling pathways that mediate the actions of urocortin are not fully understood. We and others have shown that urocortin effects on cardiac contractility and relaxation are mediated through modulation of intracellular Ca2+ handling via several independent pathways including the cAMP/PKA, Ca2+/calmodulin-dependent protein kinases II (CaMKII), and exchange protein activated by cAMP (Epac) signaling, resulting in an increase of L-type Ca2+ current and sarcoplasmic reticulum Ca2+ content (5, 60, 61). UcnII also modulates cardiac contractility via phosphoinositide 3-kinase (PI3K)/Akt signaling resulting in phosphorylation of endothelial nitric oxide (NO) synthase (eNOS) and an increase of NO production (55, 56). Moreover, a prohypertrophic effect of urocortins involves an Akt/GSK3β signaling pathway-mediated increase in cell size and protein-to-DNA ratio as demonstrated in neonatal and adult rat cardiomyocytes (7, 22).
Cardiac hypertrophy and HF are the result of complex electrical and structural remodeling processes in response to mechanical and neurohumoral stimuli (34). Structural and functional remodeling in hypertrophy and HF is the result of altered gene expression that is regulated by activation of specific transcription factors. Members of the family of nuclear factor of activated T-cells (NFAT) transcription factors play a crucial role in hypertrophic signaling (3, 34). Four different NFAT isoforms (c1 to c4) are expressed in the heart (53). Isoforms NFATc3 and NFATc4 have established roles in hypertrophic signaling, whereas NFATc1 plays a key role in cardiac development (valve formation and morphogenesis of the heart), and it has been suggested that a basal activity of NFATc1 in cardiac myocytes is required to maintain the differentiated phenotype of adult myocytes (52). NFAT transcription factors are activated by dephosphorylation by the Ca2+-sensitive phosphatase calcineurin (CaN) resulting in NFAT translocation to the nucleus, nuclear accumulation of activated NFAT, and transcriptional activity. Rephosphorylation of NFAT by several kinases such as p38 mitogen-activated kinase (p38), glycogen synthase kinase-3β (GSK3β), and c-Jun NH2-terminal kinases (JNKs) causes inactivation of NFAT, nuclear export, and release of transcriptional effects (3, 46, 47, 52, 59).
Despite the facts that urocortins and NFAT transcription factors play important roles in cardiac hypertrophy and HF, little is known whether these signaling pathways are interconnected. In chromaffin cells, for example, it was shown that the CaN/NFAT pathway mediates the effect of urocortins on catecholamine synthesis (16); however, no interplay between urocortin and NFAT signaling is known for cardiac tissue. However, CaN/NFAT signaling has been directly linked to NO and the cGMP-dependent protein kinase (PKG) activity (18), and we have shown previously that UcnII stimulates NO production in cardiac myocytes via UcnII-dependent eNOS phosphorylation and activation of cAMP/PKA and PI3K/Akt signaling cascades (55, 56).
Therefore, the goal of the present study was to investigate whether and how UcnII regulates NFAT activity in cardiac myocytes and whether these previously established signaling pathways that can be activated by UcnII are involved. We aimed to elucidate the signaling pathway mediating UcnII-dependent changes of the cytosolic-nuclear distribution of NFAT (and NFAT-dependent transcriptional activity) in adult normal myocytes and myocytes from failing hearts. Furthermore, we also aimed to contrast the behavior of two isoforms of NFAT, one being representative of a transcription factor with an established and well-documented role in cardiac disease (NFATc3), the other not being related to disease in adult hearts but linked to maintenance of a normal cardiac phenotype (NFATc1). UcnII-induced NFATc1 and NFATc3 distribution and activity in quiescent myocytes using adenoviral expression of NFATc1- and NFATc3-green fluorescent fusion proteins (GFP) as well as the transcriptional activity of endogenous NFAT were measured. We found that UcnII induced NFATc1 translocation to the cytosol and a reduction of NFATc1-mediated transcriptional activity in adult rabbit ventricular myocytes, whereas NFATc3 was unaffected in normal hearts. In myocytes from failing hearts, a decreased basal nuclear localization of NFATc1 was observed, in contrast to an increased nuclear localization of NFATc3, consistent with our earlier observations (52). In failing hearts, UcnII facilitated nuclear export of NFATc1 and NFATc3 and diminished NFAT-mediated transcriptional activity. The regulation of NFAT by UcnII is mediated by a PI3K/Akt/eNOS/NO pathway that converges on the activation of several NFAT kinases, leading NFAT inactivation.
MATERIAL AND METHODS
Solutions and chemicals.
All chemicals and reagents were purchased from Sigma-Aldrich (St. Louis, MO), unless noted otherwise. BCA Protein Assay was from Thermo Fisher Scientific (Waltham, MA). Liberase TH was obtained from Roche Diagnostic (Indianapolis, IN), the Luciferase Assay System E1500 was from Promega (Sunnyvale, CA), and penicillin and streptomycin were purchased from Molecular Probes/Life Technologies (Grand Island, NY). During all experiments, cells were bathed in Tyrode solution containing the following (in mmol/l): 136 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 10 HEPES, and 10 glucose pH 7.4 with NaOH. Agonists, antagonists, and pharmacological inhibitors were added to the culture medium for 2 or 24 h concomitant with viral infections.
Rabbit HF model.
Studies were performed on control male New Zealand White rabbits (Harlan Laboratories, Indianapolis, IN), and rabbits with surgically induced hypertrophy and HF. HF was induced by combined volume [aortic valve insufficiency (AI)] and pressure (aortic constriction) overload as previously described (2, 39, 40). AI was performed by inserting a beveled 5-Fr micropuncture introducer, connected to a pressure transducer, via the left carotid artery and pushing it abruptly through the aortic valve several times under transthoracic echocardiography guidance. The severity of aortic valve insufficiency was assessed subsequently by two-dimensional echocardiography. Aortic constriction surgery was performed 2 wk later. The abdominal aorta was surgically prepared proximally to the renal arteries, and a 4/0-silk tie ligature was tightened around the aorta, resulting in a reduction of aortic diameter of ∼25–40% (depending on the severity of AI). Each rabbit underwent echocardiography examination before AI surgery to record baseline parameters and at 2- to 4-wk intervals after surgeries. Hypertrophy followed by HF developed over the course of ∼5–8 mo. Rabbits with developed HF were killed for single cell isolation when a marked left-ventricular dilation (>50% increase in left ventricular end-diastolic diameter) and systolic dysfunction (decrease of left-ventricular fractional shortening by >40%) had developed. All protocols for animal surgery, handling, and cell isolation (see below) were approved by the Institutional Animal Care and Use Committee of Rush University Medical Center.
Cell isolation.
Left ventricular myocytes were isolated from normal male control New Zealand White rabbits (32 animals) and from rabbits with chronic nonischemic HF as described above (16 animals). Rabbits were anaesthetized with sodium pentobarbital (50 mg/kg), and hearts were quickly excised, mounted on a Langendorff apparatus and retrogradely perfused via the aorta. After an initial washing step with oxygenated Ca2+-free Tyrode solution (in mmol/l: 140 NaCl, 4 KCl, 10 d-glucose, 5 HEPES, 1 MgCl2, and 10 BDM pH 7.4 with NaOH), the heart was perfused with MEM solution containing 20 μmol/l Ca2+ and 22.5 μg/mg Liberase TH for 20 min at 37° C. The left ventricle was cut from the heart and minced, filtered, and washed in a MEM solution containing 50 μmol/l Ca2+ and 10 mg/ml bovine serum albumin. Isolated cells were kept in MEM solution with 50 μmol/l Ca2+ at room temperature (22–24°C) until used for experimentation. Cell surface area of cultured normal and HF myocytes were measured from two-dimensional confocal images recorded from a central plane. HF myocytes revealed an increased surface area by ∼40% [normal myocytes: 932 ± 88 μm2 (n = 22); HF myocytes: 1,310 ± 48 μm2 (n = 31); P < 0.0005 vs. normal].
Cell culture and viral transduction.
Isolated cells were plated on sterile, laminin-coated glass coverslips in Medium 199, supplemented with penicillin and streptomycin (50 μg/ml). Adenoviral gene transfer in isolated myocytes that were kept in short-term culture (24 h; multiplicity of infection of 500) was used to overexpress NFATc1-GFP, NFATc3-GFP, Luc-NFAT-GFPl, and IL2-red fluorescence protein (RFP) (50–52). Experiments were performed 24 h after infection.
Fluorescence measurements of NFAT-GFP and IL2-RFP.
Fluorescence measurements were performed with single cell confocal microscopy (Bio-Rad Radiance 2000/MP). GFP was exited with an argon ion laser line at 488 nm, and emitted fluorescence was collected at 500–520 nm. Images were background subtracted, and the mean fluorescence of defined regions of interest (ROIs) was analyzed using ImageJ (Wayne Rasband, National Institutes of Health, Bethesda, MD). The subcellular distribution of NFAT-GFP was analyzed by measuring the mean fluorescence of a ROI covering the entire nucleus (NFATnuc fluorescence) and a cytoplasmic ROI of the same size (NFATcyt fluorescence). The subcellular NFAT distribution was quantified as the ratio (R) of NFATnuc to NFATcyt fluorescence (RNFAT) and specified for NFAT isoforms (RNFATc1 and RNFATc3).
IL2-RFP (in vivo NFAT transcriptional assay, also referred to as NFAT-RFP) (51) was excited at 535 nm (green He-Ne laser), and emission was collected at ≥570 nm. The RFP signal was averaged over the entire surface area of the cell.
