Abstract
Chronic heart failure (CHF) impairs nitric oxide (NO)-mediated regulation of skeletal muscle O2 delivery-utilization matching such that microvascular oxygenation falls faster (i.e., speeds PO2mv kinetics) during increases in metabolic demand. Conversely, exercise training improves (slows) muscle PO2mv kinetics following contractions onset in healthy young individuals via NO-dependent mechanisms. We tested the hypothesis that exercise training would improve contracting muscle microvascular oxygenation in CHF rats partly via improved NO-mediated function. CHF rats (left ventricular end-diastolic pressure = 17 ± 2 mmHg) were assigned to sedentary (n = 11) or progressive treadmill exercise training (n = 11; 5 days/wk, 6–8 wk, final workload of 60 min/day at 35 m/min; −14% grade downhill running) groups. PO2mv was measured via phosphorescence quenching in the spinotrapezius muscle at rest and during 1-Hz twitch contractions under control (Krebs-Henseleit solution), sodium nitroprusside (SNP; NO donor; 300 μM), and NG-nitro-l-arginine methyl ester (L-NAME, nonspecific NO synthase blockade; 1.5 mM) superfusion conditions. Exercise-trained CHF rats had greater peak oxygen uptake and spinotrapezius muscle citrate synthase activity than their sedentary counterparts (p < 0.05 for both). The overall speed of the PO2mv fall during contractions (mean response time; MRT) was slowed markedly in trained compared with sedentary CHF rats (sedentary: 20.8 ± 1.4, trained: 32.3 ± 3.0 s; p < 0.05), and the effect was not abolished by L-NAME (sedentary: 16.8 ± 1.5, trained: 31.0 ± 3.4 s; p > 0.05). Relative to control, SNP increased MRT in both groups such that trained CHF rats had slower kinetics (sedentary: 43.0 ± 6.8, trained: 55.5 ± 7.8 s; p < 0.05). Improved NO-mediated function is not obligatory for training-induced improvements in skeletal muscle microvascular oxygenation (slowed PO2mv kinetics) following contractions onset in rats with CHF.
Keywords: kinetics, microcirculation, myocardial infarction, oxygen delivery, oxygen utilization
chronic heart failure (CHF) induces multiple alterations in cardiovascular structure and function that culminate in reduced exercise capacity and poor prognosis (8, 51, 52, 54). Derangements in the skeletal muscle O2 transport pathway are major features of this disease (54). Within the microcirculation, impaired hemodynamic control (35, 56) compromises the dynamic matching between O2 delivery and utilization (Q̇O2 and V̇o2, respectively) and reduces muscle microvascular oxygenation (PO2mv) during transitions in metabolic demand (3, 11, 14, 19, 45). As dictated by Fick's law of diffusion, lower PO2mv reduces the driving pressure for blood-myocyte O2 flux and impairs oxidative metabolism and contractile performance (29, 64).
Impaired nitric oxide (NO)-mediated function is a hallmark of CHF (16, 32, 37). Notwithstanding the plethora of factors modulating muscle blood flow during exercise (including neural, mechanical, and humoral components), deterioration of NO-mediated function plays a key role in the blunted functional hyperemic response characteristic of CHF (28, 34). Accordingly, these perturbations contribute to the pathological PO2mv profiles (characterized by lower PO2mv and faster PO2mv fall; i.e., speeded kinetics) during muscle contractions in CHF (19). Endurance exercise training, on the other hand, is a nonpharmacological intervention capable of improving NO-mediated function in skeletal muscle of both healthy (21, 43) and CHF (24, 40, 66) individuals. Recent evidence indicates that exercise training improves muscle PO2mv (resulting in higher PO2mv and slower PO2mv kinetics) during muscle contractions in healthy young rats partly via NO-dependent mechanisms (26). Whether similar improvements in contracting muscle PO2mv occur, and, if so, whether they are mediated via enhanced NO signaling in CHF is unknown. Given the role of skeletal muscle dysfunction in the pathophysiology and limiting symptoms of CHF (8, 51, 52, 54) and the importance of PO2mv in setting the driving force for blood-myocyte O2 flux and metabolic control, a better understanding of the mechanistic basis for improving contracting muscle PO2mv with exercise training in CHF is imperative to the design of effective therapeutic treatments for this disease.
The purpose of the present investigation was to determine the effects of endurance exercise training on contracting muscle PO2mv and the contribution of NO to these responses in rats with matched central indexes of CHF. Based on the potential improvement in NO-mediated function following exercise training in CHF (24, 40, 66), we tested the hypotheses that 1) exercise training would elevate muscle PO2mv and slow PO2mv kinetics (i.e., resulting in higher microvascular oxygenation across the on-contractions transient); 2) increased NO levels [via the NO donor sodium nitroprusside (SNP)] would elevate PO2mv and slow PO2mv kinetics to a greater extent in sedentary compared with trained CHF rats; and 3) reduced NO levels [nonspecific NO synthase inhibition with NG-nitro-l-arginine methyl ester (l-NAME)] would lower PO2mv and speed PO2mv kinetics to a greater extent in trained compared with sedentary CHF rats during the transition from rest to contractions.
MATERIALS AND METHODS
A total of 26 male Sprague-Dawley rats (body mass: 346 ± 6 g; ∼2 to 3 mo old; Charles Rivers Laboratories, Boston, MA) were used in the present investigation. Rats were maintained on a 12-h:12-h light/dark cycle with food and water provided ad libitum. All experimental procedures followed guidelines established by the National Institutes of Health and were approved by the Institutional Animal Care and Use Committee of Kansas State University.
Myocardial infarction was induced in all rats via ligation of the left main coronary artery as described in detail previously (14, 48–50). Briefly, rats were anesthetized with 5% isoflurane-O2 mixture (Butler Animal Health Supply, Dublin, OH) and intubated for mechanical ventilation with a rodent respirator (model 680; Harvard Instruments, Holliston, MA) for the duration of the surgical procedure. The heart was accessed through a left thoracotomy in the fifth intercostal space and the left main coronary artery was ligated (6-0 silk suture) at ∼1 to 2 mm distal to the edge of the left atrium. The incision was then closed, and ampicillin (50 mg/kg im) was injected locally to reduce the opportunity for infection. The analgesic agents bupivacaine (1.5 mg/kg sc) and buprenorphine (0.01–0.05 mg/kg im) were administered subsequently and anesthesia and mechanical ventilation discontinued. All rats were monitored closely for ≥6 h for the development of arrhythmias and signs of undue stress (e.g., labored breathing). Rats were also monitored daily (e.g., appetite, weight loss/gain, gait, and posture) according to an intensive 10-day post-operative plan conducted in association with the university veterinary staff. The survival rate for the rats receiving the myocardial infarction operation was 88% (23 out of 26). At the end of the experimental protocol, one rat demonstrated no discernable damage to the left ventricle along with no changes in cardiac morphology or hemodynamic function and was therefore not included in the data analyses. Accordingly, results from the present investigation are presented below for a total of 22 animals. Each animal was allowed ≥3 wk of recovery before initiation of any experimental procedure. Previous reports support that this rat myocardial infarction model induces central and peripheral vascular and microvascular dysfunction consonant with CHF (10, 48–50).
Rats were then familiarized with downhill running on a custom-built motor-driven treadmill over the course of a 1-wk period (5–10 min/day at a speed of 20 m/min and −14% grade). After the familiarization phase, rats were assigned randomly to either sedentary (CHF; n = 11) or endurance exercise trained (CHF + EXT; n = 11) groups. Sedentary rats were confined to cage activities, whereas trained rats ran 5 days/wk over 6 to 7 wk on the declined treadmill (−14% grade). All rats from the CHF + EXT group underwent the same training protocol, in which treadmill running duration and speed were increased progressively from 10 min at 25 m/min to 60 min at 35 m/min. This final workload was maintained for the final 3 to 4 wk. Downhill treadmill running was selected for the present investigation given that both level and uphill treadmill running do not recruit the rat spinotrapezius muscle (31). Previous work from our laboratory has demonstrated that downhill treadmill running specifically trains the rat spinotrapezius muscle and constitutes an effective model for whole-body exercise training (23, 26). Of note, downhill running as a model of exercise training requires faster treadmill speeds than conventional level or uphill running regimens to elicit equivalent training adaptations in rats with a similar degree of left ventricular dysfunction and CHF (48). CHF and CHF + EXT rats underwent subsequent experimental procedures (described below) after ∼5 and 10 wk from the myocardial infarction surgery, respectively. This experimental design was used herein to match central cardiac function in an attempt to isolate peripheral vascular adaptations.
Peak oxygen uptake measurements.
