Abstract
Previous studies have shown a role for nitric oxide and S-nitrosylation (SNO) in postconditioning (PostC), but specific SNO proteins and sites have not been identified in the myocardium after PostC. In this study, we examined SNO signaling in PostC using a Langendorff-perfused mouse heart model. After 20 min of equilibrium perfusion and 25 min of global ischemia, PostC was applied at the beginning of reperfusion with six cycles of 10 s of reperfusion and 10 s of ischemia. The total period of reperfusion was 90 min. Compared with the ischemia-reperfusion (I/R) control, PostC significantly reduced postischemic contractile dysfunction and infarct size. PostC-induced protection was blocked by treatment with NG-nitro-l-arginine methyl ester (l-NAME) (10 μmol/l; a constitutive NO synthase inhibitor), but not by either ODQ (10 μmol/l, a highly selective soluble guanylyl cyclase inhibitor) or KT5823 (1 μmol/l, a specific protein kinase G inhibitor). Two biotin switch based methods, two dimensional CyDye-maleimide difference gel electrophoresis (2D CyDye-maleimide DIGE) and SNO-resin-assisted capture (SNO-RAC), were utilized to identify SNO-modified proteins and sites. Using 2D CyDye-maleimide DIGE analysis, PostC was found to cause a 25% or greater increase in SNO of a number of proteins, which was blocked by treatment with l-NAME in parallel with the loss of protection. Using SNO-RAC, we identified 77 unique proteins with SNO sites after PostC. These results suggest that NO-mediated SNO signaling is involved in PostC-induced cardioprotection and these data provide the first set of candidate SNO proteins in PostC hearts.
Keywords: nitric oxide, ischemic postconditioning, protein S-nitrosylation, soluble guanylyl cyclase/cGMP
ischemic postconditioning (PostC), the intermittent interruption of blood flow during the first minute of reperfusion, has been reported to effectively reduce myocardial ischemia-reperfusion (I/R) injury (30, 33, 34). Nitric oxide (NO) has been shown to be an important signaling molecule involved in PostC-induced cardioprotection (2, 20, 33). Early studies suggested that NO mediates PostC-induced protection through the classical soluble guanylyl cyclase (sGC)/cyclic guanosine monophosphate (cGMP) signaling pathway (1, 32). A specific sGC inhibitor, 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ), was shown to block the PostC-mediated reduction in infarct size in a Langendorff perfused heart model of I/R injury (8, 32). However, Cohen et al. (3) recently reported that pharmacological postconditioning with a NO donor, S-nitroso-N-acetyl- d,l-penicillamine (SNAP), was not blocked by ODQ, suggesting that a sGC/cGMP-independent NO signaling is important in mediating PostC (3).
Besides activating the sGC/cGMP signaling pathways, NO can directly modify protein sulfhydryl residues through protein S-nitrosylation (SNO), which has emerged as an important protein posttranslational modification in cardiovascular signaling (14, 23) and cardioprotection (24, 27, 28). SNO exerts cardioprotection not only through modulating protein structure and function, but also by shielding the S-nitrosylated thiol(s) from irreversible oxidative modification upon reperfusion (13, 28).
The goal of this study was to determine if PostC leads to an increase in protein SNO and to identify specific proteins and sites that undergo SNO following PostC. Using a modified biotin switch method with CyDye-maleimide monoreactive fluorescence dyes and a two-dimensional difference gel electrophoresis (2D DIGE), we found that PostC significantly increased protein SNO, and this increase was prevented with l-NAME treatment. SNO-resin-assisted capture (SNO-RAC) was utilized to identify those SNO-modified proteins and sites (i.e., 8 unique SNO-modified proteins in I/R hearts, while almost 10-fold more proteins in PostC hearts). These data are consistent with the hypothesis that PostC exerts its cardioprotection via NO-mediated protein SNO.
METHODS AND MATERIALS
Animals and compounds.
Male C57BL/6J mice obtained from Jackson Laboratories (Bar Harbor, ME) were used for all experiments. Mice were between 12 and 16 wk of age at the time of experimentation. All animals received humane treatment in accordance with National Institutes of Health guidelines and the “Guiding Principles for Research Involving Animals and Human Beings.” This study was reviewed and approved by the Institutional Animal Care and Use Committee of the National Heart, Lung, and Blood Institute. All compounds were obtained from Sigma (St. Louis, MO). The dose of each compound was based on previous studies (25, 26).
Langendorff perfused mouse hearts and I/R-PostC protocol.
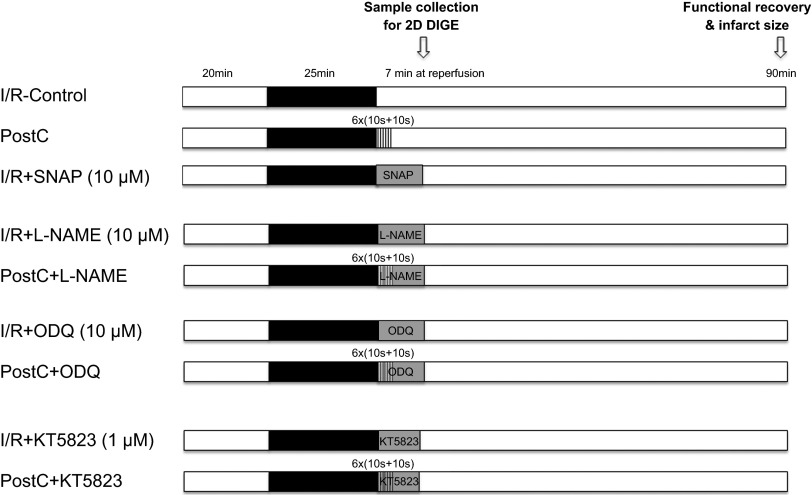
After anesthesia with pentobarbital and anticoagulation with heparin, a thoracotomy was performed and the heart was quickly excised and placed in ice-cold Krebs-Henseleit buffer (in mmol/l: 120 NaCl, 11 d-glucose, 25 NaHCO3, 1.75 CaCl2, 4.7 KCl, 1.2 MgSO4, and 1.2 KH2PO4). The aorta was cannulated and the heart was perfused with Krebs-Henseleit buffer (oxygenated with 95% O2/5% CO2 and maintained at pH 7.4) in retrograde fashion at a constant pressure of 100 cm of water at 37°C. The perfusion was performed in the dark to prevent light-induced SNO decomposition. After equilibrium perfusion for 20 min, mouse hearts were subjected to 25 min of no-flow global ischemia followed by 90 min of reperfusion. PostC was performed at the beginning of reperfusion with six cycles of 10 s of reperfusion and 10 s of ischemia. Each drug was infused during the first 7 min of reperfusion including the PostC period. Hearts that displayed a persistent irregular beating pattern after 20 min of reperfusion were excluded. The timing of drug administration is illustrated in the I/R-PostC protocol as shown in Fig. 1.
Fig. 1.
