Abstract
Zebrafish Na+/H+ exchanger 3b (zNHE3b) is highly expressed in the apical membrane of ionocytes where Na+ is absorbed from ion-poor fresh water against a concentration gradient. Much in vivo data indicated that zNHE3b is involved in Na+ absorption but not leakage. However, zNHE3b-mediated Na+ absorption has not been thermodynamically explained, and zNHE3b activity has not been measured. To address this issue, we overexpressed zNHE3b in Xenopus oocytes and characterized its activity by electrophysiology. Exposure of zNHE3b oocytes to Na+-free media resulted in significant decrease in intracellular pH (pHi) and intracellular Na+ activity (aNai). aNai increased significantly when the cytoplasm was acidified by media containing CO2-HCO3− or butyrate. Activity of zNHE3b was inhibited by amiloride or 5-ethylisopropyl amiloride (EIPA). Although the activity was accompanied by a large hyperpolarization of ∼50 mV, voltage-clamp experiments showed that Na+/H+ exchange activity of zNHE3b is electroneutral. Exposure of zNHE3b oocytes to medium containing NH3/NH4+ resulted in significant decreases in pHi and aNai and significant increase in intracellular NH4+ activity, indicating that zNHE3b mediates the Na+/NH4+ exchange. In low-Na+ (0.5 mM) media, zNHE3b oocytes maintained aNai of 1.3 mM, and Na+-influx was observed when pHi was decreased by media containing CO2-HCO3− or butyrate. These results provide thermodynamic evidence that zNHE3b mediates Na+ absorption from ion-poor fresh water by its Na+/H+ and Na+/NH4+ exchange activities.
Keywords: Na+/H+ exchange, Na+/NH4+ exchange, NHE3, zebrafish, Xenopus oocyte electrophysiology, ammonium, intracellular pH
teleost fishes in fresh water (FW) maintain a body fluid osmolarity of ∼300 mosM (34). This value is similar to those of mammals but more than 300 times higher than those of their ion-poor environments. To balance loss of ions mainly from the gill and urine, FW fishes actively absorb ions from the surrounding water against a concentration gradient. Ion absorption is mediated by ionocytes that are mitochondrion-rich epithelial cells scattered on the surface of gills of adult fishes and the skin of larvae. Moreover, the mechanisms whereby ionocytes actively absorb ions have received considerable attention (13, 17–18, 26, 46).
Na+/H+ exchanger 3 (NHE3; Slc9a3) was first identified as a homolog of NHE1 in mammals. Its transcript is highly expressed in the gastrointestinal tract stomach, small intestine, large intestine, and the kidney (11, 37). NHE3 is localized at the brush-border (apical) membranes of the intestinal epithelium (jejunum, ileum, and colon) and the renal tubule (proximal tubule and thick ascending limb). It is involved in intestinal electroneutral Na+ absorption and renal reabsorption of Na+ and HCO3− (41). In teleost and elasmobranch fishes, an ortholog or a species-specific paralog of NHE3 has been found to be localized in the apical membrane of ionocytes of acid-tolerant Osorezan dace (Tribolodon hakonensis) (16), euryhaline Atlantic stingray (Dasyatis sabina) (9), zebrafish (49), Mozambique tilapia (Oreochromis mossambicus) (19), medaka (Oryzias latipes) (30), and rainbow trout (Oncorhynchus mykiss) (21). NHE3 expression in the gill or larvae increased when 1) Osorezan dace, Mozambique tilapia, and medaka were acclimated to acidic water (15–16, 30), 2) Atlantic stingray and medaka were transferred from seawater (SW) to FW (9), and 3) zebrafish and medaka were acclimated to low-Na+ FW (48–49). Treating zebrafish larvae with an NHE inhibitor (5-ethylisopropyl amiloride, EIPA) reduced intracellular Na+ activity (aNai) of ionocytes (12), reduced 22Na+ uptake by the larvae (25), and increased ionocyte-surface Na+ activity (i.e., reduced Na+ absorption) (42). In zebrafish larvae, knockdown of NHE3b, the ionocyte-specific paralog of NHE3, reduced whole body Na+ content and increased ionocyte surface Na+ activity (42). Exposure of zebrafish larvae to acidic (pH 4) water stimulated 22Na+ uptake by zebrafish larvae, and knockdown of NHE3b inhibited acid-water stimulated increase of 22Na+ uptake (25). These results suggest that NHE3 is involved in the system that mediates Na+ absorption and H+ excretion in ionocytes.
NHE3-mediated Na+ absorption and H+ excretion by FW fishes have also been questioned by a thermodynamic reason (38). In mammals, members of the NHE family mediate electroneutral exchange of intracellular H+ for external Na+ with a 1:1 stoichiometry (10, 33). With this activity, NHE3-mediated Na+ absorption is reasonable in the intestinal epithelium and renal proximal tubule, where luminal Na+ is abundantly present and drives Na+-influx mode of NHE3 in the apical membrane. In contrast, the apical membrane of ionocytes of FW fishes is directly exposed to environmental water in which Na+ is less than 1 mM. As such, thermodynamic calculations concluded that NHE3-mediated Na+ absorption in ionocytes of FW fishes is unfeasible and NHE3 can “leak” Na+. It is unreasonable that fishes upregulate Na+-leak transporter in a Na+-poor environment; the above conclusion was made under following assumptions: 1) Fish NHE3 activity is the same as that of mammalian NHE3, though detailed fish NHE3 activity remains uncharacterized. 2) Local pH around the inner surface of the plasma membrane is similar to that of whole cytosol. 3) There is no other molecule that drives NHE3.
Seven decades ago, Krogh proposed that Na+ absorption by FW fish is linked with NH4+ excretion (14, 24, 47). As NH4+ is the major end product of nitrogen metabolism in teleosts that accumulates in the bloodstream, teleosts likely utilize the NH4+-efflux gradient as a driving force to absorb Na+. In mammals, NHE3 is hypothesized to mediate Na+/NH4+ exchange based on experiments on membrane preparation of tissues (23) and is considered a major mechanism of apical plasma membrane NH4+ secretion in the proximal tubule (44), particularly as part of ammoniagenesis. As NHE3 is highly expressed in the apical membrane of ionocytes and is involved in the Na+ absorption system, NHE3 is hypothesized to mediate the exchange of internal NH4+ for Na+ in FW. However, there is no direct evidence for Na+/NH4+ exchange activity of exogenously expressed NHE3 in vitro. Recently, a fish homolog of the rhesus (Rh) glycoprotein NH3 channel (Rhcg1) and carbonic anhydrases, which catalyze the reversible reaction of CO2 + H2O ↔ HCO3− + H+, were shown to be colocalized with NHE3 in the apical membrane of ionocytes. These studies proposed another hypothesis that Rhcg1 and/or carbonic anhydrases drive NHE3 and are involved in the NHE3-mediated Na+-absorption system (the metabolon or transportsome hypothesis) (1, 20, 25, 30–31, 36, 42). At this time, there is no experimental evidence for this metabolon hypothesis in vitro.
To understand the NHE3-mediated Na+-uptake machinery in ionocyte of FW fish, we analyzed the activity of zebrafish NHE3b (zNHE3b) in Xenopus oocytes as a first clue. The exogenously expressed zNHE3b was found to be highly active, and its Na+/H+ and Na+/NH4+ exchange activities were characterized using pH, Na+, and NH4+ microelectrodes. Our results provide the first thermodynamic evidence for the zNHE3b-mediated Na+ absorption from ion-poor FW by characterizing its activity in vitro, indicating that Xenopus oocyte electrophysiology is a powerful tool to analyze components of the zNHE3b-mediated Na+-absorption system in ionocytes.
MATERIALS AND METHODS
Expression and immunohistochemistry of zebrafish NHE3b (zNHE3b) in Xenopus oocytes.
Full-length cDNAs of zebrafish Nhe3b, 5′-TTCTGATATGGCGTTTTCTACTCTTC-3′ and 5′-AACCCTATCACATGTTCAGCAA-3′, from the gill were amplified and inserted into the pGEMHE Xenopus laevis expression vector (29). The plasmid was linearized with NotI restriction enzyme and transcribed into cRNA in vitro using T7 RNA polymerase and mMESSAGE mMACHINE kits (Ambion, Austin, TX). Xenopus laevis oocytes were dissociated with collagenase as described previously (39) and injected with 50 nl of water or a solution containing cRNA at 0.2 ng/nl (10 ng/oocyte), using a Nanoject-II injector (Drummond Scientific, Broomall, PA). Oocytes were incubated at 16°C in OR3 medium (39) and studied 2–4 days after injection.
