Abstract
Exaggerated GLP-1 and PYY secretion is thought to be a major mechanism in the reduced food intake and body weight after Roux-en-Y gastric bypass surgery. Here, we use complementary pharmacological and genetic loss-of-function approaches to test the role of increased signaling by these gut hormones in high-fat diet-induced obese rodents. Chronic brain infusion of a supramaximal dose of the selective GLP-1 receptor antagonist exendin-9–39 into the lateral cerebral ventricle significantly increased food intake and body weight in both RYGB and sham-operated rats, suggesting that, while contributing to the physiological control of food intake and body weight, central GLP-1 receptor signaling tone is not the critical mechanism uniquely responsible for the body weight-lowering effects of RYGB. Central infusion of the selective Y2R-antagonist BIIE0246 had no effect in either group, suggesting that it is not critical for the effects of RYGB on body weight under the conditions tested. In a recently established mouse model of RYGB that closely mimics surgery and weight loss dynamics in humans, obese GLP-1R-deficient mice lost the same amount of body weight and fat mass and maintained similarly lower body weight compared with wild-type mice. Together, the results surprisingly provide no support for important individual roles of either gut hormone in the specific mechanisms by which RYGB rats settle at a lower body weight. It is likely that the beneficial effects of bariatric surgeries are expressed through complex mechanisms that require combination approaches for their identification.
Keywords: Roux-en-Y gastric bypass; gut hormones; brain; GLP-1R knockout; food intake; exendin-(9–39), BIIE0246; high-fat diet
the number of bariatric surgeries performed has steadily increased because for many obese patients, it is the last hope for significant and enduring body weight loss and general improvement of health. There have been an increasing number of clinical and preclinical studies with the goal to unravel the mechanisms underlying these beneficial effects of bariatric surgeries, but there has not yet been a breakthrough. A major hypothesis is that changes in gut hormone release are crucial. Drastically increased postprandial circulating levels of GLP-1 and PYY have been demonstrated in clinical studies (18, 32, 33, 36, 38, 44, 47, 50) and in rodent models (13, 16, 37, 56) for both Roux-en-Y gastric bypass and vertical sleeve gastrectomy.
GLP-1 is a powerful hormone that acts both in the periphery and brain to stimulate insulin secretion, inhibit gastric emptying, and suppress food intake (for recent reviews, see Refs. 7, 12, 43). Exogenous administration of GLP-1 or its stable analog exendin-4, as well as PYY(3–36), has been shown in numerous preclinical and clinical studies to suppress food intake and lower body weight (e.g., (2, 4, 5, 9, 15, 19, 24, 27, 40, 57, 59) or to suppress hepatic glucose production (52), in some of them by directly acting on the brain. The stable GLP-1 receptor agonist exendin-4 is widely used by Type 2 diabetic patients to stabilize glucose levels and reduce body weight (30, 43). Although GLP-1 gains easy access to the brain (26, 29), its short half-life makes unclear whether endogenous GLP-1 from the gut reaches the brain in high enough concentrations to affect food intake and glucose homeostasis under normal conditions. The greatly increased circulating levels after gastric bypass or sleeve gastrectomy may affect the brain directly or indirectly via GLP-1 receptors on sensory vagal fibers assumed to innervate the intestinal mucosa and hepatic portal vein (42, 62). In addition, proglucagon is expressed in neurons of the solitary nucleus in the caudal brain stem, the origin of extensive local and forebrain projections of GLP-1 and GLP-2 immunoreactive fibers (61).
Although exaggerated postprandial GLP-1 and PYY responses are widely believed to play a major role in the beneficial effects of RYGB on body weight and glucose homeostasis, direct evidence has been lacking. Strong indirect evidence comes from studies in RYGB patients, with appetite and body weight negatively correlated with elevated PYY and GLP-1 concentrations (38). Furthermore, food intake increased upon treatment of RYGB patients (38) and rats after RYGB (22) with the nonspecific inhibitor of gut hormone secretion octreotide. There have been only two attempts to mechanistically and directly test the role of endogenous GLP-1 and PYY in the weight-reducing effects of bariatric surgeries. In a study in PYY-deficient mice, the initial body weight loss after a modified gastric bypass surgery was greatly attenuated compared with wild-type mice, suggesting a role for exaggerated circulating levels of this gut peptide (14). In a study in GLP-1 receptor-deficient mice, sleeve gastrectomy was similarly effective compared with wild-type mice, suggesting that exaggerated circulating GLP-1 levels are not required for VSG's effects on weight loss and improvement of glycemic control (64). This latter finding was surprising, given the similarly elevated circulating levels of postprandial GLP-1 and PYY in humans (44) and rodents (13, 56).
Here, we examined the potential roles of brain GLP-1 and Y2-receptor signaling in the body weight-lowering effects of RYGB. We were specifically interested in mechanisms that defend the lower levels of body weight after the initial weight loss phase. Unlike calorie restriction-induced weight loss, RYGB does not induce strong counter-regulatory responses such as increased hunger, allowing most RYGB patients to resist weight regain. We tested the hypothesis that central GLP-1 and Y2 receptor signaling contribute to the defense of the lower body weight level after RYGB. To this end, we used chronic pharmacologic blockade of central GLP-1 or Y2 receptor signaling by their respective antagonists exendin-(9-39) (Ex9) or BIIE0246. Having recently established a murine model of RYGB with a small gastric pouch that closely mimics surgery in humans and leads to sustained loss of body weight by selective reduction of excess fat mass (25), we then tested the effectiveness of RYGB to lower food intake and body weight in GLP-1 receptor-null mice.
MATERIALS AND METHODS
Animals
Rats.
Male Sprague-Dawley rats initially weighing ∼200 g (Harlan Industries, Indianapolis, IN) were housed individually in wire-mesh cages at a constant temperature of 21–23°C with a 12:12-h light-dark cycle (lights on 0700, off at 1900). Food and water were provided ad libitum unless otherwise indicated. Animals were made obese by putting them on a two-choice diet for 20 wk consisting of normal laboratory chow (kcal%: carbohydrates, 58; fat, 13.5; protein, 28.5; no. 5001, Purina LabDiet, Richmond IN) and high-sucrose, high-fat diet (sweet HF diet; kcal%: carbohydrates, 35; fat, 45; protein, 20; D12451, Research Diets, New Brunswick, NJ), with each of the diets containing sufficient minerals and vitamins. They were then randomly assigned to either RYGB or sham surgery. Liquid Ensure diet (kcal%: carbohydrates, 64; fat, 21.6; protein, 14.4; Abbott Laboratories, Columbus, OH) was provided for the first 5 days after surgery or longer if needed. A lean control group without surgery was placed on a regular chow diet throughout the experiment. Four rats, two with RYGB and two with sham surgery were shipped from the Surgical Core of Harvard Medical School (Dr. Lee Kaplan).
Mice.
GLP-1R-deficient mice were originally generated on a 129/J, CD1, and C57BL/6J background and backcrossed six times on a pure C57BL/6J background by Dr. Daniel Drucker in Toronto, Canada (54). A breeding colony with this background was then transferred to the Pennington Biomedical Research Center, where they were maintained by sister-brother breeding. Genotyped male GLP-1R−/− and C57/BL6J wild-type mice were made obese on a high-fat diet (58% of energy from fat, Research Diets D12331) for 12 wk and were 4 mo old at the time of surgery. After surgery or sham surgery, they were given a medium-fat diet (27% of energy from fat, no. 8626 Teklad mouse breeder diet), except for postsurgical weeks 10–16, when they were given a two-choice cafeteria diet consisting of two complete diets, one low-fat (regular chow, 4.4% energy from fat, Teklad 7001) and one high-fat (58% energy from fat, Research Diets no. D12331). The reason for switching to medium-fat diet after surgery was twofold. First, in earlier studies in both mice and rats, we noted that after RYGB, animals increased preference for low-fat regular chow and that some RYGB animals showed signs of morbidity, if provided only a high-fat diet. Second, reduced intake of high-fat foods is an important component of postsurgical behavioral counseling in gastric bypass patients. However, we did not provide a choice of low- and high-fat diets throughout the postsurgical period because it would complicate the nterpretation of energy expenditure data and the respiratory exchange ratio.
All protocols involved in this study were approved by the Institutional Animal Care and Use Committee at the Pennington Biomedical Research Center or at Harvard University in accordance with guidelines established by the National Institutes of Health.
Roux-en-Y Gastric Bypass Surgery
Rats.
