Abstract
SK channels are upregulated in human patients and animal models of heart failure (HF). However, their activation mechanism and function in ventricular myocytes remain poorly understood. We aim to test the hypotheses that activation of SK channels in ventricular myocytes requires Ca2+ release from sarcoplasmic reticulum (SR) and that SK currents contribute to reducing triggered activity. SK2 channels were overexpressed in adult rat ventricular myocytes using adenovirus gene transfer. Simultaneous patch clamp and confocal Ca2+ imaging experiments in SK2-overexpressing cells demonstrated that depolarizations resulted in Ca2+-dependent outward currents sensitive to SK inhibitor apamin. SR Ca2+ release induced by rapid application of 10 mM caffeine evoked repolarizing SK currents, whereas complete depletion of SR Ca2+ content eliminated SK currents in response to depolarizations, despite intact Ca2+ influx through L-type Ca2+ channels. Furthermore, voltage-clamp experiments showed that SK channels can be activated by global spontaneous SR Ca2+ release events Ca2+ waves (SCWs). Current-clamp experiments revealed that SK overexpression reduces the amplitude of delayed afterdepolarizations (DADs) resulting from SCWs and shortens action potential duration. Immunolocalization studies showed that overexpressed SK channels are distributed both at external sarcolemmal membranes and along the Z-lines, resembling the distribution of endogenous SK channels. In summary, SR Ca2+ release is both necessary and sufficient for the activation of SK channels in rat ventricular myocytes. SK currents contribute to repolarization during action potentials and attenuate DADs driven by SCWs. Thus SK upregulation in HF may have an anti-arrhythmic effect by reducing triggered activity.
Keywords: small conductance calcium activated potassium channel, heart failure, arrhythmia, delayed afterdepolarization
the complex interaction between intracellular calcium (Ca2+) dynamics and membrane potential is critical for the function of cardiomyocytes (CMs) (7). By providing a direct coupling between Ca2+ and voltage, electrogenic Ca2+-dependent ion transporting mechanisms such as the sodium-calcium exchanger (Na+/Ca2+ exchanger, or NCX1) play important roles in both normal CM physiology and arrhythmogenesis (3). Recent discovery of the expression of small conductance Ca2+-activated potassium channels (SK, KCa2, or KCNN) in CMs implicates these channels as a novel pathway for translation of intracellular Ca2+ signals into changes of membrane potential (39, 42). Previous work by our group and others established that SK channels are rapidly activated by submicromolar range cytosolic [Ca2+] through Ca2+ binding to their constitutively associated calmodulin (1, 30, 31, 40). SK channels can conceivably provide a uniquely efficient feedback from changes in Ca2+ cycling to membrane repolarization in CMs. Indeed, several recent studies have implicated functional roles of SK channels in cardiac excitability and arrhythmogenesis (14, 17, 18, 34, 36). However, cellular mechanisms for activation of SK channels in CMs are largely unknown, and their exact role in cardiac arrhythmia remains controversial (21, 24).
SK channels were initially considered atria-specific because of their higher expression level in atria than ventricles (42) and because under normal conditions they could neither be activated nor regulate repolarization in ventricular myocytes (33). However, importance of SK channels in ventricular physiology and arrhythmia was suggested by several recent studies demonstrating their upregulation and functional contribution to repolarization in failing ventricles from human patients and animal models of heart failure (HF) (10, 12, 14, 23).
Enhanced proclivity to arrhythmia in HF has been attributed to increased triggered activity presented by early and delayed afterdepolarizations (EADs and DADs, respectively). These aberrations in cardiac electrical activity arise as a consequence of untimely activation of inward INCX1 by spontaneous Ca2+ release from the SR mediated by SR Ca2+ release channels known as ryanodine receptors (RyR2s) (2, 16, 25). SK channels, with the ability of translating Ca2+ elevation into enhanced repolarization, may play an important role in arrhythmogenesis associated with abnormal Ca2+ handling in HF by acting as a direct antagonism of NCX1. SK inhibition prevents a specific form of arrhythmia, i.e., after shock spontaneous ventricular fibrillation in a rabbit HF model with tachycardia-induced cardiomyopathy (TICM) (14). Additionally, potential anti-arrhythmic effect of SK upregulation as a compensatory mechanism for the downregulation of multiple repolarizing potassium currents in HF has been suggested by recent optical mapping studies (11, 23). However, direct cellular mechanisms for the arrhythmogenic roles of SK channels are not clearly understood. Repolarizing SK currents can conceptually be activated by spontaneous Ca2+ waves (SCW) and reduce the amplitudes of DADs resulting from depolarizing NCX1 currents, thus providing an anti-arrhythmic effect. However, until now it is not clear how SK channels are activated in cardiomyocytes and whether they respond to SCWs.
Of particular interest is the question of how SK channels respond to global versus local Ca2+ rise from different sources (32). In theory SK channels can be activated by Ca2+ entering the myocytes via plasmalemmal L-type Ca2+ channels (LTCCs) that open during action potential (AP) and close at rest. Alternatively SK channels can respond to SR Ca2+ release, which in HF occurs not only in systole but in diastole as well in the form of pro-arrhythmic global propagating SCWs (22, 25, 35). Consequently, mechanism of activation of SK channels (i.e., source of Ca2+) and their contribution to repolarization at the cellular level are important factors determining their involvement in arrhythmogenesis.
SK channels are expressed at minimal levels in normal ventricular myocytes, and their upregulation is highly variable among myocytes in HF (12, 14, 33). In addition, interpretation of the effects of SK upregulation obtained with myocytes from failing hearts is expected to be complicated due to broad remodeling of multiple cellular signaling pathways and ion channels, which is a hallmark of HF. To establish a reliable system for understanding the activation mechanism of SK channels and the direct functional effect of SK upregulation, we overexpressed the SK2 channel in cultured adult rat ventricular cardiomyocytes (ARCM). Our studies revealed that RyR2-mediated SR Ca2+ release is the main Ca2+ source for activation of SK channels in ventricular myocytes. Importantly, we demonstrated that SK channels are effectively recruited by SCWs and able to attenuate DADs. These findings present the first direct evidence at the cellular level that upregulation of SK channels could serve as an anti-arrhythmic mechanism in ventricular myocytes of failing hearts.
