Abstract
Chronic alcohol abuse increases lung oxidative stress and susceptibility to respiratory infections by impairing alveolar macrophage (AM) function. NADPH oxidases (Nox) are major sources of reactive oxygen species in AMs. We hypothesized that treatment with the critical antioxidant glutathione (GSH) attenuates chronic alcohol-induced oxidative stress by downregulating Noxes and restores AM phagocytic function. Bronchoalveolar lavage (BAL) fluid and AMs were isolated from male C57BL/6J mice (8–10 wk) treated ± ethanol in drinking water (20% wt/vol, 12 wk) ± orally gavaged GSH in methylcellulose vehicle (300 mg·kg−1·day−1, during week 12). MH-S cells, a mouse AM cell line, were treated ± ethanol (0.08%, 3 days) ± GSH (500 μM, 3 days or last 1 day of ethanol). BAL and AMs were also isolated from ethanol-fed and control mice ± inoculated airway Klebsiella pneumoniae (200 colony-forming units, 28 h) ± orally gavaged GSH (300 mg/kg, 24 h). GSH levels (HPLC), Nox mRNA (quantitative RT-PCR) and protein levels (Western blot and immunostaining), oxidative stress (2′,7′-dichlorofluorescein-diacetate and Amplex Red), and phagocytosis (Staphylococcus aureus internalization) were measured. Chronic alcohol decreased GSH levels, increased Nox expression and activity, enhanced oxidative stress, impaired phagocytic function in AMs in vivo and in vitro, and exacerbated K. pneumonia-induced oxidative stress. Although how oral GSH restored GSH pools in ethanol-fed mice is unknown, oral GSH treatments abrogated the detrimental effects of chronic alcohol exposure and improved AM function. These studies provide GSH as a novel therapeutic approach for attenuating alcohol-induced derangements in AM Nox expression, oxidative stress, dysfunction, and risk for pneumonia.
Keywords: glutathione, alveolar macrophage, nicotinamide adenine dinucleotide phosphate oxidases, oxidative stress, phagocytic function
chronic alcohol abuse increases susceptibility to acute respiratory distress syndrome (ARDS) (54), a severe form of lung injury with a 26% mortality rate (23), and increases risk of developing respiratory infections, such as pneumonia (49). ARDS is characterized by the development of pulmonary inflammation and edema in response to aspiration, sepsis, or trauma, resulting in systemic proinflammatory cascade activation (68). Compared with a 22% incidence of ARDS in nonalcoholic patients at risk for lung injury, alcoholic patients have a 43% incidence of ARDS (52). Alcohol predisposes to ARDS development through multiple mechanisms, including oxidative stress (11, 33, 35, 37, 52). Furthermore, subjects with a history of alcohol use disorders have a higher prevalence of community-acquired pneumonia (20), resulting in worse clinical outcomes than nonalcoholics. Chronic alcohol ingestion increases oxidative stress, which primes the lung for respiratory infections and injury (34).
Chronic alcohol consumption depleted levels of the critical antioxidant glutathione (GSH) in the bronchoalveolar lavage (BAL) fluid of human subjects (53) and in the alveolar macrophages (AMs) of rats (14). In clinical and animal studies, decreased GSH in the alveolar space is associated with chronic oxidative stress and AM dysfunction (12, 14, 37, 70). AMs are critical in innate and acquired immunity (66) since they phagocytize and clear apoptotic cells and infectious particles (47). The binding and internalization of inactive Staphylococcus aureus by AMs from chronic ethanol-exposed animal models is impaired (12), and oral treatments with GSH precursors l-2-oxothiaxolidine-4-carboxylate (Procysteine; Transcend Therapeutics) or N-acetylcysteine improved AM phagocytosis in vitro (14). Collectively, these studies demonstrate that chronic alcohol ingestion decreases GSH levels and stimulates oxidative stress in the alveolar space, leading to AM dysfunction (16) that could contribute to the increased risks of pneumonia or ARDS. However, because l-2-oxothiaxolidine-4-carboxylate and N-acetylcysteine are additionally involved in the synthesis of the antioxidant cysteine, the therapeutic effects of these molecules may not solely be due to their role as GSH precursors. To our knowledge, the studies presented herein are the first to delineate the unique and critical role of GSH in attenuating alcohol-induced AM dysfunction in a study of in vivo bacterial phagocytosis and clearance.
Reactive oxygen species (ROS) are involved in complex physiological processes, including cell signaling and apoptosis (26, 65), and play important roles in disease pathogenesis. NADPH oxidases (Nox) are a major source of ROS production in the lungs under physiological conditions (57). The primary ROS generated by Nox proteins in AMs is superoxide, a reactive species essential to the respiratory burst involved in the killing of microbes following phagocytosis (28).
Nox proteins, including Nox1–5 and Duox1–2, are membrane-associated, multicomponent enzymes that catalyze the reduction of molecular oxygen to superoxide and hydrogen peroxide (H2O2), using NADPH as an electron donor (10). Nox1, Nox2, and Nox4 (32, 51, 59) are expressed in the human lung, and Duoxes are expressed in the airway. Along with p22phox, these components comprise the catalytically active cytochrome-like moiety of Noxes (1, 36, 41). Activation of the Nox-p22phox complex may require additional subunits. For example, Nox1 is activated primarily through interactions with the cytosolic subunits GTP-Rac, NoxO1, and NoxA1 (17, 64), the latter two of which can be replaced by p47phox and p67phox, respectively (4, 64). Activation of Nox2 requires association with p40phox, GTP-Rac, p47phox, and p67phox (8), and Nox2 is responsible for respiratory burst in AMs (59). Nox4 generates ROS upon association with p22phox (22) and has been implicated in differentiation (18, 19, 44, 69), cellular senescence (62), and oxygen sensing (43). Nox4 is constitutively active producing H2O2, but its activity can be augmented by factors that increase its expression or by Poldip2 (48). In mouse embryos, Nox1, Nox2, and Nox4 are key sources of ROS production in response to ethanol exposure (21), and chronic ethanol exposure increased the expression of these Noxes in the rat lung (59). Taken together, these findings suggest that Noxes may play critical roles in ethanol-induced oxidative stress and in the pathogenesis of lung injury (59).
The objective of the current study is to therapeutically attenuate the molecular mechanisms involved in alcohol-induced oxidative stress and dysfunction in AMs. Previous studies in our laboratories showed that, during chronic alcohol ingestion, expression of Noxes are increased in the lung (6) and in AMs, subsequently increasing oxidative stress and AM phagocytic dysfunction (71). We hypothesize that treatment with the critical antioxidant GSH can reduce alcohol-induced oxidative stress through modulation of Nox expression, to improve AM phagocytic function. The investigations presented herein extend our previous studies involving alcohol-induced AM derangements and, to our knowledge, this is the first demonstration that dietary GSH treatment provides a novel therapeutic approach for attenuating ethanol-mediated increases in Nox expression.
MATERIALS AND METHODS
Mouse model of chronic ethanol consumption.
All animal protocols were reviewed and approved by the Emory University Institutional Animal Care and Use Committee, and all animal studies were done in accordance with National Institutes of Health guidelines outlined in the Guide for the Care and Use of Laboratory Animals. Male C57BL/6J mice (Jackson Laboratory, Bar Harbor, ME), aged 8–10 wk, were given either no ethanol or ethanol (20% wt/vol) in their drinking water for 12 wk as previously reported (67, 71). This method replicates blood alcohol levels following chronic ethanol consumption in human subjects (67). During the final week of ethanol treatment, all mice were gavaged daily as previously reported (38, 55) for 7 days with either GSH (300 mg·kg−1·day−1 in 100 μl methylcellulose vehicle) or vehicle alone.
Following death, tracheas were cannulated via tracheotomy, and BAL fluid was collected with three 1-ml saline lavages. Mouse alveolar macrophages (mAM) were then isolated from the fluid by centrifugation at 8,000 rpm for 5 min. The cell pellet was resuspended in RPMI-1640 medium containing 2% fetal bovine serum (FBS) and 1% penicillin/streptomycin. The cell pellet was then cultured overnight, allowing AMs to attach to the plastic petri dishes. After being stained with Diff-Quik (Dade Behring, Newark, DE) and counted with a hemocytometer, the cell population was determined by differential staining to be ∼95% AMs (16). The macrophages were then plated overnight in RPMI-1640 medium containing 0.5% FBS and 1% penicillin/streptomycin at 37°C in 5% CO2 atmosphere before beginning experiments.
GSH and glutathione disulfide levels in mouse BAL fluid and AMs.
BAL fluid and isolated AMs were used to measure reduced glutathione and glutathione disulfide levels, GSH and GSSG, respectively. To prevent auto-oxidation before GSH and GSSG analysis, 250 μl of BAL fluid were immediately added to an equal volume of 10% perchloric acid (final 5%) preservation solution containing iodoacetic acid (6.7 μM) and boric acid (0.1 M) with 5 μM γ-glutamyl-glutamate as an internal standard and immediately frozen at −80°C (40). AM cell pellets obtained from the BAL were immediately treated with a 5% perchloric acid preservation containing iodoacetic acid (3.35 μM), boric acid (0.05 M), and γ-glutamyl-glutamate (2.5 μM). GSH and GSSG were measured in BAL fluid and AMs by HPLC after samples were derivatized with dansyl chloride as previously described (40). To control for dilution by the lavage procedure, molar concentrations of GSSG and GSH were normalized to bicinchoninic acid (BCA) assay (Thermo Scientific, Rockford, IL) protein levels, as previously reported (14). The GSH and GSSG values for the AMs were normalized to cellular protein values. The redox potential (Eh) of the GSH/GSSG thiol pair was calculated using the Nernst equation: Eh = E0 + (RT/2F)ln([GSSG]/[GSH]2). E0 is the standard potential for the redox couple, R is the gas constant, T is the absolute temperature, and F is Faraday's constant. A more positive Eh number indicates greater oxidation of the GSH/GSSG thiol pair (42).
MH-S cell culture and ethanol stimulation.