Peak oxygen uptake (V̇o2peak) was measured in CHF and CHF + EXT rats during a downhill (−14% grade) running test performed in a metabolic chamber placed on the treadmill. As described previously (9, 23, 26), the speed was set initially to 25 m/min for 2 to 3 min and then increased progressively in a ramplike fashion by ∼5–10 m/min until the rat was unable to keep pace with the treadmill belt or no further elevations in V̇o2 were observed despite continued increases in treadmill speed. At this point of the test the V̇o2peak was measured and recorded. Alterations in gait (e.g., lowering of the hindlimbs, dropping of the tail, and elevation of the snout) normally occurred immediately before termination of the test. Gas measurements were performed in real time via an inline O2 analyzer (model S-3A/I; AEI Technologies; Pittsburgh, PA). The analyzer was calibrated with precision-mixed gases that spanned the expected range of gas concentrations based on previous studies. We have reported previously highly reproducible V̇o2peak measurements using the aforementioned techniques and protocol (9).
Surgical preparation.
Rats were anesthetized initially with a 5% isoflurane-O2 mixture and maintained on 2% to 3% isoflurane-O2 and placed on a heating pad to maintain core temperature at ∼37° to 38°C as measured via rectal probe. The right carotid artery was cannulated, and a 2-Fr catheter-tipped pressure transducer (Millar Instruments; Houston, TX) was advanced into the left ventricle for measurement of systolic and diastolic pressures and left ventricular Δpressure/Δtime (LV dp/dt). Upon completion of these measurements, the transducer was removed and the carotid artery was recannulated with a catheter (PE-10 connected to PE-50; Intra-Medic Tubing; Clay Adams Brand, Sparks, MD) for continuous monitoring of mean arterial pressure (MAP; Digi-Med BPA model 200; Louisville, KY) and infusion of the phosphorescent probe palladium meso-tetra-(4-carboxyphenyl)porphyrin dendrimer (R2; 15 mg/kg; Oxygen Enterprises, Philadelphia, PA). The tail (caudal) artery was cannulated (PE-10 connected to PE-50) for blood sampling and infusion of anesthetic agents. Blood from the tail catheter was sampled at the end of the experimental protocol for determination of arterial gases, pH, and systemic hematocrit (Nova Stat Profile M; Waltham, MA).
Isoflurane-O2 mixture inhalation was discontinued progressively after catheter placement procedures, and rats were kept under anesthesia with pentobarbital sodium administered intra-arterially to effect. The level of anesthesia was monitored frequently via the blink and toe-pinch reflexes and supplemented as necessary. Overlying skin and fascia from the middorsal region of the rat were reflected surgically to expose the right spinotrapezius muscle. The spinotrapezius muscle exhibits a mixed fiber-type composition and oxidative capacity (13) that resemble closely the human quadriceps (38), thus representing a useful analog of human locomotor muscle. The muscle was moistened frequently throughout the surgery and experimental protocol via superfusion of Krebs-Henseleit bicarbonate-buffered solution, consisting of (in mM) 4.7 KCl, 2.0 CaCl2, 2.4 MgSO4, 131 NaCl, and 22 NaHCO3, equilibrated with 5% CO2 and 95% N2 (pH 7.4, warmed to 37° to 38°C). Surrounding tissue was covered with Saran wrap (Dow Brands, Indianapolis, IN). Stainless steel electrodes were sutured to the rostral (cathode) and caudal (anode) regions of the spinotrapezius muscle for electrically induced twitch contractions. A previous report from our laboratory has demonstrated that these surgical procedures do not impact the microvascular integrity and responsiveness of the rat spinotrapezius muscle (1).
Experimental protocol.
Three distinct contraction bouts were performed under control (5 ml Krebs-Henseleit), SNP (NO donor; 5 ml of a 300 μM solution), and l-NAME (nonisoform-specific NO synthase inhibitor; 5 ml of a 1.5-mM solution) superfusion conditions. Drugs were purchased from Sigma-Aldrich (St. Louis, MO), and concentrations were chosen based on previous studies from our laboratory (19, 20, 26, 27). All solutions were maintained at ∼37° to 38°C. Although superfusion order was randomized between control and SNP conditions, l-NAME was the last treatment due to its relatively long half-life. Importantly, a recent report from our laboratory demonstrated that the superfusion protocol used herein (see below) does not exert residual (i.e., post-washout) SNP alteration and/or impairment of skeletal muscle blood flow, V̇o2 or PO2mv responses either at rest or during contractions (27). The spinotrapezius was superfused with each solution (average flow rate of ∼1.5 ml/min) for a total time of 3 min, followed by ∼3 min incubation period to allow resting muscle PO2mv to stabilize. Subsequently, electrical stimulation (1 Hz, ∼7 V, 2-ms pulse duration) of the muscle was evoked via a stimulator (model s48; Grass Technologies, Quincy, MA) for 3 min. This stimulation protocol evokes an approximately four- to fivefold increase in blood flow together with approximately six- to sevenfold increase in metabolic rate (which corresponds to ∼30% spinotrapeziusV̇o2peak) above resting with either minor or no alterations in blood pH that are consistent with moderate intensity exercise (4, 27). The muscle was then allowed to recover for ∼25 min before the next condition was initiated (stimulation parameters were held constant). During the recovery period following the SNP condition, the muscle was superfused at an average flow rate of ∼1.5 ml/min with Krebs-Henseleit to wash out SNP. A >30-min recovery period between consecutive bouts (i.e., 3 min off-transition, ∼25 min passive recovery, 3 min superfusion, ∼3 min incubation) was used herein to prevent any priming and drug-ordering effects that could confound the experimental interpretation of the PO2mv responses to muscle contractions (5, 20). We have reported previously that the spinotrapezius preparation exhibits reproducible PO2mv responses from three contraction bouts separated by ∼30 min (26). At the end of the experimental protocol, rats were euthanized with intra-arterial pentobarbital sodium overdose (>50 mg/kg). For each rat, the lungs and heart were dissected, weighed, and normalized to body mass. Myocardial infarct size was determined via planimetry as described in detail previously (19).
Spinotrapezius muscle PO2mv measurement.
PO2mv was measured by phosphorescence quenching using a frequency domain phosphorometer (PMOD 5000; Oxygen Enterprises, Philadelphia, PA). As described in detail previously (4), this technique applies the Stern-Volmer relationship (59), which describes quantitatively the O2 dependence of the phosphorescent probe (R2) via the following equation:
where kQ is the quenching constant and τ and τ° are the phosphorescence lifetimes in the absence of O2 and the ambient O2 concentration, respectively. The phosphor R2 (τ° = 601 μs and kQ = 409 mmHg−1/s at pH 7.4 and temperature ∼38°C) (41) was infused ∼15 min before initiation of the first muscle contraction. R2 contains Pd-porphyrin cores that bind to biological macromolecules (primarily albumin in blood plasma) (67). This facilitates its uniform distribution in the plasma and provides a signal corresponding to the volume-weighed O2 pressure in the microvascular compartment (primarily the PO2 within capillaries, which volumetrically constitutes the major intramuscular space) (53). Restriction of the R2 probe to the microvascular space is also promoted by its negative charge (55). The common end of the bifurcated light guide was positioned ∼2–4 mm superficial to the dorsal surface of the exposed spinotrapezius muscle. The phosphorometer modulates sinusoidal excitation frequencies between 100 Hz and 20 kHz and allows phosphorescence lifetime measurements from 10 μs to ∼2.5 ms. The excitation light (524 nm) was focused on a randomly selected area of ∼2 mm diameter of exposed muscle and has a penetration depth of ∼500 μm. PO2mv was measured continuously and recorded at 2-s intervals throughout the duration of the experimental protocol.
Analysis of muscle PO2mv kinetics.
The kinetics of PO2mv were described by nonlinear regression analysis using the Marquardt-Levenberg algorithm (SigmaPlot 11.2; Systat software, San Jose, CA) for the onset of contractions. Transient PO2mv responses were fit with either a one- or two-component model as follows.
One-component:
Two-component:
where PO2mv(t) is the PO2mv at a given time t; PO2mv(BL) corresponds to the precontracting resting PO2mv; Δ1 and Δ2 are the amplitudes for the first and second components, respectively; TD1 and TD2 are the independent time delays for each component; and τ1 and τ2 are the time constants (i.e., time to 63% of the response) for each component. Goodness of fit was determined using three criteria: the coefficient of determination, sum of squared residuals, and visual inspection.
The mean response time (MRT) was used to describe the overall dynamics of the PO2mv fall following the onset of muscle contractions:
where TD and τ are defined above. The MRT analysis was limited to the first component of the PO2mv response given that inclusion of an emergent second component underestimates the actual speed of the PO2mv fall for the onset of contractions (26).
Movement of the light guide and/or animal was avoided to monitor the same sampling site in a given animal throughout all experimental conditions. However, alteration of the PO2mv measurement plane (e.g., due to deep sighs of the animal, etc.) during muscle contractions precluded kinetic curve fitting in some instances. Therefore, PO2mv kinetics from the present investigation are given for the following animal numbers under the specified conditions: CHF control (10/11), CHF SNP (10/11), CHF l-NAME (10/11), CHF + EXT control (11/11), CHF + EXT SNP (7/11) and CHF + EXT l-NAME (11/11).