MATERIALS AND METHODS
Construction of SK2 adenovirus.
Adenovirus encompassing rat SK2 sequence was constructed with the ViraPower adenoviral Gateway expression system. Briefly, coding region of rSK2 was first cloned into pENTRTM1A vector, and then recombined into pAD/CMV/V5-DESTTM vector with LR recombination reaction. Verified plasmid of the expression construct was digested with PacI and used to transfect 293A cells with Lipofectamine (Invitrogen) for adenoviral stock and amplification. Titer of the amplified adenoviral stock was determined using 293A cells overlaid with media containing 4% agarose. A viral construct with no coding sequence following a CMV promoter was used as the control virus.
Cardiomyocyte isolation and primary culture.
Isolation of adult rat ventricular myocytes was carried out with 9- to 12-wk-old Sprague-Dawley male rats following procedures approved by Rhode Island Hospital Animal Care and Use Committee as previously described (5). Rats were anesthetized with a cocktail of 50% Sleepaway pentobarbital sodium (100 μl/100 g of animal weight) and 50% heparin (1,000 USP units/ml). After rats were unresponsive to rear paw and tail pinching, hearts were excised and plunged into ice cold Tyrode's solution. The hearts were then perfused by gravity on a Langendorff apparatus with 37°C Tyrode's solution followed by collagenase solution for 16 to 17 min. Both ventricles were then coarsely minced and placed in a 37°C water shaker in collagenase solution. Isolated myocytes were plated into 24-well containers on laminin-coated glass coverslips in serum-free medium 199 (Life Technologies) supplemented with (in mM) 25 NaHCO3, 10 HEPES, 5 creatine, and 5 taurine and 10 U/ml penicillin, 10 μg/ml streptomycin, and 10 μg/ml gentamycin (pH 7.3). Ventricular myocytes were cultured at 37°C in humidified atmosphere with 95% air/5% CO2. After 2 to 3 h, unattached cells were removed and myocytes were transduced with adenoviruses at multiplicity of infection (MOI) = 10 and cultured for 36–48 h before analysis. At such conditions culture-dependent structural and functional remodeling of myocytes are minimized (4).
Whole-cell patch clamp, Ca2+ imaging and analysis.
Whole-cell patch clamp recordings of transmembrane currents and membrane potentials were performed with an Axopatch 200B amplifier (Axon Instruments, MDS, Sunnyvale, CA). For all recordings, the bath solution contained (in mM) 140 NaCl, 5.4 KCl, 1.0 CaCl2, 1.0 MgCl2, 10 HEPES, and 5.6 glucose (pH 7.3). Pipette solution contained (in mM) 90 K-aspartate, 50 KCl, 5 MgATP, 5 NaCl, 1 MgCl2, 0.1 Tris GTP, 10 HEPES, and 0.1 Rhod-2 K+-salt (Invitrogen) (pH 7.2). Depolarizing voltage steps to record SK currents were applied at 10-s intervals under voltage clamp. Action potentials were elicited by injection of short current pulse at 1.2× threshold under current clamp. Intracellular Ca2+ imaging was simultaneously performed using Leica SP5 II confocal microscope equipped with 63× 1.4 numerical aperture oil objective in line-scan mode at a rate of 5 or 10 ms per line and synchronized with electrophysiology setup using a trigger at the beginning of the scan. Rhod-2 was excited by 543-nm line of HeNe laser, and fluorescence was acquired at wavelengths of 560–660 nm. All experiments were performed at room temperature. Space-averaged Ca2+ transient time profiles were obtained using ImageJ (National Institutes of Health) software. Statistical analysis of electrophysiological and Ca2+ imaging data was performed using Origin 8.0 (OriginLab).
Western blot analysis and immunocytochemistry.
For Western blot analysis Radioimmunoprecipitation assay buffer supplemented with cocktail of calpain inhibitors and protease inhibitors (Sigma) was directly added to the cells after 48 h in culture. Cell lysate proteins were subjected to 4% to 20% SDS-PAGE and blotted onto nitrocellulose membranes (Bio-Rad Labs). Primary antibodies used were anti-SK2 (Sigma) and anti-GAPDH (Abcam). Protein bands were visualized using the Super Signal West Pico kit (Pierce). For immunolocalization studies, myocytes were fixed on glass coverslips with 4% paraformaldehyde and permeabilized with 0.2% Triton X-100/PBS (pH 7.2) containing 1% BSA. Specimens were incubated with anti-SK2 (1:200; Sigma) and anti-RyR2 (1:200; Thermo Fisher Scientific) or anti-CaV1.2 (1:200; Thermo Fisher Scientific) primary antibodies followed by fluorophore-conjugated, highly cross-adsorbed IgGs (anti-goat Alexa Fluor-488 and anti-mouse Alexa Fluor-546; 1:200; Invitrogen). Images were acquired using Leica SP5 II confocal system equipped with 63× 1.4 numerical aperture oil objective using 488-nm line of argon-ion laser and 546-nm line of HeNe laser for excitation. Emitted fluorescence was collected at 500–530 nm and 560–660 nm wavelengths.
Statistical analysis.
Data are presented as means ± SE. Statistical significance was evaluated by paired or unpaired Student's t-test as appropriate. P < 0.05 was considered significant.
RESULTS
Functional expression of SK channels in adult rat ventricular cardiomyocytes.