Under adequate isoflurane anesthesia, the stomach was first freed from all the ligaments that connect it to the liver and the spleen. Then, the focus of the surgeon turned to the gastric artery that emerges from the celiac artery. The gastric artery is in very close approximation with the gastroesophageal junction and then runs parallel to the lesser curvature of the stomach branching into one anterior and one posterior vessel that, in turn, give rise to several smaller branches on the anterior and posterior wall. Before transecting the stomach, the gastric artery was gently dissected off the gastric wall using a microhook. Using a bipolar cautery probe, the first anterior branch that crosses the anterior wall of the stomach, just millimeters from the gastroesophageal junction was cauterized, allowing for transection of the stomach wall with fine scissors without bleeding. After repairing the distal stomach using a running 6–0 silk suture, an end-to-end gastrojejunostomy was constructed with a 7–0 silk suture. This surgery resulted in a gastric pouch of no more than about 5% of the gastric volume, as well as Roux, biliopancreatic, and common limbs of about 20, 22, and 60 cm. Sham surgery consisted of laparotomy and mobilization of the stomach and small intestine. For analgesia, buprenorphine, meloxicam (1–2 mg/kg sc), and/or carprofen (5 mg/kg sc) were administered as necessary. To overcome potential deficits in iron absorption and development of anemia, rats were administered a macromolecular dextran-iron complex (iron dextran injectable, catalog no. 93963, 5 mg sc; Town and Country, Ashland, OH) once a week for the first 2 wk after RYGB surgery. Additional doses were administered to individual anemic animals if indicated by a hematocrit of less than 40%.
In addition to the high-fat-fed RYGB and sham-operated rats, a nonsurgical, age-matched group was fed standard laboratory chow throughout the study and served as lean, never-obese, control group.
Mice.
As described in detail earlier (25), surgery resulted in a small gastric pouch of less than 5% of the stomach volume, and Roux-, biliopancreatic, and common limbs of about 6, 6, and 12 cm, respectively. By taking care to preserve gastric vasculature as much as possible, the mortality rate is <10%, and the animals typically consume significant amounts of food 2 days after surgery. Sham surgery consisted in cutting the jejunum followed by reanastomosis and by mobilizing the stomach and placing a metal clip on the greater curvature.
Measurement of Body Weight and Body Composition
Body weight was monitored daily for the first 2 wk and then was recorded weekly. Body composition was measured before the introduction of the high-fat diet (23 wk before surgery), after 20 wk of the high-fat diet (3 wk before surgery), 6 wk after surgery, as well as before and after the chronic pharmacological blockade, by using a Minispec LF 90 NMR Analyzer (Bruker, The Woodlands, TX). This method uses whole body magnetic resonance relaxometry in unanesthetized rodents with excellent linearity and reproducibility (34).
Experimental Protocol
Rats.
Eight weeks after surgery, all rats, including the chow-fed controls were equipped with intracerebroventricular cannulas. After recovery from this second surgery, rats were adapted to an automated system (PhenoMaster/LabMaster, TSE Systems, Chesterfield MO), continuously monitoring food and water intake, oxygen consumption, and locomotor activity. At this time, access of RYGB and sham-operated rats was restricted to the high-fat diet only. After adaptation with training lids, animals were monitored for a 4–6-day baseline period, at which time ALZET Minipumps (2 ml/14 days, Durect, Cupertino, CA) containing either the GLP-1 receptor antagonist Ex9, or the Y2 receptor antagonist BIIE0246, or the respective vehicle were implanted under the back skin and connected to the intracerebroventricular cannulas under isoflurane anesthesia. After monitoring all parameters continuously for another 16–18 days, the rats were transferred back to regular cages, and the pumps were removed.
Mice.
At the time of surgery, mice were switched from high-fat (45%) to medium-fat diet (27% energy from fat). Seven weeks after surgery, body composition was measured as described above. At 12–14 wk after surgery, the medium-fat diet was replaced with a two-choice cafeteria diet consisting of two complete diets, one low-fat (regular chow, 4.4%) and one high-fat (58% energy from fat. Intake of both components was measured daily for the first 10 days and then for 3 days every week. Body composition was measured before and after 6 wk on this high-fat cafeteria diet.
Chronic Intracerebroventricular Infusions
Intracerebroventricular cannulas (Plastics One, Roanoke, VA) were aimed at the left lateral ventricle as described in detail earlier (65). The lateral ventricle was chosen because the infusion reaches the entire brain and to avoid any inadvertent damage to the roof of the third ventricle and dorsal hypothalamus. After recovery, cannula placements were verified by monitoring the acute drinking response to intracerebroventricular injection of ANG II. All animals drank at least 5 ml of water within 10 min of ANG II administration.
Infusions started with the implantation and connection with ALZET minipumps loaded with either Ex9 (100 μg·rat−1·day−1, dissolved in sterile saline (Twentyfirst Century Biochemicals, Marlboro, MA), BIIE0246 (100 μg·rat−1·day−1, dissolved in 40% DMSO; Tocris Bioscience, Minneapolis MN), or sterile saline as vehicle control (Veh), in separate groups of 8–10 rats. All infusions lasted for 14 days (nominally) at a rate of 0.5 μl/h, and the pumps were promptly removed at 16–18 days after the start of infusion. The dose of Ex9 was based on its effectiveness to increase food intake and body weight in both chow and high-fat diet-fed rats reported in an earlier study by Barrera et al. (6). At an average body weight of about 450–500 g, this amounts to about 42–46 pmol·kg−1·min−1. Because the much lower dose of 0.5 pmol·kg−1·min−1 was effective in increasing food intake in mice (31), we consider our dose as clearly supramaximal. The dose of BIIE0246 was based on earlier reports with brain injections (1, 45) and a separate experiment with single injections to test its effectiveness to prevent suppression of food intake induced by injection of PYY(3–36). To this end, male Sprague-Dawley rats (n = 5) on regular chow with fourth ventricular cannulas were food-deprived overnight and received an acute injection of BIIE0246 (1 μg/3 μl in 5% DMSO; Tocris) or vehicle alone, followed 15 min later by an acute injection of PYY(3–36) (1.5 μg/3 μl in sterile saline; Tocris) or saline, and food intake was measured 30 min after the last injection. One rat that did not decrease food intake after PYY(3–36) injection was not included in the analysis. On the basis of the effectiveness of 1 μg BIIE0246 in this acute study, we used a chronic infusion rate of 2.1 μg/30 min (100 μg/day) in the chronic infusion study.
Measurement of Food Intake and V̇o2 Consumption
Up to the time rats were placed in the metabolic chambers, food intake was measured manually, taking into account spillage. In the chambers, food and water consumption was measured automatically, without taking spillage into account. All food intake is reported in kilocalories per day or kilocalories per 12-h dark or light period. V̇o2 consumption was measured throughout the baseline and 14-day infusion period in metabolic chambers (PhenoMaster/LabMaster, TSE Systems) and expressed either per rat or per kilogram body mass.
Statistical Analysis
All data were analyzed with appropriate two-way ANOVAs. Where appropriate, time was used as within-subjects repeated measure. Selective preplanned comparisons of individual means were made by using Bonferroni-corrected multiple comparison tests. Raw food intake data were collected in grams and multiplied by values for metabolizable energy content as provided by the supplier. Values expressed as means ± SE are reported throughout.
RESULTS
Effects of Pharmacological Blockade of Central GLP-1R Signaling in Rats
RYGB-induced effects on body weight and body composition.
The effect of RYGB and sham surgery on body weight and body composition is shown in Fig. 1. RYGB rats showed a rapid initial weight loss and stayed at that significantly lower body weight level throughout the study (Fig. 1A). At the time of brain cannulation, they weighed significantly less, and the fat mass and adiposity index were significantly lower compared with sham-operated controls, and similar to age-matched, chow-fed control rats (Fig. 1, B and C). Total and percent lean mass after RYGB was similar to chow-fed lean controls, and significantly lower compared with sham-operated rats (Fig. 1, D and E).
Fig. 1.