The mAM cell line MH-S (ATCC, Manassas, VA) was used as a model system for studying effects of ethanol in vitro. Cells were cultured in RPMI-1640 media containing 10% FBS and 1% penicillin/streptomycin. After plating (24 h), MH-S cells were cultured in media containing 0.5% FBS, and selected wells were treated for 3 days with a clinically relevant ethanol concentration of 0.08% in a modular incubation chamber (Billups-Rothenberg, Del Mar, CA) to prevent alcohol evaporation as reported (60). During either the last 24 h or for the duration of exposure, selected wells were also treated with 500 μM GSH (ICN Nutritional Biochemicals, Cleveland, OH), the GSH concentration present in the alveolar lining fluid of control mice. GSH levels were measured in lysed MH-S cells by HPLC methods described above.
RNA isolation and quantitative RT-PCR.
Total RNA was extracted from mAMs or MH-S cells using TRIzol reagent (Invitrogen, Carlsbad, CA). mRNA expression was determined and quantified using specific mRNA primers given in Table 1, according to protocols previously described (71). Values for each target are expressed relative to mRNA levels of 9S in the same sample.
Table 1.
Mouse primer sequences to measure mRNA levels using quantitative RT-PCR
| Primer | Forward Sequence (5′-3′) | Reverse Sequence (5′-3′) |
|---|---|---|
| Nox1 | CGCTCCCAGCAGAAGGTCGTGATTACCAAG | GGAGTGACCCCAATCCCTGCCCCAACCA |
| Nox2 | GTCACACCCTTCGCATCCATTCTCAAGTCAGT | CTGAGACTCATCCCAGCCAGTGAGGTAG |
| Nox4 | CTGGAGGAGCTGGCTCGCCAACGAAG | GTGATCATGAGGAATAGCACCACCACCATGCAG |
| p22phox | AACGAGCAGGCGCTGGCGTCCG | CACAGTGGTATTTCGGCGCC |
| p47phox | CCAGCACTATGTGTACATGT | AAGGAGATGTTCCCCATTGA |
| p67phox | GCCTTCTGGTAAGCACCTAA | GAGGCCTTCAGCGAGGTGCA |
| 9S | GAGCTAGCCTCTGCCAGAGG | TCCAAGCCTCAAGACAGGAA |
Nox, NADPH oxidase.
Western blot analysis for MH-S cells.
Proteins were isolated from MH-S cells using a cell lysis buffer as previously described (67). Whole cell extracts prepared from untreated and ethanol-treated MH-S cells (40 μg/lane) were resolved in 4–12% bis-Tris polyacrylamide gels (Invitrogen), followed by transfer to nitrocellulose membranes. Membranes were probed with primary antibodies for Nox1 (1:1,000; Santa Cruz Biotechnology, Santa Cruz, CA), Nox2 (1:1,000; Abcam, Cambridge, MA), Nox4 (1:2,500, gift from Dr. David Lambeth, Emory University), or cyclin-dependent kinase 4 (CDK4, 1:1,000; Santa Cruz Biotechnology). Proteins were incubated with peroxidase-coupled anti-rabbit or anti-goat IgG (1:2,000) and visualized in the presence of LumiGlo reagent when exposed in a Bio-Rad Chemidoc XRS/HQ. Densitometric analysis was performed using Bio-Rad Quantity One (version 4.5.0) software. Values are expressed as the relative protein expression normalized to CDK4 protein.
Confocal immunostaining of mAMs.
AMs were isolated from the BAL fluid of control and ethanol-fed mice treated with or without dietary GSH. The cell pellet of AMs was resuspended in RPMI-1640 medium containing 2% FBS and 1% penicillin/streptomycin and cultured for 24 h. Cells were then fixed to chamber slides with 4% paraformaldehyde. Cells were incubated with primary antibodies for Nox1 (1:100), Nox2 (1:100), Nox4 (1:100; Santa Cruz Biotechnology), and TGF-β1 (1:100), followed by incubation with fluorescently labeled secondary antibodies. Fluorescence of the confocal microscopic images was measured using FluoView (Olympus) via quantitative digital analysis. Values are normalized to DAPI nuclear stain and are expressed as mean relative fluorescent units per cell ± SE.
Cellular oxidative stress production.
mAMs or MH-S cells were cultured in RPMI-1640 media containing 0.5% FBS for 24 h before the start of the experiment. Cells were then incubated with 5 μM 2′,7′-dichlorofluorescein-diacetate (DCFH-DA; Invitrogen) in RPMI at 37°C for 30 min in the dark to determine intracellular ROS production as described previously (56). Cells were then washed with phosphate-buffered saline three times to remove excess dye, and fluorescence was measured using FluoView (Olympus, Melville, NY) via quantitative digital analysis. ROS production values are expressed as means ± SE, relative to average control values.
H2O2 released in media collected from mAMs or MH-S cells was determined using Amplex Red assay (Invitrogen), according to the manufacturer's protocol. In brief, cells were incubated with 500 μl Amplex Red (20 μM) and horseradish peroxidase (0.1 U/ml) at 37°C for 30 min in the dark. The reaction mixtures were then measured for fluorescence in duplicate (excitation 540 nm, emission 590 nm), and H2O2 concentrations were calculated utilizing standard curves generated with reagent H2O2. Cell cultures were then lysed in 100 μl cell lysis buffer and centrifuged at 12,000 rpm for 10 min. Supernatant protein concentrations were determined using BCA assay. H2O2 concentrations were normalized to cellular protein concentrations and expressed as means ± SE, relative to average control values.
Phagocytosis.
Phagocytic ability of AMs was assessed as previously described (16). In brief, mAMs were incubated with 1 × 106 particles of pH-sensitive pHrodo S. aureus BioParticles conjugate (Invitrogen) for 2 h and then fixed with 4% paraformaldehyde. Phagocytosis of bacteria was analyzed using an Olympus confocal microscope containing an argon/krypton laser. Cells from 10 fields/experimental condition were assessed using quantitative digital fluorescence imaging software (FluoView 300, version 4.3; Olympus). To measure S. aureus internalization, laser confocal microscopy was performed at 50% of cell depth using identical background and gain settings. AMs with any internalized bacteria were considered positive for phagocytosis. Phagocytosis was quantified by phagocytic index, which was calculated as the percentage of cells positive for phagocytosis multiplied by the relative fluorescence units of S. aureus per cell.
Lung Klebsiella pneumoniae clearance in vivo.
As previously described (50), Klebsiella pneumoniae (ATCC no. 43816, Manassas, VA) was grown in 100 ml of trypticase soy broth at 37°C in a shaker for 18 h. Bacteria were pelleted by centrifugation (1,200 g) and resuspended in 10 ml of sterile phosphate-buffered saline to achieve a stock concentration of 2 × 1010 colony-forming units (CFU)/ml. Serial dilutions were performed to make a final concentration of 2 × 103 CFU/ml for inoculation of mice, where a total volume of 100 μl was inoculated in the airway of each mouse. K. pneumoniae were plated on MacConkey agar (Remel, Lenexa, KS) to confirm their presence and quantify their concentration.
Male C57BL/6J mice were anesthetized with ketamine (6 mg/kg) and xylazine (2 mg/kg), and tracheotomy was performed (29). An inoculating syringe was used to deliver 100 μl of the K. pneumoniae inoculum intratracheally. After 4 h, selected mice were gavaged as previously reported (38, 55) with 300 mg/kg GSH in 100 μl methylcellulose vehicle or vehicle alone and killed after 24 h. BAL fluid and mAMs (lysed in 0.01% Triton X) collected from these mice were plated on MacConkey agar to determine the concentration of K. pneumoniae.
Statistical analysis.
Data are presented as means ± SE. Statistical significance was calculated using one-way ANOVA followed by Tukey-Kramer test to detect differences between individual groups using GraphPad Prism version 5 (GraphPad, San Diego, CA). P < 0.05 was considered statistically significant.
RESULTS
Chronic alcohol exposure decreased GSH levels in the BAL fluid and AMs of mice.
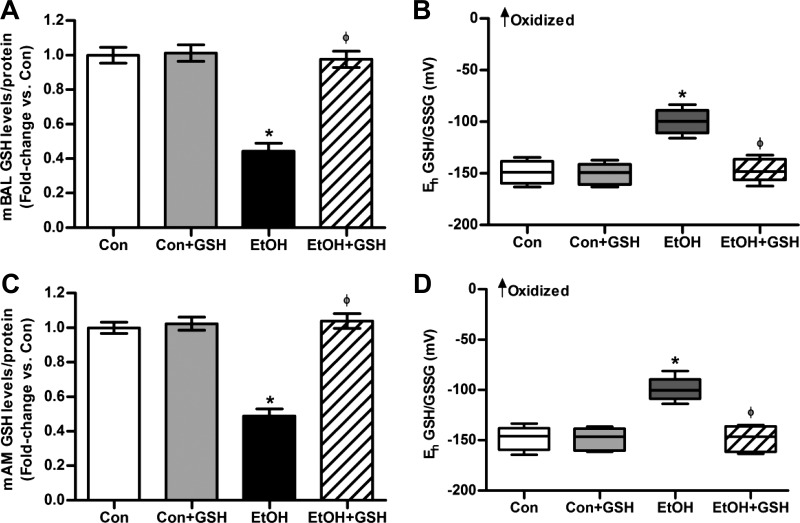
BAL fluid and AMs were isolated from control and ethanol-fed mice (20% wt/vol ethanol for 12 wk) to evaluate the effects of ethanol on GSH and GSSG levels in vivo. For the BAL fluid, the samples were derivatized with dansyl chloride, and GSH and GSSG levels were measured using HPLC and normalized to protein concentrations [control (Con), 67.3 μg protein/ml; Con + GSH, 68.6 μg/ml; ethanol (EtOH), 70.1 μg/ml; and EtOH + GSH, 67.7 μg/ml]. Compared with control mice, the BAL fluid from ethanol-fed mice showed a 55 ± 4.6% decrease in GSH levels (Fig. 1A). The redox potential became more positive, indicating that the redox potential of the GSH/GSSG thiol pair was oxidized by ∼50 mV (Fig. 1B). Similarly, the AMs from ethanol-fed mice showed decreased GSH levels (Fig. 1C) and increased oxidation of the GSH/GSSG thiol pair (Fig. 1D). GSH treatment did not alter the GSH pool or the redox potential of the controls. However, oral GSH treatment restored the GSH pool to control values and normalized the redox potential of the BAL fluid and AMs of the ethanol group.