Luciferase assay for transcriptional activity of NFAT.
Cardiac myocytes were infected with the Luc-NFAT-GFP (NFAT-sensitive luciferase) alone or in combination with NFATc1-GFP or NFATc3-GFP as described above. Twenty-four hours after infection, cells were lysed and luciferase activity was measured using a Luciferase Assay System with a plate-reading luminometer (type VICTOR3 V; Perkin Elmer, Waltham, MA) according to manufacturer instructions. The BCA Protein Assay was used to determine the concentration of protein used in the luciferase assays. Luciferase luminescence was normalized to protein concentration.
Data analysis and presentation.
Data are presented as individual observations or as means ± SE and were analyzed using Student t-test and one-way ANOVA. Unless stated otherwise n represents the number of individual cells, and differences were considered significant at P < 0.05.
RESULTS
UcnII effects on basal subcellular NFAT distribution.
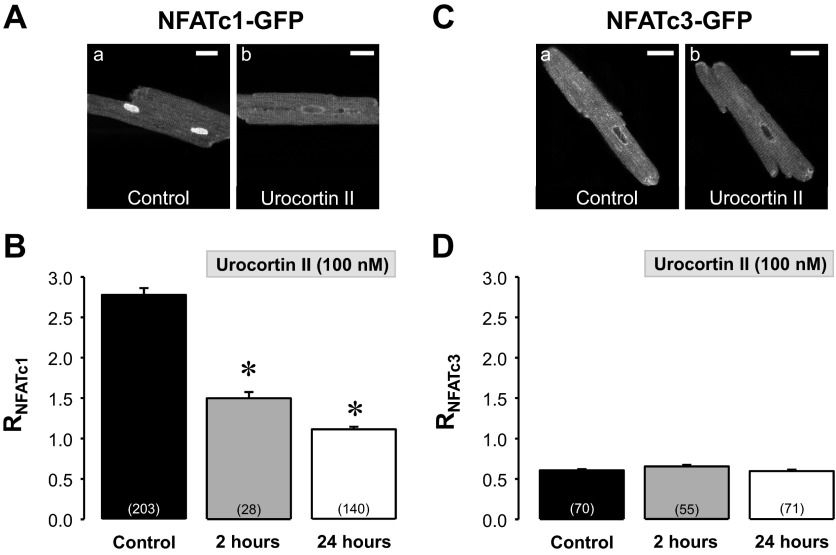
We first investigated whether UcnII was able to induce a cytosolic-nuclear translocation of NFAT in normal rabbit ventricular cardiomyocytes. NFATc1 and NFATc3 isoforms were adenovirally expressed using NFATc1-GFP and NFATc3-GFP fusion proteins. Myocytes were either left untreated (control) or exposed to a maximally effective concentration of UcnII (100 nmol/l), as determined previously (60), for 2 and 24 h. NFAT translocation was quantified as changes of RNFAT (ratio of NFATnuc to NFATcyt fluorescence). Figure 1A shows representative confocal images of an untreated (a) myocyte and a cell treated for 24 h with UcnII (Fig. 1Ab). NFATc1 revealed a primarily nuclear localization under basal conditions, whereas exposure to UcnII induced nuclear export of NFATc1. Average results in Fig. 1B show that UcnII decreased RNFATc1 significantly from 2.78 ± 0.08 to 1.49 ± 0.08 after 2 h of UcnII exposure and to 1.11 ± 0.03 (or by ∼60%) after 24 h of UcnII exposure, i.e., after 24 h of UcnII treatment NFATc1 was essentially evenly distributed between cytosol and nucleus. This time point was used in all of the following experiments. In contrast to NFATc1, the basal localization of NFATc3 was cytosolic (RNFATc3: 0.61 ± 0.12; Fig. 1C) and remained unchanged during UcnII treatment (Fig. 1D).
Fig. 1.
Urocortin II (UcnII) induced nuclear factor of activated T-cells (NFAT)c1 and NFATc3 translocation. A: confocal images of subcellular distribution of NFATc1-green fluorescent protein (GFP) in ventricular myocytes in the absence (a; control) and presence (24 h) of UcnII (b). B: average NFATc1 ratio (RNFATc1) in control and after 2 and 24 h UcnII treatment. C: confocal images of NFATc3-GFP distribution in control (a) and UcnII-treated (b) myocytes. D: average RNFATc3 in control and after 2 and 24 h UcnII treatment. Number in parentheses indicate number of individual cells. *P < 0.001 vs. control. Scale bar = 20 μm.
Since in normal ventricular myocytes UcnII did only affect the translocation of NFATc1, in the following experiments we focused on the NFATc1 isoform to investigate the underlying signaling pathways.
Signaling pathway responsible for UcnII-induced NFATc1 translocation to the cytosol.
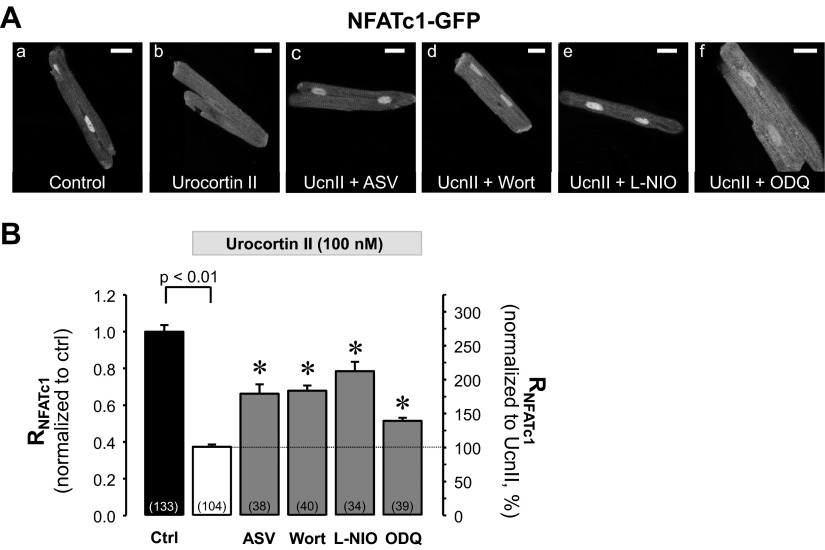
Based on the notion that in cardiac myocytes cGMP-dependent protein kinase type 1 (PKG1) can inhibit calcineurin/NFAT signaling (18), and our own observation that UcnII can increase NO production via Akt/eNOS phosphorylation (55, 56), we tested the hypothesis that NFATc1 is regulated by the UcnII-stimulated PI3K/Akt/eNOS/NO pathway. Akt is activated through PI3K/PDK-dependent phosphorylation at Ser473 and Thr308 (54) and stimulates eNOS by phosphorylation at Ser1177 resulting in NO production (17, 63). NO activates soluble guanylyl cyclase (sGC), which causes an increase of cGMP resulting in activation of PKG1 (23, 32). In previous work we demonstrated that this signaling pathway is activated by UcnII. In rabbit ventricular myocytes UcnII activated Akt via phosphorylation (Ser473 and Thr308), resulting in eNOS activation (via phosphorylation at Ser1177) and an increase of NO (55, 56), followed by a rise of cGMP levels (unpublished results). To test the hypothesis that this signaling cascade also regulates NFAT, we stimulated ventricular myocytes expressing NFATc1-GFP with the agonist UcnII (Fig. 2Ab) with or without various inhibitors present. Inhibitors used were as follows: CRFR2: antisauvagine-30 (ASV; 10 nmol/l; Fig. 2Ac); PI3K: wortmannin (Wort; 300 nmol/l; Fig. 2Ad); eNOS: N5-(1-iminoethyl)-l-ornithine (l-NIO; 10 μmol/l; Fig. 2Ae); and sGC: 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ; 10 μmol/l; Fig. 2Af). In our earlier studies we demonstrated the desired effects of these inhibitors in rabbit ventricular myocytes: ASV abolished UcnII-mediated positive inotropic effects (60, 61), whereas UcnII-induced phosphorylation and activation of Akt and eNOS, NO production (55, 56), sGC activation, and increase of cGMP were abolished by Wort, l-NIO, and ODQ, respectively. In the current study, inhibitors were applied together with UcnII for 24 h. Average data for RNFATc1 are shown in Fig. 2B. Data were normalized to untreated control myocytes (Fig. 2B, left ordinate) and also expressed as percent change relative to RNFATc1 in the presence of UcnII (Fig. 2B, right ordinate). The average data shown in Fig. 2B demonstrate that all inhibitors induced a significant reduction of the UcnII-mediated NFATc1 export to the cytoplasm. Compared with UcnII treatment alone all inhibitors allowed translocation of NFATc1 back into the nucleus and increased RNFATc1 between 38 and 110% (ASV: 77 ± 14%; wortmannin: 82 ± 8%: l-NIO: 110 ± 13%; ODQ: 38 ± 5%; all P < 0.001 vs. UcnII). None of the inhibitors, however, was capable of fully restoring the basal NFATc1 distribution, suggesting that UcnII may affect NFATc1 distribution through additional pathways and/or incomplete inhibition by the applied blockers. Furthermore, the effect of UcnII on NFATc1 translocation appeared to be Ca2+ independent. In the presence of a membrane-permeable Ca2+ chelator (BAPTA-AM; 25 μmol/l) or the inositol-1,4,5-trisphosphate (IP3) receptor blocker 2-aminoethoxydiphenyl borate (2-APB; 3 μmol/l), the UcnII-induced nuclear export of NFATc1 was unchanged, whereby the latter result further indicated that a putative CRFR2/PLC/IP3/Ca2+ pathway was not involved (data not shown). In conclusion, the data shown in Fig. 2 support the hypothesis that UcnII facilitates nuclear export of NFATc1 by a Ca2+-independent mechanism that involves a CRFR2/PI3K/Akt/eNOS pathway and sGC/PKG signaling.
