Abstract
The factors that contribute to pulmonary embolism (PE), a potentially fatal complication of deep vein thrombosis (DVT), remain poorly understood. Whereas fibrin clot structure and functional properties have been implicated in the pathology of venous thromboembolism and the risk for cardiovascular complications, their significance in PE remains uncertain. Therefore, we systematically compared and quantified clot formation and lysis time, plasminogen levels, viscoelastic properties, activated factor XIII cross-linking, and fibrin clot structure in isolated DVT and PE subjects. Clots made from plasma of PE subjects showed faster clot lysis times with no differences in lag time, rate of clot formation, or maximum absorbance of turbidity compared with DVT. Differences in lysis times were not due to alterations in plasminogen levels. Compared with DVT, clots derived from PE subjects showed accelerated establishment of viscoelastic properties, documented by a decrease in lag time and an increase in the rate of viscoelastic property formation. The rate and extent of fibrin cross-linking by activated factor XIII were similar between clots from DVT and PE subjects. Electron microscopy revealed that plasma fibrin clots from PE subjects exhibited lower fiber density compared with those from DVT subjects. These data suggest that clot structure and functional properties differ between DVT and PE subjects and provide insights into mechanisms that may regulate embolization.
Keywords: deep vein thrombosis, pulmonary embolism, fibrin, lysis
vascular coagulation disorders that target the lung include pulmonary embolism (PE) and excessive alveolar fibrin deposition following systemic sepsis or trauma. PE is a prevalent and serious complication of deep vein thrombosis (DVT), a thrombotic disease that mainly affects the large veins of the lower extremities and pelvis. Together, PE and DVT comprise the common thrombotic disease, venous thromboembolism (VTE). Small pulmonary emboli can impair pulmonary circulation in limited segments of the lungs, whereas large emboli lodged within the main pulmonary artery or trunk can completely obstruct pulmonary circulation (6). The result of large emboli is often death (7) and has been estimated to account for an in-hospital case fatality rate of 23% (1). Despite a common pathology relating PE to DVT, the mechanisms that underlie embolization remain mostly unknown.
Recent studies have implicated alterations in mechanical properties of fibrin clots, as well as clot structure, in the development and outcome of vascular diseases (4, 11, 19). For example, fibrin clot stiffness, which was associated with reduced fibrinolytic rates and increased fiber density, was found to be an independent predictor of premature atherothrombotic events (4). This relationship has been extended in vivo to thrombi removed from myocardial infarction patients, where fibrin clot properties and thrombus structure were related to ischemic time (19). Similar knowledge for DVT and PE is limited to one study that showed increased permeability and faster lysis of fibrin clots formed in vitro from the plasma of PE subjects compared with DVT (23). Although this study offered an indication that fibrin clots may differ between DVT and PE, it did not provide a comprehensive evaluation of the biochemical, mechanical, and structural properties of fibrin clots in DVT and PE. Importantly, this study lacked examinations of both clot structure and viscoelastic properties, both of which may determine a clot's ability to deform in response to shear stress, a characteristic that may dictate the propensity for embolization. Therefore, we evaluated for the first time clot formation and lysis time, plasminogen levels, viscoelastic properties, and activated factor XIIIa (FXIIIa) cross-linking of fibrin in clots prepared in vitro from the plasma of subjects with acute isolated DVT or acute PE. In addition, plasma fibrin clot structure, including fiber density and fiber bundles, were evaluated by scanning electron microscopy. Together, differences in fibrin clot properties and clot structure between DVT and PE subjects may provide important insights into the mechanisms that regulate embolization.
MATERIALS AND METHODS
Study population.
We performed a prospective cohort study from January 2010 to March 2012 of subjects 18 years of age or older presenting to the Hospital of the University of Pennsylvania emergency department with isolated acute DVT or PE. Exclusion criteria included history of VTE within the prior 4 wk, unavailable for 90-day follow-up, current use of anticoagulants, or inability to provide informed consent. DVT was diagnosed by compression ultrasonography and PE by computed tomographic pulmonary angiography. All subjects were classified in one of two groups, isolated DVT or PE. Subjects diagnosed with both DVT and PE were classified as PE. Subjects with isolated DVT were further classified according to the anatomic location of their thrombus (proximal vs. distal). DVT below the popliteal vein was considered distal and involving the popliteal vein or above, proximal. Subjects with both distal and proximal DVT were classified as proximal since DVT is thought to progress from a distal to proximal location, from where it is more likely to embolize (9). Demographic information, thrombotic risk factors, comorbidities, and medications were collected from all subjects.
Before initiation of anticoagulant therapy, blood was drawn from subjects in 3.2% sodium citrate (BD Vacutainer, Franklin Lakes, NJ), and platelet-poor plasma was prepared as described previously (12) and stored at −80°C in 0.1-ml aliquots for single use. The target sample size was calculated based on preliminary clot formation and lysis time assays in initial DVT and PE subjects collected. However, due to use of these samples in another study (12), there were some limitations on sample volume and thus not every patient sample could be included in every assay. Because functional properties may provide insights about embolization, fibrinogen concentration, clot formation and lysis time assays and viscoelastic properties were given priority for sample analysis, respectively. All available samples were studied using these assays. Plasminogen levels were measured in all remaining samples that had previously been assessed for clot formation and lysis time. FXIIIa and scanning electron microscopy were performed on a randomly selected subset of samples from the DVT and PE cohorts. These samples were representative of the overall study population with respect to demographic and clinical characteristics. In addition, the mean clot formation and lysis time variables and viscoelastic property curves for the subset of FXIIIa and scanning electron microscopy samples were within one standard deviation of the entire sample population for each of these assays. The study design was approved by the Institutional Review Board Involving Human Subjects at the University of Pennsylvania, and written informed consent was obtained from all study participants.
Clot formation and lysis time.