Fig. 1.
Chronic ethanol consumption reduced bronchoalveolar lavage (BAL) and alveolar macrophage (AM) glutathione (GSH) levels in vivo. BAL fluid and AMs were collected from control (Con) and ethanol-fed (EtOH) mice (n = 5, in duplicate) given oral GSH (300 mg·kg−1·day−1 in 100 μl methylcellulose vehicle) or vehicle alone for the last 7 days of ethanol feeding. In the BAL fluid, GSH levels (A) and redox potential of the GSH/glutathione disulfide (GSSG) thiol pair (B) were determined by HPLC. In isolated AMs, GSH levels (C) and redox potential of the GSH/GSSG thiol pair (D) were measured by HPLC. GSH and GSSG levels in BAL fluid and AMs were normalized to the appropriate protein concentration. Eh, redox potential. Values are expressed as means ± SE, relative to control. Box plots are shown with medians and the upper and lower quartiles. *P < 0.05, vs. Con; ΦP < 0.05, vs. EtOH.
GSH treatment attenuated ethanol-induced MH-S and mAM Nox expression in vitro and in vivo.
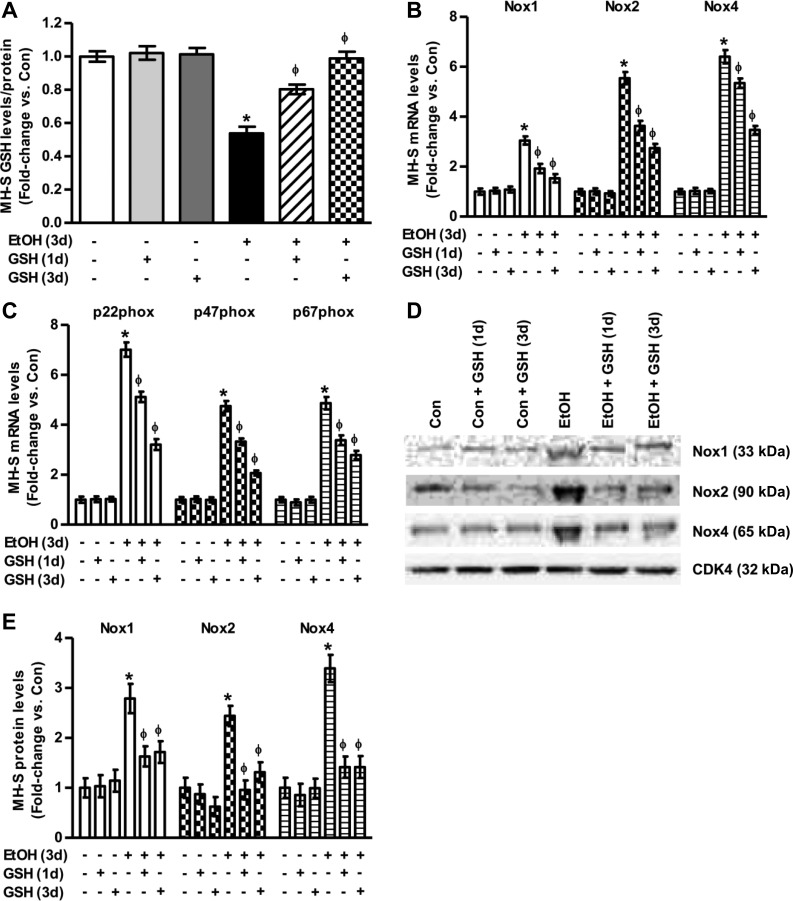
To evaluate the effects of ethanol on GSH levels in vitro, MH-S cells either untreated or treated with ethanol (0.08%, 3 days) ± GSH (500 μM, 3 days or 1 day) were lysed, and GSH levels were measured using HPLC. Similar to in vivo experiments, ethanol-treated MH-S cells showed a 46 ± 3.9% decrease in GSH levels (Fig. 2A) compared with controls. Whereas GSH treatment did not alter the GSH pool of the controls, GSH treatment restored the intracellular GSH pool of the ethanol group to control values.
Fig. 2.
Ethanol induced NADPH oxidase (Nox) mRNA and protein expression levels in vitro. Cultured MH-S cells were either untreated (Con) or ethanol-treated (EtOH, 0.08%) for 3 days ± GSH for either the last 24 h of ethanol exposure, referred to as GSH 1 day. In other experiments, GSH was added throughout the exposure and referred to as GSH 3 days. A: intracellular GSH levels in these MH-S cells (n = 3, in duplicate), as measured by HPLC. GSH levels were normalized to protein concentration. Values are expressed as means ± SE, relative to control. B and C: mRNA levels of Nox1, Nox2, Nox4, p22phox, p47phox, and p67phox were measured in these MH-S cells (n = 3, in duplicate). All mRNA values were measured by quantitative RT-PCR (qRT-PCR), normalized to 9S mRNA, and expressed as means ± SE, relative to no treatment. D and E: protein expression was assessed by Western blotting (D) and densitometric analysis (E) for Nox1, Nox2, and Nox4 in these MH-S cells (n = 6). Protein values were normalized to cyclin-dependent kinase 4 (CDK4) protein levels, and expressed as means ± SE, relative to no treatment. *P < 0.05 vs. Con; ΦP < 0.05 vs. EtOH.
Compared with untreated MH-S cells, ethanol (0.08%, 3 days) increased mRNA levels of Nox1 (3.0-fold), Nox2 (5.5-fold), and Nox4 (6.4-fold) (Fig. 2B). Ethanol also increased MH-S cell mRNA levels of p22phox (7.0-fold), p47phox (4.8-fold), and p67phox (4.9-fold) (Fig. 2C). Additionally, as measured by Western blotting analysis (Fig. 2D) and densitometry (Fig. 2E), ethanol exposure increased the protein levels of Nox1, Nox2, and Nox4. Treatment with GSH (500 μM, 1 or 3 days) had no effects on controls but caused time-dependent attenuation of ethanol-induced increases in mRNA and protein levels of Noxes and their associated proteins in MH-S cells (Fig. 2, B–E).
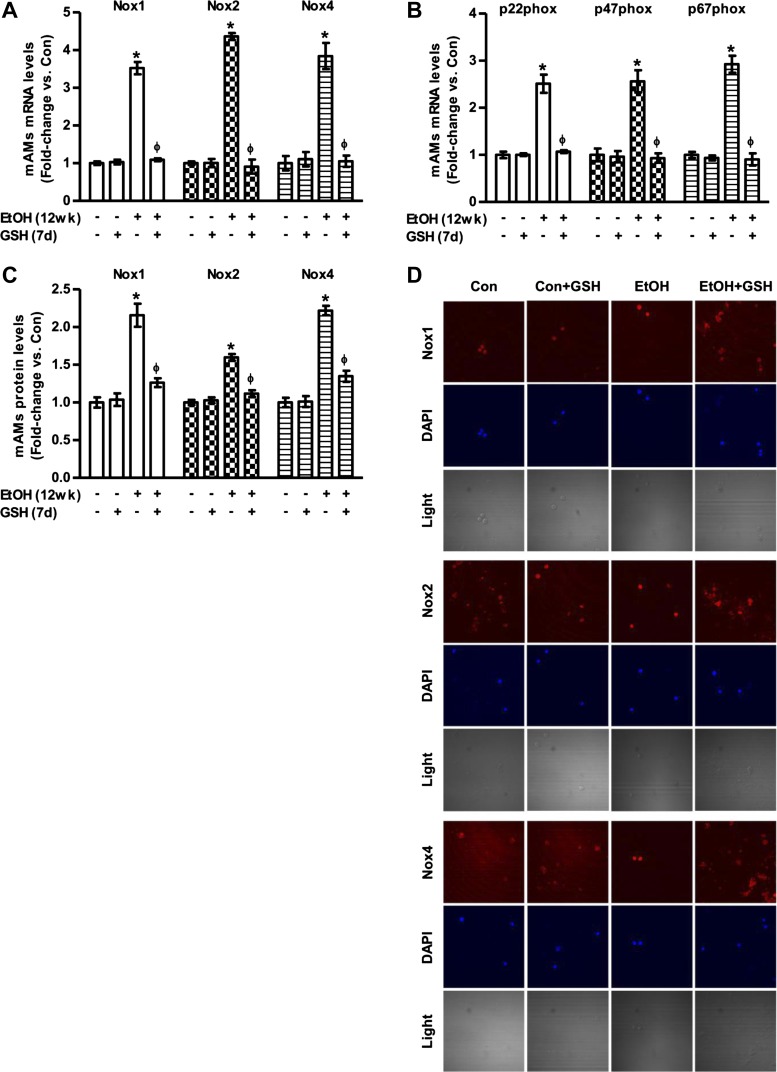
As shown in Fig. 3, A and B, in vivo ethanol consumption also increased mAM mRNA expression levels of Nox1 (3.5-fold), Nox2 (4.4-fold), Nox4 (3.8-fold), p22phox (2.5-fold), p47phox (2.6-fold), and p67phox (2.9-fold). Furthermore, chronic ethanol feeding increased the protein levels of Nox1, Nox2, and Nox4, as measured by computer analysis (Fig. 3C) of confocal microscopic images (Fig. 3D). Treatment with GSH (300 mg·kg−1·day−1, during week 12 of ethanol feeding) attenuated ethanol-induced increases in mRNA and protein levels of Noxes, and their affiliated proteins in mAMs (Fig. 3, A–D). Collectively, these data indicate that chronic ethanol exposure increased mRNA and protein levels of Noxes and Nox-associated proteins in mAMs and that oral GSH treatment in vivo can reverse these ethanol-induced increases.
