Abstract
KCNQ (Kv7 family) potassium (K+) channels were recently found in airway smooth muscle cells (ASMCs) from rodent and human bronchioles. In the present study, we evaluated expression of KCNQ channels and their role in constriction/relaxation of rat airways. Real-time RT-PCR analysis revealed expression of KCNQ4 > KCNQ5 > KCNQ1 > KCNQ2 > KCNQ3, and patch-clamp electrophysiology detected KCNQ currents in rat ASMCs. In precision-cut lung slices, the KCNQ channel activator retigabine induced a concentration-dependent relaxation of small bronchioles preconstricted with methacholine (MeCh; EC50 = 3.6 ± 0.3 μM). Bronchoconstriction was also attenuated in the presence of two other structurally unrelated KCNQ channel activators: zinc pyrithione (ZnPyr; 1 μM; 22 ± 7%) and 2,5-dimethylcelecoxib (10 μM; 24 ± 8%). The same three KCNQ channel activators increased KCNQ currents in ASMCs by two- to threefold. The bronchorelaxant effects of retigabine and ZnPyr were prevented by inclusion of the KCNQ channel blocker XE991. A long-acting β2-adrenergic receptor agonist, formoterol (10 nM), did not increase KCNQ current amplitude in ASMCs, but formoterol (1–1,000 nM) did induce a time- and concentration-dependent relaxation of rat airways, with a notable desensitization during a 30-min treatment or with repetitive treatments. Coadministration of retigabine (10 μM) with formoterol produced a greater peak and sustained reduction of MeCh-induced bronchoconstriction and reduced the apparent desensitization observed with formoterol alone. Our findings support a role for KCNQ K+ channels in the regulation of airway diameter. A combination of a β2-adrenergic receptor agonist with a KCNQ channel activator may improve bronchodilator therapy.
Keywords: airway smooth muscle, KCNQ voltage-activated potassium channel, precision-cut lung slice, bronchoconstrictor signal transduction, bronchodilator therapy
hypercontraction of airway smooth muscle cells (ASMCs), which contributes to pathological narrowing of the airways in asthma, is widely ascribed to both an increase in local concentrations of various bronchoconstrictor agonists and increased sensitivity to these agonists (41). Locally released bronchoconstrictor agonists include acetylcholine (ACh), leukotriene D4, endothelin, and histamine, all of which activate Gq/11-coupled receptors on ASMCs (41), resulting in elevation of cytosolic calcium concentration ([Ca2+]cyt) and ASMC contraction (22, 27, 42, 43).
We previously provided evidence that cholinergic bronchoconstriction in rat precision-cut lung slices (PCLS) is dependent on activation of L-type voltage-sensitive Ca2+ channels (VSCCs) at submaximal agonist concentrations (5). Activation of VSCCs, and hence the influx of Ca2+, is tightly controlled by the voltage drop across the plasma membrane. L-Type VSCCs generally open in a steeply voltage-dependent manner at voltages positive to −40 mV (23). In resting ASMCs, opening of L-type VSCCs is opposed by negative transmembrane voltages of −45 to −60 mV, maintained predominantly by the efflux of potassium ions (K+) via plasma membrane K+ channels (22).
It is well established that changes in K+ channel activity have a dramatic effect on ASMC membrane voltage; inhibition of K+ channels results in membrane depolarization, and activation of K+ channels causes membrane hyperpolarization (18). Hence, regulation of K+ channel activity is an important mechanism for adjustment of membrane voltage to control the activity of VSCCs. Ca2+ influx through L-type VSCCs can activate smooth muscle contraction and in turn, induce bronchoconstriction. Thus the activity of K+ channels opposes airway smooth muscle contraction, providing an important autoregulatory mechanism that prevents excessive airway constriction.
We previously found that KCNQ voltage-activated K+ (Kv) channels (Kv7 family) are expressed in ASMCs from guinea pig and human lungs (3), and they were also recently reported to be expressed in murine and rat ASMCs (10). Among the many types of K+ channels expressed in ASMCs (2, 31, 45, 50, 51), KCNQ channels are perhaps the most likely to contribute significantly to their negative resting transmembrane voltages. KCNQ channels are unique among the Kv channels in that they are active at the very negative voltages measured in relaxed ASMCs, and they do not inactivate during sustained membrane depolarization (3). Furthermore, unlike Ca2+-activated K+ channels, which are primarily activated in response to L-type VSCC-mediated Ca2+ influx or agonist-induced release of intracellular Ca2+ stores (51), KCNQ channels are active at low [Ca2+]cyt of resting ASMCs. Thus these channels may be uniquely suited to stabilize negative resting voltages to oppose activation of L-type VSCCs and thereby, function as negative regulators of ASMC contraction.
In the present study, we provide evidence that KCNQ channels are expressed and functional in rat ASMCs and further demonstrate that pharmacological activators of these channels act as bronchodilators by opposing methacholine (MeCh)-induced suppression of KCNQ currents. These bronchodilatory effects of KCNQ channel activators are compared with those of a long-acting β2-adrenergic receptor agonist, formoterol, to provide evidence for a distinct and perhaps complementary mechanism of bronchodilation that could have clinical relevance for combination therapy.
MATERIALS AND METHODS
Precision-cut lung slices.
All animal studies were approved by the Loyola University Chicago Institutional Animal Care and Use Committee and were conducted in accordance with the 1996 Guide for the Care and Use of Laboratory Animals (National Academy of Sciences, Washington, DC). Male Sprague-Dawley rats (300–400 g) were euthanized with sodium pentobarbital. The trachea was cut below the larynx and cannulated with a 3-mm outer-diameter polypropylene-barbed tubing connector (Cole-Parmer, Vernon Hills, IL), and the lungs together with the heart were surgically removed and placed into a modified Hank’s balanced salt solution (mHBSS; in mM: NaCl 137.9, KCl 5.33, CaCl2 1.26, MgCl2 0.49, MgSO4 0.41, HEPES 20, KH2PO4 0.44, Na2HPO4 0.34, d-glucose 5, pH 7.4, NaOH at 37°C, 298–300 mosM), preheated to 37°C. The lungs were inflated with 15 ml of 2% low-melting temperature agarose in mHBSS at 37°C, followed by injection of 5 ml air and then cooled at 4°C for 30 min to solidify the agarose. Individual lobes were separated and trimmed perpendicular to the direction of the bronchus. Lung slices (0.35–0.5 mm thick) containing cross-sections of airways were made using a rat brain slicer (Zivic Instruments, Pittsburgh, PA) or a Vibratome (Leica Microsystems, Buffalo Grove, IL). Lung slices were incubated for at least 12 h in serum-free tissue-culture medium F-12/DMEM, supplemented with insulin-transferin-selenium (Cellgro; Mediatech, Manassas, VA) and antibiotic antimycotic solution (10,000 units penicillin, 10 mg streptomycin, and 25 μg amphotericin B/ml; Sigma-Aldrich, St. Louis, MO) at 37°C in 5% CO2, and used for up to 4 days after preparation.
Slices containing airways were used only if: 1) the airways were approximately circular; 2) beating cilia were observed (indicating an intact epithelium); and 3) the airway wall and all parenchymal attachments were intact. A lung slice was mounted in a perfusion chamber on the stage of an inverted microscope (Olympus IX-71) and visualized via a 10× objective. A cross-section of a small bronchiole (0.04–0.2 mm in diameter) was positioned in the center of the microscopic field, and lung slices were equilibrated in control medium (in mM: 140 NaCl, 5.36 KCl, 1.2 MgCl2, 2 CaCl2, 10 HEPES, 10 d-glucose, pH 7.3, 298 mosM) at room temperature for at least 30 min after mounting. Control medium ± drugs was superfused continuously over the lung slice via a gravity-fed perfusion system at a rate of ∼5 ml/min. Experiments were conducted at room temperature. Images were captured with a 12-bit digital camera (Orca; Hamamatsu, Hamamatsu Photonics, Hamamatsu, Japan) at 5-s intervals. The lumenal area was measured by image analysis using Simple PCI software (Hamamatsu Photonics). To ensure stability of the preparation, the resting luminal area was recorded for 20 min before drug application. Summarized measurements of the lumenal area of the airway in each experiment represent the average for the last 1 min of drug application or washout (total 12 measurements in each case) and for 5 min of control recording (total 60 measurements).
