Abstract
Reprogramming somatic cells to induced pluripotent stem cells (iPSCs) eliminates many epigenetic modifications that characterize differentiated cells. In this study, we tested whether functional differences between chronic obstructive pulmonary disease (COPD) and non-COPD fibroblasts could be reduced utilizing this approach. Primary fibroblasts from non-COPD and COPD patients were reprogrammed to iPSCs. Reprogrammed iPSCs were positive for oct3/4, nanog, and sox2, formed embryoid bodies in vitro, and induced teratomas in nonobese diabetic/severe combined immunodeficient mice. Reprogrammed iPSCs were then differentiated into fibroblasts (non-COPD-i and COPD-i) and were assessed either functionally by chemotaxis and gel contraction or for gene expression by microarrays and compared with their corresponding primary fibroblasts. Primary COPD fibroblasts contracted three-dimensional collagen gels and migrated toward fibronectin less robustly than non-COPD fibroblasts. In contrast, redifferentiated fibroblasts from iPSCs derived from the non-COPD and COPD fibroblasts were similar in response in both functional assays. Microarray analysis identified 1,881 genes that were differentially expressed between primary COPD and non-COPD fibroblasts, with 605 genes differing by more than twofold. After redifferentiation, 112 genes were differentially expressed between COPD-i and non-COPD-i with only three genes by more than twofold. Similar findings were observed with microRNA (miRNA) expression: 56 miRNAs were differentially expressed between non-COPD and COPD primary cells; after redifferentiation, only 3 miRNAs were differentially expressed between non-COPD-i and COPD-i fibroblasts. Interestingly, of the 605 genes that were differentially expressed between COPD and non-COPD fibroblasts, 293 genes were changed toward control after redifferentiation. In conclusion, functional and epigenetic alterations of COPD fibroblasts can be reprogrammed through formation of iPSCs.
Keywords: COPD, IPSCs, microarray, miRNA
the reprogramming of somatic cells by defined transcription factors has allowed the derivation of induced pluripotent stem cells (iPSCs) with similar functional and molecular phenotypic characteristics to embryonic stem cells (ESCs). Like ESCs, iPSCs have the capacity for self-renewal and can differentiate into mature cell types. iPSCs promise to advance regenerative medicine in several distinct ways: as therapeutic agents themselves (39), as a source of differentiated cells (9, 16, 17), or, as in the present study, as a model system to explore the role of epigenetic reprogramming in abnormally functioning cells.
Chronic obstructive pulmonary disease (COPD) is a progressive inflammatory disease of the lungs, the third leading cause of death in the United States, and the only major cause of death that is increasing. Existing therapies for COPD modestly improve airflow and symptoms, but no currently available treatment meaningfully slows disease progression or reverses the structural changes that compromise lung function. These structural alterations result from lung damage in the face of a compromised ability to mediate repair (31, 42). Restoration of normal tissue repair could slow the progression of disease and, potentially, restore lost lung function and is, therefore, an appealing novel approach for the therapy of COPD (35). Fibroblasts are the major cells responsible for production and remodeling of extracellular matrix. In addition, they are potent sources of growth factors and can modulate the proliferation and function of epithelial and endothelial cells. Moreover, they respond vigorously to a variety of mediators that are released at sites of injury (31, 32).
We and others have shown that fibroblasts isolated from COPD patients have abnormal repair functions and gene expression compared with fibroblasts of matched controls (14, 20, 27, 28, 42). Importantly, the differences between COPD and non-COPD fibroblasts persist with extended culture in vitro, suggesting an alteration in the differentiated phenotype (14, 20, 28). Alternatively, the differences between COPD and non-COPD fibroblasts could reflect underlying genetic differences, or both genetic and epigenetic mechanisms could contribute.
Fibroblasts isolated from various tissues are well recognized as having phenotypic differences, much as epithelial cells isolated from different tissues do. Unfortunately, there are few well-characterized molecular or surface antigens that serve as markers to distinguish different fibroblast phenotypes. Recent studies have used global gene expression to characterize fibroblast populations (21). However, distinct populations of fibroblasts are most commonly identified by functional characteristics (29), as has been done with fibroblasts from non-COPD and COPD patients. These studies have identified a number of differences between fibroblasts from non-COPD and COPD patients. Among these, fibroblasts from COPD patients contract collagen gels less and migrate toward a chemotactic stimulus less robustly and differ in gene expression (27, 42). The present study tested the hypothesis that reprogramming fibroblasts into iPSCs followed by redifferentiation into fibroblasts would reverse differences between COPD and non-COPD fibroblasts.
MATERIALS AND METHODS
All experiments were done in triplicates.
Cell lines.
Primary lung fibroblasts from three patients with COPD and three patients without clinical or functional signs of COPD (non-COPD) were included (Table 1). Fibroblasts were obtained from normal-appearing areas of the pulmonary parenchyma regions of surgical specimens from patients with COPD. Non-COPD fibroblasts were obtained from specimens from patients with similar age and smoking history who had normal lung function. Surgery for all patients was performed for the removal of isolated tumors suspected of being malignant. Normal tissue was excised from an area outside the perimeter of the mass and initiated into culture. Acquisition of samples was approved by the Human Studies Committee of the Medical Board of the State of Schleswig-Holstein, and all patients provided written, informed consent for the acquisition of material for research. The cells were cultured on 100-mm tissue culture dishes (Falcon; Becton-Dickinson Labware, Lincoln Park, NJ) with Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS; Biofluid, Rockville, MD), 50 U/ml penicillin G sodium, 50 μg/ml streptomycin sulfate (penicillin-streptomycin; GIBCO, Life Technologies, Grand Island, NY), and 1 μg/ml amphotercin B (Pharma-Tek, Huntington, NY). Cells were cultured at 37°C in a humidified atmosphere of 5% CO2 and passaged every 4–5 days.
Table 1.
Clinical data and gold stage for COPD and non-COPD subjects
| Patient Condition | Age, yr | Sex | FEV1, % | FVC, % | FEV/FVC, % | Smoking, pack·yr | Current |
|---|---|---|---|---|---|---|---|
| Non-COPD1 | 67 | M | 96.7 | 116 | 91.9 | 15 | Nonsmoking 20 yr |
| Non-COPD2 | 74 | F | 87 | 107.4 | 84.1 | 0 | Nonsmoking |
| Non-COPD3 | 72 | M | 95.8 | 77.1 | 94.6 | 50–100 | Unknown |
| COPD1 Gold 4 | 54 | M | 17.8 | 62.6 | 27.9 | 46 | Nonsmoking 17 yr |
| COPD2 Gold 2 | 67 | F | 72.6 | 98 | 59 | 42 | Nonsmoking 7 yr |
| COPD3 Gold 3 | 60 | M | 45.2 | 79 | 57.6 | 43–60 | Smoking |
COPD, chronic obstructive pulmonary disease; M, male; F, female; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity.
Induction of iPSCs.
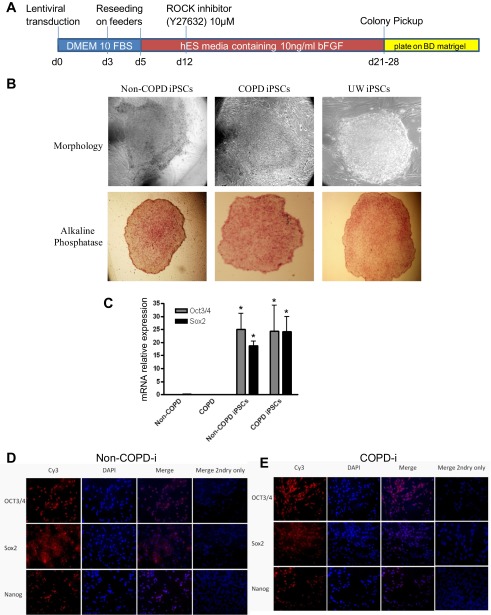
A commercial kit containing lentiviral particles with the four genes, Oct4, Sox2, Klf4, and c-Myc (Stemgent, Cambridge, MA), as described by Takahashi et al. (40), was used according to the manufacturer's recommendation. Briefly, 105 lung fibroblasts from COPD and non-COPD subjects at passage 6 (three subjects each) were seeded in six-well plates. After 24 h, cells were infected with the four lentiviral particles at multiplicity of infection of 10, and Polybrene was added at 6 μg/ml in DMEM. Twenty-four hours postinfection, media were replaced with fresh DMEM with 10% FBS. Cells were trypsinized 48 h later and were seeded on six-well plates containing a feeder layer of mitomycin-C (Fisher Scientific, Swedesboro, NJ) treated mouse embryonic fibroblasts, in DMEM with 10% FBS. Media were then switched to DMEM/F12 supplemented with 10 ng/ml FGF and 20% serum replacement (Invitrogen, Grand Island, NY), and plates were incubated at 37°C at 5% CO2. Media were changed every 48 h, and, at day 12 postinfection, Rock inhibitor (Y27632) was added at 10 μM for 24 h to enhance the colony formation (11). Colonies were picked at days 21–28 and were reseeded on matrigel (BD Bioscience, San Jose, CA), and media were changed to mTESR1 (Stemcell Technologies, Vancouver, Canada). The time line for iPSC generation is shown in Fig. 1A.
Fig. 1.