Citrate synthase measurement.
The activity of the mitochondrial enzyme citrate synthase (a marker of oxidative capacity) from the spinotrapezius and select individual hindlimb muscles or muscle parts (soleus, red gastrocnemius, and white gastrocnemius) was measured in duplicate from muscle homogenates by a modification of the method described by Srere (62). Upon termination of the experimental protocol and euthanasia, muscles were removed, dissected free of connective tissue, and weighed. Citrate synthase activity was measured spectrophotometrically (Spectramax M5 microplate; Molecular Devices, Sunnyvale, CA) in 300 μl aliquots at 30°C.
Statistical analysis.
Data comparison was performed using unpaired Student's t-test or two-way repeated-measures ANOVA where appropriate. F-statistics were calculated using Type III (adjusted) sums of squares due to the unbalanced nature of the data. Student-Newman-Keuls post hoc tests were used to determine where the differences were located. A one-tailed test was performed when a priori directional hypotheses were tested (19, 20, 27). The level of significance was set at p < 0.05. Results are presented as means ± SE.
RESULTS
Morphological and hemodynamic variables revealed impaired ventricular function consistent with the presence of a substantial myocardial infarction and LV wall destruction in both groups (Table 1). CHF and CHF + EXT rats showed similar central indexes of moderate compensated heart failure (Table 1; p > 0.05 for all).
Table 1.
Morphological and hemodynamic characteristics of CHF and CHF + EXT rats
| CHF | CHF + EXT | |
|---|---|---|
| LVEDP, mmHg | 17 ± 2 | 18 ± 2 |
| LV dp/dt, mmHg/s | 6,300 ± 347 | 5,982 ± 282 |
| RV, mg | 350 ± 23 | 378 ± 26 |
| RV/body mass, mg/g | 0.74 ± 0.06 | 0.70 ± 0.05 |
| Lung, mg | 2,606 ± 304 | 2,420 ± 304 |
| Lung/body mass, mg/g | 5.55 ± 0.75 | 4.51 ± 0.61 |
| Infarct size, % | 38 ± 2 | 37 ± 2 |
Values are means ± SE. LVEDP, left ventricular end-diastolic pressure; LV dp/dt, left ventricular Δpressure/Δtime; RV, right ventricle; CHF, chronic heart failure; EXT, endurance exercise trained.
The greater body weight in CHF + EXT compared with CHF rats (CHF: 479 ± 11; CHF + EXT: 541 ± 10 g; p < 0.05) can be ascribed to the experimental design used herein, i.e., endpoint was ∼5 and 10 weeks post-myocardial infarction for CHF and CHF + EXT rats, respectively. CHF + EXT rats had higher V̇o2peak than CHF rats (CHF: 63 ± 2; CHF + EXT: 68 ± 2 ml·kg−1·min−1; p < 0.05). Citrate synthase activity was higher in the soleus (CHF: 29.0 ± 1.0; CHF + EXT: 36.9 ± 1.7 μmol·g−1·min−1), red gastrocnemius (CHF: 39.4 ± 1.4; CHF + EXT: 55.1 ± 1.3 μmol·g−1·min−1) and spinotrapezius (CHF: 19.7 ± 0.7; CHF + EXT: 21.6 ± 0.8 μmol·g−1·min−1) muscles from CHF + EXT rats (p < 0.05 for all). Citrate synthase activity from the white gastrocnemius was not different between CHF and CHF + EXT (10.6 ± 0.8 and 12.2 ± 1.0 μmol·g−1·min−1, respectively; p > 0.05).
No differences in relative spinotrapezius muscle mass were found between CHF and CHF + EXT (0.89 ± 0.03 and 0.94 ± 0.02 mg/g, respectively; p > 0.05).
There were no differences in arterial O2 saturation (CHF: 90.9 ± 1.3; CHF + EXT: 93.8 ± 0.6%), pH (CHF: 7.42 ± 0.01; CHF + EXT: 7.43 ± 0.01) or systemic hematocrit (CHF: 34.3 ± 1.3; CHF + EXT: 36.0 ± 0.6%) between groups (p > 0.05 for all).
Effects of exercise training on muscle PO2mv in CHF.
MAP was not different in CHF compared with CHF + EXT rats either before or after Krebs-Henseleit superfusion (Table 2; p > 0.05 for all). The stimulation protocol used herein did not evoke significant changes in MAP in CHF or CHF + EXT rats in any of the distinct superfusion conditions (p > 0.05 for all). The mean difference in MAP between the onset and offset of contractions were as follows: CHF control: 2 ± 1; CHF SNP: 8 ± 2; CHF l-NAME: 2 ± 1; CHF + EXT control: 0 ± 3; CHF + EXT SNP: 6 ± 2; CHF + EXT l-NAME: −1 ± 1 mmHg.
Table 2.
Mean arterial pressure (expressed in mmHg) pre- and postsuperfusion of Krebs-Henseleit (control), SNP, and l-NAME in CHF and CHF + EXT rats
| Control |
SNP |
l-NAME |
||||
|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | |
| CHF | 105 ± 3 | 104 ± 3 | 110 ± 3 | 101 ± 3* | 112 ± 5 | 113 ± 5 |
| CHF + EXT | 115 ± 7 | 115 ± 7 | 114 ± 9 | 106 ± 7 | 121 ± 6 | 123 ± 7 |
Values are means ± SE. SNP, sodium nitroprusside. Significantly different from:
CHF post- NG-nitro-l-arginine methyl ester (l-NAME) superfusion.
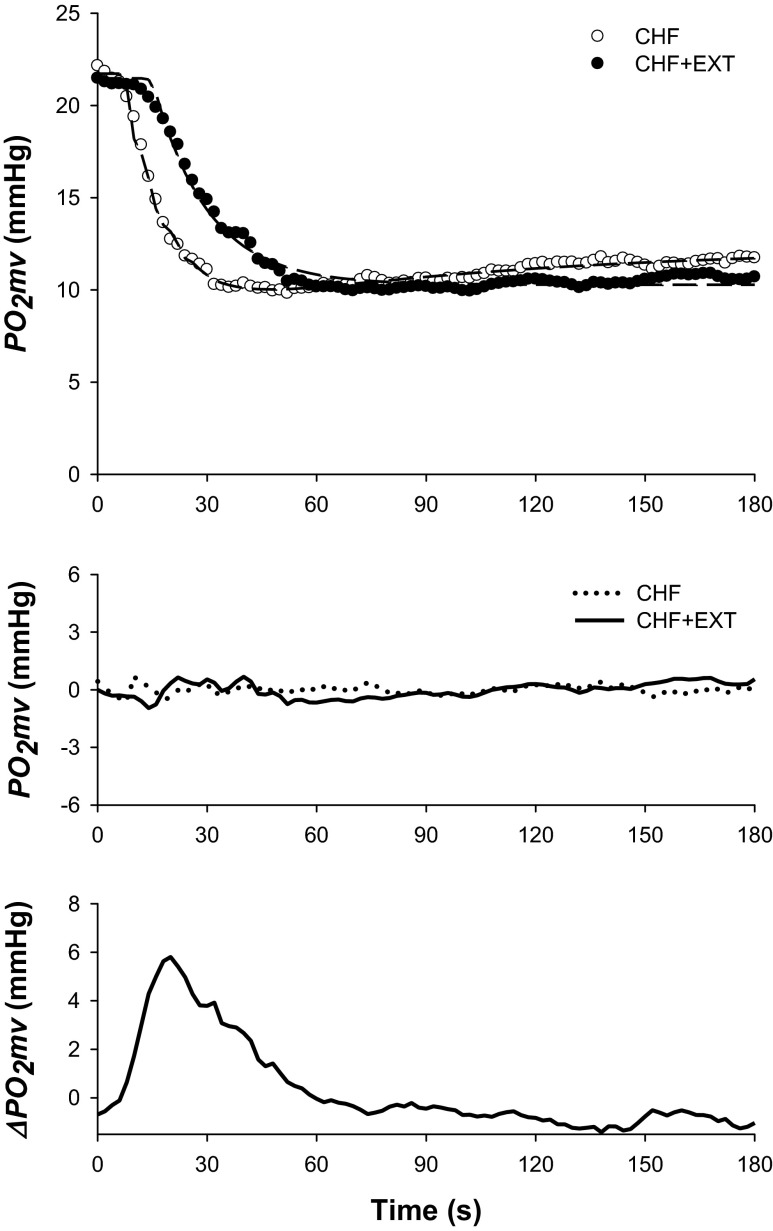
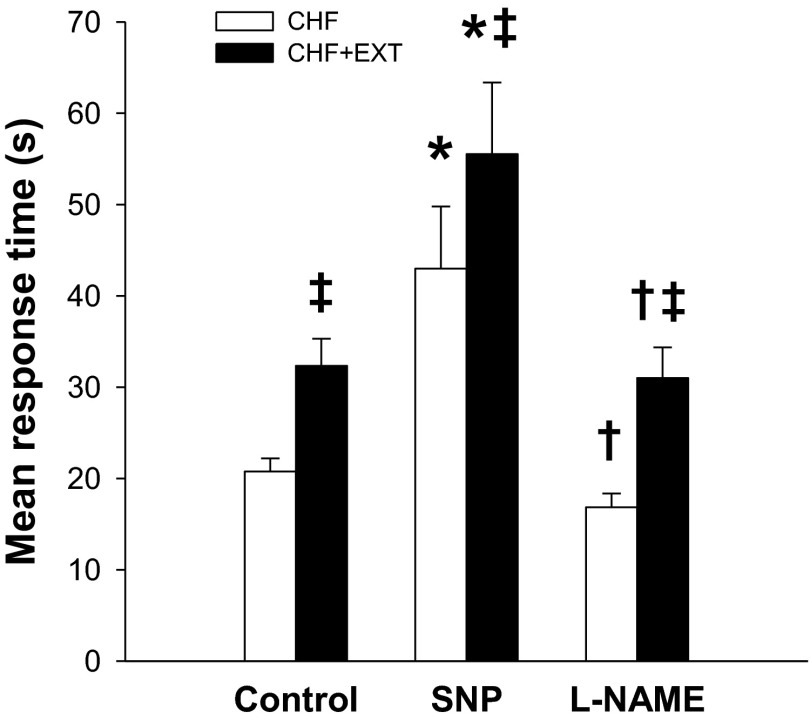
Although resting and contracting steady-state PO2mv [PO2mv(BL) and PO2mv(SS), respectively] were not different between groups (Fig. 1 and Table 3; p > 0.05 for both), exercise training induced significant differences in the time course of PO2mv following the onset of contractions under the control condition (Figs. 1–4 and Table 3). Specifically, the overall speed of PO2mv fall during contractions (MRT and T63) was markedly slowed in CHF + EXT compared with CHF rats (Fig. 4 and Table 3; p < 0.05 for all).