Transduction of adult rat ventricular cardiomyocytes (ARCMs) with SK2 virus (SK group) resulted in transient outward currents in response to depolarizing voltage steps under voltage clamp (Fig. 1A). Several lines of evidence establish that these currents are carried by overexpressed SK channels: 1) membrane potential was held at −50 mV such that the majority of Ito, another transient outward current in CMs, is expected to be inactivated; 2) these outward currents were absent in ARCMs transduced with control adenovirus (control group) (Fig. 1B); 3) importantly, 100 nM apamin, a specific SK channel blocker, inhibited the vast majority of the outward currents in the SK group but had no effect on the small outward currents in control cells (Fig. 1, A–C); and 4) Western blot showed that SK2 protein is dramatically increased in cells transduced with SK2 virus compared with control (Fig. 1D). Additionally, contribution by Ca2+-activated chloride channels to the outward currents is expected to be minimal in light of the very little outward currents in control cells and the small size of these currents previously reported in ventricular myocytes (38, 43). Magnified views of the currents in control cells reveal the inward Ca2+ currents (Fig. 1B, insets) typical of rat ventricular myocytes. Application of 100 nM apamin led to 12.2 ± 4.8% (means ± SE; n = 4) reduction in peak Ca2+ current level at 0 mV in control cells, although this blocking effect on ICa is likely an overestimate because time-dependent Ca2+ current rundown may also contribute to this reduction. Ca2+ currents are not clearly resolvable in SK overexpressing cells due to the large outward SK currents, even after the majority of SK currents were blocked by apamin (Fig. 1A, inset). Although SK2 channels were reported to have subnanomolar sensitivity to apamin in heterologous system (26) and mouse atrial cardiomyocytes (42), 100 nM apamin was necessary for its effect on ventricular action potential durations (APDs) in failing rabbit hearts (14). We noticed that inhibition of SK channels in ARCMs is slow and incomplete even with 100 nM apamin [higher concentrations were not tested in light of its potential blocking effect on ICa (9)], likely reflecting the slow diffusion of the peptide toxin into transverse tubules (T tubules). At 0 mV, 84.5 ± 2.2% (n = 6) of the peak outward currents was inhibited within the time of our experiments (5–10 min). The outward currents in SK-overexpressing myocytes could also be effectively blocked by another SK channel blocker, UCL1684, at 0.5–1 μM (data not shown). On average, overexpression resulted in ∼24 pA/pF apamin-sensitive outward SK current at 0 mV (Fig. 1C). For comparison, SK upregulation resulted in ∼8 pA/pF SK current in epicardial ventricular myocytes from rabbit HF model (14) and ∼7 pA/pF in endocardial myocytes from human HF patients (12).
Fig. 1.

Functional expression of SK channels in cultured adult rat cardiomyocytes. A: representative whole cell current traces from a myocyte transduced with SK2 virus in response to depolarizing voltage steps (−40 mV to +40 mV) from a holding potential of −50 mV before (left) and after 100 nM apamin (right). Insets are magnified views of current traces (with different scales) at the beginning of the depolarizations to reveal inward Ca2+ currents. B: representative current traces from a myocyte transduced with control virus under the same conditions as in A. All currents are normalized to cell capacitance. C: normalized peak outward current amplitudes are averaged and plotted as a function of depolarizing membrane potentials for control and SK transduced myocytes before and after 100 nM apamin. Error bars represent SE. *Statistically significant difference between baseline and apamin for SK group (P < 0.05, paired Student's t-test). D: Western blots of SK2 in cultured ventricular myocytes from 3 different rats. Cells from each rat transduced with either control virus (“C”) or SK2 virus (SK2) were compared side by side. Under our experimental conditions the antibody labeling revealed a primary band at ∼230 KDa, suggestive of SK channel migration in gels as predominantly the tetramer form, since the predicted molecular weight for SK2 monomers is between 60 and 70 KDa. GAPDH was used for loading control.
Ca2+ release from SR is necessary and sufficient for activation of SK currents in ventricular myocytes.
The transient nature of SK currents during voltage steps (Fig. 1A) likely results from dynamic changes in cytosolic [Ca2+] because SK channels do not spontaneously inactivate (26). We explored the dependence of SK activation on Ca2+ cycling with simultaneous recordings of SK currents and Ca2+ transients (Fig. 2A). SK currents rapidly activate at the beginning of depolarization and decay during the voltage step, following the rise and decay of [Ca2+], suggesting that change in [Ca2+] determines the profile of SK currents. A closer look at the time courses reveals that SK currents decay faster than Ca2+ transients (e.g., at 0 mV, SK currents: half decay time t1/2 = 72 ± 7 ms, n = 6; Ca2+: t1/2 = 125 ± 12 ms, n = 6; means ± SE). This discrepancy in kinetics between SK currents and Ca2+ transients is expected because of the steeper dependence of SK activation on [Ca2+] (Hill coefficient n = 3–5) (1) than Ca2+ dye Rhod-2 (Hill coefficient n = 1) (19).
Fig. 2.

Activation of SK channels requires Ca2+ release from SR. A: representative current traces (left) and simultaneously recorded Ca2+ transients (right) from an SK-transduced myocyte with the same voltage protocol as in Fig. 1. Only 4 traces at indicated potentials are shown for clarity. B: current traces (left) and Ca2+ transients (right) from the same myocyte in A in the persistent presence of 10 mM caffeine. Inset provides a magnified view of the remaining currents to reveal inward Ca2+ currents. C: average amplitudes of normalized peak outward current (left) and Ca2+ transients (right) are plotted as a function of depolarizing membrane potentials for baseline condition (black) and after unloading of Ca2+ from SR with 10 mM caffeine (red). Error bars are SE. *Statistically significant difference between baseline and caffeine (P < 0.05, paired Student's t-test).
Elevations of intracellular [Ca2+] in CMs are primarily conferred by two key mechanisms, influx through plasmalemmal LTCCs and release from RyR2s predominantly localized on the membranes of junctional SR (8). Results in Fig. 2A do not allow us to distinguish Ca2+ from which of the two closely coupled sources directly activates SK channels. To evaluate the contribution of each Ca2+ source to SK activation in CMs, we first asked whether Ca2+ release from the SR is necessary for SK activation, i.e., whether influx through LTCCs alone is sufficient. To that end, we treated the SK-overexpressing cells with 10 mM caffeine to fully deplete SR Ca2+ content. As shown in Fig. 2, B and C, the transient outward SK currents were completely abolished in the persistent presence of caffeine, along with the much reduced Ca2+ transients. Interestingly, incubation with caffeine resulted in more complete inhibition of outward currents than apamin (93.3 ± 1.6% at 0 mV, n = 6; P < 0.05; compare Fig. 2 with Fig. 1). A magnified view of the remaining currents revealed Ca2+ currents comparable with the control cells (Fig. 1B and 2B, insets), suggesting intact gating of LTCCs in the presence of caffeine. We also used 10 μM thapsigargin as an alternative approach to deplete the SR Ca2+ content. Prolonged exposure or pretreatment with thapsigargin (>20 min) also effectively abolished outward SK currents in response to depolarizing voltage steps, even in the presence of 100 nM β-adrenergic agonist isoproterenol (Iso) to increase the magnitude of Ca2+ currents (Fig. 3). These experiments demonstrate that Ca2+ influx through LTCCs alone is not sufficient to activate SK currents, whereas SR Ca2+ release is critical.