Reprogramming of non-chronic obstructive pulmonary disease (COPD) and COPD fibroblasts into induced pluripotent stem cells (iPSCs). A: schematic diagram of the reprogramming protocol. Fibroblasts (3 non-COPD and 3 COPD fibroblasts) were transduced with lentiviral vectors of oct3/4, sox2, klf-4, and C-myc at multiplicity of infection of 10, then seeded at day (d) 3 on feeders. At day 5, media was switched to human embryonic stem (hES) media, and at day 12 Rho kinase (ROCK) inhibitor was added. At days 21–28, colonies were picked up and moved to six-well plates coated with matrigel that contained feeder-free media. B: morphology and alkaline phosphatase staining of iPSCs. The reprogrammed cell lines were morphologically similar to iPSCs obtained from University of Wisconsin (UW; positive control) and expressed alkaline phosphatase. C: real-time PCR for oct3/4, sox2, and nanog in primary fibroblasts and reprogrammed iPSCs from COPD and non-COPD individuals. Genes were normalized to 18S rRNA (N = 3 in each group). *Significant difference from COPD and non-COPD primary fibroblasts, P < 0.05. D and E: immunocytochemistry staining of iPSCs. The nuclei of cells are stained with DAPI. Oct3/4, sox2, and Nanog specific antigens are detected with CY3 (red) labeled antibodies in iPSCs from non-COPD (D) and COPD (E). COPD-i, reprogrammed COPD.
Immunocytochemistry.
iPSCs were cultured until subconfluence in eight-chamber slides (Nunc, Naperville, IL) in DMEM/F12 and fixed in 4% paraformaldehyde for 30 min. Cells were washed in phosphate-buffered saline before permeabilization and following each subsequent step. Blocking was performed using horse serum for 1 h. Permeabilization was performed in 0.1% sodium citrate buffer with 0.1% Triton at 4°C for 5 min. Cells were incubated with rabbit polyclonal anti-oct3/4, anti-sox2, or anti-nanog (Stemgent) at 1:200 dilution at 4°C overnight. Cells were then incubated with Cy3 conjugated anti-rabbit IgG antibody (Stemgent) and stained with propidium iodide after washing. Stained cells were visualized and photographed using a Nikon Eclipse TE300 microscope (Nikon, Tokyo, Japan) equipped with a DP71 digital camera (Olympus, Tokyo, Japan).
Real-time PCR.
Total RNA was extracted using Trizol reagent (Invitrogen), and 1 μg of total RNA was treated with DNase I to eliminate potential genomic DNA contamination. For cDNA synthesis, total RNA was transcribed using high-capacity cDNA reverse transcription kits (Applied Biosystems, Foster City, CA). Real-time PCR was performed for oct3/4, sox2, and nanog. Detection of messenger RNA (mRNA) was conducted in a total volume of 20 μl using Taqman Gene Expression Assays and the ABI Prism 7500 (Applied Biosystems) following the manufacturer's instructions. As an internal control, the rRNA control kit (Applied Biosystems) was used.
Differentiation of iPSCs to hepatocytes (endoderm).
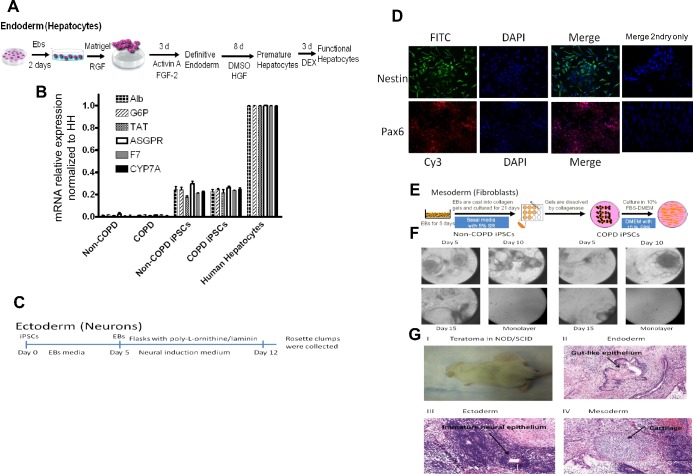
Cells were differentiated toward hepatocytes following the protocol established by our group (5). Briefly, iPSCs were placed in low-attachment petri dishes for 48 h in DMEM/F12 supplemented with 15% knockout serum replacement, 1 mmol/l nonessential amino acids, and l-glutamine (all from Invitrogen). For differentiation, embryoid bodies (EBs) were plated on 5% Matrigel-Growth Factor Reduced (BD Bioscience), and maintained for 3 days in DMEM/F12 media supplemented with 100 ng/ml recombinant activin-A (R&D Systems) and 100 ng/ml FGF-2 (Invitrogen). Cells were then grown for 8 days in DMEM/F12 containing 100 ng/ml hepatocyte growth factor (R&D Systems), followed by culture for 3 additional days in DMEM/F12 containing 10−7 mol/l dexamethasone (Sigma-Aldrich, St. Louis, MO). To prove that cells were differentiated to hepatocyte-like cells, real-time PCR for hepatic markers, albumin (Alb), glucose-6-phosphatase (G6P), tyrosine transaminase (TAT), coagulation factor 7 (F7), cytochrome P-450 7A (CYP7A), and asialoglycoprotein receptor (ASGPR) was performed.
Differentiation of iPSCs to neurons (ectoderm).
To initiate differentiation, we followed the protocol by Delaloy et al. (12). Briefly, iPSCs colonies were detached and grown as aggregates or EBs in suspension for 5 days in iPSCs medium without FGF-2. On day 5, EBs were transferred to flasks precoated with poly-l-ornithine/laminin (20 μg/ml) in neural induction medium (DMEM/F12/N2) consisting of 33% F12, 66% DMEM, 1× N2 supplement (25 mg/ml insulin, 50 mg/ml transferrin, 100 mM putrescine, 30 nM selenium chloride, and 20 nM progesterone), 1% nonessential amino acids solution, 10 ng/ml FGF-2 (all from Invitrogen), and 2 mg/ml heparin (Sigma-Aldrich). After a few days in the neural induction medium, elongated cells appeared and formed rosettes. After 12 days, neuroepithelial cells in the rosettes were isolated from the surrounding cells with 0.2 mg/ml dispase. Rosette clumps were collected and transferred to a flask for 2–3 h to allow nonneural cells to attach. The floating cells were transferred to a flask coated with poly-HEME (Sigma-Aldrich) to prevent cell attachment. Neural markers Nestin and Pax6 were then assessed by immunohistochemistry. Cells were incubated with primary antibodies (Nestin and Pax6) at 1:200 dilution for 1 h. FITC and CY3 secondary antibodies were added and stained with DAPI after washing.
Differentiation of iPSCs to fibroblasts (mesoderm).
Fibroblast differentiation was done following the protocol established in our lab using three-dimensional (3D) collagen gels (43). Briefly, to prepare EBs, iPSCs were treated with 1 mg/ml collagenase, and cells were collected by centrifugation at 200 g for 2 min. The pellet was resuspended in differentiation medium containing 90% DMEM/F12, 10% serum replacement, 1% nonessential amino acids, and 1 mM l-glutamine. Cells were then placed into a bacteriological petri dish (Sarstedt, Nümbrecht, Germany) and cultured for 5 days. Floating EBs were collected into a 50-ml polypropylene conical tube (Falcon; Beckton-Dickinson Labware, Franklin Lakes, NJ) and precipitated without centrifugation.
Collagen gels were prepared as described previously (26). Briefly, rat tail tendon collagen, distilled water, and 4× concentrated DMEM were combined so that the final mixture resulted in 0.75 mg/ml collagen, with a physiological ionic strength of 1× DMEM and a pH of 7.4. EBs were then suspended in the neutralized collagen solution. Aliquots (1.0 ml/well) of the mixture of EBs in collagen were then cast into each well of a 12-well tissue culture plate (Falcon) and allowed to gel. After gelation was completed, normally within 20 min at room temperature, basal media (1:1 mixture of differentiation media and DMEM/F12) were added on the top of gels in a 12-well plate (1.0 ml/well). The basal medium was changed every 2–3 days, and EBs were cultured for 21 days in the three-dimensional type I collagen gels. The cultured gels, into which differentiated fibroblasts had migrated, were then dissolved with 1 mg/ml collagenase at 37°C in a 5% CO2 atmosphere for 1 h. The resulting cells were resuspended with DMEM containing 10% FBS (10% FBS-DMEM) and centrifuged at 200 g for 5 min. The cells, including the EBs, were cultured in a 100-mm tissue culture dish (Falcon) with 10% FBS-DMEM, 50 U/ml penicillin, 50 μg/ml streptomycin, and 1 μg/ml amphotericin B. When the fibroblasts, which grew as a monolayer, were near confluent, the cells were trypsinized and passaged in 10% FBS-DMEM. Differentiated fibroblast functions were assessed by gel contractions and chemotaxis, while fibroblast marker vimentin was assessed by immunohistochemistry. Cells were incubated with primary antibodies (vimentin and desmin) for 1 h. Cells were then incubated with FITC secondary antibodies and stained with propidium iodide after washing.
Teratoma formation.