Ischemia-reperfusion (I/R) and postconditioning (PostC) protocol. Mouse hearts were Langendorff perfused with Krebs-Henseleit buffer (oxygenated with 95% O2/5% CO2 and maintained at pH 7.4) at a constant pressure of 100 cm of water at 37°C in the dark. After equilibrium perfusion for 20 min, mouse hearts were subjected to 25 min of no-flow ischemia. PostC (6 cycles of 10 s of ischemia and 10 s of reperfusion) was applied immediately upon reperfusion, followed by an additional 90 min of reperfusion. Drug administration is illustrated for each PostC or I/R protocol. SNAP, S-nitroso-N-acetyl-d,l-penicillamine. l-NAME, NG-nitro-l-arginine methyl ester.
Cardiac contractile function and infarct size measurements.
A latex balloon connected to a pressure transducer was inserted into the left ventricle of Langendorff perfused mouse hearts to monitor left ventricular developed pressure (LVDP). LVDP was recorded and digitized using a PowerLab system (ADInstruments, Colorado Springs, CO). The rate-pressure product (RPP = LVDP × heart rate) was used as an index of cardiac contractile function. The postischemic functional recovery was expressed as percentage of the preischemic RPP during the equilibrium period. For measurement of myocardial infarct size, at the end of the 90 min of reperfusion, hearts were perfused with 1% (wt/vol) of 2,3,5-triphenyltetrazolium chloride (TTC) and incubated in TTC at 37°C for 15 min, followed by fixation in 10% (wt/vol) formaldehyde. Infarct size was expressed as the percentage of the total cross-sectional area of the ventricles.
Total heart homogenate preparation.
Total heart homogenate was prepared for 2D DIGE and SNO-RAC proteomic analyses, and sample preparations were carried out in the dark to prevent SNO decomposition. Each snap-frozen mouse heart was powdered on liquid nitrogen followed by homogenization with a tight-fitting glass Dounce homogenizer on ice in 1.5 ml homogenate buffer containing (in mmol/l) 300 sucrose, 250 HEPES-NaOH (pH 7.8), 1 EDTA, and 0.1 neocuproine (a copper chelating agent). An EDTA-free protease inhibitor tablet (Roche Diagnostics, Indianapolis, IN) was added to the homogenate buffer just before use. Protein concentration of total heart homogenate was determined using the Bradford protein assay. Total heart homogenates were aliquoted in amber tubes, snap-frozen on dry ice, and stored at −80°C.
Identification of SNO proteins by 2D CyDye-maleimide DIGE.
We used a modified biotin switch method (10) with CyDye maleimide monoreactive sulfhydryl-reactive fluorescent dyes (GE Healthcare Life Sciences, Piscataway, NJ) to identify SNO proteins. After the CyDye-maleimide switch and 2D DIGE, each gel was scanned at the unique excitation/emission wavelength of each dye using a Typhoon 9400 imager (GE Healthcare Life Sciences) at a resolution of 100 μm. Images from each gel were aligned using the two internal anchor spots and analyzed with Progenesis Discovery software (Nonlinear Dynamics, Newcastle upon Tyne, UK). The gel was poststained with SYPRO Ruby (Sigma), and the protein spots that corresponded to the CyDye-maleimide fluorescent intensity with 25% or higher difference were picked. The Ettan Spot Handling Workstation (GE Healthcare Life Sciences) was used for automated extraction of the selected protein spots followed by in-gel trypsin digestion. After sample extraction from the spot handling workstation, each sample was manually desalted using Millipore (Billerica, MA) C18 Ziptips following the manufacturer's recommendation in preparation for liquid chromatography-tandem MS (LC-MS/MS) analysis.
SNO protein and site identification with SNO-resin assisted capture (SNO-RAC).
A modified version of the SNO-RAC protocol was developed to identify SNO-modified proteins and sites (5, 11, 12). All buffers were degassed before use with the SNO-RAC protocol to prevent oxidation of the thiopropyl Sepharose resin (GE Healthcare, Piscataway, NJ). In brief, after blocking free sulfhydryl group with 20 mmol/l N-ethylmaleimide (NEM), each total heart homogenate (0.25 mg) was loaded onto preequilibrated resin in the presence of 20 mmol/l Na-ascorbate, and incubated for 4 h at room temperature. After washing, resin-bound proteins were then subjected to trypsin digestion (sequencing grade modified; Promega) overnight at 37°C with rotation in the digestion buffer containing (in mmol/l) 50 NH4HCO3 and 1 EDTA. After washing, peptides were eluted for 30 min at room temperature in elution buffer containing (in mmol/l) 20 DTT, 10 NH4HCO3, and 50% methanol (vol/vol), followed by two volumes of H2O. All fractions were combined and concentrated via Speedvac. Samples were then resuspended in 0.1% formic acid and cleaned with Millipore C18 Ziptips for LC-MS/MS analysis.
LC-MS/MS analysis and database search.
LC-MS/MS was performed using an Eksigent nanoLC-Ultra 1D plus system (Dublin, CA) coupled to an LTQ Orbitrap Elite mass spectrometer (Thermo Fisher Scientific, San Jose, CA) using CID fragmentation. Peptides were first loaded onto an Zorbax 300SB-C18 trap column (Agilent, Palo Alto, CA) at a flow rate of 6 μl/min for 6 min, and then separated on a reversed-phase PicoFrit analytical column (New Objective, Woburn, MA) using a short 15-min linear gradient of 5–40% acetonitrile for 2D gel spots and 40-min gradient for SNO-RAC in 0.1% formic acid at a flow rate of 250 nl/min. LTQ-Orbitrap Elite settings were as follows: spray voltage 1.5 kV; full MS mass range m/z 300 to 2,000. The LTQ-Orbitrap Elite was operated in a data-dependent mode; i.e., one MS1 high resolution (60,000) scan for precursor ions followed by six data-dependent MS2 scans for precursor ions above a threshold ion count of 500 with collision energy of 35%. The raw file generated from the LTQ Orbitrap Elite was analyzed using Proteome Discoverer v1.3 software (Thermo Fisher Scientific, LLC) using our six-processor Mascot cluster at NIH (v.2.4) search engine. The search criteria was set to: database, Swiss Institute of Bioinformatics (Sprot_103112, 16573 sequences); taxonomy, Mus musculus (mouse); enzyme, trypsin; miscleavages, 2; variable modifications, Oxidation (M), Deamidation (NQ), Acetyl (protein N-term), NEM (C); MS peptide tolerance 20 ppm; MS/MS tolerance as 0.8 Da. Protein identifications were accepted based on two or more unique peptides with a false discovery rate (FDR) of 99% or higher. Raw mass spectrometry data for SNO sites identified via SNO-RAC in PostC hearts (Table 3) can be accessed at Peptide Atlas (http://www.peptideatlas.org/PASS/PASS00392).
Table 3.
