Abstract
Cardiac conduction is the process by which electrical excitation spreads through the heart, triggering individual myocytes to contract in synchrony. Defects in conduction disrupt synchronous activation and are associated with life-threatening arrhythmias in many pathologies. Therefore, it is scarcely surprising that this phenomenon continues to be the subject of active scientific inquiry. Here we provide a brief review of how the conceptual understanding of conduction has evolved over the last century and highlight recent, potentially paradigm-shifting developments.
Keywords: cardiac conduction, ephaptic coupling, gap junctions, modeling, myocardium
this article is part of a collection on Standards and Guidelines. Other articles appearing in this collection, as well as a full archive of all collections, can be found online at http://ajpheart.physiology.org/.
Background
Cardiac conduction is the process by which electrical activation is communicated between myocytes, triggering their synchronous contraction. Impulses originating in the sinoatrial node spread to the atria and via the specialized His-Purkinje conduction system to the ventricles. The sequence of activation thus achieved is key in translating the lengthwise contraction of individual myocytes into the complex three-dimensional pumping motion of the heart.
It is well established that aberrant ventricular conduction is associated with a high risk of sudden cardiac death, presumably due to ventricular arrhythmias (67). The prevention of arrhythmias caused by conduction abnormalities remains a topic of intense research in part because there are many factors that are thought to govern cardiac conduction. This review will focus on canonical determinants of conduction: cellular excitability, gap junctions, and tissue architecture in the context of the historical development of the theoretical understanding of conduction in the ventricular myocardium. Additionally, long theorized and new experimental evidence will be discussed concerning alternative modes of action potential propagation from myocyte to myocyte.
The Study of Conduction
Dr. Theodor Wilhelm Engelmann is credited with determining in 1875 that electrical activity in strips of frog atrial muscle spread through muscular tissue (27). It took 39 years before the first direct measurements of cardiac conduction velocity were reported, when Dr. Thomas Lewis and his colleagues first quantified the velocity of cardiac conduction from canine myocardium (61). Conduction velocity is still the primary metric for quantifying the spread of electrical activity in cardiac muscle in part because of its conceptual simplicity and the ease of measuring the time it takes for the electrical wavefront to travel a known distance. More importantly, velocity as a metric of conduction has yielded insights into the mechanisms of electrical activity spread, as conduction velocity is not uniform in all directions from the point of stimulation. This issue of direction-specific conduction spread has provided the foundation of scientific inquiry and debate. But before researchers could even begin to understand the biophysical mechanisms governing cardiac conduction velocity, they first had to determine what caused the action potential, and to do that, the determinants of tissue excitability had to be understood.
Tissue Excitability
The definition of the term “excitability” has evolved with our understanding of the mechanisms underlying this phenomenon. For the purpose of this review, it is convenient to think of excitability in terms of the probability that a propagated action potential will be triggered in response to some quantity of charge entering a cell over a period of time. In neurons, Drs. Wallace Fenn and Doris Cobb (28) suggested in 1936 that a propagated action potential was caused by sodium ions entering an excitable cell and depolarizing the membrane. Subsequently, and perhaps more famously, Drs. Alan Lloyd Hodgkin, Andrew Huxley and Bernard Katz published a series of manuscripts on the topic of neuronal excitation (39–46). Finally, Drs. Alan Lloyd Hodgkin and Paul Horowicz demonstrated that excitability in cardiac myocytes is based on similar mechanisms of sodium entry (38). These early studies, demonstrated that sodium conductance through the cell membrane is very low when the cell is electrically quiescent but increases dramatically during the depolarization phase of the action potential. These and other findings prompted Hodgkin and Huxley to suggest that there may be specialized “pores” or channels through which Na+ permeates the cell membrane (44). In 1964, Dr. Toshio Narahashi and colleagues reported on the sodium current blocking properties of tetrodotoxin (72), and the use of pharmacological blockers to elucidate properties of the sodium current began in earnest. This line of investigation culminated in the landmark 1976 paper by Drs. Erwin Neher and Bert Sackmann's in which currents were recorded from single ion channels (73), and finally, the identification of the cardiac isoform of the voltage-gated sodium current (Nav1.5) (30) responsible for depolarizing the preponderance of cardiac myocytes. Overall, it is now well accepted that sodium channel availability is an important determinant of cellular excitability (95).
Potassium channels and excitability.
Potassium channels provide outward current that acts to set the resting membrane potential and repolarize the membrane when it is depolarized. Thus these channels shape the action potential after the upstroke, and their relevance to conduction has been thought to be limited to an influence on resting membrane potential. Under pathological conditions such as following ischemia, changes in resting membrane potential are determined by potassium currents, particularly the inward rectifier current (IK1) and the ATP-sensitive potassium current (IKATP). Since the resting membrane potential is a key determinant of sodium channel availability, potassium currents have a significant influence on conduction under such conditions. However, in recent years it has been demonstrated that modulating potassium currents can also affect conduction velocity and its dependence on the sodium current, independent of changes in resting membrane potential: partial inhibition of IK1 can speed up conduction under normal physiological conditions but not when sodium channel availability is compromised (119). On the other hand, pharmacological activation of IKATP and the slow component of the delayed rectifier potassium current (IKs) both slow conduction under normal physiological conditions; however, only the latter slows conduction when sodium channel availability is reduced (118). Overall, these findings suggest that modulating voltage-dependent K+ currents affects conduction independent of Na+ channel availability, whereas modulating K+ currents that do not display voltage-dependent kinetics only affect conduction when Na+ channel availability is not reduced.
Furthermore, ongoing studies suggest a codependence between the membrane expression levels of inward rectifier potassium channels (Kir2.1) and sodium channels (Nav1.5) (68). An additional line of evidence suggesting an interrelationship between the two channel types comes from the recently identified long QT syndrome type 9, where mutations in the scaffolding protein caveolin-3 was associated with alterations in the biophysical properties of both Kir2.1 and Nav1.5 channels. It is therefore likely that potassium channels will continue to be a significant area of research focus in coming years.
Early Revisions and Models of Conduction
Cable theory.
In 1952, Dr. Silvio Weidmann demonstrated that electrical communication between cardiac myocytes could be described using a theory first developed in the 19th century by William Thomson (a.k.a. Lord Kelvin) to account for the transmission of electrical signals through a transatlantic telegraph cable (113, 114). Importantly, the application of what is known as cable or the continuous core conductor theory supposes that myocardial tissue is a syncytium coupled through purely resistive pathways. While the theory was initially applied to understand action potential propagation through nerves, which are readily conceived as a core of conductive material surrounded by a nonconductive sheath, Weidman extended these treatments to understand electrical impulse propagation through cardiac Purkinje fibers, which are cable-like in structure (124).
Briefly, for a fiber of radius a with intracellular resistance per unit length ri and extracellular resistance per unit length re bounded by a membrane with resistance per unit length rm and capacitance per unit length cm, the transmembrane potential vm can be described by:
By extension, it was demonstrated that conduction velocity is related to ri and re by:
where k is a constant representing membrane properties (101). In the heart, ri and re are determined by various anatomical structures that compose the electric current path inside, outside, and between myocytes.
Gap junctions.
Up until 1954, many theorized cytoplasmic continuity between myocytes, but then Drs. Fritiof S. Sjostrand and Ebba Andersson showed by use of electron microscopy that myocytes are fully bounded by a membrane (96). Therefore, the physical nature of electrical connectivity between cells remained speculative. In the 1960s, Dr. Lloyd Barr demonstrated the existence of low resistance pathways between cardiac myocytes (5, 21), which he termed “the nexus.” These structures have come to be known more widely as “gap junctions,” the name having been coined by Drs. Milton W. Brightman and Thomas S. Reese in 1969 (10).