Isolation of airway myocytes.
Methods for isolation of rat ASMCs were essentially as described previously (3). Briefly, lungs were dissected immediately posteuthanasia; connective tissue, vasculature, and innervation were removed from primary and secondary bronchi. Bronchial segments, 3–5 mm in length, were transferred to ice-cold physiological saline solution (PSS), containing (in mM): 140 NaCl, 5.36 KCl, 0.34 Na2HPO4, 0.44 KH2PO4, 1.2 MgCl2, 0.05 CaCl2, 10 HEPES, 10 d-glucose, pH adjusted to 7.2, with NaOH on ice, 298 mosM. The segments were cut open, and the epithelium was removed using a cotton-tipped applicator. Each bronchial segment was then cut into strips, ∼1 mm wide. The bronchial strips were transferred into PSS (pH adjusted to 7.2 with NaOH at 37°C), supplemented with BSA (1 mg/ml), collagenase type VIII (800 units/ml; Sigma-Aldrich), papain (30 units/ml; Worthington Biochemical, Lakewood, NJ), and DL-dithiothreitol (1 mM). The strips were then incubated for 45 min at 37°C. After enzymatic digestion, the tissue was washed three to five times with ice-cold PSS and gently triturated with a fire-polished Pasteur pipette to release individual myocytes.
Quantitative real-time RT-PCR.
For RNA isolation, smooth muscle strips were dissected from rat trachea and cleared of connective tissue and epithelium. Each strip was cut perpendicularly into pieces, approximately 1–2 mm wide, and used for cell isolation as described above. The cell suspension containing predominantly smooth muscle cells (verified by cell appearance) was filtered through a 100-μm nylon mesh (Becton Dickinson, Franklin Lakes, NJ) and centrifuged at 1,000 g for 5 min. The cell pellet was resuspended in RNeasy Plus lysis buffer, and total RNA was extracted using the Qiagen RNeasy Plus Mini Kit (Qiagen, Valencia, CA). The mRNA was reverse transcribed using the Bio-Rad iScript cDNA synthesis kit (Bio-Rad, Hercules, CA). The cDNA thus obtained was amplified in a real-time RT-PCR reaction using primers specific for rat KCNQ1–5 (Kv7.1–Kv7.5) subtypes (SABiosciences, Frederick, MD). For quantitative estimates of KCNQ1–5 mRNA expression in ASMCs, standard curves were constructed with known quantities of cDNA target, and PCR results (threshold cycle) were extrapolated to the standard curve, expressed as nanogram target RNA/nanogram input RNA.
Patch clamp.
The whole-cell perforated patch configuration was used to measure membrane currents under voltage-clamp conditions. All experiments were performed at room temperature with continuous perfusion of bath solution, as described previously (3). The standard bath solution for ASMCs contained (in mM): 140 NaCl, 5.36 KCl, 1.2 MgCl2, 2 CaCl2, 10 HEPES, 10 d-glucose, pH 7.3, 298 mosM. Standard internal (pipette) solution contained (in mM): 135 KCl, 5 NaCl, 10 HEPES, 0.05 K2EGTA, 1 MgCl2, 20 d-glucose, pH 7.2, 298 mosM. To isolate KCNQ currents, 100 μM GdCl3 was added to bath solutions (3).
Voltage-clamp command voltages were generated using an Axopatch 200B amplifier under control of pCLAMP 9 software (Axon Instruments, Inverurie, Scotland). Amphotericin B (120 μg/ml) was added to the internal solution for membrane patch perforation. Whole-cell currents were digitized at 2 kHz and filtered at 1 kHz. KCNQ currents in ASMCs were recorded by application of 5-s voltage steps from −4 mV holding voltage to test voltages ranging from −84 mV to +16 mV. To obtain current-voltage curves, the last 2,000 data points recorded during each voltage step (corresponding to 1,000 ms recording time) were averaged and normalized by cell capacitance. Stable currents were recorded for at least 15 min before drug application. Time courses before and during drug applications were recorded at −20 mV holding voltage.
Statistics.
SigmaStat (Systat Software, Point Richmond, CA) was used for all statistical analyses. Paired Student's t-test was used for comparisons of parameters measured before and after treatments. Comparisons among multiple treatment groups were evaluated by ANOVA, followed by a Holm-Sidak post hoc test (12). Differences associated with P ≤ 0.05 were considered statistically significant.
Materials.
Collagenase, acetyl-β-methylcholine chloride (methacholine), zinc pyrithione (ZnPyr), isoproterenol, formoterol fumarate, and verapamil were from Sigma-Aldrich. Retigabine dihydrochloride was from LGM Pharma (Boca Raton, FL). XE991 dihydrochloride was from Ascent Scientific (Princeton, NJ). Amphotericin B was from Calbiochem (San Diego, CA). Papain was from Worthington Biochemical. Low melting-point agarose was from Gibco (Invitrogen, Carlsbad, CA). 2,5-Dimethyl-celecoxib was generously provided by Dr. Axel Schönthal (University of Southern California, Los Angeles, CA).
RESULTS
Expression and function of KCNQ K+ channels in rat ASMCs.
To determine whether KCNQ (Kv7) K+ channels are expressed and functional in rat airway myocytes, a combination of real-time RT-PCR and patch-clamp electrophysiology was used.
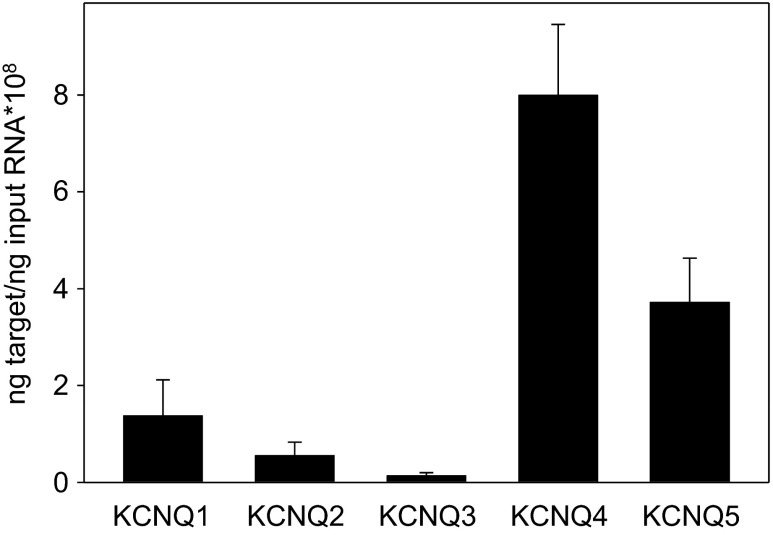
With the use of quantitative real-time RT-PCR, mRNAs for all five mammalian KCNQ subtypes (KCNQ1–5) were detected in cells isolated from rat tracheal smooth muscle strips. Based on mean expression data from n = 4 rats, this relatively pure population of ASMCs expresses KCNQ4 > KCNQ5 > KCNQ1 > KCNQ2 > KCNQ3 (Fig. 1).
Fig. 1.
Multiple KCNQ subtypes are expressed in rat airway smooth muscle cells (ASMCs). Expression levels of mRNAs for KCNQ1–5 were estimated using quantitative real-time RT-PCR in rat airway myocytes. The mRNA levels of KCNQ1–5 were averaged from 4 rats (2 rats for KCNQ4), with each reaction performed in duplicate.