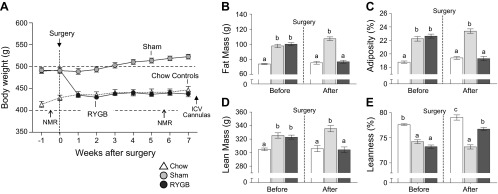
Effect of RYGB surgery in high-fat diet-induced obese rats on body weight and body composition. A: male Sprague-Dawley rats were maintained on a high-fat diet throughout the study, and body composition was assessed before and after surgery. In addition, an age-matched, nonsurgical control group was fed regular low-fat chow throughout. Body weight of RYGB rats rapidly decreased within the first 2 wk and remained at that reduced level throughout the observation period, closely matching body weight of chow-fed controls. B–E: effects of RYGB on total fat mass (B), relative fat mass (adiposity; C), total lean mass (D), and relative lean mass (E) in sham-operated (light gray bars), RYGB rats (dark gray bars), and chow-fed, unoperated, age-matched lean controls (open bars). Note that RYGB completely reversed excess high-fat diet-induced fat mass, and lean mass gain to levels found in chow-fed rats. Values are expressed as means ± SE of 15–20 rats/group. Bars that do not share the same letters denote significant differences at P < 0.05 (based on ANOVA followed by Bonferroni-adjusted multiple comparisons).
Effects of Chronic Intracerebroventricular Ex9 Infusion on Body Weight and Body Composition
In RYGB rats, infusion of the GLP-1 receptor antagonist Ex9 resulted in a slow but steady body weight increase, which became significant compared with saline infusion around day 12 (Fig. 2A). At the end of the infusion period on day 16, Ex9 infused RYGB rats had gained significantly (t[9] = 3.56, P < 0.01) more body weight compared with vehicle-infused RYGB rats (Fig. 2B). However, sham-operated rats gained body weight more rapidly with Ex9 compared with vehicle infusion, with the first significant difference on day 6 (t[9] = 3.38, P < 0.01), and more rapidly than RYGB rats with Ex9 infusion. Analysis of body weight gain from pump implantation to day 16 revealed significant main effects of infusion [F(1,41) = 58.48, P < 0.0001] and surgery [F(2,41) = 6.00, P < 0.005] but no significant interaction (Fig. 2B). Follow-up comparisons showed that Ex9 significantly increased weight gain vs. vehicle in all groups. Vehicle infusion did not differentially affect body weight gain in the three groups, although RYGB rats gained the least.
Fig. 2.
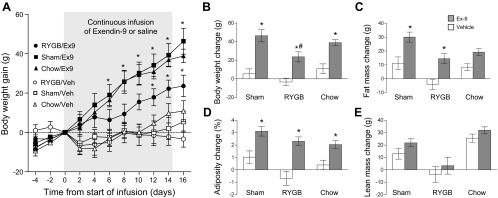
Effect of chronic pharmacological blockade of central GLP-1 receptor signaling on body weight and composition in rats after RYGB or sham operation. A: 2–4 mo after RYGB or sham surgery, exendin-(9–39) (Ex9), (100 μg·rat−1·day−1 for 14 days) or vehicle (Veh, 0.5 μl/h) was infused intracerebroventricularly into the lateral ventricle. Ex9 significantly increased body weight gain in all three groups compared with their respective vehicle controls. Values are expressed as means ± SE of 7 or 8 rats/group. *P < 0.05, RYGB/Ex9 vs. RYGB/Veh and Sham/Ex9 vs. Sham/Veh. B–E: change of body weight (B), fat mass (C), adiposity (D), and lean mass (E), as measured by NMR before and after infusion. Values are expressed as means ± SE of 7 or 8 rats/group. *P < 0.05, Ex9 vs. vehicle. #P < 0.05, RYGB vs. Sham.
Analysis of changes in body composition was based on measurements at 8.7 ± 1.0 days before and 8.8 ± 1.0 days after metabolic chamber housing, an average total period of 38 days. For fat mass gain, it revealed significant main effects of infusion [F(1,37) = 33.07, P < 0.0001] and surgery [F(2,37) = 9.50, P < 0.001], but no significant interaction (Fig. 2C). Direct comparisons showed that Ex9 significantly increased fat mass gain vs. vehicle in RYGB and sham-operated rats (RYGB: +18.6 g, t = 3.54, P < 0.01; sham: +18.96 g, t = 4.02, P < 0.01), but not in chow-fed rats [+10.8 g, t = 2.37, not significant (n.s.)]. As shown in Fig. 2D, the adiposity index was similarly affected, except that only the main effect of infusion was significant [F(1,37) = 42.79, P < 0.0001] and that in follow-up comparisons, the adiposity-promoting effect of Ex9 was significant in RYGB (t = 4.65, P < 0.001), sham (t = 3.61, P < 0.01), and chow-fed rats (t = 2.96, P < 0.05). Finally, analysis of lean mass revealed only a significant main effect of surgery, reflecting the significantly lower lean mass gain in RYGB rats compared with sham-operated and chow-fed rats (Fig. 2E).
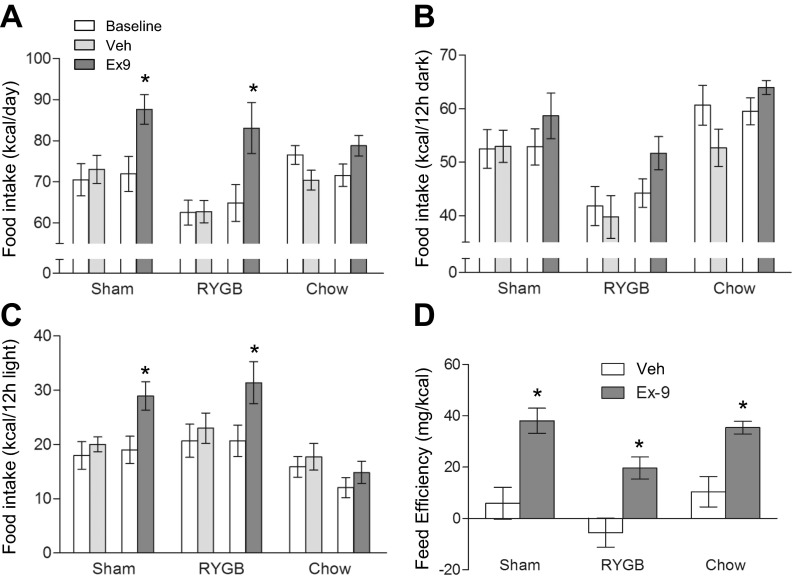
Effects of Ex9 on Food and Water Intake and Feed Efficiency
Analysis of food intake data assessed during the baseline and infusion periods in the metabolic chambers revealed a significant main effect of infusion [F(3,82) = 11.02, P < 0.0001] and surgery [F(2,82) = 4.77, P < 0.05] (Fig. 3A). Follow-up multiple comparisons showed that in both RYGB and sham-operated rats, but not in chow-fed controls, food intake was significantly higher under conditions of GLP-1 blockade compared with the same rats before infusion and with vehicle-infused rats. The increase of food intake in RYGB rats was of similar magnitude as in sham-operated rats. As expected, RYGB rats consumed about 12% less calories compared with sham-operated rats during baseline and vehicle infusion.
Fig. 3.
Effects of chronic pharmacological blockade of central GLP-1 receptor signaling on food intake and feeding efficiency of rats 2–4 mo after RYGB surgery. A: average daily food intake during preinfusion baseline period (open bars) and days 4–14 of infusion with either Ex9 (dark gray) or vehicle (light gray). Average dark (B) and light (C) period food intake. D: feed efficiency calculated during days 4–14 of Ex9 or vehicle infusion. Note that Ex9 significantly increases 24-h and 12-h light period food intake in RYGB and sham rats and feed efficiency in all three groups. Values are expressed as means ± SE of 6–8 rats/group. *P < 0.05, Ex9 vs. vehicle.
Focusing on the dark period food intake, ANOVA that was applied to only RYGB and sham-operated rats revealed a significant main effect of surgery [F(1,54) = 15.76, P < 0.001] and a marginally significant effect of infusion [F(3,54) = 2.63, P = 0.06]. Follow-up tests showed RYGB rats generally consuming fewer calories (∼20%) during baseline and vehicle infusion compared with sham-operated rats and significantly higher intake with Ex9 vs. saline in RYGB rats (Fig. 3B). Focusing on light period intake, there was a significant main effect of infusion F[3,54] = 6.48, P < 0.001), with both RYGB and sham rats, but not chow rats, showing significant Ex9-induced increases. In the absence of Ex9, food intake was not different between RYGB and sham rats.
Feed efficiency calculated as weight gain per calorie eaten in the 11-day period from days 4–14 after the start of infusion showed that RYGB rats are slightly less efficient under saline conditions, but that sham-operated, RYGB, and chow-fed lean rats significantly increase feed efficiency under the influence of Ex9 (Fig. 3D).
Finally, Ex9 infusion resulted in small increases in water intake that seemed secondary to the increased food intake (not shown).