Thrombin (0.25 U/ml) (American Diagnostica, Stamford, CT), CaCl2 (20 mM) (Fisher Scientific, Pittsburgh, PA), and tissue plasminogen activator (tPA) (1.25 μg/ml) (American Diagnostica) combined in 50 mM Tris (Sigma, St. Louis, MO) and 140 mM NaCl (Macron, Center Valley, PA), pH 7.4 (TBS), were simultaneously added (0.004 ml) to plasma (0.1 ml). Changes in turbidity were monitored spectrophotometrically at 350 nm for 6 h. Lag time was defined as the time for absorbance to change to 0.01 (16). The rate of clot formation was measured as the slope of the linear part of the clot formation curve during the zero to maximum absorbance transition. Fibrinogen concentration is proportional to the maximum absorbance and also affects the rate of clot formation (3, 18). Because of this, the rate of clot formation was normalized for maximum absorbance. Lysis time was defined as the time from the maximum absorbance to the zero absorbance transition. Lysis time is also affected by the maximum absorbance. For example, for two clots that each lyse in 10 min, a clot with a maximum absorbance of 2.0 lyses two times as fast as the clot with maximum absorbance of 1.0. Thus, lysis time was normalized for maximum absorbance. Each sample was measured in duplicate.
Plasma levels of fibrinogen and plasminogen.
Plasma fibrinogen levels were measured using an ELISA as previously described (12). For plasminogen quantification, total plasma protein levels from DVT and PE subjects were quantified using bicinchoninic acid assay (Thermo Scientific, Rockford, IL). From each subject, 40 μg protein were loaded on a NuPAGE 12% Bis-Tris gel (Invitrogen, Carlsbad, CA) and transferred to polyvinylidene difluoride (PVDF) membrane (Millipore, Billerica, MA). The membrane was probed with a goat anti-human plasminogen antibody (1:2,000) (Abcam, Cambridge, MA) followed by rabbit anti-goat IgG conjugated to IRDye680 (Rockland, Gilbertsville, PA). The blots were viewed and quantified using the Odyssey infrared imaging system (LI-COR, Lincoln, NE). Band signal intensity is reported in arbitrary units.
Viscoelastic properties.
Viscoelastic properties were measured with a rheometer (AR-G2; TA Instruments, New Castle, DE) during plasma clot formation at 25°C. As described above, plasma clots (0.1 ml) were generated between the rheometer stage and a 40-mm parallel plate. To avoid drying, the outside of the forming clot was surrounded by mineral oil (3.52 mPa·s) (Cannon Instrument, State College, PA). A time-sweep test was performed for 1 h under an oscillation procedure of 2% strain at an angular frequency of 5 radians/s. The storage modulus and the loss modulus, measures of elastic and viscous properties, respectively, were measured at 3-s intervals. Each sample was measured in duplicate. Curves were averaged using Origin 6 by averaging Y-values for each X-value time point.
Factor XIIIa cross-linking.
Plasma in 10-μl aliquots was clotted as above, and the reaction was quenched with lithium dodecyl sulfate sample buffer (4×, 25 μl) followed by boiling at 100°C for 5 min. Samples were diluted 100-fold and run on a NuPAGE 4–12% Bis-Tris gel (Invitrogen), followed by transfer of proteins to PVDF membrane (Millipore) and probing with polyclonal rabbit anti-human fibrinogen antibody (1:2,000) (Dakocytomation, Glostrup, Denmark) and goat anti-rabbit IgG conjugated to IRDye680 (Rockland). The blots were viewed and quantified by the Odyssey system as described above. γ-Dimer and α-polymer formation were normalized for the sum total γ-chain and γ-dimer and α-chain and α-polymer at each time point, respectively.
Scanning electron microscopy.
Plasma clots were generated as described above (0.1 ml) for 2 h and were then washed, fixed, dehydrated with serial ethanol dilutions followed by hexamethyldisilazane (Acros Organics, Fair Lawn, NJ), and sputter-coated with gold palladium as described previously (25). High-definition micrographs were taken on a Philips/FEI XL20 scanning electron microscope (FEI, Hillsboro, OR). Nine to 12 micrographs were taken for each sample. Fiber density and fiber bundling were quantified using Image J (W. S. Rasband, ImageJ, U. S. National Institutes of Health, Bethesda, MD http://imagej.nih.gov/ij/, 1997–2011). For fiber density, micrographs were overlaid with a 6 × 6 rectangular grid, with each rectangle being 3 × 4 μm2 in size. Values were normalized for the number of images taken and are reported as the mean number of fibers per 3 × 4 μm2 rectangle ± SD. Fiber bundles and the number of fibers per bundle were counted for each image, resulting in a total area of 432 μm2. These values were normalized for number of images and are reported as the mean number of bundles or number of fibers per bundle ± SD.
Statistics.
All continuous variables were tested for Gaussian distribution by the D'Agostino and Pearson omnibus normality test. For parametric distributions comparing two groups, an unpaired t-test was performed. For nonparametric distributions, Mann Whitney U-test was performed. For comparisons between more than two groups, one-way ANOVA was used for parametric data, followed by Tukey's multiple-comparison tests. Nonparametric data were compared by Kruskal Wallis test followed by Dunn's multiple-comparison test. Values are expressed as means ± SD for parametric data and the median and the interquartile range for nonparametric data. Correlations were assessed by Spearman rank correlation analysis. The presence of statistical outliers was determined by Grubb's outlier test. All rates are reported as 95% confidence intervals (CI). P values are reported for comparison between groups and not for multiple-comparison tests. A P value of 0.05 or less was considered statistically significant. All statistical calculations, including linear regression analysis, were generated and analyzed using GraphPad Prism (GraphPad Software, La Jolla, CA).
RESULTS
Study population characteristics.
Over the study period, 35 subjects with acute isolated DVT and 40 subjects with acute PE were enrolled. Of the 35 DVT subjects, 27 had proximal and 8 had distal DVT. Patient demographics, disease history, risk factors, and medications are shown in Table 1. Demographic variables, comorbidities, risk factors, and medication use were similar between DVT and PE subjects with the exceptions that more DVT subjects were male compared with PE, more DVT subjects had a previous history of peripheral artery disease, which is a reported risk factor for VTE (20), and immunosuppressant use was increased in DVT subjects, including chemotherapeutics and glucocorticosteroids.