Since their discovery, gap junctions have been intensely studied, with over 14,000 publications in the literature as of 2013. Each gap junction channel consists of two hexameric connexon hemichannels, each in turn composed of six molecules from the connexin protein family (97). In the heart, three isoforms connexins 40, 43, and 45 are expressed (8, 9, 53, 122) which have different conductances, permeabilities, and cardiac tissue-specific expression patterns (15, 35). Furthermore, different connexin isoforms can combine to form heterotypic and heteromultimeric gap junctions which demonstrate composition-dependent properties (25, 69). However, the situation in mammalian ventricular myocardium is somewhat simplified by the fact that it predominantly expresses connexin 43 (Cx43). Moreover, in the ventricle of humans, and many other mammals, Cx43 is almost exclusively localized to the intercalated disks (34). Thus myocytes are coupled by gap junction channels in primarily end-to-end fashion (49).
Since the majority of early conduction measurements in myocardial tissue were performed macroscopically on Purkinje fibers, which resemble a cable, it is unsurprising that cable theory continued to fit the available data well. To reconcile the discovery of gap junctions with what appeared to be continuous conduction, many deemed gap junctional conductance to be sufficiently high as to render the cytoplasms of coupled myocytes electrically contiguous (123, 125). Gap junctional conductance was therefore, often incorporated into the intracellular resistance term ri.
Anisotropic conduction.
Although thought to be a true syncitium through the first half of the 20th century, ultrastructural studies in the 1950s revealed the cellular nature of cardiac muscle (78, 81, 96). Atrial and ventricular myocardium came to be seen as brick wall-like structures composed of myocytes 100–150 μm long and 10–20 μm wide (19). Therefore, cardiac tissue is anisotropic, with lengthwise orientation of cardiac myocytes and predominantly end-to-end gap junctional coupling (49). This tissue architecture suggests that cardiac conduction should be different parallel to the long axis relative to the short axis of myocytes. Drs. J. Walter Woodbury and Wayne E. Crill showed in the late 1950s that myocardium exhibited a direction-dependent spatial decay of current injected into a point of myocardium, with the longest decay, or space constant, occurring parallel to the long axis of moycytes and the shortest decay occurring perpendicular to myocytes (16). Continuous cable theory was quickly updated to two and three-dimensional models of anisotropic tissue to incorporate the vectors of directional resistivity both inside (ri) and outside (re) cells (50, 74, 75). From here, these models as a group came to be referred to as “bidomain models” and predicted that cardiac conduction velocity in two- and three-dimensional tissue should be anisotropic. By assuming that cardiac myocardium is a continuous but anisotropic medium, Dr. L. Clerc demonstrated that conduction velocity parallel and transverse to fibers is predicted by an inverse square relationship to total axial resistance, similar to what would be anticipated from cable theory (12). Microelectrode recordings of conduction velocity from multiple sites of myocardial tissue agreed well with these mathematical treatments, and therefore, anisotropic conduction was linked to fiber orientation (22, 93). In this context, it is important to note that the continuous anisotropic resistivity envisaged by bidomain models is a theoretical approximation. In tissue, end-to-end contacts between myocytes can mediate both longitudinal and transverse coupling patterns.
Discontinuous conduction.
As technology improved to allow for electrical measurements at greater spatial and temporal resolution, studies in the 1970s revealed yet more complications with the understanding of cardiac conduction. Were the myocardium to behave as a syncytium, the time constant of slow depolarization preceding sodium channel activation (τfoot) should depend on only axial and membrane resistance and, therefore, be independent of conduction velocity (θ). The model also predicted that the maximal rate of rise of the transmembrane potential (dV/dtmax) should depend only on sodium channel availability; therefore, faster conduction should be associated with a larger dV/dtmax (100). However, ground-breaking microscopic measurements made by Dr. Madison Spach and colleagues in both atrial and ventricular myocardium revealed a very different picture: longitudinal conduction, which is faster, was associated with a longer τfoot and smaller dV/dtmax, relative to slower transverse conduction (105, 106). These experiments began to call into question the use of models based on continuous cable theory to describe anisotropic conduction.
To explain these new discrepancies between measurements made in tissue and those mathematically modeled, the concept of discontinuous conduction was proposed since gap junctions may represent high-resistance pathways between myocytes, rather than the low-resistance structures previously assumed. In fact, direct measurements of gap junctional resistance revealed that resistance at the intercalated disks is approximately equal to the axial resistance of a myocyte (87). Importantly, discontinuous conduction suggested that conduction transverse to the myocyte axis might be slower and also more discontinuous relative to conduction parallel to myocytes, and further experimental evidence supported this assessment (100, 106).
The development of discontinuous conduction theory needed to account for a more complex type of axial resistance that included the following important parameters. First, the mechanisms underlying the resistance of the intercalated disks needed to be explored since the number and function of gap junctions can dramatically affect gap junctional conductance. Second, a conduction wavefront will encounter more discontinuities caused by gap junctions when it travels transverse to myocytes relative to their longitudinal axis, as myocytes are shorter than they are long. Since myocytes organize in a roughly brick-like structure in a sheet or bundle and the orientation of these sheets and bundles maintain a three-dimensional geometry that is optimized for the efficient propulsion of blood, tissue geometry could not be treated as a simple two- or three-dimensional sheet (101). Case in point, myocyte orientation changes from the epicardium to the endocardium (60, 80, 110), and this complex rotational anisotropy has been shown to produce important differences when measuring cardiac conduction (111).
Contemporary Understanding of Anisotropic Conduction
Myocyte geometry.
Cardiac myocytes do not maintain the same size and geometry over an organism's lifetime (63, 103). Changes in cell size and composition can alter cytoplasmic and extracellular resistances in direction-dependent manners, leading to altered conduction anisotropy (92, 106) (the ratio of longitudinal to transverse conduction velocities). Once again, changes in myocyte geometry will also alter the number of gap junctions encountered by the electrical wavefront over a given distance (58, 87, 89). Additionally, cellular hypertrophy over postnatal life is accompanied by changes in GJ localization from uniform distribution around the cell to predominantly intercalated disk localization (3, 36), further confounding the problem of understanding growth-related changes in conduction.
It is noteworthy though that there remain open questions regarding the relationship between cell size and conduction velocity. Some in silico studies have suggested a positive correlation between cell size and conduction velocity (32, 103), whereas experiments in hypertrophied myocardium have suggested a negative correlation between myocyte diameter and conduction velocity (65). A combined experimental and simulation study by Dr. Rob F. Wiegerinck and colleagues found increased longitudinal, but not transverse, conduction velocity in failing rabbit hearts; however, the degree of increase was insufficient to compensate for the increased path length resulting from hypertrophy resulting in an increased total activation time (126). To complicate matters further, a recent study by Dr. Thomas Seidel and colleagues suggests that myocyte shape may be as important as myocyte size, if not more so, with respect to modulating conduction velocity in a predictable manner (94).
It continues to be a challenge to understand the role that heterogeneous cell geometry has on conduction in diseases such as cardiac hypertrophic cardiomyopathy (121), because these conditions often also affect the expression and localization of membrane proteins, especially connexins. Additionally, myocyte geometry can also change in the acute time scale in response to ischemia (115) or osmolarity, for example (18).
Nonmyocytes.
In addition to cardiac myocytes, the heart also consists of fibroblasts and other nonmyocyte cells. Indeed, these cells outnumber the myocytes although the latter account for the majority of the heart's volume (128). It has been proposed that fibroblasts could influence cardiac conduction by either acting as passive obstacles or coupling to myocytes and/or each other and acting as either a current sink or even participating actively in conduction. Additionally, under pathophysiological conditions, fibroblasts can transform into myofibroblasts that participate in inflammatory signaling and have also been proposed to modulate conduction (6). However, the extent to which fibroblasts and myofibroblasts electrotonically couple with myocytes in the ventricular myocardium remains a topic of ongoing inquiry (51, 91, 127). For more detailed discussion of the role of nonmyocytes in conduction, the reader is referred to reviews by Drs. Peter Kohl and (55, 56) and Heather Duffy (6, 23).
