Abstract
Signals from the vestibular system, area postrema, and forebrain elicit nausea and vomiting, but gastrointestinal (GI) vagal afferent input arguably plays the most prominent role in defense against food poisoning. It is difficult to determine the contribution of GI vagal afferent input on emesis because various agents (e.g., chemotherapy) often act on multiple sensory pathways. Intragastric copper sulfate (CuSO4) potentially provides a specific vagal emetic stimulus, but its actions are not well defined in musk shrews (Suncus murinus), a primary small animal model used to study emesis. The aims of the current study were 1) to investigate the effects of subdiaphragmatic vagotomy on CuSO4-induced emesis and 2) to conduct preliminary transneuronal tracing of the GI-brain pathways in musk shrews. Vagotomy failed to inhibit the number of emetic episodes produced by optimal emetic doses of CuSO4 (60 and 120 mg/kg ig), but the effects of lower doses were dependent on an intact vagus (20 and 40 mg/kg). Vagotomy also failed to affect emesis produced by motion (1 Hz, 10 min) or nicotine administration (5 mg/kg sc). Anterograde transport of the H129 strain of herpes simplex virus-1 from the ventral stomach wall identified the following brain regions as receiving inputs from vagal afferents: the nucleus of the solitary tract, area postrema, and lateral parabrachial nucleus. These data indicate that the contribution of vagal pathways to intragastric CuSO4-induced emesis is dose dependent in musk shrews. Furthermore, the current neural tracing data suggest brain stem anatomical circuits that are activated by GI signaling in the musk shrew.
Keywords: taste aversion, pica, vagus, Suncus murinus
nausea and vomiting are common symptoms in numerous medical conditions, including advanced cancer and diabetic gastroparesis (e.g., 12, 45) and are prominent side effects of medical treatments such as cancer chemotherapy, inhalational anesthetics, and opioid analgesics (e.g., 13, 29). Research focused on these clinical conditions and chemical stimuli provide little insight into the normal physiology of nausea and emesis. Neural inputs from four sources have been identified as eliciting nausea and vomiting: 1) gastrointestinal (GI) vagal afferents, 2) area postrema (AP), 3) vestibular system, and 4) forebrain circuits. These neural systems are activated by the presence of toxins in the GI tract (vagal afferents) and vascular system (AP), provocative motion (vestibular nuclear complex), and conditioned stimuli (forebrain; e.g., conditioned food aversion) (see Review in 15). The vagal afferent pathway from the GI tract is positioned to serve a primary role in the initial defense against food poisoning. Nevertheless, it is difficult to determine the contribution of GI vagal afferent input to emetic stimulation because various chemical agents used in mechanistic research studies often act on multiple pathways to provoke nausea and emesis. For example, cisplatin chemotherapy can stimulate vagal afferent fibers and the AP to produce emesis (1, 35).
The traditional stimulus for investigating the GI vagal afferent component of emesis is intragastric administration of copper sulfate (CuSO4), a gastric irritant (25, 50); however, its action in musk shrews (Suncus murinus), the primary small animal model for emesis research, is not well defined (e.g., 17, 20, 26, 31, 34, 47). Furthermore, although viral tracing techniques have been used to identify brain regions that receive abdominal vagal afferent inputs in nonemetic rodents (37), parallel studies in emetic species have not been conducted. To assess the mechanisms of action of CuSO4 in musk shrews, we needed to identify the brain regions that receive inputs from abdominal vagal afferents in this species. The aims of the current study were 1) to investigate the effects of subdiaphragmatic vagotomy on CuSO4-induced emesis and 2) to conduct preliminary transneuronal tracing experiments to determine the anatomy of the stomach-to-brain connection in musk shrews. We also assessed completeness of vagotomy by using the gold standard from studies in the rat, retrograde transport of the neuronal tracer Fluoro-Gold from the peritoneal cavity to the dorsal motor nucleus (DMN) (36). The H129 strain of herpes simplex virus was used for stomach-to-brain tracing because it is primarily transported in an anterograde direction to label ascending sensory pathways from the periphery to the brain (2, 4, 37, 43). H129 virus was microinjected into the ventral surface muscle of the stomach, and the centrally transported virus was localized immunohistochemically in the brain.
MATERIALS AND METHODS
Animals.
Studies included 63 adult musk shrews (>45 days of age), offspring from breeding stock obtained from the Chinese University of Hong Kong (a strain originating from Taiwan) (49) or the University of Virginia (a strain originating from Guam) (11), and housed individually. Musk shrews were fed a mixture of 75% Purina Cat Chow Complete Formula and 25% Complete Gro-Fur mink food pellets (38, 46). Six adult male Sprague-Dawley rats (Charles River; Wilmington, MA) were also used as controls for viral tracing studies. Rats were pair-housed and fed standard chow (5P76 Pro-Lab IsoPro RMH3000). All animals were maintained on a 12-h light/12-h dark cycle (0700–1900 h light period), with free access to food and water. Experiments were approved by the University of Pittsburgh's Institutional Animal Care and Use Committee. Animals were housed in an animal care facility accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International.
Study 1: effects of vagotomy on nicotine, CuSO4, and motion-induced emesis.
Study 1 included 8 sham-operated (64.5 ± 1.3 g; means ± SE) and 8 vagotomized (68.5 ± 1.9 g) male musk shrews (Taiwan strain). Shrews were anesthetized with isoflurane (2 to 3%) using an induction chamber (10 × 8.5 cm, height and diameter of cylinder) followed by a nose cone during surgery. The ventral abdominal surface was shaved from the costal margin to the inguinal ligament. The skin was subsequently sterilized with betadine surgical scrub and 70% isopropyl alcohol. A midline 1.5-cm laparotomy incision was made, and the ventral and dorsal vagal trunks running along the esophagus were bluntly dissected and transected using a thermal cautery. The peritoneum was sutured (2–0 silk; Ethicon), and the skin was closed with surgical staples (7.5 × 1.75 mm, Michel). Sham operations were performed using a similar approach as vagotomy surgery, except that the vagi were not manipulated or lesioned. Animals were weighed daily after surgery to assess body weight changes and were allowed to recover for 1 wk before behavioral testing. After surgery, ketoprofen was administered for 2 days (2 mg/kg sc, twice daily).
Three emetic tests were conducted with 1 wk between each test (first test = nicotine, second test = CuSO4, and final test = motion). Nicotine [(-)-nicotine, catalog no. 36733] and CuSO4 [copper (II) sulfate pentahydrate, catalog no. 209198] were obtained from Sigma-Aldrich (St. Louis, MO). Nicotine was made as a 2.5 mg/ml solution in sterile saline (0.15 M NaCl; subcutaneous injection = 5 mg·kg−1·2 ml−1), and CuSO4 was dissolved in filtered water (Milli-Q) at a concentration of 24 mg/ml (gavage injection = 120 mg·kg−1·5 ml−1). Chemical doses were based on those previously demonstrated to induce peak levels of emesis (7, 40, 54). For motion exposure, the test chambers (28 × 17 × 12 cm; length by width by height) were covered with a clear acrylic lid placed directly on the top and were placed on a reciprocating shaker (Taitec, Double Shaker R-30, Taiyo Scientific Industrial). Horizontal motion (4 cm displacement; 2 cm left and 2 cm right; 1 Hz) was applied for 10 min. These motion exposure parameters were previously shown to be optimal for inducing emesis in musk shrews (22). Up to four animals were tested simultaneously between 0800 and 1600 h (light phase). For all tests, animals had 15 min of adaption in the test chambers (28 × 17 × 12 cm; length by width by height) before injection of chemicals or the start of motion and 30 min after these manipulations. All animal behavior was recorded with a digital video camera (Sony DCR-SR300 or HDR-XR550V, wide field lenses) placed above each test chamber and connected to a computer for storage (Media Recorder; Noldus Information Technology). A trained observer was positioned outside the transparent test chambers to record the occurrence of an emetic episode (with or without a vomit), abdominal contraction, or a swaying movement using a notebook computer installed with coding software (JWatcher; http://www.jwatcher.ucla.edu/). Abdominal contractions and swaying motions (moving the center of body from side to side) are commonly associated with emesis in musk shrews (19). An emetic episode was recognized as a sequence of contractions of the abdomen and head movements (retching).
To verify the completeness of subdiaphragmatic vagotomy, animals were injected with 0.2 ml (ip) of the retrograde tracer Fluoro-Gold (1% solution in 0.15 M NaCl; Fluorochrome, Denver, CO) to label the abdominal vagal projection from the DMN. This procedure has been used in musk shrews and rats (10, 36). Five days after injection with Fluoro-Gold, animals were euthanized with an overdose of Beuthanasia-D solution (0.03 ml, containing 390 mg/ml pentobarbital sodium; Intervet/Merck). Following lack of respiration, animals were transcardially perfused with 0.15 M NaCl rinse and then 4% paraformaldehyde fixative. After an overnight fixation in 4% paraformaldehyde and 24 h cryoprotection in 20% sucrose, brains were frozen on dry ice and sectioned at 30 μm directly to microscope slides using a cryostat (−20°C; Microm HM500). Microscope slides were allowed to dry overnight and then rinsed in Milli-Q water, 70% ethanol, 95% ethanol, 100% ethanol, and Histoclear. Slides were coverslipped with DPX mountant (VWR).