S-nitrosylation sites identified via SNO-RAC proteomic analysis in PostC hearts
| Protein Name | ID | Peptide Sequence | SNO-Cys |
|---|---|---|---|
| Extracellular matrix and cell membrane | |||
| Galectin-1 | P16045 | ACGLVASNLNLKPGECLK | 3, 17 |
| Long-chain fatty acid transport protein 1 | Q60714 | VGSCGFNSR | 406 |
| PDZ and LIM domain protein 5 | Q8CI51 | ACTGSLNMTLQR | 73 |
| Protein-glutamine gamma-glutamyltransferase 2 | P21981 | YSGCLTESNLIK | 553 |
| Cytoplasm and cytoskeleton | |||
| 6-Phosphofructokinase, muscle type | P47857 | LPLMECVQVTK | 351 |
| IFANTPDSGCVLGMR | 709 | ||
| Alpha-enolase* | P17182 | VNQIGSVTESLQACK | 357 |
| Annexin A6 | P14824 | GTVCAANDFNPDADAK | 358 |
| Cytoplasmic dynein 1 heavy chain 1 | Q9JHU4 | VQYPQSQACK | 631 |
| LQGATCSNNK | 4568 | ||
| Destrin | Q9R0P5 | ASGVQVADEVCR | 12 |
| Dihydropyrimidinase-related protein 2 | O08553 | GLYDGPVCEVSVTPK | 504 |
| E3 ubiquitin-protein ligase UBR4 | A2AN08 | AVQCLNTSSK | 2552 |
| Elongation factor 2 | P58252 | ETVSEESNVLCLSK | 591 |
| Filamin-B | Q80X90 | VAVTEGCQPSR | 1326 |
| Fructose-bisphosphate aldolase A* | P05064 | ALANSLACQGK | 339 |
| Glutaredoxin-1 | Q9QUH0 | AQEFVNCK | 8 |
| Glyceraldehyde-3-phosphate dehydrogenase* | P16858 | IVSNASCTTNCLAPLAK | 150, 154 |
| VPTPNVSVVDLTCR | 245 | ||
| Heat shock protein HSP 90-β | P11499 | FENLCK | 564 |
| l-Lactate dehydrogenase A chain | P06151 | VIGSGCNLDSAR | 163 |
| Myosin-binding protein C, cardiac-type | O70468 | ATNLQGEAQCECR | 1260, 1262 |
| Myosin light chain kinase, smooth muscle | Q6PDN3 | VAGTQPITCK | 1291 |
| Obscurin | A2AAJ9 | QADTGTVCATSPK/ | 3060 |
| Rab GDP dissociation inhibitor β | Q61598 | TDDYLDQPcCETINR | 203 |
| Selenium-binding protein 1 | P17563 | GGSVQVLEDQELTCQPEPLVVK | 371 |
| Serine/threonine-protein phosphatase 2A 55 kDa regulatory subunit B α isoform | Q6P1F6 | AGAGGGNDIQWCFSQVK | 13 |
| Titin* | A2ASS6 | VSECYVAR | 25303 |
| VLDSPGPCGK | 30933 | ||
| Trifunctional purine biosynthetic protein adenosine-3 | Q64737 | QVLVAPGNAGTACAGK | 41 |
| Triosephosphate isomerase | P17751 | IAVAAQNCYK | 117 |
| IIYGGSVTGATCK | 268 | ||
| Tripartite motif-containing protein 72 | Q1XH17 | MQLQEACMR | 144 |
| FCLVTSR | 242 | ||
| Tubulin β-4A chain | Q9D6F9 | NMMAACDPR | 303 |
| TAVCDIPPR | 354 | ||
| Ubiquitin-like modifier-activating enzyme 1 | Q02053 | DNPGVVTCLDEAR | 234 |
| Endo/sarcoplasmic reticulum, nucleus, ribosome | |||
| Bifunctional glutamate/proline-tRNA ligase | Q8CGC7 | VACQGEVVR | 910 |
| Cellular nucleic acid-binding protein | P53996 | TSEVNCYR | 159 |
| eIF4E-binding protein 1 | Q60876 | SAGSSCSQTPSR | 7 |
| Nascent polypeptide-associated complex subunit α | P70670 | GTVVCLADSSLDTSVSASK | 755 |
| Poly(rC)-binding protein 1 | P60335 | INISEGNCPER | 54 |
| 40S ribosomal protein S3 | P62908 | GCEVVVSGK | 134 |
| 40S ribosomal protein S28 | P62858 | TGSQGQCTQVR | 27 |
| 60S ribosomal protein L10 | Q6ZWV3 | MLSCAGADR | 105 |
| 60S ribosomal protein L27a | P14115 | NQSFCPTVNLDK | 70 |
| Sarco/endoplasmic reticulum calcium ATPase 2 | O55143 | SLPSVETLGCTSVICSDK | 344, 349 |
| TGTLTTNQMSVCR | 364 | ||
| Mitochondria | |||
| 2-Oxoglutarate dehydrogenase | Q60597 | ICEEAFTR | 566 |
| 3-Ketoacyl-CoA thiolase | Q8BWT1 | YAVGSACIGGGQGIALIIQNTA | 382 |
| Acetyl-CoA acetyltransferase | Q8QZT1 | QATLGAGLPISTPCTTVNK | 116 |
| ATP synthase subunit ϵ | P56382 | FSQICAK | 19 |
| Aconitate hydratase | Q99KI0 | VGLIGSCTNSSYEDMGR | 385 |
| Aspartate aminotransferase | P05202 | VGAFTVVCK | 295 |
| Carnitine O-acetyltransferase | P47934 | IYGQACATYESASLR | 449 |
| Carnitine O-palmitoyltransferase 1 | Q924X2 | SCTNESAAFVQAMMK | 608 |
| Citrate synthase | Q9CZU6 | LPCVAAK | 211 |
| Creatine kinase S-type | Q6P8J7 | GLSLPPACSR | 180 |
| Cytochrome b-c1 complex subunit 1* | Q9CZ13 | LCTSATESEVTR | 380 |
| Dihydrolipoyllysine-residue acetyltransferase component of pyruvate dehydrogenase complex | Q8BMF4 | DVPLGAPLCIIVEK | 290 |
| Dynamin-1-like protein | Q8K1M6 | FATEYCNTIEGTAK | 351 |
| Electron transfer flavoprotein-ubiquinone oxidoreductase | Q921G7 | ASCDAQTYGIGLK | 265 |
| Enoyl-CoA hydratase | Q8BH95 | TFQDCYSSK | 111 |
| LVEEAIQCAEK | 225 | ||
| Glutathione S-transferase κ 1 | Q9DCM2 | LIENTDAACK | 176 |
| Iron-sulfur cluster assembly 2 homolog | Q9DCB8 | LTDSCVQR | 56 |
| Isocitrate dehydrogenase [NADP]* | P54071 | SSGGFVWACK | 308 |
| VCVQTVESGAMTK | 402 | ||
| Isocitrate dehydrogenase [NAD] subunit α | Q9D6R2 | IEAACFATIK | 331 |
| cSDFTEEICR | 359 | ||
| Lactation elevated protein 1 | Q3V384 | VVQCLQK | 100 |
| Leucine-rich PPR motif-containing protein | Q6PB66 | LIAAYCNVGDIEGASK | 207 |
| Malate dehydrogenase* | P08249 | GYLGPEQLPDCLK | 89 |
| EGVVECSFVQSK | 275 | ||
| Methylmalonate-semialdehyde dehydrogenase | Q9EQ20 | VCNLIDSGTK | 368 |
| Mitochondrial tRNA-specific 2-thiouridylase 1 | Q9DAT5 | TPNPDINCNK | 101 |
| Mitofusin-1 | Q811U4 | LCQQVDVTQK | 681 |
| NADH-ubiquinone oxidoreductase 75 kDa subunit | Q91VD9 | LSVAGNCR | 75 |
| AVTEGAQAVEEPSIC | 727 | ||
| NADH-ubiquinone oxidoreductase chain 3 | P03899 | ANPYECGFDPTSSAR | 39 |
| Propionyl-CoA carboxylase α chain | Q91ZA3 | MADEAVCVGPAPTSK | 107 |
| Short/branched chain specific acyl-CoA dehydrogenase | Q9DBL1 | ASSTCQLTFENVK | 261 |
| Succinate dehydrogenase cytochrome b560 subunit | Q9CZB0 | SLCLGPTLIYSAK | 107 |
| Succinate dehydrogenase [ubiquinone] flavoprotein subunit | Q8K2B3 | TLNEADCATVPPAIR | 654 |
| Succinate-semialdehyde dehydrogenase | Q8BWF0 | NAGQTCVCSNR | 328, 330 |
| Succinyl-CoA ligase subunit α | Q9WUM5 | LIGPNCPGVINPGECK | 172, 181 |
| Succinyl-CoA ligase subunit β | Q9Z2I9 | IcNQVLVCER | 158 |
| ILACDDLDEAAK | 430 | ||
| Voltage-dependent anion-selective channel-1 | Q60932 | YQVDPDACFSAK | 245 |
| Voltage-dependent anion-selective channel-2* | Q60930 | SCSGVEFSTSGSSNTDTGK | 48 |
| Voltage-dependent anion-selective channel-3 | Q60931 | cNTPTYCDLGK | 8 |
S-nitrosylation (SNO) cysteine residues (SNO-Cys) are labeled in upper case and underlined (C); cysteine residues blocked by N-ethylmaleimide (NEM) are labeled in lower case (c). Protein identifications were accepted based on two or more unique peptides with a FDR of 99% or higher. *SNO-modified proteins and peptides were also identified from 2 of 3 SNO-RAC/LC-MS/MS proteomic analyses. SNO-Cys indicates the amino acid in the protein that is SNO.