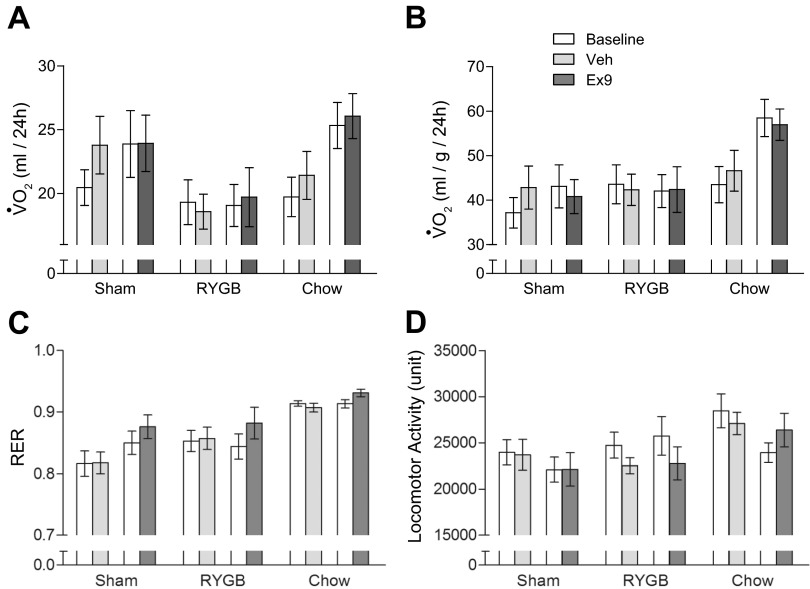
Effects of Ex9 on Energy Expenditure, Respiratory Exchange Ratio, and Locomotor Activity
Analysis of V̇o2 consumption in milliliters per rat during the baseline infusion periods in the metabolic chambers for all three groups (RYGB, sham, and chow) revealed a significant main effect of surgery [F(2,82) = 5.59, P < 0.01], but not infusion [F(3,96) = 1.93, n.s.) or interaction [F(6,82) = 0.72, n.s.] (Fig. 4A). The main effect of surgery was due to generally lower V̇o2 consumption in RYGB rats compared with sham-operated and chow-fed rats. Expression of V̇o2 consumption per kilogram total body mass (Fig. 4B), revealed a significant main effect of surgery [F(2,82) = 7.13, P < 0.005], which was due to higher energy expenditure of chow-fed rats with Ex9 infusions. If the ANOVA only included RYGB and sham-operated rats, the main effect of surgery was no longer significant [F(1,54) = 0.28, n.s.]. Thus, V̇o2 consumption was not significantly different between RYGB and sham-operated rats. Finally, when energy expenditure was corrected for lean body mass, it was slightly higher in RYGB vs. sham-operated rats, but the main effect of surgery did not reach statistical significance (data not shown).
Fig. 4.
Effects of chronic pharmacological blockade of central GLP-1 receptor signaling on energy expenditure, respiratory exchange ratio, and locomotor activity of rats 2–4 mo after RYGB surgery. A: average daily energy expenditure during preinfusion baseline period (white bars) and days 4–14 of infusion with either Ex9 (dark gray) or vehicle (light gray). B: energy expenditure corrected for total body mass. C: respiratory exchange ratio. Note that chow-fed rats show the expected higher RER compared with the high-fat-fed RYGB and sham rats. D: locomotor activity. Values are expressed as means ± SE of 6–8 rats/group.
As expected, the respiratory exchange ratio was significantly higher for chow-fed lean rats, as reflected by the significant main effect of surgery [F(2,82) = 23.10, P < 0.0001; ANOVA across all three groups] (Fig. 4C). There was also a marginally significant main effect of infusion [F(3,82) = 3.14, P < 0.05], reflecting the consistently higher respiratory exchange ratio (RER) during Ex9 vs. vehicle infusion in all three groups. However, ANOVA across only RYGB and sham groups yielded no significant main effect of surgery [F(1,54) = 1.8, n.s.], revealing that there was no difference in RER between RYGB and sham-operated rats.
Locomotor activity was generally higher in chow-fed controls as indicated by the highly significant main effect of surgery [F(2,82) = 5.32, P < 0.01] (Fig. 4D). However, ANOVA applied only to RYGB and sham-operated rats did not reveal a significant main effect of surgery [F(1,54) = 0.73, n.s.], and there was no significant effect of Ex9 on locomotor activity.
Effects of Pharmacological Blockade of Central Y2R-Signaling in Rats with BIIE0246
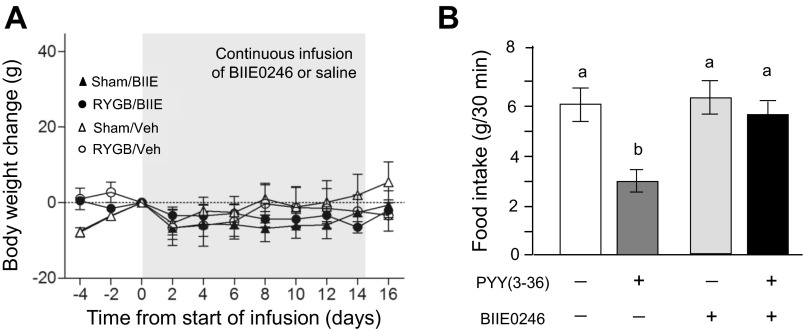
In contrast to Ex9, chronic infusion of BIIE0246 had no significant effects on body weight compared with vehicle infusion (Fig. 5A). There was a slight decrease of body weight over the 14-day infusion period in both RYGB and sham-operated rats. To verify potency of BIIE0246, the Y2 receptor blocker or vehicle was injected intracerebroventricularly 15 min before injection of PYY(3–36) or saline in a separate group of rats (Fig. 5B). Two-way ANOVA yielded significant effects of pretreatment [blocker vs. vehicle, F(1,12) = 10.48, P < 0.01], posttreatment [PYY(3–36) vs. vehicle, F(1,12) = 13.26, P < 0.010], and a significant interaction [F(1,12) = 8.83, P < 0.05], meaning that the food intake-suppressing effect of PYY(3–36) was almost completely abolished by BIIE0246, without having an effect of its own, confirming efficacy of BIIE0246 to acutely block the anorexic effects of intracerebroventricular PYY(3–36).
Fig. 5.
Effects of chronic pharmacological blockade of PYY/Y-2 receptor signaling on body weight. A: 5 mo after RYGB or sham surgery, BIIE0246 (BIIE, 100 μg·rat−1·day−1 for 14 days) or vehicle (Veh, 0.5 μl/h) was infused intracerebroventricularly into the lateral ventricle. Note that vehicle-treated groups are the same as in Fig. 2 and that BIIE did not have any significant effects on body weight in either surgical group. Values are expressed as means ± SE of 6–8 rats/group. B: intracerebroventricular infusion of PYY(3–36) significantly suppresses 30-min food intake in overnight food-deprived rats, and coinfusion of BIIE0246 completely antagonizes the effect of PYY(3–36), demonstrating short-term potency of the antagonist. Values are expressed as means ± SE of four rats. Bars that do not share the same letters denote significant differences at P < 0.05 (based on ANOVA followed by Bonferroni-adjusted multiple comparisons).
Effect of RYGB on Body Weight and Composition in GLP-1R-Null and Wild-Type Mice
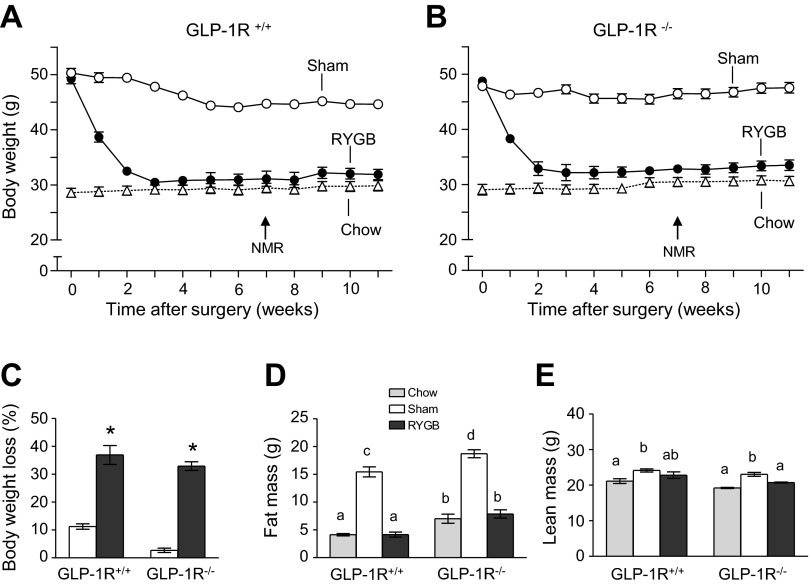
Our mouse model of RYGB closely matches human surgery in the size of the gastric pouch and relative lengths of the three limbs (25) and produces sustained body weight loss in wild-type animals (Fig. 6A). Here, we show that RYGB-induced weight loss was almost identical in whole body GLP-1 receptor knockout mice (Fig. 6B). Maximal body weight loss of 30–35% was reached after about 3 wk and was sustained for 8 wk after surgery in both genotypes (Fig. 6C). Weight loss was entirely accounted for by fat mass loss in both genotypes, so that fat mass of RYGB animals was not different from chow-fed lean controls (Fig. 6, D and E).