Fig. 3.
Ethanol augmented Nox mRNA and protein expression levels in vivo. mAMs were collected from control (Con) and ethanol-fed (EtOH; 12 wk) mice ± oral GSH for the last 7 days of ethanol feeding. A and B: mRNA levels of Nox1, Nox2, Nox4, p22phox, p47phox, and p67phox were measured in these mAMs (n = 5, in duplicate). All mRNA values were measured by qRT-PCR, normalized to 9S mRNA, and expressed as means ± SE, relative to control. C and D: protein expression of Nox1, Nox2, and Nox4 was determined in these mAMs (n = 5), as measured by computer analysis (quantification of fluorescence intensity, C) of confocal fluorescence microscopic images (representative images, D). Quantification of fluorescence values are normalized to DAPI nuclear stain and expressed as mean relative fluorescent units (RFU) per cell ± SE, relative to Con. *P < 0.05 vs. Con; ΦP < 0.05 vs. EtOH.
GSH treatment reduced ethanol-induced increases in mAM oxidative stress and phagocytic dysfunction.
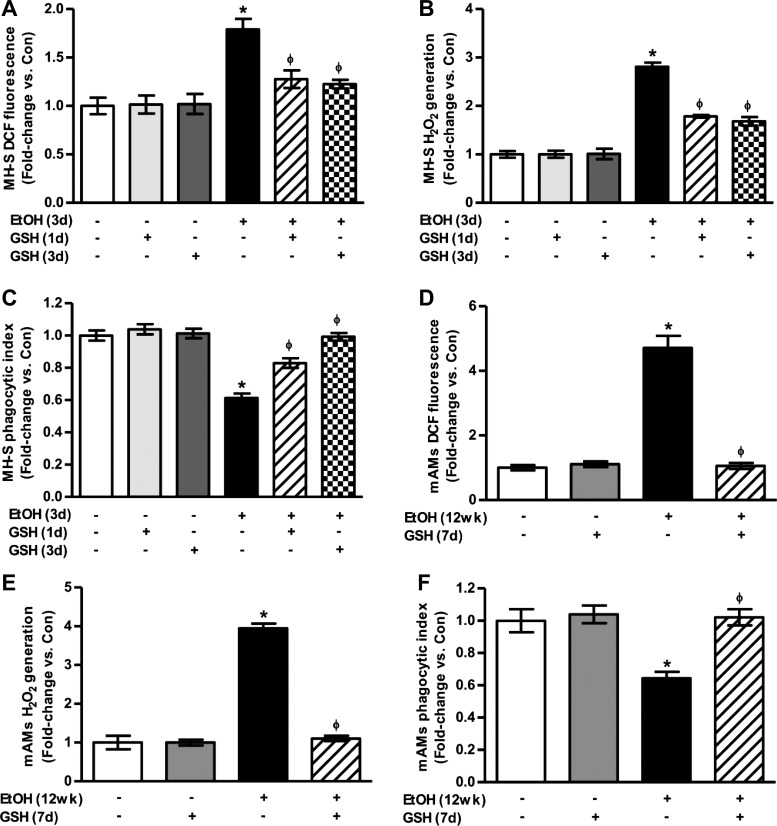
MH-S cells were used to evaluate the effects of ethanol and GSH on AM oxidative stress in vitro. Oxidative stress was determined using DCFH-DA fluorescence to measure intracellular ROS production and Amplex Red to determine extracellular H2O2. Compared with control MH-S cells, ethanol exposure stimulated a 1.8-fold increase in DCF fluorescence (Fig. 4A), a 2.8-fold increase in H2O2 generation (Fig. 4B), and a 39 ± 2.9% reduction in phagocytic capacity (Fig. 4C). Treatment with GSH for only the last day of in vitro ethanol exposure or for the duration of ethanol exposure attenuated AM oxidative stress and phagocytic dysfunction in these MH-S cells.
Fig. 4.
Ethanol increased oxidative stress and phagocytic dysfunction in mouse alveolar macrophages (mAMs). In cultured MH-S cells that were either untreated (Con) or ethanol-treated (EtOH, 0.08%) for 3 days ± GSH for either the last 24 h (GSH, 1 day) or for the duration of ethanol exposure (GSH, 3 days) (n = 3, in duplicate), intracellular reactive oxygen species (ROS) production was measured as 2′,7′-dichlorofluorescein (DCF) fluorescence (A), and extracellular H2O2 was measured by Amplex Red assay (B). Phagocytic ability of these MH-S cells was assessed by phagocytosis assay (n = 3, 10 fields/experimental condition) (C). In mAMs collected from control (Con) and ethanol-fed (EtOH) mice ± GSH for the last 7 days of ethanol feeding (n = 5, in duplicate), DCF fluorescence (D) and H2O2 generation (E) were measured. Phagocytic ability of these mAMs was assessed by phagocytosis assay (n = 5, 10 fields/experimental condition) (F). Phagocytic index was calculated from the percentage of phagocytic cells multiplied by the relative fluorescence units of Staphylococcus aureus per cell. All values are expressed as means ± SE, relative to control. *P < 0.05 vs. Con; ΦP < 0.05 vs. EtOH.
For in vivo experiments, mAMs were isolated from control and ethanol-fed mice, with each group randomly assigned to vehicle or GSH (300 mg·kg−1·day−1) during week 12 of feeding. Similarly to in vitro experiments using MH-S cells, chronic ethanol ingestion increased in vivo mAM DCF fluorescence by 4.7-fold (Fig. 4D) and H2O2 generation by 3.9-fold (Fig. 4E) compared with AMs from control animals. In parallel studies, isolated mAMs treated with PEG-catalase showed a 80 ± 2.3% decrease in H2O2 generation compared with untreated cells (data not shown). However, dietary GSH completely attenuated ethanol's effects on AM oxidative stress in these animals.
Furthermore, phagocytic index was lower in mAMs from ethanol-fed animals (Fig. 4F), and GSH treatment reversed ethanol-mediated AM dysfunction. These findings not only demonstrate that ethanol increases mAM oxidative stress in vivo and in vitro but also illustrate that late intervention with GSH supplementation attenuates ethanol-induced oxidative stress and phagocytic dysfunction in AMs.
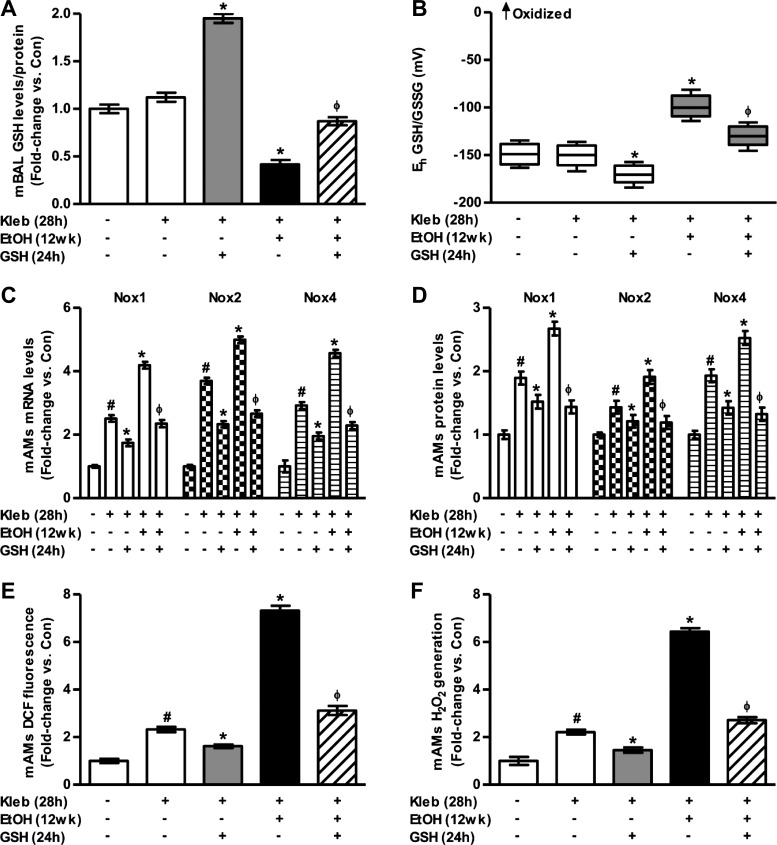
GSH attenuates combined ethanol and K. pneumoniae-induced AM oxidative stress in vivo.
Subjects with alcohol use disorders have an increased risk of developing respiratory infections, such as pneumonia (49). We next examined whether the ethanol-induced suppression of AM phagocytosis was physiologically relevant, resulting in an increased risk of respiratory infections, and whether GSH reversed these effects. To evaluate the effects of combined ethanol and K. pneumoniae on GSH and GSSG levels in vivo, BAL fluid was isolated from control and ethanol-fed mice challenged with K. pneumoniae (200 CFU inoculated intratracheally). Samples were derivatized with dansyl chloride, and GSH and GSSG levels were measured using HPLC and normalized to protein concentrations [Con, 68.4 μg protein/ml; K. pneumoniae (Kleb), 68.1 μg/ml; Kleb + GSH, 69.7 μg/ml; Kleb + EtOH, 73.0 μg/ml; and Kleb + EtOH + GSH, 72.6 μg/ml]. Compared with control mice (Fig. 1, A and B), GSH levels and oxidation of the redox potential for the GSH/GSSG thiol pair were unchanged with a 24-h K. pneumoniae challenge alone (Fig. 5, A and B). However, compared with control K. pneumoniae mice or the ethanol-fed mice (no bacterial treatment), the BAL fluid from ethanol-fed mice treated with K. pneumoniae showed further decreases in GSH levels (Fig. 5A) and increases in oxidation of the redox potential for the GSH/GSSG thiol pair (Fig. 5B). These findings indicate that chronic alcohol exposure, combined with bacterial challenge, exacerbated the oxidative stress in the alveolar space with further decreases in GSH and increases in GSSG levels resulting in further oxidation of the BAL fluid in vivo. GSH treatment of the K. pneumoniae group (without ethanol) increased GSH availability in the alveolar lining fluid and increased the reducing capacity of this thiol pair. Dietary GSH (300 mg/kg for 24 h during bacterial exposure) partially reversed the combined effects of alcohol and K. pneumoniae on the redox oxidative stress in the BAL fluid (Fig. 5, A and B).