Data analysis.
Results are expressed as means ± SE. Statistical significance was determined by one-way ANOVA followed by a post hoc Bonferroni test.
RESULTS
Ischemic PostC exerted a protective effect.
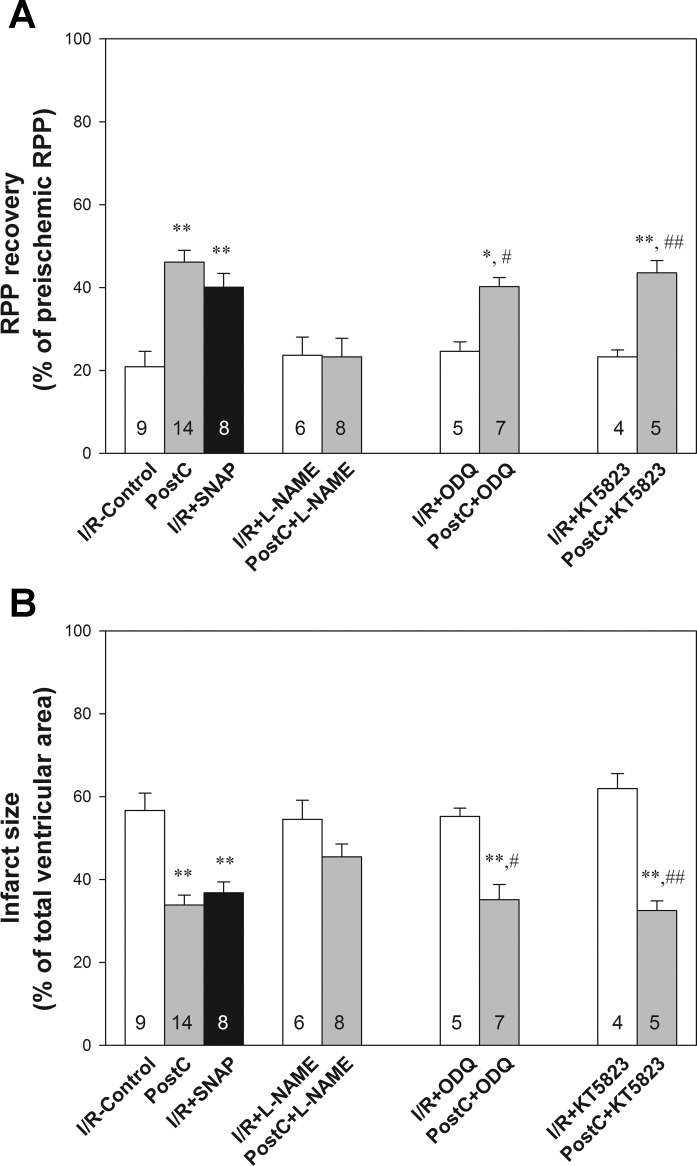
We first confirmed that a protocol of PostC with six cycles of 10 s of reperfusion and 10 s of ischemia applied at the beginning of reperfusion after 25 min of global no-flow ischemia (Fig. 1) was cardioprotective. As shown in Table 1 and Fig. 2, PostC significantly increased the postischemic functional recovery, as the rate-pressure product (RPP) recovery was 46.1 ± 2.9% (n = 14) in PostC hearts compared with 20.9 ± 3.7% (n = 9) in I/R-control hearts. The postischemic myocardial infarction was 33.8 ± 2.4% (n = 14), significantly smaller than I/R-control hearts (56.7 ± 4.2%, n = 9).
Table 1.