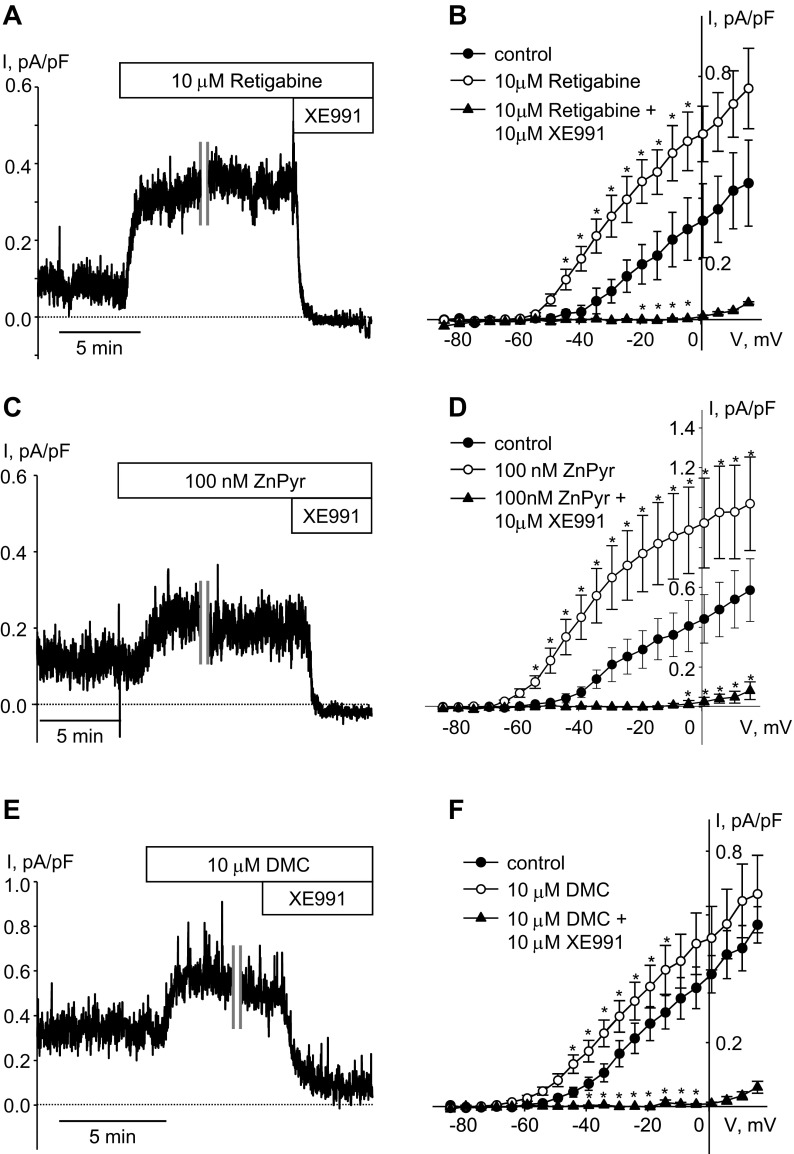
Functional expression of KCNQ channels is most definitively determined by measuring K+ currents with the expected electrophysiological and pharmacological characteristics. We therefore measured K+ currents in enzymatically dispersed rat airway myocytes using patch-clamp electrophysiology. Noninactivating K+ currents, recorded at −20 mV holding voltage in rat ASMCs, were enhanced in the presence of selective KCNQ channel activators retigabine (10 μM) and ZnPyr (100 nM) and inhibited upon application of the KCNQ channel blocker XE991 (10 μM), indicating that sustained current at that voltage was mediated predominantly by KCNQ channels (Fig. 2, A and C). With the use of a voltage-step protocol, both retigabine and ZnPyr increased KCNQ currents recorded at voltages positive to −40 mV and shifted the threshold of channel activation to more negative voltages (Fig. 2, B and D). We had previously found that 2,5-dimethylcelecoxib (DMC), a structural analog of the cyclooxygenase-2 inhibitor, celecoxib, acts as a KCNQ channel activator (similar to celecoxib) in vascular smooth muscle cells and guinea pig ASMCs (3, 4). Here, we found that DMC is also effective as an activator of KCNQ currents in rat ASMCs (Fig. 2, E and F).
Fig. 2.
Pharmacology of KCNQ currents in rat ASMCs. Representative time courses of retigabine [A; 10 μM, cell capacitance (C) = 23.6 pF]-, zinc pyrithione (ZnPyr; C; 100 nM, C = 14.3 pF)-, and 2,5-dimethylcelecoxib (DMC; E; 10 μM, C = 20.2 pF)-induced enhancement of endogenous KCNQ currents recorded in ASMCs at −20 mV holding voltage. A break in the recording (10 min) is indicated by 2 vertical gray lines. B, D, and F: current-voltage (I–V) relationships of KCNQ currents recorded in ASMCs before (control, filled circles); during treatment with 10 μM retigabine (B; open circles, n = 3), 100 nM ZnPyr (D; open circles, n = 4), and 10 μM DMC (F; open circles, n = 7); and after 5-min treatment with the KCNQ channel blocker XE991 in the presence of each activator (10 μM, filled triangles). *Significant difference from control (one-way repeated-measures ANOVA, P < 0.05).
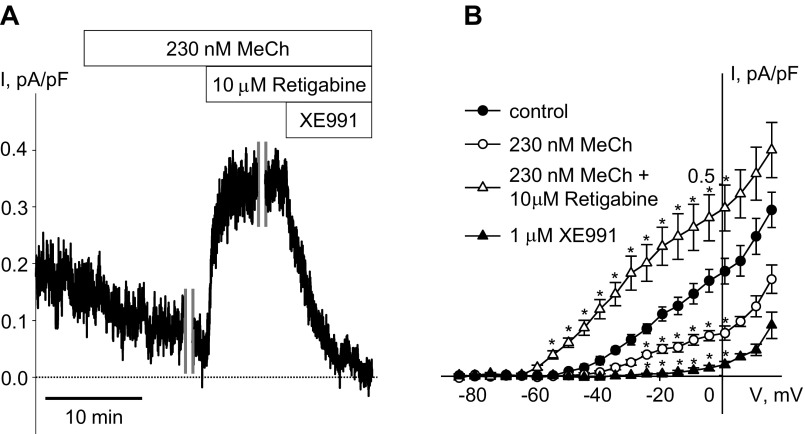
Treatment with the muscarinic cholinergic agonist MeCh (230 nM) significantly reduced KCNQ currents recorded at −20 mV holding voltage in rat ASMCs (55.1 ± 6.2% reduction; n = 7, P < 0.05, paired t-test), in agreement with our previous finding in guinea pig ASMCs (3). Application of retigabine (10 μM) in the continued presence of MeCh (230 nM) more than fully restored the currents, resulting in an increase in current amplitude to greater than the control level; the currents were completely abolished on subsequent application of XE991 (1 μM; Fig. 3).
Fig. 3.
Suppression of KCNQ currents by methacholine (MeCh) in rat ASMCs and their restoration by the KCNQ channel activator retigabine. A: representative time course of KCNQ current inhibition during treatment of an ASMC (C = 20.0 pF) with MeCh (230 nM for 20 min), recorded in an ASMC at −20 mV holding voltage. After 10 min of MeCh treatment, the time course recording was interrupted for 10 min for measurement of the steady-state I–V relationship (time break indicated by vertical gray lines). Then, after an additional 1-min recording at −20 mV holding voltage, retigabine (10 μM) was applied in the presence of MeCh for 5 min. The time course recording was interrupted again for 10 min for measurement of the steady-state I–V relationship, and after an additional 1-min time course recording, XE991 (1 μM) was applied in the presence of MeCh and retigabine for 10 min. B: I–V relationships of KCNQ currents recorded in ASMCs before (control, filled circles, n = 7), during treatment with 230 nM MeCh (open circles, n = 7), in the presence of retigabine (10 μM) applied with 230 nM MeCh (open triangles, n = 7), and in the presence of XE991 (1 μM, filled triangles, n = 7). *Significant difference from control (one-way repeated-measures ANOVA, P < 0.05).
KCNQ channel activators attenuated MeCh-induced constriction of airways.
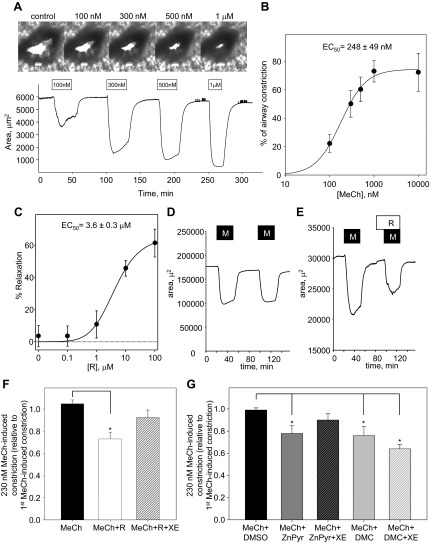
MeCh induced concentration-dependent constriction of rat bronchioles in PCLS, which were superfused with increasing concentrations of MeCh in the range of 100 nM to 1 μM; each concentration was applied for 30 min, followed by 45-min washout before application of the next MeCh concentration (Fig. 4, A and B). Percent of airway constriction, relative to the cross-sectional area measured before the initial MeCh treatment, was calculated for each MeCh concentration and plotted against concentration of MeCh. Concentration-response curves for each experiment were fitted by the Hill equation with a mean EC50 of 248 ± 49 nM, Hill coefficient of 1.6 ± 0.2, and maximal constriction of 81 ± 7% (n = 5; Fig. 4B).