Fig. 6.
RYGB-induced reduction in weight loss and food intake is similar in GLP-1 receptor-deficient and wild-type mice. Body weight of wild-type (A) and GLP-1R−/− mice (B) made obese with high-fat diet for 12 wk and subjected to either RYGB or sham surgery. High-fat diet was replaced with medium-fat diet after surgery. Note that the RYGB-induced weight-loss curves are almost identical for the two genotypes. C: percent weight loss at 8 wk after surgery. Note that percent weight loss after RYGB was not significantly different between the two genotypes but significantly greater than after sham operation. The small weight loss in sham-operated animals is due to the switch from high- to medium-fat diet after surgery. Fat mass (D) and lean mass (E) of lean (chow-fed), sham-operated (high-fat obese), and RYGB rats at 7 wk after surgery. Note that all excess fat mass but no lean mass is lost after RYGB. Values are expressed as means ± SE; n = 8–10 mice. Bars that do not share the same letter are significantly different from each other. *P < 0.05 vs. sham.
Exposure to a high-fat diet did not reveal a differential response in GLP-1R−/− compared with wild-type mice. Sham-operated mice of both genotypes significantly gained body weight during the 6 wk of high-fat feeding, but this weight gain was significantly less in GLP-1R−/− mice (Fig. 7, A and B). In contrast, RYGB mice of both genotypes did not gain any weight; they initially even lost some weight. Because some GLP-1R−/− mice exhibited reduced food intake and body weight and showed signs of morbidity on a single high-fat diet, we offered them a two-choice diet composed of regular chow and high-fat pellets. In addition to measuring total food intake, this allowed us to determine a preference ratio for the high-fat diet. Compared with regular chow as the only diet, access to this choice diet significantly increased total calorie intake in RYGB and sham-operated mice of both genotypes, but the increase was smaller in knockout mice (Fig. 7D). On the choice diet, while sham-operated mice showed a high preference for the high-fat pellets, RYGB mice consumed just as many calories from low (regular chow) compared with high-fat (Fig. 7, C and E). There was no effect of genotype on this RYGB-induced loss of high-fat preference.
Fig. 7.
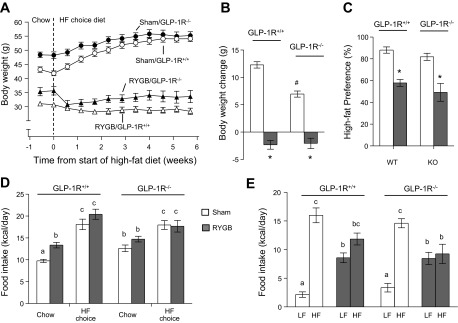
Food intake and body weight response of wild-type (GLP-1R+/+) and GLP-1R−/− mice with RYGB or sham surgery to high-fat diet exposure. Mice were switched from regular chow diet to a two-choice diet consisting of regular chow (low-fat) and high fat. A and B: sham but not RYGB mice of both genotypes significantly increased body weight during 6 wk of high-fat choice diet (*P < 0.05 vs. sham; #P < 0.05 vs. GLP-1R+/+). C–E: mice of all groups increased food intake on the high-fat choice diet (D), but while sham mice consumed significantly more of the high-fat, RYGB mice consumed equal amounts of low-fat and high-fat (E). Bars that do not share the same letter are significantly different from each other. Thus, RYGB mice of both phenotypes had a significantly lower preference ratio for a high-fat diet (C). *P < 0.05 vs. sham. Values are expressed as means ± SE; n = 7 or 8.
DISCUSSION
There is considerable evidence that both GLP-1 and PYY physiologically inhibit food intake (e.g., 7, 8, 17, 19, 57). Because both of these gut hormones are hypersecreted after RYGB and sleeve gastrectomy, they are prime candidates for the weight-lowering effects of these two types of bariatric surgeries (e.g., 14, 38). To test the hypothesis that either exaggerated GLP-1 or Y2 receptor signaling in the brain is responsible for the maintenance of a low body weight level after RYGB, we chronically infused the respective antagonists Ex9 or BIIE0246 into the brain over a period of 2 wk. Infusion of Ex9, but not BIIE0246, significantly increased body weight and adiposity of RYGB rats by increasing food intake and feed efficiency, suggesting that central GLP-1 receptor signaling but not Y2 receptor signaling contributes to the lower weight level defended in rats after RYGB. Although it is not clear how Ex9 significantly increased feeding efficiency in the absence of significantly reduced energy expenditure, a similar observation was made in rats with knockdown of brain stem proglucagon (6) and in rats with acute peripheral treatment with Ex9 (3). We speculate that it may be due to a combination of changes in energy intake, absorption, and partitioning, together with nonsignificant changes in energy expenditure. As weight gain with Ex9 infusion did not slow down at the end of the 2-wk infusion, RYGB rats may well have reached presurgical body weight levels if blockade would have continued.
However, interpretation of our findings is complicated because of the same or even greater anabolic response to Ex9 infusion in sham-operated obese and chow-fed normal rats. This outcome suggests that central GLP-1 signaling is involved in normal physiological body weight control, but is not uniquely involved in the effects on body weight of RYGB. Ideally, for demonstrations of the effectiveness of a compound to specifically antagonize a mechanism that suppresses food intake, a dose is chosen that by itself does not significantly increase food intake (55). However, choosing a lower dose of Ex9 that would produce a smaller or no anabolic effect in sham-operated rats could be expected to produce even less of an effect in RYGB rats, and it is questionable whether a dose could be found that increases body weight in RYGB rats more than in sham-operated rats. On the other hand, considering the exaggerated circulating postprandial GLP-1 levels after RYGB, we cannot rule out that a higher dose of Ex9 may have been more effective in increasing food intake and reversing suppressed body weight levels after RYGB. However, we consider the dose of 100 μg·day−1·rat−1 as supramaximal, because a more than 100-fold lower dose (per body weight) still significantly changed food intake and glucose tolerance in mice (31). On a per animal basis, our dose of Ex9 was more than 1,000-fold higher and in all likelihood blocked even the presumptively high levels of central GLP-1 receptor signaling after RYGB in the rat. While only a full dose-response study could provide definitive answers, this is not practical for this type of long-term infusion with a costly peptide.
Because we infused the blockers 3–5 mo after surgery, we cannot rule out different outcomes at earlier time points, particularly, the initial acute weight loss phase. Given that in human subjects, GLP-1 and PYY levels were elevated and associated with appetite and weight loss as soon as 2 days after RYGB (38), blockade during this phase in our paradigm would be expected to prevent or reduce initial weight loss. However, the complicating factor with similar anabolic effects in control rats would remain. We were particularly interested in the mechanisms defending the relatively stable lower body weight level after surgery because this is potentially the most remarkable feature of bariatric surgeries. After voluntary or forced calorie restriction, strong counter-regulatory responses, such as heightened hunger and reduced energy expenditure, make it difficult to stay at the lower body weight level for most dieters (48). These counter-regulatory responses seem to be absent or masked after RYGB. The possibility that central GLP-1 receptor signaling is involved in this process is intriguing and should be further investigated.
By infusing the blockers centrally, we were not able to affect peripheral GLP-1 and Y2 receptor signaling. Particularly, GLP-1 receptor signaling via vagal afferents has been implicated in the control of food intake (28, 35, 46, 51, 58). It is possible that peripheral infusions would have provided clearer results, perhaps with a larger anabolic effect in RYGB compared with sham-operated rats. This is suggested by a study with acute subcutaneous injections of Ex9 in RYGB and sham-operated rats at about 6 wk after surgery, where Ex9 significantly increased 2-h food intake in RYGB but not sham-operated rats (3). We chose to infuse the blockers centrally, mainly for economic reasons, but also because of the clear implication of the central GLP-1 signaling system in food intake and energy homeostasis (6, 63), and recent demonstrations of GLP-1 receptor-mediated effects on food intake and food reward in areas outside the classical homeostatic areas of hypothalamus and brain stem, in the mesolimbic dopamine system (4, 20, 21). A corollary of central infusion of antagonists is that our experiment does not distinguish between GLP-1 and PYY originating from the gut and from respective neurons in the brain. If GLP-1 from the gut does not reach the brain in sufficient concentrations even after RYGB, the observed effect must be due to antagonism of neuronal GLP-1. It would, thus, be interesting to carry out gastric bypass in animals with selective loss of neuronal GLP-1.