Fig. 3.

Inhibition of SERCA to deplete SR Ca2+ stores eliminates SK currents. A: examples of current traces (left) and simultaneously recorded Ca2+ transients (right) from an SK-transduced myocyte with the same voltage protocol as in Fig. 1. Only 4 traces at indicated potentials are shown for clarity. Isoproterenol (Iso; 100 nM) was added to the bath solution to increase the magnitude of L-type Ca2+ currents. B: current traces (left) and Ca2+ transients (right) from the same myocyte in A 25 min after treatment with 10 μM thapsigargin. C: average amplitudes of normalized peak outward current (left) and Ca2+ transients (right) are plotted as a function of depolarizing membrane potentials for SK transduced cells pretreated with 10 μM thapsigargin for >20 min (red). Iso (100 nM) was added to the bath solution to increase the magnitudes of L-type Ca2+ currents. For comparison, data for baseline conditions from Fig. 2C were replotted as the control for SK transduced cells without thapsigargin pretreatment (gray). Error bars are SE. *Statistically significant difference between control and thapsigargin pretreated myocytes (P < 0.05, unpaired Student's t-test).
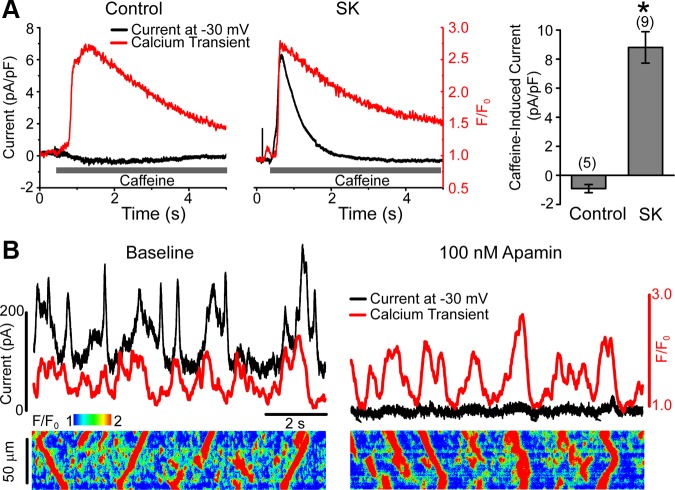
We next asked whether SR Ca2+ release independent of LTCCs is sufficient for SK activation. Caffeine (10 mM) was rapidly applied to SK-overexpressing myocytes under voltage clamp with membrane potential held at −30 mV, under which condition open probability of LTCCs is minimal. As shown in Fig. 4A, coinciding with the Ca2+ transient induced by caffeine, a transient outward current was activated in SK-transduced cells, indicating that Ca2+ release from SR is sufficient to activate SK current. This outward current is carried by SK channels because in control cells caffeine typically induces a small inward current at −30 mV (Fig. 4A), likely due to activation of NCX1, and because −30 mV is near the predicted reversal potential for Ca2+-activated chloride current. When compared with Fig. 2, the difference in the kinetics between SK current and Ca2+ transient became more pronounced in this case, because Ca2+ removal from the cytosol was dramatically slowed in the presence of caffeine (SK current: t1/2 = 0.48 ± 0.10 s, n = 8; Ca2+: t1/2 = 1.36 ± 0.23 s, n = 8; means ± SE).
Fig. 4.
Ca2+ release from SR is sufficient for activation of SK channels. A: representative current traces (black) are shown together with simultaneous line-scanning Ca2+ transient profile (red) recorded from a control (left) and an SK-transduced (middle) myocyte under whole cell voltage clamp. Cells were first stimulated by 10 depolarizing steps (500 ms) to −10 mV at 0.5 Hz to load the SR before membrane potential was held at −30 mV during rapid application of 10 mM caffeine. Peak amplitudes of caffeine-induced currents normalized to cell capacitance from indicated number of cells are shown on the right (means ± SE). B: representative outward currents (black) and time-dependent Ca2+ profile (red) under baseline condition (left) and after 100 nM apamin (right) are shown on top of Ca2+ transient images simultaneously recorded from an SK-transduced myocyte under whole cell voltage clamp with membrane potential held at −30 mV. Spontaneous Ca2+ waves were facilitated with 100 nM Iso + 100 μM caffeine.
Importantly, spontaneous global Ca2+ release (Ca2+ wave, or SCW) can also activate SK currents. Figure 4B shows examples of transient SK currents at −30 mV in response to SCWs facilitated by 100 nM Iso and 100 μM caffeine. However, in this case outward currents are less faithfully coincidental with the Ca2+ profile, likely because our line-scanning approach to monitor propagating SCWs reports Ca2+ transient at the line of choice, whereas SK currents are measured from the whole cell. The identity of SK currents was verified with application of 100 nM apamin, which diminished the outward currents in response to SCWs in the same cell (Fig. 4B, right).
SK currents contribute to repolarization and attenuate DADs.
Activation of SK channels is expected to enhance repolarization of membrane potential with outward K+ currents. We performed current-clamp experiments to evaluate the cellular function of SK channels in ventricular myocytes. To assess the effect of SK expression on DADs, we perfused the cells with 100 nM Iso plus 100 μM caffeine and paced with current injection at 2 Hz for 30 s to promote SCWs. Figure 5A shows that in control myocytes Ca2+ waves result in large depolarizations of membrane potential attributable to activation of NCX1 (35), which occasionally reach threshold and trigger APs. Apamin does not significantly affect the average amplitude of DADs in control cells (Fig. 5, A and B). Strikingly, Ca2+ waves of similar amplitudes in SK overexpressing cells result in very little depolarizations and rather often small hyperpolarizations (Fig. 5, A and B), indicating that activation of SK channels can reduce or reverse DADs in response to SCWs. Inhibition of SK channels with apamin in these cells effectively reversed the direction of wave-induced changes in membrane potential, although the average size of DADs was still significantly smaller than in control cells (P < 0.05, Student's t-test; Fig. 5, A and B), which likely reflects the incomplete block of SK channels. Action potentials recorded at the end of the 30 s/2 Hz pacing (in 100 nM Iso and 100 μM caffeine) were analyzed to evaluate the effect of SK channels on APD. SK overexpression resulted in very short APs compared with control cells (Fig. 5C) without affecting the resting membrane potential (SK: −74.1 ± 0.9 mV, n = 27; control: 74.1 ± 0.6 mV, n = 12). Inhibition of SK channels by 100 nM apamin led to prolongation of APD50 by more than threefold, although still significantly shorter compared with control myocytes (P < 0.05), likely a result of incomplete SK inhibition with 100 nM apamin as shown in Fig. 1. The small prolongation of APD by apamin in control cells was not statistically significant.