In the normal heart, fibroblasts are thought to provide structural support directly, as well as by laying down the extracellular matrix (6). However, under pathophysiological conditions, excessive deposition of extracellular collagen can occur, electrically isolating myocytes from each other, forming barriers to conduction. Indeed, in an elegant study in aging human atrial fiber bundles, Dr. Madison Spach demonstrated how collagenous septa deposited between myocytes create a tortuous electrical path and facilitate reentrant arrhythmias (104). Likewise, in the ventricles, fibrosis can create resistive barriers to conduction and act as an arrhythmogenic substrate. While a certain degree of fibrosis occurs in aging hearts, it can be greatly exacerbated in a variety of pathological conditions (11, 33, 48).
Gap junctions.
Given the role gap junctions play in electrically coupling myocytes and because they are remodeled so frequently in cardiac disease, they have received the preponderance of experimental attention. While slowed conduction is well correlated with an increased risk of arrhythmias, there is disagreement in the literature concerning the relationship between the degree of gap junctional uncoupling and conduction velocity changes. It is well established that pharmacologically uncoupling gap junctions slow cardiac conduction (57, 87, 88). With this observation comes an important experimental caveat: most articles only report data from doses of gap junction uncouplers that measurably slow conduction (120). This binary data representation therefore excludes the linear correlation between the degree of gap junction uncoupling and conduction slowing, as well as nonspecific (i.e., non-gap junction related) effects of the drugs. Importantly, pharmacological gap junction inhibition studies have provided sufficient evidence that gap junctions at the ends of myocytes constitute the primary source of delay during microscopic conduction (58, 89). Thus modulating gap junctional coupling preferentially impacts transverse conduction velocity, leading to altered anisotropy (90, 92, 106).
Contrast pharmacological gap junction manipulation with genetically manipulating gap junction functional expression. There are a variety of studies on cardiac conduction using the same transgenic mouse lineage expressing 50% of the wild-type levels of Cx43, the primary ventricular gap junction protein. Some groups have reported significantly slowed conduction in mice with a 50% reduction in Cx43 expression relative to wild-type mice (26, 37), whereas others could not measure a difference between them (7, 17, 71, 109, 112, 116, 117).
These very basic studies are critically important to understanding cardiac arrhythmias because gap junction remodeling is a hallmark of cardiac disease. To make matters worse, in disease, the relationship between gap junction remodeling and conduction is even less clear. For example, a reduction of total Cx43 expression in a canine pacing-induced heart failure model was associated with aberrant ventricular conduction and increased arrhythmogenesis (79). In a similar model, gap junction remodeling (relocation) and conduction slowing preceded loss of Cx43 expression (2).
The phrase “gap junctional remodeling” is a catchall term to describe connexin redistribution at the cellular level, posttranslational modification, and total protein expression changes. While it has been demonstrated that cellular redistribution of connexins to the lateral membrane is associated with altered conduction, in hypertrophy, for example, lateralization occurs concomitantly with changes in myocyte geometry (102). Whether or not lateralized Cx43 in the pathological myocardium forms functional gap junctions remains an important and complex question (24). The connexin life cycle is also dynamically regulated by a variety of biochemical pathways including phosphorylation and dephosphorylation of specific amino acid residues of the channel (4, 82, 98, 99). Therefore, gap junctions do not exist in isolation and are part of larger macromolecular complexes and biological pathways.
Dynamic determinants of conduction.
In addition to the structural substrate, conduction is also modulated by dynamic functional changes. Primarily, these dynamics result from the interplay between the strength of the excitatory impulse (the source) and the electrical load represented by the tissue it must excite (the sink). In the adult canine ventricular myocardium, each myocyte is coupled to an average of 11 ± 3 other myocytes (92). As an activation wavefront spreads through the myocardium, the amount of source available per unit mass of tissue is determined by its excitability, whereas the balance between source and sink is determined by the curvature of the wavefront and its interaction with the architecture of the myocardium: intercellular coupling, fiber orientation, rotational anisotropy, branching tissue geometry, etc. (58, 88, 92, 106). Since local excitability in tissue is dynamically modulated by changes in the shape and duration of action potentials, mismatch between source and sink can arise locally and dynamically, creating a functional substrate for arrhythmogenic conduction defects. Pathophysiological gap junction remodeling and fibrosis can exacerbate source-sink mismatch and thereby the propensity for arrhythmias. For a more detailed discussion of source-sink mismatch, the reader is referred to the in depth review of electrotonic conduction by Drs. Andre Kleber and Yoram Rudy (54).
Ion Channels at the Intercalated Disk: Functional Implications
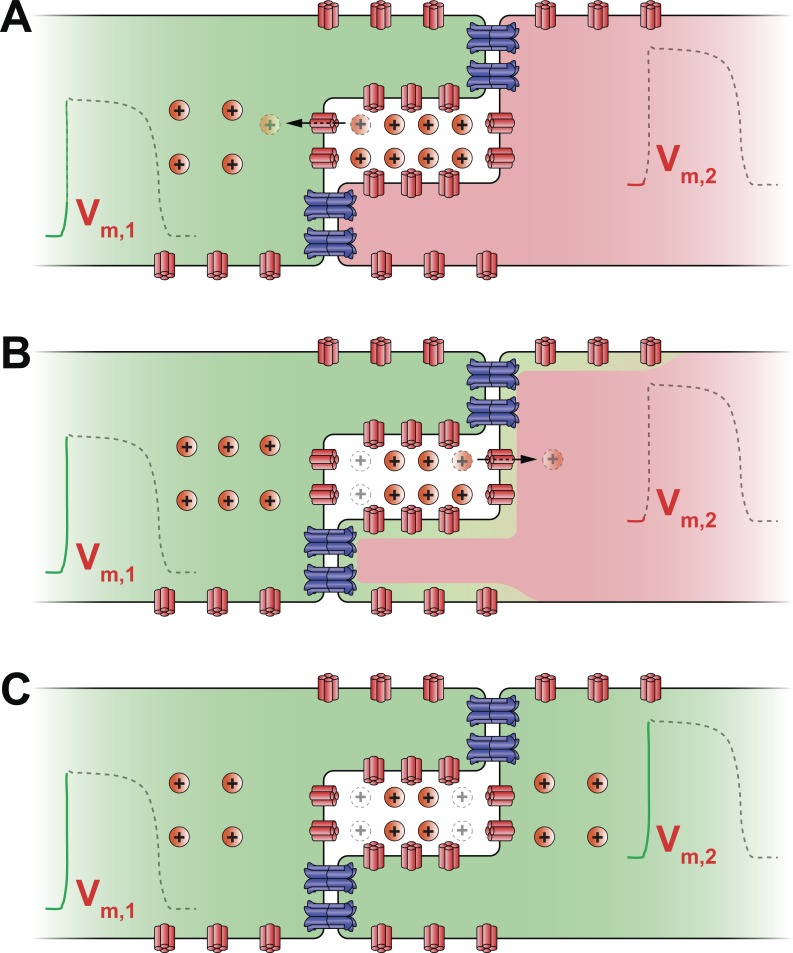
The major impetus for reassessing our understanding of conduction arises from structural insights into the subcellular localization of ion channels. In 1996, Dr. Sidney A. Cohen published the first immunofluorescence images of rat tetrodotoxin-resistant sodium channels (rH1), demonstrating their strong localization at the intercalated disk of cardiac myocytes (13). While the importance of ion channels at the intercalated disk has been long postulated as a mechanism of non-gap junction-mediated coupling, this work was mostly the domain of mathematical models (14, 59, 66, 70, 107, 108, 129). These models envision intercellular coupling as occurring thusly: a depolarized myocyte withdraws sodium ions from the restricted junctional cleft via its intercalated disk-localized Nav1.5 channels (Fig. 1A). The resulting depletion of positive charge from the junctional cleft would render the local extracellular electrical potential more negative. Consequently, the transmembrane potential across the apposed membrane of the neighboring myocyte becomes more positive, causing the activation of Nav1.5 channels (Fig. 1B). Thus electrical activation is communicated from one cell to another without the direct transfer of ions between them (Fig. 1C).
