Abstract
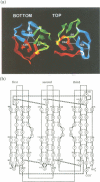
The crystal structure of vitelline membrane outer layer protein I (VMO-I), which is isolated from the vitelline membrane outer layer of hen's eggs, has been determined by the multiple isomorphous replacement method and refined to an R-factor of 18.8% at 2.2 A resolution. The main chain folds into an unusual structure that consists of three beta-sheets forming Greek key motifs, which are related by an internal pseudo three-fold symmetry. The internal portion surrounded by these three beta-sheets is filled with hydrophobic side chains. This conformational feature coincides with three internal repeats in the sequence. Although a similar fold exists in the second domain of delta-endotoxin, there are significant structural differences between the two proteins, with the three-fold symmetry being most regular in VMO-I.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Back J. F., Bain J. M., Vadehra D. V., Burley R. W. Proteins of the outer layer of the vitelline membrane of hen's eggs. Biochim Biophys Acta. 1982 Jul 12;705(1):12–19. doi: 10.1016/0167-4838(82)90329-6. [DOI] [PubMed] [Google Scholar]
- Bain J. M., Hall J. M. Observations on the development and structure of the vitelline membrane of the hen's egg: an electron microscope study. Aust J Biol Sci. 1969 Jun;22(3):653–665. doi: 10.1071/bi9690653. [DOI] [PubMed] [Google Scholar]
- Bass S. H., Mulkerrin M. G., Wells J. A. A systematic mutational analysis of hormone-binding determinants in the human growth hormone receptor. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4498–4502. doi: 10.1073/pnas.88.10.4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowie J. U., Lüthy R., Eisenberg D. A method to identify protein sequences that fold into a known three-dimensional structure. Science. 1991 Jul 12;253(5016):164–170. doi: 10.1126/science.1853201. [DOI] [PubMed] [Google Scholar]
- Brünger A. T., Krukowski A., Erickson J. W. Slow-cooling protocols for crystallographic refinement by simulated annealing. Acta Crystallogr A. 1990 Jul 1;46(Pt 7):585–593. doi: 10.1107/s0108767390002355. [DOI] [PubMed] [Google Scholar]
- Brünger A. T., Kuriyan J., Karplus M. Crystallographic R factor refinement by molecular dynamics. Science. 1987 Jan 23;235(4787):458–460. doi: 10.1126/science.235.4787.458. [DOI] [PubMed] [Google Scholar]
- Chothia C. Proteins. One thousand families for the molecular biologist. Nature. 1992 Jun 18;357(6379):543–544. doi: 10.1038/357543a0. [DOI] [PubMed] [Google Scholar]
- Cleary S., Mulkerrin M. G., Kelley R. F. Purification and characterization of tissue plasminogen activator kringle-2 domain expressed in Escherichia coli. Biochemistry. 1989 Feb 21;28(4):1884–1891. doi: 10.1021/bi00430a068. [DOI] [PubMed] [Google Scholar]
- Cura V., Krishnaswamy S., Podjarny A. D. Heavy-atom refinement against solvent-flattened phases. Acta Crystallogr A. 1992 Sep 1;48(Pt 5):756–764. doi: 10.1107/s0108767392003416. [DOI] [PubMed] [Google Scholar]
- Day L. A. Circular dichroism and ultraviolet absorption of a deoxyribonucleic acid binding protein of filamentous bacteriophage. Biochemistry. 1973 Dec 18;12(26):5329–5339. doi: 10.1021/bi00750a017. [DOI] [PubMed] [Google Scholar]
- Hutchinson E. G., Thornton J. M. The Greek key motif: extraction, classification and analysis. Protein Eng. 1993 Apr;6(3):233–245. doi: 10.1093/protein/6.3.233. [DOI] [PubMed] [Google Scholar]
- Jensen C. Ultrastructural changes in the avian vitelline membrane during embryonic development. J Embryol Exp Morphol. 1969 Jun;21(3):467–484. [PubMed] [Google Scholar]