Study 2: effects of vagotomy on emesis induced by low doses of CuSO4.
Study 2 included 8 sham-operated (77.2 ± 3.0 g) and 6 vagotomized (79.7 ± 4.4 g) male musk shrews (Taiwan strain). Procedures were the same as in study 1, with the exception that a different dose of intragastric CuSO4 was administered during each weekly testing session (first test = 20 mg/kg, second test = 40 mg/kg, and final test = 60 mg/kg; each administered in a volume of 5 ml/kg).
Study 3: intraperitoneal versus ventral stomach injection of H129.
Study 3 included 25 musk shrews (24.4 ± 0.9 g, females and 36.3 ± 1.2 g, males; Guam strain). Six Sprague-Dawley male rats (344 ± 14.5 g) were also used as a control condition because H129 labeling of the vagal system is documented in this species (37). Surgery and recovery were performed in a Biosafety Level 2 facility. Animals were anesthetized with either isoflurane inhalation (1–3% in oxygen) or pentobarbital sodium (40–50 mg/kg ip). A midline incision was made to open the abdominal wall, and the stomach was gently retracted to reveal the ventral gastric surface, where virus injections were made adjacent to the arterial branches. In each animal, four to six injections (5 to 10 μl of total volume per animal; 18 shrews and 6 rats) were made using a 10-μl Hamilton syringe with a 33- to 36-gauge needle (Nanofil; World Precision Instruments). The H129 virus stock (4.7 × 107 pfu/ml) was stored in 100-μl aliquots at −80°C and thawed only once, immediately before use. H129 virus was obtained from the Center for Neuroanatomy with Neurotropic Viruses (www.cnnv.pitt.edu). In additional shrews (n = 7), H129 virus (5 to 10 μl of total volume) was administered by dripping the H129 onto the stomach (after opening the peritoneal cavity), to determine whether central viral labeling in stomach-injected cases could result from leakage of virus from the stomach wall into the abdominal cavity. After surgery, ketoprofen was administered for 2 days (2 mg/kg sc, twice daily). All animals were weighed before surgery and daily after surgery.
Musk shrews were euthanized 5 or 9 days postinjection; rats were euthanized 3 or 5 days postinjection. Animals were given an overdose of pentobarbital sodium (Nembutal; 100 mg/kg ip; shrews and rats) or Beuthanasia-D (0.03 ml ip; shrews only) and transcardially perfused with 0.2 M phosphate-buffered saline (PBS) followed by 4% paraformaldehyde (in 0.1 M PB). The brain and spinal cord (from T8 to T10) were postfixed for 24 to 48 h and then placed in 30% sucrose for 24 to 48 h. Tissue was then frozen on dry ice and stored at −80°C, cut at 40 μm using a cryostat (−20°C), and collected into cryopreservant solution (51) in three (shrew) or six (rat) serial sets, and stored at −20°C until immunolabeling.
For immunohistochemistry, sections were removed from cryopreservant and rinsed in 0.1 M PB for 1 h, incubated in 0.3% hydrogen peroxide in 0.1 M PB, and then rinsed with 0.1 M PB. The tissue was placed in primary antibody overnight with gentle agitation at room temperature. The primary antibody (polyclonal rabbit anti-HSV-1, Dako, Lot no. 00050463) was diluted 1:20 K in 0.1 M PB containing 1% normal donkey serum and 0.3% Triton X-100. Sections were rinsed with 0.1 M PB for an hour and then placed in the secondary antibody for 1 h at room temperature with agitation. The secondary antibody (biotinylated donkey anti-rabbit IgG; Jackson Immuno) was diluted to 1:500 in 0.1 M PB with 1% donkey serum and 0.3% Triton X-100. The tissue was then rinsed with 0.1 M PB for 30 min and placed in AB complex (Vectastain Elite kit, Vector Laboratories) for 90 min at room temperature with agitation. The AB complex was made 30 min before use by combining 4.5 μl of “A” and 4.5 ul of “B” in 1 ml of 0.1 M PB containing 0.3% Triton X-100. The tissue was then rinsed with 0.1 M PB for 30 min followed by 0.1 M Tris buffer for 10 min. For the chromogen reaction, tissues were placed in 0.1 M Tris buffer containing 0.1% diaminobenzidine and 0.01% hydrogen peroxide for 15 min. Finally, the tissues were rinsed in 0.1 M Tris buffer for 10 min and 0.1 M PB for 20 min. Anti-HSV-1 antibody specificity was confirmed by the complete absence of immunolabeling in brain tissue sections from shrews that were not injected with H129 but were processed in the same manner. Immunoreacted tissue sections were mounted onto microscope slides and allowed to dry overnight and then rinsed in Milli-Q water, 70% ethanol, 95% ethanol, 100% ethanol, and Histoclear. Slides were coverslipped with DPX mountant.
Study 4: ventral stomach injection of a higher titer of H129 virus.
Study 4 was conducted to 1) assess if more labeling of the forebrain of shrews could be produced by using a higher titer of H129 virus, 2) determine whether a shorter time frame of transport would produce more labeling (i.e., 3 days), and 3) to confirm that the results from study 3 were not specific to Guam-derived musk shrews. Study 4 included eight male musk shrews (66.5 ± 1.8 g; Taiwan strain). All procedures were the same as study 3 except that a new virus stock of H129 was used with a titer of 1.95 × 1010 pfu/ml. Shrews were euthanized at 3 (n = 4) or 5 days (n = 4) postinjection of virus into the ventral stomach wall.
Data analysis.
For behavioral data, JWatcher data files containing emesis and other behavioral time stamps were processed using custom scripts written in Matlab (R2010a; The Mathworks). Extracted variables included the total of all emetic episodes, total episodes with vomiting, total episodes without vomiting, the duration of emesis (time from first to last episode; min), emetic rate (episodes/min), standard deviation of the emetic episode interval (SD-I; min), total abdominal contractions, total swaying movements, and latency to the first emetic episode (min) (19). Total number of emetic episodes (all, with vomiting, and without vomiting), duration, rate, SD-I, abdominal contractions, and swaying were analyzed using two-way ANOVA (stimulus by surgery condition). Tukey's tests were used to compare means between sham and vagotomy conditions after ANOVA. We applied survival plots and Cox regression analysis to latency data, permitting the use of all of the data, including censored values (i.e., animals without emesis during the test period). P < 0.05 was used to determine statistical significance.
Visual verification of subdiaphragmatic vagotomy is not reliable because of growth of connective tissue at the surgical site during recovery. The presence of Fluoro-Gold in the DMN was determined by fluorescent detection. Images were collected using a Nikon epifluorescence microscope equipped with a wide-band UV filter and analyzed with ImageJ software (NIH; http://rsb.info.nih.gov/ij/) to assess the density of staining (gray level) in the left and right DMN relative to the adjacent nucleus tractus solitarus (NTS; higher percentage values indicate more fluorescent staining in the DMN compared with the NTS). NTS labeling indicated the level of background fluorescence in the hindbrain. Data values were collected by an experimenter naïve to the surgical condition of the samples.
For viral tracing analysis, every third section through the brain and spinal cord (i.e., one section every 120 μm) was analyzed for labeling by an experimenter naïve to treatment condition. A Zeiss Axiostar Plus light microscope was used for semiquantitative evaluation of viral labeling (brown immunoperoxidase) within the caudal, middle, and rostral NTS, AP, DMN, parabrachial nucleus (PBN), paraventricular nucleus of the hypothalamus (PVN), amygdala, bed nucleus of the stria terminalis (BNST), and insular cortex. These brain areas were selected a priori based on a report of central transneuronal labeling after H129 injections into the stomach wall in rats (37). Digital images for presentation were collected using an Olympus BX51 microscope with a Hamamatsu Photonics ORCA-ER camera. H129 viral labeling was scored using a three-point scale: + = 1 to 2 cells in an area; ++ = partial labeling of an area (i.e., more than 1 or 2 cells); +++ = moderate to dense labeling throughout the area (cells and processes fill most or all of the area) (Table 1).
Table 1.