Fig. 1.
Schematic cartoon illustrating the mechanism of ephaptic coupling. A: sodium channels (shown in red) on the depolarized (green) myocyte's membrane activate and withdraw sodium ions (Na+) from the restricted extracellular cleft at the intercalated disk. As a result, the transmembrane potential (Vm,1) of the first myocyte is elevated. B: concomitant depletion of positive charge from the extracellular cleft lowers the local extracellular potential (Φe). This leads to an increase in the second, resting (red) myocyte's transmembrane potential (Vm,2), defined as the difference between its intracellular potential and the extracellular potential (Φe). In turn, sodium channels located at or near the intercalated disk of the second myocyte activate. C: sodium enters the second myocyte via these channels further depolarizing it and triggering an action potential. Thus activation is communicated ephaptically from cell to cell without the direct transfer of ions between them.
Dr. Nicholas Sperelakis is perhaps best known for championing these mechanisms, which he summarized in a 2002 review (108). Later that year, Drs. Jan P. Kucera, Stephan Rohr, and Yoram Rudy confirmed the presence of both sodium channels and connexins at the intercalated disc (59). More importantly, they revised mathematical models of cardiac conduction in a one-dimensional strand, demonstrating that the high density of sodium channels at the intercalated disk could impact cardiac conduction in previously unappreciated ways. Specifically, they concluded that gap junctions are still likely the principal mechanism of electrical transmission between cells, but sodium channels at the intercalated disk could modulate the conduction velocity, gap junction relationship particularly if the space between the myocytes were very small and densely packed with sodium channels.
Since then much evidence has been uncovered to support the existence at the intercalated disk of a macromolecular complex containing the gap junction protein Cx43, as well as cardiac sodium channels (Nav1.5). Indeed, Cx43 and Nav1.5 were found to coimmunoprecipitate from mouse heart lysates (64) and more recently to colocalize at the intercalated disk (76). Work from the group of Dr. Mario Delmar has demonstrated that mechanical adhesion proteins first localize to sites of cell-cell contact followed by recruitment of Cx43 gap junctions and ankyrin-G, a submembrane adapter protein involved in localizing cardiac sodium channels (Nav1.5) in the membrane (31). Recent results from the laboratory of Dr. Mario Delmar demonstrate a loss of Nav1.5 from the membrane in conditional Cx43 knockout mice (52) and even suggest that Cx43 is important for the recruitment of Nav1.5 channels into the membrane at the intercalated disk (1, 20).
In addition to sodium channels, evidence has also emerged placing various potassium channel isoforms at the intercalated disk, specifically, the inward rectifier potassium channel (Kir2.1) (68), the ATP-sensitive potassium channel (Kir6.2) (47), the delayed rectifier potassium channel [KvLQT1 encoded by KQT-like subfamily, member 1, gene (KCNQ1)] (83) and the “rapid” delayed rectifier potassium channel [Kv11.1 encoded by human ether-a-go-go-related gene (hERG)] (130). As previously discussed, potassium channels can have significant effects on conduction. Additionally, being localized at the intercalated disk, they could also play a role in intercellular coupling via a potassium-mediated ephaptic mechanism. Briefly, potassium efflux from a depolarized myocyte could lead to a transient accumulation of potassium in the narrow junctional cleft, causing the membrane of the neighboring myocyte to depolarize via an inward potassium current (108).
Although the presence of ion channels at the intercalated disk is suggestive, the ephaptic coupling hypothesis has yet to be experimentally tested in the heart. A key missing element has been the identification of a well-defined structure that could serve as a functional unit of ephaptic coupling, an ephapse. The localization of ion channels at the intercalated disk could have important implications in this regard given the necessity of close apposition between membranes of adjacent cells for ephaptic coupling (62, 70). Taking the experimental evidence together with predictions made by the models, intermembrane spacing could be a key variable in identifying specific microdomains within the intercalated disk that could function as an ephapse.
Interstitial volume.
As with any electrical circuit, one must not only consider electrical conduction “forward” through the circuit, but also how the circuit is completed by a current return path. In tissue, the return path can be the interstitial space between the myocytes. At this point, it is useful to revisit cable theory for a moment, as there is no other mathematical theory that has been applied so rigorously to cardiac conduction. Since myocyte geometry is anisotropic, the interstitial space outside the cells is also anisotropic and can affect axial resistance and thereby conduction in an anisotropic manner (77, 101). Cable theory-based models predict a direct proportionality between the volume of the extracellular space and conduction velocity (32, 81), and this has been experimentally supported in the cable-like papillary muscle (29).
However, one of our recent manuscripts provides evidence that modulating the extracellular volume in a heart changes epicardial conduction in a manner inconsistent with bidomain models of cardiac conduction that lack ephaptic coupling (120). Additionally, we demonstrated that small degrees of gap junction uncoupling that did not alter conduction normally, significantly slowed conduction when the interstitial space was increased. This study suggested that the effects of small degrees of gap junctional uncoupling on cardiac conduction can be unmasked by increasing the interstitial volume. What remains unknown though is how increasing interstitial volume produces changes in cardiac conduction inconsistent with bidomain models.
As mentioned earlier, non-gap junction-mediated coupling or “ephaptic” coupling had been proposed by mathematical models, and the distance between cells at the intercalated disk might be an important factor for mediating this type of coupling. A candidate structure that could serve as a functional ephaptic unit as definitive as a synapse or gap junction emerges from recent work by an author of this review together with his colleague Dr. J. Matthew Rhett. In these studies a new feature of cardiac ultrastructure was described: the perinexus, a juxta-gap junction membrane microdomain rich in undocked connexin hemichannels wherein interaction between Cx43 and the cardiac sodium channel (Nav1.5) occurs. Given the close (0–20 nm) apposition of membranes from adjacent myocytes in the vicinity of the gap junction, the perinexus emerges as a strong candidate structure for the cardiac ephapse (84–86).
As it stands, there is evidence from both experiments and mathematical models to suggest that ephaptic coupling could be important to cardiac conduction. While previously viewed as a possible alternative to electrotonic coupling, ephaptic coupling has since come to be viewed as operating in tandem with gap junctions, helping sustain conduction when gap junctional coupling is compromised. To fully appreciate the role of ephaptic coupling, a multipronged strategy will be necessary: 1) further whole heart experiments will need to be performed to generate additional examples of complex conduction; 2) the biochemical and functional ultrastructure of the ephaptic machinery will need to be dissected by high-resolution microscopic, molecular, and physiological methods; and 3) multidimensional mathematical models will need to be revised to incorporate ephaptic coupling and tested against the new experimental data.
Conduction: A New Multifactorial Understanding
Like all science, the understanding of cardiac conduction has undergone revisions and refinements over the last 130 years, and it appears that discoveries will only continue to accelerate. The picture that is emerging though is that cardiac conduction is not a simple phenomenon mechanistically determined by a few independent biophysical parameters. Rather, cellular excitability, gap junctional conductance, cell size, gap junction localization, subcellular architecture, and ion channel localization are important and interrelated determinants of the conduction phenomenon. While these factors were initially studied vis-à-vis conduction in isolation using a reductionist approach, we are beginning to appreciate that changes in one determinant can potentiate the effects of altering another. This type of higher-level understanding of conduction is critical for determining why certain therapies developed to treat conduction defects are sometimes ineffective or even produce deleterious effects. While it could be argued the interrelated nature and complexity of biological processes means that we will never completely prevent conduction-related arrhythmias, we would suggest that understanding the myriad factors underpinning conduction could eventually provide individualized therapeutic targets that might provide for improved treatment of patients suffering from disease of the heart.