Fig. 1.
Top: Spinotrapezius muscle PO2mv profiles (circles) and their model fits (dashed lines) from representative chronic heart failure (CHF) and CHF + endurance exercise trained (EXT) rats in the control condition. Note that exercise training slowed the overall speed of the PO2mv fall during contractions. Middle: PO2mv residuals demonstrate excellent model fits. Bottom: absolute PO2mv difference between CHF + EXT and CHF responses shown at top. Note that the greatest effect of exercise training on PO2mv kinetics occurred during the rest-contraction transient (i.e., 0–60 s). Time zero denotes the onset of muscle contractions.
Table 3.
Spinotrapezius muscle PO2mv kinetics following the onset of contractions under control, SNP, and l-NAME conditions in CHF and CHF + EXT rats
| Control |
SNP |
l-NAME |
||||
|---|---|---|---|---|---|---|
| CHF | CHF + EXT | CHF | CHF + EXT | CHF | CHF + EXT | |
| PO2mv(BL), mmHg | 24.3 ± 2.3 | 22.6 ± 1.9 | 33.7 ± 2.3* | 35.3 ± 2.9* | 23.5 ± 2.4† | 21.3 ± 1.0† |
| Δ1PO2mv, mmHg | 10.3 ± 1.0 | 9.5 ± 1.1 | 8.8 ± 0.8 | 10.4 ± 2.1 | 11.9 ± 1.6 | 11.6 ± 1.0 |
| Δ2PO2mv, mmHg | 1.9 ± 0.3 | 2.2 ± 0.4 | 2.4 ± 0.4 | 2.3 ± 0.4 | ||
| ΔTotalPO2mv, mmHg | 8.8 ± 1.0 | 8.7 ± 1.0 | 8.8 ± 0.8 | 10.4 ± 2.1 | 9.7 ± 1.3 | 10.6 ± 0.8 |
| PO2mv(SS), mmHg | 15.5 ± 1.8 | 13.9 ± 1.2 | 24.9 ± 2.2* | 24.9 ± 2.2* | 13.7 ± 1.2† | 10.7 ± 0.6*† |
| TD1, s | 8.9 ± 1.5 | 12.6 ± 1.3# | 4.0 ± 1.7* | 5.7 ± 2.1*# | 6.7 ± 0.7† | 11.9 ± 2.1†‡ |
| TD2, s | 45.7 ± 5.7 | 83.3 ± 10.5 | 35.4 ± 4.7 | 60.5 ± 15.5 | ||
| τ1, s | 11.9 ± 1.3 | 19.7 ± 3.0# | 39.0 ± 6.5* | 49.8 ± 8.3*‡ | 10.1 ± 1.4† | 19.1 ± 2.9†# |
| τ2, s | 41.2 ± 12.6 | 57.0 ± 9.9 | 59.3 ± 9.1 | 38.0 ± 13.5 | ||
| T63, s | 20.4 ± 1.2 | 31.2 ± 2.3‡ | 42.2 ± 7.7* | 55.0 ± 7.1*‡ | 17.4 ± 1.6† | 31.3 ± 3.4†‡ |
Values are means ± SE. PO2mv(BL), resting PO2mv; Δ1PO2mv, amplitude of the first component; Δ2PO2mv, amplitude of the second component; ΔTotalPO2mv, overall amplitude regardless of one- or two-component model fit; PO2mv(SS), contracting steady-state PO2mv; TD1, time delay for the first component; TD2, time delay for the second component; τ1, time constant for the first component; τ2, time constant for the second component; T63, time to reach 63% of the primary amplitude as determined independent of modeling procedures. The one-component exponential model was used to analyze the PO2mv kinetics in the following conditions: CHF control (2/10), CHF SNP (10/10), CHF l-NAME (1/10), CHF + EXT control (7/10), CHF + EXT SNP (7/7), CHF + EXT l-NAME (5/11). Significantly different from:
control within group;
SNP within group;
CHF within superfusion condition;
P = 0.05–0.1 vs. CHF within condition.
Fig. 4.
Spinotrapezius muscle PO2mv kinetics (mean response time, which describes the overall dynamics of PO2mv fall) following the onset of contractions in the CHF and CHF + EXT groups under control, SNP, and l-NAME superfusion conditions. Significantly different from: *control within group; †SNP within group; ‡CHF within superfusion condition.
Effects of altered NO on muscle PO2mv in sedentary and trained CHF rats.
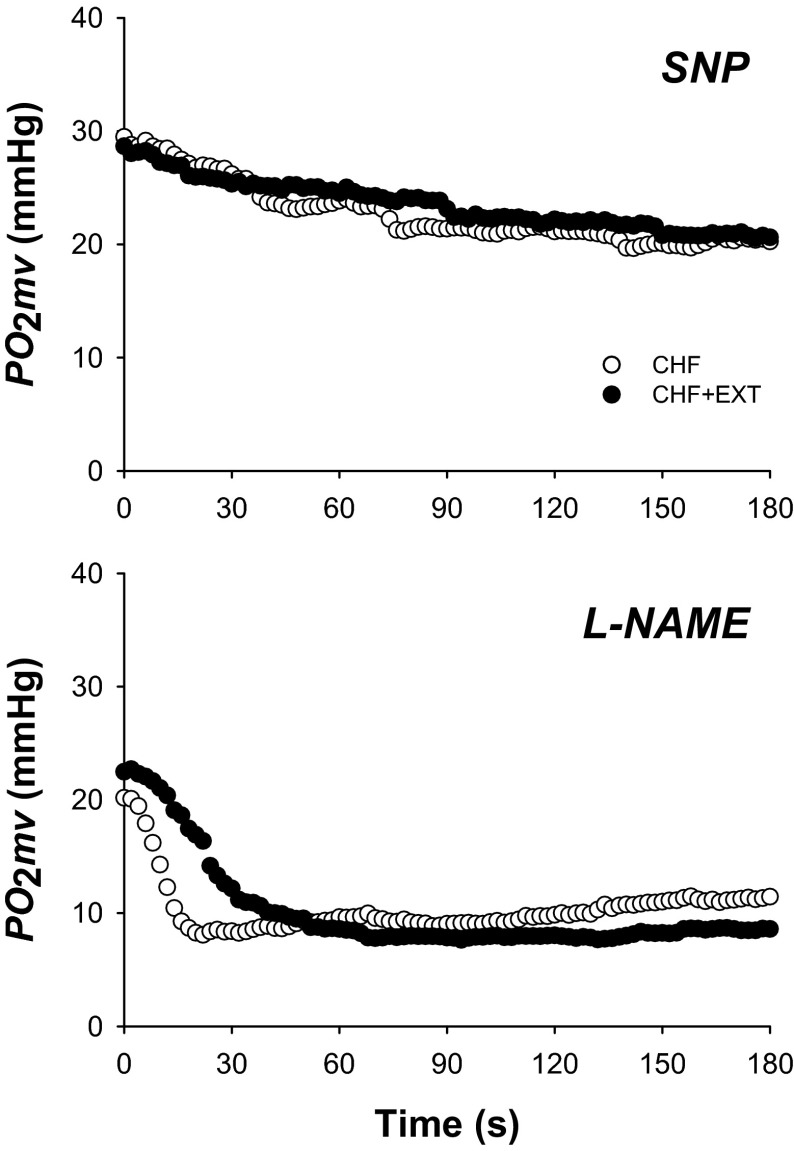
SNP superfusion did not change MAP in either CHF or CHF + EXT rats (Table 2; p > 0.05 for both). Relative to the control condition, SNP elevated PO2mv(BL) and PO2mv(SS) in both groups to similar values (Figs. 1 and 2 and Table 3; p < 0.05 for all). No differences in the amplitude of the PO2mv response following the onset of contractions (Δ1PO2mv and ΔTotalPO2mv) were observed between CHF and CHF + EXT rats with SNP (Table 3; p > 0.05 for all). SNP slowed MRT, T63, and τ1 in both groups such that CHF + EXT rats continued to display slower PO2mv kinetics when compared with their sedentary counterparts (Fig. 4 and Table 3; p < 0.05 for all). It is important to note that the absolute and relative changes promoted by SNP in MRT, T63, and τ1 were not different when comparing CHF and CHF + EXT rats (data not shown; p > 0.05).