For the immunohistochemical study, oocytes were washed in ND96 saline solution [96 mM NaCl, 2 mM KCl, 1 mM MgCl2, 1.8 mM CaCl2, and 5 mM HEPES (pH 7.5)], fixed in 2% paraformaldehyde in 100 mM phosphate buffer (pH 7.4) at 4°C for 1 h, and washed in ND96. The oocytes were then quickly frozen in an optimum cutting temperature compound (Sakura Finetek, Tokyo, Japan). Frozen sections (6 μm) were prepared, permeabilized with 0.1% Triton X-100 in PBS at 20°C for 10 min, incubated with 5% fetal bovine serum (FBS; Invitrogen, Carlsbad, CA) in PBS at 20°C for 1 h, and incubated with rat polyclonal antiserum against 838–851 of zNHE3b (ETPEEKPATHHTRL) (1:1,000) (20) in PBS containing 5% FBS at 20°C for 16 h. After being washed with PBS, the sections were incubated with Alexa Fluor 488-labeled secondary antibody (1:2,000 dilution; Invitrogen) in PBS containing 5% FBS at 20°C for 1 h. The sections were mounted on antifade glycerol (90% glycerol, 10% 10× PBS, and 0.1% 1,4-phenylenediamine at pH 7.4). Fluorescence images were obtained with a laser confocal microscope (TCS-SPE; Leica, Wetzlar, Germany) and processed with LAS AF software (Leica).
Oocyte electrophysiology using H+ ion- and Na+ ion-selective microelectrodes.
To measure pHi or aNai of oocytes, H+ ion- or Na+ ion-selective microelectrodes were prepared with a H+ ionophore I-mixture B ion-selective resin (Fluka Chemical, Ronkonkoma, NY) or a Na+ ionophore cocktail A (Fluka), respectively, as described previously (7, 39). pHi or aNai was measured as the difference between the pH or Na+ electrode and a KCl voltage electrode impaled into the oocyte, and the membrane potential (Vm) was measured as the difference between the KCl microelectrode and an extracellular calomel. pH electrodes were calibrated using pH 6.0 and 8.0 buffers (Fisher), followed by a point calibration in ND96 (pH 7.5). Na+ electrodes were calibrated with 10 and 100 mM NaCl, and the specificity was checked using 100 mM KCl, followed by point calibration in ND96 (96 mM Na+).
The oocyte was held on a nylon mesh in a chamber and perfused with solution. Vm and pHi or aNai were constantly monitored and recorded at 0.4 Hz. Na+-free (0Na) solutions were prepared by substituting NaCl with choline chloride. Osmolarity and pH of all media were adjusted to ∼200 mosM and 7.5, respectively. In solutions containing HCO3−-CO2, 33 mM NaCl was replaced by 33 mM NaHCO3, and the solutions were equilibrated with 5% CO2-95% O2 to pH 7.5. Butyrate solutions were prepared by substituting 30 mM NaCl with 30 mM butyric acid and were equilibrated with NaOH to pH 7.5. In solutions containing NH3/NH4+, 10 mM NaCl was replaced by 10 mM NH4Cl. Na+ gradient-dependent activity was analyzed by changing the bath solution from ND96 to 0Na-ND96. pHi-dependent activity was analyzed by changing the bath solution from ND96 to HCO3−-CO2 or butyrate solution. NH3/NH4+-dependent activity was analyzed by changing the bath solution from ND96 to NH3/NH4+ solution. To analyze the reverse mode of Na+ gradient-dependent activity, oocytes were preincubated in ND96 solution containing 2 mM Na+ (2Na-ND96) for 2–4 h. At steady state, the bath solution was changed from 2 Na+- ND96 to ND96.
Quantitative data were presented as means ± SE. Values for initial pHi, aNai, Vm, ΔpHi/dt, ΔaNai/dt, and ΔVm were compared between zNHE3b and control oocytes, and the statistical significances (P values) were calculated by unpaired two-sided Student's t-test using GraphPad Prism software (Version 5, GraphPad, San Diego, CA).
Oocyte electrophysiology using NH4+ ion-selective microelectrode.
NH4+ ion-selective microelectrodes were prepared with ammonium ionophore I, cocktail A (Fluka) (4). 1 mM NH4Cl was used as a backfill solution. Intracellular NH4+ activity (aNH4i) of oocytes was measured as the difference between the NH4+ electrode and a KCl voltage electrode impaled into the oocyte, and the Vm was measured as described above. NH4+ electrodes were calibrated with 10 and 100 mM NH4Cl solutions containing 100 mM KCl and 5 mM HEPES (pH 7.5), followed by point calibration in 1 mM NH4Cl solutions containing 100 mM KCl and 5 mM HEPES (pH 7.5).
The oocyte was held on a nylon mesh in a chamber and perfused with solution. Vm and aNH4i were constantly monitored and recorded at 0.4 Hz. High-NH4+ solution was prepared by substituting NaCl with NH4Cl (96NH4-ND96). NH4+ gradient-dependent activity was analyzed by changing the bath solution from ND96 to 96NH4-ND96.
Quantitative data were presented as means ± SE. Values for aNH4i/dt were compared between zNHE3b and control oocytes, and the statistical significances (P values) were calculated by unpaired two-sided Student's t-test using GraphPad Prism software.
Effect of inhibitors.
Amiloride and EIPA (Sigma-Aldrich, St. Louis, MO) were dissolved in DMSO to prepare 500 mM stock solutions. The oocytes were perfused with ND96 and then perfused with 0Na-ND96 containing DMSO. After the maximum ΔpHi/dt was recorded (∼2 min after the buffer change), the oocytes were incubated in 0Na-ND96 containing 0.1–1 mM amiloride or 0.01–0.1 mM EIPA for ∼5 min. Final concentrations of DMSO with or without the inhibitors were adjusted to 0.2%. DMSO, 0.2%, alone did not inhibit the activity of zNHE3b (data not shown). The inhibitory rate was calculated by comparing ΔpHi/dt in 0Na-ND96 the presence or absence of inhibitor.
Niflumic acid (2-{[3-(trifluoromethyl)phenyl]amino}nicotinic acid, Sigma-Aldrich) was dissolved in DMSO to prepare a 500 mM stock solution and then added to ND96 and 0Na-ND96, whose pH values were adjusted to 7.5 with NaOH and choline base, respectively. A 500 mM ZnCl2 suspension was prepared in water and then dissolved in ND96 and 0Na-ND96. The oocyte was perfused with ND96 and then perfused with 0Na-ND96 to record the activity in the absence of inhibitor. The same oocyte was continuously perfused in ND96 containing inhibitor for 7–10 min and then incubated in 0Na-ND96 containing inhibitor for 10 min to compare the rate of pH change and degree of hyperpolarization.
Quantitative data are presented as means ± SE. Values for ΔpHi/dt in 0Na-ND96 solution with or without inhibitors were compared and the statistical significances (P values) calculated by unpaired two-sided Student's t-test using GraphPad Prism software.
Two-electrode voltage clamp.
Current-voltage (I–V) relationships of zNHE3b or water-injected oocytes were analyzed by a two-electrode voltage clamp as described previously (7). During the periods when the I–V protocols were not being run, oocytes were not clamped. The oocytes were perfused with ND96 medium and then perfused with Na+-free medium or medium containing CO2-HCO3− or butyrate. Oocytes were clamped at a holding potential of −30 mV in ND96 solution or at −80 mV in 0Na, CO2-HCO3−, or butyrate solutions. The I–V protocols consisted of 200-ms steps from a holding potential in 20-mV steps between −140 and +60 mV. I–V relations were recorded once in ND96 solution and then in 0Na, CO2-HCO3−, or butyrate solutions after the hyperpolarizations were maximized.
pHi measurements during Vm clamping.
Two KCl electrodes and one pH electrode were inserted into an oocyte, and then it was clamped to a holding potential (Vh) of −60 mV. Current and pHi were monitored constantly and recorded at 0.5 Hz as described previously (7). At steady state, the bath solution was changed from ND96 to 0Na+-ND96 solution. After the maximum ΔpHi/dt was recorded (∼2 min after the buffer change), Vh was changed to 0 or −120 mV and ΔpHi/dt were recorded for 2–3 min.
Quantitative data were presented as means ± SE. Values for ΔpHi/dt in 0Na-ND96 at various Vh levels were compared, and the statistical significances (P values) were analyzed using ANOVA followed by the Tukey-Kramer test with GraphPad Prism software.
RESULTS
Immunofluorescence of zNHE3b expressed in Xenopus oocytes.
Expression of zNHE3b in Xenopus oocytes was confirmed by immunofluorescence staining using anti-zNHE3b polyclonal antibody. The immunoreactivity was concentrated at the cell surface of the zNHE3b oocytes and was not observed in the water-injected (control) oocytes (Fig. 1A).