Fig. 5.
GSH attenuates AM oxidative stress in Klebsiella pneumoniae-exposed alcohol-fed mice. Mice fed either no ethanol (Con) or ethanol (EtOH) were inoculated with K. pneumoniae [Kleb, 200 colony-forming units (CFU)]. After 4 h, animals were gavaged with GSH or vehicle and then euthanized 24 h later (n = 4–5). GSH levels (A) and redox potential of the GSH/GSSG thiol pair (B) were determined in BAL fluid collected from mice, as measured by HPLC. GSH and GSSG levels were normalized to protein concentration. Box plots are shown with medians and the upper and lower quartiles. C: mRNA levels of Nox1, Nox2, and Nox4 were measured in mAMs isolated from these mice. All mRNA values were measured by qRT-PCR and normalized to 9S mRNA. D: protein levels of Nox1, Nox2, and Nox4 were determined in these mAMs, as measured by computer analysis of confocal fluorescence microscopic images, and quantification of fluorescence values are expressed. DCF fluorescence (E) and H2O2 generation (F) were measured in these mAMs. All values are expressed as means ± SE, relative to untreated controls without bacterial challenge and where fold-change = 1.0. #P < 0.05 vs. Con; *P < 0.05 vs. Con + Kleb; ΦP < 0.05 vs. EtOH + Kleb.
Compared with control mice (Fig. 3, A–C), mRNA and protein levels of Nox1, Nox2, and Nox4 were increased with the K. pneumoniae challenge alone (Fig. 5, C–D) as expected. Compared with the group with ethanol alone, the added treatment with K. pneumoniae mice exacerbated the increases in the mRNA levels of Nox1, Nox2, and Nox4 (Fig. 5C) associated with ethanol alone (P ≤ 0.05, Fig. 3, A and B). Furthermore, ethanol feeding with bacterial challenge increased the protein levels of Nox1, Nox2, and Nox4, as measured by confocal microscopy (Fig. 5D). Treatment with GSH (300 mg/kg for 24 h during K. pneumoniae exposure) attenuated alcohol and bacteria-induced mRNA and protein levels of Noxes in mAMs (Fig. 5, C–D). Therefore, chronic alcohol exposure, combined with bacterial challenge, further enhanced the mRNA and protein levels of Noxes in mAMs. Despite the bacterial challenge, in vivo GSH treatment remained an effective strategy to reverse ethanol-induced alterations in gene expression and AM dysfunction.
AM oxidative stress was determined using DCFH-DA fluorescence to measure intracellular ROS production and Amplex Red to determine extracellular H2O2. Compared with control mice (Fig. 4, D and E), oxidative stress was increased with K. pneumoniae challenge alone but was blocked by oral GSH treatments (Fig. 5, E and F). For the ethanol plus K. pneumoniae group, the increase in DCF fluorescence (Fig. 5E) and H2O2 (Fig. 5F) was statistically greater than the control plus K. pneumoniae group or the ethanol alone group. Oral GSH given 4 h after K. pneumoniae exposure reduced oxidative stress production. These findings indicate that alcohol, combined with bacterial challenge, stimulated oxidative stress and that intervention with GSH attenuates alcohol- and bacteria-induced mAM oxidative stress in vivo.
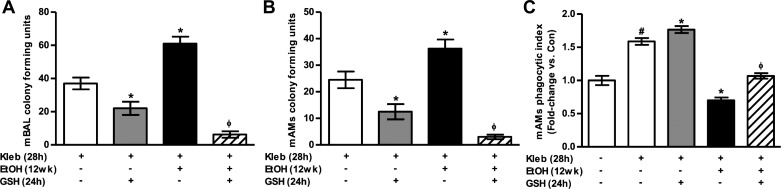
GSH improves clearance of K. pneumoniae from the lung in vivo.
BAL fluid and mAMs were isolated from control and ethanol-fed mice that were challenged with K. pneumoniae. Oral GSH in the control-fed mice attenuated K. pneumoniae infection in BAL and mAMs. Compared with control mice, in vivo ethanol exposure impaired clearance of K. pneumoniae bacteria from BAL fluid (Fig. 6A) and mAMs (Fig. 6B). Oral GSH treatments improved bacterial clearance in BAL fluid and mAMs from both control and ethanol-fed mice (Fig. 6, A and B). The phagocytic ability to clear S. aureus bacteria was enhanced in mAMs isolated from K. pneumoniae-challenged mice (Fig. 6C) compared with control mice (Fig. 4F). However, phagocytosis and clearance of S. aureus was impaired in mAMs isolated from K. pneumoniae-challenged ethanol-fed mice. GSH treatment reversed ethanol-mediated AM dysfunction to rescue phagocytic ability in vivo. Collectively, these data suggested that dietary GSH treatments can attenuate ethanol-induced AM dysfunction and improve K. pneumoniae clearance in alcohol-exposed lungs.
Fig. 6.
GSH promotes lung bacterial clearance of K. pneumoniae in alcohol-fed mice. Mice fed either no ethanol (Con) or ethanol (EtOH) were inoculated for 4 h with K. pneumoniae (Kleb, 200 CFU) ± 24 h with GSH during bacterial exposure (n = 4–5). A and B: clearance of K. pneumoniae was assessed in BAL fluid (A) and lysed mAMs (B) that were plated on MacConkey agar where the concentration of K. pneumoniae bacteria was quantified as CFU. CFU in BAL fluid was normalized to number of isolated macrophages. C: phagocytic ability of these mAMs to clear S. aureus following K. pneumoniae challenge was assessed by phagocytosis assay (10 fields/experimental condition). Phagocytic index was calculated from the percentage of phagocytic cells multiplied by the relative fluorescence units of S. aureus per cell. All values are expressed as means ± SE, relative to untreated controls without bacterial challenge and where fold-change = 1.0. #P < 0.05 vs. Con; *P < 0.05 vs. Con + Kleb; ΦP < 0.05 vs. EtOH + Kleb.
DISCUSSION
Chronic alcohol abuse is a comorbid variable that increases the risk of respiratory infections and ARDS (54). Chronic alcohol ingestion depleted GSH levels in BAL fluid (9) and AMs (10) and caused oxidative stress and AM dysfunction in clinical and animal studies (12, 14, 37, 70). AMs are important to innate and acquired immunity (66) due to their ability to phagocytize and clear apoptotic cells and infectious particles from the lung (47). Chronic ethanol exposure causes AM dysfunction (12) through mechanisms that may involve alcohol-induced oxidative stress (16). One potential mechanism for increased oxidative stress involves upregulation of the Nox family of proteins that comprise membrane-associated, multicomponent Nox enzymes that generate ROS (10). Under physiological conditions, the primary sources of ROS production are the Noxes (28, 57). Nox1 (32), Nox2 (59), and Nox4 (51) are expressed in the lung, with Nox2 being the classical phagocytic oxidase necessary for ROS production during respiratory burst (59). Although not involved in respiratory burst, the expression of Nox4, a constitutively active Nox isoform, was enhanced in the lung tissue of ethanol-fed rats (15). Our previous studies demonstrated that chronic ethanol ingestion increased Nox1, Nox2, and Nox4 expression in whole lung tissue (67) and in mAMs (71). Because redox balance in the airway is critical for lung immunity and Noxes are central to the clearance of microbes through respiratory burst, the current study examined the therapeutic effects of restoring the GSH pool in the alveolar lining fluid on attenuating AM oxidative stress and dysfunction associated with chronic ethanol ingestion.
Confirming our previous studies (71), mAMs exposed to alcohol in vitro and in vivo demonstrated upregulation of p22phox, a catalytic subunit for Nox1, Nox2, and Nox4, as well as p47phox and p67phox, regulatory proteins for Nox1 and Nox2. Ethanol exposure also increased the expression of Nox1, Nox2, and Nox4. Whereas chronic ethanol exposure augmented Nox expression in AMs, the mechanisms responsible for these increases remain undefined. Several factors that increase Nox expression are stimulated by alcohol. For example, previous studies showed that ethanol enhanced angiotensin II activity (6), a potent stimulus for Nox expression (63). The role of angiotensin II in alcohol-induced oxidative stress in the lung was further supported by studies demonstrating that chronic alcohol-induced increases in rat lung Nox could be attenuated by angiotensin-converting enzyme inhibitors (59). We have previously reported that either Nox1 or Nox2 is needed for Nox4 expression (71), which could contribute to the upregulation of TGF-β1 through increased oxidative stress (58). Chronic ethanol ingestion also increased TGF-β1 expression (5), which has been implicated in the regulation of Nox4 (10). The current studies identified alcohol-induced Noxes as modulators contributing to AM oxidative stress; however, further studies are warranted to elucidate the molecular mechanisms involved in ethanol-mediated Nox expression, which may include TGF-β1 regulation.
Recent studies suggest that oxidative stress can stimulate positive feedback loops that promote further ROS generation (7). Hypoxia activated hypoxia-inducible factor-1 and upregulated Nox1 through increased ROS (32). Lipopolysaccharide (LPS)/interferon-γ-induced ROS upregulated redox-sensitive NF-κB to activate Nox2 expression in monocytes (2). The transcription factors NF-κB and hypoxia-inducible factor-1 are induced by ROS generated by thrombin-activated Nox4 (9). Furthermore, the promoters of Nox1, Nox2, and Nox4 contain response elements for several oxidative stress-activated transcription factors, such as phosphatidylinositol 3-kinase and protein kinase C in Nox1 (24), interferon regulatory factors-1 and -2 and NF-κB in Nox2 (8), and nuclear respiratory factor-1 and NF-κB in Nox4 (46). Collectively, these studies suggest that alcohol-induced oxidative stress may stimulate that activity of redox-regulated transcription factors that promote increased expression of Noxes. Furthermore, we have previously shown that knockdown of Noxes attenuated alcohol-induced oxidative stress in AMs (71). Whereas our current studies show that chronic ethanol ingestion upregulated Noxes, the specific mechanisms by which alcohol mediates these effects are the focus of our future studies.