GRANTS
This work was supported in part by National Institutes of Health Grants R01-HL-102298-01A1 (to S. Poelzing), R01-HL-56728-10A2 (to R. Gourdie), and R01-1DE019355-01 (R. Gourdie subcontract) and an American Heart Association Postdoctoral Fellowship (to R. Veeraraghavan).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
R.V. and S.P. drafted manuscript; R.V., R.G., and S.P. edited and revised manuscript; R.V., R.G., and S.P. approved final version of manuscript.
REFERENCES
- 1.Agullo-Pascual E, Lin X, Pfenniger A, Lubkemeier I, Willecke K, Rothenberg E, Delmar M. A novel noncanonical role of cx43 in the heart: ensuring the arrival of nav1.5 to the intercalated disk. Heart Rhythm 10: 1742, 2013 [Google Scholar]
- 2.Akar FG, Nass RD, Hahn S, Cingolani E, Shah M, Hesketh GG, DiSilvestre D, Tunin RS, Kass DA, Tomaselli GF. Dynamic changes in conduction velocity and gap junction properties during development of pacing-induced heart failure. Am J Physiol Heart Circ Physiol 293: H1223–H1230, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Angst BD, Khan LU, Severs NJ, Whitely K, Rothery S, Thompson RP, Magee AI, Gourdie RG. Dissociated spatial patterning of gap junctions and cell adhesion junctions during postnatal differentiation of ventricular myocardium. Circ Res 80: 88–94, 1997 [DOI] [PubMed] [Google Scholar]
- 4.Axelsen LN, Stahlhut M, Mohammed S, Larsen BD, Nielsen MS, Holstein-Rathlou NH, Andersen S, Jensen ON, Hennan JK, Kjolbye AL. Identification of ischemia-regulated phosphorylation sites in connexin43: a possible target for the antiarrhythmic peptide analogue rotigaptide (ZP123). J Mol Cell Cardiol 40: 790–798, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Barr L, Dewey MM, Berger W. Propagation of action potentials and the structure of the nexus in cardiac muscle. J Gen Physiol 48: 797–823, 1965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baum J, Duffy HS. Fibroblasts and myofibroblasts: what are we talking about? J Cardiovasc Pharmacol 57: 376–379, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beauchamp P, Choby C, Desplantez T, de Peyer K, Green K, Yamada KA, Weingart R, Saffitz JE, Kleber AG. Electrical propagation in synthetic ventricular myocyte strands from germline connexin43 knockout mice. Circ Res 95: 170–178, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Beyer EC. Molecular cloning and developmental expression of two chick embryo gap junction proteins. J Biol Chem 265: 14439–14443, 1990 [PubMed] [Google Scholar]
- 9.Beyer EC, Paul DL, Goodenough DA. Connexin43: a protein from rat heart homologous to a gap junction protein from liver. J Cell Biol 105: 2621–2629, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brightman MW, Reese TS. Junctions between intimately apposed cell membranes in the vertebrate brain. J Cell Biol 40: 648–677, 1969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calkins H. Arrhythmogenic right ventricular dysplasia. Curr Probl Cardiol 38: 103–123, 2013 [DOI] [PubMed] [Google Scholar]
- 12.Clerc L. Directional differences of impulse spread in trabecular muscle from mammalian heart. J Physiol 255: 335–346, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cohen SA. Immunocytochemical localization of rH1 sodium channel in adult rat heart atria and ventricle. Presence in terminal intercalated disks. Circulation 94: 3083–3086, 1996 [DOI] [PubMed] [Google Scholar]
- 14.Copene ED, Keener JP. Ephaptic coupling of cardiac cells through the junctional electric potential. J Math Biol 57: 265–284, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Coppen SR, Dupont E, Rothery S, Severs NJ. Connexin45 expression is preferentially associated with the ventricular conduction system in mouse and rat heart. Circ Res 82: 232–243, 1998 [DOI] [PubMed] [Google Scholar]
- 16.Crill WE, Rumery RE, Woodbury JW. Effects of membrane current on transmembrane potentials of cultured chick embryo heart cells. Am J Physiol 197: 733–735, 1959 [DOI] [PubMed] [Google Scholar]
- 17.Danik SB, Liu F, Zhang J, Suk HJ, Morley GE, Fishman GI, Gutstein DE. Modulation of cardiac gap junction expression and arrhythmic susceptibility. Circ Res 95: 1035–1041, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Mello WC. Cell swelling, impulse conduction, and cardiac arrhythmias in the failing heart. Opposite effects of angiotensin II and angiotensin (1–7) on cell volume regulation. Mol Cell Biochem 330: 211–217, 2009 [DOI] [PubMed] [Google Scholar]
- 19.De Mello WC. Electrical Phenomena in the Heart. New York: Academic Press, 1972 [Google Scholar]
- 20.Delmar M. Connexin43 regulates sodium current; ankyrin-G modulates gap junctions: the intercalated disc exchanger. Cardiovasc Res 93: 220–222, 2012 [DOI] [PubMed] [Google Scholar]
- 21.Dewey MM, Barr L. Intercellular connection between smooth muscle cells: the nexus. Science 137: 670–672, 1962 [DOI] [PubMed] [Google Scholar]
- 22.Draper MH, Mya-Tu M. A comparison of the conduction velocity in cardiac tissues of various mammals. Q J Exp Physiol Cogn Med Sci 44: 91–109, 1959 [DOI] [PubMed] [Google Scholar]
- 23.Duffy HS. Fibroblasts, myofibroblasts, and fibrosis: fact, fiction, and the future. J Cardiovasc Pharmacol 57: 373–375, 2011 [DOI] [PubMed] [Google Scholar]
- 24.Duffy HS. The molecular mechanisms of gap junction remodeling. Heart Rhythm 9: 1331–1334, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elenes S, Martinez AD, Delmar M, Beyer EC, Moreno AP. Heterotypic docking of Cx43 and Cx45 connexons blocks fast voltage gating of Cx43. Biophys J 81: 1406–1418, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eloff BC, Lerner DL, Yamada KA, Schuessler RB, Saffitz JE, Rosenbaum DS. High resolution optical mapping reveals conduction slowing in connexin43 deficient mice. Cardiovasc Res 51: 681–690, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Engelmann TW. Ueber die Leitung der Erregung im Herzmuskel. Pflügers Arch 11: 465–480, 1875 [Google Scholar]
- 28.Fenn W, Cobb D. Electrolyte changes in muscle during activity. Am J Physiol 115: 345–356, 1936 [Google Scholar]
- 29.Fleischhauer J, Lehmann L, Kleber AG. Electrical resistances of interstitial and microvascular space as determinants of the extracellular electrical field and velocity of propagation in ventricular myocardium. Circulation 92: 587–594, 1995 [DOI] [PubMed] [Google Scholar]
- 30.Fozzard HA. Cardiac sodium and calcium channels: a history of excitatory currents. Cardiovasc Res 55: 1–8, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Geisler SB, Green KJ, Isom LL, Meshinchi S, Martens JR, Delmar M, Russell MW. Ordered assembly of the adhesive and electrochemical connections within newly formed intercalated disks in primary cultures of adult rat cardiomyocytes. J Biomed Biotechnol 2010: 624719, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ghaly HA, Boyle PM, Vigmond EJ, Shimoni Y, Nygren A. Simulations of reduced conduction reserve in the diabetic rat heart: response to uncoupling and reduced excitability. Ann Biomed Eng 38: 1415–1425, 2010 [DOI] [PubMed] [Google Scholar]