Table 1.
Study population demographics, history, and medication use
| DVT (n = 35) | PE (n = 40) | P Value | |
|---|---|---|---|
| Age, yr | 57 (42–71) | 56 (41–65) | |
| Sex, %male | 21 (60.0) | 9 (22.5) | 0.0019† |
| BMI, kg/m2 | 28.0 (23.6–33.7) | 28.0 (23.7–32.1) | |
| Race and ethnicity | |||
| Caucasian | 17 (48.6) | 21 (52.5) | |
| African American | 17 (48.6) | 18 (45.0) | |
| Other | 1 (2.9) | 1 (2.5) | |
| Hispanic | 2 (5.7) | 0 (0.0) | |
| Disease history and risk factors | |||
| H/O DVT | 7 (20.0) | 5 (12.5) | |
| H/O PE | 3 (8.6) | 6 (15.0) | |
| H/O CAD/MI/angina | 3 (8.6) | 1 (2.5) | |
| H/O stroke | 3 (8.6) | 1 (2.5) | |
| Liver disease | 1 (2.9) | 3 (7.5) | |
| Rheumatoid arthritis | 2 (5.7) | 0 (0.0) | |
| Peripheral artery disease | 6 (17.1) | 1 (2.5) | 0.0455† |
| Active cancer | 13 (37.1) | 17 (42.5) | |
| Recent surgery‡ | 8 (22.9) | 9 (22.5) | |
| Immobilization >6 wk | 7 (20.0) | 6 (15.0) | |
| Air travel# | 1 (2.9) | 2 (5.0) | |
| Tobacco use | 12 (34.3) | 14 (35.0) | |
| Pregnancy | 1 (2.9) | 2 (5.0) | |
| Trauma | 2 (5.7) | 3 (7.5) | |
| Infection | 2 (5.7) | 0 (0.0) | |
| Medications | |||
| Antiplatelet | 8 (22.9) | 5 (12.5) | |
| Statins | 5 (14.3) | 6 (15.0) | |
| Hormonal contraception | 1 (7.1) | 6 (19.4) | |
| Hormone therapy | 3 (21.4) | 1 (3.2) | |
| Antioxidants | 2 (5.7) | 4 (10.0) | |
| Immunosuppressants | 7 (20.0) | 1 (2.5) | 0.0219† |
Age and body mass index (BMI) are shown as median and interquartile range. Comparisons were made by Mann Whitney U-test. All other variables are shown as no. and percentage within each group. H/O, "history of"; CAD, coronary artery disease; MI, myocardial infarction.
Comparisons were made by Fischer's exact test.
Within past 6 wk.
Greater than 6 h within past 6 wk. Because of sex differences between deep vein thrombosis (DVT) and pulmonary embolism (PE), hormonal contraception and therapy were compared among female subjects only.
Plasma fibrinogen concentration.
Fibrinogen levels were similar between isolated DVT and PE subjects [DVT: n = 34, 4.61 (3.50–6.76) mg/ml; PE: n = 38, 5.50 (3.91–6.46) mg/ml; P = 0.3021] and did not differ between distal DVT, proximal DVT, or PE [distal: n = 9, 4.15 (3.50–6.48) mg/ml; proximal: n = 25, 4.65 (3.47–6.81) mg/ml; PE: n = 38, 5.50 (3.91–6.46) mg/ml; P = 0.4500].
Clot formation and lysis time.
Clot formation and lysis time were measured in clots made from the plasma of 28 DVT and 28 PE subjects. The lag time, rate of clot formation, and maximum absorbance were similar for both DVT and PE samples (Table 2). Consistent with previous studies (23), lysis time was faster in clots from PE subjects compared with DVT (DVT: 225.50 ± 131.40 mg/ml; PE: 158.70 ± 83.25 mg/ml; P = 0.0292; Table 2).
Table 2.
Clot formation, lysis time, and plasminogen levels in DVT and PE subjects
| DVT† (n = 28) |
||||
|---|---|---|---|---|
| Distal (n = 7) | Proximal (n = 21) | PE (n = 28) | P Value | |
| Lysis time, min | 225.50 ± 131.40† | 158.70 ± 83.25‡ | 0.0292*† | |
| 284.50 ± 142.80‡ | 205.90 ± 124.80‡ | 0.0249*‡ | ||
| AbsMax | 0.64 ± 0.27† | 0.76 ± 0.25‡ | 0.0825† | |
| 0.63 ± 0.21‡ | 0.65 ± 0.29‡ | 0.2208‡ | ||
| Lag time, min | 14.70 (12.49–20.01)† | 13.11 (6.52–18.09)‡ | 0.2037† | |
| 14.83 (9.45–20.05)‡ | 14.57 (12.99–20.64)‡ | 0.3858‡ | ||
| Rate of clot formation, Abs/min | 0.16 (0.14–0.19)† | 0.13 (0.11–0.17)‡ | 0.1194† | |
| 0.13 (0.10–0.19)‡ | 0.16 (0.12–0.21)‡ | 0.2661‡ | ||
| Plasminogen, AU | 2.59 (1.98–3.26)† | 2.75 (2.23–3.85)‡ | 0.3561† | |
| 2.46 (2.06–3.16)‡ | 2.59 (1.98–3.01)‡ | 0.4915‡ | ||
Lag time is the time for absorbance (Abs) to change to 0.01 (16). The rate of clot formation is the slope of the linear part of the clot formation curve during the zero to maximum absorbance transition normalized for maximum absorbance (Absmax). It is reported as the median slope with 95% confidence interval (CI). Lysis time is the time from the maximum absorbance to the zero absorbance transition normalized for maximum absorbance. All variables were assessed for parametric distribution by D'Agostino and Pearson omnibus normality test. Lysis and maximum absorbance exhibited parametric distributions, whereas lag time, rate of clot formation, and plasminogen levels did not. For parametric data, DVT and PE were compared by unpaired t-test, and distal DVT, proximal DVT, and PE were compared by one-way ANOVA, followed by Tukey's multiple-comparison test. For nonparametric data, DVT and PE were compared by Mann Whitney U-test, and distal DVT, proximal DVT, and PE were compared by Kruskal-Wallis test, followed by Dunn's multiple-comparison test. Plasminogen levels were measured in all available DVT (distal: n = 4; proximal: n = 14) and PE (n = 28) samples. AU, arbitrary units. P values are reported for comparison between groups and not for multiple-comparison tests.