Fig. 2.
Spinotrapezius muscle PO2mv responses from representative CHF and CHF + EXT rats under sodium nitroprusside (SNP) and NG-nitro-l-arginine methyl ester (l-NAME) superfusion conditions. Time zero denotes the onset of muscle contractions. Note that SNP slowed PO2mv kinetics to a greater extent in CHF + EXT compared with CHF rats. l-NAME did not alter PO2mv kinetics in either group but reduced the contraction steady-state PO2mv only in CHF + EXT rats when compared with control. Therefore, CHF + EXT rats continued to exhibit slower PO2mv kinetics than their sedentary counterparts during the l-NAME condition. PO2mv kinetics parameters are shown in Table 3 and Fig. 4.
l-NAME superfusion did not change MAP in either CHF or CHF + EXT rats (Table 2; p > 0.05 for both). Relative to the control condition, l-NAME had no effects on PO2mv(BL) in either group but decreased PO2mv(SS) only in CHF + EXT rats (Figs. 1 and 2 and Table 3; p < 0.05). No differences in the amplitude of the PO2mv response following the onset of contractions (Δ1PO2mv, Δ2PO2mv, and ΔTotalPO2mv) were observed between CHF and CHF + EXT rats with l-NAME (Table 3; p > 0.05 for all). Notably, l-NAME did not alter the time course of PO2mv fall at the onset of contractions (i.e., TD1, τ1, MRT, and T63) in CHF or CHF + EXT rats when compared with the control condition (Figs. 3 and 4 and Table 3; p > 0.05 for all). Thus CHF + EXT rats exhibited slower PO2mv on-kinetics (i.e., TD1, MRT, and T63) than their sedentary counterparts during the l-NAME condition (Fig. 4 and Table 3; p<0.05 for all).
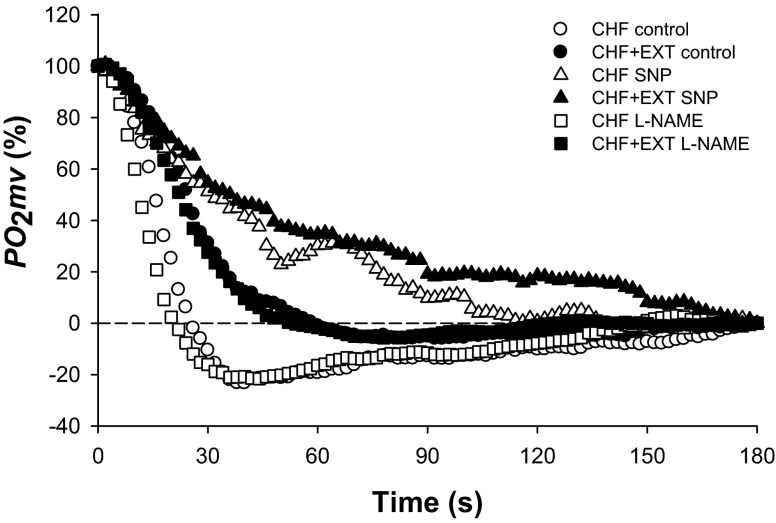
Fig. 3.
Mean relative spinotrapezius muscle PO2mv responses in CHF and CHF + EXT rats under control, SNP, and l-NAME superfusion conditions. Time zero denotes the onset of muscle contractions. SE bars were omitted for clarity. Note slower PO2mv kinetics in CHF + EXT compared with CHF rats during the control condition. SNP slowed PO2mv kinetics in CHF + EXT compared with CHF rats, whereas l-NAME had no effects on PO2mv kinetics in either group relative to the control condition. PO2mv kinetics parameters are shown in Table 3 and Fig. 4.
DISCUSSION
The present investigation demonstrates that endurance exercise training fundamentally changes the microvascular oxygenation profile (i.e., slows PO2mv kinetics) following the onset of contractions in the spinotrapezius muscle of CHF rats. Relative to the control condition, increased NO with SNP slowed PO2mv kinetics in both groups (such that CHF + EXT rats had greater MRT of the PO2mv fall), whereas decreasing NO bioavailability with l-NAME had no effects on PO2mv kinetics in either group. Contrary to our hypothesis, enhanced NO-mediated function may not be obligatory for training-induced improvements in contracting muscle microvascular oxygenation in CHF.
Exercise training and muscle microvascular oxygenation in CHF.
Skeletal muscle PO2mv kinetics reflect the dynamic matching between Q̇O2 and V̇o2 within the microvascular space during transitions in metabolic demand (4). Therefore, slowed PO2mv kinetics following the onset of contractions in CHF + EXT rats (Figs. 1–4 and Table 3) indicates an improved Q̇O2/V̇o2 matching secondary to faster adjustment in muscle Q̇O2 compared with CHF rats (14). Importantly, enhanced microvascular Q̇O2 responses across the rest-contraction transient after exercise training oppose the predations of CHF on muscle conductive and diffusive microvascular Q̇O2 [i.e., mainly reduced red blood cell flux (fRBC) and proportion of red blood cell-flowing capillaries] (35, 56) and reduce fractional O2 extraction (thus increasing effectively the pressure gradient driving O2 from the blood into the myocyte). Moreover, improvements in contracting muscle microvascular Q̇O2 are crucial to support a faster rate of oxidative phosphorylation (i.e., faster V̇o2 kinetics; as suggested by higher spinotrapezius citrate synthase activity seen herein and reported previously) (46, 58) following completion of an exercise training program in CHF individuals. At any given submaximal contractile activity, these training-induced adaptations in microvascular O2 transport would be expected to attenuate perturbations of the intracellular milieu (e.g., changes in ADP, PCr, and Cr concentrations), reduce the rate of anaerobic glycolysis and reliance on finite energy sources, and contribute to increased exercise tolerance as documented previously (29, 30, 64, 65), thus providing a mechanistic basis for these effects.
The improvement (slowing) in muscle PO2mv kinetics seen herein after training derives principally from adaptations in peripheral microvascular control given that no differences in central indexes of heart failure were observed between CHF and CHF + EXT groups (Table 1). This finding is consistent with our experimental design and with the notion that training-induced adaptations to left ventricular pump function (i.e., improved systolic volume and cardiac output) are a function of training frequency, intensity, and duration, such that central cardiac adaptations in CHF rats might only be observed following high-intensity sprint training regimens (47).
The peripheral adaptations observed herein are also consistent with the fact that CHF patients exhibit substantial plasticity within their skeletal muscle O2 transport pathway (conductive and diffusive components) in response to exercise training (17) and the major role of skeletal muscle dysfunction in the pathophysiology of CHF (8, 51, 52, 54). As discussed previously (26, 54), there is an interdependence between muscle conductive and diffusive O2 transport components setting fractional O2 extraction (57): O2 extraction = 1 − e−Do2/βQm, where DO2 is muscle O2 diffusing capacity (dictated largely by capillary hematocrit and the volume density of red blood cell-flowing capillaries) (18, 22), β is the slope of the O2 dissociation curve in the physiologically relevant range, and Q̇m is muscle blood flow. Because β is unlikely to be affected appreciably by exercise training, alterations in O2 extraction (and thus PO2mv) will depend primarily on the DO2/Q̇m ratio (57). It is interesting to note that although CHF induces peripheral microvascular dysfunction via impairments in both muscle DO2 (reduced proportion of red blood cell-flowing capillaries) and Q̇m (reduced fRBC) (35, 56), exercise training can promote beneficial adaptations in both DO2 (2, 44, 57) and Q̇m (60). In this sense, slower PO2mv kinetics following the onset of contractions in CHF + EXT rats (Figs. 1–4 and Table 3) is evidence for a lower DO2/Q̇m ratio (i.e., reduced fractional O2 extraction) and suggests that adaptations in conductive (Q̇m; mainly faster fRBC kinetics) rather than diffusive O2 transport are of relatively greater importance in improving contracting muscle microvascular oxygenation in this disease.