- 33.Goldsmith EC, Bradshaw AD, Spinale FG. Cellular mechanisms of tissue fibrosis. 2. Contributory pathways leading to myocardial fibrosis: moving beyond collagen expression. Am J Physiol Cell Physiol 304: C393–C402, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gourdie RG, Green CR, Severs NJ. Gap junction distribution in adult mammalian myocardium revealed by an anti-peptide antibody and laser scanning confocal microscopy. J Cell Sci 99: 41–55, 1991 [DOI] [PubMed] [Google Scholar]
- 35.Gourdie RG, Green CR, Severs NJ, Anderson RH, Thompson RP. Evidence for a distinct gap-junctional phenotype in ventricular conduction tissues of the developing and mature avian heart. Circ Res 72: 278–289, 1993 [DOI] [PubMed] [Google Scholar]
- 36.Gourdie RG, Green CR, Severs NJ, Thompson RP. Immunolabelling patterns of gap junction connexins in the developing and mature rat heart. Anat Embryol (Berl) 185: 363–378, 1992 [DOI] [PubMed] [Google Scholar]
- 37.Guerrero PA, Schuessler RB, Davis LM, Beyer EC, Johnson CM, Yamada KA, Saffitz JE. Slow ventricular conduction in mice heterozygous for a connexin43 null mutation. J Clin Invest 99: 1991–1998, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hodgkin AL, Horowicz P. Movements of Na and K in single muscle fibres. J Physiol 145: 405–432, 1959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hodgkin AL, Huxley AF. The components of membrane conductance in the giant axon of Loligo. J Physiol 116: 473–496, 1952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hodgkin AL, Huxley AF. Currents carried by sodium and potassium ions through the membrane of the giant axon of Loligo. J Physiol 116: 449–472, 1952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hodgkin AL, Huxley AF. The dual effect of membrane potential on sodium conductance in the giant axon of Loligo. J Physiol 116: 497–506, 1952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hodgkin AL, Huxley AF. Movement of sodium and potassium ions during nervous activity. Cold Spring Harb Symp Quant Biol 17: 43–52, 1952 [DOI] [PubMed] [Google Scholar]
- 43.Hodgkin AL, Huxley AF. Propagation of electrical signals along giant nerve fibers. Proc R Soc Lond B Biol Sci 140: 177–183, 1952 [DOI] [PubMed] [Google Scholar]
- 44.Hodgkin AL, Huxley AF. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol 117: 500–544, 1952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hodgkin AL, Huxley AF, Katz B. Measurement of current-voltage relations in the membrane of the giant axon of Loligo. J Physiol 116: 424–448, 1952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hodgkin AL, Katz B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol 108: 37–77, 1949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hong M, Bao L, Kefaloyianni E, Agullo-Pascual E, Chkourko H, Foster M, Taskin E, Zhandre M, Reid DA, Rothenberg E, Delmar M, Coetzee WA. Heterogeneity of ATP-sensitive K+ channels in cardiac myocytes: enrichment at the intercalated disk. J Biol Chem 287: 41258–41267, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hoogendijk MG, Opthof T, Postema PG, Wilde AA, de Bakker JM, Coronel R. The Brugada ECG pattern: a marker of channelopathy, structural heart disease, or neither? Toward a unifying mechanism of the Brugada syndrome. Circ Arhythm Electrophysiol 3: 283–290, 2010 [DOI] [PubMed] [Google Scholar]
- 49.Hoyt RH, Cohen ML, Saffitz JE. Distribution and three-dimensional structure of intercellular junctions in canine myocardium. Circ Res 64: 563–574, 1989 [DOI] [PubMed] [Google Scholar]
- 50.Jack JJB, Noble D, Tsien RW. Electric Current Flow in Excitable Cells. Oxford: Clarendon, 1975 [Google Scholar]
- 51.Jacquemet V, Henriquez CS. Loading effect of fibroblast-myocyte coupling on resting potential, impulse propagation, and repolarization: insights from a microstructure model. Am J Physiol Heart Circ Physiol 294: H2040–H2052, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jansen JA, Noorman M, Musa H, Stein M, de Jong S, van der Nagel R, Hund TJ, Mohler PJ, Vos MA, van Veen TA, de Bakker JM, Delmar M, van Rijen HV. Reduced heterogeneous expression of Cx43 results in decreased Nav1.5 expression and reduced sodium current that accounts for arrhythmia vulnerability in conditional Cx43 knockout mice. Heart Rhythm 9: 600–607, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kanter HL, Saffitz JE, Beyer EC. Cardiac myocytes express multiple gap junction proteins. Circ Res 70: 438–444, 1992 [DOI] [PubMed] [Google Scholar]
- 54.Kleber AG, Rudy Y. Basic mechanisms of cardiac impulse propagation and associated arrhythmias. Physiol Rev 84: 431–488, 2004 [DOI] [PubMed] [Google Scholar]
- 55.Kohl P, Camelliti P. Cardiac myocyte-nonmyocyte electrotonic coupling: implications for ventricular arrhythmogenesis. Heart Rhythm 4: 233–235, 2007 [DOI] [PubMed] [Google Scholar]
- 56.Kohl P, Camelliti P. Fibroblast-myocyte connections in the heart. Heart Rhythm 9: 461–464, 2012 [DOI] [PubMed] [Google Scholar]
- 57.Kojodjojo P, Kanagaratnam P, Segal OR, Hussain W, Peters NS. The effects of carbenoxolone on human myocardial conduction: a tool to investigate the role of gap junctional uncoupling in human arrhythmogenesis. J Am Coll Cardiol 48: 1242–1249, 2006 [DOI] [PubMed] [Google Scholar]
- 58.Kucera JP, Kleber AG, Rohr S. Slow conduction in cardiac tissue: insights from optical mapping at the cellular level. J Electrocardiol 34, Suppl: 57–64, 2001 [DOI] [PubMed] [Google Scholar]
- 59.Kucera JP, Rohr S, Rudy Y. Localization of sodium channels in intercalated disks modulates cardiac conduction. Circ Res 91: 1176–1182, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.LeGrice IJ, Smaill BH, Chai LZ, Edgar SG, Gavin JB, Hunter PJ. Laminar structure of the heart: ventricular myocyte arrangement and connective tissue architecture in the dog. Am J Physiol Heart Circ Physiol 269: H571–H582, 1995 [DOI] [PubMed] [Google Scholar]
- 61.Lewis T, Meakins J, White PD. The excitatory process in the dog's heart. Part I. The auricles. Philos Trans R Soc Lond B Biol Sci 205: 375–420, 1914 [Google Scholar]
- 62.Lin J, Keener JP. Ephaptic coupling in cardiac myocytes. IEEE Trans Biomed Eng 60: 576–582, 2013 [DOI] [PubMed] [Google Scholar]
- 63.Litchenberg WH, Norman LW, Holwell AK, Martin KL, Hewett KW, Gourdie RG. The rate and anisotropy of impulse propagation in the postnatal terminal crest are correlated with remodeling of Cx43 gap junction pattern. Cardiovasc Res 45: 379–387, 2000 [DOI] [PubMed] [Google Scholar]
- 64.Malhotra JD, Thyagarajan V, Chen C, Isom LL. Tyrosine-phosphorylated and nonphosphorylated sodium channel beta1 subunits are differentially localized in cardiac myocytes. J Biol Chem 279: 40748–40754, 2004 [DOI] [PubMed] [Google Scholar]
- 65.McIntyre H, Fry CH. Abnormal action potential conduction in isolated human hypertrophied left ventricular myocardium. J Cardiovasc Electrophysiol 8: 887–894, 1997 [DOI] [PubMed] [Google Scholar]