Fig. 5.
Functional effects of SK upregulation in ventricular myocytes. A: simultaneous recordings of membrane potential (black) and Ca2+ profile (red) are shown on top of line-scan images under baseline condition (left) and after 100 nM apamin (right). Control (top) and SK- (bottom) transduced myocytes were treated with 100 nM Iso and 100 μM caffeine and periodically stimulated at 2 Hz for 30 s (blue trace) under current clamp to induce waves upon cessation of pacing. B: average amplitudes of wave-induced voltage changes from each group with indicated number of waves (3–7 cells, means ± SE). *Statistically significant difference between baseline and apamin for SK group (P < 0.05, unpaired Student's t-test). C: representative action potentials recorded under whole cell current clamp from control (left) and SK transduced myocyte (middle) stimulated at 2 Hz in 100 nM Iso and 100 μM caffeine before (black) and after 100 nM apamin (red). Dashed lines indicate 0 mV level. At right, the average action potential duration at 50% repolarization (APD50) for each group is shown. *Statistically significant difference between baseline and apamin for SK group (P < 0.05, paired Student's t-test).
Subcellular distribution of SK channels in ventricular myocytes.
Distance between SK channels and their Ca2+ sources determines the specificity, level, and speed of activation (20). Our immunolocalization experiments demonstrate that the overexpressed SK channels are distributed in a similar fashion as endogenous channels, despite the apparently higher expression level (Fig. 6). Fluorescence profiles indicate that SK channels are concentrated on the external sarcolemmal membrane and along the Z-lines in rat ventricular myocytes, which is consistent with patterns previously reported in mouse and dog ventricular cells (33, 39, 42). In contrast, LTCCs (CaV1.2; Fig. 6A) and RyR2s (Fig. 6B) are expressed primarily along the Z-lines and much less at external sarcolemma, also consistent with previous findings (13, 15, 28). Regular periodicity of the fluorescence profiles in the longitudinal direction suggests largely intact T tubule structures in cultured cells (Fig. 6).
Fig. 6.
Subcellular distribution of SK channels in relation to L-type Ca2+ channels and ryanodine receptors (RyR2s). A: confocal images of representative control (left) and SK (right) transduced myocytes labeled with anti-SK2 (green) and anti-CaV1.2 (red) antibodies. Bottom: plots of fluorescence profiles corresponding to the areas and directions indicated by white boxes and arrows in the images above. B: myocytes were labeled, imaged, and analyzed as in A except that anti-RyR2 antibody was used instead of anti-CaV1.2. AU, arbitrary units.
DISCUSSION
SK channels have recently emerged as a novel cardiac channel type with important functions in ventricular electrophysiology and arrhythmogenesis, particularly in HF (10, 12, 23). Investigation of the activation mechanism and cellular function of SK channels in cardiomyocytes is needed to understand thoroughly their pro- or anti-arrhythmic roles. In the present study, we used the experimental system of adult rat ventricular myocytes with adenovirally overexpressed SK2 channels to investigate their role in the regulation of repolarization and arrhythmogenic DADs induced by spontaneous Ca2+ waves (SCWs). Our main findings are 1) that RyR2-mediated SR Ca2+ release is both necessary and sufficient for the activation of repolarizing SK currents and 2) that enhanced expression of SK channels reduces the amplitudes of pro-arrhythmic DADs in response to SCWs. Our results suggest that upregulation of SK channels may present an adaptive mechanism to attenuate arrhythmogenic Ca2+-dependent disturbances in membrane potential in conditions associated with aberrant Ca2+ homeostasis.
Functional expression of SK channels in ventricular myocytes.
Studies with rabbit and canine HF models (TICM) as well as human patients demonstrated that inhibition of upregulated SK channels with apamin prolongs APD at the whole heart level and that apamin-sensitive ISK can be directly recorded in ventricular myocytes from failing hearts (10, 12, 14, 23). In contrast, APD and currents in rat and dog ventricular myocytes from healthy hearts were found insensitive to apamin despite the immunodetection of SK channels (33). Until now it is not clear why SK channels in healthy ventricular myocytes were not activated or contribute to repolarization. Our experiments with control rat ventricular myocytes confirmed the previous finding (33), with little effect of apamin on either outward currents or APD (Figs. 1 and 5C), despite the immunodetection of endogenous SK channels (Figs. 1D and 6). In contrast, voltage-clamp experiments showed that overexpressed SK channels are efficiently activated by intracellular Ca2+ elevation (Figs. 2, 3, and 4). In addition, current clamp experiments showed that SK currents following overexpression contribute to repolarization and shorten APD (Fig. 5C). Immunolocalization experiments demonstrate that the expression pattern of overexpressed SK channels resembles that of endogenous channels, suggesting that adenovirus-mediated overexpression does not significantly affect trafficking and subcellular distribution of SK channels and that in vitro overexpression in ARCMs is a physiologically relevant mimic of the in vivo SK upregulation observed in HF. Together with the large difference in protein levels revealed by Western blot between control and SK transduced myocytes, these data suggest that the lack of SK currents and contribution to repolarization in control ventricular myocytes is because SK expression level is too low for functional measurements.
Ca2+ sources for SK activation in ventricular myocytes.