In contrast to the effect of Ex9, BIIE0246 did not increase body weight of RYGB and sham-operated rats, suggesting that under the conditions of our paradigm, central Y2 receptor signaling does not play a role in the effects of RYGB on body weight. Absence of any effect of the blocker on body weight of sham-operated obese and chow-fed lean controls questioned its efficacy, prompting us to test the ability of BIIE0246 to antagonize PYY(3–36)-induced suppression of food intake. We found pretreatment with BIIE0246 abolished the acute food intake-reducing effects of fourth ventricular PYY(3–36). However, it is possible that the drug lost efficacy over the 2 wk in the minipumps at body temperature. The lack of effect of Y2 receptor blockade in RYGB rats is in contrast to findings with a modified gastrointestinal bypass model in PYY-deficient mice (14). In that study, wild-type, but not mice with modified gastrointestinal bypass weighed significantly less 10 days after surgery compared with sham-operated mice, supporting a role for PYY in the acute effects of gastric bypass on body weight. There are many experimental differences that could explain the apparent discrepancy. As for GLP-1, PYY signaling through the Y2 receptor has both a peripheral and central component (2, 8, 9, 49, 53, 58). However, in contrast to the GLP-1 receptor antagonist Ex9, the Y2 receptor antagonist BIIE0246 does not easily cross the blood-brain barrier (11) and may not reach Y2 receptors on arcuate nucleus neurons in sufficient concentrations. Chronic infusions into the lateral ventricle are highly likely to penetrate to all areas of the brain, including critical neurons in the basomedial hypothalamus (2).
Given these limitations with semichronic central blockade of GLP-1 and Y2 receptors, we then took advantage of an available GLP-1 receptor-deficient mouse generated by the laboratory of Daniel Drucker (54) and a murine model of RYGB recently developed in our laboratory (25). Unlike other models, our mouse RYGB model, which is characterized by a very small gastric pouch without using a metal clip, has a very low mortality rate of <10% and a rapid postsurgical recovery, allowing animals to ingest solid food as soon as 1–3 days after surgery (25).
RYGB was just as effective in reducing body weight and fat mass in GLP-1 receptor-deficient compared with wild-type mice, suggesting that signaling through this receptor, whether centrally or peripherally, is not required for the beneficial effects on body weight of RYGB. The same conclusion was recently reached in a mouse model of vertical sleeve gastrectomy (64), a surgical intervention that is drastically different from RYGB, but produces equal weight loss and similarly elevated levels of postprandial circulating GLP-1 levels in rodents and humans (13, 44). Therefore, surprisingly, GLP-1R signaling alone does not seem to be required for the beneficial effects of these two weight loss surgeries and other mechanisms that operate independently or in cooperation with GLP-1 signaling must be responsible. An inherent potential problem of germline knockout models is the presence of compensatory developmental changes. This could account for the unexpected outcome of these two types of surgery. It is conceivable that other mechanisms involved in the control of food intake and regulation of energy balance at least partially take over the role of GLP-1 signaling. Such redundant mechanisms may include signaling through the GLP-2 receptor, known to play a role not just in intestinal plasticity (41), but also in the neural control of appetite and energy balance (23, 39, 60). In fact, the capacity of acute intracerebroventricular injections of GLP-1 to suppress food intake is greatly enhanced if the GLP-1 receptor is blocked by coinjection of Ex-9 and in GLP-1R−/− mice (39), and plastic adaptations in the interaction between GLP-1R and GLP-2R signaling could be the reason that no overt perturbations of appetite and energy balance are observed in GLP-1R−/− mice (54). It is also possible that the contribution of critical sites of GLP-1 signaling were masked by the whole body knockout approach. Inducible and targeted knockout strategies will be necessary to rule out these possibilities of false negatives. Another limitation of our study is the absence of functional verification of GLP-1R knockout by testing known effects of GLP-1R agonist administration. However, homozygous knockout was verified by genotyping the mice before surgery and was previously demonstrated to result in functional loss of GLP-1R signaling (54). Finally, we cannot rule out the possibility that GLP-1R-deficiency was unable to change the outcome of RYGB because GLP-1 secretion was not increased in our particular mouse model. However, this is highly unlikely in view of the overwhelming evidence of such GLP-1 hypersecretion across many rodent models and the human literature involving both RYGB and sleeve gastrectomy.
Taken together, the present findings from two complementary approaches surprisingly do not support a major role of GLP-1R in the effects of RYGB on body weight control. However, the results do not rule out a role for the GLP-1R in other beneficial effects of RYGB, such as weight loss-independent improvements in glycemic control. In addition, our study also does not support an important role for central Y2 receptor signaling, at least under the limited conditions tested and without a semichronic positive control for the effectiveness of the Y2R antagonist.
Perspectives and Significance
Given the complexity of gut-brain signaling (10), it is likely that a number of pathways are involved, operating synergistically and in a fashion reflecting the dynamic changes taking place over time in the gut and other peripheral organs. Thus, interrupting only one signaling mechanism as done here may not reveal its real contribution to the overall effect. This argument is supported by recent studies highlighting the redundancy of mechanisms in the control of food intake and energy balance—the demonstration that combinations of drugs often have a larger effect than each of the individual drugs has alone. Therefore, it might be important to target more than one suspected pathway in interventional studies to obtain significant results.
GRANTS
Supprot was provided by National Institutes of Health Grants DK-047348 (HRB), DK-068036, and DK-085495 (to J. Ye).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: J.Y., H.M., C.D.M., and H.-R.B. interpreted results of experiments; J.Y., M.B.M., L.M.P., H.M., C.D.M., and H.-R.B. edited and revised manuscript; J.Y., N.S., H.M., C.D.M., D.J.D., and H.-R.B. approved final version of manuscript; Z.H., M.B.M., R.L.T., L.M.P., N.S., and D.J.D. performed experiments; M.B.M., R.L.T., and H.-R.B. analyzed data; M.B.M. and H.-R.B. prepared figures; H.M., C.D.M., and H.-R.B. conception and design of research; H.-R.B. drafted manuscript.
ACKNOWLEDGMENTS
We thank Jennifer Terrebone and the Pennington Behavioral and Metabolic Phenotyping Core for help with the metabolic chambers.