P ≤ 0.05.
Subjects with DVT.
Subgroups in DVT distal and proximal.
The anatomic location of DVT is a well-established risk factor for PE. One early study reported that 44% of patients with proximal DVT developed PE, whereas 0% with distal DVT did (9). Therefore, we explored differences in the in vitro fibrin clot lysis time between subjects with distal (n = 7) or proximal (n = 21) DVT and PE (n = 28). Lysis time was significantly faster in PE compared with distal DVT subjects (Table 2). However, lysis times were similar between proximal DVT and PE (Table 2). No other clot formation and lysis time variables differed between distal DVT, proximal DVT, or PE clots (Table 2).
Plasma levels of plasminogen.
Within the samples that were evaluated for clot formation and lysis time, all available DVT (n = 18) and PE (n = 28) samples were assessed for plasminogen levels by Western blot analysis. No differences in plasminogen levels were found between DVT and PE subjects (Table 2). In addition, no differences were observed between distal (n = 4) or proximal DVT (n = 14) and PE (n = 28). Spearman's rank correlation analysis was used to evaluate the effect of plasminogen on lysis time. No relationship was observed between these two variables (r = 0.03169; P = 0.8363). Therefore the differences in lysis time are not attributed to changes in plasminogen levels.
Viscoelastic properties of fibrin clots.
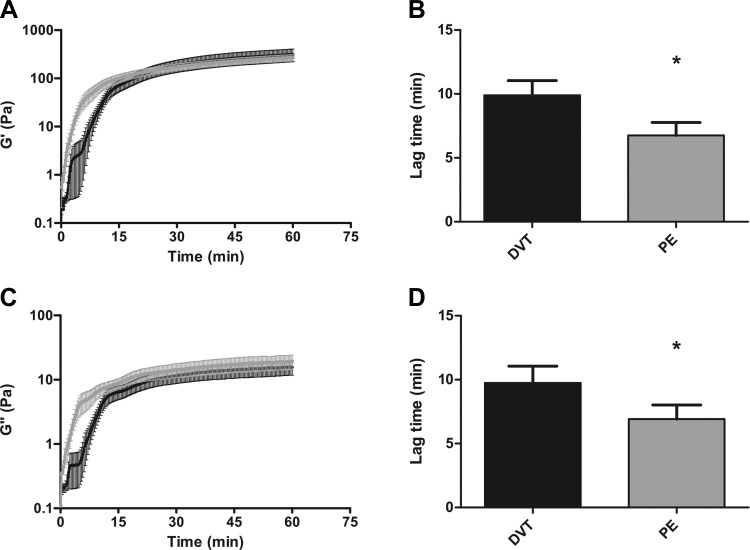
Fibrin is a viscoelastic polymer and experiences shear stress within the vessel wall due to blood flow (17). Alterations in viscoelastic behavior may affect the ability of thrombi to deform appropriately in response to shear stress and may thus contribute to embolization. The viscoelastic properties are comprised of two components, the elastic component or storage modulus, which is a measure of clot stiffness, and the inelastic component or loss modulus, which is a measure of plasticity. Averaged viscoelastic property curves for clots made from 21 DVT and 28 PE subjects are shown in Fig. 1, A and C. Initial establishment of viscoelastic properties was accelerated in plasma fibrin clots of PE subjects compared with DVT. Evaluation of individual elastic property curves obtained from PE subjects showed a shorter lag time compared with DVT [DVT: 9.42 (6.59–14.91) min; PE: 6.59 (2.43–10.25) min; P = 0.0186; Fig. 1, A and B]. Linear regression analysis of averaged curves demonstrated an increase in the rate of elastic property formation in clots from PE subjects compared with DVT [DVT: 7.35 (95% CI, 6.85–7.85) Pa/min; PE: 8.02 (95% CI, 7.92–8.12) Pa/min; P = 0.0087; Fig. 1A; Table 3]. Similar results were obtained with viscous property curves (Fig. 1, C and D, and Table 3). For both the elastic and inelastic components, neither lag time nor the rate of property formation differed between distal (n = 3) and proximal DVT (n = 18) and PE (n = 28). Of note, mean individual lag times (Fig. 1, B and D) do not appear to coincide with lag times from the viscoelastic property curves due to averaging effects. Despite differences in the initial phases of viscoelastic property formation, the final elastic and viscous properties were similar between isolated DVT and PE subjects.
Fig. 1.
Viscoelastic properties in deep vein thrombosis (DVT) and pulmonary embolism (PE) subjects. A: formation of the clot elastic property (G′) as a function of time in DVT (black, n = 21) and PE (gray, n = 28) subjects. B: lag time is significantly faster in PE subjects compared with DVT (*P = 0.0186). C: formation of the clot viscous property (G") as a function of time in DVT and PE subjects. D: lag time is significantly faster in PE subjects compared with DVT (*P = 0.0299). Both the elastic and viscous properties are measured in Pascals (Pa). Error bars represent the SD of the mean.
Table 3.
Kinetic analysis of viscoelastic properties of fibrin clots in DVT and PE subjects
| DVT (n = 21) | PE (n = 28) | P Value | |
|---|---|---|---|
| G′ | |||
| Lag time, min | 9.42 (6.59–14.91) | 6.59 (2.43–10.25) | 0.0186* |
| Rate of formation, Pa/min | 7.35 (6.85–7.85) | 8.02 (7.92–8.12) | 0.0087§ |
| Final, Pa | 227.3 ± 176.1 | 273.0 ± 224.5 | 0.4468 |
| G′′ | |||
| Lag time, min | 8.34 (6.59–14.03) | 6.09 (2.26–11.25) | 0.0299* |
| Rate of formation, Pa/min | 0.51 (0.74–0.84) | 0.60 (0.57–0.62) | <0.0019§ |
| Final, Pa | 12.22 ± 9.74 | 15.25 ± 10.59 | 0.3184 |
Storage modulul (G′) and loss modulus (G′′) are measured in Pascals (Pa).