To determine the pluripotent capacity of iPSCs, 100 μl of cell suspension containing approximately one million cells of each generated iPSC line were injected subcutaneously into the dorsal flank of nonobese diabetic/severe combined immunodeficient mice (two animals/cell line). Human ESCs from University of Wisconsin were used as a positive control, and PBS was used as negative control. Animals were checked every week for tumors, which were observed and recovered 5–8 wk after injection. The tumors were fixed overnight in 10% formalin, dehydrated, cleared, and paraffin embedded. Tissues were then cut into 5-μm sections and stained with hematoxylin and eosin. All procedures performed on animals were approved by the University of Nebraska Medical Center Animal Care and Use Committee and were within the guidelines for humane care of laboratory animals.
Collagen gel contraction assay.
Collagen gels were prepared as described previously (26). Fibroblasts were trypsinized and mixed with the neutralized collagen solution so that the final cell density in the collagen solution was 3 × 105 cells/ml. Aliquots (0.5 ml/well) of the mixture of cells in collagen were cast into each well of 24-well tissue culture plates (Falcon), and the mixture was allowed to gel. After gelation was completed, the gels were gently released from the 24-well tissue culture plates and transferred into 60-mm tissue culture dishes (three gels in each dish), which contained 5 ml of freshly prepared serum-free DMEM (SF-DMEM). The gels were then incubated at 37°C in a 5% CO2 atmosphere for 3 days. Gel contraction was quantified using an Optomax V image analyzer (Optomax, Burlington, MA) daily. Data were expressed as a percentage of the initial gel size from three gels per cell line and in three different experiments. Human fetal lung fibroblasts (HFL-1) were used as a positive control for both gel contraction and chemotaxis assays (38). HFL-1 cells were purchased from American Type Culture Collection (Rockville, MD). The cells were cultured in 100 mm tissue culture dishes (FALCON) in DMEM supplemented with 10% FBS, 50 U/ml penicillin G sodium, 50 mg/ml streptomycin sulfate, and 1 μg/ml fungizone, and maintained at 37°C in a humidified 5% CO2 incubator.
Chemotaxis assay.
Cell migration was assessed using the Boyden blindwell chamber (Neuro Probe, Gaithersburg, MD), as previously described (7). Briefly, 26 μl of SF-DMEM containing human fibronectin (5 μg/ml) were placed into the lower wells. Eight-micrometer pore polycarbonate membranes (Neuro Probe) were used. Cells were trypsinized and suspended in SF-DMEM at a density of 1 × 106 cells/ml. Fifty microliters of cell suspension were then added into each upper well. Cells were allowed to migrate at 37°C in a 5% CO2 atmosphere for 6 h. Cells that had not migrated were scraped off the upper surface of the membrane, and the membranes were air-dried. Cells were then stained with PROTOCOL (Fisher Scientific) and mounted on a glass microscope slide. Chemotaxis was assessed by counting the number of cells in five high-power fields from three different experiments.
mRNA and microRNA preparation and microarray assay.
mRNA and microRNAs (miRNAs) were extracted using mirVana miRNA Isolation Kit (Ambion, Applied Biosystems, Austin, TX) following the manufacturer's instructions. Hybridizations and microarray analysis for mRNA (Affymetrix Human Gene 1.0 ST Array) and miRNA (Affymetrix miRNAs Array), following the manufacturer's recommendations, were performed in the University of Nebraska Medical Center microarray core facility. Analysis of Affymetrix data were conducted with BRB Array Tools version 3.8.0 stable release, developed by Dr. Richard Simon (34) and the R programming language with Bioconductor project (30). Low-level analysis, which converts probe level data to a gene level expression data as well as normalization, was done using RMA. Expression levels were analyzed on the log2 scale. To visualize the data, we used hierarchical clustering to cluster genes and samples of significant gene lists. Results are displayed in heatmaps and dendrograms (25). Genes were compared between groups with random variance t-tests (using paired tests if appropriate), and genes were considered differentially expressed by 0.5-fold or 2-fold with a false discovery rate of 10%.
Sample size and statistical analysis.
All experiments were done in triplicate on separate occasions. For this study, we planned to perform independent tests between COPD and non-COPD groups and paired tests between the derived cell groups. With three arrays per group (6 total), we had 85% power to detect a twofold change between groups with an α-level of 0.01 and a two-sided paired t-test. This calculation assumes the log2 standard deviation (SD) is 0.2 (based on our prior experiments), and the correlation between samples is 0.8.
The random-variance t-tests allows for sharing information among genes about variation without assuming that all genes have the same variance, which gives a more accurate estimate of the variability when sample sizes are small (45). For real-time PCR data, collagen contraction and chemotaxis, Mann-Whitney test was used, and P > 0.05 was considered significant.
Ingenuity pathway analysis.
To further investigate the potential functional significance of the expression pattern of mRNA in COPD fibroblasts compared with the reprogrammed and re-differentiated COPD-i cells, we compared the differentially expressed genes in the parent cells that were no longer differentially expressed in the reprogrammed cells, or vice versa. We sought to identify COPD-associated gene networks from these candidate genes to determine the cellular pathways that contained the genes that were differentially expressed in COPD compared with control fibroblasts. We used Ingenuity Pathway Analysis (IPA) software (version 7.6, Ingenuity Systems), a web-based application, which links the uploaded differentially expressed genes to a database of gene functions gleaned from the biomedical research literature that enables identifying relationships, biological mechanisms, functions, and pathways of relevance associated with the molecules under study (15). Briefly, a set of COPD genes that were differentially expressed by more or less than twofold from non-COPD and also from COPD-i was uploaded into the web-delivered application, and each gene identifier was mapped and placed in relevant molecular networks.
RESULTS
The first aim of the present study was to examine whether non-COPD and COPD fibroblasts could be reprogrammed to a pluripotent state. We utilized lentiviral transduction to induce iPSCs from lung fibroblasts of patients with or without COPD. Our initial attempt resulted in cells that formed colonies that resembled iPSCs and that expressed Oct3/4 and Nanog but not Sox2. These cells formed EBs but failed to differentiate into fibroblasts. We then modified the method by adding rock inhibitor and plating the picked iPSCs colonies on matrigel (BD) (Fig. 1A). As shown in Fig. 1B, the iPSCs that formed under these conditions were very small and differ markedly from the characteristic spindle shape of fibroblasts. They formed colonies characteristic of stem cells and closely resembled iPSCs obtained from the University of Wisconsin (47). Colonies expressed alkaline phosphatase, a pluripotency marker of stem cells. Furthermore, cells significantly expressed the message for markers of undifferentiation oct3/4, sox2, and nanog, measured by real-time PCR, compared with their corresponding fibroblasts (Fig. 1C). No stain was detected using rabbit ire-immune sera (data not shown). In the same manner, colonies induced from both non-COPD and COPD also produced oct3/4 and sox2 proteins assessed by immunocytochemistry (Fig. 1, D and E).
Pluripotency iPSCs derived from COPD and non-COPD fibroblasts.
To confirm the pluripotency and self-renewal capacity of our iPSCs, we induced EBs in vitro and differentiated iPSCs in vitro toward cells that represent the three germ layers and in vivo through formation of teratomas in immune-compromised animals. iPSCs derived from all non-COPD and COPD primary cells were differentiated toward hepatocyte-like cells utilizing the protocol outlined in Fig. 2A. These differentiated cells expressed Alb, G6P, TAT, ASGPR, F7, and CYP7A, markers of hepatic phenotype (Fig. 2B). The differentiated cells also produced Alb levels detected by Western blot (data not shown). Similarly, iPSCs derived from all primary fibroblasts differentiated toward ectoderm (Fig. 2C), as assessed by Nestin and Pax6 immunocytochemistry (Fig. 2D). Finally, iPSCs derived from all primary fibroblasts differentiated into fibroblasts in the 3D collagen gel system (Fig. 2, E and F). We further confirmed the pluripotency of the iPSCs derived from each primary fibroblast by formation of teratomas in immune-compromised animals. Eight of twelve animals injected with the six iPSCs generated (two animals each) were positive for teratomas verified by hematoxylin and eosin staining, which demonstrated that all three germ layers, mesoderm, endoderm, and ectoderm, were in the tumors (Fig. 2G). Each of the iPSCs resulted in at least one animal that developed a teratoma.
Fig. 2.
In vitro and in vivo differentiation of iPSCs (3 non-COPD and 3 COPD) toward endoderm, ectoderm, and mesoderm germ layers. A: strategy for differentiation of iPSCs cells to endoderm (hepatocytes). RGF, reduced growth factor; FGF-2, fibroblast growth factor-2; HGF, hepatocyte growth factor; DEX, dexamethasone. B: real-time analysis for expression of lineage-specific hepatic markers [albumin (Alb), glucose-6-phosphatase (G6P), asialoglycoprotein receptor (ASGPR), tyrosine aminotransferase (TAT), cytochrome P-450 7A (CYP7A), and coagulation factor 7 (F7)] compared with human hepatocytes (HH). Genes were normalized to 18S rRNA (N = 3 in each group). C: protocol used for neurons precursor differentiation representing ectoderm germ cells. D: immunocytochemistry of nestin and pax6 markers of ectoderm germ cells where nucleus was stained with DAPI (blue), neural progenitor markers nestin antibodies labeled with FITC (green), and pax6 antibodies labeled with Cy3 (red). E: strategy used to differentiate reprogrammed iPSCs into fibroblasts representing the mesoderm germ cells. SR, serum replacement; FBS, fetal bovine serum. F: morphology of different stages of iPSCs differentiation after embryoid bodies (EBs) were placed in three-dimensional culture into fibroblast-like cells at days 5, 10, and 15 and on monolayer outgrowth of fibroblasts. G: teratoma formation in immunodeficient nonobese diabetic/severe combined immunodeficient (NOD/SCID) mice, showing teratoma in the animal (I); gutlike epithelium representing endoderm (II); immature neural epithelium representing ectoderm (III); and cartilage-like cells representing mesoderm (IV).