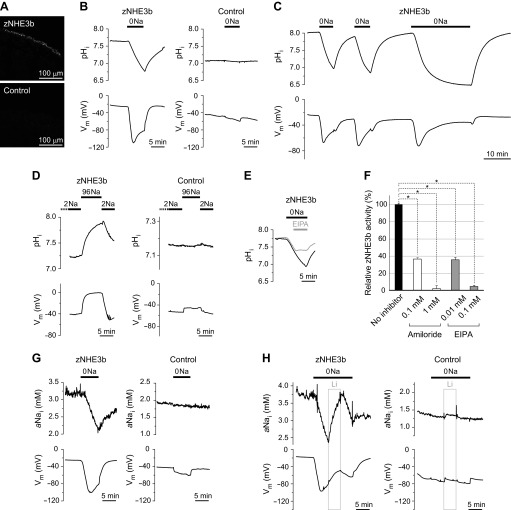
Fig. 1.
Na+-free medium-elicited H+-influx and Na+-efflux activities of zebrafish Na+/H+ exchanger 3b (zNHE3b) expressed in Xenopus oocytes. A: surface expression of zNHE3b in Xenopus oocytes. Immunofluorescence images of oocytes injected with zNHE3b cRNA or water (negative control) are shown. Scale bars, 100 μm. B: Na+-free medium-elicited H+-influx activity of zNHE3b. Representative traces of intracellular pH (pHi) and membrane potential (Vm) of zNHE3b and control oocytes are shown. 0Na indicates incubation in solution in which all Na+ (∼96 mM) was replaced with choline. C: experiment with a longer period of incubation time in 0Na solution. D: experiment with reversed conditions. Oocytes were preincubated in low-Na+ solution (2 mM Na+:2Na) and then incubated in ND96 saline solution. E: inhibition of zNHE3b-mediated H+ influx activity in Na+-free solution by 5-ethylisopropyl amiloride (EIPA). F: inhibition of zNHE3b activity by amiloride and EIPA. Rates of pHi changes (ΔpHi/dt) in 0Na solution in the presence or absence of inhibitors are shown as relative zNHE3b activity. G: Na+-free medium-elicited Na+-efflux activity of zNHE3b. Representative traces of intracellular Na+ activity (aNai) and Vm of zNHE3b and control oocytes are shown. H: Li+-transport activity of zNHE3b. Li+-influx was detected by Na+ electrode as increased aNai in solution in which all Na+ was replaced with Li+ [0Na(Li)]. Values are expressed as means ± SE and zNHE3b and relative activity (ΔpHi/dt) of zNHE3b with or without inhibitors were compared (F). Statistical significances were calculated by unpaired two-sided Student's t-test. *P < 0.001.
Basal pHi, aNai, and Vm of zNHE3b oocytes.
Basal pHi, aNai, and Vm of zNHE3b in ND96 saline solution were analyzed using microelectrodes, and their averages were compared with those of the water-injected (control) oocytes (Table 1). pHi in zNHE3b oocytes was 7.79, which was significantly higher than that of the water-injected oocytes (7.22, P < 0.001), i.e., [H+]i of zNHE3b oocytes was nearly four times lower than that of control oocytes. aNai of zNHE3b oocytes was 6.01 mM, which was 2.4-fold higher than that of control oocytes (2.47 mM, n = 6, P < 0.001). Vm of zNHE3b oocytes was more positive than that of control oocytes (P < 0.001). These high-aNai and low-[H+]i characteristics of zNHE3b oocytes indicate that zNHE3b was active in oocytes in the culture medium and ND96 saline solution.
Table 1.
pHi, aNai, and Vm measurements of zNHE3b and water-injected control oocytes
| zNHE3b |
Control |
||||
|---|---|---|---|---|---|
| Measurement (Solution) | Units | Means ± SE | n | Means ± SE | n |
| Initial pHi (96Na) | 7.79 ± 0.03‡ | 31 | 7.22 ± 0.03 | 21 | |
| ΔpHi (0Na) | −1.27 ± 0.08‡ | 4 | −0.02 ± 0.01 | 8 | |
| ΔpHi (CO2-HCO3−, 96Na) | −0.71 ± 0.01‡ | 9 | −0.47 ± 0.02 | 7 | |
| ΔpHi (butyrate, 96Na) | −0.39 ± 0.02 | 4 | −0.37 ± 0.00 | 3 | |
| ΔpHi/dt (0Na) | × 10−5 pH units/s | −195 ± 27‡ | 14 | −5 ± 2 | 9 |
| ΔpHi/dt (CO2-HCO3−, 96Na) | × 10−5 pH units/s | −432 ± 28‡ | 9 | −243 ± 14 | 7 |
| ΔpHi/dt (butyrate, 96Na) | × 10−5 pH units/s | −233 ± 27 | 4 | −231 ± 14 | 4 |
| ΔpHi/dt (NH3/NH4+, 86Na) | × 10−5 pH units/s | −133 ± 14‡ | 10 | −35 ± 8 | 6 |
| Initial aNai (96Na) | mM | 6.01 ± 0.46‡ | 18 | 2.47 ± 0.28 | 18 |
| ΔaNai/dt (0Na) | mM/min | −0.249 ± 0.027‡ | 7 | −0.030 ± 0.011 | 6 |
| ΔaNai/dt (CO2-HCO3−, 96Na) | mM/min | 0.171 ± 0.021† | 4 | 0.005 ± 0.007 | 4 |
| ΔaNai/dt (butyrate, 96Na) | mM/min | 0.202 ± 0.019‡ | 5 | 0.001 ± 0.002 | 5 |
| ΔaNai/dt (NH3/NH4+, 86Na) | mM/min | 0.115 ± 0.022† | 11 | 0.017 ± 0.009 | 6 |
| Initial Vm (96Na) | mV | −30.1 ± 1.2 | 49 | −58.1 ± 1.9 | 39 |
| ΔVm (0Na) | mV | −55.2 ± 5.5‡ | 12 | −11 ± 1 | 5 |
| ΔVm (CO2-HCO3−, 96Na) | mV | −44.2 ± 4.7‡ | 9 | 12.3 ± 3.0 | 11 |
| ΔVm (butyrate, 96Na) | mV | −49.1 ± 6.0‡ | 9 | 9.5 ± 2.9 | 9 |
| ΔVm (NH3/NH4+, 86Na) | mV | 16.9 ± 1.6‡ | 19 | 44.8 ± 4.1 | 12 |
| Initial pHi (0.5Na) | 7.33 ± 0.06 | 6 | 7.21 ± 0.09 | 6 | |
| ΔpHi (CO2-HCO3−, 0.5Na) | −0.49 ± 0.04 | 3 | −0.44 ± 0.02 | 3 | |
| ΔpHi (butyrate, 0.5Na) | −0.39 ± 0.01 | 3 | −0.34 ± 0.04 | 3 | |
| ΔpHi/dt (CO2-HCO3−, 0.5Na) | × 10−5 pH units/s | −276 ± 33 | 3 | −264 ± 8 | 3 |
| ΔpHi/dt (butyrate, 0.5Na) | × 10−5 pH units/s | −145 ± 10 | 3 | −164 ± 16 | 3 |
| Initial aNai (0.5Na) | mM | 1.23 ± 0.05† | 6 | 1.70 ± 0.13 | 6 |
| ΔaNai/dt (CO2-HCO3−, 0.5Na) | mM/min | 0.056 ± 0.001† | 3 | −0.007 ± 0.004 | 3 |
| ΔaNai/dt (butyrate, 0.5Na) | mM/min | 0.058 ± 0.005‡ | 3 | −0.001 ± 0.003 | 3 |
| Initial Vm (0.5Na) | mV | −50.9 ± 1.5* | 12 | −56.4 ± 1.8 | 11 |
| ΔVm (CO2-HCO3−, 0.5Na) | mV | 11.4 ± 0.5 | 3 | 13.0 ± 2.4 | 3 |
| ΔVm (butyrate, 0.5Na) | mV | 4.2 ± 0.8 | 3 | 7.7 ± 1.6 | 3 |
zNHE3b, zebrafish Na+/H+ exchanger 3b; n, number of experiments; pHi, intracellular pH; aNai, intracellular Na+ activity; Vm, membrane potential; statistical significances were calculated by unpaired two-sided Student's t-test,
P < 0.05, †P < 0.01,
P < 0.001.
Na+-free medium-elicited Na+/H+ exchange activity (Na+-efflux mode) of zNHE3b.
Na+/H+ exchange activity of zNHE3b was analyzed as changes of pHi or aNai of zNHE3b oocytes when exposed to a Na+-free solution. Figure 1 shows representative traces of pHi, aNai, and Vm of each oocyte. In water-injected control oocytes, removal of extracellular Na+ from ND96 caused slight decreases in pHi (−5 × 10−5 pH units/s) and aNai (−0.03 mM/min) (Table 1), showing that Xenopus oocytes exhibit a weak endogenous Na+/H+ exchange activity when exposed to Na+-free solution.