Effects of altered NO on PO2mv kinetics in CHF.
NO and its derivatives are important modulators of skeletal muscle vascular control (63). Impairments in NO-mediated function contribute to diminished functional hyperemia (28, 34) and temporal Q̇O2/V̇o2 mismatch (19) during muscle contractions in CHF. Conversely, exercise training potentially ameliorates NO-induced vasodilation in CHF (24, 40, 66) and improves (slows) contracting muscle PO2mv kinetics in healthy individuals (26). We therefore hypothesized initially that endurance exercise training would enhance muscle microvascular oxygenation following the onset of contractions (i.e., slow PO2mv kinetics) in CHF partly via improved NO-mediated function. Surprisingly, SNP slowed the overall dynamics of the PO2mv fall (mean response time; MRT) in CHF and CHF + EXT rats, whereas l-NAME did not alter MRT in either group (Fig. 4). Despite the small but significant reduction in the contracting steady-state PO2mv [PO2mv(SS)] in CHF + EXT rats with l-NAME (Table 3), our results suggest that improved NO-mediated function is not obligatory for training-induced enhancement of muscle PO2mv kinetics following contractions onset in rats with CHF.
These responses contrast markedly with those seen previously in healthy rats, in which SNP slowed PO2mv kinetics to a greater extent in sedentary animals and l-NAME abolished the differences in PO2mv kinetics between sedentary and trained animals that were evident during the control condition (26). It is therefore crucial to appreciate that in trained CHF skeletal muscle, enhanced NO-mediated function may not be obligatory to slow PO2mv kinetics (and consequently to increase the pressure head for O2 diffusion) at a time when V̇o2 is rising at its fastest rate and improve muscle O2 supply and oxidative function following contractions onset (4, 14, 29, 54, 64).
Potential mechanisms underlying slowed PO2mv kinetics with training in CHF.
Although endurance exercise training improves muscle PO2mv kinetics (potentially via a speeding of the fRBC response as discussed above) following the onset of contractions in both healthy (26) and CHF (present investigation) rats, enhanced NO signaling does not appear to be intrinsic to training-induced adaptations in muscle microvascular O2 exchange in CHF + EXT (Figs. 1–4 and Table 3). Despite evidence supporting an improvement in NO-induced vasodilation in CHF after exercise training (24, 40, 66), it is interesting to note that Lindsay et al. (39) reported no effects of training on acetylcholine-induced relaxation of aortic rings in CHF rats and Yi et al. (68) found preserved prostaglandin-induced relaxation of coronary arteries from trained CHF dogs. Together with the fact that impairments in NO-mediated function with CHF might be compensated by increased contribution of other vasodilators such as endothelium-derived hyperpolarizing factors (EDHF) (33, 42), these data highlight the redundancy and synergism of mechanisms regulating muscle O2 delivery (25) and suggest that exercise training may alter the relative contribution of distinct mechanisms governing microvascular oxygenation in CHF.
Among the myriad of training-induced adaptations that might enhance contracting muscle PO2mv kinetics in CHF + EXT are improved neurohumoral (e.g., ↓ catecholamines, arginine vasopressin, angiotensin II, endothelin-1), autonomic (↓ sympathetic tone and ↑ vagal tone), and inflammatory (e.g., ↓ tumor necrosis factor-α, interleukin-6) responses; reduced oxidative stress (e.g., ↑ superoxide dismutase, catalase, glutathione peroxidase); and structural alterations (↑ capillarity) (7, 12, 15, 51, 52, 54). Training might also produce alterations in the regional distribution of Q̇m (49) and attenuate spatial heterogeneities in contracting muscle microvascular oxygenation (36). These and other potential adaptations may shift the balance between vasodilators and vasoconstrictors in favor of the former without obligatory improvements in NO-mediated function (e.g., ↑ EDHF and ↓ endothelium-dependent contracting factors, respectively) and thus improve muscle Q̇O2/V̇o2 matching (slow PO2mv kinetics) during transitions in metabolic demand in trained CHF individuals. Future studies designed to address the relative contribution of the abovementioned factors in improving skeletal muscle PO2mv kinetics following exercise training in CHF will be valuable.
Clinical implications.
Resolution of the mechanisms underlying the improvements in muscle microvascular oxygenation following exercise training in CHF is crucial to the development of effective therapeutic strategies targeting this disease. The current results carry important clinical implications for CHF populations by demonstrating that enhanced NO-mediated function may not be obligatory for slowed PO2mv kinetics across the rest-contractions transient after training. Moreover, endurance exercise training might constitute a more powerful intervention than some contemporary pharmacological therapies (e.g., angiotensin-converting enzyme inhibitors and β-adrenergic blockers), which have been reported not to abrogate microvascular oxygenation deficits during muscle contractions in CHF patients (61).
Experimental considerations.
As mentioned above, downhill treadmill running was used herein as a model of endurance exercise training given that this paradigm effectively trains the rat spinotrapezius muscle (as evidenced by slowed PO2mv kinetics, greater citrate synthase activity, and resistance to fatigue) and induces training adaptations such as increased whole-body V̇o2peak and hindlimb muscle citrate synthase activity (present results; refs. 23, 26). In fact, the effects of downhill training on whole-body V̇o2peak and muscle oxidative enzyme capacity observed in the current investigation are similar to those reported previously by Musch et al. (48) in male CHF rats with similar left ventricular infarct size and LVEDP subjected to uphill training (10% grade).
It could be argued that reduced driving pressure (MAP) with SNP compared with l-NAME in CHF rats (Table 2) potentially constrains blood flow dynamics and influences PO2mv kinetics during metabolic transitions. However, previous reports from our laboratory (6) indicate that this effect is negligible or nonexistent when MAP is greater than ∼70 mmHg as herein.
Further experimental considerations involving the utilization of downhill treadmill running as a model of endurance exercise training, differences in vascular adaptations to training based on muscle fiber type composition, and potential muscle damage following eccentric exercise (downhill running) have been discussed in detail previously (26).
Conclusions
The current novel findings indicate that endurance exercise training is a powerful nonpharmacological treatment capable of improving muscle microvascular oxygenation (resulting in slowed PO2mv kinetics) during metabolic transitions in CHF. Contrary to what was found previously in healthy skeletal muscle (26), enhanced NO-mediated function may not be obligatory for these training-induced microvascular adaptations in CHF.
GRANTS
This project was supported in part by a Fellowship from the Brazilian Ministry of Education/CAPES-Fulbright and a Doctoral Student Research Grant from the American College of Sports Medicine Foundation (to D. M. Hirai); American Heart Association Heartland Affiliate Grant 0750090Z (to T. I. Musch); Kansas State University SMILE grant; and National Heart, Lung, and Blood Institute Grant HL-108328 (to D. C. Poole).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: D.M.H., S.W.C., C.T.H., S.K.F., D.J.M., B.J.B., T.I.M., and D.C.P. conception and design of research; D.M.H., S.W.C., C.T.H., S.K.F., D.J.M., B.J.B., T.I.M., and D.C.P. performed experiments; D.M.H., S.W.C., C.T.H., S.K.F., D.J.M., B.J.B., T.I.M., and D.C.P. analyzed data; D.M.H., S.W.C., C.T.H., S.K.F., D.J.M., B.J.B., T.I.M., and D.C.P. interpreted results of experiments; D.M.H., S.W.C., C.T.H., S.K.F., D.J.M., B.J.B., T.I.M., and D.C.P. prepared figures; D.M.H., S.W.C., C.T.H., S.K.F., D.J.M., B.J.B., T.I.M., and D.C.P. drafted manuscript; D.M.H., S.W.C., C.T.H., S.K.F., D.J.M., B.J.B., T.I.M., and D.C.P. edited and revised manuscript; D.M.H., S.W.C., C.T.H., S.K.F., D.J.M., B.J.B., T.I.M., and D.C.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank K. Sue Hageman, Gabrielle E. Sims, and Dr. Tadakatsu Inagaki for excellent technical assistance.