Reprogrammed iPSCs differentiated toward functional fibroblasts.
We next assessed the function of the reprogrammed fibroblasts derived from iPSCs from non-COPD (non-COPD-i) and from COPD patients (COPD-i) and compared their function to that of the primary cells from which they were derived. As demonstrated in Fig. 3A, COPD primary fibroblasts contracted 3D gels less robustly than primary non-COPD cells (areas of the gels at day 3 were 77.3 ± 3.8 and 49.00 ± 1.2%. respectively, P < 0.002). Differentiated COPD-i and non-COPD-i fibroblasts contracted 3D gels to a similar degree (areas of gels at day 3 were 56.3 ± 3.2 and 58.0 ± 3.2%, respectively, P > 0.73). Importantly, the re-differentiated non-COPD-i and COPD-i fibroblasts contracted collagen gels to an extent similar to each other and to non-COPD parent cells (Fig. 3A).
Fig. 3.
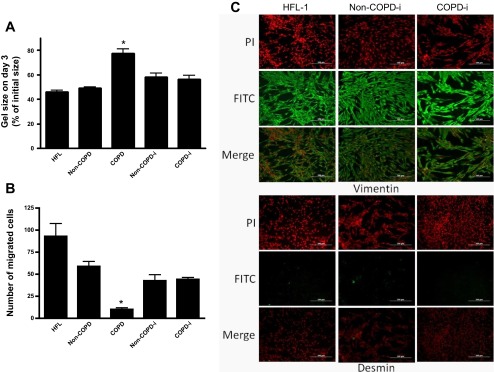
A: collagen gel contraction (N = 3 in each group). Fibroblasts were cast into collagen gels and maintained in floating culture in serum-free media. The size of gels was measured on day 3 and shown as percentage of initial area. B: chemotaxis toward fibronectin in the Boyden blindwell chemotaxis assay (N = 3 in each group). Cells were trypsinized, and chemotaxis toward fibronectin was assessed by counting the number of migrated cells in five high-power fields. Human fetal lung fibroblasts (HFL-1) served as positive control in both assays. Values are means ± SE. *P < 0.05. C: immunophenotyping of differentiated fibroblasts from COPD and non-COPD which are positive for vimentin and negative for desmin. PI, propidium iodide.
Chemotaxis of fibroblasts toward fibronectin demonstrated similar results. While primary COPD fibroblasts migrated less robustly than non-COPD (10.2 ± 1.9 and 58.9 ± 5.6, respectively, P < 0.001), COPD-i and non-COPD-i fibroblasts migrated to a similar degree (44.0 ± 2.1 and 42.3 ± 6.7 cells/5 high-power fields, respectively). This was significantly greater than that for parent COPD fibroblasts (P < 0.01, all comparisons). Chemotaxis of the re-differentiated fibroblasts was not as robust as that of parent non-COPD cells (75 and 72% for non-COPD-i and COPD-i, respectively), but this difference did not achieve statistical significance. These data suggest that differentiation of reprogrammed iPSCs from both COPD and non-COPD fibroblasts yield cells that are functionally very similar to each other for both gel contraction and chemotaxis, and that these cells resemble the functional activity of parental non-COPD cells.
As shown in Fig. 3C, COPD-i and non-COPD-i differentiated cells were positive for vimentin, a marker for mesenchymal phenotype, and negative for desmin. HFL-1 cells served as a positive control.
Global gene and miRNA expression.
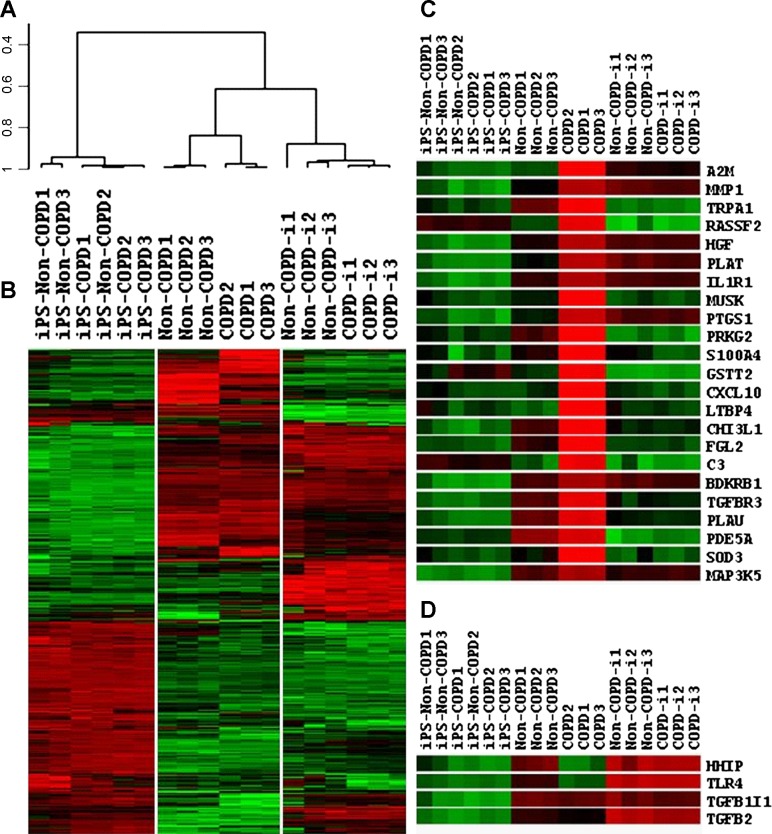
To further characterize the functional differences in the reprogrammed fibroblasts, we assessed global gene expression in the primary fibroblasts, iPSCs, and re-differentiated fibroblasts. All comparisons were done at false discovery rate of 1% and P value < 0.001. As demonstrated by both dendrogram (Fig. 4A) and heatmap for clustering (Fig. 4B), primary cells from both COPD and non-COPD patients were clustered together. However, COPD and non-COPD fibroblasts were distinguishable with 1,881 differentially expressed genes. A more stringent cutoff of less than 0.5-fold and higher than 2-fold revealed a total of 605 differentially expressed genes. At these cutoffs, COPD fibroblasts underexpressed 245 genes and overexpressed 360 genes compared with non-COPD fibroblasts. For the iPSCs as a group, there was markedly similar gene expression with a correlation value of 0.97. In iPSCs derived from non-COPD fibroblasts, only 13 genes were differentially expressed, with no genes overexpressed by 2-fold and only one gene underexpressed at 0.5-fold compared with iPSCs derived from COPD. Following redifferentiation, the fibroblasts derived from both COPD and non-COPD were more similar than the primary fibroblasts with differential expression of only 112 genes. COPD-i fibroblasts underexpressed three genes by 0.5-fold, and no genes were overexpressed by 2-fold compared with non-COPD-i. However, the differentiated fibroblasts were similar with a correlation in gene expression of 0.94. The differentiated fibroblasts were different from primary cells with a correlation value of 0.82. This suggests that our in vitro differentiation resulted in fibroblasts, but these fibroblasts differ from “authentic” lung fibroblasts. As expected, the iPSCs were quite distinct from both primary and differentiated fibroblasts with a correlation value of ∼0.33.
Fig. 4.
A and B: dendrogram and heatmap, respectively, of mRNA microarrays in non-COPD and COPD fibroblasts, reprogrammed iPSCs, and differentiated fibroblasts non-COPD-i and COPD-i. C and D: heatmap of differentially expressed genes related to COPD. (N = 3 in each group). See Table 2 for definition of acronyms.
To confirm that the reprogramming might restore abnormalities that are characteristic of COPD, we assessed the genes that were differentially expressed in parental cells that were no longer differentially expressed after reprogramming into iPSCs and redifferentiation. A total of 293 genes were differentially expressed between COPD and non-COPD that were no longer differentially expressed between COPD-i and non-COPD-i. Intriguingly, of 201 genes that were overexpressed in COPD compared with non-COPD parental fibroblasts and were reduced in COPD-i, 62 have been linked to lung diseases, and 23 have been related to COPD in the published literature (Fig. 4C, Table 2, top). Of the 92 genes that were underexpressed in COPD parental fibroblasts compared with non-COPD parental fibroblasts and were increased in COPD-i, 4 genes have been related to COPD (Fig. 4D, Table 2, bottom).
Table 2.