In clinical studies, an alcohol use disorder was associated with decreased GSH and an oxidation of the GSH/GSSG redox potential by ∼50 mV, independent of smoking history (70). In the current study, chronic ethanol ingestion in the mouse model produced similar decreases in the GSH levels and oxidation of the redox potential of the GSH/GSSG thiol pair in the BAL fluid and AMs. Previous studies demonstrated that treatment with l-2-oxothiaxolidine-4-carboxylate restored GSH levels in the BAL and AMs from ethanol-fed rats and attenuated defects in phagocytosis (12), implicating alcohol-induced GSH depletion and chronic oxidative stress in AM dysfunction. l-2-Oxothiaxolidine-4-carboxylate is a precursor to cysteine, which is required for downstream GSH synthesis. To determine the unique and critical role that GSH itself plays in attenuating alcohol-induced AM phagocytic dysfunction, ethanol-exposed mAMs were treated with GSH as an intervention in vitro and in vivo. Although there were no effects in the BAL or AMs of the control group, limited GSH uptake by intestinal epithelial cells and tightly controlled GSH homeostasis may prevent spikes in GSH in the alveolar lining fluid under control conditions, even in the presence of oral supplements. In contrast, GSH treatment during the last week of ethanol ingestion restored the GSH pool and the reducing capacity of the BAL fluid and intracellular AM environment. The mechanisms by which oral GSH restored the GSH pool in the BAL of ethanol-fed mice remain to be determined, but these results are similar to our previous observations where intraperitoneal injections in the preterm rabbit exposed to hyperoxia improved the GSH pool in BAL and protected against lung injury (13). Alternatively, one positive aspect of ethanol with LPS-induced increases in lung permeability (67) may result in increased systemic GSH through oral supplements and increased GSH leak in the alveolar space. Another potential mechanism may be that increases in systemic GSH and oxidative stress in the alveolar space may upregulate GSH transport in the lung and alveolar space (3).
Although oral GSH supplements were associated with normalization of the GSH pool in the BAL, the underlying mechanisms for improved AM phagocytosis are unclear. One mechanism could be through restoration of the GSH pool, as shown in the studies presented herein, or cysteine pool in the AM. By breaking down extracellular GSH, γ-glutamyltransferase (GGT) provides cysteine, the rate-limiting amino acid for GSH resynthesis (45). As such, GGT is critical for maintaining GSH and cysteine homeostasis. Thus, GGT with its active site directed outward is critical for AMs to use extracellular GSH to increase their intracellular GSH pool (27). In a genetic model of GGT deficiency, GSH in the BAL was decreased approximately sixfold and its oxidation was increased approximately sevenfold (39). In previous studies, we demonstrated that ethanol-induced decreases in GSH in the alveolar lining fluid are concomitant with decreases in GSH in the AM (14). Correspondingly, increases in GSH in the alveolar lining fluid through dietary GSH precursors improve the GSH content of AMs. Therefore, dietary GSH undoubtedly improved the extracellular GSH pool and the intracellular AM pool. Increased GSH in the epithelial lining fluid may also directly react with ROS in the lining fluid, thereby decreasing ROS that react with AMs. In addition to restoring the AM pools, reversible oxidative modifications of proteins in the plasma membrane, especially the cysteine moieties, can alter their function at the molecular level of catalytic activity, signaling and macromolecular interactions (30). Therefore, oxidation of critical proteins located in the AM plasma membrane may render them inactive. By restoring the extracellular GSH pool, these critical cysteines facing the lining fluid may then be reduced and restored to their active state for catalytic activity or signaling related to AM phagocytosis (25, 61). Likewise, intracellular proteins also contain redox-sensitive sites. For example, actin is sensitive to oxidation, and restoration of the redox state would permit its polymerization (31), a critical step in phagocytosis. Therefore, dietary GSH restored both the extracellular and intracellular GSH pools after chronic ethanol ingestion. This undoubtedly restored multiple extracellular and intracellular cascades that resulted in restoration of AM bacterial clearance.
With the use of ethanol-exposed MH-S cells, AMs isolated from mice following chronic ethanol ingestion, or AMs from subjects with an alcohol use disorder, we demonstrated that alcohol upregulated Nox1, Nox2, Nox4, and associated Nox regulatory proteins (71). In those models, knockdown of Noxes improved alcohol-induced AM phagocytic dysfunction, suggesting a central role for oxidative stress in impaired AM phagocytosis. In vitro ethanol exposure increased TGF-β1 and IL-13 production in AMs, which led to alternative activation and impaired phagocytosis (15). Treatment with GSH during ethanol exposure attenuated oxidative stress, TGF-β1 and IL-13 production, alternative activation, and impaired phagocytosis. The current study extends those findings by demonstrating that GSH treatment reversed ethanol-mediated decreases in intracellular GSH levels and attenuated ethanol-induced expression of Noxes and Nox regulatory proteins in ethanol-exposed mAMs in vitro and in vivo. We have previously shown that expression of Nox1 and Nox2 begins to increase after 6 h of ethanol exposure and that Nox4 expression begins to increase after 12 h, remaining elevated for the duration of ethanol exposure (3 days) (71). Because GSH was also effective during the last 24 h of ethanol exposure, the data support that intervention with oral GSH can reverse ethanol-induced Nox4 expression even after 12 wk of ethanol ingestion. As expected with attenuation of Nox proteins, ethanol-mediated increases in oxidative stress were also abrogated with GSH treatment in mAMs. Equally important, ethanol-induced impairments in AM phagocytic function were reversed by GSH treatment in vitro or in vivo. Thus, the ethanol-induced upregulation of Noxes and their regulatory proteins and subsequent oxidative stress were central to impaired AM phagocytosis. Although we have shown herein that oral GSH prevention treatment (3 days) and intervention treatment (1 day) can attenuate ethanol-induced AM derangements, dysregulation of the GSH pathway may not be the only mechanism by which alcohol induces its detrimental effects. For example, we have previously demonstrated that activation of peroxisome proliferator-activated receptor-γ with its ligand rosiglitazone can attenuate alcohol-induced lung Nox expression, oxidative stress, and barrier dysfunction (67). Although additional studies are needed to determine the underlying mechanisms, these data highlight an etiological role for GSH depletion in the alveolar space in the upregulation of Nox expression, chronic oxidative stress, and impaired AM phagocytosis. Furthermore, these data suggest that strategies to improve GSH availability in the alveolar space and AMs will attenuate the ethanol-induced Nox expression and oxidative stress to restore bacterial clearance by AMs.
We next examined whether GSH treatments could attenuate the risk of experimentally induced bacterial infections in vivo. In the control group, infection with K. pneumoniae did not significantly alter the GSH pool or the redox state of the alveolar lining fluid. However, GSH treatment superimposed on K. pneumoniae treatment significantly increased the GSH pool and the reducing capacity of the BAL fluid. Whether the increased GSH in the epithelial lining fluid was due to increased transport, leak, or secretion by alveolar cells remains to be determined. However, GSH treatment decreased the induced expression of Nox proteins and corresponding increased ROS generation by AMs associated with K. pneumoniae treatments. Equally important, GSH treatment improved K. pneumoniae clearance from the alveolar space of control animals. Although there was decreased K. pneumonia in the AM, improved phagocytic capacity suggested that decreased K. pneumonia was due to increased killing.
As expected, oral GSH normalized the GSH pool and reducing capacity even when chronic ethanol ingestion was superimposed on K. pneumonia treatments. Correspondingly, oral GSH normalized the expression of various Nox isoforms and ROS production in the ethanol- and K. pneumoniae-treated group. This was accompanied by improved phagocytic function of the AM as well as clearance of K. pneumoniae from the airspace and AM. To our knowledge, this is the first study to report the therapeutic potential of dietary GSH in an in vivo model of bacterial phagocytosis and clearance.
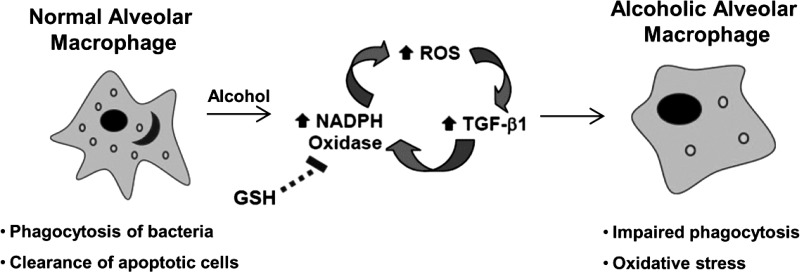
Whereas ROS generation through Nox2 is critical for microbicidal functions, these data suggested that oxidative stress and GSH depletion in the BAL exacerbate infection. Excessive ROS generation through ethanol-induced upregulation of Nox isoforms (71) and TGF-β1 (15) with accompanying chronic oxidative stress was central to ethanol-induced phagocytic dysfunction in mAMs (conceptual summary Fig. 7). However, the specific mechanisms by which GSH reduced ethanol-mediated increases in oxidative stress and improved AM function remain to be defined. In previous studies, ethanol feeding increased the expression of alternative activation markers and immunosuppressive TGF-β1, resulting in attenuated phagocytosis in AMs from rats (15). Furthermore, TGF-β1 upregulated Nox protein expression, particularly that of Nox4 (10), suggesting a vicious cycle of ROS generation and TGF-β1 expression. Therefore, GSH could improve AM function through downregulation of TGF-β1 expression, thereby attenuating Nox levels and oxidative stress, reversing alternative activation, and ultimately improving the capacity to combat a K. pneumoniae infection.
Fig. 7.