Subcellular distribution of SK channels and their colocalization with Ca2+ sources have important implications for their activation and specific functions. Elevations of intracellular [Ca2+] in CMs are primarily conferred by two key proteins, plasmalemmal LTCCs and RyR2s predominantly localized on the membranes of junctional SR (8). Previous studies in mice revealed that SK channels closely colocalize with, and structurally couple to, CaV1.3, an LTCC isoform primarily expressed in atrial myocytes, but to a much lesser degree with CaV1.2, the predominant isoform in ventricular myocytes (32). Our functional data show that Ca2+ influx through LTCCs alone is not sufficient to activate SK channels (Figs. 2 and 3), also arguing against a close coupling between SK and CaV1.2 channels in ventricular myocytes. In consistence, our immunocytochemistry experiments showed that a larger fraction of SK channels is expressed on the external sarcolemma in contrast with LTCCs and RyR2s (Fig. 6). Based on these experiments it is reasonable to conclude that SK channels in ventricular myocytes respond to global changes in intracellular [Ca2+] rather than local changes in the vicinity of specific Ca2+ sources. This conclusion is consistent with the micromolar Ca2+ affinity of SK channels (1) and supported directly by the functional correlation between SK currents and our measurements of global [Ca2+] (Figs. 2, 3, and 4).
It is known that influx through LTCCs contributes a larger fraction to the global Ca2+ elevation during Ca2+ transients in ventricular myocytes from large animals (e.g., rabbits and dogs) and humans than in those from rats (∼30% vs. <10%) (8). Consequently, it is possible that opening of LTCCs in the absence of SR Ca2+ release can activate some SK channels in ventricular myocytes from large animals in contrast with rats, even if there is no direct functional coupling between SK and LTCCs, as suggested by our present study. Nonetheless, because SR Ca2+ release represents the primary source of Ca2+ elevation in ventricular myocytes from all species, we expect the observed SK activation by SCWs and its effect on DADs to be preserved in large animals and human.
Anti/pro-arrhythmic roles of SK channels in HF.
Previous studies on SK channels in ventricular myocytes were primarily focused on their potential pro-arrhythmic effects related to shortened APD, steepened APD restitution, and promotion of late phase 3 EADs (14, 23). We showed in the present study that overexpression of SK channels indeed shortens APD but did not observe evidence of late phase 3 EADs. Arrhythmogenic effect of SK channels is apparently complex and has remained a matter of debate (21, 23, 29). Anti-arrhythmic effects of SK channels in failing ventricles have been recently suggested with optical mapping of the whole hearts (11, 12, 23) but not directly investigated at the cellular level. Our study has provided the first direct cellular evidence for an anti-arrhythmic mechanism of SK channels.
Enhanced NCX1 function and hyperactive RyR2s in HF are thought to promote arrhythmogenic DADs induced by SCWs (2, 16, 22, 25, 27, 35, 37, 41). Recent discovery of SK upregulation in human patients and animal models of HF (10, 12, 14, 23) raises the possibility that SK channels may influence the amplitude of DADs by antagonizing depolarizing NCX1 current. This hypothesis was not directly tested in previous studies of cardiac SK channels. In this study we used overexpressed channels in cultured myocytes to demonstrate that indeed SK channels can attenuate DADs via their activation by SCWs. Our findings suggest that upregulation of SK channels in HF may serve to effectively decouple the link between SCWs and DADs, thus attenuating triggered activity and arrhythmias. With a dog HF model (TICM) we recently discovered an intriguing decoupling between SCWs and diastolic membrane potential changes at late stages of HF (6). Coincidentally, SK channels were found upregulated in ventricles from the same animal model (10). It is therefore tempting to hypothesize that SK channel activity contributes to this decoupling phenomenon.
Therefore, a balanced view of the arrhythmogenic involvement of SK channels in HF has started to emerge in which they can be both pro- and anti-arrhythmic. Our characterization of the activation and function of SK channels in ARCMs will facilitate the further elucidation of the roles and mechanisms by which they contribute to arrhythmogenesis.
Summary.
In conclusion, our data demonstrate that SK channels in ventricular myocytes are not functionally coupled to LTCCs, but rather respond to global increase in [Ca2+] largely attributable to Ca2+ release from SR. We directly demonstrate for the first time that upregulation of SK channels provides an efficient mechanism to couple RyR2-mediated SR Ca2+ release and repolarization to oppose the depolarizing force of NCX1 and thus reduce arrhythmogenic oscillations of membrane potential. Our findings establishing the anti-arrhythmic capacity of SK channels have important implications for the ongoing efforts in pursuing SK channels as a target for treatment of cardiac rhythm disorders.
Limitations.
SK channels were adenovirally overexpressed in cardiomyocytes to directly test their effect on DADs. The average apamin-sensitive current level in our overexpression is about three times as high as the reported level of SK upregulation in rabbit HF model and human patients (12, 14). Additionally, it is well known that many ionic currents and Ca2+-handling proteins are remodeled in HF. Therefore, the quantitative effect of SK channels on the amplitudes of DADs remains to be determined in the actual context of HF. However, our experiments have provided the proof of principle that SK channels expressed in ventricular myocytes can indeed respond to SCWs and attenuate DADs. Given the pronounced effect of SK channels in our overexpression system, we expect qualitatively similar findings in myocytes from failing hearts. The well-established experimental system of cultured ARCM (4) provides a physiologically appropriate environment to study the activation and function of SK channels in ventricular myocytes. Although our experiments with immunocytochemistry, patch clamp, and Ca2+ imaging all suggest that structural, electrical, and Ca2+ handling features are well preserved in the culture, potential remodeling resulting from culturing needs to be considered when extrapolating our findings to the in vivo system.
GRANTS
D. Terentyev is supported by a Rhode Island Foundation Grant. J. A. Rochira and W. Li are supported by the National Heart, Lung, and Blood Institute Grant T32-HL094300-03. G. Koren is supported by the National Heart, Lung, and Blood Institute Grants R01-HL-046005-18 and R01-HL-093205-01.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: D.T., G.K., and W.L. conception and design of research; D.T., J.A.R., R.T., K.R., and W.L. performed experiments; D.T., R.T., and W.L. analyzed data; D.T., R.T., and W.L. interpreted results of experiments; D.T. and W.L. prepared figures; D.T. and W.L. drafted manuscript; D.T., J.A.R., K.R., G.K., and W.L. edited and revised manuscript; D.T., J.A.R., R.T., K.R., G.K., and W.L. approved final version of manuscript.