Levels of expression of genes related to COPD pathogenesis
| No. | GenBank Accession No. | Gene Symbol | Description | COPD/non-COPD | COPD/COPD-i | Literature Related to COPD |
|---|---|---|---|---|---|---|
| Genes expressed significantly higher in COPD compared with non-COPD and significantly reduced in COPD-i | ||||||
| 1 | NM_000014 | A2M | Homo sapiens alpha-2-macroglobulin (A2M), mRNA | 20.41 | 7.14 | http://www.ncbi.nlm.nih.gov/pubmed/22209925 |
| 2 | NM_002421 | MMP1 | Homo sapiens matrix metallopeptidase 1 (interstitial collagenase) (MMP1), transcript variant 1, mRNA | 12.99 | 4.76 | http://www.ncbi.nlm.nih.gov/pubmed/20075389 |
| 3 | NM_007332 | TRPA1 | Homo sapiens transient receptor potential cation channel, subfamily A, member 1 (TRPA1), mRNA | 12.05 | 100.00 | http://www.ncbi.nlm.nih.gov/pubmed/21848366 |
| 4 | NM_014737 | RASSF2 | Homo sapiens Ras association (RalGDS/AF-6) domain family member 2 (RASSF2), transcript variant 1, mRNA | 11.90 | 18.18 | http://www.ncbi.nlm.nih.gov/pubmed/20887155 |
| 5 | NM_000601 | HGF | Homo sapiens hepatocyte growth factor (hepapoietin A; scatter factor) (HGF), transcript variant 1, mRNA | 7.69 | 4.35 | http://www.ncbi.nlm.nih.gov/pubmed/19281086 |
| 6 | NM_000930 | PLAT | Homo sapiens plasminogen activator, tissue (PLAT), transcript variant 1, mRNA | 6.67 | 3.03 | http://www.ncbi.nlm.nih.gov/pubmed/20075389 |
| 7 | NM_000877 | IL1R1 | Homo sapiens interleukin 1 receptor, type I (IL1R1), mRNA | 6.25 | 4.76 | http://www.ncbi.nlm.nih.gov/pubmed/22163019 |
| 8 | NM_005592 | MUSK | Homo sapiens muscle, skeletal, receptor tyrosine kinase (MUSK), mRNA | 5.88 | 7.14 | http://www.ncbi.nlm.nih.gov/pubmed/22424883 |
| 9 | NM_000962 | PTGS1 | Homo sapiens prostaglandin-endoperoxide synthase 1 (prostaglandin G/H synthase and cyclooxygenase) (PTGS1), transcript variant 1, mRNA | 5.56 | 2.63 | http://www.ncbi.nlm.nih.gov/pubmed/21798652 |
| 10 | NM_006259 | PRKG2 | Homo sapiens protein kinase, cGMP-dependent, type II (PRKG2), mRNA | 4.76 | 14.08 | http://www.ncbi.nlm.nih.gov/pubmed/7592887 |
| 11 | NM_019554 | S100A4 | Homo sapiens S100 calcium binding protein A4 (S100A4), transcript variant 2, mRNA | 3.70 | 5.88 | http://www.ncbi.nlm.nih.gov/pubmed/21970519 |
| 12 | NM_000854 | GSTT2 | Homo sapiens glutathione S-transferase theta 2 (GSTT2), mRNA | 3.70 | 4.76 | http://www.ncbi.nlm.nih.gov/pubmed/21349909/ |
| 13 | NM_001565 | CXCL10 | Homo sapiens chemokine (C-X-C motif) ligand 10 (CXCL10), mRNA | 3.70 | 3.85 | http://www.ncbi.nlm.nih.gov/pubmed/22452977 |
| 14 | NM_001042544 | LTBP4 | Homo sapiens latent transforming growth factor beta binding protein 4 (LTBP4), transcript variant 1, mRNA | 3.57 | 2.86 | http://www.ncbi.nlm.nih.gov/pubmed/20106877 |
| 15 | NM_001276 | CHI3L1 | Homo sapiens chitinase 3-like 1 (cartilage glycoprotein-39) (CHI3L1), mRNA | 3.23 | 5.00 | http://www.ncbi.nlm.nih.gov/pubmed/22554524/ |
| 16 | NM_006682 | FGL2 | Homo sapiens fibrinogen-like 2 (FGL2), mRNA | 3.23 | 4.76 | http://www.ncbi.nlm.nih.gov/pubmed/20438701/ |
| 17 | NM_000064 | C3 | Homo sapiens complement component 3 (C3), mRNA | 3.23 | 3.57 | http://www.ncbi.nlm.nih.gov/pubmed/21846943 |
| 18 | NM_000710 | BDKRB1 | omo sapiens bradykinin receptor B1 (BDKRB1), mRNA | 2.94 | 2.78 | http://www.ncbi.nlm.nih.gov/pubmed/20451601 |
| 19 | NM_003243 | TGFBR3 | Homo sapiens transforming growth factor, beta receptor III (TGFBR3), mRNA | 2.63 | 4.35 | http://www.ncbi.nlm.nih.gov/pubmed/19131638 |
| 20 | NM_002658 | PLAU | Homo sapiens plasminogen activator, urokinase (PLAU), transcript variant 1, mRNA | 2.50 | 5.26 | http://www.ncbi.nlm.nih.gov/pubmed/17975202 |
| 21 | NM_001083 | PDE5A | Homo sapiens phosphodiesterase 5A, cGMP-specific (PDE5A), transcript variant 1, mRNA | 2.13 | 13.51 | http://www.ncbi.nlm.nih.gov/pubmed/16458289 |
| 22 | NM_003102 | SOD3 | Homo sapiens superoxide dismutase 3, extracellular (SOD3), mRNA | 2.13 | 2.56 | http://www.ncbi.nlm.nih.gov/pubmed/21621610 |
| 23 | NM_005923 | MAP3K5 | Homo sapiens mitogen-activated protein kinase kinase kinase 5 (MAP3K5), mRNA | 1.89 | 2.04 | http://www.ncbi.nlm.nih.gov/pubmed/18281606 |
| Genes expressed significantly higher in non-COPD compared with COPD and significantly elevated in COPD-i | ||||||
| 1 | NM_022475 | HHIP | Homo sapiens hedgehog interacting protein (HHIP), mRNA. | 0.34 | 0.25 | http://www.ncbi.nlm.nih.gov/pubmed/22080838 |
| 2 | NR_024168 | TLR4 | Homo sapiens toll-like receptor 4 (TLR4), transcript variant 3, transcribed RNA | 0.38 | 0.14 | http://www.ncbi.nlm.nih.gov/pubmed/22983353 |
| 3 | NM_000660 | TGFB1 | Homo sapiens transforming growth factor, beta 1 (TGFB1), mRNA | 0.69 | 0.67 | http://www.ncbi.nlm.nih.gov/pubmed/22937864 |
| 4 | NM_001135599 | TGFB2 | Homo sapiens transforming growth factor, beta 2 (TGFB2), transcript variant 1, mRNA | 0.72 | 0.21 | http://www.ncbi.nlm.nih.gov/pubmed/22452977 |
Top: levels of genes that were expressed significantly higher in COPD by at least twofold at P value <0.001 compared with non-COPD and significantly reduced in the differentiated cells after reprogramming (COPD-i) by at least twofold at P value <0.001. Bottom: levels of genes that were expressed significantly higher in non-COPD by at least twofold at P value <0.001 compared with COPD and significantly elevated in the COPD-i cells by at least twofold at P value <0.001.
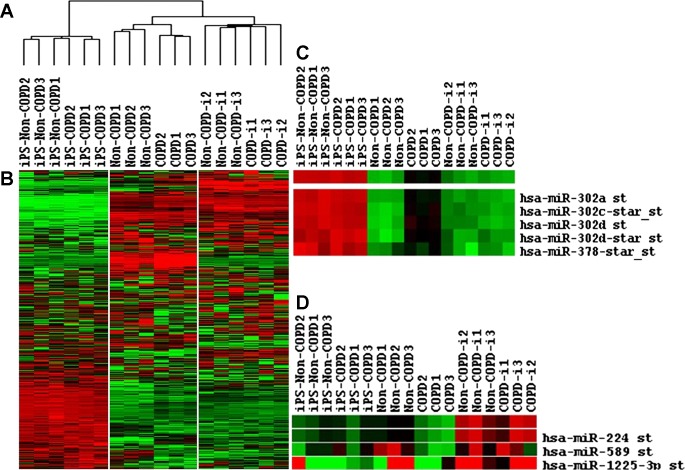
Expression of microRNA demonstrated similar relationships. Parental COPD and non-COPD fibroblasts clustered together, the iPSCs derived from both types of parental cells resembled each other, and the re-differentiated fibroblasts derived from the iPSCs were more similar than the parental cells (Fig. 5A). Figure 5B illustrates the heatmap for miRNA expression in all cells. Primary cells from both COPD and non-COPD patients were distinguishable with 56 differentially expressed miRNAs. However, only 4 miRNAs were underexpressed by 0.5-fold, and 25 miRNAs were overexpressed by 2-fold in primary fibroblasts derived from COPD patients compared with non-COPD patients. Reprogrammed iPSCs from both cells were similar in their miRNA expression, with only 13 miRNAs differentially expressed. After redifferentiation, the fibroblasts derived from both COPD and non-COPD primary fibroblasts were similar with only three miRNAs differentially expressed.
Fig. 5.
A and B: dendrogram and heatmap, respectively, of micro-RNA (miR) microarrays in non-COPD and COPD fibroblasts, reprogrammed iPSCs (iPS), and differentiated fibroblasts non-COPD-i and COPD-i. C and D: heatmap of differentially expressed micro-RNA in COPD compared with non-COPD and COPD-i. (N = 3 in each group).