- 66.Medvinskii AB, Pertsov AM. [Fiber interaction during impulse propagation in smooth muscle and myocardial tissues. Electrotonic interaction.] Biofizika 24: 135–140, 1979 [PubMed] [Google Scholar]
- 67.Mehra R. Global public health problem of sudden cardiac death. J Electrocardiol 40: S118–122, 2007 [DOI] [PubMed] [Google Scholar]
- 68.Milstein ML, Musa H, Balbuena DP, Anumonwo JM, Auerbach DS, Furspan PB, Hou L, Hu B, Schumacher SM, Vaidyanathan R, Martens JR, Jalife J. Dynamic reciprocity of sodium and potassium channel expression in a macromolecular complex controls cardiac excitability and arrhythmia. Proc Natl Acad Sci USA 109: E2134–E2143, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Moreno AP. Biophysical properties of homomeric and heteromultimeric channels formed by cardiac connexins. Cardiovasc Res 62: 276–286, 2004 [DOI] [PubMed] [Google Scholar]
- 70.Mori Y, Fishman GI, Peskin CS. Ephaptic conduction in a cardiac strand model with 3D electrodiffusion. Proc Natl Acad Sci USA 105: 6463–6468, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Morley GE, Vaidya D, Samie FH, Lo C, Delmar M, Jalife J. Characterization of conduction in the ventricles of normal and heterozygous Cx43 knockout mice using optical mapping. J Cardiovasc Electrophysiol 10: 1361–1375, 1999 [DOI] [PubMed] [Google Scholar]
- 72.Narahashi T, Moore JW, Scott WR. Tetrodotoxin blockage of sodium conductance increase in lobster giant axons. J Gen Physiol 47: 965–974, 1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Neher E, Sakmann B. Single-channel currents recorded from membrane of denervated frog muscle fibres. Nature 260: 799–802, 1976 [DOI] [PubMed] [Google Scholar]
- 74.Peskoff A. Electric potential in cylindrical syncytia and muscle fibers. Bull Math Biol 41: 183–192, 1979 [DOI] [PubMed] [Google Scholar]
- 75.Peskoff A. Electric potential in three-dimensional electrically syncytial tissues. Bull Math Biol 41: 163–181, 1979 [DOI] [PubMed] [Google Scholar]
- 76.Petitprez S, Zmoos AF, Ogrodnik J, Balse E, Raad N, El-Haou S, Albesa M, Bittihn P, Luther S, Lehnart SE, Hatem SN, Coulombe A, Abriel H. SAP97 and dystrophin macromolecular complexes determine two pools of cardiac sodium channels Nav1.5 in cardiomyocytes. Circ Res 108: 294–304, 2011 [DOI] [PubMed] [Google Scholar]
- 77.Plonsey R, Barr RC. Bioelectricity: A Quantitative Approach. New York: Springer, 2007 [Google Scholar]
- 78.Poche R, Lindner E. [Study of the intercalated discs of heart muscle tissue in warm-blooded and cold-blooded animals.] Zeitschrift fur Zellforschung und mikroskopische Anatomie 43: 104–120, 1955 [PubMed] [Google Scholar]
- 79.Poelzing S, Rosenbaum DS. Altered connexin43 expression produces arrhythmia substrate in heart failure. Am J Physiol Heart Circ Physiol 287: H1762–H1770, 2004 [DOI] [PubMed] [Google Scholar]
- 80.Poelzing S, Roth BJ, Rosenbaum DS. Optical measurements reveal nature of intercellular coupling across ventricular wall. Am J Physiol Heart Circ Physiol 289: H1428–H1435, 2005 [DOI] [PubMed] [Google Scholar]
- 81.Price KC, Weiss JM, Hata D, Smith JR. Experimental needle biopsy of the myocardium of dogs with particular reference to histologic study by electron microscopy. J Exp Med 101: 687–694, 1955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Procida K, Jorgensen L, Schmitt N, Delmar M, Taffet SM, Holstein-Rathlou NH, Nielsen MS, Braunstein TH. Phosphorylation of connexin43 on serine 306 regulates electrical coupling. Heart Rhythm 6: 1632–1638, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rasmussen HB, Moller M, Knaus HG, Jensen BS, Olesen SP, Jorgensen NK. Subcellular localization of the delayed rectifier K+ channels KCNQ1 and ERG1 in the rat heart. Am J Physiol Heart Circ Physiol 286: H1300–H1309, 2004 [DOI] [PubMed] [Google Scholar]
- 84.Rhett JM, Gourdie RG. The perinexus: a new feature of Cx43 gap junction organization. Heart Rhythm 9: 619–623, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rhett JM, Ongstad EL, Jourdan J, Gourdie RG. Cx43 associates with Na(v)1.5 in the cardiomyocyte perinexus. J Membr Biol 245: 411–422, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rhett JM, Veeraraghavan R, Poelzing S, Gourdie RG. The perinexus: Sign-post on the path to a new model of cardiac conduction? Trends Cardiovasc Med 23: 222–228, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rohr S. Role of gap junctions in the propagation of the cardiac action potential. Cardiovasc Res 62: 309–322, 2004 [DOI] [PubMed] [Google Scholar]
- 88.Rohr S, Kucera JP, Kleber AG. Slow conduction in cardiac tissue, I: effects of a reduction of excitability versus a reduction of electrical coupling on microconduction. Circ Res 83: 781–794, 1998 [DOI] [PubMed] [Google Scholar]
- 89.Rohr S, Salzberg BM. Discontinuities in action potential propagation along chains of single ventricular myocytes in culture: multiple site optical recording of transmembrane voltage (MSORTV) suggests propagation delays at the junctional sites between cells. Biol Bull 183: 342–343, 1992 [DOI] [PubMed] [Google Scholar]
- 90.Rohr S, Salzberg BM. Multiple site optical recording of transmembrane voltage (MSORTV) in patterned growth heart cell cultures: assessing electrical behavior, with microsecond resolution, on a cellular and subcellular scale. Biophys J 67: 1301–1315, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sachse FB, Moreno AP, Abildskov JA. Electrophysiological modeling of fibroblasts and their interaction with myocytes. Ann Biomed Eng 36: 41–56, 2008 [DOI] [PubMed] [Google Scholar]
- 92.Saffitz JE, Kanter HL, Green KG, Tolley TK, Beyer EC. Tissue-specific determinants of anisotropic conduction velocity in canine atrial and ventricular myocardium. Circ Res 74: 1065–1070, 1994 [DOI] [PubMed] [Google Scholar]
- 93.Sano T, Takayama N, Shimamoto T. Directional difference of conduction velocity in the cardiac ventricular syncytium studied by microelectrodes. Circ Res 7: 262–267, 1959 [DOI] [PubMed] [Google Scholar]
- 94.Seidel T, Salameh A, Dhein S. A simulation study of cellular hypertrophy and connexin lateralization in cardiac tissue. Biophys J 99: 2821–2830, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shaw RM, Rudy Y. Ionic mechanisms of propagation in cardiac tissue. Roles of the sodium and L-type calcium currents during reduced excitability and decreased gap junction coupling. Circ Res 81: 727–741, 1997 [DOI] [PubMed] [Google Scholar]
- 96.Sjostrand FS, Andersson E. Electron microscopy of the intercalated discs of cardiac muscle tissue. Experientia 10: 369–370, 1954 [DOI] [PubMed] [Google Scholar]
- 97.Sohl G, Willecke K. Gap junctions and the connexin protein family. Cardiovasc Res 62: 228–232, 2004 [DOI] [PubMed] [Google Scholar]
- 98.Solan JL, Lampe PD. Connexin43 phosphorylation: structural changes and biological effects. Biochem J 419: 261–272, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Solan JL, Lampe PD. Key connexin 43 phosphorylation events regulate the gap junction life cycle. J Membr Biol 217: 35–41, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Spach MS. Transition from a continuous to discontinuous understanding of cardiac conduction. Circ Res 92: 125–126, 2003 [DOI] [PubMed] [Google Scholar]