ACKNOWLEDGMENT
We thank Dr. Richard Clements for critically reading the manuscript.
REFERENCES
- 1.Adelman JP, Maylie J, Sah P. Small-conductance Ca2+-activated K+ channels: form and function. Annu Rev Physiol 74: 245–269, 2012 [DOI] [PubMed] [Google Scholar]
- 2.Aiba T, Tomaselli GF. Electrical remodeling in the failing heart. Curr Opin Cardiol 25: 29–36, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antoons G, Willems R, Sipido KR. Alternative strategies in arrhythmia therapy: evaluation of Na/Ca exchange as an anti-arrhythmic target. Pharmacol Ther 134: 26–42, 2012 [DOI] [PubMed] [Google Scholar]
- 4.Banyasz T, Lozinskiy I, Payne CE, Edelmann S, Norton B, Chen B, Chen-Izu Y, Izu LT, Balke CW. Transformation of adult rat cardiac myocytes in primary culture. Exp Physiol 93: 370–382, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Belevych AE, Sansom SE, Terentyeva R, Ho HT, Nishijima Y, Martin MM, Jindal HK, Rochira JA, Kunitomo Y, Abdellatif M, Carnes CA, Elton TS, Gyorke S, Terentyev D. MicroRNA-1 and -133 increase arrhythmogenesis in heart failure by dissociating phosphatase activity from RyR2 complex. PLoS One 6: e28324, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belevych AE, Terentyev D, Terentyeva R, Nishijima Y, Sridhar A, Hamlin RL, Carnes CA, Gyorke S. The relationship between arrhythmogenesis and impaired contractility in heart failure: role of altered ryanodine receptor function. Cardiovasc Res 90: 493–502, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bers DM. Calcium cycling and signaling in cardiac myocytes. Annu Rev Physiol 70: 23–49, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Bers DM. Excitation-Contraction Coupling and Cardiac Contractile Force. Dordrecht: Kiuwer Academic Publishers, 2001 [Google Scholar]
- 9.Bkaily G, Sculptoreanu A, Jacques D, Economos D, Menard D. Apamin, a highly potent fetal L-type Ca2+ current blocker in single heart cells. Am J Physiol Heart Circ Physiol 262: H463–H471, 1992 [DOI] [PubMed] [Google Scholar]
- 10.Bonilla IM, Long VL, Vargas-Pinto P, Green J, Yoo J, Allen D, Wright P, Mohler PJ, Carnes CA. Calcium-activated potassium current modulates ventricular (but not atrial) repolarization in chronic heart failure. Circulation 126: A16846, 2012 [Google Scholar]
- 11.Chang PC, Hsieh YC, Hsueh CH, Weiss JN, Lin SF, Chen PS. Apamin induces early afterdepolarizations and torsades de pointes ventricular arrhythmia from failing rabbit ventricles exhibiting secondary rises in intracellular calcium. Heart Rhythm 10: 1516–1524, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang PC, Turker I, Lopshire JC, Masroor S, Nguyen BL, Tao W, Rubart M, Chen PS, Chen Z, Ai T. Heterogeneous upregulation of apamin-sensitive potassium currents in failing human ventricles. J Am Heart Assoc 2: e004713, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen-Izu Y, McCulle SL, Ward CW, Soeller C, Allen BM, Rabang C, Cannell MB, Balke CW, Izu LT. Three-dimensional distribution of ryanodine receptor clusters in cardiac myocytes. Biophys J 91: 1–13, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chua SK, Chang PC, Maruyama M, Turker I, Shinohara T, Shen MJ, Chen Z, Shen C, Rubart-von der Lohe M, Lopshire JC, Ogawa M, Weiss JN, Lin SF, Ai T, Chen PS. Small-conductance calcium-activated potassium channel and recurrent ventricular fibrillation in failing rabbit ventricles. Circ Res 108: 971–979, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crossman DJ, Ruygrok PN, Soeller C, Cannell MB. Changes in the organization of excitation-contraction coupling structures in failing human heart. PLoS One 6: e17901, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cutler MJ, Rosenbaum DS, Dunlap ME. Structural and electrical remodeling as therapeutic targets in heart failure. J Electrocardiol 40: S1–S7, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Diness JG, Sorensen US, Nissen JD, Al-Shahib B, Jespersen T, Grunnet M, Hansen RS. Inhibition of small-conductance Ca2+-activated K+ channels terminates and protects against atrial fibrillation. Circ Arrhythm Electrophysiol 3: 380–390, 2010 [DOI] [PubMed] [Google Scholar]
- 18.Ellinor PT, Lunetta KL, Glazer NL, Pfeufer A, Alonso A, Chung MK, Sinner MF, de Bakker PI, Mueller M, Lubitz SA, Fox E, Darbar D, Smith NL, Smith JD, Schnabel RB, Soliman EZ, Rice KM, Van Wagoner DR, Beckmann BM, van Noord C, Wang K, Ehret GB, Rotter JI, Hazen SL, Steinbeck G, Smith AV, Launer LJ, Harris TB, Makino S, Nelis M, Milan DJ, Perz S, Esko T, Kottgen A, Moebus S, Newton-Cheh C, Li M, Mohlenkamp S, Wang TJ, Kao WH, Vasan RS, Nothen MM, MacRae CA, Stricker BH, Hofman A, Uitterlinden AG, Levy D, Boerwinkle E, Metspalu A, Topol EJ, Chakravarti A, Gudnason V, Psaty BM, Roden DM, Meitinger T, Wichmann HE, Witteman JC, Barnard J, Arking DE, Benjamin EJ, Heckbert SR, Kaab S. Common variants in KCNN3 are associated with lone atrial fibrillation. Nat Genet 42: 240–244, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Escobar AL, Velez P, Kim AM, Cifuentes F, Fill M, Vergara JL. Kinetic properties of DM-nitrophen and calcium indicators: rapid transient response to flash photolysis. Pflügers Arch 434: 615–631, 1997 [DOI] [PubMed] [Google Scholar]
- 20.Fakler B, Adelman JP. Control of KCa channels by calcium nano/microdomains. Neuron 59: 873–881, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Grunnet M, Bentzen BH, Sorensen US, Diness JG. Cardiac ion channels and mechanisms for protection against atrial fibrillation. Rev Physiol Biochem Pharmacol 162: 1–58, 2012 [DOI] [PubMed] [Google Scholar]