In zNHE3b oocytes, significant influx of H+ was observed as a significant decrease in pHi (−195 × 10−5 pH units/s, P < 0.001 compared with water-injected oocyte) when Na+ was removed from the bath solution (Fig. 1B, Table 1). The maximal rate of intracellular acidification (ΔpHi/s) continued for ∼5 min, and then the acidification rate decayed slowly. When zNHE3b oocytes were incubated in Na+-free solution for a longer period of time (∼20 min), pHi dropped from ∼7.8 to ∼6.5 (Fig. 1C, Table 1). When Na+ was readded to the bath solution, significant H+ efflux was observed as significant increase in pHi (Fig. 1, B and C). Na+ efflux of zNHE3b oocytes was observed as a significant decrease in aNai (−0.249 mM/min, P < 0.001 compared with water-injected oocyte) when exposed to Na+-free solution (Fig. 1G, Table 1). Interestingly, H+ influx and Na+ efflux activities of zNHE3b oocytes in Na+-free solution were accompanied by marked hyperpolarization (−55.2 mV, Fig. 1, B, C, and G, and Table 1).
High [Na+]o-elicited Na+/H+ exchange activity (Na+-influx mode) of zNHE3b.
Similar experiments were performed in a reverse condition. zNHE3b and control oocytes were preincubated in low-Na+ (2 mM) solution (2Na-ND96) for 1–2 h; then Na+-influx and H+-efflux activities were monitored when extracellular Na+ was increased to ∼96 mM. The basal pHi and Vm of zNHE3b oocytes in 2Na-ND96 were 7.31 ± 0.12 and −56.5 ± 4.0 mV, respectively (n = 4). When the bath solution was changed from 2Na-ND96 to ND96, H+-efflux activity of zNHE3b was observed as a significant increase in pHi (+227 ± 53 × 10−5 pH units/s, n = 4, P < 0.01 compared with water-injected oocyte) (Fig. 1D). The H+-efflux activity of zNHE3b oocytes solution was accompanied by a marked depolarization (+38.4 ± 6.7 mV, n = 4, P < 0.01 compared with water-injected oocyte).
Inhibition of zNHE3b with amiloride and EIPA.
Inhibition of zNHE3b by amiloride and EIPA was analyzed by monitoring H+-influx activity of zNHE3b oocytes in a Na+-free medium with inhibitors at various concentrations. Taking advantage of the fact that the maximal rate of intracellular acidification (ΔpHi/s) of zNHE3b oocytes in Na+-free medium continues for ∼5 min, inhibitor was added 1 min after the acidification rate was maximized. The reduced rate of the intracellular acidification was observed for 3–4 min (Fig. 1E), and the relative activity is shown in Fig. 1F. Activity of zNHE3b was partially inhibited by 0.1 mM amiloride or 0.01 mM EIPA (∼65% inhibition, n = 3–8, P < 0.001 compared with zNEH3b activity with no inhibitor) and was almost completely inhibited by 1 mM amiloride or 0.1 mM EIPA (95–98% inhibition, n = 3–5, P < 0.001).
Li+/H+-exchange activity of zNHE3b.
Li+ is known to be detected by Na+ microelectrodes (43), and Li+ influx is measured as increase of aNai in Na+-free solution in which all Na+ is replaced with Li+ [0Na(Li)-ND96] (7). In a 0Na(Li)-ND96 solution, acute increase in aNai was observed in zNHE3b oocytes, indicating that zNHE3b mediates Li+/H+ exchange (Fig. 1H).
Intracellular acidification-stimulated Na+/H+-exchange activity (Na+-influx mode) of zNHE3b.
The Na+/H+ exchange activity of zNHE3b was further analyzed using other protocols. The above results described the Na+ gradient-driven activity of zNHE3b. Here, we analyzed intracellular acidification-stimulated Na+-influx activity in oocytes. We used solutions containing 33 mM HCO3−-5% CO2 or 30 mM butyrate, which are known to decrease pHi of oocytes. Exposure of water-injected oocytes to solution containing CO2-HCO3− or butyrate reduced pHi at rates of −243 and −231 × 10−5 pH units/s, respectively (Fig. 2, A and C, Table 1). No changes in aNai and Vm of control oocytes were observed in these solutions (Fig. 2, B and D, Table 1).
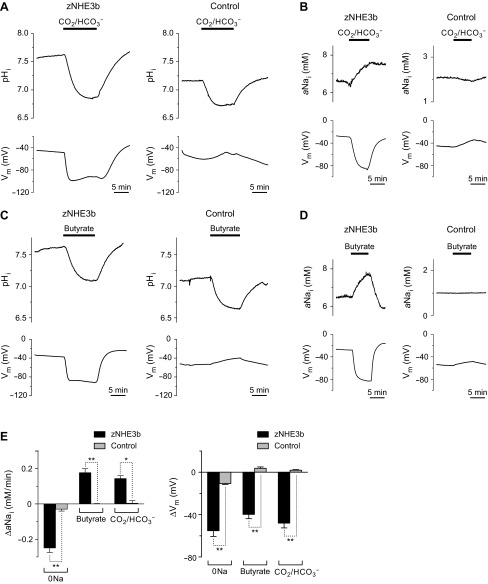
Fig. 2.
Intracellular acidification-stimulated Na+-influx activity of zNHE3b. Representative traces of pHi, aNai, and Vm are shown (A–D). A: intracellular acidification of oocytes in 33 mM HCO3−-5% CO2 solution. B: CO2-elicited Na+-influx activity of zNHE3b. C: intracellular acidification of oocytes in 30 mM butyrate solution. D: butyrate-elicited Na+-influx activity of zNHE3b. E: comparison of changes of aNai (ΔaNai/dt) and membrane potential (ΔVm) in 0Na, 33 mM HCO3−-5% CO2, and 30 mM butyrate solutions. 33 mM HCO3−/5% CO2 and 30 mM butyrate solutions contain 96 mM Na+. Values are expressed as means ± SE and compared between zNHE3b and control oocytes (E). Statistical significances were calculated by unpaired two-sided Student's t-test. **P < 0.001; *P < 0.01.
In zNHE3b oocytes, exposure to solutions containing CO2-HCO3− or butyrate reduced pHi at rates of −432 and −233 × 10−5 pH units/s, respectively, and induced hyperpolarization (ΔVm, −44.2 and −49.1 mV, respectively, P < 0.001 compared with water-injected oocytes) (Figs. 2, A and C, Table 1). In addition, aNai levels were increased at the rate of +0.171 and +0.202 mM/min, respectively (P < 0.001 compared with water-injected oocytes) (Fig. 2, B and D, Table 1). These results indicate that intracellular acidification drives Na+ absorption by zNEH3b.
Na+ absorption by zNHE3b oocytes in low Na+ media.
The intracellular acidification-stimulated Na+-influx activity of zNHE3b described above were observed in media containing 96 mM Na+, which is much higher than those of cytosol. To observe whether zNHE3b mediates Na+ absorption from Na+-poor media, we performed similar experiments in media containing 0.5 mM Na+ (0.5Na-ND96). Oocytes were preincubated in 0.5Na-ND96 for 2–4 h and then exposed to media containing 0.5 mM Na+ and CO2-HCO3− or butyrate. In 0.5Na-ND96, steady-state pHi, aNai, and Vm of zNHE3b oocytes were 7.33, 1.23 mM, and −50.9 mV, respectively (Table 1). Exposure of zNHE3b oocytes to 0.5Na media containing 5% CO2-33 mM HCO3− or 30 mM butyrate increased aNai at the rate of +0.056 and +0.058 mM/min, respectively (P < 0.01 compared with water-injected oocytes) (Fig. 3, Table 1), showing that zNHE3b has the ability to absorb Na+ from a Na+-poor environment when appropriate driving forces are present. Hyperpolarization was not observed in CO2-HCO3− or butyrate solutions containing 0.5 mM Na+.
Fig. 3.
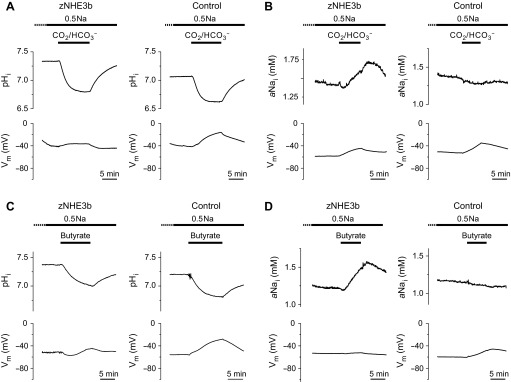
Na+-influx activity of zNHE3b in low-Na+ environment. Representative traces of pHi, aNai, and Vm are shown. Experiments were performed using zNHE3b or control oocytes preincubated in low-Na+ solution (0.5 mM Na+:0.5Na) for 2–4 h and solutions containing 0.5 mM Na+. A and C: intracellular acidification of oocytes in 33 mM HCO3−-5% CO2 or 30 mM butyrate solution. B and D: CO2 or butyrate-elicited Na+-influx activity of zNHE3b.
Na+/H+ exchange activity of zNHE3b is electroneutral.