Fig. 4.
Attenuation of MeCh-induced airway constriction by KCNQ potassium channel activators. A, top: representative images of a small airway before treatment (control) and in the presence of MeCh at increasing concentrations (100 nM, 300 nM, 500 nM, 1 μM); bottom: corresponding representative time course of changes in the lumenal area of the same small airway. MeCh, at each concentration (as indicated by the open bars), was applied for 30 min (1 μM MeCh was applied for 20 min), followed by a 45-min washout in each case. B: concentration dependence of airway constriction in response to MeCh fitted to the Hill equation (n = 5). C: mean dose-response curve for retigabine (R)-induced relaxation of rat airways preconstricted with 230 nM MeCh fitted to the Hill equation (n = 6). MeCh was applied to each slice for 30 min before addition of retigabine for 30 min in the continued presence of 230 nM MeCh. A single concentration of retigabine was used for each precision-cut lung slice (PCLS; 6 slices total from 4 different animals for each concentration). D: representative time course of changes in the lumenal area of a small airway on repetitive application of 230 nM MeCh (M; 30 min), with 45 min washout between applications. E: representative time course of changes in the lumenal area of a small airway on application of 230 nM MeCh, followed by a 30-min washout, 15-min application of retigabine (R; 10 μM) alone, and then a second application of 230 nM MeCh for 30 min in the presence of retigabine (10 μM). F: summarized bar graph of 2nd MeCh-induced constriction, relative to 1st MeCh-induced constriction, when MeCh was applied alone (black bar, n = 5), in the presence of retigabine (10 μM, white bar, n = 8), and in the presence of retigabine (10 μM) together with XE991 (XE; 10 μM, striped bar, n = 7). *Significant difference from MeCh alone (ANOVA on ranks, P < 0.01). G: summarized bar graph of 2nd MeCh-induced constriction relative to 1st MeCh-induced constriction when MeCh (230 nM) was applied in the presence of vehicle (0.1% of DMSO, black bar, n = 5), in the presence of ZnPyr (1 μM, dark-gray bar, n = 6), in the presence of ZnPyr (1 μM) together with XE991 (10 μM, dark-gray striped bar, n = 5), in the presence of DMC (10 μM, light-gray bar, n = 6), and in the presence of DMC (10 μM) together with XE991 (10 μM, light-gray striped bar, n = 5). *Significant difference from MeCh applied in the presence of DMSO (one-way ANOVA, P < 0.05).
Retigabine induced dose-dependent, sustained relaxation of airways that had been preconstricted for 30 min with 230 nM MeCh (EC50 = 3.6 ± 0.3 μM; maximum relaxation, 61 ± 9%; Fig. 4C). Bronchorelaxant effects were assessed further by measuring the extent of bronchoconstriction when MeCh was applied in the absence or presence of different structurally unrelated KCNQ channel activators (or vehicle). Application of 230 nM MeCh produced similar degrees of airway constriction on repetitive exposures (Fig. 4D). However, MeCh-induced airway constriction was reduced by 31% in the presence of 10 μM retigabine (MeCh + R; n = 8; Fig. 4, E and F). DMC and ZnPyr also significantly attenuated MeCh-induced airway constriction relative to vehicle controls: 10 μM DMC by 23% (MeCh + DMC; n = 6) and 1 μM ZnPyr by 21% (MeCh + ZnPyr; n = 6; Fig. 4G). The actions of both retigabine and ZnPyr were prevented by inclusion of the KCNQ channel blocker XE991 (10 μM; Fig. 4, F and G), providing evidence for the specific targeting of KCNQ channels to elicit their bronchorelaxant effects. The bronchorelaxant effects of DMC were not reduced significantly in the presence of XE991 (Fig. 4F).
β-Adrenoceptor-mediated relaxation of airways: not mediated by KCNQ channel activation but enhanced by KCNQ channel activator retigabine.
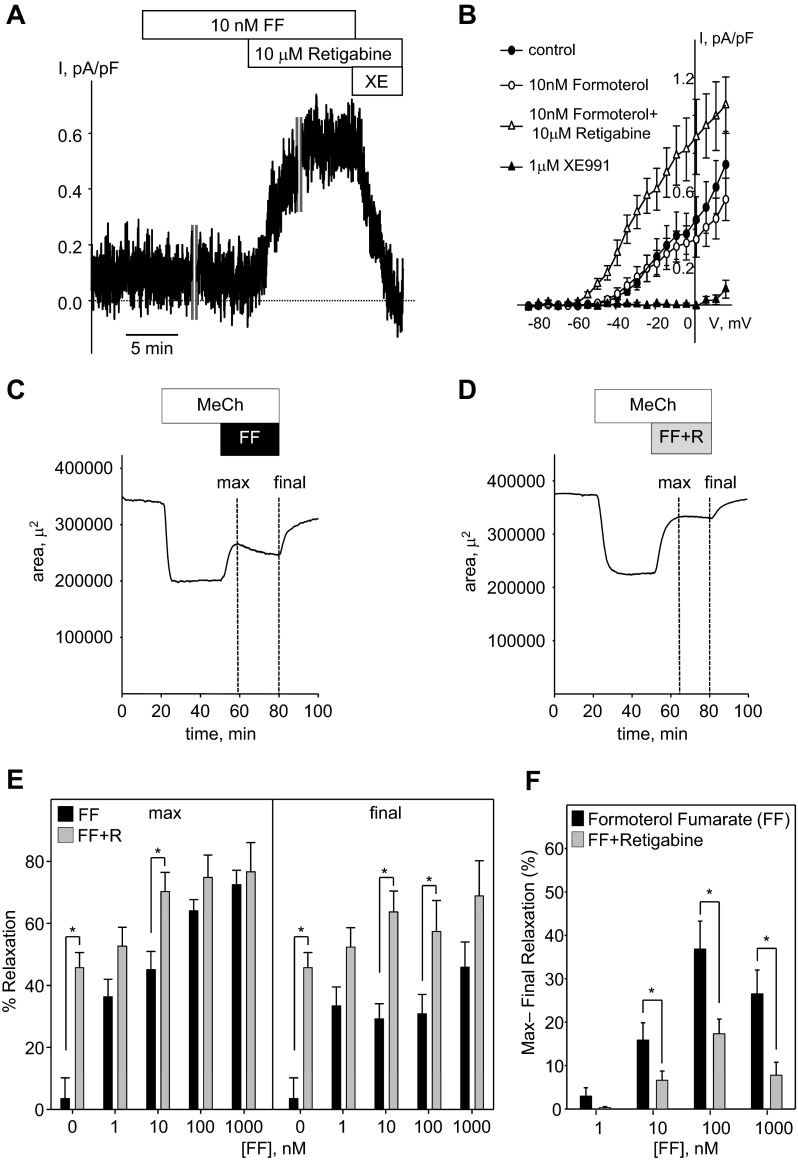
β-Adrenergic receptor agonists are commonly used for bronchodilator therapy. We examined whether the bronchorelaxant effects of the long-acting β2-adrenergic receptor agonist formoterol are mediated via activation of KCNQ channels in rat airway myocytes. Formoterol, at a concentration sufficient to produce significant relaxation of rat airways (10 nM, see below), did not enhance KCNQ currents recorded at −20 mV holding voltage in rat ASMCs (Fig. 5, A and B). However, subsequent application of retigabine (10 μM) in the presence of formoterol significantly increased the current, and the current was abolished effectively by application of 1 μM of XE991 (Fig. 5, A and B). Another β-adrenergic receptor agonist, isoproterenol (100 nM), was also ineffective in enhancement of KCNQ currents (data not shown).