Evaluation of cardiac contractile function in Langendorff-perfused mouse hearts
| Preischemic Equilibration |
End of Reperfusion |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Heart Samples | n | BW, g | FR, ml/min | HR, beats/min | LVDP, cmH2O | +dP/dt, cmH2O/ms | −dP/dt, cmH2O/ms | FR, ml/min | HR, beats/min | LVDP, cmH2O | +dP/dt, cmH2O/ms | −dP/dt, cmH2O/ms |
| I/R-Control | 9 | 27.2 ± 0.6 | 2.6 ± 0.2 | 346 ± 18 | 144 ± 8 | 8.1 ± 0.5 | −5.8 ± 0.2 | 1.7 ± 0.2 | 338 ± 10 | 33 ± 6 | 4.2 ± 0.4 | −3.4 ± 0.3 |
| PostC | 14 | 26.6 ± 0.5 | 2.5 ± 0.2 | 351 ± 12 | 135 ± 4 | 8.5 ± 0.3 | −5.9 ± 0.2 | 1.7 ± 0.1 | 320 ± 11 | 67 ± 5† | 5.6 ± 0.3 | −4.3 ± 0.2 |
| I/R-SNAP | 8 | 27.9 ± 0.3 | 2.7 ± 0.2 | 389 ± 12 | 125 ± 9 | 8.0 ± 0.3 | −5.2 ± 0.3 | 1.8 ± 0.1 | 345 ± 11 | 54 ± 3* | 4.7 ± 0.2 | −3.9 ± 0.3 |
| I/R-l-NAME | 6 | 27.2 ± 0.5 | 2.6 ± 0.1 | 380 ± 20 | 147 ± 8 | 9.1 ± 0.4 | −6.2 ± 0.2 | 2.1 ± 0.2 | 301 ± 19 | 44 ± 8 | 4.2 ± 0.5 | −3.7 ± 0.4 |
| l-NAME + PostC | 8 | 26.5 ± 0.6 | 2.9 ± 0.2 | 374 ± 11 | 143 ± 4 | 9.0 ± 0.4 | −6.2 ± 0.4 | 1.8 ± 0.3 | 309 ± 13 | 47 ± 6 | 4.2 ± 0.5 | −3.6 ± 0.4 |
| I/R-ODQ | 5 | 27.3 ± 0.3 | 2.7 ± 0.3 | 386 ± 26 | 129 ± 7 | 8.1 ± 0.6 | −5.5 ± 0.4 | 1.7 ± 0.2 | 344 ± 13 | 30 ± 6 | 4.0 ± 0.5 | −3.3 ± 0.2 |
| ODQ + PostC | 7 | 28.3 ± 0.5 | 2.5 ± 0.2 | 379 ± 20 | 130 ± 7 | 9.0 ± 0.6 | −5.7 ± 0.4 | 2.0 ± 0.2 | 334 ± 10 | 57 ± 4‡ | 4.7 ± 0.3 | −3.9 ± 0.3 |
| I/R-KT5823 | 4 | 27.6 ± 0.3 | 2.5 ± 0.1 | 416 ± 24 | 124 ± 6 | 8.4 ± 0.6 | −5.6 ± 0.3 | 1.9 ± 0.1 | 314 ± 11 | 39 ± 5 | 3.9 ± 0.3 | −3.1 ± 0.4 |
| KT5823 + PostC | 5 | 27.6 ± 0.6 | 2.4 ± 0.1 | 408 ± 21 | 127 ± 5 | 8.5 ± 0.4 | −6.3 ± 0.4 | 1.9 ± 0.1 | 344 ± 19 | 65 ± 3§ | 5.5 ± 0.3 | −4.0 ± 0.2 |
Values are means ± SE; n = no. of hearts. BW, body weight; FR, flow rate; HR, heart rate; LVDP, left ventricular developed pressure; ±dP/dt, rates of pressure rise and fall, respectively; I/R, ischemia-reperfusion; SNAP, S-nitroso-N-acetyl-d,l-penicillamine. l-NAME, NG-nitro-l-arginine methyl ester; ODQ, 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one; PostC, postconditioning;.
P < 0.05,
P < 0.01 vs. I/R-control;
P < 0.05 vs. I/R-ODQ;
P < 0.05 vs. I/R- KT5823.
Fig. 2.
PostC-induced cardioprotection was dependent upon nitric oxide (NO)/S-nitrosylation (SNO) signaling. A: postischemic left ventricular rate-pressure product (RPP) functional recovery. B: infarct size, measured at the end of reperfusion by 1% TTC staining. Results are expressed as means ± SE. Statistical significance was determined by one-way ANOVA followed by a post hoc Bonferroni test. *P < 0.05; **P < 0.01 vs. I/R-control; #P < 0.05 vs. I/R + 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ); ##P < 0.01 vs. I/R + KT5823. The number of animals in each group is indicated in the column.
Reperfusion with SNAP protected the heart against I/R injury.
Cohen et al. (3) recently demonstrated that infusion of an NO/SNO donor SNAP at reperfusion has a protective effect in an intact rabbit coronary artery occlusion and reperfusion model. To test whether an NO donor can mimic PostC in our Langendorff perfused mouse heart I/R model, we infused the NO donor, SNAP (10 μmol/l), into the perfusate during the first 7 min of reperfusion. As shown in Fig. 2, treatment with SNAP upon reperfusion significantly improved cardiac postischemic functional recovery (40.0 ± 3.4%, n = 8) and decreased postischemic myocardial infarct size (36.8 ± 2.7%, n = 8).
PostC increased myocardial SNO.
To test whether PostC increased SNO, PostC hearts were collected and snap frozen in liquid N2 after 2 min of PostC plus 5 min of additional reperfusion and I/R-control hearts after 7 min of reperfusion. The total heart homogenate was prepared in the dark as described in methods and materials. A modified biotin switch method using CyDye-maleimide monoreactive fluorescence dyes and 2D DIGE proteomic analysis was carried out for SNO detection (25, 26). As shown in Fig. 3, SNO proteins in I/R-control hearts were labeled by Cy3-maleimide (pseudocolored in green) and PostC hearts were labeled with Cy5-maleimide (pseudocolored in red). SNO protein spots showing a change of at least 25% or higher in PostC hearts compared with I/R-control were picked for identification via mass spectrometry. As shown in Table 2, PostC-treated hearts showed an increase in SNO for a number of proteins, and most of these SNO proteins were previously found in IPC hearts (25, 26), including aconitase, ATP synthase subunit α, creatine kinase S/M type, α-cardiac muscle actin, cytoplasmic malate dehydrogenase, electron transfer flavoproteins α/β, myosin light chain 1, and myoglobin.
Fig. 3.
PostC increased myocardial protein SNO. Top: representative two-dimensional (2D) CyDye-maleimide difference gel electrophoresis (DIGE) gel from three independent experiments was scanned at each of the distinct wavelengths of the fluors, showing a pattern of protein SNO for that particular treatment group. Bottom: overlaid image of Cy3-maleimide (I/R control, green) vs. Cy5-maleimide (PostC, red). Protein spots (1–13) showing a change of at least 25% or higher in PostC hearts compared with I/R-control were picked for MS/MS analysis and are listed in Table 2.
Table 2.
Proteins identified by 2D CyDye-maleimide DIGE with increased SNO level in PostC hearts
| SNO Level (Arbitrary Ratio) |
||||||
|---|---|---|---|---|---|---|
| Spots | Protein Name | Accession No. | MW, kDa | Protein pI | PostC vs. I/R | PostC vs. PostC + l-NAME |
| 1 | 2-Oxoglutarate dehydrogenase | Q60597 | 116.4 | 6.83 | 1.48 ± 0.15 | 1.40 ± 0.16 |
| 2 | Aconitate hydratase, mitochondrial | Q99KI0 | 85.4 | 8.08 | 1.50 ± 0.19 | 1.38 ± 0.14 |
| 3 | Mitochondrial F1-ATPase subunit α | Q03265 | 59.7 | 9.22 | 1.47 ± 0.18 | 1.50 ± 0.13 |
| 4 | Creatine kinase S-type | Q6P8J7 | 47.8 | 8.64 | 1.45 ± 0.18 | 1.53 ± 0.14 |
| 5 | Creatine kinase M-type | P07310 | 43.0 | 6.58 | 1.51 ± 0.28 | 1.41 ± 0.11 |
| 6 | α-Cardiac muscle actin | P68033 | 42.3 | 5.23 | 1.45 ± 0.17 | 1.44 ± 0.16 |
| 7 | Malate dehydrogenase, cytoplasmic | P14152 | 36.5 | 6.16 | 1.46 ± 0.15 | 1.49 ± 0.19 |
| 8 | Glyceraldehyde-3-phosphate dehydrogenase | P16858 | 35.8 | 8.25 | 1.28 ± 0.04 | 1.35 ± 0.09 |
| 9 | Malate dehydrogenase, mitochondrial | P08249 | 35.6 | 8.68 | 1.57 ± 0.14 | 1.45 ± 0.08 |
| 10 | Electron transfer flavoprotein α | Q99LC5 | 35.0 | 8.62 | 1.54 ± 0.12 | 1.55 ± 0.14 |
| 11 | Electron transfer flavoprotein β | Q9DCW4 | 27.8 | 8.24 | 1.46 ± 0.12 | 1.49 ± 0.13 |
| 12 | Myosin light chain 1 | P09542 | 22.5 | 5.03 | 1.43 ± 0.13 | 1.50 ± 0.13 |
| 13 | Myoglobin | P04247 | 17.1 | 7.07 | 1.30 ± 0.05 | 1.31 ± 0.07 |
Values are means ± SE. Protein identifications were accepted based on two or more unique peptides with a false discovery rate (FDR) of 99% or higher and a correct molecular mass identification. SNO protein spots showed a change of at least 25% or higher in PostC hearts compared with I/R-control or PostC + l-NAME hearts (P < 0.05, n = 3 in each group).