Fig. 2.
Pharmacological manipulation of UcnII-induced NFATc1 redistribution via phosphatidylinositol-3 kinase (PI3K)/Akt kinase (Akt)/endothelial nitric oxide synthase (eNOS)/nitric oxide (NO) signaling pathway. A: confocal images of subcellular NFATc1-GFP distribution in control (a), after UcnII treatment (b), and UcnII treatment in combination with blockers antisauvagine-30 (ASV; c), wortmannin (Wort; d), N5-(1-iminoethyl)-l-ornithine (l-NIO; e), and 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ; f). B: average data of RNFATc1 normalized to control (left ordinate) and expressed as %change relative to UcnII (right ordinate). *P < 0.001 vs. UcnII. Scale bar = 20 μm.
Exogenous NO-mediated NFATc1 translocation to the cytoplasm.
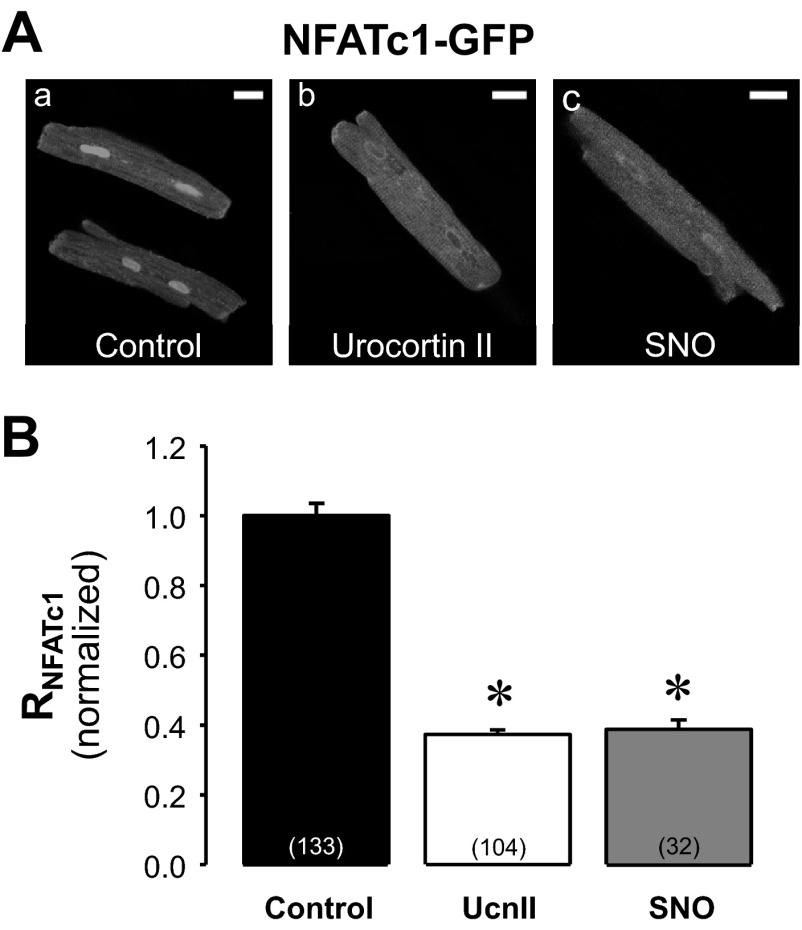
Together with our previous findings (55, 56) the data presented in Fig. 2 suggest that eNOS activation and endogenous NO production is involved in the UcnII-dependent transport of NFATc1 out of the nucleus. To test this hypothesis further, we challenged NFATc1-GFP-expressing cardiac myocytes with the NO-donor spermine NONOate (SNO; 100 μmol/l). Cells were treated with SNO for 24 h to allow for direct comparison with UcnII. Fig. 3A shows confocal images of untreated (control, Fig. 3Aa), UcnII (Fig. 3Ab)-treated, and SNO-treated (Fig. 3Ac) myocytes. Mean values of RNFATc1 normalized to control are presented in Fig. 3B. In the presence of SNO NFATc1 export to the cytoplasm was enhanced and normalized RNFATc1 decreased to 0.39 ± 0.03 (P < 0.001 vs. control). This decrease was similar to the effect of UcnII (normalized RNFATc1 = 0.37 ± 0.001). The results indicate that NO can induce a translocation of NFATc1 to the cytoplasm and lend further support to the hypothesis that the UcnII-mediated NO increase (as shown directly in our previous studies; see Refs. 55, 56) indeed is involved in NFATc1 translocation.
Fig. 3.
Effect of exogenous NO on subcellular NFATc1 distribution. A: confocal images of NFATc1-GFP distribution in control (a), UcnII (b), and spermine NONOate (SNO; c)-treated ventricular myocytes. B: average normalized RNFATc1 data in control, UcnII, and SNO. *P < 0.001 vs. control. Scale bar = 20 μm.
The role of kinases for UcnII-induced NFATc1 translocation to the cytoplasm.
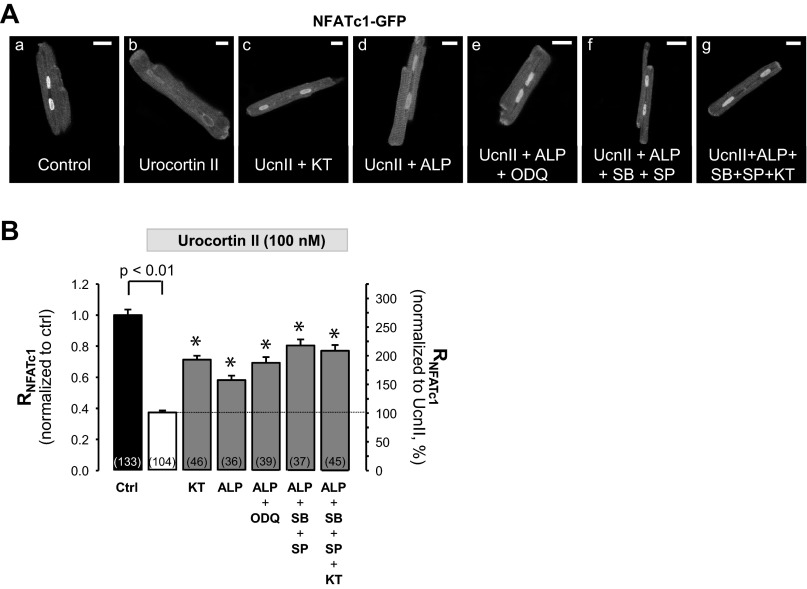
The subcellular localization of NFAT is determined by the balance between activities of NFAT kinases and the NFAT phosphatase calcineurin. GSK3β, JNKs, and p38 kinases have been identified of being capable of NFAT phosphorylation, which results in reduced nuclear occupancy (1, 9, 20, 41, 62). We sought to determine a putative contribution of these kinases to the UcnII-induced NFATc1 translocation out of the nucleus, together with a potential role of PKG in this process. For this purpose, cells were treated with the appropriate inhibitors in the presence of UcnII. Inhibitors used were as follows: KT5823 (KT; 0.1 μmol/l, Fig. 4, Ac and Ag) for PKG; alsterpaullone (ALP; 1 μmol/l, Fig. 4, Ad-Ag) for GSK3β; SP600125 (SP; 10 μmol/l; Fig. 4, Af and Ag) for JNK2; and SB203580 (SB; 10 μmol/l; Fig. 4, Af and Ag) for p38. The average effects of these inhibitors on UcnII-induced nuclear export of NFATc1 are summarized in Fig. 4B. Inhibition of PKG with KT significantly reversed the UcnII-induced NFATc1 export and increased normalized RNFATc1 from 0.37 ± 0.01 to 0.71 ± 0.02 (P < 0.001 vs. UcnII). These data suggest a potential novel role of PKG for NFAT phosphorylation either directly or through a downstream target of PKG. This notion was further supported by the observation that KT alone increased the normalized RNFATc1 to 1.41 ± 0.07 (n = 26; P < 0.001 vs. control), suggesting that basal sGC/PKG activity limits nuclear occupancy of NFATc1 under control conditions. This is reminiscent of our earlier observation that inhibition of basal activity of NFAT kinases alone (demonstrated for inhibition of GSK3β with ALP, which increased normalized RNFATc1 to 1.78; see Ref. 50) can enhance nuclear accumulation of NFATc1. Here we show that inhibition of GSK3β with ALP also significantly attenuated nuclear export of NFATc1 induced by UcnII. The inhibition of nuclear export by ALP was further enhanced in the presence of ODQ (to inhibit sGC and its downstream target PKG), lending further support to a potential involvement of PKG. During simultaneous GSK3β and sGC inhibition normalized RNFATc1 increased to 0.69 ± 0.04 (P < 0.001 vs. UcnII). To test for additive inhibitory effects of various kinase inhibitors, three (ALP, SB, and SP: inhibition of all “classical” NFAT kinases) and four (ALP, SB, SP, and KT) blockers were applied simultaneously. The presence of three or four inhibitors further reduced the UcnII-induced nuclear export of NFATc1 (normalized RNFATc1 increased to 0.80 ± 0.04 and 0.77 ± 0.04, respectively); however, the inhibitors were not capable of fully restoring control conditions. This may be in part due to incomplete inhibition of kinase activities by the blockers and/or the involvement of additional kinases in NFAT phosphorylation. Nuclear export of phosphorylated NFAT is regulated by the nuclear export protein Crm1 (exportin 1) and can be blocked by leptomycin B (28, 64). In the presence of leptomycin (40 nmol/l), the UcnII-induced NFATc1 export was significantly attenuated and normalized RNFATc1 was 0.71 ± 0.03 (n = 29) compared with 0.39 ± 0.03 in UcnII alone (P < 0.001; data not shown). The data suggest that UcnII-induced NFATc1 export occurs predominantly via the exportin 1 pathway.