Lag time values are reported as median with 95% CI and were compared by Mann Whitney U-test.
Rates of G′ and G′′ formation are reported as mean slope with 95% CI and were analyzed by linear regression analysis. The final G′ and G′′ values are reported as means ± SD and were compared by unpaired t-test.
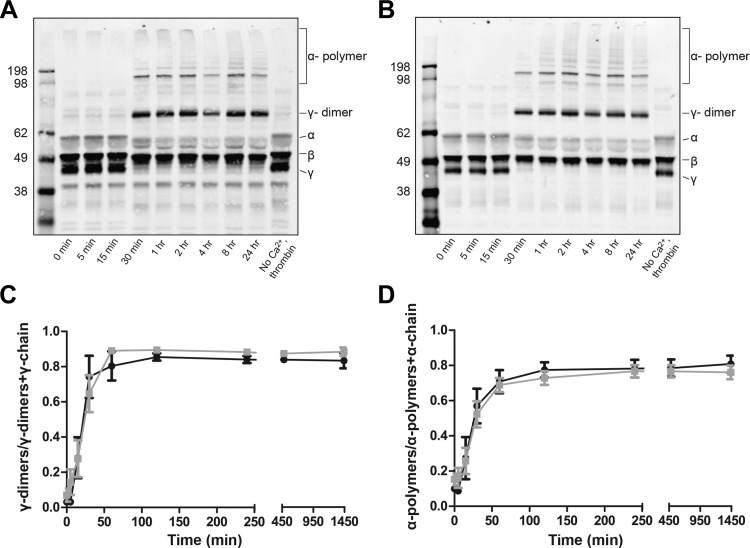
Factor XIIIa cross-linking of fibrin.
FXIIIa cross-linking, as determined by the rate and extent of γ-dimers and α-polymers formed, was measured in the plasma of DVT (n = 6), one of whom was distal, and PE (n = 12) subjects over a 24-h period by Western blot analysis (Fig. 2, A and B). The formation of γ-dimers was normalized by the sum total of the γ-chain and γ-dimers at each time point. A similar normalization was performed for α-polymers. The rate and extent of γ-dimer formed, as determined by linear regression analysis, and as the final point in the FXIIIa cross-linking curves, respectively, was similar between clots from DVT and PE subjects (Fig. 2C). Similar results were found for α-polymer (Fig. 2D). Correspondingly, loss of γ- and α-chains of fibrinogen resulting from incorporation into γ-dimers and α-polymers, respectively, was similar between DVT and PE clots (data not shown). Therefore, FXIIIa cross-linking of fibrin, which has been shown to increase the storage modulus and decrease the loss modulus (18) and delay lysis time (10), did not account for the differences in viscoelastic properties and faster lysis in PE clots.
Fig. 2.
Factor XIIIa cross-linking of fibrin fibers within plasma clots as a function of time in DVT and PE subjects. Representative Western blots from DVT (A) and PE (B) subjects. γ-Dimers and α-polymers both appear in DVT and PE at 30 min. The γ-chain is completely cross-linked by 1 h, whereas some α-chain remains at 24 h. C: formation of γ-dimers normalized to total γ-chain over time in DVT (black, n = 6) and PE (gray, n = 12) subjects. The rate {DVT: 0.03 [95% confidence interval (CI), 0.02–0.04] γ-dimers/min; PE: 0.02 (95% CI, 0.01–0.03) γ-dimers/min; P = 0.3332}, as determined by linear regression analysis, and extent (DVT: 0.83 ± 0. 10 γ-dimers; PE: 0.82 ± 0. 15 γ-dimers; P = 0.8809) of γ-dimer formation was similar between clots from DVT and PE subjects. D: formation of α-polymers normalized total α-chain over time in DVT and PE subjects. The rate [DVT: 0.02 (95% CI, 0.01–0.03) α-polymers/min; PE: 0.01 (95% CI, 0.01–0.02) α-polymers/min; P = 0.4216] and extent (DVT: 0.78 ± 0. 10 α-polymers; PE: 0.67 ± 0. 12 α-polymers; P = 0.0767) of α-polymer formation were similar between clots from DVT and PE subjects.
Scanning electron microscopy to evaluate fibrin clot structure.
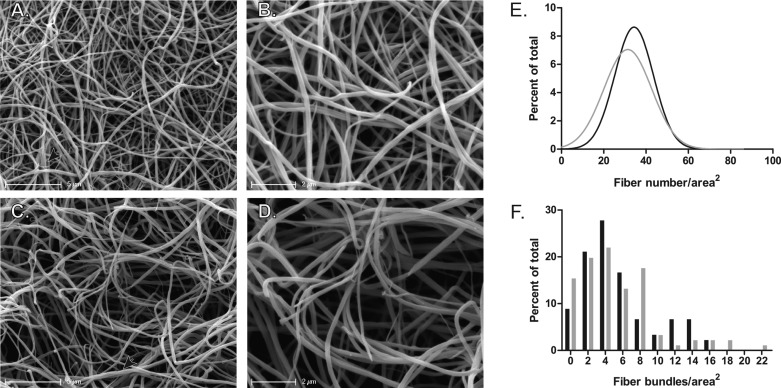
Fibrin clot structure was evaluated in seven DVT (all with proximal DVT) and eight PE subjects (Fig. 3, A–D). Histograms of fiber density displayed a Gaussian distribution and showed that clots from DVT subjects had a mean of 34.36 ± 9.14 fibers/area2, whereas PE fibrin clots had a mean of 31.38 ± 11.29 fibers/area2 (P < 0.0001; Fig. 3E). Histograms of fiber bundling and the number of fibers per bundle were both asymmetric and could therefore not be fit with Gaussian distributions. Comparison of means showed that neither fiber bundling (DVT: 3.73 ± 2.55 bundles/area2; PE: 3.36 ± 4.32 bundles/area2; P = 0.1554; Fig. 3F) nor the number of fibers per bundle were significantly different between clots from DVT and PE subjects (DVT: 4.25 ± 0.41 fibers/bundle; PE: 3.85 ± 0.38 fibers/bundle; P = 0.0686). Neither fiber density (r = 0.0071; P = 0.9798) nor fiber bundling (r = −0.1265; P = 0.6407) was related to fibrinogen concentration by Spearman rank correlation.