As described above, changes in pHi and aNai in zNHE3b oocytes were accompanied by a hyperpolarization of the oocyte. Interestingly, Na+-free solution-elicited Na+-efflux and intracellular acidification-stimulated Na+-influx activities of zNHE3b oocytes were accompanied by hyperpolarization (Fig. 2E), which was not observed in the low-Na+ solutions (Fig. 3, Table 1). These results suggest two possible mechanisms: 1) zNHE3b is an electrogenic transporter that switches stoichiometry between an nNa+/1H+ exchange mode (0Na solution) and a 1Na+/nH+ exchange mode (CO2-HCO3− and butyrate solutions), or 2) zNHE3b is an electroneutral transporter that stimulates another unknown activity of zNHE3b per se or an endogenous transporter/channel in Xenopus oocytes.
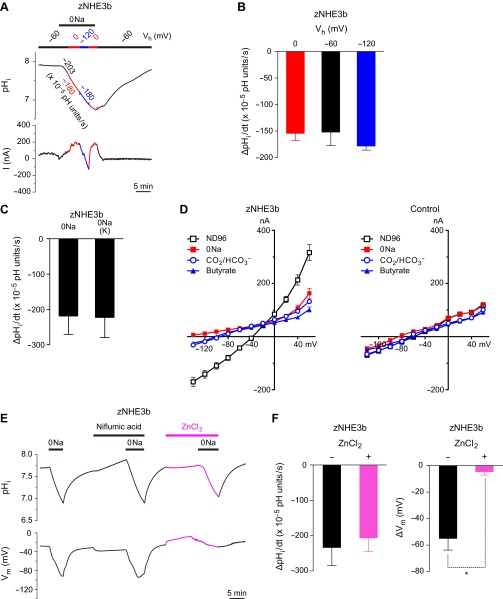
To clarify whether the Na+/H+ exchange activity of zNHE3b is electroneutral or electrogenic, we examined whether the activity is dependent on membrane potential. zNHE3b oocytes were voltage clamped, and the intracellular-acidification rates (ΔpHi/dt) in Na+-free solution were measured at various holding potentials (Fig. 4, A and B). Although membrane currents were shifted by change in Vh between 0 and −120 mV, intracellular-acidification rates were constant. When unclamped, zNHE3b oocytes were incubated in a Na+-free medium in which all Na+ was replaced with choline (0Na) or potassium [0Na(K)]; however, the intracellular-acidification rates were similar (Fig. 4C). In these experiments, 0Na(choline) and 0Na(K) solutions elicited hyperpolarization (Fig. 1) and depolarization, respectively, (data not shown). These results indicate that the Na+/H+ exchange activity is independent of membrane potential or membrane current and suggests that the Na+/H+ exchange activity is electroneutral.
Fig. 4.
Evidence indicating the electroneutral nature of zNHE3b. A: holding potential (Vh)-independent H+-influx activity of zNHE3b. Representative traces of pHi and membrane current (I) of voltage-clamped zNHE3b oocyte are shown. B: comparison of Na+-free medium-elicited ΔpHi/dt of voltage-clamped oocytes at the indicated holding potentials. C: comparison of ΔpHi/dt of unclamped oocytes in Na+-free medium in which all Na+ was replaced with choline (0Na) or potassium [0Na(K)]. D: current-voltage (I–V) relationships of zNHE3b and control oocytes in ND96, 0Na-ND96, ND96 containing 33 mM HCO3−-5% CO2, and ND96 containing 30 mM butyrate. E: inhibitory effect of 0.2 mM niflumic acid or 1.5 mM ZnCl2. Representative traces of pHi and Vm of unclamped zNHE3b oocyte is shown. F: hyperpolarization (ΔVm)-specific inhibition of zNHE3b activity by 1.5 mM ZnCl2. Na+-free medium-elicited H+-influx activity (ΔpHi/dt) was not inhibited by 1.5 mM ZnCl2. Values are expressed as means ± SE. Statistical significances were calculated by ANOVA followed by the Tukey-Kramer test (B) or unpaired two-sided Student's t-test (F). *P < 0.01.
I–V relationships of oocytes in various solutions are shown in Fig. 4D. When compared with control oocytes, zNHE3b oocytes showed only small currents in 0Na, CO2-HCO3− and butyrate solutions though the reversal potentials shifted negatively, suggesting that the hyperpolarization or negative shift of the reversal potential is independent of the Na+/H+ exchange activity.
zNHE3b oocytes did not show pHi changes in Cl−-free solutions in which all Cl− was replaced with gluconate, and intracellular Cl− activity of zNHE3b oocytes did not change in the Na+-free solution (data not shown). Niflumic acid (0.2 mM) did not inhibit Na+-free medium-elicited hyperpolarization (Fig. 4E). In contrast, 1.5 mM ZnCl2 (H+ channel inhibitor) inhibited Na+-free medium-elicited hyperpolarization of zNHE3b oocytes (n = 3, P < 0.01 compared with ΔVm of zNEH3b with no inhibitor), although Na+/H+ exchange activity was not altered (Fig. 4, E and F).
Na+/NH4+ exchange activity of zNHE3b.
Na+/NH4+ exchange activity of zNHE3b was analyzed as changes in aNai, pHi, and aNH4i of oocytes by using Na+-, H+-, and NH4+-selective microelectrode, respectively, when exposed to a medium containing 10 mM or 96 mM NH3/NH4+. In 10 mM NH3/NH4+ solution (pH 7.5), the calculated concentration of NH4+ was 9.9 mM as the pKa of NH4+ is 9.25.
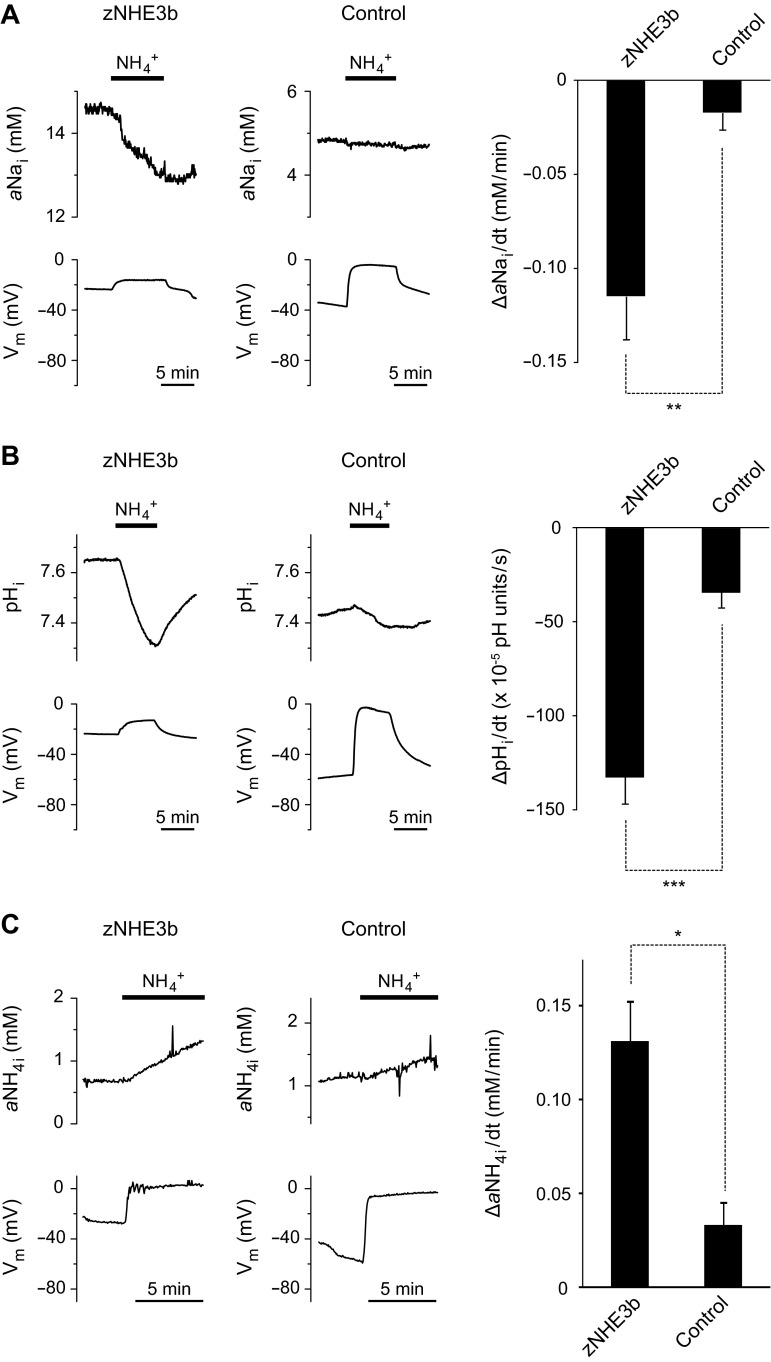
Exposure of water-injected oocytes to the solution containing 10 mM NH3/NH4+ reduced pHi at the rate of −35 × 10−5 pH units/s, which was accompanied by a depolarization of the oocyte (ΔVm, +45 mV) (Fig. 5B, Table 1), as previously reported (2, 5, 22). A medium of 10 mM NH3/NH4+ caused slight change in aNai of control oocytes at the rate of −0.02 mM/min (Fig. 5A, Table 1), and 96 mM NH3/NH4+ medium caused slight change in aNH4i of control oocytes at the rate of 0.03 ± 0.01 mM/min (n = 3) (Fig. 5C).