l-NAME abolished the PostC-induced increase in protein SNO.
To confirm that the changes in SNO observed in Fig. 3 and Table 2 are involved in the protection associated with PostC, we performed additional experiments using l-NAME to determine whether the inhibition of NOS with l-NAME, which has been previously shown to block PostC-induced protection, would also block the PostC-mediated increase in protein SNO. Langendorff-perfused mouse hearts were treated with 10 μmol/l l-NAME during the first 7 min of reperfusion. Compared with I/R-control, perfusion of non-PostC hearts with l-NAME had no significant effect on postischemic functional recovery (Table 1, Fig. 2A) or infarct size (Fig. 2B). However, treatment with l-NAME blocked PostC-induced cardioprotection (Fig. 2). After confirming that PostC-induced protection was blocked by l-NAME, we compared the SNO level of PostC hearts with and without l-NAME treatment to determine whether the PostC-induced increase in SNO could be prevented by l-NAME. As shown in Table 2, PostC + l-NAME hearts have significantly lower SNO levels compared with PostC hearts, suggesting that inhibition of NOS with l-NAME prevented the PostC-induced increase in SNO.
S-nitrosylation site identification in PostC hearts via SNO-RAC analysis.
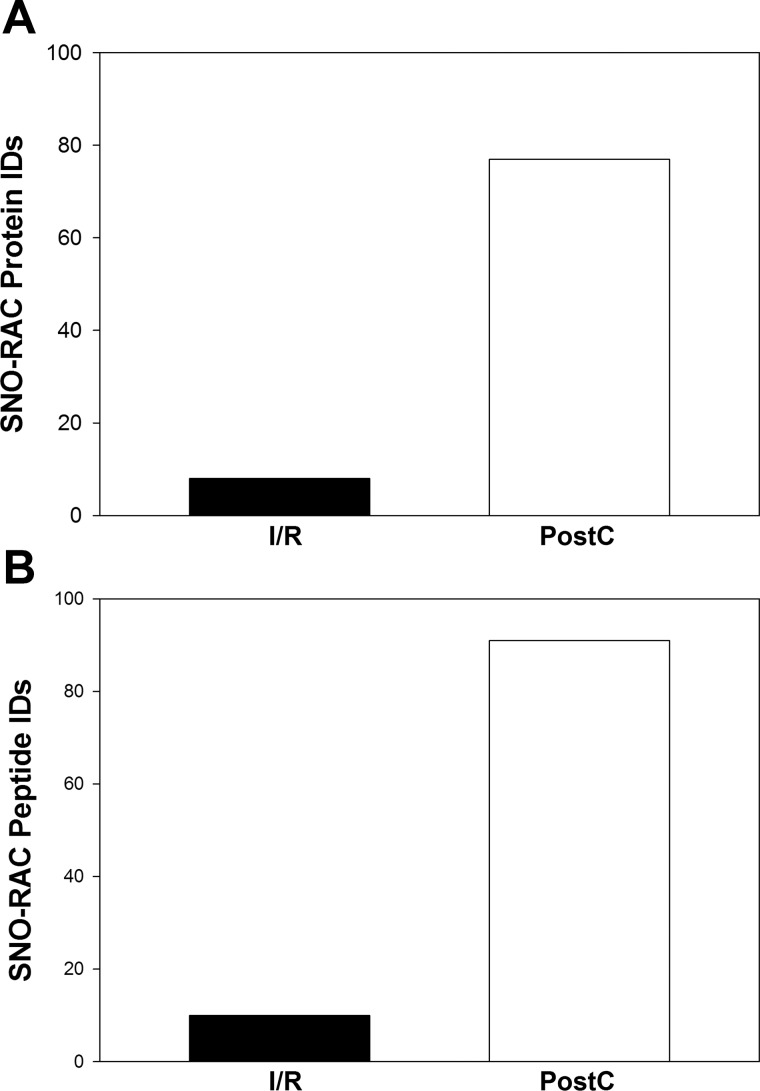
Because of dynamic range issues and the limited amount of protein that can be loaded onto the 2D gel, the SNO proteins identified in 2D DIGE are biased toward detection of high-abundance proteins (Table 2). Furthermore, although 2D fluor-maleimide DIGE is useful in screening and quantifying SNO proteins, unfortunately we have not been able to identify SNO sites with this method (12). Therefore, we utilized a SNO-RAC protocol to identify SNO proteins and sites in total heart homogenates. As shown in Fig. 4, SNO-RAC detected only 10 SNO peptides from 8 unique proteins in I/R hearts, while 91 SNO peptides from 77 proteins were identified in PostC hearts. Almost half (37 of 77) of the identified SNO proteins are mitochondrial, suggesting mitochondria are a major organelle targeted by PostC-mediated SNO. Most of these proteins contain only one SNO modified cysteine, while ∼20% of them contain two or more SNO sites (Table 3).
Fig. 4.
PostC increased total number of SNO-modified proteins and sites. Total number of SNO-modified proteins (A) and sites (B) from SNO-RAC were identified via LC-MS/MS for I/R and PostC hearts (n = 3 in each group). Protein identifications were accepted based on two or more unique peptides with a false discovery rate (FDR) of 99% or higher. Each protein/peptide was identified from at least 2 of 3 SNO-RAC/LC-MS/MS proteomic analyses.
ODQ or KT5823 treatment did not block PostC-mediated protection.
In non-PostC hearts, perfusion with either ODQ (a sGC inhibitor) or KT5823 (a specific protein kinase G inhibitor) during the first 7 min of reperfusion did not significantly affect postischemic functional recovery or infarct size (Fig. 2). In contrast to l-NAME treatment, which abolished the protection of PostC, 10 μmol/l ODQ or 1 μmol/l KT5823 treatment did not block PostC-induced cardioprotection, i.e., postischemic RPP recovery was 40.2 ± 2.2% (n = 7) for PostC + ODQ and 43.5 ± 3.0% (n = 5) for PostC + KT5823, infarct size was 35.1 ± 3.7% (n = 7) for PostC + ODQ and 32.5 ± 2.3% (n = 5) for PostC + KT5823, which were comparable to the protective effect induced by PostC.