Fig. 3.
Scanning electron micrographs of clots formed from the plasma of DVT and PE subjects. A and B: representative micrographs from an individual DVT subject. Magnification bars represent 5 (A) and 2 (B) μm. C and D: representative micrographs from an individual PE subject. Magnification bars represent 5 (C) and 2 (D) μm. Bundles are indicated by arrowheads. E: nonlinear regression fit of Gaussian distributions of fiber density for DVT (black, n = 7) and PE (gray, n = 8) subjects. F: histograms for the distributions of fiber bundles in DVT (black, n = 7) and PE (gray, n = 8) subjects. Histograms for fiber density and fiber bundling were normalized to the total number of fibers or bundles, respectively, and are represented as the percent of total.
DISCUSSION
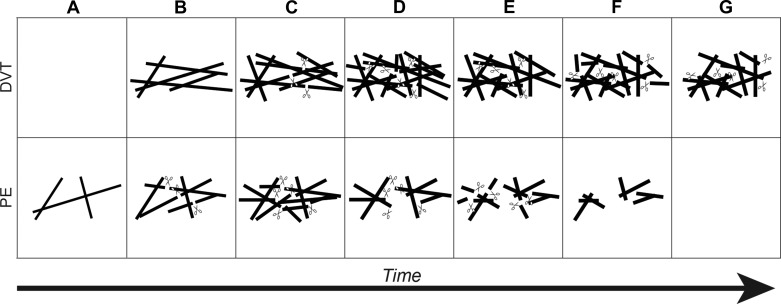
This study compared and quantified for the first time the biochemical, mechanical, and structural properties of plasma fibrin clots from subjects with isolated DVT and PE. A significant difference was observed in the establishment of mechanical properties in PE fibrin clots. Both the storage and loss moduli were established earlier and at a faster rate in clots from PE subjects compared with DVT (Fig. 1 and Table 3). This may alter the structure of the clot and may also predispose the clots to earlier lysis, as described below. The accelerated acquisition of viscoelastic properties occurred in the absence of any observable changes in the rate of clot formation or lag time by spectrophotometry (Table 2). Formation of an initial fibrin fiber network that would acquire viscoelastic properties more quickly, but may not thicken immediately to cause an earlier increase in turbidity, may explain this observation (Fig. 4). Alternatively, these results might reflect the increased sensitivity of rheometry to detect changes in clot formation compared with spectrophotometry.
Fig. 4.
Schematic representation of clot formation and lysis in DVT and PE subjects. Fibrin fibers are denoted by solid lines and plasmin by scissors. A: in DVT clots, fibrin fibers have not formed. In PE clots, thin fibers and an initial network begin to develop. B: DVT clots now form fibrin fibers and a network. In PE clots, tissue plasminogen activator (tPA) and plasminogen begin to bind and lyse fibers. Concomitantly, new fibers are added to the network. C: new fibers are added to DVT clots, and tPA and plasminogen begin to bind and lyse fibers. New fibers continue to be added to the PE clot, and existing fibers thicken. More plasmin is generated due to additional fibrin formation. D: in DVT clots, new fibers are added to the network, existing fibers are thickened, and more plasmin is generated. The fibrin network is fully formed in both DVT and PE clots. Here, plasmin continues to lyse the clots. Fibers reach their final diameter and are comparable between DVT and PE, resulting in similar maximum turbidity during clot formation and lysis time assays. E: for both DVT and PE clots, plasmin lysis continues. However, lysis proceeds at a faster rate in PE clots due to decreased fiber density. F: lysis continues in DVT clots, whereas lysis is mostly complete in PE clots, with small fibrin fragments and fibrin degradation products produced. G: lysis advances in DVT clots, but significant clot structure remains due to increased fiber density. Plasmin has completely degraded the fibrin clot in PE subjects, and all degradation products and fibrin structures have been removed by flowing blood.
Plasma fibrin clots formed in vitro from PE subjects exhibited faster lysis times compared with DVT (Table 2). This difference was not related to alterations in plasminogen levels, since DVT and PE subjects had similar plasma levels of plasminogen. Previous studies have shown that, compared with other coagulation components, plasminogen appears to play a minor role in clot formation and lysis time assays (13). In addition, studies investigating the relationship between plasminogen levels in DVT compared with healthy controls have not reported differences between the two groups (13), nor is there strong evidence for increased risk of thrombosis in heterozygous or homozygous plasminogen-deficient individuals (22). Differences in lysis times may instead relate to the decrease in fiber density in PE subjects quantified from the scanning electron micrographs (Fig. 3). Decreased fibrin density may also account for the increased permeability in PE clots compared with DVT that has been reported previously (23). Clot formation and lysis are dynamic processes that occur simultaneously (8). Compared with fibrin monomer, polymerized fibrin substantially accelerates activation of plasmin by tPA and, thus, lysis of clots (8, 21). Therefore, faster establishment of a fibrin network might predispose clots from PE subjects to premature lysis compared with isolated DVT (Fig. 4). Moreover, tPA concentration is the limiting factor during plasmin activation and fibrin lysis (2). Hence, clots with fewer fibers will have a higher tPA-to-fiber ratio, resulting in increased fibrinolysis. This may account for the accelerated rate of lysis in PE clots, which have decreased fiber density compared with DVT (5). Therefore, the faster lysis of fibrin clots in PE may contribute to instability and a tendency toward embolization before full clot formation. Currently, there is no animal model of PE from an existing thrombus. However, recent studies in FVIII null mice that used intravital microscopy to monitor real-time thrombus formation in a laser-induced injury model have shown that embolization can occur as the thrombus is forming, before full clot formation, and also that defects in clotting may promote embolization (15). These animal studies support the suggestions that a shift toward lysis and away from clotting may contribute to embolization in PE subjects.