Relationships between differentially expressed mRNA and miRNA.
To further assess the relationship between miRNA expression and their target in primary fibroblasts and the differentiated fibroblasts after reprogramming, we used the Target scan website to determine whether significantly underexpressed miRNAs in COPD fibroblasts have target genes that were significantly overexpressed compared with non-COPD and COPD-i fibroblasts, and vice versa. Table 3 presents 14 miRNAs that were significantly overexpressed and targeted three genes that were significantly underexpressed in COPD fibroblasts. In the same manner, three miRNAs were underexpressed and targeted six genes that were overexpressed in COPD fibroblasts (Table 3). Interestingly, after redifferentiation, six miRNAs were overlapped and were corrected toward non-COPD values by reprogramming (Table 4). These miRNAs and their targets are potential candidates for further studies to determine whether epigenetic factors could modulate differential gene expression in COPD by modulating microRNA expression.
Table 3.
Expression levels of miRNAs and their target genes in COPD compared with non-COPD fibroblasts
| miRNA ID | Fold Change COPD/non-COPD | Gene Symbol with Fold Change in Gene Expression COPD/non-COPD |
|---|---|---|
| hsa-miR-1208 | 3.01 | TRL4 0.38; TGFB2 0.72 |
| hsa-miR-1225-5p | 2.352 | TRL4 0.38 |
| hsa-miR-127-5p | 2.046 | TRL4 0.38 |
| hsa-miR-146a | 2.256 | TRL4 0.38 |
| hsa-miR-150 | 2.245 | TRL4 0.38 |
| hsa-miR-199b-5p | 2.522 | TGFB2 0.72 |
| hsa-miR-200c | 3.603 | TRL4 0.38 |
| hsa-miR-302a | 4.736 | TRL4 0.38 |
| hsa-miR-302c | 2.888 | TRL4 0.38 |
| hsa-miR-302d | 14.19 | TRL4 0.38 |
| hsa-miR-328 | 2.272 | TGFB2 0.72 |
| hsa-miR-885-3p | 2.48 | TRL4 0.38; HHIP 0.34 |
| hsa-miR-92b | 3.714 | HHIP 0.34; TGFB2 0.72 |
| hsa-miR-943 | 2.141 | TRL4 0.38; TGFB2 0.72 |
| hsa-miR-1184 | 0.474 | RASSF2 11.9; IL1R1 5.88; PTGS1 4.76; TGFBR3 2.63 |
| hsa-miR-224 | 0.464 | RASSF2 11.9; IL1R1 5.88; PTGS1 4.76; TGFBR3 2.63 |
| hsa-miR-27a-star | 0.51 | TRPA1 12.05; RASSF2 11.9; PTGS1 4.76; TGFBR3 2.63; PLAU 6.25; FGL2 3.23 |
miRNA, micro-RNA.
Table 4.
Expression levels of miRNAs that were differentially expressed in COPD compared with non-COPD and COPD-i
| Probeset ID | COPD/non-COPD | COPD/COPD-i |
|---|---|---|
| hsa-miR-127-5p_st | 2.05 | 3.14 |
| hsa-miR-200c_st | 3.6 | 1.98 |
| hsa-miR-302a_st | 4.74 | 5.87 |
| hsa-miR-302c_st | 2.89 | 2.32 |
| hsa-miR-302d_st | 14.19 | 13.17 |
| hsa-miR-224_st | 0.46 | 0.23 |
Functional and pathway analysis in IPA.
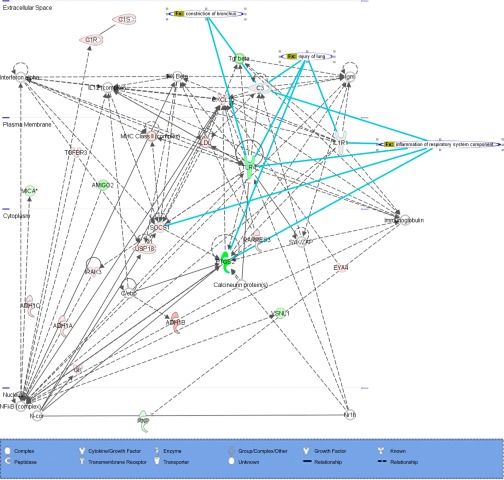
Genes that were significantly expressed (P < 0.001) in COPD with twofold change compared with non-COPD and COPD-i were further analyzed using IPA to determine the biological pathways in which genes that were differentially expressed were involved. The differentially expressed genes were involved in the pathways, “Cellular Movement, Cell to Cell Signaling, and Inflammatory Response.” Figure 6 represents the network for 293 genes that were significantly expressed in COPD primary fibroblasts by more or less than twofold compared with non-COPD and COPD-i.
Fig. 6.
Ingenuity Pathway Analysis of differentially expressed genes in COPD compared with non-COPD and COPD-i. Dashed line, indirect interaction with other molecules; solid line, direct interaction with other molecules; bold lines, correlation with different lung diseases. Red indicates expression of genes by more than 2-fold in COPD, and green indicates expression of genes by less than 0.5-fold in COPD.
IPA analysis suggested several gene networks that involve activation of the inflammatory pathways, such as NF-κB and interferon-α,β pathways in COPD fibroblasts. IL-1R1, prostaglandin-endoperoxide synthase 2 (PTGS2), complement component 3 (C3), and toll-like receptor 4 (TLR4) were mapped in correlation to the inflammation of the respiratory system component as well as lung injury. Also, TLR4, transforming growth factor-β, and C3 were mapped in correlation with construction of the bronchus.
DISCUSSION
Takahashi and Yamanaka's landmark study in 2006 (41) demonstrated that just four transcription factors (Oct3/4, Sox2, Klf4, and C-Myc) were sufficient to reprogram differentiated somatic cells into pluripotent stem cells. Fibroblasts from the lungs of patients with COPD are functionally different from fibroblasts from the lungs of non-COPD patients (31, 32, 42). Whether an acquired phenotypic alteration due to “programming” of fibroblasts in COPD patients plays a role, or whether the differences reflect an underlying set of genetic abnormalities, or if both mechanisms play roles remains unknown. We used the reprogramming method developed by Takahashi and Yamanaka to address this question.
To determine whether epigentic factors play a role in determining the alteration in COPD fibroblasts phenotype, we generated iPSCs from fibroblasts from the lungs of three separate COPD patients and three separate non-COPD patients of similar age and smoking history and characterized these in terms of pluripotency, both in vitro and in vivo. Several studies have successfully generated iPSCs from a variety of tissues, including lung (36), and from patients with different diseases (3, 8, 13, 24). The present study demonstrates the generation of iPSCs from lung fibroblasts from non-COPD and COPD patients. Interestingly, to generate authentic iPSCs, we needed to slightly modify the method described by Takahashi and Yamanaka, suggesting that lung fibroblast programming may differ from the more commonly used skin fibroblasts.
Several lines of evidence support our generation of iPSCs from lung fibroblasts. In concordance with previous studies, morphology, immunostaining, and expression of markers associated with pluripotency, including oct3/4, sox2, nanog, and alkaline phosphatase, were all consistent with the generation of iPSCs. Furthermore, under appropriate conditions, our lung-derived iPSCs readily formed EBs and differentiated in vitro into cells expressing markers of ectoderm, mesoderm, and endoderm. Moreover, when injected into immunodeficient mice, our iPSCs induced tumors containing differentiated derivatives of the three germ layers (4, 18, 23, 39, 40).
Several studies from our laboratory and from others have compared lung fibroblasts from COPD patients to fibroblasts from the lungs of non-COPD subjects. These studies demonstrated a variety of functional differences and differences in gene expression (14, 20, 27, 28, 42). These differences persist in culture, suggesting that they are not solely an acute response to the inflammatory milieu in the lung in COPD, but reflect a difference in differentiated phenotype. A major question is whether these differences reflect underlying genetic differences or represent an acquired alteration, or both.
Cellular differentiation frequently involves epigenetic alterations that modify gene expression and cellular function. Reprogramming of somatic cells to iPSCs largely erases this epigenetic programming. In the present study, the process of reprogramming of lung fibroblasts into iPSCs followed by redifferentiation into fibroblasts, largely eliminated the functional and gene expression differences that were present in COPD compared with non-COPD fibroblasts. This is consistent with epigenetic mechanisms being responsible for the functional differences in COPD fibroblasts. Importantly, reprogramming and redifferentiation did result in very similar fibroblasts morphologically at the different stages of reprogramming and redifferentiation, but they were not completely identical in terms of mRNA and miRNA expression. Whether this is due to incomplete reprogramming of epigenetic modifications or to underlying genetic differences remains to be determined.
The genes that were differentially expressed and were reprogrammed through the process of iPSC formation and redifferentiation are consistent with current concepts of COPD pathogenesis. Several genes in inflammatory pathways associated with COPD, including the NF-κB, the P38 MAPK, and the IFN-β pathways, were identified. The IPA analysis revealed particular genes, IL1R1, PTGS2, C3, and TLR4, that are connected to lung injury. The expression levels of these genes were in accordance with previous studies (1, 2, 6, 33). In addition, we found significant reduction of hedgehog interacting protein (HHIP) in COPD compared with non-COPD fibroblasts and to COPD-i fibroblasts. HHIP expression at both mRNA and protein levels is reduced in COPD lung tissues (10, 48). In addition, HHIP has been identified as a COPD-related gene in genome-wide association studies, and the single nucleotide polymorphisms involved appear to modify HHIP expression. While the role of HHIP in the development of COPD is undefined, our results suggest that both genetic and epigenetic mechanisms could contribute to altered HHIP expression.