Summary of labeling after H129 injection into the ventral stomach of musk shrews
| NTS |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Animal | Days | Spinal Cord | Caudal | Middle | Rostral | AP | DMN | RF | PBN | Cerebellum |
| Study 3 | ||||||||||
| 38 | 5 | − | + | − | ++ | − | + | − | − | − |
| 165 | 5 | + | ++ | ++ | ++ | − | ++ | ++ | − | − |
| 42 | 5 | + | + | + | ++ | − | ++ | ++ | ++ | + |
| 170 | 5 | − | ++ | ++ | ++ | − | ++ | ++ | ++ | + |
| 179 | 5 | + | ++ | ++ | +++ | − | ++ | ++ | ++ | + |
| 35 | 5 | − | ++ | ++ | +++ | + | +++ | ++ | ++ | + |
| 103 | 5 | ++ | ++ | +++ | +++ | ++ | ++ | ++ | ++ | + |
| 39 | 5 | − | ++ | +++ | +++ | ++ | +++ | +++ | +++ | + |
| 40 | 9 | + | − | − | − | − | − | − | − | − |
| 51 | 9 | + | − | − | − | − | + | + | − | − |
| 68 | 9 | − | − | − | − | − | − | ++ | − | − |
| 21 | 9 | + | − | ++ | ++ | − | +++ | − | − | − |
| Study 4 | ||||||||||
| 91 | 3 | − | + | + | − | − | − | − | − | − |
| 104 | 3 | − | − | + | ++ | − | +++ | + | − | − |
| 92 | 3 | − | + | + | ++ | − | +++ | + | − | − |
| 103 | 5 | ++ | − | + | − | − | − | − | − | − |
| 97 | 5 | ++ | ++ | ++ | +++ | − | ++ | ++ | + | ++ |
| 119 | 5 | ++ | ++ | +++ | +++ | + | ++ | +++ | +++ | +++ |
+, 1 to 2 cells; ++, partial area; +++, all of area. See text for key to abbreviations.
RESULTS
Study 1: effects of vagotomy on nicotine, CuSO4, and motion-induced emesis.
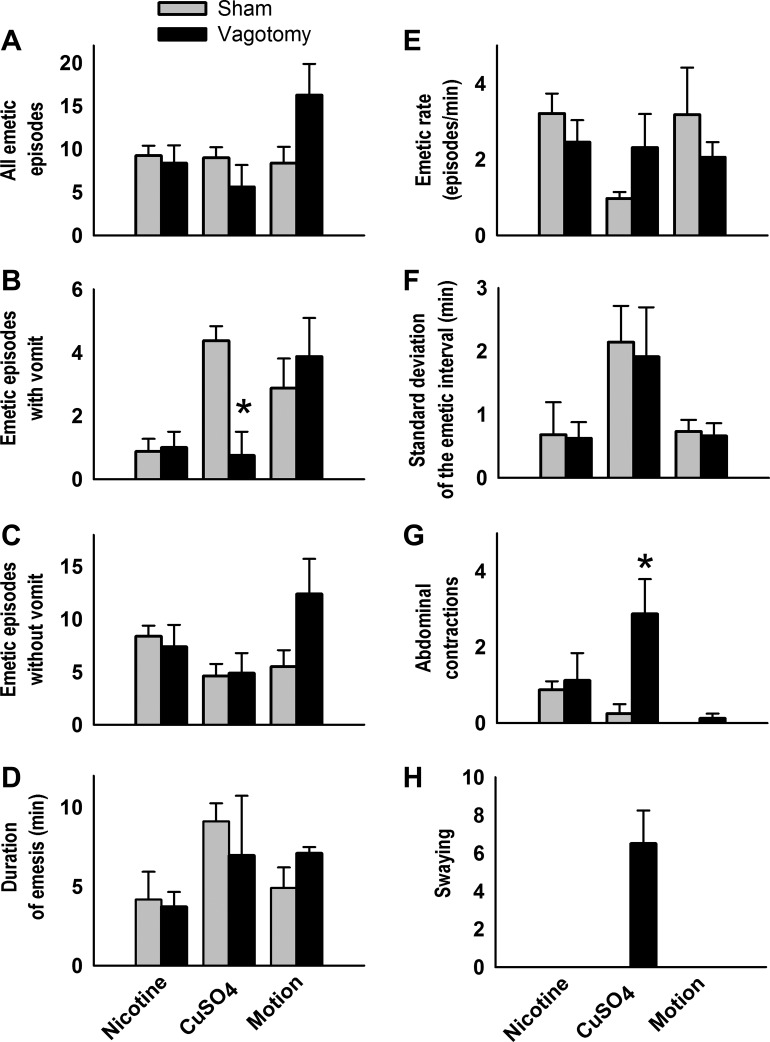
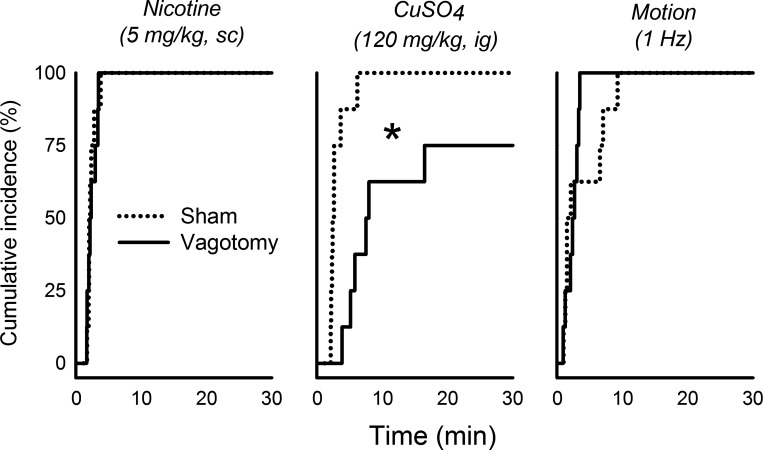
There were significant interactions between surgical condition and emetic stimulus for total number of emetic episodes, episodes with vomiting, episodes without vomiting, and abdominal contractions [Fs(2,28) ≥ 3.6, p ≤ 0.05, interaction effects, ANOVA, Fig. 1, A–C and G]. CuSO4 injection in vagotomized shrews, compared with sham-operated controls, produced a significant decrease in vomiting and increase in abdominal contractions (P < 0.05, Tukey's tests; Fig. 1, B and G). SD-I was increased in the CuSO4 condition compared with nicotine or motion exposure [F(2,24) = 6.7, P < 0.005, main effect of stimulus, ANOVA, Fig. 1F]. There were no significant differences in cumulative emetic latency after nicotine injection or motion exposure between surgical conditions, but vagotomized shrews did show a significantly longer latency to the first emetic episode compared with sham-operated controls after injection of CuSO4 (P < 0.05, Cox regression; Fig. 2).
Fig. 1.
Effects of nicotine injection (5 mg/kg sc), copper sulfate injection (CuSO4, 120 mg/kg ig), and motion exposure (10 min, 1 Hz) on the total number of emetic episodes (A), emetic episodes with vomiting (B), emetic episodes without vomiting (C), duration of emesis (D), emetic rate (E), standard deviation of the emetic interval (F), abdominal contractions (G), and swaying (H) in sham-operated compared with subdiaphragmatic vagotomized musk shrews. *P < 0.05, Tukey's test, sham vs. vagotomy. Data represent means ± SE.
Fig. 2.
Effects of nicotine injection (5 mg/kg sc), CuSO4 injection (120 mg/kg ig), and motion exposure (10 min, 1 Hz) on the cumulative latency (incidence) to the first emetic episode in sham-operated compared with subdiaphragmatic vagotomized musk shrews. *P < 0.05, Cox regression.
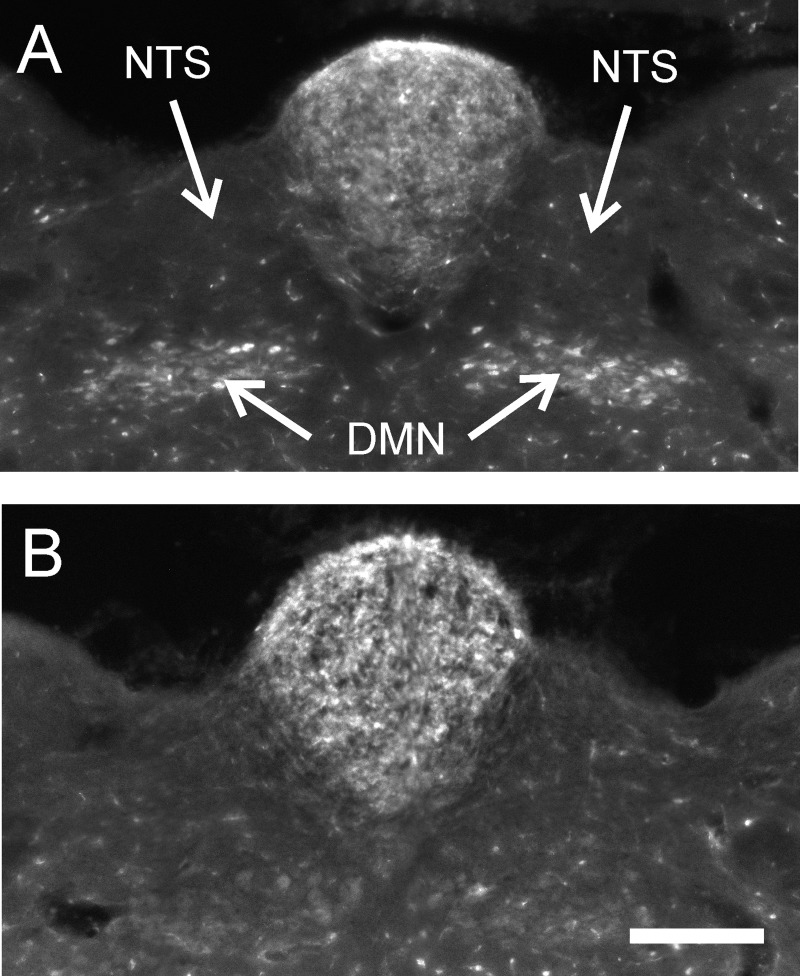
Subdiaphragmatic vagotomy eliminated fluorescent labeling of motor neurons in the DMN (see samples in Fig. 3). The percentage gray level of the DMN relative to the adjacent NTS was significantly different between sham and vagotomized animals [52.1 ± 7.6% vs. 12.9 ± 2.7%, respectively; t(14) = 4.6, P = 0.0005, one-tailed test]. Notably there are low levels of autofluorescence of the brain using this methodology, and therefore percentage values for the DMN, which has relatively larger cell bodies than the adjacent NTS, will not be zero. Body weights were slightly reduced (percentage of presurgery weight) in the vagotomized condition compared with sham-operated controls at 6 days after surgery compared with before the first emesis test [−6.9 ± 1.2% compared with −2.0 ± 1.5%, respectively; t(14) = 2.2, P < 0.03]; but the groups displayed identical increases in body weight by the time of tissue collection 1 wk after the last emesis test (7.4 ± 1.9% compared with 7.2 ± 1.2%, respectively).