FXIIIa cross-linking increases clot stiffness and also delays lysis (10, 18). However, FXIIIa cross-linking did not account for the differences observed between DVT and PE subjects in viscoelastic properties and lysis (Fig. 2). These findings suggest that the timing and process of fibrin fiber formation differs between DVT and PE subjects, resulting in dissimilar clot structures.
Among subjects presenting with acute DVT, identifying those at highest risk for PE could inform treatment decisions. Individuals with proximal DVT are at higher risk for PE than those with distal DVT (9, 14). We observed faster lysis times in clots from PE subjects than those with DVT. This difference is confined to the comparison between PE and distal DVT, although the number of samples evaluated was small (Table 2). Whether lysis time is a predictor of PE independent of anatomic DVT location will require evaluation in a larger study.
Due to insufficient sample volume, we were unable to perform clot formation and lysis time and viscoelastic studies on samples from all subjects. A smaller subset of samples was analyzed by scanning electron microscopy and FXIIIa cross-linking. These limitations constrained the ability to compare distal DVT, proximal DVT, and PE in all assays. The subject samples randomly chosen for FXIIIa cross-linking and scanning electron microscopy have representative clinical and demographic features of the study population at large. Furthermore, the mean clot formation and lysis time variables and viscoelastic property curves for this subset were within one standard deviation of the entire sample population for each of these assays. Although this subset of samples may represent our population accurately, future validation of the findings that includes a larger study population of subjects with proximal and distal DVT is required.
The originating location of all thromboses in PE remains unclear. Previous research has demonstrated that roughly 40% of patients diagnosed with PE do not concomitantly have DVT (26). This indicates that either the entire thrombus has embolized, leaving no trace upon diagnosis, or that there is an alternate originating point such as the superficial veins, arterial system, or within the lungs themselves. Although there is currently no way to assess the source of the thrombus in PE, thrombi originating from these alternative points may indeed have differing structural and functional properties compared with PE arising from DVT.
It is possible that some of the subjects classified as having isolated DVT in our study may have had silent PE. Indeed, as many as 40% of DVT patients without chest symptoms are found to have PE upon lung imaging (14). This may include patients initially diagnosed as DVT that later presented, after sample collection, with PE. However, this is unlikely, since median duration of symptoms at diagnosis in our population was 2 days in subjects with PE and 4 days in DVT. Nevertheless, misclassification of some PE subjects as having isolated DVT would be expected to bias our results toward the null and should not account for the observed differences in the isolated DVT and PE cohorts.
Previous studies have indicated that the structural characteristics of fibrin clots formed from platelet-poor plasma may replicate the fibrin structure of thrombi obtained from thrombosis patients (4, 19). Our study demonstrates that there are differences between the structure and functional characteristics of fibrin clots formed in vitro from the plasma of DVT and PE subjects. However, these findings may not be indicative of in vivo thrombi, since they are lacking important cellular components, including endothelial cells, neutrophils, and platelets (24). Additional studies that obtain thrombi from DVT and PE subjects would be valuable in assessing the relationship between in vitro and in vivo fibrin clot properties.
In summary, compared with DVT, in vitro clots from PE subjects exhibit faster lysis times, possibly due to lower fiber density, as well as earlier establishment of viscoelastic properties. These properties distinguish individuals with PE from those with isolated DVT and may define structural characteristics that underlie embolization.
GRANTS
The work was supported by National Institutes of Health Grants HL-54926, HL-103918, and ES-013508 to H. Ischiropoulos, HL-090774 to J. W. Weisel, and HL-112903 to A. Cuker. M. R. Martinez is supported by the Hemostasis and Thrombosis Training Rrant T32 HL-07971.
DISCLOSURES
No conflicts of interest, financial or otherwise are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: M.R.M., A. Cuker, A.M.M., A. Crichlow, J.W.W., and H.I. conception and design of research; M.R.M., A. Crichlow, R.T.L., I.N.C., and C.N. performed experiments; M.R.M. analyzed data; M.R.M., A. Cuker, J.W.W., and H.I. interpreted results of experiments; M.R.M. prepared figures; M.R.M. and H.I. drafted manuscript; M.R.M., A. Cuker, A.M.M., J.W.W., and H.I. edited and revised manuscript; M.R.M., A. Cuker, A.M.M., A. Crichlow, R.T.L., I.N.C., C.N., J.W.W., and H.I. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. K. Hawkins for generously allowing us to use the AR-G2 rheometer and Dr. R. Litvinov for help on FXIIIa assays.