Hypothetical schematic of alcohol-induced AM oxidative stress and dysfunction. AMs that are exposed to chronic alcohol exhibit a feed-forward loop of increased ROS and augmented expression of TGF-β1 and Noxes. We propose that treatment with GSH leads to reduced Nox expression, decreased oxidative stress, and improved phagocytic function in AMs.
In summary, chronic ethanol ingestion increased AM oxidative stress and impaired phagocytosis through upregulation of Nox1, Nox2, Nox4, and Nox regulatory proteins. Oral GSH restored the GSH pool, the reducing capacity of the alveolar lining fluid and within the AM, and subsequent detrimental effects on the AM. Whereas the beneficial effects of oral GSH may be secondary to positive systemic effects, the ability of GSH to restore phagocytosis in the ethanol-exposed cell line suggests that restoration of the extracellular GSH pool is pivotal to restoring AM bacterial clearance. These beneficial effects were physiologically relevant and associated with decreased K. pneumoniae infection. To our knowledge, this is the first report showing the ability of oral GSH to attenuate ethanol-induced expression of Noxes, oxidative stress, and impaired phagocytic function in AMs. Additional studies are needed to determine if GSH treatments will be an effective novel therapeutic approach for attenuating increased AM oxidative stress, impaired phagocytic capacity, and increased susceptibility to lung infection and injury associated with an alcohol use disorder.
GRANTS
This work was supported in part by a National Institute on Alcohol Abuse and Alcoholism (NIAAA) T32 training grant (5T32-AA-013528-08), by the Emory Alcohol and Lung Biology Center (1P50-AA-135757), by a Merit Review Award (1I01BX001910) from the Department of Veterans Affairs (Biomedical Laboratory Research and Development), by NIAAA NRSA (1F32-AA-020724-01), and by NIAAA R01 (5R01 012197).
DISCLOSURES
The contents of this report do not represent the views of the Department of Veterans Affairs or the United States Government. The authors have no conflicts of interest to disclose.
AUTHOR CONTRIBUTIONS
Author contributions: S.M.Y., C.M.H., and L.A.S.B. conception and design of research; S.M.Y. and F.L.H. performed experiments; S.M.Y. and F.L.H. analyzed data; S.M.Y., F.L.H., C.M.H., and L.A.S.B. interpreted results of experiments; S.M.Y. prepared figures; S.M.Y. drafted manuscript; S.M.Y., C.M.H., and L.A.S.B. edited and revised manuscript; S.M.Y., F.L.H., C.M.H., and L.A.S.B. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge the contributions of Leandrea Burwell for measuring glutathione levels in BAL fluid from mice.
REFERENCES
- 1.Ambasta RK, Kumar P, Griendling KK, Schmidt HH, Busse R, Brandes RP. Direct interaction of the novel Nox proteins with p22phox is required for the formation of a functionally active NADPH oxidase. J Biol Chem 279: 45935–45941, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Anrather J, Racchumi G, Iadecola C. NF-kappaB regulates phagocytic NADPH oxidase by inducing the expression of gp91phox. J Biol Chem 281: 5657–5667, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Bai C, Brown LA, Jones DP. Glutathione transport by type II cells in perfused rat lung. Am J Physiol Lung Cell Mol Physiol 267: L447–L455, 1994 [DOI] [PubMed] [Google Scholar]
- 4.Banfi B, Clark RA, Steger K, Krause KH. Two novel proteins activate superoxide generation by the NADPH oxidase NOX1. J Biol Chem 278: 3510–3513, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Bechara RI, Brown LA, Roman J, Joshi PC, Guidot DM. Transforming growth factor beta1 expression and activation is increased in the alcoholic rat lung. Am J Respir Crit Care Med 170: 188–194, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Bechara RI, Pelaez A, Palacio A, Joshi PC, Hart CM, Brown LA, Raynor R, Guidot DM. Angiotensin II mediates glutathione depletion, transforming growth factor-β1 expression, and epithelial barrier dysfunction in the alcoholic rat lung. Am J Physiol Lung Cell Mol Physiol 289: L363–L370, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Becker LB. New concepts in reactive oxygen species and cardiovascular reperfusion physiology. Cardiovasc Res 61: 461–470, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 87: 245–313, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Bonello S, Zahringer C, BelAiba RS, Djordjevic T, Hess J, Michiels C, Kietzmann T, Gorlach A. Reactive oxygen species activate the HIF-1alpha promoter via a functional NFkappaB site. Arterioscler Thromb Vasc Biol 27: 755–761, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Brown DI, Griendling KK. Nox proteins in signal transduction. Free Radic Biol Med 47: 1239–1253, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown LA, Harris FL, Bechara R, Guidot DM. Effect of chronic ethanol ingestion on alveolar type II cell: glutathione and inflammatory mediator-induced apoptosis. Alcohol Clin Exp Res 25: 1078–1085, 2001 [PubMed] [Google Scholar]
- 12.Brown LA, Harris FL, Ping XD, Gauthier TW. Chronic ethanol ingestion and the risk of acute lung injury: a role for glutathione availability? Alcohol 33: 191–197, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Brown LA, Perez JA, Harris FL, Clark RH. Glutathione supplements protect preterm rabbits from oxidative lung injury. Am J Physiol Lung Cell Mol Physiol 270: L446–L451, 1996 [DOI] [PubMed] [Google Scholar]
- 14.Brown LA, Ping XD, Harris FL, Gauthier TW. Glutathione availability modulates alveolar macrophage function in the chronic ethanol-fed rat. Am J Physiol Lung Cell Mol Physiol 292: L824–L832, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Brown SD, Brown LA. Ethanol induced TGF-β1 and ROS production are necessary for ethanol induced alveolar macrophage dysfunction and induction of alternative activation Alcohol Clin Exp Res 36: 1952–1962, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown SD, Gauthier TW, Brown LA. Impaired terminal differentiation of pulmonary macrophages in a Guinea pig model of chronic ethanol ingestion. Alcohol Clin Exp Res 33: 1782–1793, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng G, Diebold BA, Hughes Y, Lambeth JD. Nox1-dependent reactive oxygen generation is regulated by Rac1. J Biol Chem 281: 17718–17726, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Clempus RE, Sorescu D, Dikalova AE, Pounkova L, Jo P, Sorescu GP, Schmidt HH, Lassegue B, Griendling KK. Nox4 is required for maintenance of the differentiated vascular smooth muscle cell phenotype. Arterioscler Thromb Vasc Biol 27: 42–48, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cucoranu I, Clempus R, Dikalova A, Phelan PJ, Ariyan S, Dikalov S, Sorescu D. NAD(P)H oxidase 4 mediates transforming growth factor-beta1-induced differentiation of cardiac fibroblasts into myofibroblasts. Circ Res 97: 900–907, 2005 [DOI] [PubMed] [Google Scholar]
- 20.de Roux A, Cavalcanti M, Marcos MA, Garcia E, Ewig S, Mensa J, Torres A. Impact of alcohol abuse in the etiology and severity of community-acquired pneumonia. Chest 129: 1219–1225, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Dong J, Sulik KK, Chen SY. The role of NOX enzymes in ethanol-induced oxidative stress and apoptosis in mouse embryos. Toxicol Lett 193: 94–100, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ellmark SH, Dusting GJ, Fui MN, Guzzo-Pernell N, Drummond GR. The contribution of Nox4 to NADPH oxidase activity in mouse vascular smooth muscle. Cardiovasc Res 65: 495–504, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Erickson SE, Martin GS, Davis JL, Matthay MA, Eisner MD, Network NNA. Recent trends in acute lung injury mortality: 1996–2005. Crit Care Med 37: 1574–1579, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fan C, Katsuyama M, Nishinaka T, Yabe-Nishimura C. Transactivation of the EGF receptor and a PI3 kinase-ATF-1 pathway is involved in the upregulation of NOX1, a catalytic subunit of NADPH oxidase. FEBS Lett 579: 1301–1305, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Fitzpatrick AM, Jones DP, Brown LA. Glutathione redox control of asthma: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal 17: 375–408, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fleury C, Mignotte B, Vayssiere JL. Mitochondrial reactive oxygen species in cell death signaling. Biochimie 84: 131–141, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Forman HJ, Skelton DC. Protection of alveolar macrophages from hyperoxia by gamma-glutamyl transpeptidase. Am J Physiol Lung Cell Mol Physiol 259: L102–L107, 1990 [DOI] [PubMed] [Google Scholar]
- 28.Forman HJ, Torres M. Reactive oxygen species and cell signaling: respiratory burst in macrophage signaling. Am J Respir Crit Care Med 166: S4–S8, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Gauthier TW, Young PA, Gabelaia L, Tang SM, Ping XD, Harris FL, Brown LA. In utero ethanol exposure impairs defenses against experimental group B streptococcus in the term Guinea pig lung. Alcohol Clin Exp Res 33: 300–306, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Go YM, Jones DP. The redox proteome. J Biol Chem 288: 26512–26520, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Go YM, Park H, Koval M, Orr M, Reed M, Liang Y, Smith D, Pohl J, Jones DP. A key role for mitochondria in endothelial signaling by plasma cysteine/cystine redox potential. Free Radic Biol Med 48: 275–283, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goyal P, Weissmann N, Grimminger F, Hegel C, Bader L, Rose F, Fink L, Ghofrani HA, Schermuly RT, Schmidt HH, Seeger W, Hanze J. Upregulation of NAD(P)H oxidase 1 in hypoxia activates hypoxia-inducible factor 1 via increase in reactive oxygen species. Free Radic Biol Med 36: 1279–1288, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Guidot DM, Brown LA. Mitochondrial glutathione replacement restores surfactant synthesis and secretion in alveolar epithelial cells of ethanol-fed rats. Alcohol Clin Exp Res 24: 1070–1076, 2000 [PubMed] [Google Scholar]