Fig. 3.
Retrograde labeling of the dorsal motor nucleus (DMN) after intraperitoneal injection of Fluoro-Gold. Labeling in a sham-operated (A) and a vagotomized musk shrew (B). NTS, nucleus of the solitary tract. The scale bar = 200 μm.
Study 2: effects of vagotomy on emesis induced by low doses of CuSO4.
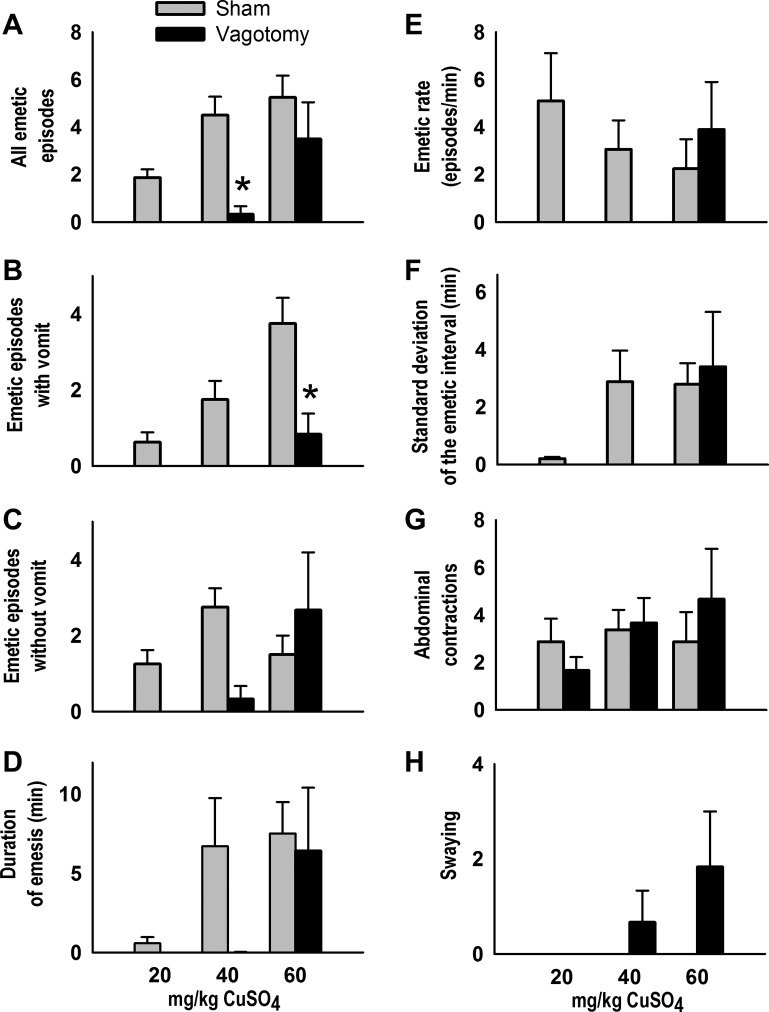
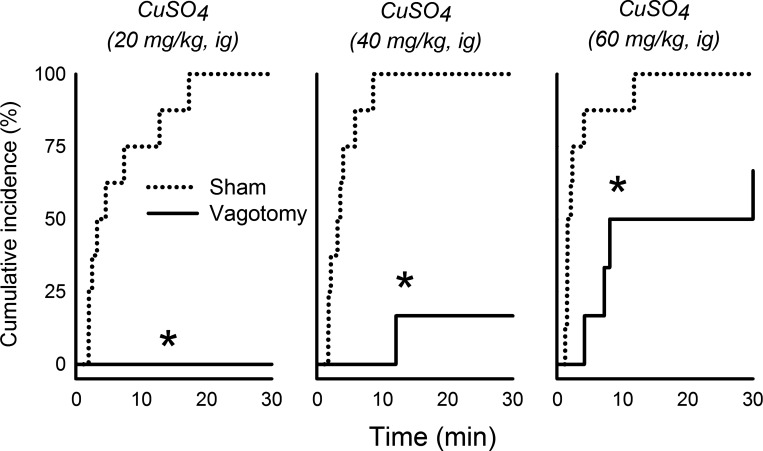
The effects of CuSO4 dose on emetic episodes with and without vomiting was dependent on the surgical condition [Fs(2,24) ≥ 3.6, P ≤ 0.05, interaction effects, ANOVA, Fig. 4, B and C]. Vagotomized shrews displayed significantly fewer total emetic episodes and emetic episodes with vomiting after intragastric injection of 40 and 60 mg/kg CuSO4, respectively, compared with sham-operated controls (P < 0.05, Tukey's test; Fig. 4, A and B). No vagotomized shrews administered a 20 mg/kg dose of CuSO4 exhibited any emetic episodes. CuSO4 administration also produced significantly more total emetic episodes in sham-operated versus vagotomized animals [F(1,24) = 11.9, P < 0.005, main effect of surgical condition, ANOVA, Fig. 4A]. Duration of emesis was significantly longer with increased dosage of CuSO4 [F(2,24) = 5.0, P < 0.02, main effect of stimulus dose, ANOVA; Fig. 4D]. Vagotomized shrews demonstrated a longer emetic latency compared with sham-operated controls for all three doses of CuSO4 (P < 0.05, Cox regression; Fig. 5).
Fig. 4.
Effects of CuSO4 injection (20, 40, or 60 mg/kg ig) on the total number of emetic episodes (A), emetic episodes with vomiting (B), emetic episodes without vomiting (C), duration of emesis (D), emetic rate (E), standard deviation of the emetic interval (F), abdominal contractions (G), and swaying (H) in sham-operated compared with subdiaphragmatic vagotomized musk shrews. *P < 0.05, Tukey's test, sham vs. vagotomy. Data represent means ± SE.
Fig. 5.
Effects of CuSO4 injection (20, 40, or 60 mg/kg ig) on the cumulative latency (incidence) to the first emetic episode in sham-operated compared with subdiaphragmatic vagotomized musk shrews. *P < 0.05, Cox regression.
Body weights were not significantly different (percentage of presurgery weight) between vagotomized and sham-operated controls at 6 days after surgery (−8.3 ± 1.9% compared with −5.2 ± 1.7%, respectively) or at the time of tissue collection (−6.2 ± 2.0% compared with −2.8 ± 0.9%, respectively). Similar to study 1, the percentage gray level of the DMN relative to the adjacent NTS was significantly different between sham and vagotomized animals [75.3 ± 15.6% versus 33.1 ± 8.8%, respectively; t(14) = 2.1, P = 0.03, one-tailed test].
Study 3: intraperitoneal versus ventral stomach injection of H129.
Shrews were euthanized 5 days (n = 10) or 9 days (n = 8) after H129 virus was injected into the ventral stomach wall. Twelve of these 18 shrews displayed viral immunolabeling in the central nervous system (Table 1), whereas 6 shrews displayed no detectable labeling. All shrews were asymptomatic on the day of euthanization, and the percentage change in body weight was not significantly different between shrews with (−7.7 ± 1.6%) or without central viral labeling (−8.9 ± 2.3%).