Fig. 4.
Role of kinases for UcnII-induced nuclear export of NFATc1. A: confocal images of subcellular NFATc1-GFP distribution in control (a), in the presence of UcnII (b), and UcnII plus kinase inhibitors KT5823 (PKG1 inhibition; c), asterpaullone [ALP; glycogen synthase kinase-3β (GSK3β) inhibition; d), ALP + ODQ (sGC inhibition; e), ALP + SB203580 (p38 kinase inhibition) + SP600125 [c-Jun NH2-terminal kinase (JNK) inhibition; f], and ALP + SB203580 + SP600125 + KT5823 (g). B: average data of RNFATc1 normalized to control (left ordinate) and expressed as %change relative to UcnII (right ordinate). *P < 0.001 vs. UcnII. Scale bar = 20 μm.
UcnII-induced NFAT translocation in HF.
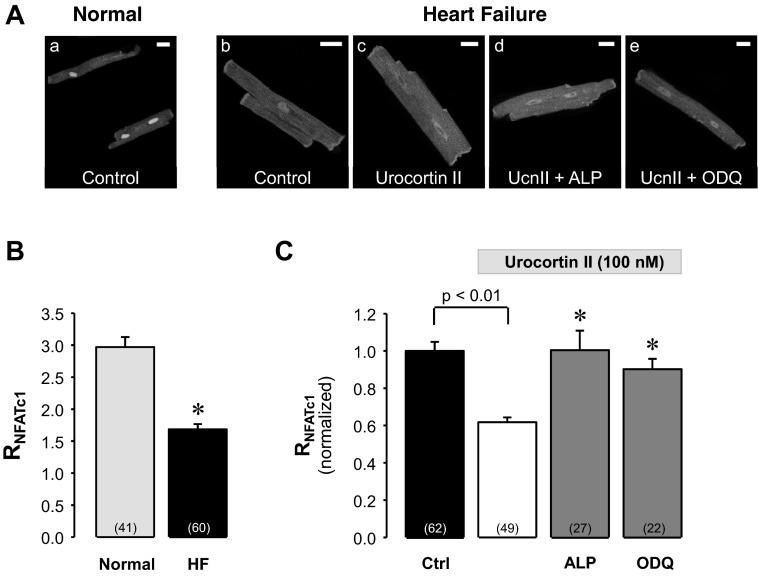
NFAT transcription factors (including the isoform NFATc3) have been implicated to play a crucial role in gene transcription underlying the profound structural and functional remodeling processes that occur in cardiac hypertrophy and HF (24, 35, 52). Furthermore, urocortins are known to exert beneficial effects in HF (13, 14, 43–45) but also have been shown to be prohypertrophic (7, 22). Therefore, we investigated the subcellular distribution of NFATc1 and NFATc3 and the effect of UcnII thereon in ventricular myocytes from a nonischemic rabbit HF model, induced by combined volume and pressure overload (39, 40). As shown in Fig. 5, the nuclear localization of NFATc1 in myocytes from failing hearts was less pronounced compared with normal cells (Fig. 5, Aa vs. Ab). As summarized in Fig. 5B in HF myocytes RNFATc1 was 1.68 ± 0.08, compared with 2.97 ± 0.16 in normal myocytes (P < 0.0001).
Fig. 5.
UcnII-induced nuclear export of NFATc1 in heart failure (HF). A: confocal images of subcellular NFATc1-GFP distribution in normal (a) and HF ventricular myocytes (b). Subcellular NFATc1-GFP distribution in HF ventricular myocytes in the presence of UcnII (c), UcnII + ALP (d), and UcnII + ODQ (e). B: average control RNFATc1 in normal and HF myocytes. *P < 0.001 vs. normal. C: average normalized RNFATc1 data from HF myocytes in control, UcnII, UcnII + ALP, and UcnII + ODQ. *P < 0.001 vs. UcnII. Scale bar = 20 μm.
In HF myocytes UcnII (Fig. 5Ac) induced further redistribution of NFATc1 out of the nucleus and decreased RNFATc1 by an additional 38 ± 2.6% (P < 0.001 vs. HF; Fig. 5C). This UcnII-induced nuclear export of NFATc1 was completely prevented by ALP (Fig. 5Ad) and by ODQ (Fig. 5Ae; both inhibitors P < 0.001 vs. UcnII, Fig. 5C) and suggests that the same regulatory pathways are involved that are responsible for UcnII-induced NFATc1 export in normal cells (Figs. 2 and 4).
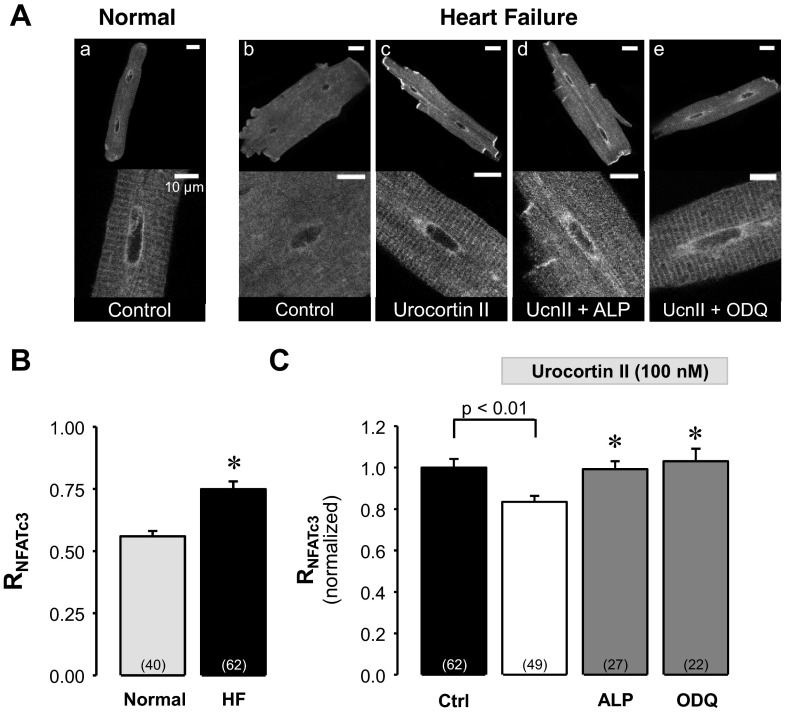
In contrast to NFATc1, nuclear localization of NFATc3 increased in HF cells (Fig. 6Ab) compared with normal cells (Fig. 6Aa) with an increase of RNFATc3 by 34% from 0.56 ± 0.02 to 0.75 ± 0.03 (P < 0.0001 vs. normal; Fig. 6B), consistent with our previous results (52) using an immunohistochemical approach.
Fig. 6.
UcnII-induced nuclear export of NFATc3 in HF. A, top: confocal images of subcellular NFATc3-GFP distribution in normal (a) and HF ventricular myocytes (b). Subcellular NFATc3-GFP distribution in HF ventricular myocytes in the presence of UcnII (c), UcnII + ALP (d), and UcnII + ODQ (e). Scale bar = 20 μm. A, bottom: nuclear region shown at higher magnification. Scale bar = 10 μm. B: average control RNFATc3 in normal and HF myocytes. *P < 0.001 vs. normal. C: average normalized RNFATc3 data from HF myocytes in control, UcnII, UcnII + ALP, and UcnII + ODQ. *P < 0.001 vs. UcnII.
Since HF myocytes revealed an enhanced nuclear NFATc3 occupancy, we investigated whether UcnII is capable of influencing the nuclear distribution of NFATc3. UcnII (Fig. 6Ac) stimulation resulted in a translocation of NFATc3 back to the cytoplasm and decreased RNFATc3 by 17 ± 3% (P < 0.001 vs. HF; Fig. 6C). This UcnII-induced nuclear export of NFATc3 was completely prevented by ALP (Fig. 6Ad) and ODQ (Fig. 6Ae) (P < 0.001 vs. UcnII; Fig. 6C).
In summary, these results indicate that UcnII affects not only the distribution of NFATc1 in the healthy heart but also in failing hearts. Furthermore, while NFATc3 distribution was not affected by UcnII in normal hearts, UcnII gained a regulatory role for NFATc3 translocation in failing hearts that appeared to involve similar signaling pathways as for NFATc1.
UcnII-induced NFAT-regulated transcriptional activity in normal and HF myocytes.