DISCUSSION
NO signaling has been suggested to play an important role in PostC-induced protection. Inhibition of NOS by l-NAME has been shown to block protection in a variety of postconditioning models (18, 29, 33). Furthermore PostC was blocked by reducing agents such as N-acetyl-l-cysteine or 2-mercaptopropionylglycine (19), suggesting that a redox-sensitive mechanism is also involved in the protection afforded by PostC. In addition, a recent study has suggested that PostC prolongs early acidosis, and this would favor the formation of protein SNO (22). Therefore, all of these studies suggest a possible role for protein S-nitrosylation in PostC-induced cardioprotection.
The results contained herein provide the first demonstration that PostC leads to an increase in protein SNO. We further show that this PostC mediated increase in protein SNO is blocked with l-NAME, which also blocks the protective effects of PostC. Comparing the SNO proteins measured by SNO-RAC in PostC hearts (Table 3) with the proteins that show SNO in IPC hearts [Table 1 from Kohr et al. (13)], we find that ∼50% of those proteins that were SNO with IPC also show SNO with PostC (25, 26), suggesting that there might be a common set of proteins targeted by NO/SNO signaling with both IPC and PostC. Therefore, the increase in SNO in IPC and PostC may play a similar role in cardioprotection against I/R injury. For example, we have shown that IPC led to an increase in SNO of the mitochondrial F1-ATPase subunit α. In this study, we also found that PostC induced an increase in SNO of the mitochondrial F1-ATPase (Table 2). In addition, the IPC-induced increase in SNO could shield critical cysteine residue(s) from further oxidative damage upon reperfusion (13). Interestingly, a similar finding has been reported in a recent study, in which Cys294 of the mitochondrial F1-ATPase was found to form a disulfide bond with another cysteine residue in dyssynchronous heart failure, while cardiac resynchronization therapy leads to SNO of Cys294 and prevents disulfide formation (31).
The sGC/cGMP/protein kinase G (PKG) signaling pathway has been suggested to mediate PostC-induced cardioprotection (4, 15), and the main supportive evidence is that inhibition of the cGMP-dependent signaling pathway with selective inhibitors such as ODQ or KT5823 blocks the protection induced by PostC (8, 18, 32). However, a recent study questioned the role of NO-mediated sGC/cGMP/PKG-dependent signaling in PostC by demonstrating that the addition of SNAP, an NO donor, at reperfusion produced protection that was not blocked by ODQ (3). In addition, a role for cGMP-independent NO-induced cardioprotection against I/R injury has also been demonstrated in studies using isolated cardiomyocytes (6, 9). In the present study, treatment of perfused mouse hearts with either ODQ or KT5823 did not alter postischemic functional recovery or infarct size in non-PostC hearts. However, mouse hearts treated with either ODQ or KT5823 were still protected by PostC, suggesting that blockade of the sGC/cGMP/PKG pathway does not abolish NO-dependent PostC-induced cardioprotection. In addition, Methner et al. (16) have shown that protection through postconditioning is unaffected by cardiomyocyte-selective ablation of protein kinase G.
Cardioprotection similar to that obtained with PostC could be also achieved with pharmacological agents given upon reperfusion, i.e., pharmacological postconditioning. By using an intact I/R heart model, Cohen et al. (3) showed that SNAP, an S-nitrosothiol agent, was protective when administered upon reperfusion. In this study, we found that SNAP also elicits pharmacological postconditioning effects in Langendorff perfused mouse hearts (Fig. 2 and Table 1). A mitochondria-targeted S-nitrosothiol agent, mito-SNO, has also been shown to be protective when given at reperfusion in a recent study with an open-chest mouse I/R model. Furthermore, the protection afforded by PostC or mito-SNO has been found to be unaffected by cardiomyocyte-selective ablation of PKG, suggesting an important role for SNO signaling in PostC (16). In addition, Penna et al. (21) showed that pharmacological PostC by diazoxide induced mitochondrial protein S-nitrosylation.
Limitations and perspectives.
The data in this paper demonstrate that PostC leads to an increase in SNO and myocardial protection. Further, the protection afforded by PostC was abolished by l-NAME, suggesting a key role for NO/SNO signaling in PostC-induced protection. However, treatment with either ODQ (a specific sGC inhibitor) or KT5823 (a specific PKG inhibitor) did not block PostC-induced protection, suggesting that NO-mediated protein SNO, rather than activation of the sGC/cGMP/PKG signaling pathway, plays an essential role in PostC. These results together with similar findings from IPC hearts (25) suggest that NO-mediated protein SNO plays a common protective role in the myocardium. These studies have identified a number of proteins that undergo SNO with PostC. However, future studies will be needed to demonstrate the functional impact of SNO on these protein targets and their specific role in PostC.
GRANTS
This work was supported by the National Institutes of Health Intramural Program (A. M. Aponte, J. Sun, and E. Murphy), the American Heart Association (12BGIA11780030, M. J. Kohr), and National Heart, Lung, and Blood Institute Grants 5R-01-HL-039752 (C. Steenbergen) and 1K99-HL-114721 (M. J. Kohr). G. Tong was supported by the China Scholarship Council No. 2011659007.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: G.T., A.M.A., M.J.K., C.S., E.M., and J.S. conception and design of research; G.T. and J.S. performed experiments; G.T. and J.S. analyzed data; G.T., A.M.A., M.J.K., C.S., E.M., and J.S. interpreted results of experiments; G.T. and J.S. prepared figures; G.T. and J.S. drafted manuscript; G.T., A.M.A., M.J.K., C.S., E.M., and J.S. edited and revised manuscript; G.T., A.M.A., M.J.K., C.S., E.M., and J.S. approved final version of manuscript.