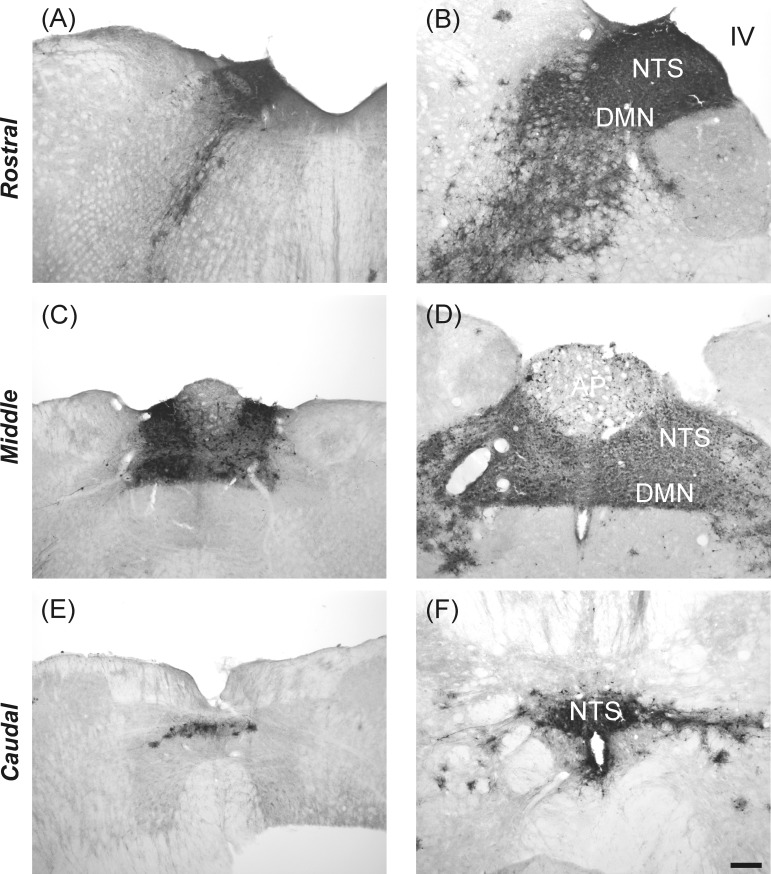
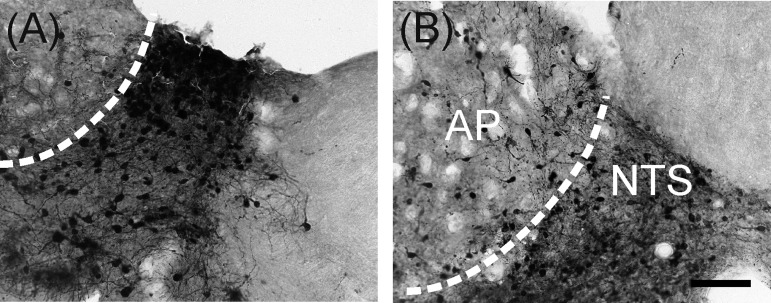
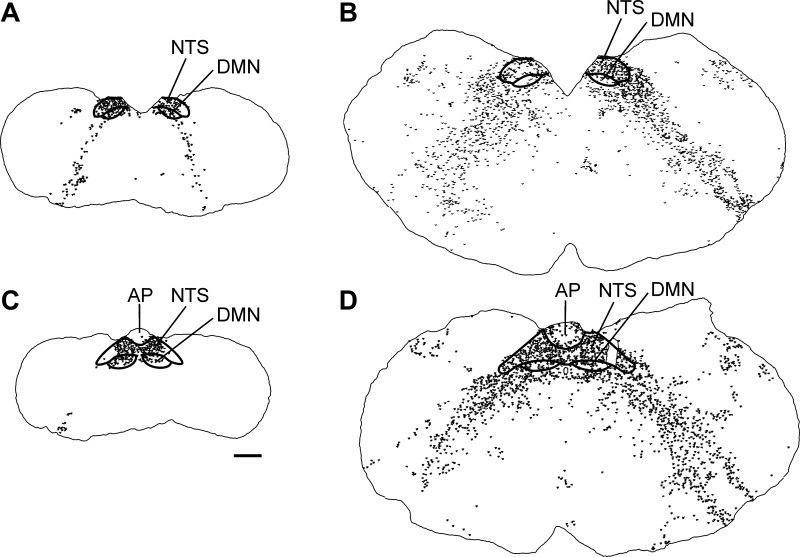
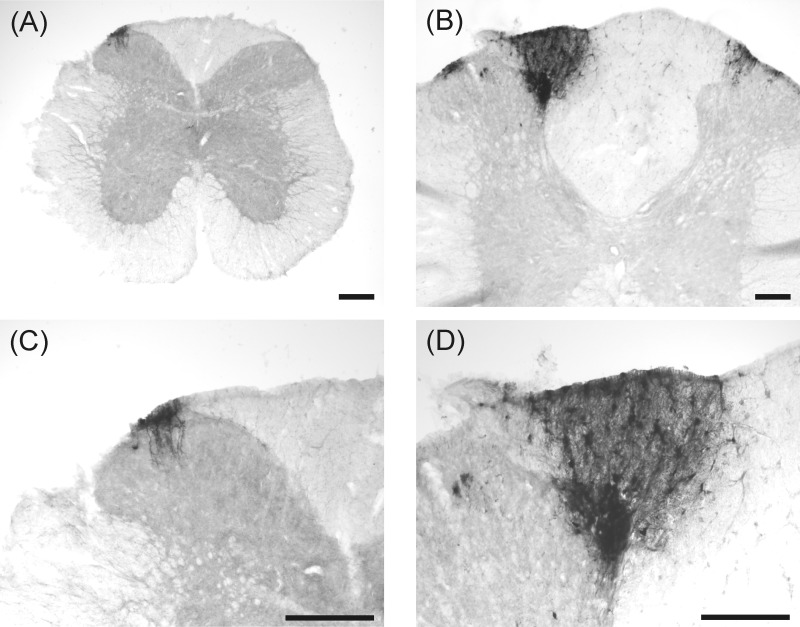
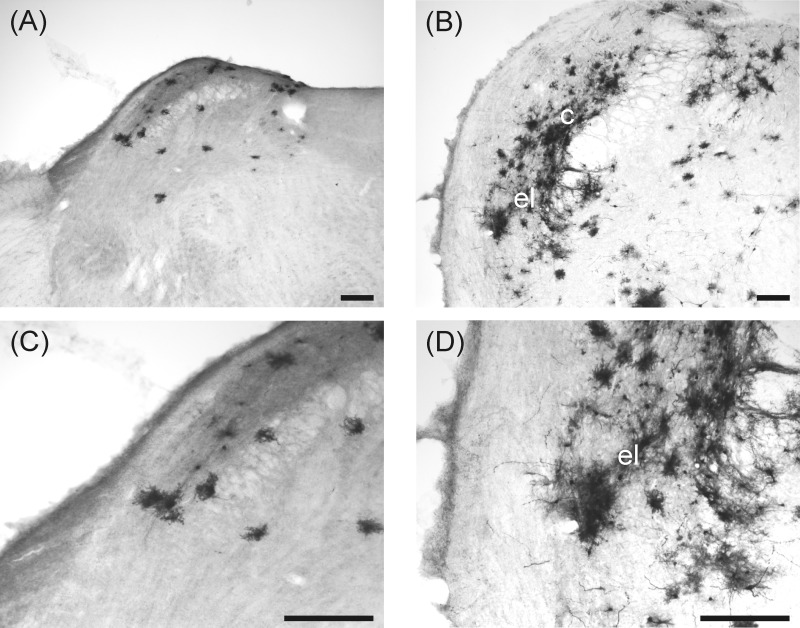
Viral immunoperoxidase labeling appeared bilaterally in the brain. Viral labeling was more prevalent in shrews euthanized at 5 days versus 9 days postinjection (Table 1). Immunoreactive cells were present within the NTS at levels caudal to the AP, at the level of the AP, and rostral to the AP (Table 1 and Fig. 6; left, shrew and right, rat). Labeling also extended into the AP, DMN, and hindbrain reticular formation, including parts of the nucleus ambiguus (Table 1; Figs. 6, 7, and 8). Labeling within the dorsal horn of the spinal cord occurred in seven shrews (Table 1 and Fig. 9), whereas no preganglionic sympathetic neurons were labeled in the intermediolateral cell column. Labeled cells appeared in the medial and lateral PBN and adjacent levels of the cerebellum (Fig. 10 and Table 1). Forebrain labeling was much less prominent than hindbrain or midbrain labeling. We detected labeling within the PVN in only three shrews, one of which also displayed a few labeled cells in the amygdala and BNST (data not shown).
Fig. 6.
Labeling of H129 infection at three levels of the NTS of musk shrews (left: A, C, and E) and rats (right: B, D, and F) after inoculation of the ventral stomach wall. The scale bar = 200 μm.
Fig. 7.
High magnification images of NTS and area postrema (AP) showing H129 labeling in musk shrews (A) and rats (B) after inoculation of the ventral stomach wall. The scale bar = 100 μm.
Fig. 8.