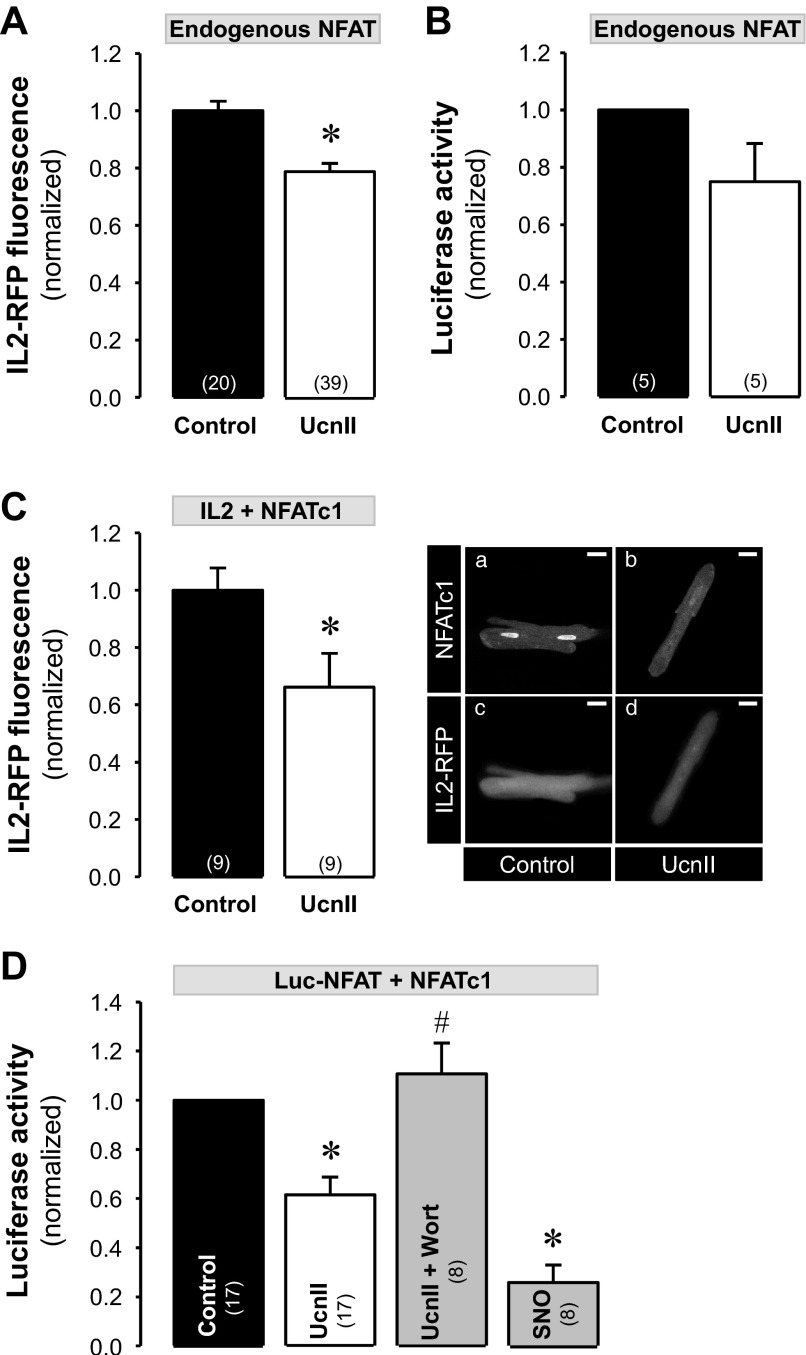
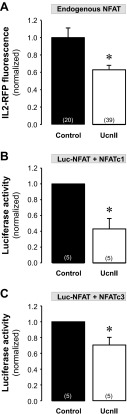
We further tested whether UcnII stimulation not only induced nuclear export of NFAT but also affected NFAT-regulated transcriptional activity. For this purpose we used two different methods 1) a RFP reporter tool where RFP is expressed under the control of NFAT (51) referred to here as “IL2-RFP reporter,” and 2) a standard luciferase assay where luciferase is expressed under NFAT control and luciferase activity is quantified with a luminescence assay (51). First, we investigated transcriptional activity in normal ventricular myocytes. To study transcriptional activity of endogenous NFAT, cells were either infected with the IL2-RFP reporter or with NFAT-sensitive luciferase (Luc-NFAT-GFP). The IL2-RFP reporter (also referred to as NFAT-RFP) has the advantage of allowing monitoring of transcriptional activity of NFAT continuously in single intact living cells (51). Both approaches yielded qualitatively similar results. As shown in Fig. 7A, 24-h exposure of normal myocytes to UcnII significantly reduced the transcriptional activity of endogenous NFAT, resulting in a 22% decrease in RFP fluorescence compared with untreated myocytes (average normalized IL2-RFP fluorescence decreased from 1.00 ± 0.03 in control to 0.78 ± 0.03 in the presence of UcnII; P < 0.001 vs. control). Similarly, the transcriptional activity quantified as endogenous NFAT-sensitive luciferase expression was decreased by 25% in the presence of UcnII compared with untreated cells (normalized luciferase activity decreased from 1.00 to 0.75 ± 0.13; Fig. 7B).
Fig. 7.
UcnII-induced NFAT-regulated transcriptional activity in normal myocytes. A: averaged normalized IL2-red fluorescence protein (RFP) fluorescence data for in vivo measurements of endogenous NFAT activity in control and UcnII-treated myocytes. B: averaged normalized data of endogenous NFAT-dependent luciferase activity in control and UcnII-treated cells. C: normal cardiac myocytes were coinfected with IL2-RFP and NFATc1-GFP for in vivo measurements of NFATc1-regulated transcriptional activity. Right: confocal images of NFATc1 (a and b) and IL2-RFP (c and d) fluorescence in untreated and UcnII-treated myocytes. Left: averaged normalized IL2-RFP fluorescence data in control and in the presence of UcnII. D: average normalized NFATc1-dependent luciferase activity in normal myocytes in control and after treatment with UcnII, UcnII + Wort, and SNO. *P < 0.05 vs. control; #P < 0.05 vs. UcnII. Numbers in parentheses indicate number of individual cells treated (A and C) or number of hearts (B and D). In B and D, data for treatment effects were normalized to control values obtained from the same heart.
Furthermore, we studied changes of transcriptional activity mediated specifically by the NFATc1 isoform. For this purpose, cells were coinfected with either IL2-RFP or Luc-NFAT-GFP and NFATc1-GFP viruses. Figure 7C shows cardiac myocytes coinfected with NFATc1-GFP (a and b) and IL2-RFP (c and d) in the presence and absence (control) of UcnII. UcnII reduced NFATc1-mediated IL2-RFP transcriptional activity, resulting in a 34% decrease in RFP fluorescence compared with untreated myocytes (average normalized IL2-RFP fluorescence decreased from 1.00 ± 0.08 in control to 0.66 ± 0.12 in the presence of UcnII; P < 0.05 vs. control). Figure 7, Ca and Cb, shows that concomitantly with a reduction in RFP fluorescence (Fig. 7, Cc and Cd), UcnII caused the expected nuclear export of NFATc1 that amounted to a ∼60% decrease of RNFATc1 compared with untreated control cells.
In normal myocytes, coinfected with Luc-NFAT-GFP and NFATc1-GFP, UcnII (24 h) decreased NFATc1 mediated luciferase expression by 39% compared with untreated cells (normalized luciferase activity decreased from 1.00 to 0.62 ± 0.07; P < 0.01 vs. control; Fig. 7D). Furthermore, inhibition of PI3K with Wort, for the purpose of blocking the downstream Akt/eNOS/NO/PKG pathway, reversed the UcnII-dependent reduction in luciferase activity (normalized luciferase activity in the presence UcnII + Wort was 1.11 ± 0.12; P < 0.05 vs. control; Fig. 7D). The NO donor SNO decreased NFATc1-dependent transcriptional activity (normalized luciferase activity decreased to 0.26 ± 0.07; P < 0.001 vs. control; Fig. 7D). These data are in line with the UcnII-induced NFATc1 translocation to the cytoplasm (reduction of RNFATc1 by ∼60%; see Fig. 1B) and demonstrate that the same PI3K/Akt/eNOS/NO pathway is involved (cf. Figs. 2 and 3).
Effects of UcnII on NFAT-mediated transcriptional activity were also investigated in HF myocytes. The results were qualitatively identical with the translocation experiments (Figs. 5 and 6). As shown in Fig. 8A UcnII reduced endogenous NFAT transcriptional activity. In HF cells expressing the IL2-RFP reporter, the normalized RFP fluorescence signal decreased by 37% in the presence of UcnII (from 1.00 ± 0.11 to 0.63 ± 0.05; P < 0.005 vs. control). In HF myocytes expressing NFATc1 (Fig. 8B) or NFATc3 (Fig. 8C), UcnII treatment caused a significant decrease in transcriptional activity determined with the luciferase assay. In NFATc1-expressing cells, UcnII decreased normalized luciferase activity by nearly 60% to 0.43 ± 0.13 (P < 0.05 vs. control), whereas in NFATc3-expressing HF cells transcriptional activity decreased by ∼30% to a normalized value of 0.71 ± 0.10 (P < 0.05 vs. control). In summary, in HF myocytes UcnII not only reduced transcriptional activity in cells overexpressing NFATc1 or NFATc3 but also transcriptional activity regulated by endogenous NFAT. Furthermore, the transcriptional activity results paralleled the UcnII-dependent effects on NFAT translocation.
Fig. 8.