REFERENCES
- 1.Bailey JK, Kindig CA, Behnke BJ, Musch TI, Schmid-Schoenbein GW, Poole DC. Spinotrapezius muscle microcirculatory function: effects of surgical exteriorization. Am J Physiol Heart Circ Physiol 279: H3131–H3137, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Bebout DE, Hogan MC, Hempleman SC, Wagner PD. Effects of training and immobilization on VO2 and DO2 in dog gastrocnemius muscle in situ. J Appl Physiol 74: 1697–1703, 1993 [DOI] [PubMed] [Google Scholar]
- 3.Behnke BJ, Delp MD, McDonough P, Spier SA, Poole DC, Musch TI. Effects of chronic heart failure on microvascular oxygen exchange dynamics in muscles of contrasting fiber type. Cardiovasc Res 61: 325–332, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Behnke BJ, Kindig CA, Musch TI, Koga S, Poole DC. Dynamics of microvascular oxygen pressure across the rest-exercise transition in rat skeletal muscle. Respir Physiol 126: 53–63, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Behnke BJ, Kindig CA, Musch TI, Sexton WL, Poole DC. Effects of prior contractions on muscle microvascular oxygen pressure at onset of subsequent contractions. J Physiol 539: 927–934, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Behnke BJ, Padilla DJ, Ferreira LF, Delp MD, Musch TI, Poole DC. Effects of arterial hypotension on microvascular oxygen exchange in contracting skeletal muscle. J Appl Physiol (1985) 100: 1019–1026, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Brum PC, Bacurau AV, Medeiros A, Ferreira JC, Vanzelli AS, Negrao CE. Aerobic exercise training in heart failure: impact on sympathetic hyperactivity and cardiac and skeletal muscle function. Braz J Med Biol Res 44: 827–835, 2011 [DOI] [PubMed] [Google Scholar]
- 8.Coats AJ, Clark AL, Piepoli M, Volterrani M, Poole-Wilson PA. Symptoms and quality of life in heart failure: the muscle hypothesis. Br Heart J 72: S36–S39, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Copp SW, Davis RT, Poole DC, Musch TI. Reproducibility of endurance capacity and VO2peak in male Sprague-Dawley rats. J Appl Physiol 106: 1072–1078, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Copp SW, Hirai DM, Ferguson SK, Holdsworth CT, Musch TI, Poole DC. Effects of chronic heart failure on neuronal nitric oxide synthase-mediated control of microvascular O2 pressure in contracting rat skeletal muscle. J Physiol 590: 3585–3596, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Copp SW, Hirai DM, Ferreira LF, Poole DC, Musch TI. Progressive chronic heart failure slows the recovery of microvascular O2 pressures after contractions in the rat spinotrapezius muscle. Am J Physiol Heart Circ Physiol 299: H1755–H1761, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crimi E, Ignarro LJ, Cacciatore F, Napoli C. Mechanisms by which exercise training benefits patients with heart failure. Nat Rev Cardiol 6: 292–300, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Delp MD, Duan C. Composition and size of type I, IIA, IID/X, and IIB fibers and citrate synthase activity of rat muscle. J Appl Physiol (1985) 80: 261–270, 1996 [DOI] [PubMed] [Google Scholar]
- 14.Diederich ER, Behnke BJ, McDonough P, Kindig CA, Barstow TJ, Poole DC, Musch TI. Dynamics of microvascular oxygen partial pressure in contracting skeletal muscle of rats with chronic heart failure. Cardiovasc Res 56: 479–486, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Downing J, Balady GJ. The role of exercise training in heart failure. J Am Coll Cardiol 58: 561–569, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Drexler H, Lu W. Endothelial dysfunction of hindquarter resistance vessels in experimental heart failure. Am J Physiol Heart Circ Physiol 262: H1640–H1645, 1992 [DOI] [PubMed] [Google Scholar]
- 17.Esposito F, Reese V, Shabetai R, Wagner PD, Richardson RS. Isolated quadriceps training increases maximal exercise capacity in chronic heart failure: the role of skeletal muscle convective and diffusive oxygen transport. J Am Coll Cardiol 58: 1353–1362, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Federspiel WJ, Popel AS. A theoretical analysis of the effect of the particulate nature of blood on oxygen release in capillaries. Microvasc Res 32: 164–189, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferreira LF, Hageman KS, Hahn SA, Williams J, Padilla DJ, Poole DC, Musch TI. Muscle microvascular oxygenation in chronic heart failure: role of nitric oxide availability. Acta Physiol (Oxf) 188: 3–13, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Ferreira LF, Padilla DJ, Williams J, Hageman KS, Musch TI, Poole DC. Effects of altered nitric oxide availability on rat muscle microvascular oxygenation during contractions. Acta Physiol (Oxf) 186: 223–232, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Green DJ, Maiorana A, O'Driscoll G, Taylor R. Effect of exercise training on endothelium-derived nitric oxide function in humans. J Physiol 561: 1–25, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Groebe K, Thews G. Calculated intra- and extracellular PO2 gradients in heavily working red muscle. Am J Physiol Heart Circ Physiol 259: H84–H92, 1990 [DOI] [PubMed] [Google Scholar]
- 23.Hahn SA, Ferreira LF, Williams JB, Jansson KP, Behnke BJ, Musch TI, Poole DC. Downhill treadmill running trains the rat spinotrapezius muscle. J Appl Physiol 102: 412–416, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Hambrecht R, Fiehn E, Weigl C, Gielen S, Hamann C, Kaiser R, Yu J, Adams V, Niebauer J, Schuler G. Regular physical exercise corrects endothelial dysfunction and improves exercise capacity in patients with chronic heart failure. Circulation 98: 2709–2715, 1998 [DOI] [PubMed] [Google Scholar]
- 25.Hellsten Y, Nyberg M, Jensen LG, Mortensen SP. Vasodilator interactions in skeletal muscle blood flow regulation. J Physiol 590: 6297–6305, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hirai DM, Copp SW, Ferguson SK, Holdsworth CT, McCullough DJ, Behnke BJ, Musch TI, Poole DC. Exercise training and muscle microvascular oxygenation: functional role of nitric oxide. J Appl Physiol 113: 557–565, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hirai DM, Copp SW, Ferguson SK, Holdsworth CT, Musch TI, Poole DC. The NO donor sodium nitroprusside: evaluation of skeletal muscle vascular and metabolic dysfunction. Microvasc Res 85: 104–111, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hirai T, Zelis R, Musch TI. Effects of nitric oxide synthase inhibition on the muscle blood flow response to exercise in rats with heart failure. Cardiovasc Res 30: 469–476, 1995 [PubMed] [Google Scholar]
- 29.Hogan MC, Arthur PG, Bebout DE, Hochachka PW, Wagner PD. Role of O2 in regulating tissue respiration in dog muscle working in situ. J Appl Physiol 73: 728–736, 1992 [DOI] [PubMed] [Google Scholar]
- 30.Holloszy JO, Coyle EF. Adaptations of skeletal muscle to endurance exercise and their metabolic consequences. J Appl Physiol 56: 831–838, 1984 [DOI] [PubMed] [Google Scholar]
- 31.Kano Y, Padilla D, Hageman KS, Poole DC, Musch TI. Downhill running: a model of exercise hyperemia in the rat spinotrapezius muscle. J Appl Physiol 97: 1138–1142, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Katz SD, Biasucci L, Sabba C, Strom JA, Jondeau G, Galvao M, Solomon S, Nikolic SD, Forman R, LeJemtel TH. Impaired endothelium-mediated vasodilation in the peripheral vasculature of patients with congestive heart failure. J Am Coll Cardiol 19: 918–925, 1992 [DOI] [PubMed] [Google Scholar]
- 33.Katz SD, Krum H. Acetylcholine-mediated vasodilation in the forearm circulation of patients with heart failure: indirect evidence for the role of endothelium-derived hyperpolarizing factor. Am J Cardiol 87: 1089–1092, 2001 [DOI] [PubMed] [Google Scholar]
- 34.Katz SD, Krum H, Khan T, Knecht M. Exercise-induced vasodilation in forearm circulation of normal subjects and patients with congestive heart failure: role of endothelium-derived nitric oxide. J Am Coll Cardiol 28: 585–590, 1996 [DOI] [PubMed] [Google Scholar]