REFERENCES
- 1.Abbott CR, Monteiro M, Small CJ, Sajedi A, Smith KL, Parkinson JR, Ghatei MA, Bloom SR. The inhibitory effects of peripheral administration of peptide YY(3–36) and glucagon-like peptide-1 on food intake are attenuated by ablation of the vagal-brainstem-hypothalamic pathway. Brain Res 1044: 127–131, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Abbott CR, Small CJ, Kennedy AR, Neary NM, Sajedi A, Ghatei MA, Bloom SR. Blockade of the neuropeptide Y Y2 receptor with the specific antagonist BIIE0246 attenuates the effect of endogenous and exogenous peptide YY(3–36) on food intake. Brain Res 1043: 139–144, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Abegg K, Schiesser M, Lutz TA, Bueter M. Acute peripheral GLP-1 receptor agonism or antagonism does not alter energy expenditure in rats after Roux-en-Y gastric bypass. Physiol Behav 121: 70–78, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alhadeff AL, Rupprecht LE, Hayes MR. GLP-1 neurons in the nucleus of the solitary tract project directly to the ventral tegmental area and nucleus accumbens to control for food intake. Endocrinology 153: 647–658, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baraboi ED, Michel C, Smith P, Thibaudeau K, Ferguson AV, Richard D. Effects of albumin-conjugated PYY on food intake: the respective roles of the circumventricular organs and vagus nerve. Eur J Neurosci 32: 826–839, 2010 [DOI] [PubMed] [Google Scholar]
- 6.Barrera JG, Jones KR, Herman JP, D'Alessio DA, Woods SC, Seeley RJ. Hyperphagia and increased fat accumulation in two models of chronic CNS glucagon-like peptide-1 loss of function. J Neurosci 31: 3904–3913, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barrera JG, Sandoval DA, D'Alessio DA, Seeley RJ. GLP-1 and energy balance: an integrated model of short-term and long-term control. Nat Rev Endocrinol 7: 507–516, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Batterham RL, Cowley MA, Small CJ, Herzog H, Cohen MA, Dakin CL, Wren AM, Brynes AE, Low MJ, Ghatei MA, Cone RD, Bloom SR. Gut hormone PYY(3–36) physiologically inhibits food intake. Nature 418: 650–654, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Batterham RL, ffytche DH, Rosenthal JM, Zelaya FO, Barker GJ, Withers DJ, Williams SC. PYY modulation of cortical and hypothalamic brain areas predicts feeding behaviour in humans. Nature 450: 106–109, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Berthoud HR, Shin AC, Zheng H. Obesity surgery and gut-brain communication. Physiol Behav 105: 106–119, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brothers SP, Saldanha SA, Spicer TP, Cameron M, Mercer BA, Chase P, McDonald P, Wahlestedt C, Hodder PS. Selective and brain penetrant neuropeptide y y2 receptor antagonists discovered by whole-cell high-throughput screening. Mol Pharmacol 77: 46–57, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brubaker PL. Minireview: update on incretin biology: focus on glucagon-like peptide-1. Endocrinology 151: 1984–1989, 2010 [DOI] [PubMed] [Google Scholar]
- 13.Chambers AP, Jessen L, Ryan KK, Sisley S, Wilson-Perez HE, Stefater MA, Gaitonde SG, Sorrell JE, Toure M, Berger J, D'Alessio DA, Woods SC, Seeley RJ, Sandoval DA. Weight-independent changes in blood glucose homeostasis after gastric bypass or vertical sleeve gastrectomy in rats. Gastroenterology 141: 950–958, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chandarana K, Gelegen C, Karra E, Choudhury AI, Drew ME, Fauveau V, Viollet B, Andreelli F, Withers DJ, Batterham RL. Diet and gastrointestinal bypass-induced weight loss: the roles of ghrelin and peptide YY. Diabetes 60: 810–818, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chelikani PK, Haver AC, Reeve JR, Jr, Keire DA, Reidelberger RD. Daily, intermittent intravenous infusion of peptide YY(3–36) reduces daily food intake and adiposity in rats. Am J Physiol Regul Integr Comp Physiol 290: R298–R305, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Chelikani PK, Shah IH, Taqi E, Sigalet DL, Koopmans HH. Comparison of the effects of Roux-en-Y gastric bypass and ileal transposition surgeries on food intake, body weight, and circulating peptide YY concentrations in rats. Obes Surg 20: 1281–1288, 2010 [DOI] [PubMed] [Google Scholar]
- 17.Dailey MJ, Moghadam AA, Moran TH. Jejunal linoleic acid infusions require GLP-1 receptor signaling to inhibit food intake: implications for the effectiveness of Roux-en-Y gastric bypass. Am J Physiol Endocrinol Metab 301: E1184–E1190, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dar MS, Chapman WH, 3rd, Pender JR, Drake AJ, 3rd, O'Brien K, Tanenberg RJ, Dohm GL, Pories WJ. GLP-1 response to a mixed meal: what happens 10 years after Roux-en-Y gastric bypass (RYGB)? Obes Surg 22: 1077–1083, 2012 [DOI] [PubMed] [Google Scholar]
- 19.De Silva A, Salem V, Long CJ, Makwana A, Newbould RD, Rabiner EA, Ghatei MA, Bloom SR, Matthews PM, Beaver JD, Dhillo WS. The gut hormones PYY 3–36 and GLP-1 7–36 amide reduce food intake and modulate brain activity in appetite centers in humans. Cell Metab 14: 700–706, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dickson SL, Shirazi RH, Hansson C, Bergquist F, Nissbrandt H, Skibicka KP. The glucagon-like peptide 1 (GLP-1) analogue, exendin-4, decreases the rewarding value of food: a new role for mesolimbic GLP-1 receptors. J Neurosci 32: 4812–4820, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dossat AM, Lilly N, Kay K, Williams DL. Glucagon-like peptide 1 receptors in nucleus accumbens affect food intake. J Neurosci 31: 14453–14457, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fenske WK, Bueter M, Miras AD, Ghatei MA, Bloom SR, le Roux CW. Exogenous peptide YY3–36 and Exendin-4 further decrease food intake, whereas octreotide increases food intake in rats after Roux-en-Y gastric bypass. Int J Obes (Lond) 36: 379–384, 2012 [DOI] [PubMed] [Google Scholar]
- 23.Guan X, Shi X, Li X, Chang B, Wang Y, Li D, Chan L. GLP-2 receptor in POMC neurons suppresses feeding behavior and gastric motility. Am J Physiol Endocrinol Metab 303: E853–E864, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Habegger KM, Heppner KM, Amburgy SE, Ottaway N, Holland J, Raver C, Bartley E, Muller TD, Pfluger PT, Berger J, Toure M, Benoit SC, Dimarchi RD, Perez-Tilve D, D'Alessio DA, Seeley RJ, Tschop MH. GLP-1R responsiveness predicts individual gastric bypass efficacy on glucose tolerance in rats. Diabetes In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hao Z, Zhao Z, Berthoud HR, Ye J. Development and verification of a mouse model for Roux-en-Y gastric bypass surgery with a small gastric pouch. PLoS One 8: e52922, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hassan M, Eskilsson A, Nilsson C, Jonsson C, Jacobsson H, Refai E, Larsson S, Efendic S. In vivo dynamic distribution of 131I-glucagon-like peptide-1 (7–36) amide in the rat studied by gamma camera. Nucl Med Biol 26: 413–420, 1999 [DOI] [PubMed] [Google Scholar]
- 27.Hayes MR, Bradley L, Grill HJ. Endogenous hindbrain glucagon-like peptide-1 receptor activation contributes to the control of food intake by mediating gastric satiation signaling. Endocrinology 150: 2654–2659, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kanoski SE, Fortin SM, Arnold M, Grill HJ, Hayes MR. Peripheral and central GLP-1 receptor populations mediate the anorectic effects of peripherally administered GLP-1 receptor agonists, liraglutide and exendin-4. Endocrinology 152: 3103–3112, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kastin AJ, Akerstrom V, Pan W. Interactions of glucagon-like peptide-1 (GLP-1) with the blood-brain barrier. J Mol Neurosci 18: 7–14, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Kelly AS, Rudser KD, Nathan BM, Fox CK, Metzig AM, Coombes BJ, Fitch AK, Bomberg EM, Abuzzahab MJ. The effect of glucagon-like peptide-1 receptor agonist therapy on body mass index in adolescents with severe obesity: a randomized, placebo-controlled, clinical trial. JAMA Pediatr 167: 355–360, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Knauf C, Cani PD, Ait-Belgnaoui A, Benani A, Dray C, Cabou C, Colom A, Uldry M, Rastrelli S, Sabatier E, Godet N, Waget A, Penicaud L, Valet P, Burcelin R. Brain glucagon-like peptide 1 signaling controls the onset of high-fat diet-induced insulin resistance and reduces energy expenditure. Endocrinology 149: 4768–4777, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Korner J, Bessler M, Inabnet W, Taveras C, Holst JJ. Exaggerated glucagon-like peptide-1 and blunted glucose-dependent insulinotropic peptide secretion are associated with Roux-en-Y gastric bypass but not adjustable gastric banding. Surg Obes Relat Dis 3: 597–601, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Korner J, Inabnet W, Febres G, Conwell IM, McMahon DJ, Salas R, Taveras C, Schrope B, Bessler M. Prospective study of gut hormone and metabolic changes after adjustable gastric banding and Roux-en-Y gastric bypass. Int J Obes (Lond) 33: 786–795, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kunnecke B, Verry P, Benardeau A, von Kienlin M. Quantitative body composition analysis in awake mice and rats by magnetic resonance relaxometry. Obes Res 12: 1604–1615, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Labouesse MA, Stadlbauer U, Weber E, Arnold M, Langhans W, Pacheco-Lopez G. Vagal afferents mediate early satiation and prevent flavour avoidance learning in response to intraperitoneally infused exendin-4. J Neuroendocrinol 24: 1505–1516, 2012 [DOI] [PubMed] [Google Scholar]