Fig. 5.
Formoterol effects on KCNQ currents and on relaxation of rat airways: no effect on currents but enhanced bronchodilator responses when combined with retigabine. A: representative time course of KCNQ current recording in an ASMC (C = 13.9 pF) at −20 mV holding voltage before (5 min) and during treatment with formoterol fumarate (FF; 10 nM FF for 5 min). The time course recording was interrupted for 10 min for measurement of the steady-state I–V relationship (time break indicated by vertical gray lines). After an additional 5-min recording, retigabine (10 μM) was applied in the continued presence of formoterol. The time course recording was interrupted again for 10 min for recording of the steady-state I–V curves, and after an additional 5-min recording, XE991 (1 μM) was applied in the presence of retigabine. B: I–V relationships of KCNQ currents recorded in ASMCs before (control, filled circles, n = 4), during treatment with 10 nM formoterol (open circles, n = 4), in the presence of retigabine (10 μM) applied with 10 nM formoterol (open triangles, n = 3), and in the presence of XE991 (1 μM, filled triangles, n = 3). C: representative time course of changes in the lumenal area of a small airway in rat PCLS on application of 230 nM MeCh (30 min), followed by application of 230 nM MeCh in the presence of 10 nM formoterol (30 min). Dashed, vertical lines marked “max” and “final” indicate 1-min time intervals where the maximum or final relaxation was measured relative to the MeCh-induced constriction (measured during the last minute of MeCh alone). D: representative time course of changes in the lumenal area of a small airway treated as in C, except that 10 μM retigabine was included with the formoterol treatment (FF + R, 30 min). E: summarized bar graph of formoterol-induced relaxation, with or without 10 μM retigabine (gray or black bars, respectively), measured at the maximum and final time points, as indicated in C and D (n = 6–10). F: summarized bar graph of the maximum minus the final percent relaxation depicted in E. E and F: *significant difference between formoterol treatments alone vs. formoterol plus retigabine treatments (Student's t-test, n = 6–10, P < 0.05).
In rat PCLS, formoterol (1–1,000 nM) induced concentration-dependent relaxation of airways constricted with 230 nM MeCh, to a maximum of 72 ± 5% at 1 μM (Fig. 5E). However, there was a noticeable, time-dependent decline in the relaxation response at concentrations of formoterol ≥10 nM (compare maximal relaxation with final relaxation in Fig. 5, C and E). We then tested the combination of varying concentrations of formoterol with a submaximal concentration of retigabine (10 μM; a concentration that produced 45.7 ± 4.9% relaxation when applied alone; Fig. 4C). In the presence of retigabine, both maximum and sustained airway relaxation were increased relative to formoterol alone, although the extent of relaxation for each drug administered alone was not additive when combined (Fig. 5E). There was, however, a notable reduction in time-dependent desensitization to formoterol when it was combined with retigabine (Fig. 5D). The amount of desensitization for each concentration of formoterol in the absence and presence of 10 μM retigabine was estimated by subtraction of final relaxation (after a 30-min treatment) from maximal relaxation. Desensitization to formoterol (10–1,000 nM) was decreased significantly in the presence of 10 μM retigabine (Fig. 5F).
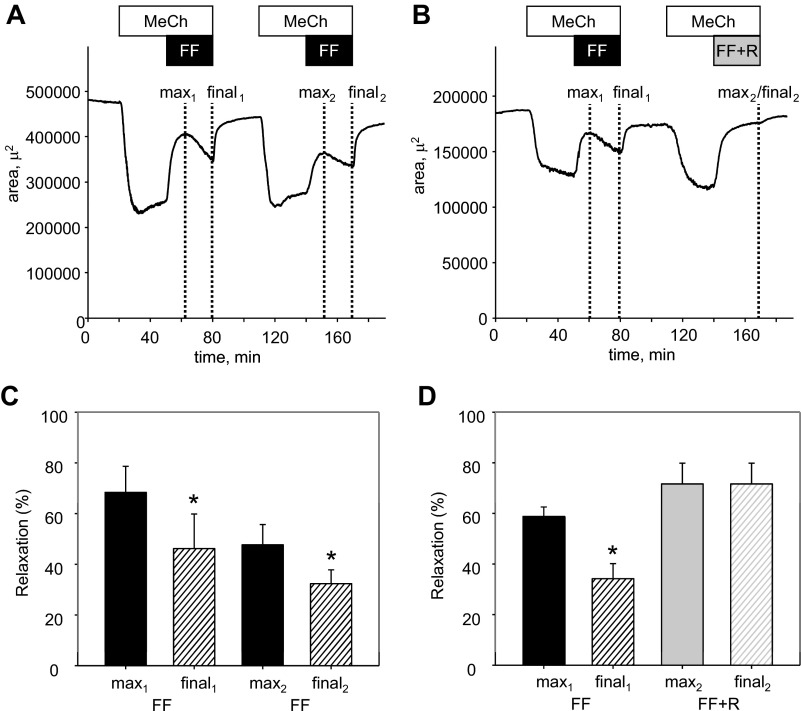
To investigate further the desensitization to formoterol in rat PCLS, formoterol (10 nM) was applied twice, at a 90-min interval, to airways constricted repetitively with 230 nM MeCh. As observed previously, formoterol (10 nM; 30 min) induced acute relaxation of airways constricted with 230 nM MeCh, peaking at 68 ± 10% but declining significantly to a final relaxation of only 46 ± 14% after 30 min of formoterol treatment (Fig. 6, A and C). After washout of both MeCh and formoterol, a second application of MeCh produced an airway constriction of comparable magnitude with the initial application, whereas formoterol-induced relaxation was reduced on the second application (peak relaxation declined from 68 ± 10% to 48 ± 8%, and final relaxation declined from 46 ± 14% to 32 ± 6%; Fig. 6, A and C). When retigabine (10 μM) was combined with the second application of formoterol, there was a significantly greater relaxation without any detectable, time-dependent desensitization (72 ± 8% peak and 72 ± 8% final relaxation after 30 min with combined formoterol and retigabine treatment compared with only 59 ± 4% peak relaxation and 34 ± 6% final relaxation when the same slices were exposed acutely to formoterol alone; Fig. 6, B and D).
Fig. 6.
Combination of retigabine with formoterol prevents desensitization in airway relaxation responses. A: representative time course of changes in the luminal area of a small airway on application of 230 nM MeCh (30 min), followed by application of 230 nM MeCh in the presence of 10 nM formoterol (30 min), with a 45-min washout between MeCh applications. Dashed, vertical lines indicate 1-min time intervals, where maximal relaxation (“max”) or final relaxation at the end of the 30 min treatment were measured relative to the preceding MeCh-induced constriction (measured during the last min of MeCh alone). B: representative time course of changes in the luminal area of a small airway, treated as in A, except that 10 nM formoterol plus 10 μM retigabine (30 min) were added after the second MeCh treatment. C: summarized bar graph of 10 nM formoterol-induced relaxation measured at the time points indicated in A. *Significant difference from max1 (one-way repeated-measures ANOVA, P < 0.05, n = 6). D: summarized bar graphs of 10 nM formoterol- and 10 nM formoterol plus 10 µM retigabine-induced relaxation measured at the time points indicated in B. *Significant difference from max1, max2, and final2 (one-way repeated-measures ANOVA, P < 0.05, n = 6).
DISCUSSION
In the present study, we have found that isolated rat ASMCs express KCNQ K+ channels, which produce noninactivating, outward currents that are suppressed in response to bronchoconstrictor concentrations of MeCh. These same currents can be enhanced by KCNQ channel activators, including retigabine, ZnPyr, and DMC, which may overcome the suppression induced by MeCh. MeCh-induced constriction of rat bronchioles was significantly attenuated by each of these KCNQ K+ channel activators. Moreover, in combination with the long-acting β2-adrenergic receptor agonist formoterol, retigabine opposed the time- and use-dependent desensitization of formoterol-induced bronchorelaxation.