REFERENCES
- 1.Anderson FA, Jr, Wheeler HB, Goldberg RJ, Hosmer DW, Patwardhan NA, Jovanovic B, Forcier A, Dalen JE. A population-based perspective of the hospital incidence and case-fatality rates of deep vein thrombosis and pulmonary embolism. The Worcester DVT Study. Arch Intern Med 151: 933–938, 1991 [PubMed] [Google Scholar]
- 2.Bannish BE, Keener JP, Fogelson AL. Modelling fibrinolysis: a 3D stochastic multiscale model. Math Med Biol In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carr ME, Jr, Hermans J. Size and density of fibrin fibers from turbidity. Macromolecules 11: 46–50, 1978 [DOI] [PubMed] [Google Scholar]
- 4.Collet JP, Allali Y, Lesty C, Tanguy ML, Silvain J, Ankri A, Blanchet B, Dumaine R, Gianetti J, Payot L, Weisel JW, Montalescot G. Altered fibrin architecture is associated with hypofibrinolysis and premature coronary atherothrombosis. Arterioscler Thromb Vasc Biol 26: 2567–2573, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Collet JP, Park D, Lesty C, Soria J, Soria C, Montalescot G, Weisel JW. Influence of fibrin network conformation and fibrin fiber diameter on fibrinolysis speed: dynamic and structural approaches by confocal microscopy. Arterioscler Thromb Vasc Biol 20: 1354–1361, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Corrin B, Nicholson AG. Pathology of the Lungs. London, UK: Churchill Livingston, 2011, p. 778 [Google Scholar]
- 7.Gorham LW. A study of pulmonary embolism. I. A clinicopathological investigation of 100 cases of massive embolism of the pulmonary artery; diagosis by physical signs and differentiation from acute myocardial infarction. Arch Intern Med 108: 8–22, 1961 [DOI] [PubMed] [Google Scholar]
- 8.Hoylaerts M, Rijken DC, Lijnen HR, Collen D. Kinetics of the activation of plasminogen by human tissue plasminogen activator. Role of fibrin. J Biol Chem 257: 2912–2919, 1982 [PubMed] [Google Scholar]
- 9.Kakkar VV, Howe CT, Flanc C, Clarke MB. Natural history of postoperative deep-vein thrombosis. Lancet 2: 230–232, 1969 [DOI] [PubMed] [Google Scholar]
- 10.Lorand L, Jacobsen A. Accelerated lysis of blood clots. Nature 195: 911–912, 1962 [DOI] [PubMed] [Google Scholar]
- 11.Machlus KR, Cardenas JC, Church FC, Wolberg AS. Causal relationship between hyperfibrinogenemia, thrombosis, and resistance to thrombolysis in mice. Blood 117: 4953–4963, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martinez M, Cuker A, Mills A, Lightfoot R, Fan Y, Tang WH, Hazen SL, Ischiropoulos H. Nitrated fibrinogen is a biomarker of oxidative stress in venous thromboembolism. Free Radic Biol Med 53: 230–236, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meltzer ME, Lisman T, de Groot PG, Meijers JC, le Cessie S, Doggen CJ, Rosendaal FR. Venous thrombosis risk associated with plasma hypofibrinolysis is explained by elevated plasma levels of TAFI and PAI-1. Blood 116: 113–121, 2010 [DOI] [PubMed] [Google Scholar]
- 14.Moser KM, Fedullo PF, LitteJohn JK, Crawford R. Frequent asymptomatic pulmonary embolism in patients with deep venous thrombosis. J Am Med Assoc 271: 223–225, 1994 [PubMed] [Google Scholar]
- 15.Neyman M, Gewirtz J, Poncz M. Analysis of the spatial and temporal characteristics of platelet-delivered factor VIII-based clots. Blood 112: 1101–1108, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pieters M, Covic N, van der Westhuizen FH, Nagaswami C, Baras Y, Toit Loots D, Jerling JC, Elgar D, Edmondson KS, van Zyl DG, Rheeder P, Weisel JW. Glycaemic control improves fibrin network characteristics in type 2 diabetes: a purified fibrinogen model. Thromb Haemost 99: 691–700, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roberts WW, Kramer O, Rosser RW, Nestler FH, Ferry JD. Rheology of fibrin clots. I. Dynamic viscoelastic properties and fluid permeation. Biophys Chem 1: 152–160, 1974 [DOI] [PubMed] [Google Scholar]
- 18.Ryan EA, Mockros LF, Weisel JW, Lorand L. Structural origins of fibrin clot rheology. Biophys J 77: 2813–2826, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Silvain J, Collet J, Nagaswami C, Beygui F, Edmondson KE, Bellemain-Appaix A, Cayla G, Pena A, Brugier D, Barthelemy O, Montalescot G, Weisel JW. Composition of coronary thrombus in acute myocardial infarction. J Am Coll Cardiol 57: 1359–1367, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sorensen HT, Horvath-Puho E, Sogaard KK, Christensen S, Johnsen SP, Thomsen RW, Prandoni P, Baron JA. Arterial cardiovascular events, statins, low-dose aspirin and subsequent risk of venous thromboembolism: a population-based case-control study. J Thromb Haemost 7: 521–528, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Suenson E, Petersen LC. Fibrin and plasminogen structures essential to stimulation of plasmin formation by tissue-type plasminogen activator. Biochim Biophys Acta 870: 510–519, 1986 [DOI] [PubMed] [Google Scholar]
- 22.Tait RC, Walker ID, Conkie JA, Islam SI, McCall F. Isolated familial plasminogen deficiency may not be a risk factor for thrombosis. Thromb Haemost 76: 1004–1008, 1996 [PubMed] [Google Scholar]
- 23.Undas A, Zawilska K, Ciesla-Dul M, Lehmann-Kopydlowska A, Skubiszak A, Ciepluch K, Tracz W. Altered fibrin clot structure/function in patients with idiopathic venous thromboembolism and in their relatives. Blood 114: 4272–4278, 2009 [DOI] [PubMed] [Google Scholar]
- 24.von Bruhl ML, Stark K, Steinhart A, Chandraratne S, Konrad I, Lorenz M, Khandoga A, Tirniceriu A, Coletti R, Kollnberger M, Byrne RA, Laitinen I, Walch A, Brill A, Pfeiler S, Manukyan D, Braun S, Lange P, Riegger J, Ware J, Eckart A, Haidari S, Rudelius M, Schulz C, Echtler K, Brinkmann V, Schwaiger M, Preissner KT, Wagner DD, Mackman N, Engelmann B, Massberg S. Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J Exp Med 209: 819–835, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weisel JW, Nagaswami C. Computer modeling of fibrin polymerization kinetics correlated with electron microscope and turbidity observations: clot structure and assembly are kinetically controlled. Biophys J 63: 111–128, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamaki T, Nozaki M, Sakurai H, Takeuchi M, Soejima K, Kono T. Presence of lower limb deep vein thrombosis and prognosis in patients with symptomatic pulmonary embolism: preliminary report. Eur J Vasc Endovasc Surg 37: 225–231, 2009 [DOI] [PubMed] [Google Scholar]