UcnII-induced NFAT-regulated transcriptional activity in HF myocytes. A: HF cardiac myocytes were infected with IL2-RFP to measure endogenous NFAT-dependent transcriptional activity. Averaged normalized data of IL2-RFP fluorescence in control and after UcnII treatment. B: HF cells were coinfected with Luc-NFAT-GFP and NFATc1-GFP for measurements of NFATc1-regulated transcriptional activity. Averaged normalized relative luciferase activity in control and in the presence of UcnII. C: average normalized NFATc3-dependent relative luciferase activity in HF myocytes in control and after UcnII treatment. *P < 0.05 vs. control. Numbers in parentheses indicate numbers of individual cells treated (A) or numbers of hearts (B and C). In B and C, data for UcnII effects were normalized to control values obtained from the same heart.
DISCUSSION
In this study, we investigated the regulation of the transcription factor isoforms NFATc1 and NFATc3 in ventricular myocytes from normal and failing hearts by the cardioactive peptide urocortin II. The key findings of our investigation were as follows: first, NFAT isoforms c1 and c3, expressed by adenoviral transfer, revealed a distinctly different basal subcellular distribution in ventricular myocytes from normal healthy and from failing hearts: NFATc1 was localized to the nucleus (RNFATc1 > 1) whereas NFATc3 was predominantly found in the cytosol (RNFATc3 < 1). This basal pattern of NFAT distribution was preserved in HF myocytes; however, RNFATc1 was decreased and RNFATc3 was increased relative to normal myocytes. Second, in normal myocytes UcnII induced a nuclear export of NFATc1 and attenuated NFAT-dependent transcriptional activity but did not affect the subcellular distribution of NFATc3. In contrast, in HF myocytes UcnII facilitated nuclear export and inhibited transcriptional activity of both isoforms. Third, the regulation of NFAT translocation and transcriptional activity was mediated by a PI3K/Akt/eNOS/NO signaling cascade that converged on the activation of several kinases, including GSK3β, JNK, p38, and PKG, resulting in phosphorylation, deactivation, and nuclear export of NFAT. Fourth, UcnII attenuated transcriptional activity of endogenous NFAT in normal and HF myocytes. While our experimental approach precluded the identification of the endogenous NFAT isoform(s) responsible for the UcnII-mediated reduction in transcriptional activity, our observation that UcnII had no effect on translocation in NFATc3 expressing normal cells suggests a critical involvement of endogenous NFATc1.
Urocortins, peptides of the corticotropin-releasing factor family, have been associated with a number of cardiovascular functions and effects, ranging from positive chronotropic, inotropic, and lusitropic effects to vasodilation and increased coronary blood flow (5, 8, 12, 13, 60, 61). Urocortins also appear to play important roles in cardiac disease. Urocortins and their cellular receptors are upregulated in cardiac disease, with important functional consequences. On the one hand the peptide has been shown to attenuate ischemia-reperfusion injury (4, 6) and to have beneficial effects in HF by reducing total peripheral resistance, left ventricular filling pressure and cardiac afterload, and increasing cardiac output and left ventricular ejection fraction (13, 14, 43–45). On the other hand urocortins can also have arrhythmogenic effects (61) and have been shown to be prohypertrophic (7, 22).
Cardiac hypertrophy is induced by pathological (e.g., pressure or volume overload) or physiological stimuli (e.g., exercise training or developmental growth) (33). Pathological hypertrophy is associated with cardiac fibrosis, left ventricular dilation, contractile dysfunction, and HF. Concomitant with these profound structural and functional changes, commonly referred to as hypertrophic remodeling, are altered gene expression and transcriptional activity that govern these remodeling processes (10, 29, 33). Members of the NFAT family have been identified as important transcription factors in the heart. The isoforms NFATc3 and NFATc4 have been directly associated with cardiac hypertrophic remodeling and transcriptional activity related to HF (53). In contrast, the isoform NFATc1 has been assigned a role in early cardiac development (15, 58) and possibly the maintenance of a normal differentiated adult cardiac phenotype (50); however, it appears to play no or only a minor role in hypertrophy and HF. We propose that NFATc1 and NFATc3 mediate (at least in part and in conjunction with other cardiac transcription factors, however, those potential interactions with other transcription factors will not be discussed here) opposite effects on the cardiac phenotype and that the balance between NFATc1- and NFATc3-mediated effects determines the degree of hypertrophic and HF remodeling. For the following discussion we will view disturbances of this equilibrium between NFATc1- and NFATc3-mediated effects as crucial for whether the outcomes of remodeling processes are beneficial or adverse for the progression and outcome of cardiac disease. In line with the reported effects of urocortins in hypertrophy and HF, we found differential effects of UcnII on the subcellular distribution and transcriptional activity of the NFAT isoform c1 and c3. Under basal conditions NFATc1 was preferentially located in the nucleus of normal ventricular myocytes, consistent with its “maintenance” function as described above. UcnII led to nuclear export of NFATc1 and reduced NFAT-dependent transcriptional activity. These observations could explain qualitatively a prohypertrophic effect of UcnII. By curtailing NFATc1-mediated transcriptional activity per se, the above postulated equilibrium between NFATc1 and NFATc3 signaling shifts away from the maintenance function in favor of hypertrophic signaling. A different picture emerges in fully developed HF. Here, the nuclear occupancy by NFATc1 was diminished in exchange to an enhanced nuclear localization of NFATc3, i.e., the isoform that has been linked to cardiac hypertrophy and HF now shows enhanced nuclear presence, whereas the maintenance isoform NFATc1 has a reduced nuclear occupancy. Within the above postulated framework, the equilibrium is shifted towards hypertrophic and HF remodeling mediated by NFATc3 activity. The effects of UcnII on NFAT translocation are in line with the reported beneficial effects of UcnII in HF. First, the enhanced nuclear export of “hypertrophic” NFATc3 diminishes prohypertrophic signaling. Second, the UcnII-mediated nuclear export of NFATc1 is significantly less in HF. Together, these two consequences of UcnII stimulation shifts the equilibrium away from prohypertrophic signaling in favor of regaining a more normal cardiac phenotype. We therefore propose, based on the analysis of distribution and activity of NFAT isoforms, that UcnII can have prohypertrophic effects, consistent with previous reports (7, 22). In established HF, however, urocortins may mediate antihypertrophic (as defined above) NFAT signaling that is consistent with the reported beneficial clinical effects of urocortins in cardiac disease. The differential effects of urocortins in healthy and diseased hearts maybe related to reported altered circulating plasma levels of urocortins (36) and urocortin receptor expression (26, 37).
Our previous work on UcnII signaling in the heart established (55, 56) a signaling pathway that entailed UcnII binding to the CRFR2 (60, 61), followed by G protein-linked activation of PI3K and Akt, phosphorylation of eNOS, NO production and sGC activation (referred to here as PI3K/Akt/eNOS/NO pathway). Expanding on this previous work (55, 56) we examined whether the same PI3K/Akt/eNOS/NO pathway was involved in the regulation of UcnII-mediated translocation of NFAT. Inhibition of key steps of the PI3K/Akt/eNOS/NO cascade, ranging from inhibition of CRFR2 to blocking PI3K, eNOS, and sGC, resulted in a significant reduction of UcnII-induced nuclear NFAT export and NFAT-mediated transcriptional activity, suggesting that the same signaling pathway was involved. Furthermore, exogenous NO mimicked the effect of UcnII confirming a central role of NO in this process. The central role of NO was further supported by the experimental observation that inhibition of PI3K and exogenous NO not only affected NFATc1 translocation but also NFATc1-mediated transcriptional activity.
With respect to NFAT regulation by UcnII the PI3K/Akt/eNOS/NO cascade appears to converge on the activation of NFAT kinases. The term “NFAT kinase” refers to the group of kinases that are capable of rephosphorylation of NFAT proteins and inducing nuclear export (21). Selective or combined inhibition of the classical NFAT kinases (GSK3β, JNK, and p38 kinase) attenuated UcnII-induced nuclear NFAT export. In addition, our results assign a novel potential role to PKG as an NFAT kinase. Selective inhibition of PKG strongly attenuated UcnII induced NFATc1 export (Fig. 4), and inhibition of basal PKG activity enhanced nuclear occupancy of NFATc1. Thus PKG appears to shift aforementioned equilibrium away from prohypertophic signaling towards maintenance (mediated by NFATc1 signaling) of a normal cardiac phenotype. This is consistent with recent evidence for a distinct role of PKG in inhibition of NFAT hypertrophy signaling (18). Whether the action of PKG consists of direct phosphorylation of NFAT or activation of downstream NFAT kinases remains to be determined. Indeed, there is evidence from cardiac and other tissues that GSK3β (11) and p38 kinase (31) are PKG phosphorylation targets. Furthermore, NFAT isoforms contain complete or incomplete potential PKG consensus sequences of the type Arg-Arg-X-Ser/Thr (cf. Ref. 19), which opens the possibility of direct NFAT phosphorylation by PKG. Alternatively, the PI3K/Akt/eNOS/NO signaling pathway could also activate NFAT kinases in a parallel PKG-independent fashion as has been demonstrated for NO-dependent p38 activation in the heart (42).