Fig. 5.
Na+/NH4+ exchange activity of zNHE3b. Extracellular NH4+-stimulated Na+-efflux and NH4+-influx activities were analyzed using Na+, H+, and NH4+ electrodes. Representative traces of pHi, aNai, NH4+ activity (aNH4i), and Vm of zNHE3b and control oocytes in response to a medium containing NH4+ were shown in left. A: comparison of 10 mM NH3/NH4+-stimulated Na+-efflux activity between zNHE3b and control oocytes. B: comparison of 10 mM NH3/NH4+-stimulated NH4+-influx activity between zNHE3b and control oocytes measured by pH electrode. C: comparison of 96 mM NH3/NH4+-stimulated NH4+-influx activity between zNHE3b and control oocytes measured by NH4+ electrode. Values are expressed as means ± SE. Statistical significances were calculated by unpaired two-sided Student's t-test. ***P < 0.001; **P < 0.01; *P < 0.05.
Exposure of zNHE3b oocytes to the solution containing 10 mM NH3/NH4+ elicited significant decrease in pHi (−133 × 10−5 pH units/s, P < 0.001 compared with water-injected oocytes) (Fig. 5B, Table 1), showing that NH4+-influx rate was increased and depolarization decreased in the oocytes (ΔVm, +17 mV, P < 0.001 compared with water-injected oocytes). Enhanced NH4+-influx rate was also confirmed as increased aNH4i of zNHE3b oocytes in medium containing 96 mM NH3/NH4+ (0.13 ± 0.02 mM/min, n = 3, P < 0.05 compared with water-injected oocytes) (Fig. 5C). Exposure to a medium containing 10 mM NH3/NH4+ elicited significant decrease in aNai of zNHE3b oocytes (−0.11 mM/min, P < 0.01 compared with water-injected oocytes) (Fig. 5A, Table 1) indicating that zNHE3b acted as a Na+/NH4+ exchanger.
DISCUSSION
In this study, we characterized the function of zNHE3b expressed in Xenopus oocyte. Importantly, zNHE3b was localized to the plasma membrane of Xenopus oocytes and showed strong activity. These results contrast those of NHE3s of Atlantic stingray (Dasyatis sabina) and banded houndshark (Triakis scyllium), which were intracellularly localized when expressed in Xenopus oocyte (9, 28) and showed only a weak function (data not shown). In vivo, all these NHE3s are localized to the apical membrane of epithelial cells (8, 20, 28, 49). However, the intracellular localization in Xenopus oocytes was different as described above. These results suggest that the surface expression of elasmobranch NHE3s are negatively regulated in Xenopus oocytes, whereas zNHE3b avoids the negative regulation by unknown reason. This property of zNHE3b is advantageous to characterize the activity in vitro to demonstrate the Na+-uptake machinery of FW fishes.
With the use of electrophysiological techniques, Na+/H+ exchange activity of zNHE3b was observed as large changes in pHi or aNai of oocytes, which were monitored directly by ion-selective microelectrodes in real time, in response to Na+-free medium or media that induce intracellular acidification. When zNHE3b oocytes were exposed in Na+-free medium, zNHE3b activity was observed as a large reduction in pHi that exceeded more than 1 pH unit after a 10-min incubation. This response could be reversed and repeated when extracellular Na+ was readded and removed. By monitoring the rate of reduction in pHi (ΔpHi/dt) in 0Na+-solutions containing inhibitors, one can easily assess the effect of inhibitors in real time and quantify the effect by comparing ΔpHi/dt. When pHi of zNHE3b oocytes was acidified by exposing them to media containing CO2-HCO3− and butyrate, zNHE3b activity was clearly observed as a sharp increase in aNai. In 0Na(Li+) solution, Li+ influx was measured as increased aNai in zNHE3b oocytes. These results clearly demonstrate that zNHE3b has Na+/H+ and Li+/H+ exchange activity, and indicate that this system is therefore a powerful tool to analyze the activity and regulation of zNHE3b.
The large hyperpolarization of zNHE3b oocytes in 0Na+, CO2-HCO3−, and butyrate solutions initially raised the possibility that zNHE3b mediates electrogenic exchange of nNa+/1H+ (0Na solution) and 1Na+/nH+ (CO2-HCO3− and butyrate solutions). However, subsequent studies using voltage-clamp, high K+ solution, and ZnCl2 showed no evidence that Na+/H+ exchange activity is electrogenic. This phenomenon is similar to Na+/H+ exchange-associated currents in murine macrophages, which were explained as resulting from coupling of NHE to a separate endogenous H+ conductive pathway and inhibited by 1 mM ZnCl2 (10). In zNHE3b oocytes, the coupling activity that elicits hyperpolarization is unclear. However, it is not a Ca2+-activated nonselective anion channel because the inhibitor niflumic acid had no effect on the hyperpolarization. The H+ channel is known to be inhibited by ZnCl2 (32), but probably not the coupling partner, because the resulting H+ gradient is not thermodynamically adequate to explain the hyperpolarization (data not shown).
Amiloride (0.1–1 mM) or (EIPA 0.01–0.1 mM) partially or almost completely inhibited the Na+/H+ exchange activity of zNHE3b. The EIPA sensitivity of zNHE3b expressed in Xenopus oocytes is similar to that of Osorezan dace NHE3 expressed in PS120 cells and is less sensitive than human NHE3 expressed in PS120 cells (16). The present results provided information that complements those of prior in vivo studies on EIPA. EIPA 0.1 mM inhibited 22Na+ uptake by whole zebrafish larvae in “Fish Water” (pH 6.7–7.4) (12) described in “The Zebrafish Book” (45) and acidified Ottawa tap water (pH 3.9–4.0) but not in normal Ottawa tap water (pH 7.3–7.5) (25). EIPA 1 mM increased ionocyte-surface Na+ activity (i.e., reduction of Na+ uptake) (42). EIPA 0.1 mM is an adequate concentration to inhibit zNHE3b, thus, the present study reconfirmed that these effects of EIPA are caused by the inhibition of zNHE3b in vivo. EIPA 0.01 mM and amiloride 0.1 mM reduced intracellular Na+ activity (intensity of sodium green fluorescence) of ionocytes in zebrafish larvae (12). The inhibitory effects of EIPA and amiloride on the intensity of sodium green fluorescence seem to be more sensitive than those on zNHE3b oocytes. In adult zebrafish, 0.1 mM amiloride reduced Na+ influx rate in hard water but not in soft water, and 0.05 mM EIPA apparently increased Na+ influx rate in soft water but not in hard water (1). This effect is worth analyzing, using higher concentrations of EIPA and amiloride in a future study.
In mammalian cells or squid giant axons, extracellular NH3/NH4+ triggers influx of NH3 that consumes intracellular H+ and raises pHi quickly (NH3-induced alkalinization) (2, 3). When extracellular NH3/NH4+ is removed after a preload of NH3/NH4+, efflux of NH3 leads to a fall in pHi. Imposed by NH3/NH4+ prepulse, this acid load method is commonly used in culture cells as a procedure for intracellular acidification and is also used in the selection of culture cells with altered NHE activity (H+-killing method) (16, 27, 40). In Xenopus oocytes, 10–20 mM extracellular NH3/NH4+ causes pHi to fall (NH4+-induced acidification), because the plasma membrane has low NH3 permeability (2, 5, 22). This phenomenon is likely due to endogenous NH4+ conduction of the plasma membrane of oocytes and is known as an NH3/NH4+-elicited paradoxical fall in pHi of Xenopus oocyte (16, 27, 40). In the present study, NH3/NH4+-elicited pHi fall of zNHE3b oocytes was enhanced approximately four times that of control oocytes. In addition, we confirmed that NH3/NH4+ solution significantly increased aNH4i of zNHE3b oocytes. These results demonstrated that NH4+ influx rate is increased by zNHE3b expression.
In the event that the increased NH4+ influx is not related to Na+/NH4+ exchange activity, then aNai level is likely increased in NH3/NH4+ solution by intracellular acidification-induced Na+/H+ exchange activity (Na+-influx mode) of zNHE3b, in the same manner seen in experiments using CO2-HCO3− or butyrate solution as described above. If zNHE3b has Na+/NH4+ exchange activity, the Na+-efflux mode of zNHE3b may be driven by extracellular NH4+ resulting in aNai fall. As shown in Fig. 5A, the NH4+ medium elicited significant “decrease” in aNai of zNHE3b oocytes, showing that zNHE3b acts as Na+/NH4+ exchanger. Na+/NH4+ exchange activity of NHEs was found in mammals by analyzing tissue membrane preparations (23), but there is no direct evidence for Na+/NH4+ exchange mediated by exogenously expressed NHEs. To our knowledge, this is the first demonstration that exogenously expressed NHE acts as a Na+/NH4+ exchanger.