Drawings of rostral and middle NTS sections from a musk shrew (left: A and C) and a rat (right: B and D) (see Table 1) showing the locations of H129-immunopositive cell bodies throughout the hindbrain. The scale bar = 500 μm.
Fig. 9.
Labeling of H129 infection in the spinal cord (T8-T10) of a musk shrew (left: A and C) and a rat (right: B and D) after inoculation of the ventral stomach wall. The scale bar = 200 μm. The bottom row is a higher magnification of the top row.
Fig. 10.
Labeling of H129 infection in the PBN of a musk shrew (left: A and C) and a rat (right: B and D) after inoculation of the ventral stomach wall. el, external lateral and c, central subnuclear regions of the PBN. The scale bar = 200 μm. The bottom row is a higher magnification of the top row.
Musk shrews that received an abdominal cavity injection of H129 virus (dripping on the stomach surface) were euthanized 9 days posttreatment. No central viral labeling was observed in 5 of these control animals, whereas 2 animals displayed sparse immunolabeling in the brain, including 1 to 2 labeled cells in the rostral NTS and PBN, and cerebellum in one animal (data not shown). There was no H129 immunolabeling in the spinal cord or forebrain. All 7 control shrews were asymptomatic 9 days postinjection, and the percentage reduction in body weight was not significantly different between animals with (−8.4 ± 2.2%) or without positive labeling (−3.8 ± 2.0%).
Control rats were euthanized either 3 days (n = 2) or 4 days (n = 4) after H129 virus was injected into the ventral stomach wall. Only three of these six rats displayed viral labeling within the central nervous system. All rats were asymptomatic before tissue collection, and the percentage change in body weight was not significantly different between animals with labeling (−3.1 ± 3.6%) versus those without labeling (+0.3 ± 3.6%). One rat had sparse labeling confined to the NTS. The other two rats displayed strong labeling of the NTS, AP, DMN, reticular formation, and spinal cord (Figs. 6–9). One of these two animals also displayed viral labeling within the PVN, SON, central amygdala, BNST, and insular cortex, which was consistent with a previous report (37).
Study 4: ventral stomach injection of a higher titer of H129 virus.
Shrews were euthanized at 3 (n = 4) or 5 days (n = 4) after H129 virus was injected into the ventral stomach wall. Six of these 8 shrews displayed viral immunolabeling in the central nervous system (Table 1), whereas 2 shrews displayed no detectable labeling. There was more labeling at 5 days postinjection compared with 3 days (Table 1), and labeled brain areas were similar to study 3. Only one animal at 5 days postinjection showed one or two labeled cells in the PVN and amygdala but none in the BNST or insular cortex. All shrews were asymptomatic on the day of euthanization, and the percentage change in body weight was not significantly different between shrews with (7.7 ± 2.7%) or without central viral labeling (14 ± 6.3%).
DISCUSSION
Subdiaphragmatic vagotomy failed to inhibit the total number of emetic episodes produced by intragastric administration of 60 or 120 mg/kg CuSO4, but the emetic effects of lower doses were more dependent on an intact vagus (20 and 40 mg/kg). Vagotomy also failed to affect motion or nicotine-induced emesis, presumably because these stimuli activate emesis via the vestibular system and AP, respectively (5, 23, 55). Injections of the H129 strain of herpes simplex virus-1 into the ventral stomach wall of shrews produced labeling of neurons in ascending viscerosensory pathways, including the NTS, AP, and lateral PBN. A similar pattern of labeling was observed in rats, showing that the regions that process sensory inputs from the stomach are similar in emetic and nonemetic species.
In prior studies using musk shrews, intragastric administration of 120 mg/kg CuSO4 was used to produce a peak level of acute emesis (7, 24, 40, 54). The current data verified that the cumulative incidence of emesis was systematically escalated by increasing the dose of CuSO4. The 120 and 60 mg/kg doses of CuSO4 generated emetic effects that were not dependent on an intact abdominal vagus (Figs. 1A and 4A); however, the latency to the first emetic episode after CuSO4 injections increased after vagotomies (Figs. 2 and 5). In addition, vagotomy reduced the number of emetic episodes with vomiting after administration of 60 or 120 mg/kg CuSO4. This decrease in vomiting could indicate that vagotomized animals had impaired reverse peristalsis (i.e., the giant retrograde contraction), which serves to move intestinal contents toward the gastric compartment for expulsion (27). Total subdiaphragmatic vagotomy destroys vagal efferent fibers that are required for generating the giant retrograde contraction (28). The increase in abdominal contractions in vagotomized animals injected with 120 mg/kg CuSO4 might also indicate that these animals attempted to position GI contents for expulsion (Fig. 1G). The most robust effects of vagotomy on the reduction of CuSO4-induced emesis occurred after injection of a dose of 40 mg/kg, when the total number of emetic episodes was significantly decreased (Fig. 4A); therefore, this dose could serve as the best approach for investigating the vagal contribution to emesis in studies using musk shrews. Similarly, in rats, vagotomy blocks the acquisition of a conditioned taste avoidance (CTA) to saccharin using a low dose of intragastric CuSO4 (5 mg/kg) (9). CTA is a potential marker of nausea in animal and human studies (41, 42, 44). Overall, the behavioral data suggest that intragastric CuSO4 can produce emesis by actions on pathways other than abdominal vagal afferent fibers; potentially these additional targets would include GI spinal afferent fibers or the AP. The latency to emesis after administration of CuSO4 was increased after vagotomy, suggesting that the additional mechanisms (i.e., spinal afferents and/or AP) required a longer latency for activation than vagal afferents. Furthermore, plasticity of the nervous system is a common problem in the interpretation of lesion effects, and this has been observed in emesis experiments in which spinal afferents begin to play a larger role in the stimulation of emesis after abdominal vagotomy (1). The present data raise the likelihood that inputs carried by both spinal and vagal afferents participate in triggering emesis.
Studies in humans, rats, and mice demonstrate that oral or gastric delivery of copper compounds produces an elevation of copper levels in the blood (3, 14). This indicates that high doses of CuSO4 in the GI tract could be absorbed into the circulation and act on the AP to produce emesis. Indeed, ablation of the AP in rats can block the conditioned taste aversion produced by intravenous CuSO4 (8), which suggests that circulating levels of CuSO4 can act directly on the brain to stimulate nausea. Copper sulfate is absorbed into the bloodstream in the intestine, but not the stomach (57). It is thus unclear whether sufficient time was available for CuSO4 to reach the intestinal transporters in vagotomized animals and reach sufficient concentration in the blood to affect the AP during the time period that emesis was observed.
Our data indicate that H129 injection in the ventral stomach of musk shrews produces a pattern of hind- and midbrain (and spinal cord) labeling that is largely similar to that reported for the rat, whereas forebrain labeling appears to be much more limited in shrews compared with rats (37). In the present study, central viral infection was present in only a subset of shrews (63%) and rats (50%) after H129 injection into the ventral stomach wall. There was significant variability in the extent of labeling achieved across individual shrews and rats. This variability does not seem to be due to the titer of H129 viral stock used because study 4 included a titer that was similar to the prior report (i.e., 1010 pfu/ml) (37). Despite the observed variability, musk shrews and rats appear to have comparable patterns of labeling within the brain stem, including the dorsal vagal complex, regions of the reticular formation, and the PBN (Table 1; Figs. 6–8 and 10). H129 is transported retrogradely to first-order motor neurons after peripheral organ injection (37), which accounts for labeling of motor neurons within the DMN. The nucleus ambiguus does not receive direct vagal (or spinal) sensory input. The absence of transneuronal retrograde labeling of sympathetic preganglionic neurons is expected, since this would require transsynaptic retrograde transport through prevertebral sympathetic ganglia, and transneuronal transport apparently proceeds only in the anterograde direction (37).
The sparse forebrain viral labeling achieved in shrews is unexpected, given that anterograde transport of phaseolus vulgaris leucoagglutinin from the NTS of the musk shrew produces substantial labeling of the PVN and BNST, with additional sparse labeling of the amygdala (21). There are at least four possible explanations for the lack of more extensive labeling following H129 virus injections in musk shrews. First, cytopathogenesis of neurons might be more pronounced in musk shrews. This could limit the transport of virus to higher order cells, as has been reported in ferrets after injection of pseudorabies virus (56). Second, synaptic density is known to influence the amount of virus transported from one infected neuron to its synaptically linked partners (6). Low levels of H129 infection within the hypothalamus and other forebrain regions could reflect a low density of synaptic inputs from ascending visceral sensory projections arising in the dorsal vagal complex and/or PBN. Third, certain cells types within circuits, although infected with another neutropic virus (i.e., pseudorabies virus), do not always produce infectious virus (39). Fourth, an effective immune response might have cleared H129 virus from infected regions of the central nervous system (e.g., 30); indeed, in the present study a 9-day postinoculation interval produced less central H129 viral labeling in shrews compared with central labeling observed after a 5-day postinoculation interval. These data suggest that, at least for musk shrews, the time window to observe H129 transneuronal labeling is relatively narrow. There was also no difference in labeling observed between strains of musk shrews.