Our approach to reprogramming was to induce iPSCs. It is possible that reprogramming strategies that are not as complete could also eliminate the epigenetic modifications that contribute to the functional differences between COPD and non-COPD fibroblasts. Defining the mechanisms required to reprogram and to eliminate these functional differences could have therapeutic implications. In this regard, deficient repair functions in lung fibroblasts have been suggested to contribute to the pathogenesis. If reprogramming of lung cells is possible and could restore lung repair, it may be possible to slow or reverse the progression of emphysema.
How fibroblasts are reprogrammed in COPD remains to be defined. However, cigarette smoke is reported to lead to a variety of epigenetic alterations (22, 46). In addition, aging is associated with epigenetic modification. Epigenetic modifications due to such pathways could contribute to the persistent inflammation and progressive disease that characterizes COPD, even after smoking cessation (19, 37, 44). The possibility that epigenetic modifications can be reprogrammed suggests a novel strategy to target this problem.
Our study has several limitations. First, we focused on lung fibroblasts. Other structural cells, including endothelial cells and airway and alveolar epithelial cells, are also altered in COPD. Our study did not address whether epigenetic alterations that are potentially reprogrammable are present in these cells as well, but our study suggests a strategy to address these questions. Second, primary cultures of lung cells are heterogeneous populations. The iPSCs are derived clonally from single cells. It is not possible, with the current technology, to ascertain the function of the individual cell from which each iPSC clone was derived. It is possible, therefore, that the iPSCs were derived from a cell that differs from the majority of the fibroblasts that contribute to the functional features present in COPD. Our results do demonstrate, however, that cells from COPD patients, if reprogrammed by forming iPSCs, can result in fibroblasts that more closely resemble fibroblasts from normal and support the concept that COPD patient fibroblasts express acquired alterations in function.
In conclusion, the present study demonstrates that human lung fibroblasts from COPD and non-COPD subjects could be reprogrammed to form iPSCs and then be redifferentiated into fibroblasts. Redifferentiated fibroblasts from COPD and non-COPD were morphologically and functionally very similar, with significantly fewer mRNAs and miRNA differences compared with their corresponding primary fibroblasts. The present study, therefore, suggests that epigenetic changes, which can be reprogrammed, contribute to the functional alterations present in fibroblasts derived from COPD patients.
GRANTS
This work was supported by a grant from Nebraska Department of Health and Human Services LB606 (36-5237-2203-001). We acknowledge The University of Nebraska Microarray Core, which received partial support from the National Center for Research Resources (5P20RR016469, RR018788-08) and the National Institute of General Medical Sciences (8P20-GM-103427, GM-103471-09).
DISCLAIMER
This publication's contents are the sole responsibility of the authors and do not necessarily represent the official views of the NIH or NIGMS.
DISCLOSURES
In the last three years, S. I. Rennard has consulted or been a member of an advisory board for Able Associates, Adelphi Research, Almirall/Prescott, APT Pharma/Britnall, Aradigm, AstraZeneca, Boehringer Ingelheim, Chiesi, CommonHealth, Consult Complete, COPDForum, DataMonitor, Decision Resources, Defined Health, Dey, Dunn Group, Eaton Associates, Equinox, Gerson, GlaxoSmithKline, Infomed, KOL Connection, M. Pankove, MedaCorp, MDRx Financial, Mpex, Novartis, Nycomed, Oriel Therapeutics, Otsuka, Pennside Partners, Pfizer (Varenicline), Pharma Ventures, Pharmaxis, Price Waterhouse, Propagate, Pulmatrix, Reckner Associates, Recruiting Resources, Roche, Schlesinger Medical, Scimed, Sudler and Hennessey, TargeGen, Theravance, UBC, Uptake Medical, and VantagePoint Mgmt. He has lectured for American Thoracic Society, AstraZeneca, Boehringer Ingelheim, California Allergy Society, Creative Educational Concept, France Foundation, Information TV, Network for Continuing Ed, Novartis, Pfizer, and SOMA. He has received industry-sponsored grants from AstraZeneca, Biomarck, Centocor, Mpex, Nabi, Novartis, and Otsuka.
AUTHOR CONTRIBUTIONS
Author contributions: H.B., Y.G., S.I., A.N., M.F., J. Ikari, X.L., X.W., J.M., R.E.B., W.W.W., O.H., K.-C.M., H.M., K.F.R., P.J.C., and S.I.R. conception and design of research; H.B., S.Y., and D.R. performed experiments; H.B., L.M.S., and J. Iqbal analyzed data; H.B., Y.G., S.I., M.F., J. Ikari, X.L., X.W., J.M., R.E.B., W.W.W., and S.I.R. interpreted results of experiments; H.B. prepared figures; H.B. and S.I.R. drafted manuscript; H.B. and S.I.R. edited and revised manuscript; H.B., Y.G., S.I., A.N., M.F., J. Ikari, X.L., X.W., J.M., L.M.S., J. Iqbal, R.E.B., W.W.W., S.Y., D.R., O.H., K.-C.M., H.M., K.F.R., P.J.C., and S.I.R. approved final version of manuscript.
REFERENCES
- 1.Al Mutairi SS, Mojiminiyi OA, Shihab-Eldeen A, Al Rammah T, Abdella N. Putative roles of circulating resistin in patients with asthma, COPD and cigarette smokers. Dis Markers 31: 1–7, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.An CH, Wang XM, Lam HC, Ifedigbo E, Washko GR, Ryter SW, Choi AM. TLR4 deficiency promotes autophagy during cigarette smoke-induced pulmonary emphysema. Am J Physiol Lung Cell Mol Physiol 303: L748–L757, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.An MC, Zhang N, Scott G, Montoro D, Wittkop T, Mooney S, Melov S, Ellerby LM. Genetic correction of Huntington's disease phenotypes in induced pluripotent stem cells. Cell Stem Cell 11: 253–263, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aoi T, Yae K, Nakagawa M, Ichisaka T, Okita K, Takahashi K, Chiba T, Yamanaka S. Generation of pluripotent stem cells from adult mouse liver and stomach cells. Science 321: 699–702, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Basma H, Soto-Gutiérrez A, Yannam GR, Liu L, Ito R, Yamamoto T, Ellis E, Carson SD, Sato S, Chen Y, Muirhead D, Navarro-Álvarez N, Wong RJ, Roy-Chowdhury J, Platt JL, Mercer DF, Miller JD, Strom SC, Kobayashi N, Fox IJ. Differentiation and transplantation of human embryonic stem cell-derived hepatocytes. Gastroenterology 136: 990–999, e994, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Botelho FM, Bauer CM, Finch D, Nikota JK, Zavitz CC, Kelly A, Lambert KN, Piper S, Foster ML, Goldring JJ, Wedzicha JA, Bassett J, Bramson J, Iwakura Y, Sleeman M, Kolbeck R, Coyle AJ, Humbles AA, Stampfli MR. IL-1alpha/IL-1R1 expression in chronic obstructive pulmonary disease and mechanistic relevance to smoke-induced neutrophilia in mice. PLos One 6: e28457, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boyden S. The chemotactic effect of mixtures of antibody and antigen on polymorphonuclear leucocytes. J Exp Med 115: 453–466, 1962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cayo MA, Cai J, Delaforest A, Noto FK, Nagaoka M, Clark BS, Collery RF, Si-Tayeb K, Duncan SA. JD induced pluripotent stem cell-derived hepatocytes faithfully recapitulate the pathophysiology of familial hypercholesterolemia. Hepatology 56: 2163–2171, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen YF, Tseng CY, Wang HW, Kuo HC, Yang VW, Lee OK. Rapid generation of mature hepatocyte-like cells from human induced pluripotent stem cells by an efficient three-step protocol. Hepatology 55: 1193–1203, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cho MH, Castaldi PJ, Wan ES, Siedlinski M, Hersh CP, Demeo DL, Himes BE, Sylvia JS, Klanderman BJ, Ziniti JP, Lange C, Litonjua AA, Sparrow D, Regan EA, Make BJ, Hokanson JE, Murray T, Hetmanski JB, Pillai SG, Kong X, Anderson WH, Tal-Singer R, Lomas DA, Coxson HO, Edwards LD, MacNee W, Vestbo J, Yates JC, Agusti A, Calverley PM, Celli B, Crim C, Rennard S, Wouters E, Bakke P, Gulsvik A, Crapo JD, Beaty TH, Silverman EK. A genome-wide association study of COPD identifies a susceptibility locus on chromosome 19q13. Hum Mol Genet 21: 947–957, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Claassen DA, Desler MM, Rizzino A. ROCK inhibition enhances the recovery and growth of cryopreserved human embryonic stem cells and human induced pluripotent stem cells. Mol Reprod Dev 76: 722–732, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delaloy C, Liu L, Lee JA, Su H, Shen F, Yang GY, Young WL, Ivey KN, Gao FB. MicroRNA-9 coordinates proliferation and migration of human embryonic stem cell-derived neural progenitors. Cell Stem Cell 6: 323–335, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ebert AD, Yu J, Rose FF, Mattis VB, Lorson CL, Thomson JA, Svendsen CN. Induced pluripotent stem cells from a spinal muscular atrophy patient. Nature 457: 277–280, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holz O, Zuhlke I, Jaksztat E, Muller KC, Welker L, Nakashima M, Diemel KD, Branscheid D, Magnussen H, Jorres RA. Lung fibroblasts from patients with emphysema show a reduced proliferation rate in culture. Eur Respir J 24: 575–579, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Ingenuity Systems Ingenuity Pathway Analysis, version 7.6 (Online). Ingenuity Systems. http://www.ingenuity.com [2012]. [Google Scholar]