Na+/NH4+ exchange activity of zNHE3b most likely a major pathway whereby Na+ is absorbed from FW with body fluid NH4+ as a driving force. Teleosts are ammonotelic animals, thus internal NH4+ is an ideal driving force especially in acidic water. This result also suggests that the Na+/NH4+ exchange activity of zNHE3b alone mediates Na+ absorption in ionocytes without forming metabolon. One may ask, whether metabolon and the Na+/H+ exchange activity of zNHE3b are not essential in the absorption of Na+ from FW. Experiments on zNHE3b oocytes in media containing only 0.5 mM Na+ demonstrated that zNHE3b mediates Na+ absorption from a low Na+ environment against the concentration gradient by its Na+/H+ exchange activity when the cytoplasm is acidified. Although the metabolon hypothesis remains to be tested in vitro, these data suggest that the Na+/H+ exchange activity of zNHE3b also mediates Na+ absorption in ionocytes when intracellular H+ is supplied from a metabolon complex containing Rhcg1 and/or carbonic anhydrases.
Activities of recombinant NHEs in mammals have been demonstrated in cell lines lacking NHE activity (e.g., PS120 and SP-1) (10, 27, 35, 40). When NHEs were stably transfected in such NHE-null cell lines, the activities were detected as increased uptake of 22Na or changes of pHi in response to intracellular acidification or changes of extracellular Na+ concentration, respectively. Similar protocol was applied for the analyses of NHEs from Xenopus laevis and the acid-tolerant Osorezan dace (6, 16). The present study demonstrated that zNHE3b activity consists of significant changes in pHi and aNai of Xenopus oocytes. The Na+/H+ exchange activity of zNHE3b was electroneutral, and this conclusion is similar to those of previous reports on NHEs from other species. However, zNHE3b oocytes showed a Na+/NH4+ exchange activity that has not been demonstrated. Our method is advantageous for the following reasons: 1) H+ and Na+ movements across the plasma membrane are monitored and quantified in real time and responses to various solutions can be compared in the same oocytes; 2) analysis of many combinations of expression is easy by coinjection of cRNAs to oocytes, which is contrastive to mammalian culture cells that require preparation of a stable transfectant; 3) influx of NH4+ can be analyzed in oocytes as enhanced NH3/NH4+-elicited pHi fall or aNH4i increase, whereas mammalian cells have NH3 permeability that is too high to monitor induced NH3/NH4+ flux as pHi change, and 4) microelectrodes can analyze net movement of Na+ across the plasma membrane in Na+ solutions regardless of concentration, whereas the 22Na method requires low extracellular Na+ concentration to increase the relative radioactivity and can only analyze movement of extracellular 22Na but not intracellular nonradioactive Na+.
Perspectives and Significance
Analyses of zNHE3b with other molecules in Xenopus oocytes will answer the following questions in future studies: 1) Are Rhcg1 and carbonic anhydrases really drive zNHE3b activity by forming metabolon? 2) Is the metabolon requires any scaffold proteins? 3) If the metabolon is constituted in Xenopus oocyte, is the activity thermodynamically reasonable to explain the function of the ionocytes of fishes in FW or acidic water? 4) Which domain or region is structurally responsible for the protein-protein interaction in the metabolon and how it is regulated? Finally, 5) which components are required by the signaling pathway that regulates zNHE3b (26)? This system is also ideal to determine whether the cytosolic H+-binding site is shared with the NH4+ site.
GRANTS
This work was supported by the Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT) Grants-in-Aid for Scientific Research 18059010, 22370029, 25650114, and the 21st Century and Global Center of Excellence Program of MEXT. Work in the Romero lab was supported by the NIH Grants EY017732, DK083007, and DK092408.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: Y.I., A.K., T.H., S.H., and M.F.R. conception and design of research; Y.I., A.K., and T.H. performed experiments; Y.I., A.K., and T.H. analyzed data; Y.I., A.K., T.H., and M.F.R. interpreted results of experiments; Y.I., A.K., and T.H. prepared figures; Y.I. and A.K. drafted manuscript; Y.I., A.K., T.H., S.H., and M.F.R. approved final version of manuscript; A.K., S.H., and M.F.R. edited and revised manuscript.
ACKNOWLEDGMENTS
We thank Dr. Min-Hwang Chang and Dr. An-Ping Chen for discussing the electrophysiology; Dr. Kazuyuki Hoshijima for discussing physiology of zebrafish; Heather L. Holmes and Elyse M. Scileppi for injecting the oocytes; Dr. Keijiro Munakata, Dr. Shanshan Li, Yuuki Takahashi, Kayoko Ookata, Noriko Isoyama, Keiko Kawai, Yoko Yamamoto, Ayako Takada, and Nana Shinohara for technical assistance; and Yuriko Ishii for secretarial assistance.
REFERENCES
- 1.Boisen AM, Amstrup J, Novak I, Grosell M. Sodium and chloride transport in soft water and hard water acclimated zebrafish (Danio rerio). Biochim Biophys Acta 1618: 207–218, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Boron WF. Sharpey-Schafer lecture: gas channels. Exp Physiol 95: 1107–1130, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boron WF, De Weer P. Intracellular pH transients in squid giant axons caused by CO2, NH3, and metabolic inhibitors. J Gen Physiol 67: 91–112, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buhrer T, Peter H, Simon W. NH4+ ion-selective microelectrode based on the antibiotics nonactin/monactin. Pflügers Arch 412: 359–362, 1988 [DOI] [PubMed] [Google Scholar]
- 5.Busch S, Burckhardt BC, Siffert W. Expression of the human sodium/proton exchanger NHE-1 in Xenopus laevis oocytes enhances sodium/proton exchange activity and establishes sodium/lithium countertransport. Pflügers Arch 429: 859–869, 1995 [DOI] [PubMed] [Google Scholar]
- 6.Busch S, Rosskopf D, Lang HJ, Weichert A, Siffert W. Expression, functional characterization and tissue distribution of a Na+/H+ exchanger cloned from Xenopus laevis oocytes (XL-NHE). Pflügers Arch 436: 828–833, 1998 [DOI] [PubMed] [Google Scholar]
- 7.Chang MH, Plata C, Kurita Y, Kato A, Hirose S, Romero MF. Euryhaline pufferfish NBCe1 differs from nonmarine species NBCe1 physiology. Am J Physiol Cell Physiol 302: C1083–C1095, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choe KP, Evans DH. Compensation for hypercapnia by a euryhaline elasmobranch: effect of salinity and roles of gills and kidneys in fresh water. J Exp Zool A Comp Exp Biol 297: 52–63, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Choe KP, Kato A, Hirose S, Plata C, Sindic A, Romero MF, Claiborne JB, Evans DH. NHE3 in an ancestral vertebrate: primary sequence, distribution, localization, and function in gills. Am J Physiol Regul Integr Comp Physiol 289: R1520–R1534, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Demaurex N, Orlowski J, Brisseau G, Woodside M, Grinstein S. The mammalian Na+/H+ antiporters NHE-1, NHE-2, and NHE-3 are electroneutral and voltage independent, but can couple to an H+ conductance. J Gen Physiol 106: 85–111, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Donowitz M, Ming Tse C, Fuster D. SLC9/NHE gene family, a plasma membrane and organellar family of Na+/H+ exchangers. Mol Aspects Med 34: 236–251, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Esaki M, Hoshijima K, Kobayashi S, Fukuda H, Kawakami K, Hirose S. Visualization in zebrafish larvae of Na+ uptake in mitochondria-rich cells whose differentiation is dependent on foxi3a. Am J Physiol Regul Integr Comp Physiol 292: R470–R480, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Evans DH. Freshwater fish gill ion transport: August Krogh to morpholinos and microprobes. Acta Physiol (Oxf) 202: 349–359, 2011 [DOI] [PubMed] [Google Scholar]