REFERENCES
- 1.Burley DS, Ferdinandy P, Baxter GF. Cyclic GMP and protein kinase-G in myocardial ischaemia-reperfusion: opportunities and obstacles for survival signaling. Br J Pharmacol 152: 855–869, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen MV, Downey JM. Ischemic postconditioning: from receptor to end-effector. Antioxid Redox Signal 14: 821–831, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Cohen MV, Yang XM, Liu Y, Solenkova NV, Downey JM. Cardioprotective PKG-independent NO signaling at reperfusion. Am J Physiol Heart Circ Physiol 299: H2028–H2036, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Costa AD, Garlid KD, West IC, Lincoln TM, Downey JM, Cohen MV, Critz SD. Protein kinase G transmits the cardioprotective signal from cytosol to mitochondria. Circ Res 97: 329–336, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Forrester MT, Thompson JW, Foster MW, Nogueira L, Moseley MA, Stamler JS. Proteomic analysis of S-nitrosylation and denitrosylation by resin-assisted capture. Nat Biotech 27: 557–559, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garreffa AM, Woodman OL, Cao AH, Ritchie RH. Sodium nitroprusside protects adult rat cardiac myocytes from cellular injury induced by simulated ischemia: role for a non-cGMP-dependent mechanism of nitric oxide protection. J Cardiovasc Pharmacol 47: 1–8, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Grover GJ, Atwal KS, Sleph PG, Wang FL, Monshizadegan H, Monticello T, Green DW. Excessive ATP hydrolysis in ischemic myocardium by mitochondrial F1F0-ATPase: effect of selective pharmacological inhibition of mitochondrial ATPase hydrolase activity. Am J Physiol Heart Circ Physiol 287: H1747–H1755, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Inserte J, Barba I, Poncelas-Nozal M, Hernando V, Agullo L, Ruiz-Meana M, Garcia-Dorado D. cGMP/PKG pathway mediates myocardial postconditioning protection in rat hearts by delaying normalization of intracellular acidosis during reperfusion. J Mol Cell Cardiol 50: 903–909, 2011 [DOI] [PubMed] [Google Scholar]
- 9.Iwase H, Robin E, Guzy RD, Mungai PT, Vanden Hoek TL, Chandel NS, Levraut J, Schumacker PT. Nitric oxide during ischemia attenuates oxidant stress and cell death during ischemia and reperfusion in cardiomyocytes. Free Radic Biol Med 43: 590–599, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Jaffrey SR, Snyder SH. The biotin switch method for the detection of S-nitrosylated proteins. Sci STKE 86: PL1, 2001 [DOI] [PubMed] [Google Scholar]
- 11.Kohr MJ, Aponte A, Sun J, Gucek M, Steenbergen C, Murphy E. Measurement of S-nitrosylation occupancy in the myocardium with cysteine-reactive tandem mass tags. Circ Res 111: 1308–1312, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kohr MJ, Aponte AM, Sun J, Wang G, Murphy E, Gucek M, Steenbergen C. Characterization of potential S-nitrosylation sites in the myocardium. Am J Physiol Heart Circ Physiol 300: H1327–H1335, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kohr MJ, Sun J, Aponte A, Wang G, Gucek M, Murphy E, Steenbergen C. Simultaneous measurement of protein oxidation and S-nitrosylation during preconditioning and ischemia/reperfusion injury with resin-assisted capture. Circ Res 108: 418–426, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lima B, Forrester MT, Hess DT, Stamler JS. S-nitrosylation in cardiovascular signaling. Circ Res 106: 633–646, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lochner A, Marais E, Du Toit E, Moolman J. Nitric oxide triggers classic ischemic preconditioning. Ann NY Acad Sci 962: 402–414, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Methner C, Lukowski R, Grube K, Loga F, Smith RJ, Murphy M, Hofmann F, Krieg T. Protection through postconditioning or a mitochondria-targeted S-nitrosothiol is unaffected by cardiomyocyte-selective ablation of protein kinase G. Basic Res Cardiol 108: 337, 2013 [DOI] [PubMed] [Google Scholar]
- 17.Murray CI, Chung H, Uhrigshardt H, Van Eyk JE. Quantification of mitochondrial S-nitrosylation by CysTMT(6) switch assay. Methods Mol Biol 1005: 169–179, 2013 [DOI] [PubMed] [Google Scholar]
- 18.Penna C, Cappello S, Mancardi D, Raimondo S, Rastaldo R, Gattullo D, Losano G, Pagliaro P. Post-conditioning reduces infarct size in the isolated rat heart: role of coronary flow and pressure and the nitric oxide/cGMP pathway. Basic Res Cardiol 101: 168–179, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Penna C, Mancardi D, Rastaldo R, Losano G, Pagliaro P. Intermittent activation of bradykinin B2 receptors and mitochondrial KATP channels trigger cardiac postconditioning through redox signaling. Cardiovasc Res 75: 168–177, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Penna C, Mancardi M, Raimondo S, Geuna S, Pagliaro P. The paradigm of postconditioning to protect the heart. J Cell Mol Med 12: 435–458, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Penna C, Perrelli MG, Tullio F, Angotti C, Camporeale A, Poli V, Pagliaro P. Diazoxide postconditioning induces mitochondrial protein S-Nitrosylation and a redox-sensitive mitochondrial phosphorylation/translocation of RISK elements: no role for SAFE. Basic Res Cardiol 108: 371, 2013 [DOI] [PubMed] [Google Scholar]
- 22.Penna C, Perrelli MG, Tullio F, Moro F, Parisella ML, Merlino A, Pagliaro P. Post-ischemic early acidosis in cardiac postconditioning modifies the activity of antioxidant enzymes, reduces nitration, and favors protein S-nitrosylation. Pflügers Arch 462: 219–233, 2011 [DOI] [PubMed] [Google Scholar]
- 23.Schulman IH, Hare JM. Regulation of cardiovascular cellular processes by S-nitrosylation. Biochim Biophys Acta 1820: 752–762, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun J. Protein S-nitrosylation: a role of nitric oxide signaling in cardiac ischemic preconditioning. Acta Physiol Sin 59: 544–552, 2007 [PubMed] [Google Scholar]
- 25.Sun J, Aponte AM, Kohr MJ, Tong G, Steenbergen C, Murphy E. Essential role of nitric oxide in acute ischemic preconditioning: S-Nitros(yl)ation versus sGC/cGMP/PKG signaling? Free Radic Biol Med 54: 105–112, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun J, Kohr MJ, Nguyen T, Aponte AM, Connelly PS, Esfahani SG, Gucek M, Daniels MP, Steenbergen C, Murphy E. Disruption of caveolae blocks ischemic preconditioning-mediated S-nitrosylation of mitochondrial proteins. Antioxid Redox Signal 16: 45–56, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun J, Morgan M, Shen RF, Steenbergen C, Murphy E. Preconditioning results in S-nitrosylation of proteins involved in regulation of mitochondrial energetics and calcium transport. Circ Res 101: 1155–1163, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Sun J, Murphy E. Protein S-nitrosylation and cardioprotection. Circ Res 106: 285–296, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tong G, Sun Z, Wei X, Gu C, Kaye AD, Wang Y, Li J, Zhang Q, Guo H, Yu S, Yi D, Pei J. U50,488H postconditioning reduces apoptosis after myocardial ischemia and reperfusion. Life Sci 88: 31–38, 2011 [DOI] [PubMed] [Google Scholar]
- 30.Tsang A, Hausenloy DJ, Mocanu MM, Yellon DM. Postconditioning: a form of “modified reperfusion” protects the myocardium by activating the phosphatidylinositol 3-kinase-Akt pathway. Circ Res 95: 230–232, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Wang SB, Foster DB, Rucker J, O'Rourke B, Kass DA, Van Eyk JE. Redox regulation of mitochondrial ATP synthase: implications for cardiac resynchronization therapy. Circ Res 109: 750–757, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang XM, Philipp S, Downey JM, Cohen MV. Postconditioning's protection is not dependent on circulating blood factors or cells but involves adenosine receptors and requires PI3-kinase and guanylyl cyclase activation. Basic Res Cardiol 100: 57–63, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Yang XM, Proctor JB, Cui L, Krieg T, Downey JM, Cohen MV. Multiple, brief coronary occlusions during early reperfusion protect rabbit hearts by targeting cell signaling pathways. J Am Coll Cardiol 44: 1103–1110, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Zhao ZQ, Corvera JS, Halkos ME, Kerendi F, Wang NP, Guyton RA, Vinten-Johansen J. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. Am J Physiol Heart Circ Physiol 285: H579–H588, 2003 [DOI] [PubMed] [Google Scholar]