- 16.Jang J, Yoo JE, Lee JA, Lee DR, Kim JY, Huh YJ, Kim DS, Park CY, Hwang DY, Kim HS, Kang HC, Kim DW. Disease-specific induced pluripotent stem cells: a platform for human disease modeling and drug discovery. Exp Mol Med 44: 202–213, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang Y, Zhang MJ, Hu BY. Specification of functional neurons and glia from human pluripotent stem cells. Protein Cell 3: 818–825, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaji K, Norrby K, Paca A, Mileikovsky M, Mohseni P, Woltjen K. Virus-free induction of pluripotency and subsequent excision of reprogramming factors. Nature 458: 771–775, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leslie FM. Multigenerational epigenetic effects of nicotine on lung function. BMC Med 11: 27, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lewis CC, Chu HW, Westcott JY, Tucker A, Langmack EL, Sutherland ER, Kraft M. Airway fibroblasts exhibit a synthetic phenotype in severe asthma. J Allergy Clin Immunol 115: 534–540, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Lister R, Pelizzola M, Dowen RH, Hawkins RD, Hon G, Tonti-Filippini J, Nery JR, Lee L, Ye Z, Ngo QM, Edsall L, Antosiewicz-Bourget J, Stewart R, Ruotti V, Millar AH, Thomson JA, Ren B, Ecker JR. Human DNA methylomes at base resolution show widespread epigenomic differences. Nature 462: 315–322, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu F, Killian JK, Yang M, Walker RL, Hong JA, Zhang M, Davis S, Zhang Y, Hussain M, Xi S, Rao M, Meltzer PA, Schrump DS. Epigenomic alterations and gene expression profiles in respiratory epithelia exposed to cigarette smoke condensate. Oncogene 29: 3650–3664, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lowry WE, Richter L, Yachechko R, Pyle AD, Tchieu J, Sridharan R, Clark AT, Plath K. Generation of human induced pluripotent stem cells from dermal fibroblasts. Proc Natl Acad Sci U S A 105: 2883–2888, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma D, Wei H, Lu J, Ho S, Zhang G, Sun X, Oh Y, Tan SH, Ng ML, Shim W, Wong P, Liew R. Generation of patient-specific induced pluripotent stem cell-derived cardiomyocytes as a cellular model of arrhythmogenic right ventricular cardiomyopathy. Eur Heart J 34: 1122–1133, 2013 [DOI] [PubMed] [Google Scholar]
- 25.Michiel JLdH, Seiya I, Satoru M. Inferring gene regulatory networks from time-ordered gene expression data using differential equations. In: Proceedings of the 5th International Conference on Discovery Science New York: Springer-Verlag, 2002 [Google Scholar]
- 26.Mio T, Adachi Y, Romberger DJ, Ertl RF, Rennard SI. Regulation of fibroblast proliferation in three-dimensional collagen gel matrix. In Vitro Cell Dev Biol Anim 32: 427–433, 1996 [DOI] [PubMed] [Google Scholar]
- 27.Muller KC, Welker L, Paasch K, Feindt B, Erpenbeck VJ, Hohlfeld JM, Krug N, Nakashima M, Branscheid D, Magnussen H, Jorres RA, Holz O. Lung fibroblasts from patients with emphysema show markers of senescence in vitro. Respir Res 7: 32, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Plantier L, Marchand-Adam S, Marchal-Somme J, Leseche G, Fournier M, Dehoux M, Aubier M, Crestani B. Defect of hepatocyte growth factor production by fibroblasts in human pulmonary emphysema. Am J Physiol Lung Cell Mol Physiol 288: L641–L647, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Powell DW, Mifflin RC, Valentich JD, Crowe SE, Saada JI, West AB. Myofibroblasts. II. Intestinal subepithelial myofibroblasts. Am J Physiol Cell Physiol 277: C183–C201, 1999 [DOI] [PubMed] [Google Scholar]
- 30.R Foundation for Statistical Computing Language and environment for statistical computing. In: The R Project for Statistical Computing. Vienna: R Foundation for Statistical Computing, 2008 [Google Scholar]
- 31.Rennard SI. Inflammation and repair processes in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 160: S12–S16, 1999 [DOI] [PubMed] [Google Scholar]
- 32.Rennard SI. Inflammation in COPD: a link to systemic comorbidities. Eur Respir J 16: 91–97, 2007 [Google Scholar]
- 33.Roca-Ferrer J, Pujols L, Agusti C, Xaubet A, Mullol J, Gimferrer JM, Picado C. [Cyclooxigenase-2 levels are increased in the lung tissue and bronchial tumors of patients with chronic obstructive pulmonary disease]. Arch Bronconeumol 47: 584–589, 2011 [DOI] [PubMed] [Google Scholar]
- 34.Simon R, Lam A, Li MC, Ngan M, Menenzes S, Zhao Y. Analysis of gene expression data using BRB-ArrayTools. Cancer Inform 3: 11–17, 2007 [PMC free article] [PubMed] [Google Scholar]
- 35.Siniscalco D, Sullo N, Maione S, Rossi F, D'Agostino B. Stem cell therapy: the great promise in lung disease. Ther Adv Respir Dis 2: 173–177, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Somers A, Jean JC, Sommer CA, Omari A, Ford CC, Mills JA, Ying L, Sommer AG, Jean JM, Smith BW, Lafyatis R, Demierre MF, Weiss DJ, French DL, Gadue P, Murphy GJ, Mostoslavsky G, Kotton DN. Generation of transgene-free lung disease-specific human induced pluripotent stem cells using a single excisable lentiviral stem cell cassette. Stem Cells 28: 1728–1740, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Soria JC, Rodriguez M, Liu DD, Lee JJ, Hong WK, Mao L. Aberrant promoter methylation of multiple genes in bronchial brush samples from former cigarette smokers. Cancer Res 62: 351–355, 2002 [PubMed] [Google Scholar]
- 38.Sugiura H, Liu X, Ichikawa T, Ichinose M, Rennard SI. 3-Nitrotyrosine inhibits fibroblast-mediated collagen gel contraction and chemotaxis. Eur Respir J 34: 1452–1460, 2009 [DOI] [PubMed] [Google Scholar]
- 39.Sun YQ, Deng MX, He J, Zeng QX, Wen W, Wong DS, Tse HF, Xu G, Lian Q, Shi J, Fu QL. Human pluripotent stem cell-derived mesenchymal stem cells prevent allergic airway inflammation in mice. Stem Cells 30: 2692–2699, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131: 861–872, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126: 663–676, 2006 [DOI] [PubMed] [Google Scholar]
- 42.Togo S, Holz O, Liu X, Sugiura H, Kamio K, Wang X, Kawasaki S, Ahn Y, Fredriksson K, Skold CM, Mueller KC, Branscheid D, Welker L, Watz H, Magnussen H, Rennard SI. Lung fibroblast repair functions in patients with chronic obstructive pulmonary disease are altered by multiple mechanisms. Am J Respir Crit Care Med 178: 248–260, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Togo S, Sato T, Sugiura H, Wang X, Basma H, Nelson A, Liu X, Bargar TW, Sharp JG, Rennard SI. Differentiation of embryonic stem cells into fibroblast-like cells in three-dimensional type I collagen gel cultures. In Vitro Cell Dev Biol Anim 47: 114–124, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tzortzaki EG, Papi A, Neofytou E, Soulitzis N, Siafakas NM. Immune and genetic mechanisms in COPD: possible targets for therapeutic interventions. Curr Drug Targets 14: 141–148, 2013 [DOI] [PubMed] [Google Scholar]
- 45.Wright GW, Simon RM. A random variance model for detection of differential gene expression in small microarray experiments. Bioinformatics 19: 2448–2455, 2003 [DOI] [PubMed] [Google Scholar]
- 46.Xi S, Xu H, Shan J, Tao Y, Hong JA, Inchauste S, Zhang M, Kunst TF, Mercedes L, Schrump DS. Cigarette smoke mediates epigenetic repression of miR-487b during pulmonary carcinogenesis. J Clin Invest 123: 1241–1261, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science 318: 1917–1920, 2007 [DOI] [PubMed] [Google Scholar]
- 48.Zhou X, Baron RM, Hardin M, Cho MH, Zielinski J, Hawrylkiewicz I, Sliwinski P, Hersh CP, Mancini JD, Lu K, Thibault D, Donahue AL, Klanderman BJ, Rosner B, Raby BA, Lu Q, Geldart AM, Layne MD, Perrella MA, Weiss ST, Choi AM, Silverman EK. Identification of a chronic obstructive pulmonary disease genetic determinant that regulates HHIP. Hum Mol Genet 21: 1325–1335, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]