We previously reported expression and function of KCNQ channels in guinea pig and human airway myocytes (3). KCNQ expression in cells isolated from rat trachealis muscle was found to differ, to some extent, from human and guinea pig airway myocytes. In the present study, rat KCNQ4 mRNA was found to be most abundant, followed by KCNQ5 and KCNQ1 mRNAs. In human airway myocytes, KCNQ1 expression was highest, followed by KCNQ4 and KCNQ5, whereas airway myocytes in guinea pig expressed KCNQ mRNAs in the order KCNQ2 > KCNQ5 > KCNQ4, with KCNQ3 message being minor in all three species (3). The KCNQ expression pattern in rat airway myocytes reported here does not exactly match the KCNQ expression profile, recently reported for rat trachealis muscle, in which KCNQ1 mRNA was determined to be the most abundant, followed by KCNQ5 and KCNQ4 (10). Observed differences may reflect differences in RNA preparation [intact trachealis muscle strips (10) compared with our isolated myocyte preparation] or the assessment of expression relative to β-actin (10) compared with our estimation of the absolute amount of KCNQ-encoding mRNA, relative to input mRNA. It should be kept in mind that expression of KCNQ1, KCNQ3, and KCNQ5 was detected previously in epithelial cells (13, 14, 33, 38), so a small contamination of the smooth muscle preparation by epithelial cells may affect the measured expression pattern.
Functional expression of ion channels is most definitively evaluated by recording of ionic currents with the expected biophysical and pharmacological profiles. In agreement with the recent report by Evseev and colleagues (10), outward KCNQ K+ currents were activated by membrane depolarization in rat ASMCs with a threshold negative to −40 mV. As expected for KCNQ2–5 encoded channels (9), we found that KCNQ currents recorded in rat airway myocytes were enhanced by retigabine and DMC. ZnPyr, which appears to act at a different site on KCNQ channels and robustly enhances the activities of KCNQ1, KCNQ2, KCNQ4, and KCNQ5 channels (52, 53), also enhanced KCNQ currents in rat airway myocytes. XE991, an isoform-independent KCNQ channel blocker, effectively inhibited KCNQ currents in rat airway myocytes, as demonstrated previously for human, guinea pig, rat, and mouse ASMCs (3, 10).
We recently reported that the muscarinic ACh receptor agonist MeCh suppressed KCNQ currents in guinea pig airway myocytes (3). In the present study, KCNQ currents, recorded in rat airway myocytes, were also found to be sensitive to MeCh, although to a lesser extent: 55% inhibition at −20 mV by 230 nM MeCh in rat airway myocytes compared with 60% inhibition by 100 nM MeCh in guinea pig airway myocytes (3). Another muscarinic agonist, carbachol (0.5 μM), induced only 20% inhibition of KCNQ current in rat airway myocytes (10). These apparent differences in muscarinic suppression of KCNQ currents in rat and guinea pig myocytes may reflect different KCNQ expression profiles or other yet-unidentified species differences.
Activation of Gq/11-coupled receptors, including the M3 muscarinic ACh receptor, induces ASMC contraction, primarily by elevating [Ca2+]cyt (41). Sources of Ca2+ for activation of ASMC contraction include the inositol 1,4,5-trisphosphate-induced Ca2+ release from sarcoplasmic reticulum and Ca2+ entry across the plasma membrane. Among the main routes for Ca2+ entry are L-type VSCCs, which may be activated following membrane depolarization. Although there is some disagreement in the published literature (23), the vast majority of published results is consistent in providing compelling evidence that Ca2+ influx via L-type VSCCs is an important contributor to ASMC contraction at submaximal concentrations of agonists (1, 8, 11, 19, 21, 30, 36, 39, 44, 46, 49, 54).
K+ channels have recognized roles as regulators of membrane voltage, which controls the activity of L-type VSCCs in airway myocytes (32, 40). KCNQ channels, in contrast with other types of ASMC K+ channels, are active at resting voltages and low [Ca2+]cyt and so would be well suited to oppose membrane depolarization and activation of L-type VSCCs. In support of this proposed role, we found that rat airway constriction by 230 nM MeCh, which is entirely dependent on L-type VSCCs (5), was reduced significantly by three structurally unrelated KCNQ channel activators. The in vitro bronchorelaxant effects of two of these drugs—retigabine and ZnPyr—were prevented by inclusion of the KCNQ channel blocker, XE991, supporting the specificity of their actions. By comparison, DMC still induced significant relaxation in the presence of XE991, suggesting an additional bronchorelaxant mechanism. We had previously found that DMC is a very effective blocker of L-type VSCCs in vascular smooth muscle cells (4) and also found that blocking VSCCs was sufficient to relax airways that were preconstricted with 230 nM MeCh (5). Therefore, the results with DMC are consistent with its actions as an L-type Ca2+ channel blocker (CCB) superseding its actions as a KCNQ channel activator in the induction of bronchorelaxation.
Airway hyperconstriction in asthma is attributed, in part, to excessive Gq/11-coupled receptor activation (41). The apparent involvement of L-type VSCCs in airway constriction and Gq/11-coupled bronchoconstrictor signal transduction led to a number of clinical trials of CCBs to limit excessive bronchoconstriction in asthma patients. Unfortunately, these trials produced inconsistent results. Although CCBs were effective in relieving airway hyperconstriction in a subset of patients in most of the clinical trials, CCB therapy for asthma was ultimately abandoned due to adverse side effects and limitations of formulation that prevented effective inhalational administration of the commonly used CCBs, such as verapamil and nifedipine (11). Like CCBs, KCNQ channel activators were developed for clinical use in the treatment of conditions unrelated to airway diseases, predominantly neurological conditions, such as epilepsy and pain (15, 16, 24). Our findings suggest an alternative use for KCNQ channel activators as bronchodilators. These drugs may be more amenable to the development of inhalational formulations, or more selective agents may be identified that can be administered systemically to activate primarily the ASMC KCNQ channels and avoid unwanted off-target effects.
Even with direct lung-targeted inhalational therapy, KCNQ channel activators might have off-target effects, acting on cell types in the lung other than ASMCs. KCNQ channels are expressed in neurons (24), airway epithelial cells (13, 14, 33, 38), and the pulmonary vasculature (17, 25, 26). Thus ASMC-targeted KCNQ channel activator therapy could potentially lead to off-target effects on parasympathetic nerve activity, epithelial ion transport, or pulmonary blood flow. It remains to be determined to what extent such off-target effects might impact potential bronchodilator therapy.
Evseev et al. (10) recently tested the effect of inhaled retigabine on MeCh-induced bronchoconstriction in conscious mice and observed a significant, albeit transient, bronchorelaxant effect. It should be noted that a number of species differences in bronchoconstrictor pathways have been reported previously. For example, murine airway smooth muscle is less responsive to asthma-related bronchoconstrictors, such as histamine and leukotrienes, than is airway smooth muscle in other species (20, 34, 37), and unlike human airways, allergen-induced bronchoconstriction is mediated primarily by serotonin in both murine and rat airways (37). Despite these differences, it is clear that the fundamental Ca2+-dependent contraction of ASMCs is a common downstream determinant of airway diameter across mammalian species and that KCNQ channel activators have demonstrated bronchorelaxant effects, at least in vitro, in all mammalian species tested to date.
β2-Adrenergic receptor agonists are commonly used therapeutically for the relief of excessive airway constriction (6). Their bronchorelaxant mechanism involves increased formation of cyclic adenosine monophosphate and activation of protein kinase A, which then phosphorylates key regulatory proteins involved in the control of airway smooth muscle tone (6). Activation of large-conductance Ca2+-activated K+ (BKCa) channels has been proposed as one of the downstream effector pathways (28), although the possibility that KCNQ channel activation is also involved has not been explored previously. There is evidence that β-adrenergic receptor activation can enhance KCNQ1 channel activity in cardiac myocytes (35), and activation of KCNQ4 channels has been proposed as an essential mechanism in the vasorelaxant effects of the β-adrenergic receptor agonist isoproterenol in rat renal arteries (7). Our findings argue against a role of KCNQ channels in the bronchorelaxant effects of β2-adrenergic receptor activation, as we found no effect of β2-adrenergic agonists on ASMC KCNQ currents at concentrations that were effective in relaxation of rat bronchioles.