An interesting finding relates to the role of Akt/GSK3β signaling for NFAT regulation. GSK3β is a constitutively active serine/threonine protein kinase that phosphorylates numerous protein targets (including NFAT; Refs. 1, 25, 34). The activity of GSK3β is negatively regulated by Akt (1, 22), which in turn is expected to lead to an enhanced accumulation of NFAT in the nucleus (49, 50). Our results indicate a novel additional role for Akt and GSK3β for NFAT signaling that involves eNOS activation and NO production, and results in activation of GSK3β and ultimately nuclear export of NFAT. Thus Akt has a dual role in GSK3β regulation with opposite effect, depending on the participation of eNOS and NO. In the absence of eNOS activation and NO production Akt inhibits GSK3β, which would favor nuclear accumulation of NFAT. During stimulation with UcnII, however, activation of Akt leads to downstream activation of eNOS resulting in activation of GSK3β by the mechanism described in this study. We propose that under these specific conditions the stimulatory effect on GSK3β via the Akt/eNOS/NO/sGC/PKG pathway outweighs the direct inhibitory effect of Akt. The latter conclusion is also consistent with our observation that in the presence of UcnII the inhibition of GSK3β (with ALP; Fig. 4) and inhibition of PI3K/Akt (with Wort; Fig. 2) had very similar effects, i.e., ALP and Wort counteracted the nuclear export mediated by UcnII to a similar degree.
In summary, we demonstrate an important role for UcnII in the regulation of NFAT isoforms c1 and c3 that involves a PI3K/Akt/eNOS/NO signaling cascade. The PI3K/Akt/eNOS/NO signaling pathway is activated via CRFR2 and converges on the activation of NFAT kinases, including a novel role for PKG as an NFAT kinase, resulting in inactivation and nuclear export of NFAT. Although the NFAT isoforms c1 and c3 reveal distinct subcellular distribution patterns, both share common features of regulation by the UcnII-PI3K/Akt/eNOS/NO pathway, both in normal as well as in failing hearts. Furthermore, within the discussed frame work that the equilibrium between NFATc1- and NFATc3-mediated signaling is crucial for the maintenance of a normal cardiac phenotype or the initiation and progression of hypertrophic remodeling, we provide evidence from the perspective of NFAT transcription factor activity that can explain a prohypertrophic role for urocortins but also can provide evidence for the beneficial effects that are attributed to urocortins in HF.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-62231, HL-80101, and HL-101235 and the Leducq Foundation (to L. A. Blatter).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.W. and L.A.B. conception and design of research; S.W., S.A., and V.A.L. performed experiments; S.W., S.A., V.A.L., and L.A.B. analyzed data; S.W., S.A., V.A.L., and L.A.B. interpreted results of experiments; S.W. and L.A.B. prepared figures; S.W., S.A., V.A.L., and L.A.B. drafted manuscript; S.W., S.A., V.A.L., and L.A.B. edited and revised manuscript; S.W., S.A., V.A.L., and L.A.B. approved final version of manuscript.
REFERENCES
- 1.Antos CL, McKinsey TA, Frey N, Kutschke W, McAnally J, Shelton JM, Richardson JA, Hill JA, Olson EN. Activated glycogen synthase-3 beta suppresses cardiac hypertrophy in vivo. Proc Natl Acad Sci USA 99: 907–912, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asfaw ZE, Kuchay BM, Awad S, Polimenakos AC, Kime A, Curran J, Shonts S, Shannon T, Higgins RS, Lonchyna VA. Development of a two stage model of heart failure in the New Zealand white rabbit: surgical technique (Abstract). J Am Coll Surg 213: 36, 2011 [Google Scholar]
- 3.Beals CR, Sheridan CM, Turck CW, Gardner P, Crabtree GR. Nuclear export of NF-ATc enhanced by glycogen synthase kinase-3. Science 275: 1930–1934, 1997 [DOI] [PubMed] [Google Scholar]
- 4.Brar BK, Stephanou A, Knight R, Latchman DS. Activation of protein kinase B/Akt by urocortin is essential for its ability to protect cardiac cells against hypoxia/reoxygenation-induced cell death. J Mol Cell Cardiol 34: 483–492, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Calderon-Sanchez E, Delgado C, Ruiz-Hurtado G, Dominguez-Rodriguez A, Cachofeiro V, Rodriguez-Moyano M, Gomez AM, Ordonez A, Smani T. Urocortin induces positive inotropic effect in rat heart. Cardiovasc Res 83: 717–725, 2009 [DOI] [PubMed] [Google Scholar]
- 6.Calderon-Sanchez EM, Ruiz-Hurtado G, Smani T, Delgado C, Benitah JP, Gomez AM, Ordonez A. Cardioprotective action of urocortin in postconditioning involves recovery of intracellular calcium handling. Cell Calcium 50: 84–90, 2011 [DOI] [PubMed] [Google Scholar]
- 7.Chanalaris A, Lawrence KM, Townsend PA, Davidson S, Jamshidi Y, Stephanou A, Knight RD, Hsu SY, Hsueh AJ, Latchman DS. Hypertrophic effects of urocortin homologous peptides are mediated via activation of the Akt pathway. Biochem Biophys Res Commun 328: 442–448, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Charles CJ, Rademaker MT, Richards AM. Urocortins: putative role in cardiovascular disease. Curr Med Chem Cardiovasc Hematol Agents 2: 43–47, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Chow CW, Rincon M, Cavanagh J, Dickens M, Davis RJ. Nuclear accumulation of NFAT4 opposed by the JNK signal transduction pathway. Science 278: 1638–1641, 1997 [DOI] [PubMed] [Google Scholar]
- 10.Cohn JN, Bristow MR, Chien KR, Colucci WS, Frazier OH, Leinwand LA, Lorell BH, Moss AJ, Sonnenblick EH, Walsh RA, Mockrin SC, Reinlib L. Report of the National Heart, Lung, and Blood Institute Special Emphasis Panel on Heart Failure Research. Circulation 95: 766–770, 1997 [DOI] [PubMed] [Google Scholar]
- 11.Das A, Xi L, Kukreja RC. Protein kinase G-dependent cardioprotective mechanism of phosphodiesterase-5 inhibition involves phosphorylation of ERK and GSK3beta. J Biol Chem 283: 29572–29585, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis ME, Pemberton CJ, Yandle TG, Fisher SF, Lainchbury JG, Frampton CM, Rademaker MT, Richards AM. Urocortin 2 infusion in healthy humans: hemodynamic, neurohormonal, and renal responses. J Am Coll Cardiol 49: 461–471, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Davis ME, Pemberton CJ, Yandle TG, Fisher SF, Lainchbury JG, Frampton CM, Rademaker MT, Richards M. Urocortin 2 infusion in human heart failure. Eur Heart J 28: 2589–2597, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Davis ME, Pemberton CJ, Yandle TG, Lainchbury JG, Rademaker MT, Nicholls MG, Frampton CM, Richards AM. Effect of urocortin 1 infusion in humans with stable congestive cardiac failure. Clin Sci (Lond) 109: 381–388, 2005 [DOI] [PubMed] [Google Scholar]
- 15.de la Pompa JL, Timmerman LA, Takimoto H, Yoshida H, Elia AJ, Samper E, Potter J, Wakeham A, Marengere L, Langille BL, Crabtree GR, Mak TW. Role of the NF-ATc transcription factor in morphogenesis of cardiac valves and septum. Nature 392: 182–186, 1998 [DOI] [PubMed] [Google Scholar]
- 16.Dermitzaki E, Tsatsanis C, Gravanis A, Margioris AN. The calcineurin-nuclear factor of activated T cells signaling pathway mediates the effect of corticotropin releasing factor and urocortins on catecholamine synthesis. J Cell Physiol 227: 1861–1872, 2012 [DOI] [PubMed] [Google Scholar]
- 17.Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 399: 601–605, 1999 [DOI] [PubMed] [Google Scholar]
- 18.Fiedler B, Lohmann SM, Smolenski A, Linnemuller S, Pieske B, Schroder F, Molkentin JD, Drexler H, Wollert KC. Inhibition of calcineurin-NFAT hypertrophy signaling by cGMP-dependent protein kinase type I in cardiac myocytes. Proc Natl Acad Sci USA 99: 11363–11368, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Francis SH, Busch JL, Corbin JD, Sibley D. cGMP-dependent protein kinases and cGMP phosphodiesterases in nitric oxide and cGMP action. Pharmacol Rev 62: 525–563, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gomez del Arco P, Martinez-Martinez S, Maldonado JL, Ortega-Perez I, Redondo JM. A role for the p38 MAP kinase pathway in the nuclear shuttling of NFATp. J Biol Chem 275: 13872–13878, 2000 [DOI] [PubMed] [Google Scholar]
- 21.Graef IA, Chen F, Crabtree GR. NFAT signaling in vertebrate development. Curr Opin Genet Dev 11: 505–512, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Gruson D, Ginion A, Decroly N, Lause P, Vanoverschelde JL, Ketelslegers JM, Bertrand L, Thissen JP. Urocortin-induced cardiomyocytes hypertrophy is associated with regulation of the GSK-3beta pathway. Heart Vessels 27: 202–207, 2012 [DOI] [PubMed] [Google Scholar]