- 36.Laferrere B, Heshka S, Wang K, Khan Y, McGinty J, Teixeira J, Hart AB, Olivan B. Incretin levels and effect are markedly enhanced 1 month after Roux-en-Y gastric bypass surgery in obese patients with type 2 diabetes. Diabetes Care 30: 1709–1716, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.le Roux CW, Aylwin SJ, Batterham RL, Borg CM, Coyle F, Prasad V, Shurey S, Ghatei MA, Patel AG, Bloom SR. Gut hormone profiles following bariatric surgery favor an anorectic state, facilitate weight loss, and improve metabolic parameters. Ann Surg 243: 108–114, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.le Roux CW, Welbourn R, Werling M, Osborne A, Kokkinos A, Laurenius A, Lonroth H, Fandriks L, Ghatei MA, Bloom SR, Olbers T. Gut hormones as mediators of appetite and weight loss after Roux-en-Y gastric bypass. Ann Surg 246: 780–785, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Lovshin J, Estall J, Yusta B, Brown TJ, Drucker DJ. Glucagon-like peptide (GLP)-2 action in the murine central nervous system is enhanced by elimination of GLP-1 receptor signaling. J Biol Chem 276: 21489–21499, 2001 [DOI] [PubMed] [Google Scholar]
- 40.Ma X, Bruning J, Ashcroft FM. Glucagon-like peptide 1 stimulates hypothalamic proopiomelanocortin neurons. J Neurosci 27: 7125–7129, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 41.Munroe DG, Gupta AK, Kooshesh F, Vyas TB, Rizkalla G, Wang H, Demchyshyn L, Yang ZJ, Kamboj RK, Chen H, McCallum K, Sumner-Smith M, Drucker DJ, Crivici A. Prototypic G protein-coupled receptor for the intestinotrophic factor glucagon-like peptide 2. Proc Natl Acad Sci USA 96: 1569–1573, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nakagawa A, Satake H, Nakabayashi H, Nishizawa M, Furuya K, Nakano S, Kigoshi T, Nakayama K, Uchida K. Receptor gene expression of glucagon-like peptide-1, but not glucose-dependent insulinotropic polypeptide, in rat nodose ganglion cells. Auton Neurosci 110: 36–43, 2004 [DOI] [PubMed] [Google Scholar]
- 43.Nauck MA. Incretin-based therapies for type 2 diabetes mellitus: properties, functions, and clinical implications. Am J Med 124: S3–S18, 2011 [DOI] [PubMed] [Google Scholar]
- 44.Peterli R, Steinert RE, Woelnerhanssen B, Peters T, Christoffel-Courtin C, Gass M, Kern B, von Fluee M, Beglinger C. Metabolic and hormonal changes after laparoscopic Roux-en-Y gastric bypass and sleeve gastrectomy: a randomized, prospective trial. Obes Surg 22: 740–748, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pinilla L, Fernandez-Fernandez R, Roa J, Castellano JM, Tena-Sempere M, Aguilar E. Selective role of neuropeptide Y receptor subtype Y2 in the control of gonadotropin secretion in the rat. Am J Physiol Endocrinol Metab 293: E1385–E1392, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Plamboeck A, Veedfald S, Deacon CF, Hartmann B, Wettergren A, Svendsen LB, Meisner S, Hovendal C, Vilsboll T, Knop FK, Holst JJ. The effect of exogenous GLP-1 on food intake is lost in male truncally vagotomized subjects with pyloroplasty. Am J Physiol Gastrointest Liver Physiol 304: G1117–G1127, 2013 [DOI] [PubMed] [Google Scholar]
- 47.Pournaras DJ, Osborne A, Hawkins SC, Mahon D, Ghatei MA, Bloom SR, Welbourn R, le Roux CW. The gut hormone response following Roux-en-Y gastric bypass: cross-sectional and prospective study. Obes Surg 20: 56–60, 2010 [DOI] [PubMed] [Google Scholar]
- 48.Redman LM, Heilbronn LK, Martin CK, de Jonge L, Williamson DA, Delany JP, Ravussin E. Metabolic and behavioral compensations in response to caloric restriction: implications for the maintenance of weight loss. PLoS One 4: e4377, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reidelberger R, Haver A, Chelikani PK. Role of peptide YY(3–36) in the satiety produced by gastric delivery of macronutrients in rats. Am J Physiol Endocrinol Metab 304: E944–E950, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rodieux F, Giusti V, D'Alessio DA, Suter M, Tappy L. Effects of gastric bypass and gastric banding on glucose kinetics and gut hormone release. Obesity (Silver Spring) 16: 298–305, 2008 [DOI] [PubMed] [Google Scholar]
- 51.Ruttimann EB, Arnold M, Hillebrand JJ, Geary N, Langhans W. Intrameal hepatic portal and intraperitoneal infusions of glucagon-like peptide-1 reduce spontaneous meal size in the rat via different mechanisms. Endocrinology 150: 1174–1181, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sandoval DA, Bagnol D, Woods SC, D'Alessio DA, Seeley RJ. Arcuate GLP-1 receptors regulate glucose homeostasis but not food intake. Diabetes 57: 2046–2054, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Scott V, Kimura N, Stark JA, Luckman SM. Intravenous peptide YY3–36 and Y2 receptor antagonism in the rat: effects on feeding behaviour. J Neuroendocrinol 17: 452–457, 2005 [DOI] [PubMed] [Google Scholar]
- 54.Scrocchi LA, Brown TJ, MaClusky N, Brubaker PL, Auerbach AB, Joyner AL, Drucker DJ. Glucose intolerance but normal satiety in mice with a null mutation in the glucagon-like peptide 1 receptor gene. Nat Med 2: 1254–1258, 1996 [DOI] [PubMed] [Google Scholar]
- 55.Seeley RJ, Moran TH. Principles for interpreting interactions among the multiple systems that influence food intake. Am J Physiol Regul Integr Comp Physiol 283: R46–R53, 2002 [DOI] [PubMed] [Google Scholar]
- 56.Shin AC, Zheng H, Townsend RL, Sigalet DL, Berthoud HR. Meal-induced hormone responses in a rat model of Roux-en-Y gastric bypass surgery. Endocrinology 151: 1588–1597, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stadlbauer U, Arnold M, Weber E, Langhans W. Possible mechanisms of circulating PYY-induced satiation in male rats. Endocrinology 154: 193–204, 2013 [DOI] [PubMed] [Google Scholar]
- 58.Talsania T, Anini Y, Siu S, Drucker DJ, Brubaker PL. Peripheral exendin-4 and peptide YY(3–36) synergistically reduce food intake through different mechanisms in mice. Endocrinology 146: 3748–3756, 2005 [DOI] [PubMed] [Google Scholar]
- 59.Tang-Christensen M, Larsen PJ, Goke R, Fink-Jensen A, Jessop DS, Moller M, Sheikh SP. Central administration of GLP-1-(7–36) amide inhibits food and water intake in rats. Am J Physiol Regul Integr Comp Physiol 271: R848–R856, 1996 [DOI] [PubMed] [Google Scholar]
- 60.Tang-Christensen M, Larsen PJ, Thulesen J, Romer J, Vrang N. The proglucagon-derived peptide, glucagon-like peptide-2, is a neurotransmitter involved in the regulation of food intake. Nat Med 6: 802–807, 2000 [DOI] [PubMed] [Google Scholar]
- 61.Tang-Christensen M, Vrang N, Larsen PJ. Glucagon-like peptide containing pathways in the regulation of feeding behaviour. Int J Obes Relat Metab Disord 25 Suppl 5: S42–S47, 2001 [DOI] [PubMed] [Google Scholar]
- 62.Vahl TP, Tauchi M, Durler TS, Elfers EE, Fernandes TM, Bitner RD, Ellis KS, Woods SC, Seeley RJ, Herman JP, D'Alessio DA. Glucagon-like peptide-1 (GLP-1) receptors expressed on nerve terminals in the portal vein mediate the effects of endogenous GLP-1 on glucose tolerance in rats. Endocrinology 148: 4965–4973, 2007 [DOI] [PubMed] [Google Scholar]
- 63.Williams DL. Finding the sweet spot: peripheral versus central glucagon-like peptide 1 action in feeding and glucose homeostasis. Endocrinology 150: 2997–3001, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wilson-Perez HE, Chambers AP, Ryan KK, Li B, Sandoval DA, Stoffers D, Drucker DJ, Perez-Tilve D, Seeley RJ. Vertical sleeve gastrectomy is effective in two genetic mouse models of glucagon-like peptide-1 receptor deficiency. Diabetes 62: 2380–2385, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zheng H, Townsend RL, Shin AC, Patterson LM, Phifer CB, Berthoud HR. High-fat intake induced by mu-opioid activation of the nucleus accumbens is inhibited by Y1R-blockade and MC3/4R- stimulation. Brain Res 1350: 131–138, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]