Unlike the prior study in rats (37), the current study did not experimentally address whether central H129 labeling depends on anterograde and/or retrograde transneuronal transport of this virus from the ventral stomach wall. Although vagotomy can be conducted in musk shrews, selective sectioning of the afferent or efferent rootlets of the vagus as performed in rats (33, 48) would be difficult in shrews given their relatively small size. We also did not explore the relative contributions of vagal versus spinal components in the present study; however, labeling within the dorsal vagal complex in shrews was more pronounced and was observed more consistently compared with labeling within the dorsal horn of the spinal cord (Table 1). At present, testing the impact of vagotomy on viral transport to the brain would require a large number of musk shrews because of the extensive variability in H129 labeling observed.
Our results primarily highlight similarities in the GI-hindbrain axis of musk shrews and rats (21, 32, 37, 52, 53). There are clear functional differences between species because musk shrews (and ferrets, cats, dogs, etc.) possess a vomiting reflex, which rats (and other rodents) do not have (18). Thus we expected to observe transneuronal viral labeling in potentially unique regions of the shrew brain stem that might comprise neural circuits for vomiting that would be absent in rats; however, our approach labeled gastric vagal afferent projections to the brain stem, and evidence supports the view that a lack of emesis is due to deficits in a motor output circuit and not the sensory pathways from the GI tract to the brain (18). Moreover, evidence that vagotomy blocks the effects of a low dose of intragastric CuSO4 to induce emesis in musk shrews and produce a CTA in rats (9) suggests that vagal sensory pathways that produce “nausea” and emesis are similar. A better understanding of neural circuit similarities and differences between vomiting and nonvomiting species should facilitate improved experimental use of these species as animal models to evaluate the central mechanisms of nausea and vomiting.
Perspectives and Significance
A central function of GI-vagal-brain pathways is to detect the presence of toxins in the upper GI tract and to initiate an appropriate defense; in many species, including humans, an emetic reflex is activated. It is important to understand the biology of this system because it is stimulated in numerous disease conditions and with a multitude of drug treatments. To date, results from most studies are difficult to interpret due to use of nonspecific emetic stimuli that activate multiple levels of the gut-brain axis (e.g., chemotherapy), and the use of animal models that lack an emetic reflex (e.g., rats and mice, 15, 18). To overcome these limitations, we used a small animal model with an emetic reflex and a specific GI stimulus. The current studies indicate that a low dose of CuSO4 (40 mg/kg) specifically activates the GI vagal afferent pathway to produce emesis in musk shrews. We also demonstrate effective use of vagal lesion verification in this species; this is a standard retrograde tracing technique used in studies of the vagus in rats and should be applied to research studies using abdominal vagotomy in musk shrews. Our results also demonstrate the value of using multiple behavioral measures of emesis (vomiting, emetic duration, cumulative latency, etc.) to examine differences generated by different treatment or surgical conditions (19). Finally, the current H129 viral tracing data confirm brain stem circuits positioned to receive GI signaling in the musk shrew, providing the basis for future physiological studies of brain stem emetic circuitry in this species.
GRANTS
We gratefully acknowledge the funding support for this work from the Tobacco Settlement Fund (PA Department of Health) and NIH Grants R01DK065971, R01DC003732, R01MH059911, and P30 CA047904 (Cancer Center Support Grant; CCSG).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: C.C.H., L.R., and B.J.Y. conception and design of research; C.C.H., K.M., A.L., M.D., and D.P. performed experiments; C.C.H., K.M., A.L., and M.D. analyzed data; C.C.H., L.R., and B.J.Y. interpreted results of experiments; C.C.H. prepared figures; C.C.H. drafted manuscript; C.C.H., K.M., A.L., L.R., and B.J.Y. edited and revised manuscript; C.C.H., K.M., A.L., M.D., D.P., L.R., and B.J.Y. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Patrick Card (University of Pittsburgh) and Dr. Lynn Enquist (Princeton University), Center for Neuroanatomy with Neurotropic Viruses (http://www.cnnv.pitt.edu), for supplying the H129, which is supported by National Institutes of Health (NIH) Grant P40RR018604. We thank the University of Pittsburgh, Division of Laboratory Animal Research, especially Dawn Everard, Katie Leschak, Megan Lambert, and Dr. Joseph Newsome for excellent care of the musk shrew colony at the University of Pittsburgh Cancer Institute (UPCI).
REFERENCES
- 1.Andrews PL, Davis CJ, Bingham S, Davidson HI, Hawthorn J, Maskell L. The abdominal visceral innervation and the emetic reflex: pathways, pharmacology, and plasticity. Can J Physiol Pharmacol 68: 325–345, 1990 [DOI] [PubMed] [Google Scholar]
- 2.Archin NM, Atherton SS. Rapid spread of a neurovirulent strain of HSV-1 through the CNS of BALB/c mice following anterior chamber inoculation. J Neurovirol 8: 122–135, 2002 [DOI] [PubMed] [Google Scholar]
- 3.ATSDR Toxicological Profile For Copper. Atlanta, GA: US Department of Health and Human Services, 2004 [Google Scholar]
- 4.Barnett EM, Evans GD, Sun N, Perlman S, Cassell MD. Anterograde tracing of trigeminal afferent pathways from the murine tooth pulp to cortex using herpes simplex virus type 1. J Neurosci 15: 2972–2984, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beleslin DB, Krstic SK. Further studies on nicotine-induced emesis: nicotinic mediation in area postrema. Physiol Behav 39: 681–686, 1987 [DOI] [PubMed] [Google Scholar]
- 6.Card JP, Enquist LW, Moore RY. Neuroinvasiveness of pseudorabies virus injected intracerebrally is dependent on viral concentration and terminal field density. J Comp Neurol 407: 438–452, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Chan SW, Rudd JA, Lin G, Li P. Action of anti-tussive drugs on the emetic reflex of Suncus murinus (house musk shrew). Eur J Pharmacol 559: 196–201, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Coil JD, Norgren R. Taste aversions conditioned with intravenous copper sulfate: attenuation by ablation of the area postrema. Brain Res 212: 425–433, 1981 [DOI] [PubMed] [Google Scholar]
- 9.Coil JD, Rogers RC, Garcia J, Novin D. Conditioned taste aversions: vagal and circulatory mediation of the toxic unconditioned stimulus. Behav Biol 24: 509–519, 1978 [DOI] [PubMed] [Google Scholar]
- 10.De Jonghe BC, Horn CC. Chemotherapy agent cisplatin induces 48 h Fos expression in the brain of a vomiting species, the house musk shrew (Suncus murinus). Am J Physiol Regul Integr Comp Physiol 296: R902–R911, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dryden GL. Growth and development of Suncus murinus in captivity on Guam. J Mammalogy 49: 51–62, 1968 [Google Scholar]
- 12.Harris DG. Nausea and vomiting in advanced cancer. Br Med Bull 96: 175–185, 2010 [DOI] [PubMed] [Google Scholar]
- 13.Hesketh PJ. Chemotherapy-induced nausea and vomiting. N Engl J Med 358: 2482–2494, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Holtzmann NA, Haslam RH. Elevation of serum copper following copper sulfate as an emetic. Pediatrics 42: 189–193, 1968 [PubMed] [Google Scholar]
- 15.Horn CC. The medical implications of gastrointestinal vagal afferent pathways in nausea and vomiting. Curr Pharm Des. In press [DOI] [PubMed] [Google Scholar]
- 17.Horn CC, Henry S, Meyers K, Magnusson MS. Behavioral patterns associated with chemotherapy-induced emesis: a potential signature for nausea in musk shrews. Front Neurosci 5: 88, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horn CC, Kimball BA, Wang H, Kaus J, Dienel S, Nagy A, Gathright GR, Yates BJ, Andrews PLR. Why can't rodents vomit? A comparative behavioral, anatomical, and physiological study. PLos One 8: e60537, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horn CC, Wang H, Estival L, Meyers K, Magnusson M. Novel dynamic measures of emetic behavior in musk shrews. Autonom Neurosci 179: 60–67, 2013 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang D, Meyers K, Henry S, De la Torre F, Horn CC. Computerized detection and analysis of cancer chemotherapy-induced emesis in a small animal model, musk shrew. J Neurosci Meth 197: 249–258, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ito H, Seki M. Ascending projections from the area postrema and the nucleus of the solitary tract of Suncus murinus: anterograde tracing study using Phaseolus vulgaris leucoagglutinin. Okajimas Folia Anat Jpn 75: 9–31, 1998 [DOI] [PubMed] [Google Scholar]