Retigabine, by itself, was able to relax rat bronchioles preconstricted with 230 nM MeCh in PCLS (46% relaxation at 10 μM retigabine), and an even greater relaxation was observed when it was combined with the long-acting β2-adrenergic receptor agonist formoterol. However, the combination of retigabine with formoterol did not produce an additive relaxation of rat airways. This lack of additivity might be explained by the involvement of BKCa channel activation in β2-adrenoceptor-induced relaxation (28, 29, 47). Activation of BKCa channels and KCNQ channels produces the same downstream effect of membrane hyperpolarization and reduction of Ca2+ influx through L-type VSCC. The redundancy of these effects might preclude a fully additive relaxation when retigabine is combined with formoterol.
Sustained or repetitive exposure to β2-adrenergic receptor agonists is known to induce receptor desensitization, and our findings bore this out in the reduced rat airway relaxation responses to sustained or repetitive exposures to formoterol. Notably, the combination of retigabine with formoterol mitigated the apparent desensitization. A possible explanation for this is that whereas BK channel activation would fully depend on activity of the β2-adrenergic receptor and thus would be reduced upon β2-adrenoceptor desensitization, activity of KCNQ channels would not be affected by the loss of β2-adrenoceptor signaling. Thus whereas activation of KCNQ channels may be redundant when β2-adrenergic receptors are fully able to activate BKCa channels, when the latter response is reduced by β2-adrenergic receptor desensitization, the activation of KCNQ channels may be more prominent and sufficient to sustain bronchorelaxation on its own.
In summary, our findings suggest that KCNQ channel activators, which are already in clinical use for other conditions, may be repurposed as promising, new bronchodilator therapies. Previous studies have demonstrated that there are a number of added benefits in using combinations of β2-adrenergic agonists and antimuscarinic agents for the treatment of airway diseases (6, 48). Considering our findings that KCNQ channel activation can oppose both muscarinic and histaminergic bronchoconstriction [Figs. 5 and 6 and Brueggemann et al. (3)], a combination of KCNQ channel activators with β2 agonists might be even more beneficial. Our findings here provide the first evidence that such combination therapy might indeed provide a more effective bronchorelaxant effect than either treatment alone.
GRANTS
Support for this work was provided by the National Heart, Lung, and Blood Institute (R01 HL-089564 to K. L. Byron) and by intramural funds from Loyola University Chicago.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: L.I.B., J.M.H., S.T., D.R., L.L.C., and K.L.B. conception and design of research; L.I.B., J.M.H., S.N., S.T., D.R., and L.L.C. performed experiments; L.I.B., J.M.H., S.N., S.T., D.R., and L.L.C. analyzed data; L.I.B., J.M.H., S.N., S.T., D.R., L.L.C., and K.L.B. interpreted results of experiments; L.I.B., J.M.H., S.N., S.T., and L.L.C. prepared figures; L.I.B., J.M.H., and K.L.B. drafted manuscript; L.I.B., J.M.H., S.T., L.L.C., and K.L.B. edited and revised manuscript; L.I.B., J.M.H., S.N., S.T., D.R., L.L.C., and K.L.B. approved final version of manuscript.
REFERENCES
- 1.Ahmed T, Danta I. Modification of histamine- and methacholine-induced bronchoconstriction by calcium antagonist gallopamil in asthmatics. Respiration 59: 332–338, 1992 [DOI] [PubMed] [Google Scholar]
- 2.Boyle JP, Tomasic M, Kotlikoff MI. Delayed rectifier potassium channels in canine and porcine airway smooth muscle cells. J Physiol 447: 329–350, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brueggemann LI, Kakad PP, Love RB, Solway J, Dowell ML, Cribbs LL, Byron KL. Kv7 potassium channels in airway smooth muscle cells: signal transduction intermediates and pharmacological targets for bronchodilator therapy. Am J Physiol Lung Cell Mol Physiol 302: L120–L132, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brueggemann LI, Mackie AR, Mani BK, Cribbs LL, Byron KL. Differential effects of selective cyclooxygenase-2 inhibitors on vascular smooth muscle ion channels may account for differences in cardiovascular risk profiles. Mol Pharmacol 76: 1053–1061, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Byron KL, Brueggemann LI, Kakad PP, Haick JM. Kv7 (KCNQ) potassium channels and L-type calcium channels in the regulation of airway diameter. In: Calcium Signaling in Airway Smooth Muscle Cells, edited by Wang Y-X. New York: Springer, 2014, p. 21–33 [Google Scholar]
- 6.Cazzola M, Page CP, Rogliani P, Matera MG. β2-Agonist therapy in lung disease. Am J Respir Crit Care Med 187: 690–696, 2013 [DOI] [PubMed] [Google Scholar]
- 7.Chadha PS, Zunke F, Zhu HL, Davis AJ, Jepps TA, Olesen SP, Cole WC, Moffatt JD, Greenwood IA. Reduced KCNQ4-encoded voltage-dependent potassium channel activity underlies impaired β-adrenoceptor-mediated relaxation of renal arteries in hypertension. Hypertension 59: 877–884, 2012 [DOI] [PubMed] [Google Scholar]
- 8.Dai JM, Kuo KH, Leo JM, Pare PD, van Breemen C, Lee CH. Acetylcholine-induced asynchronous calcium waves in intact human bronchial muscle bundle. Am J Respir Cell Mol Biol 36: 600–608, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Du XN, Zhang X, Qi JL, An HL, Li JW, Wan YM, Fu Y, Gao HX, Gao ZB, Zhan Y, Zhang HL. Characteristics and molecular basis of celecoxib modulation on Kv7 potassium channels. Br J Pharmacol 164: 1722–1737, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Evseev AI, Semenov I, Archer CR, Medina JL, Dube PH, Shapiro MS, Brenner R. Functional effects of KCNQ K+ channels in airway smooth muscle. Front Physiol 4: 277, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fish JE. Calcium channel antagonists in the treatment of asthma. J Asthma 21: 407–418, 1984 [DOI] [PubMed] [Google Scholar]
- 12.Glantz SA. Primer of Biostatistics. New York: McGraw-Hill, 2005 [Google Scholar]
- 13.Grahammer F, Warth R, Barhanin J, Bleich M, Hug MJ. The small conductance K+ channel, KCNQ1: expression, function, and subunit composition in murine trachea. J Biol Chem 276: 42268–42275, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Greenwood IA, Yeung SY, Hettiarachi S, Andersson M, Baines DL. KCNQ-encoded channels regulate Na+ transport across H441 lung epithelial cells. Pflügers Arch 457: 785–794, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Gribkoff VK. The therapeutic potential of neuronal Kv7 (KCNQ) channel modulators: an update. Expert Opin Ther Targets 12: 565–581, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Gunthorpe MJ, Large CH, Sankar R. The mechanism of action of retigabine (ezogabine), a first-in-class K+ channel opener for the treatment of epilepsy. Epilepsia 53: 412–424, 2012 [DOI] [PubMed] [Google Scholar]
- 17.Gurney AM, Joshi S, Manoury B. KCNQ potassium channels: new targets for pulmonary vasodilator drugs? In: Membrane Receptors, Channels and Transporters in Pulmonary Circulation, edited by Yuan JX, Ward JP. New York: Humana, Springer Science + Business Media, 2010, p. 405–417 [DOI] [PubMed] [Google Scholar]
- 18.Hall IP. Second messengers, ion channels and pharmacology of airway smooth muscle. Eur Respir J 15: 1120–1127, 2000 [DOI] [PubMed] [Google Scholar]
- 19.Hay DW, Luttmann MA, Muccitelli RM, Goldie RG. Endothelin receptors and calcium translocation pathways in human airways. Naunyn Schmiedebergs Arch Pharmacol 359: 404–410, 1999 [DOI] [PubMed] [Google Scholar]