- 22.Hasenfuss G, Pieske B. Calcium cycling in congestive heart failure. J Mol Cell Cardiol 34: 951–969, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Hsieh YC, Chang PC, Hsueh CH, Lee YS, Shen C, Weiss JN, Chen Z, Ai T, Lin SF, Chen PS. Apamin-sensitive potassium current modulates action potential duration restitution and arrhythmogenesis of failing rabbit ventricles. Circ Arrhythm Electrophysiol 6: 410–418, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hsueh CH, Chang PC, Hsieh YC, Reher T, Chen PS, Lin SF. Proarrhythmic effect of blocking the small conductance calcium activated potassium channel in isolated canine left atrium. Heart Rhythm 10: 891–898, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Janse MJ. Electrophysiological changes in heart failure and their relationship to arrhythmogenesis. Cardiovasc Res 61: 208–217, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Kohler M, Hirschberg B, Bond CT, Kinzie JM, Marrion NV, Maylie J, Adelman JP. Small-conductance, calcium-activated potassium channels from mammalian brain. Science 273: 1709–1714, 1996 [DOI] [PubMed] [Google Scholar]
- 27.Laurita KR, Rosenbaum DS. Mechanisms and potential therapeutic targets for ventricular arrhythmias associated with impaired cardiac calcium cycling. J Mol Cell Cardiol 44: 31–43, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leroy J, Richter W, Mika D, Castro LR, Abi-Gerges A, Xie M, Scheitrum C, Lefebvre F, Schittl J, Mateo P, Westenbroek R, Catterall WA, Charpentier F, Conti M, Fischmeister R, Vandecasteele G. Phosphodiesterase 4B in the cardiac L-type Ca2+ channel complex regulates Ca2+ current and protects against ventricular arrhythmias in mice. J Clin Invest 121: 2651–2661, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li N, Timofeyev V, Tuteja D, Xu D, Lu L, Zhang Q, Zhang Z, Singapuri A, Albert TR, Rajagopal AV, Bond CT, Periasamy M, Adelman J, Chiamvimonvat N. Ablation of a Ca2+-activated K+ channel (SK2 channel) results in action potential prolongation in atrial myocytes and atrial fibrillation. J Physiol 587: 1087–1100, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li W, Aldrich RW. Activation of the SK potassium channel-calmodulin complex by nanomolar concentrations of terbium. Proc Natl Acad Sci USA 106: 1075–1080, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li W, Halling DB, Hall AW, Aldrich RW. EF hands at the N-lobe of calmodulin are required for both SK channel gating and stable SK-calmodulin interaction. J Gen Physiol 134: 281–293, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu L, Zhang Q, Timofeyev V, Zhang Z, Young JN, Shin HS, Knowlton AA, Chiamvimonvat N. Molecular coupling of a Ca2+-activated K+ channel to L-type Ca2+ channels via alpha-actinin2. Circ Res 100: 112–120, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Nagy N, Szuts V, Horvath Z, Seprenyi G, Farkas AS, Acsai K, Prorok J, Bitay M, Kun A, Pataricza J, Papp JG, Nanasi PP, Varro A, Toth A. Does small-conductance calcium-activated potassium channel contribute to cardiac repolarization? J Mol Cell Cardiol 47: 656–663, 2009 [DOI] [PubMed] [Google Scholar]
- 34.Ozgen N, Dun W, Sosunov EA, Anyukhovsky EP, Hirose M, Duffy HS, Boyden PA, Rosen MR. Early electrical remodeling in rabbit pulmonary vein results from trafficking of intracellular SK2 channels to membrane sites. Cardiovasc Res 75: 758–769, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pogwizd SM, Bers DM. Cellular basis of triggered arrhythmias in heart failure. Trends Cardiovasc Med 14: 61–66, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Skibsbye L, Diness JG, Sorensen US, Hansen RS, Grunnet M. The duration of pacing-induced atrial fibrillation is reduced in vivo by inhibition of small conductance Ca2+-activated K+ channels. J Cardiovasc Pharmacol 57: 672–681, 2011 [DOI] [PubMed] [Google Scholar]
- 37.Ter Keurs HE, Boyden PA. Calcium and arrhythmogenesis. Physiol Rev 87: 457–506, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trafford AW, Diaz ME, Eisner DA. Ca-activated chloride current and Na-Ca exchange have different timecourses during sarcoplasmic reticulum Ca release in ferret ventricular myocytes. Pflügers Arch 435: 743–745, 1998 [DOI] [PubMed] [Google Scholar]
- 39.Tuteja D, Xu D, Timofeyev V, Lu L, Sharma D, Zhang Z, Xu Y, Nie L, Vazquez AE, Young JN, Glatter KA, Chiamvimonvat N. Differential expression of small-conductance Ca2+-activated K+ channels SK1, SK2, and SK3 in mouse atrial and ventricular myocytes. Am J Physiol Heart Circ Physiol 289: H2714–H2723, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Xia XM, Fakler B, Rivard A, Wayman G, Johnson-Pais T, Keen JE, Ishii T, Hirschberg B, Bond CT, Lutsenko S, Maylie J, Adelman JP. Mechanism of calcium gating in small-conductance calcium-activated potassium channels. Nature 395: 503–507, 1998 [DOI] [PubMed] [Google Scholar]
- 41.Xie LH, Weiss JN. Arrhythmogenic consequences of intracellular calcium waves. Am J Physiol Heart Circ Physiol 297: H997–H1002, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu Y, Tuteja D, Zhang Z, Xu D, Zhang Y, Rodriguez J, Nie L, Tuxson HR, Young JN, Glatter KA, Vazquez AE, Yamoah EN, Chiamvimonvat N. Molecular identification and functional roles of a Ca2+-activated K+ channel in human and mouse hearts. J Biol Chem 278: 49085–49094, 2003 [DOI] [PubMed] [Google Scholar]
- 43.Zygmunt AC. Intracellular calcium activates a chloride current in canine ventricular myocytes. Am J Physiol Heart Circ Physiol 267: H1984–H1995, 1994 [DOI] [PubMed] [Google Scholar]