- 34.Guidot DM, Hart CM. Alcohol abuse and acute lung injury: epidemiology and pathophysiology of a recently recognized association. J Investig Med 53: 235–245, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Guidot DM, Modelska K, Lois M, Jain L, Moss IM, Pittet JF, Brown LA. Ethanol ingestion via glutathione depletion impairs alveolar epithelial barrier function in rats. Am J Physiol Lung Cell Mol Physiol 279: L127–L135, 2000 [DOI] [PubMed] [Google Scholar]
- 36.Hanna IR, Hilenski LL, Dikalova A, Taniyama Y, Dikalov S, Lyle A, Quinn MT, Lassegue B, Griendling KK. Functional association of nox1 with p22phox in vascular smooth muscle cells. Free Radic Biol Med 37: 1542–1549, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Holguin F, Moss I, Brown LA, Guidot DM. Chronic ethanol ingestion impairs alveolar type II cell glutathione homeostasis and function and predisposes to endotoxin-mediated acute edematous lung injury in rats. J Clin Invest 101: 761–768, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hwang J, Kleinhenz DJ, Rupnow HL, Campbell AG, Thule PM, Sutliff RL, Hart CM. The PPARgamma ligand, rosiglitazone, reduces vascular oxidative stress and NADPH oxidase expression in diabetic mice. Vascul Pharmacol 46: 456–462, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Jean JC, Liu Y, Brown LA, Marc RE, Klings E, Joyce-Brady M. Gamma-glutamyl transferase deficiency results in lung oxidant stress in normoxia. Am J Physiol Lung Cell Mol Physiol 283: L766–L776, 2002 [DOI] [PubMed] [Google Scholar]
- 40.Jones DP, Carlson JL, Samiec PS, Sternberg P, Jr, Mody VC, Jr, Reed RL, Brown LA. Glutathione measurement in human plasma Evaluation of sample collection, storage and derivatization conditions for analysis of dansyl derivatives by HPLC. Clin Chim Acta 275: 175–184, 1998 [DOI] [PubMed] [Google Scholar]
- 41.Kawahara T, Ritsick D, Cheng G, Lambeth JD. Point mutations in the proline-rich region of p22phox are dominant inhibitors of Nox1- and Nox2-dependent reactive oxygen generation. J Biol Chem 280: 31859–31869, 2005 [DOI] [PubMed] [Google Scholar]
- 42.Kirlin WG, Cai J, Thompson SA, Diaz D, Kavanagh TJ, Jones DP. Glutathione redox potential in response to differentiation and enzyme inducers. Free Radic Biol Med 27: 1208–1218, 1999 [DOI] [PubMed] [Google Scholar]
- 43.Lee YM, Kim BJ, Chun YS, So I, Choi H, Kim MS, Park JW. NOX4 as an oxygen sensor to regulate TASK-1 activity. Cell Signal 18: 499–507, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Li J, Stouffs M, Serrander L, Banfi B, Bettiol E, Charnay Y, Steger K, Krause KH, Jaconi ME. The NADPH oxidase NOX4 drives cardiac differentiation: Role in regulating cardiac transcription factors and MAP kinase activation. Mol Biol Cell 17: 3978–3988, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liang Y, Yeligar SM, Brown LA. Chronic-alcohol-abuse-induced oxidative stress in the development of acute respiratory distress syndrome (Abstract). Scientific World J 2012: 740308, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lu X, Murphy TC, Nanes MS, Hart CM. PPARγ regulates hypoxia-induced Nox4 expression in human pulmonary artery smooth muscle cells through NF-κB. Am J Physiol Lung Cell Mol Physiol 299: L559–L566, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lundahl J, Hallden G, Skold CM. Human blood monocytes, but not alveolar macrophages, reveal increased CD11b/CD18 expression and adhesion properties upon receptor-dependent activation. Eur Respir J 9: 1188–1194, 1996 [DOI] [PubMed] [Google Scholar]
- 48.Lyle AN, Deshpande NN, Taniyama Y, Seidel-Rogol B, Pounkova L, Du P, Papaharalambus C, Lassegue B, Griendling KK. Poldip2, a novel regulator of Nox4 and cytoskeletal integrity in vascular smooth muscle cells. Circ Res 105: 249–259, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mehta AJ, Guidot DM. Alcohol abuse, the alveolar macrophage and pneumonia. Am J Med Sci 343: 244–247, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mehta AJ, Joshi PC, Fan X, Brown LA, Ritzenthaler JD, Roman J, Guidot DM. Zinc supplementation restores PU.1 and Nrf2 nuclear binding in alveolar macrophages and improves redox balance and bacterial clearance in the lungs of alcohol-fed rats. Alcohol Clin Exp Res 35: 1519–1528, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mittal M, Roth M, Konig P, Hofmann S, Dony E, Goyal P, Selbitz AC, Schermuly RT, Ghofrani HA, Kwapiszewska G, Kummer W, Klepetko W, Hoda MA, Fink L, Hanze J, Seeger W, Grimminger F, Schmidt HH, Weissmann N. Hypoxia-dependent regulation of nonphagocytic NADPH oxidase subunit NOX4 in the pulmonary vasculature. Circ Res 101: 258–267, 2007 [DOI] [PubMed] [Google Scholar]
- 52.Moss M, Bucher B, Moore FA, Moore EE, Parsons PE. The role of chronic alcohol abuse in the development of acute respiratory distress syndrome in adults. J Am Med Assoc 275: 50–54, 1996 [PubMed] [Google Scholar]
- 53.Moss M, Guidot DM, Wong-Lambertina M, Ten Hoor T, Perez RL, Brown LA. The effects of chronic alcohol abuse on pulmonary glutathione homeostasis. Am J Respir Crit Care Med 161: 414–419, 2000 [DOI] [PubMed] [Google Scholar]
- 54.Moss M, Parsons PE, Steinberg KP, Hudson LD, Guidot DM, Burnham EL, Eaton S, Cotsonis GA. Chronic alcohol abuse is associated with an increased incidence of acute respiratory distress syndrome and severity of multiple organ dysfunction in patients with septic shock. Crit Care Med 31: 869–877, 2003 [DOI] [PubMed] [Google Scholar]
- 55.Nisbet RE, Bland JM, Kleinhenz DJ, Mitchell PO, Walp ER, Sutliff RL, Hart CM. Rosiglitazone attenuates chronic hypoxia-induced pulmonary hypertension in a mouse model. Am J Respir Cell Mol Biol 42: 482–490, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Patel N, Gonsalves CS, Yang M, Malik P, Kalra VK. Placenta growth factor induces 5-lipoxygenase-activating protein to increase leukotriene formation in sickle cell disease. Blood 113: 1129–1138, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Piotrowski WJ, Marczak J. Cellular sources of oxidants in the lung. Int J Occup Med Environ Health 13: 369–385, 2000 [PubMed] [Google Scholar]
- 58.Pittet JF, Griffiths MJ, Geiser T, Kaminski N, Dalton SL, Huang X, Brown LA, Gotwals PJ, Koteliansky VE, Matthay MA, Sheppard D. TGF-beta is a critical mediator of acute lung injury. J Clin Invest 107: 1537–1544, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Polikandriotis JA, Rupnow HL, Elms SC, Clempus RE, Campbell DJ, Sutliff RL, Brown LA, Guidot DM, Hart CM. Chronic ethanol ingestion increases superoxide production and NADPH oxidase expression in the lung. Am J Respir Cell Mol Biol 34: 314–319, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Polikandriotis JA, Rupnow HL, Hart CM. Chronic ethanol exposure stimulates endothelial cell nitric oxide production through PI-3 kinase-and hsp90-dependent mechanisms. Alcohol Clin Exp Res 29: 1932–1938, 2005 [DOI] [PubMed] [Google Scholar]
- 61.Rinna A, Torres M, Forman HJ. Stimulation of the alveolar macrophage respiratory burst by ADP causes selective glutathionylation of protein tyrosine phosphatase 1B. Free Radic Biol Med 41: 86–91, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schilder YD, Heiss EH, Schachner D, Ziegler J, Reznicek G, Sorescu D, Dirsch VM. NADPH oxidases 1 and 4 mediate cellular senescence induced by resveratrol in human endothelial cells. Free Radic Biol Med 46: 1598–1606, 2009 [DOI] [PubMed] [Google Scholar]
- 63.Seshiah PN, Weber DS, Rocic P, Valppu L, Taniyama Y, Griendling KK. Angiotensin II stimulation of NAD(P)H oxidase activity: upstream mediators. Circ Res 91: 406–413, 2002 [DOI] [PubMed] [Google Scholar]
- 64.Sumimoto H, Miyano K, Takeya R. Molecular composition and regulation of the Nox family NAD(P)H oxidases. Biochem Biophys Res Commun 338: 677–686, 2005 [DOI] [PubMed] [Google Scholar]
- 65.Thannickal VJ, Fanburg BL. Reactive oxygen species in cell signaling. Am J Physiol Lung Cell Mol Physiol 279: L1005–L1028, 2000 [DOI] [PubMed] [Google Scholar]
- 66.Trapnell BC, Whitsett JA. Gm-CSF regulates pulmonary surfactant homeostasis and alveolar macrophage-mediated innate host defense. Annu Rev Physiol 64: 775–802, 2002 [DOI] [PubMed] [Google Scholar]
- 67.Wagner MC, Yeligar SM, Brown LA, Hart MC. PPARgamma ligands regulate NADPH oxidase, eNOS, and barrier function in the lung following chronic alcohol ingestion. Alcohol Clin Exp Res 36: 197–206, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med 342: 1334–1349, 2000 [DOI] [PubMed] [Google Scholar]
- 69.Yang S, Zhang Y, Ries W, Key L. Expression of Nox4 in osteoclasts. J Cell Biochem 92: 238–248, 2004 [DOI] [PubMed] [Google Scholar]
- 70.Yeh MY, Burnham EL, Moss M, Brown LA. Chronic alcoholism alters systemic and pulmonary glutathione redox status. Am J Respir Crit Care Med 176: 270–276, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yeligar SM, Harris FL, Hart CM, Brown LA. Ethanol induces oxidative stress in alveolar macrophages via upregulation of NADPH oxidases. J Immunol 188: 3648–3657, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]