- 20.Held HD, Martin C, Uhlig S. Characterization of airway and vascular responses in murine lungs. Br J Pharmacol 126: 1191–1199, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hirota K, Hashiba E, Yoshioka H, Kabara S, Matsuki A. Effects of three different L-type Ca2+ entry blockers on airway constriction induced by muscarinic receptor stimulation. Br J Anaesth 90: 671–675, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Hirota S, Helli P, Janssen LJ. Ionic mechanisms and Ca2+ handling in airway smooth muscle. Eur Respir J 30: 114–133, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Janssen LJ. Ionic mechanisms and Ca2+ regulation in airway smooth muscle contraction: do the data contradict dogma? Am J Physiol Lung Cell Mol Physiol 282: L1161–L1178, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Jentsch TJ. Neuronal KCNQ potassium channels: physiology and role in disease. Nat Rev Neurosci 1: 21–30, 2000 [DOI] [PubMed] [Google Scholar]
- 25.Joshi S, Balan P, Gurney AM. Pulmonary vasoconstrictor action of KCNQ potassium channel blockers. Respir Res 7: 31, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Joshi S, Sedivy V, Hodyc D, Herget J, Gurney AM. KCNQ modulators reveal a key role for KCNQ potassium channels in regulating the tone of rat pulmonary artery smooth muscle. J Pharmacol Exp Ther 329: 368–376, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jude JA, Wylam ME, Walseth TF, Kannan MS. Calcium signaling in airway smooth muscle. Proc Am Thorac Soc 5: 15–22, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kume H, Hall IP, Washabau RJ, Takagi K, Kotlikoff MI. beta-Adrenergic agonists regulate KCa channels in airway smooth muscle by cAMP-dependent and -independent mechanisms. J Clin Invest 93: 371–379, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kume H, Takai A, Tokuno H, Tomita T. Regulation of Ca2+-dependent K+-channel activity in tracheal myocytes by phosphorylation. Nature 341: 152–154, 1989 [DOI] [PubMed] [Google Scholar]
- 30.Lei Y, Cao Y, Zhang Y, Edvinsson L, Xu CB. Enhanced airway smooth muscle cell thromboxane receptor signaling via activation of JNK MAPK and extracellular calcium influx. Eur J Pharmacol 650: 629–638, 2011 [DOI] [PubMed] [Google Scholar]
- 31.Liu X, Xu Y, Zhang Z. Changes in delayed rectifier K+ channel function and its regulation by protein kinase C pathway in bronchial myocytes from asthmatic rats. Chin Med J (Engl) 116: 1799–1803, 2003 [PubMed] [Google Scholar]
- 32.Malerba M, Radaeli A, Mancuso S, Polosa R. The potential therapeutic role of potassium channel modulators in asthma and chronic obstructive pulmonary disease. J Biol Regul Homeost Agents 24: 123–130, 2010 [PubMed] [Google Scholar]
- 33.Mall M, Wissner A, Schreiber R, Kuehr J, Seydewitz HH, Brandis M, Greger R, Kunzelmann K. Role of K(V)LQT1 in cyclic adenosine monophosphate-mediated Cl(−) secretion in human airway epithelia. Am J Respir Cell Mol Biol 23: 283–289, 2000 [DOI] [PubMed] [Google Scholar]
- 34.Martin TR, Gerard NP, Galli SJ, Drazen JM. Pulmonary responses to bronchoconstrictor agonists in the mouse. J Appl Physiol (1985) 64: 2318–2323, 1988 [DOI] [PubMed] [Google Scholar]
- 35.Marx SO, Kurokawa J, Reiken S, Motoike H, D'Armiento J, Marks AR, Kass RS. Requirement of a macromolecular signaling complex for beta adrenergic receptor modulation of the KCNQ1-KCNE1 potassium channel. Science 295: 496–499, 2002 [DOI] [PubMed] [Google Scholar]
- 36.Matsuda F, Sugahara K, Sugita M, Sadohara T, Kiyota T, Terasaki H. Comparative effect of amrinone, aminophylline and diltiazem on rat airway smooth muscle. Acta Anaesthesiol Scand 44: 763–766, 2000 [DOI] [PubMed] [Google Scholar]
- 37.Meurs H, Gosens R, Zaagsma J. Airway hyperresponsiveness in asthma: lessons from in vitro model systems and animal models. Eur Respir J 32: 487–502, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Moser SL, Harron SA, Crack J, Fawcett JP, Cowley EA. Multiple KCNQ potassium channel subtypes mediate basal anion secretion from the human airway epithelial cell line Calu-3. J Membr Biol 221: 153–163, 2008 [DOI] [PubMed] [Google Scholar]
- 39.Moura CT, Bezerra FC, de Moraes IM, Magalhaes PJ, Capaz FR. Increased responsiveness to 5-hydroxytryptamine after antigenic challenge is inhibited by nifedipine and niflumic acid in rat trachea in vitro. Clin Exp Pharmacol Physiol 32: 1119–1123, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Pelaia G, Gallelli L, Vatrella A, Grembiale RD, Maselli R, De Sarro GB, Marsico SA. Potential role of potassium channel openers in the treatment of asthma and chronic obstructive pulmonary disease. Life Sci 70: 977–990, 2002 [DOI] [PubMed] [Google Scholar]
- 41.Penn RB. Embracing emerging paradigms of G protein-coupled receptor agonism and signaling to address airway smooth muscle pathobiology in asthma. Naunyn Schmiedebergs Arch Pharmacol 378: 149–169, 2008 [DOI] [PubMed] [Google Scholar]
- 42.Penn RB, Benovic JL. Regulation of heterotrimeric G protein signaling in airway smooth muscle. Proc Am Thorac Soc 5: 47–57, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Perez-Zoghbi JF, Karner C, Ito S, Shepherd M, Alrashdan Y, Sanderson MJ. Ion channel regulation of intracellular calcium and airway smooth muscle function. Pulm Pharmacol Ther 22: 388–397, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Semenov I, Wang B, Herlihy JT, Brenner R. BK channel β1 subunits regulate airway contraction secondary to M2 muscarinic acetylcholine receptor mediated depolarization. J Physiol 589: 1803–1817, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Snetkov VA, Ward JP. Ion currents in smooth muscle cells from human small bronchioles: presence of an inward rectifier K+ current and three types of large conductance K+ channel. Exp Physiol 84: 835–8460, 1999 [PubMed] [Google Scholar]
- 46.Solway J, Fanta CH. Differential inhibition of bronchoconstriction by the calcium channel blockers, verapamil and nifedipine. Am Rev Respir Dis 132: 666–670, 1985 [DOI] [PubMed] [Google Scholar]
- 47.Tanaka Y, Yamashita Y, Yamaki F, Horinouchi T, Shigenobu K, Koike K. MaxiK channel mediates β2-adrenoceptor-activated relaxation to isoprenaline through cAMP-dependent and -independent mechanisms in guinea-pig tracheal smooth muscle. J Smooth Muscle Res 39: 205–219, 2003 [DOI] [PubMed] [Google Scholar]
- 48.Tashkin DP, Ferguson GT. Combination bronchodilator therapy in the management of chronic obstructive pulmonary disease. Respir Res 14: 49, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thirstrup S, Nielsen-Kudsk F, Dahl R. In vitro studies on the interactions of β2-adrenoceptor agonists, methylxanthines, Ca2+-channel blockers, K+-channel openers and other airway smooth muscle relaxants in isolated guinea-pig trachea. Eur J Pharmacol 326: 191–200, 1997 [DOI] [PubMed] [Google Scholar]
- 50.Waldron GJ, Sigurdsson SB, Aiello EA, Halayko AJ, Stephens NL, Cole WC. Delayed rectifier K+ current of dog bronchial myocytes: effect of pollen sensitization and PKC activation. Am J Physiol Lung Cell Mol Physiol 275: L336–L347, 1998 [DOI] [PubMed] [Google Scholar]
- 51.Wang YX, Fleischmann BK, Kotlikoff MI. Modulation of maxi-K+ channels by voltage-dependent Ca2+ channels and methacholine in single airway myocytes. Am J Physiol Cell Physiol 272: C1151–C1159, 1997 [DOI] [PubMed] [Google Scholar]
- 52.Xiong Q, Sun H, Li M. Zinc pyrithione-mediated activation of voltage-gated KCNQ potassium channels rescues epileptogenic mutants. Nat Chem Biol 3: 287–296, 2007 [DOI] [PubMed] [Google Scholar]
- 53.Xiong Q, Sun H, Zhang Y, Nan F, Li M. Combinatorial augmentation of voltage-gated KCNQ potassium channels by chemical openers. Proc Natl Acad Sci USA 105: 3128–3133, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang CH, Lifshitz LM, Uy KF, Ikebe M, Fogarty KE, ZhuGe R. The cellular and molecular basis of bitter tastant-induced bronchodilation. PLoS Biol 11: e1001501, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]