- 35.Kindig CA, Musch TI, Basaraba RJ, Poole DC. Impaired capillary hemodynamics in skeletal muscle of rats in chronic heart failure. J Appl Physiol 87: 652–660, 1999 [DOI] [PubMed] [Google Scholar]
- 36.Koga S, Poole DC, Ferreira LF, Whipp BJ, Kondo N, Saitoh T, Ohmae E, Barstow TJ. Spatial heterogeneity of quadriceps muscle deoxygenation kinetics during cycle exercise. J Appl Physiol 103: 2049–2056, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Kubo SH, Rector TS, Bank AJ, Williams RE, Heifetz SM. Endothelium-dependent vasodilation is attenuated in patients with heart failure. Circulation 84: 1589–1596, 1991 [DOI] [PubMed] [Google Scholar]
- 38.Leek BT, Mudaliar SR, Henry R, Mathieu-Costello O, Richardson RS. Effect of acute exercise on citrate synthase activity in untrained and trained human skeletal muscle. Am J Physiol Regul Integr Comp Physiol 280: R441–R447, 2001 [DOI] [PubMed] [Google Scholar]
- 39.Lindsay DC, Jiang C, Brunotte F, Adamopoulos S, Coats AJ, Rajagopalan B, Poole-Wilson PA, Collins P. Impairment of endothelium dependent responses in a rat model of chronic heart failure: effects of an exercise training protocol. Cardiovasc Res 26: 694–697, 1992 [DOI] [PubMed] [Google Scholar]
- 40.Linke A, Schoene N, Gielen S, Hofer J, Erbs S, Schuler G, Hambrecht R. Endothelial dysfunction in patients with chronic heart failure: systemic effects of lower-limb exercise training. J Am Coll Cardiol 37: 392–397, 2001 [DOI] [PubMed] [Google Scholar]
- 41.Lo LW, Vinogradov SA, Koch CJ, Wilson DF. A new, water soluble, phosphor for oxygen measurements in vivo. Adv Exp Med Biol 428: 651–656, 1997 [DOI] [PubMed] [Google Scholar]
- 42.Malmsjo M, Bergdahl A, Zhao XH, Sun XY, Hedner T, Edvinsson L, Erlinge D. Enhanced acetylcholine and P2Y-receptor stimulated vascular EDHF-dilatation in congestive heart failure. Cardiovasc Res 43: 200–209, 1999 [DOI] [PubMed] [Google Scholar]
- 43.McAllister RM, Newcomer SC, Laughlin MH. Vascular nitric oxide: effects of exercise training in animals. Appl Physiol Nutr Metab 33: 173–178, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McAllister RM, Terjung RL. Training-induced muscle adaptations: increased performance and oxygen consumption. J Appl Physiol 70: 1569–1574, 1991 [DOI] [PubMed] [Google Scholar]
- 45.McDonough P, Behnke BJ, Musch TI, Poole DC. Effects of chronic heart failure in rats on the recovery of microvascular PO2 after contractions in muscles of opposing fibre type. Exp Physiol 89: 473–485, 2004 [DOI] [PubMed] [Google Scholar]
- 46.Mezzani A, Grassi B, Jones AM, Giordano A, Corra U, Porcelli S, Della Bella S, Taddeo A, Giannuzzi P. Speeding of pulmonary VO2 on-kinetics by light-to-moderate-intensity aerobic exercise training in chronic heart failure: clinical and pathophysiological correlates. Int J Cardiol 167: 2189–2195, 2013 [DOI] [PubMed] [Google Scholar]
- 47.Musch TI, Moore RL, Hilty MR. Effects of dynamic exercise training on the metabolic and cardiocirculatory responses to exercise in the rat model of myocardial infarction and heart failure. Am J Cardiol 62: 20E–24E, 1988 [DOI] [PubMed] [Google Scholar]
- 48.Musch TI, Moore RL, Leathers DJ, Bruno A, Zelis R. Endurance training in rats with chronic heart failure induced by myocardial infarction. Circulation 74: 431–441, 1986 [DOI] [PubMed] [Google Scholar]
- 49.Musch TI, Nguyen CT, Pham HV, Moore RL. Training effects on the regional blood flow response to exercise in myocardial infarcted rats. Am J Physiol Heart Circ Physiol 262: H1846–H1852, 1992 [DOI] [PubMed] [Google Scholar]
- 50.Musch TI, Terrell JA. Skeletal muscle blood flow abnormalities in rats with a chronic myocardial infarction: rest and exercise. Am J Physiol Heart Circ Physiol 262: H411–H419, 1992 [DOI] [PubMed] [Google Scholar]
- 51.Piepoli MF, Guazzi M, Boriani G, Cicoira M, Corra U, Dalla Libera L, Emdin M, Mele D, Passino C, Vescovo G, Vigorito C, Villani G, Agostoni P. Exercise intolerance in chronic heart failure: mechanisms and therapies. Part II. Eur J Cardiovasc Prev Rehabil 17: 643–648, 2010 [DOI] [PubMed] [Google Scholar]
- 52.Piepoli MF, Guazzi M, Boriani G, Cicoira M, Corra U, Dalla Libera L, Emdin M, Mele D, Passino C, Vescovo G, Vigorito C, Villani GQ, Agostoni P. Exercise intolerance in chronic heart failure: mechanisms and therapies. Part I. Eur J Cardiovasc Prev Rehabil 17: 637–642, 2010 [DOI] [PubMed] [Google Scholar]
- 53.Poole DC, Behnke BJ, McDonough P, McAllister RM, Wilson DF. Measurement of muscle microvascular oxygen pressures: compartmentalization of phosphorescent probe. Microcirculation 11: 317–326, 2004 [DOI] [PubMed] [Google Scholar]
- 54.Poole DC, Hirai DM, Copp SW, Musch TI. Muscle oxygen transport and utilization in heart failure: implications for exercise (in)tolerance. Am J Physiol Heart Circ Physiol 302: H1050–H1063, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Poole DC, Wagner PD, Wilson DF. Diaphragm microvascular plasma PO2 measured in vivo. J Appl Physiol 79: 2050–2057, 1995 [DOI] [PubMed] [Google Scholar]
- 56.Richardson TE, Kindig CA, Musch TI, Poole DC. Effects of chronic heart failure on skeletal muscle capillary hemodynamics at rest and during contractions. J Appl Physiol 95: 1055–1062, 2003 [DOI] [PubMed] [Google Scholar]
- 57.Roca J, Agusti AG, Alonso A, Poole DC, Viegas C, Barbera JA, Rodriguez-Roisin R, Ferrer A, Wagner PD. Effects of training on muscle O2 transport at VO2max. J Appl Physiol 73: 1067–1076, 1992 [DOI] [PubMed] [Google Scholar]
- 58.Roditis P, Dimopoulos S, Sakellariou D, Sarafoglou S, Kaldara E, Venetsanakos J, Vogiatzis J, Anastasiou-Nana M, Roussos C, Nanas S. The effects of exercise training on the kinetics of oxygen uptake in patients with chronic heart failure. Eur J Cardiovasc Prev Rehabil 14: 304–311, 2007 [DOI] [PubMed] [Google Scholar]
- 59.Rumsey WL, Vanderkooi JM, Wilson DF. Imaging of phosphorescence: a novel method for measuring oxygen distribution in perfused tissue. Science 241: 1649–1651, 1988 [DOI] [PubMed] [Google Scholar]
- 60.Shoemaker JK, Phillips SM, Green HJ, Hughson RL. Faster femoral artery blood velocity kinetics at the onset of exercise following short-term training. Cardiovasc Res 31: 278–286, 1996 [PubMed] [Google Scholar]
- 61.Sperandio PA, Borghi-Silva A, Barroco A, Nery LE, Almeida DR, Neder JA. Microvascular oxygen delivery-to-utilization mismatch at the onset of heavy-intensity exercise in optimally treated patients with CHF. Am J Physiol Heart Circ Physiol 297: H1720–H1728, 2009 [DOI] [PubMed] [Google Scholar]
- 62.Srere PA. Citrate synthase. Methods Enzymol 13: 3–11, 1969 [Google Scholar]
- 63.Stamler JS, Meissner G. Physiology of nitric oxide in skeletal muscle. Physiol Rev 81: 209–237, 2001 [DOI] [PubMed] [Google Scholar]
- 64.Stary CM, Hogan MC. Effect of varied extracellular PO2 on muscle performance in Xenopus single skeletal muscle fibers. J Appl Physiol 86: 1812–1816, 1999 [DOI] [PubMed] [Google Scholar]
- 65.Tonkonogi M, Sahlin K. Physical exercise and mitochondrial function in human skeletal muscle. Exerc Sport Sci Rev 30: 129–137, 2002 [DOI] [PubMed] [Google Scholar]
- 66.Varin R, Mulder P, Richard V, Tamion F, Devaux C, Henry JP, Lallemand F, Lerebours G, Thuillez C. Exercise improves flow-mediated vasodilatation of skeletal muscle arteries in rats with chronic heart failure. Role of nitric oxide, prostanoids, and oxidant stress. Circulation 99: 2951–2957, 1999 [DOI] [PubMed] [Google Scholar]
- 67.Wilson DF, Lee WM, Makonnen S, Finikova O, Apreleva S, Vinogradov SA. Oxygen pressures in the interstitial space and their relationship to those in the blood plasma in resting skeletal muscle. J Appl Physiol 101: 1648–1656, 2006 [DOI] [PubMed] [Google Scholar]
- 68.Yi GH, Zwas D, Wang J. Chronic exercise training preserves prostaglandin-induced dilation of epicardial coronary artery during development of heart failure in awake dogs. Prostaglandins Other Lipid Mediat 60: 137–151, 2000 [DOI] [PubMed] [Google Scholar]