- 22.Javid FA, Naylor RJ. Variables of movement amplitude and frequency in the development of motion sickness in Suncus murinus. Pharmacol Biochem Behav 64: 115–122, 1999 [DOI] [PubMed] [Google Scholar]
- 23.Jovanovic-Micic D, Strbac M, Krstic SK, Japundzic N, Samardzic R, Beleslin DB. Ablation of the area postrema and emesis. Metab Brain Dis 4: 55–60, 1989 [DOI] [PubMed] [Google Scholar]
- 24.Kan KK, Jones RL, Ngan MP, Rudd JA. Action of prostanoids on the emetic reflex of Suncus murinus (the house musk shrew). Eur J Pharmacol 477: 247–251, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Kayashima N, Iwasaki M, Hayama T. Site of emetic action of oral copper sulfate in dogs. II. Importance of lower duodenum. Jpn J Pharmacol 28: 797–801, 1978 [DOI] [PubMed] [Google Scholar]
- 26.Kwiatkowska M, Parker LA, Burton P, Mechoulam R. A comparative analysis of the potential of cannabinoids and ondansetron to suppress cisplatin-induced emesis in the Suncus murinus (house musk shrew). Psychopharmacology (Berl) 174: 254–259, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Lang IM. Digestive tract motor correlates of vomiting and nausea. Can J Physiol Pharmacol 68: 242–253, 1990 [DOI] [PubMed] [Google Scholar]
- 28.Lang IM, Sarna SK, Condon RE. Gastrointestinal motor correlates of vomiting in the dog: quantification and characterization as an independent phenomenon. Gastroenterology 90: 40–47, 1986 [DOI] [PubMed] [Google Scholar]
- 29.Le TP, Gan TJ. Update on the management of postoperative nausea and vomiting and postdischarge nausea and vomiting in ambulatory surgery. Anesthesiol Clinics 28: 225–249, 2010 [DOI] [PubMed] [Google Scholar]
- 30.Mabon PJ, Weaver LC, Dekaban GA. Cyclosporin A reduces the inflammatory response to a multi-mutant herpes simplex virus type-1 leading to improved transgene expression in sympathetic preganglionic neurons in hamsters. J Neurovirol 5: 268–279, 1999 [DOI] [PubMed] [Google Scholar]
- 31.Matsuki N, Ueno S, Kaji T, Ishihara A, Wang CH, Saito H. Emesis induced by cancer chemotherapeutic agents in the Suncus murinus: a new experimental model. Jpn J Pharmacol 48: 303–306, 1988 [DOI] [PubMed] [Google Scholar]
- 32.Norgren R, Smith GP. Central distribution of subdiaphragmatic vagal branches in the rat. J Comp Neurol 273: 207–223, 1988 [DOI] [PubMed] [Google Scholar]
- 33.Norgren R, Smith GP. A method for selective section of vagal afferent or efferent axons in the rat. Am J Physiol Regul Integr Comp Physiol 267: R1136–R1141, 1994 [DOI] [PubMed] [Google Scholar]
- 34.Percie du Sert N, Chu KM, Wai MK, Rudd JA, Andrews PL. Telemetry in a motion-sickness model implicates the abdominal vagus in motion-induced gastric dysrhythmia. Exp Physiol 95: 768–773, 2010 [DOI] [PubMed] [Google Scholar]
- 35.Percie du Sert N, Rudd JA, Moss R, Andrews PL. The delayed phase of cisplatin-induced emesis is mediated by the area postrema and not the abdominal visceral innervation in the ferret. Neurosci Lett 465: 16–20, 2009 [DOI] [PubMed] [Google Scholar]
- 36.Powley TL, Fox EA, Berthoud HR. Retrograde tracer technique for assessment of selective and total subdiaphragmatic vagotomies. Am J Physiol Regul Integr Comp Physiol 253: R361–R370, 1987 [DOI] [PubMed] [Google Scholar]
- 37.Rinaman L, Schwartz G. Anterograde transneuronal viral tracing of central viscerosensory pathways in rats. J Neurosci 24: 2782–2786, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rissman EF. The musk shrew, Suncus murinus, a unique animal model for the study of female behavioral endocrinology. J Exp Zool Suppl 4: 207–209, 1990 [DOI] [PubMed] [Google Scholar]
- 39.Rotto-Percelay DM, Wheeler JG, Osorio FA, Platt KB, Loewy AD. Transneuronal labeling of spinal interneurons and sympathetic preganglionic neurons after pseudorabies virus injections in the rat medial gastrocnemius muscle. Brain Res 574: 291–306, 1992 [DOI] [PubMed] [Google Scholar]
- 40.Rudd JA, Ngan MP, Wai MK. Inhibition of emesis by tachykinin NK1 receptor antagonists in Suncus murinus (house musk shrew). Eur J Pharmacol 366: 243–252, 1999 [DOI] [PubMed] [Google Scholar]
- 41.Scalera G. Effects of conditioned food aversions on nutritional behavior in humans. Nutr Neurosci 5: 159–188, 2002 [DOI] [PubMed] [Google Scholar]
- 42.Smith JE, Friedman MI, Andrews PL. Conditioned food aversion in Suncus murinus (house musk shrew)–a new model for the study of nausea in a species with an emetic reflex. Physiol Behav 73: 593–598, 2001 [DOI] [PubMed] [Google Scholar]
- 43.Song CK, Schwartz GJ, Bartness TJ. Anterograde transneuronal viral tract tracing reveals central sensory circuits from white adipose tissue. Am J Physiol Regul Integr Comp Physiol 296: R501–R511, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sticht MA, Long JZ, Rock EM, Limebeer CL, Mechoulam R, Cravatt BF, Parker LA. The MAGL inhibitor, JZL184, attenuates LiCl-induced vomiting in the Suncus murinus and 2AG attenuates LiCl-induced nausea-like behavior in rats. Br J Pharmacol 165: 2425–2435, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tang DM, Friedenberg FK. Gastroparesis: approach diagnostic evaluation, management. Dis Mon 57: 74–101, 2011 [DOI] [PubMed] [Google Scholar]
- 46.Temple JL. The musk shrew (Suncus murinus): a model species for studies of nutritional regulation of reproduction. ILAR J 45: 25–34, 2004 [DOI] [PubMed] [Google Scholar]
- 47.Ueno S, Matsuki N, Saito H. Suncus murinus: a new experimental model in emesis research. Life Sci 41: 513–518, 1987 [DOI] [PubMed] [Google Scholar]
- 48.Walls EK, Wang FB, Holst MC, Phillips RJ, Voreis JS, Perkins AR, Pollard LE, Powley TL. Selective vagal rhizotomies: a new dorsal surgical approach used for intestinal deafferentations. Am J Physiol Regul Integr Comp Physiol 269: R1279–R1288, 1995 [DOI] [PubMed] [Google Scholar]
- 49.Wang CH. Introduction: A new experimental animal, Suncus murinus. In: Proceeding of ROC-Japan Symposium on Suncus murinus: New Experimental Animal, Its Speciality and Usefulness, edited by Saito H, Wang CH, Chen CY. Tainan, Taiwan, ROC: Chia Nan Junior College of Pharmacy Press (Chia Nan, Taiwan ROC), 1994 [Google Scholar]
- 50.Wang SC, Borison HL. Copper sulphate emesis; a study of afferent pathways from the gastrointestinal tract. Am J Physiol 164: 520–526, 1951 [DOI] [PubMed] [Google Scholar]
- 51.Watson RE, Jr, Wiegand SJ, Clough RW, Hoffman GE. Use of cryoprotectant to maintain long-term peptide immunoreactivity and tissue morphology. Peptides 7: 155–159, 1986 [DOI] [PubMed] [Google Scholar]
- 52.Won MH, Matsuo K, Jo SM, Kang TC, Oh YS, Choi CD, Kitoh J. Brainstem origin of the efferent components of the cervical vagus nerve in the house musk shrew, Suncus murinus. J Auton Nerv Syst 71: 55–63, 1998 [DOI] [PubMed] [Google Scholar]
- 53.Won MH, Matsuo K, Oh YS, Kitoh J. Brainstem topology of the vagal motoneurons projecting to the esophagus and stomach in the house musk shrew, Suncus murinus. J Auton Nerv Syst 68: 171–181, 1998 [DOI] [PubMed] [Google Scholar]
- 54.Yamamoto K, Ngan MP, Takeda N, Yamatodani A, Rudd JA. Differential activity of drugs to induce emesis and pica behavior in Suncus murinus (house musk shrew) and rats. Physiol Behav 83: 151–156, 2004 [DOI] [PubMed] [Google Scholar]
- 55.Yates BJ, Miller AD, Lucot JB. Physiological basis and pharmacology of motion sickness: an update. Brain Res Bull 47: 395–406, 1998 [DOI] [PubMed] [Google Scholar]
- 56.Yates BJ, Smail JA, Stocker SD, Card JP. Transneuronal tracing of neural pathways controlling activity of diaphragm motoneurons in the ferret. Neuroscience 90: 1501–1513, 1999 [DOI] [PubMed] [Google Scholar]
- 57.Zimnicka AM, Ivy K, Kaplan JH. Acquisition of dietary copper: a role for anion transporters in intestinal apical copper uptake. Am J Physiol Cell Physiol 300: C588–C599, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]