- 14.Evans DH. Teleost fish osmoregulation: what have we learned since August Krogh, Homer Smith, and Ancel Keys. Am J Physiol Regul Integr Comp Physiol 295: R704–R713, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Furukawa F, Watanabe S, Inokuchi M, Kaneko T. Responses of gill mitochondria-rich cells in Mozambique tilapia exposed to acidic environments (pH 4.0) in combination with different salinities. Comp Biochem Physiol A Mol Integr Physiol 158: 468–476, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Hirata T, Kaneko T, Ono T, Nakazato T, Furukawa N, Hasegawa S, Wakabayashi S, Shigekawa M, Chang MH, Romero MF, Hirose S. Mechanism of acid adaptation of a fish living in a pH 3.5 lake. Am J Physiol Regul Integr Comp Physiol 284: R1199–R1212, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Hirose S, Kaneko T, Naito N, Takei Y. Molecular biology of major components of chloride cells. Comp Biochem Physiol B Biochem Mol Biol 136: 593–620, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Hwang PP, Lee TH, Lin LY. Ion regulation in fish gills: recent progress in the cellular and molecular mechanisms. Am J Physiol Regul Integr Comp Physiol 301: R28–R47, 2011 [DOI] [PubMed] [Google Scholar]
- 19.Inokuchi M, Hiroi J, Watanabe S, Lee KM, Kaneko T. Gene expression and morphological localization of NHE3, NCC and NKCC1a in branchial mitochondria-rich cells of Mozambique tilapia (Oreochromis mossambicus) acclimated to a wide range of salinities. Comp Biochem Physiol A Mol Integr Physiol 151: 151–158, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Ito Y, Kobayashi S, Nakamura N, Miyagi H, Esaki M, Hoshijima K, Hirose S. Close association of carbonic anhydrase (CA2a and CA15a), Na+/H+ exchanger (Nhe3b), and ammonia transporter Rhcg1 in zebrafish ionocytes responsible for Na+ uptake. Front Physiol 4: 59, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ivanis G, Esbaugh AJ, Perry SF. Branchial expression and localization of SLC9A2 and SLC9A3 sodium/hydrogen exchangers and their possible role in acid-base regulation in freshwater rainbow trout (Oncorhynchus mykiss). J Exp Biol 211: 2467–2477, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Kikeri D, Sun A, Zeidel ML, Hebert SC. Cell membranes impermeable to NH3. Nature 339: 478–480, 1989 [DOI] [PubMed] [Google Scholar]
- 23.Kinsella JL, Aronson PS. Interaction of NH4+ and Li+ with the renal microvillus membrane Na+-H+ exchanger. Am J Physiol Cell Physiol 241: C220–C226, 1981 [DOI] [PubMed] [Google Scholar]
- 24.Krogh A. Osmotic Regulation in Aquatic Animals. Cambridge: Cambridge University Press, 1939 [Google Scholar]
- 25.Kumai Y, Perry SF. Ammonia excretion via Rhcg1 facilitates Na+ uptake in larval zebrafish, Danio rerio, in acidic water. Am J Physiol Regul Integr Comp Physiol 301: R1517–R1528, 2011 [DOI] [PubMed] [Google Scholar]
- 26.Kumai Y, Perry SF. Mechanisms and regulation of Na+ uptake by freshwater fish. Respir Physiol Neurobiol 184: 249–256, 2012 [DOI] [PubMed] [Google Scholar]
- 27.Levine SA, Montrose MH, Tse CM, Donowitz M. Kinetics and regulation of three cloned mammalian Na+/H+ exchangers stably expressed in a fibroblast cell line. J Biol Chem 268: 25527–25535, 1993 [PubMed] [Google Scholar]
- 28.Li S, Kato A, Takabe S, Chen AP, Romero MF, Umezawa T, Nakada T, Hyodo S, Hirose S. Expression of a novel isoform of Na+/H+ exchanger 3 in the kidney and intestine of banded houndshark, Triakis scyllium. Am J Physiol Regul Integr Comp Physiol 304: R865–R876, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liman ER, Tytgat J, Hess P. Subunit stoichiometry of a mammalian K+ channel determined by construction of multimeric cDNAs. Neuron 9: 861–871, 1992 [DOI] [PubMed] [Google Scholar]
- 30.Lin CC, Lin LY, Hsu HH, Thermes V, Prunet P, Horng JL, Hwang PP. Acid secretion by mitochondrion-rich cells of medaka (Oryzias latipes) acclimated to acidic freshwater. Am J Physiol Regul Integr Comp Physiol 302: R283–R291, 2012 [DOI] [PubMed] [Google Scholar]
- 31.Lin TY, Liao BK, Horng JL, Yan JJ, Hsiao CD, Hwang PP. Carbonic anhydrase 2-like a and 15a are involved in acid-base regulation and Na+ uptake in zebrafish H+-ATPase-rich cells. Am J Physiol Cell Physiol 294: C1250–C1260, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Lishko PV, Botchkina IL, Fedorenko A, Kirichok Y. Acid extrusion from human spermatozoa is mediated by flagellar voltage-gated proton channel. Cell 140: 327–337, 2010 [DOI] [PubMed] [Google Scholar]
- 33.Mahnensmith RL, Aronson PS. The plasma membrane sodium-hydrogen exchanger and its role in physiological and pathophysiological processes. Circ Res 56: 773–788, 1985 [DOI] [PubMed] [Google Scholar]
- 34.Marshall WS, Grosell M. Ion transport, osmoregulation, and acid-base balance. In: The Physiology of Fishes, edited by Evans DH, Claiborne JB. New York: CRC, 2005, p. 177–224 [Google Scholar]
- 35.Moe OW, Amemiya M, Yamaji Y. Activation of protein kinase A acutely inhibits and phosphorylates Na/H exchanger NHE-3. J Clin Invest 96: 2187–2194, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakada T, Hoshijima K, Esaki M, Nagayoshi S, Kawakami K, Hirose S. Localization of ammonia transporter Rhcg1 in mitochondrion-rich cells of yolk sac, gill, and kidney of zebrafish and its ionic strength-dependent expression. Am J Physiol Regul Integr Comp Physiol 293: R1743–R1753, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Orlowski J, Grinstein S. Diversity of the mammalian sodium/proton exchanger SLC9 gene family. Pflügers Arch 447: 549–565, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Parks SK, Tresguerres M, Goss GG. Theoretical considerations underlying Na+ uptake mechanisms in freshwater fishes. Comp Biochem Physiol C Toxicol Pharmacol 148: 411–418, 2008 [DOI] [PubMed] [Google Scholar]
- 39.Romero MF, Fong P, Berger UV, Hediger MA, Boron WF. Cloning and functional expression of rNBC, an electrogenic Na+-HCO3− cotransporter from rat kidney. Am J Physiol Renal Physiol 274: F425–F432, 1998 [DOI] [PubMed] [Google Scholar]
- 40.Sardet C, Franchi A, Pouyssegur J. Molecular cloning, primary structure, and expression of the human growth factor-activatable Na+/H+ antiporter. Cell 56: 271–280, 1989 [DOI] [PubMed] [Google Scholar]
- 41.Schultheis PJ, Clarke LL, Meneton P, Miller ML, Soleimani M, Gawenis LR, Riddle TM, Duffy JJ, Doetschman T, Wang T, Giebisch G, Aronson PS, Lorenz JN, Shull GE. Renal and intestinal absorptive defects in mice lacking the NHE3 Na+/H+ exchanger. Nat Genet 19: 282–285, 1998 [DOI] [PubMed] [Google Scholar]
- 42.Shih TH, Horng JL, Liu ST, Hwang PP, Lin LY. Rhcg1 and NHE3b are involved in ammonium-dependent sodium uptake by zebrafish larvae acclimated to low-sodium water. Am J Physiol Regul Integr Comp Physiol 302: R84–R93, 2012 [DOI] [PubMed] [Google Scholar]
- 43.Steiner RA, Oehme M, Ammann D, Simon W. Neutral carrier sodium ion-selective microelectrode for intracellular studies. Anal Chem 51: 351–353, 1979 [DOI] [PubMed] [Google Scholar]
- 44.Weiner ID, Verlander JW. Role of NH3 and NH4+ transporters in renal acid-base transport. Am J Physiol Renal Physiol 300: F11–F23, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Westerfield M. The Zebrafish Book: A Guide to the Laboratory Use of Zebrafish (Danio rerio). Eugene, OR: University of Oregon Press, 1995 [Google Scholar]
- 46.Wright PA, Wood CM. A new paradigm for ammonia excretion in aquatic animals: role of Rhesus (Rh) glycoproteins. J Exp Biol 212: 2303–2312, 2009 [DOI] [PubMed] [Google Scholar]
- 47.Wright PA, Wood CM. Seven things fish know about ammonia and we don't. Respir Physiol Neurobiol 184: 231–240, 2012 [DOI] [PubMed] [Google Scholar]
- 48.Wu SC, Horng JL, Liu ST, Hwang PP, Wen ZH, Lin CS, Lin LY. Ammonium-dependent sodium uptake in mitochondrion-rich cells of medaka (Oryzias latipes) larvae. Am J Physiol Cell Physiol 298: C237–C250, 2010 [DOI] [PubMed] [Google Scholar]
- 49.Yan JJ, Chou MY, Kaneko T, Hwang PP. Gene expression of Na+/H+ exchanger in zebrafish H+ -ATPase-rich cells during acclimation to low-Na+ and acidic environments. Am J Physiol Cell Physiol 293: C1814–C1823, 2007 [DOI] [PubMed] [Google Scholar]