- Jones T. A., Thirup S. Using known substructures in protein model building and crystallography. EMBO J. 1986 Apr;5(4):819–822. doi: 10.1002/j.1460-2075.1986.tb04287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones T. A., Zou J. Y., Cowan S. W., Kjeldgaard M. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr A. 1991 Mar 1;47(Pt 2):110–119. doi: 10.1107/s0108767390010224. [DOI] [PubMed] [Google Scholar]
- Kido S., Janado M., Nunoura H. Macromolecular components of the vitelline membrane of hen's egg. I. Membrane structure and its deterioration with age. J Biochem. 1975 Aug;78(2):261–268. doi: 10.1093/oxfordjournals.jbchem.a130903. [DOI] [PubMed] [Google Scholar]
- Kido S., Janado M., Nunoura H. Macromolecular components of the vitelline membrane of hen's egg. II. Physicochemical properties of glycoprotein I. J Biochem. 1976 Jun;79(6):1351–1356. doi: 10.1093/oxfordjournals.jbchem.a131189. [DOI] [PubMed] [Google Scholar]
- Kido S., Janado M., Nunoura H. Macromolecular components of the vitelline membrane of hen's egg. III. Physicochemical properties of glycoprotein II. J Biochem. 1977 May;81(5):1543–1548. [PubMed] [Google Scholar]
- Kido S., Morimoto A., Kim F., Doi Y. Isolation of a novel protein from the outer layer of the vitelline membrane. Biochem J. 1992 Aug 15;286(Pt 1):17–22. doi: 10.1042/bj2860017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J. D., Carroll J., Ellar D. J. Crystal structure of insecticidal delta-endotoxin from Bacillus thuringiensis at 2.5 A resolution. Nature. 1991 Oct 31;353(6347):815–821. doi: 10.1038/353815a0. [DOI] [PubMed] [Google Scholar]
- Nishikawa K., Matsuo Y. Development of pseudoenergy potentials for assessing protein 3-D-1-D compatibility and detecting weak homologies. Protein Eng. 1993 Nov;6(8):811–820. doi: 10.1093/protein/6.8.811. [DOI] [PubMed] [Google Scholar]
- Nishikawa K., Nakashima H., Kanehisa M., Ooi T. Detection of weak sequence homology of proteins for tertiary structure prediction. Protein Seq Data Anal. 1987;1(2):107–116. [PubMed] [Google Scholar]
- Perczel A., Park K., Fasman G. D. Deconvolution of the circular dichroism spectra of proteins: the circular dichroism spectra of the antiparallel beta-sheet in proteins. Proteins. 1992 May;13(1):57–69. doi: 10.1002/prot.340130106. [DOI] [PubMed] [Google Scholar]
- Richardson J. S. beta-Sheet topology and the relatedness of proteins. Nature. 1977 Aug 11;268(5620):495–500. doi: 10.1038/268495a0. [DOI] [PubMed] [Google Scholar]
- Rould M. A., Perona J. J., Steitz T. A. Improving multiple isomorphous replacement phasing by heavy-atom refinement using solvent-flattened phases. Acta Crystallogr A. 1992 Sep 1;48(Pt 5):751–756. doi: 10.1107/s0108767392003404. [DOI] [PubMed] [Google Scholar]
- Schnepf H. E., Tomczak K., Ortega J. P., Whiteley H. R. Specificity-determining regions of a lepidopteran-specific insecticidal protein produced by Bacillus thuringiensis. J Biol Chem. 1990 Dec 5;265(34):20923–20930. [PubMed] [Google Scholar]
- Wang B. C. Resolution of phase ambiguity in macromolecular crystallography. Methods Enzymol. 1985;115:90–112. doi: 10.1016/0076-6879(85)15009-3. [DOI] [PubMed] [Google Scholar]
- Widner W. R., Whiteley H. R. Location of the dipteran specificity region in a lepidopteran-dipteran crystal protein from Bacillus thuringiensis. J Bacteriol. 1990 Jun;172(6):2826–2832. doi: 10.1128/jb.172.6.2826-2832.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]