- 101.Spach MS, Heidlage JF, Barr RC, Dolber PC. Cell size and communication: role in structural and electrical development and remodeling of the heart. Heart Rhythm 1: 500–515, 2004 [DOI] [PubMed] [Google Scholar]
- 102.Spach MS, Heidlage JF, Dolber PC, Barr RC. Changes in anisotropic conduction caused by remodeling cell size and the cellular distribution of gap junctions and Na+ channels. J Electrocardiol 34, Suppl: 69–76, 2001 [DOI] [PubMed] [Google Scholar]
- 103.Spach MS, Heidlage JF, Dolber PC, Barr RC. Electrophysiological effects of remodeling cardiac gap junctions and cell size: experimental and model studies of normal cardiac growth. Circ Res 86: 302–311, 2000 [DOI] [PubMed] [Google Scholar]
- 104.Spach MS, Heidlage JF, Dolber PC, Barr RC. Mechanism of origin of conduction disturbances in aging human atrial bundles: experimental and model study. Heart Rhythm 4: 175–185, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Spach MS, Miller WT, 3rd, Dolber PC, Kootsey JM, Sommer JR, Mosher CE., Jr The functional role of structural complexities in the propagation of depolarization in the atrium of the dog. Cardiac conduction disturbances due to discontinuities of effective axial resistivity. Circ Res 50: 175–191, 1982 [DOI] [PubMed] [Google Scholar]
- 106.Spach MS, Miller WT, 3rd, Geselowitz DB, Barr RC, Kootsey JM, Johnson EA. The discontinuous nature of propagation in normal canine cardiac muscle. Evidence for recurrent discontinuities of intracellular resistance that affect the membrane currents. Circ Res 48: 39–54, 1981 [DOI] [PubMed] [Google Scholar]
- 107.Sperelakis N. An electric field mechanism for transmission of excitation between myocardial cells. Circ Res 91: 985–987, 2002 [DOI] [PubMed] [Google Scholar]
- 108.Sperelakis N, McConnell K. Electric field interactions between closely abutting excitable cells. IEEE Eng Med Biol Mag 21: 77–89, 2002 [DOI] [PubMed] [Google Scholar]
- 109.Stein M, van Veen TA, Remme CA, Boulaksil M, Noorman M, van Stuijvenberg L, van der Nagel R, Bezzina CR, Hauer RN, de Bakker JM, van Rijen HV. Combined reduction of intercellular coupling and membrane excitability differentially affects transverse and longitudinal cardiac conduction. Cardiovasc Res 83: 52–60, 2009 [DOI] [PubMed] [Google Scholar]
- 110.Streeter DD, Jr, Spotnitz HM, Patel DP, Ross J, Jr, Sonnenblick EH. Fiber orientation in the canine left ventricle during diastole and systole. Circ Res 24: 339–347, 1969 [DOI] [PubMed] [Google Scholar]
- 111.Taccardi B, Punske BB, Macchi E, Macleod RS, Ershler PR. Epicardial and intramural excitation during ventricular pacing: effect of myocardial structure. Am J Physiol Heart Circ Physiol 294: H1753–H1766, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Thomas SP, Kucera JP, Bircher-Lehmann L, Rudy Y, Saffitz JE, Kleber AG. Impulse propagation in synthetic strands of neonatal cardiac myocytes with genetically reduced levels of connexin43. Circ Res 92: 1209–1216, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Thomson W. On peristaltic induction of electric currents. Proceed R Soc Lond 8: 121–132, 1856 [Google Scholar]
- 114.Thomson W. On the theory of the electric telegraph. Proceed R Soc Lond 7: 382–399, 1854 [Google Scholar]
- 115.Tranum-Jensen J, Janse MJ, Fiolet WT, Krieger WJ, D'Alnoncourt CN, Durrer D. Tissue osmolality, cell swelling, and reperfusion in acute regional myocardial ischemia in the isolated porcine heart. Circ Res 49: 364–381, 1981 [DOI] [PubMed] [Google Scholar]
- 116.Vaidya D, Tamaddon HS, Lo CW, Taffet SM, Delmar M, Morley GE, Jalife J. Null mutation of connexin43 causes slow propagation of ventricular activation in the late stages of mouse embryonic development. Circ Res 88: 1196–1202, 2001 [DOI] [PubMed] [Google Scholar]
- 117.van Rijen HV, Eckardt D, Degen J, Theis M, Ott T, Willecke K, Jongsma HJ, Opthof T, de Bakker JM. Slow conduction and enhanced anisotropy increase the propensity for ventricular tachyarrhythmias in adult mice with induced deletion of connexin43. Circulation 109: 1048–1055, 2004 [DOI] [PubMed] [Google Scholar]
- 118.Veeraraghavan R, Larsen AP, Torres NS, Grunnet M, Poelzing S. Potassium channel activators differentially modulate the effect of sodium channel blockade on cardiac conduction. Acta Physiol (Oxf) 207: 280–289, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Veeraraghavan R, Poelzing S. Mechanisms underlying increased right ventricular conduction sensitivity to flecainide challenge. Cardiovasc Res 77: 749–756, 2008 [DOI] [PubMed] [Google Scholar]
- 120.Veeraraghavan R, Salama ME, Poelzing S. Interstitial volume modulates the conduction velocity-gap junction relationship. Am J Physiol Heart Circ Physiol 302: H278–H286, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Vetter C, Zweifel M, Zuppinger C, Carrel T, Martin D, Haefliger JA, Delacretaz E. Connexin 43 expression in human hypertrophied heart due to pressure and volume overload. Physiol Res 59: 35–42, 2010 [DOI] [PubMed] [Google Scholar]
- 122.Vozzi C, Dupont E, Coppen SR, Yeh HI, Severs NJ. Chamber-related differences in connexin expression in the human heart. J Mol Cell Cardiol 31: 991–1003, 1999 [DOI] [PubMed] [Google Scholar]
- 123.Weidmann S. The diffusion of radiopotassium across intercalated disks of mammalian cardiac muscle. J Physiol 187: 323–342, 1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Weidmann S. The electrical constants of Purkinje fibres. J Physiol 118: 348–360, 1952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Weidmann S. Electrical constants of trabecular muscle from mammalian heart. J Physiol 210: 1041–1054, 1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wiegerinck RF, Verkerk AO, Belterman CN, van Veen TA, Baartscheer A, Opthof T, Wilders R, de Bakker JM, Coronel R. Larger cell size in rabbits with heart failure increases myocardial conduction velocity and QRS duration. Circulation 113: 806–813, 2006 [DOI] [PubMed] [Google Scholar]
- 127.Xie Y, Garfinkel A, Camelliti P, Kohl P, Weiss JN, Qu Z. Effects of fibroblast-myocyte coupling on cardiac conduction and vulnerability to reentry: a computational study. Heart Rhythm 6: 1641–1649, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zak R. Cell proliferation during cardiac growth. Am J Cardiol 31: 211–219, 1973 [DOI] [PubMed] [Google Scholar]
- 129.Zemlin CW, Mironov S, Pertsov AM. Near-threshold field stimulation: intramural versus surface activation. Cardiovasc Res 69: 98–106, 2006 [DOI] [PubMed] [Google Scholar]
- 130.Zhang Y, Xiao J, Wang H, Luo X, Wang J, Villeneuve LR, Zhang H, Bai Y, Yang B, Wang Z. Restoring depressed HERG K+ channel function as a mechanism for insulin treatment of abnormal QT prolongation and associated arrhythmias in diabetic rabbits. Am J Physiol Heart Circ Physiol 291: H1446–H1455, 2006 [DOI] [PubMed] [Google Scholar]