- 23.Hare JM. Nitric oxide and excitation-contraction coupling. J Mol Cell Cardiol 35: 719–729, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol 7: 589–600, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Hogan PG, Chen L, Nardone J, Rao A. Transcriptional regulation by calcium, calcineurin, and NFAT. Genes Dev 17: 2205–2232, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Ikeda K, Tojo K, Tokudome G, Ohta M, Sugimoto K, Tamura T, Tajima N, Mochizuki S, Kawakami M, Hosoya T. Cardiac expression of urocortin (Ucn) in diseased heart; preliminary results on possible involvement of Ucn in pathophysiology of cardiac diseases. Mol Cell Biochem 252: 25–32, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Kimura Y, Takahashi K, Totsune K, Muramatsu Y, Kaneko C, Darnel AD, Suzuki T, Ebina M, Nukiwa T, Sasano H. Expression of urocortin and corticotropin-releasing factor receptor subtypes in the human heart. J Clin Endocrinol Metab 87: 340–346, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Kudo N, Matsumori N, Taoka H, Fujiwara D, Schreiner EP, Wolff B, Yoshida M, Horinouchi S. Leptomycin B inactivates CRM1/exportin 1 by covalent modification at a cysteine residue in the central conserved region. Proc Natl Acad Sci USA 96: 9112–9117, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med 322: 1561–1566, 1990 [DOI] [PubMed] [Google Scholar]
- 30.Lewis K, Li C, Perrin MH, Blount A, Kunitake K, Donaldson C, Vaughan J, Reyes TM, Gulyas J, Fischer W, Bilezikjian L, Rivier J, Sawchenko PE, Vale WW. Identification of urocortin III, an additional member of the corticotropin-releasing factor (CRF) family with high affinity for the CRF2 receptor. Proc Natl Acad Sci USA 98: 7570–7575, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Z, Zhang G, Feil R, Han J, Du X. Sequential activation of p38 and ERK pathways by cGMP-dependent protein kinase leading to activation of the platelet integrin alphaIIb beta3. Blood 107: 965–972, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Massion PB, Feron O, Dessy C, Balligand JL. Nitric oxide and cardiac function: ten years after, and continuing. Circ Res 93: 388–398, 2003 [DOI] [PubMed] [Google Scholar]
- 33.McMullen JR, Shioi T, Zhang L, Tarnavs.ki O, Sherwood MC, Kang PM, Izumo S. Phosphoinositide 3-kinase(p110alpha) plays a critical role for the induction of physiological, but not pathological, cardiac hypertrophy. Proc Natl Acad Sci USA 100: 12355–12360, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Molkentin JD. Calcineurin-NFAT signaling regulates the cardiac hypertrophic response in coordination with the MAPKs. Cardiovasc Res 63: 467–475, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Molkentin JD, Lu JR, Antos CL, Markham B, Richardson J, Robbins J, Grant SR, Olson EN. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell 93: 215–228, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ng LL, Loke IW, O'Brien RJ, Squire IB, Davies JE. Plasma urocortin in human systolic heart failure. Clin Sci (Lond) 106: 383–388, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Nishikimi T, Miyata A, Horio T, Yoshihara F, Nagaya N, Takishita S, Yutani C, Matsuo H, Matsuoka H, Kangawa K. Urocortin, a member of the corticotropin-releasing factor family, in normal and diseased heart. Am J Physiol Heart Circ Physiol 279: H3031–H3039, 2000 [DOI] [PubMed] [Google Scholar]
- 38.Parkes DG, Weisinger RS, May CN. Cardiovascular actions of CRH and urocortin: an update. Peptides 22: 821–827, 2001 [DOI] [PubMed] [Google Scholar]
- 39.Pogwizd SM. Nonreentrant mechanisms underlying spontaneous ventricular arrhythmias in a model of nonischemic heart failure in rabbits. Circulation 92: 1034–1048, 1995 [DOI] [PubMed] [Google Scholar]
- 40.Pogwizd SM, Qi M, Yuan W, Samarel AM, Bers DM. Upregulation of Na(+)/Ca(2+) exchanger expression and function in an arrhythmogenic rabbit model of heart failure. Circ Res 85: 1009–1019, 1999 [DOI] [PubMed] [Google Scholar]
- 41.Porter CM, Havens MA, Clipstone NA. Identification of amino acid residues and protein kinases involved in the regulation of NFATc subcellular localization. J Biol Chem 275: 3543–3551, 2000 [DOI] [PubMed] [Google Scholar]
- 42.Rabkin SW, Klassen SS, Tsang MY. Sodium nitroprusside activates p38 mitogen activated protein kinase through a cGMP/PKG independent mechanism. Life Sci 81: 640–646, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Rademaker MT, Cameron VA, Charles CJ, Richards AM. Integrated hemodynamic, hormonal, and renal actions of urocortin 2 in normal and paced sheep: beneficial effects in heart failure. Circulation 112: 3624–3632, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Rademaker MT, Cameron VA, Charles CJ, Richards AM. Urocortin 3: haemodynamic, hormonal, and renal effects in experimental heart failure. Eur Heart J 27: 2088–2098, 2006 [DOI] [PubMed] [Google Scholar]
- 45.Rademaker MT, Charles CJ, Espiner EA, Fisher S, Frampton CM, Kirkpatrick CM, Lainchbury JG, Nicholls MG, Richards AM, Vale WW. Beneficial hemodynamic, endocrine, and renal effects of urocortin in experimental heart failure: comparison with normal sheep. J Am Coll Cardiol 40: 1495–1505, 2002 [DOI] [PubMed] [Google Scholar]
- 46.Rao A. Signaling to gene expression: calcium, calcineurin and NFAT. Nat Immunol 10: 3–5, 2009 [DOI] [PubMed] [Google Scholar]
- 47.Rao A, Luo C, Hogan PG. Transcription factors of the NFAT family: regulation and function. Annu Rev Immunol 15: 707–747, 1997 [DOI] [PubMed] [Google Scholar]
- 48.Reyes TM, Lewis K, Perrin MH, Kunitake KS, Vaughan J, Arias CA, Hogenesch JB, Gulyas J, Rivier J, Vale WW, Sawchenko PE. Urocortin II: a member of the corticotropin-releasing factor (CRF) neuropeptide family that is selectively bound by type 2 CRF receptors. Proc Natl Acad Sci USA 98: 2843–2848, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rinne A, Banach K, Blatter LA. Regulation of nuclear factor of activated T cells (NFAT) in vascular endothelial cells. J Mol Cell Cardiol 47: 400–410, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rinne A, Blatter LA. Activation of NFATc1 is directly mediated by IP3 in adult cardiac myocytes. Am J Physiol Heart Circ Physiol 299: H1701–H1707, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rinne A, Blatter LA. A fluorescence-based assay to monitor transcriptional activity of NFAT in living cells. J Physiol 588: 3211–3216, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rinne A, Kapur N, Molkentin JD, Pogwizd SM, Bers DM, Banach K, Blatter LA. Isoform- and tissue-specific regulation of the Ca2+-sensitive transcription factor NFAT in cardiac myocytes and heart failure. Am J Physiol Heart Circ Physiol 298: H2001–H2009, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van Rooij E, Doevendans PA, de Theije CC, Babiker FA, Molkentin JD, de Windt LJ. Requirement of nuclear factor of activated T-cells in calcineurin-mediated cardiomyocyte hypertrophy. J Biol Chem 277: 48617–48626, 2002 [DOI] [PubMed] [Google Scholar]
- 54.Vanhaesebroeck B, Alessi DR. The PI3K-PDK1 connection: more than just a road to PKB. Biochem J 346: 561–576, 2000 [PMC free article] [PubMed] [Google Scholar]
- 55.Walther S, Renz S, Yang LZ, Spiess J, Pieske B, Kockskaemper J. Urocortin II causes phosphorylation of eNOS and stimulation of NO production in cardiac myocytes (Abstract). Biophys J 100: 257a–258a, 2011 [Google Scholar]
- 56.Walther S, Renz S, Yang LZ, Spiess J, Pieske B, Kockskaemper J. Urocortin II increases eNOS-mediated NO production in rabbit ventricular myocytes via both PKA- and Akt-dependent pathways (Abstract). Circulation 120: S1490, 2009 [Google Scholar]
- 57.Wiley KE, Davenport AP. CRF2 receptors are highly expressed in the human cardiovascular system and their cognate ligands urocortins 2 and 3 are potent vasodilators. Br J Pharmacol 143: 508–514, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu B, Wang Y, Lui W, Langworthy M, Tompkins KL, Hatzopoulos AK, Baldwin HS, Zhou B. Nfatc1 coordinates valve endocardial cell lineage development required for heart valve formation. Circ Res 109: 183–192, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu H, Peisley A, Graef IA, Crabtree GR. NFAT signaling and the invention of vertebrates. Trends Cell Biol 17: 251–260, 2007 [DOI] [PubMed] [Google Scholar]
- 60.Yang LZ, Kockskamper J, Heinzel FR, Hauber M, Walther S, Spiess J, Pieske B. Urocortin II enhances contractility in rabbit ventricular myocytes via CRF(2) receptor-mediated stimulation of protein kinase A. Cardiovasc Res 69: 402–411, 2006 [DOI] [PubMed] [Google Scholar]
- 61.Yang LZ, Kockskamper J, Khan S, Suarez J, Walther S, Doleschal B, Unterer G, Khafaga M, Machler H, Heinzel FR, Dillmann WH, Pieske B, Spiess J. cAMP- and Ca(2)(+)/calmodulin-dependent protein kinases mediate inotropic, lusitropic and arrhythmogenic effects of urocortin 2 in mouse ventricular myocytes. Br J Pharmacol 162: 544–556, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang TT, Xiong Q, Enslen H, Davis RJ, Chow CW. Phosphorylation of NFATc4 by p38 mitogen-activated protein kinases. Mol Cell Biol 22: 3892–3904, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang XP, Hintze TH. cAMP signal transduction induces eNOS activation by promoting PKB phosphorylation. Am J Physiol Heart Circ Physiol 290: H2376–H2384, 2006 [DOI] [PubMed] [Google Scholar]
- 64.Zhu J, McKeon F. NF-AT activation requires suppression of Crm1-dependent export by calcineurin. Nature 398: 256–260, 1999 [DOI] [PubMed] [Google Scholar]