Abstract
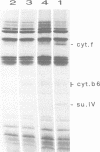
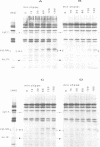
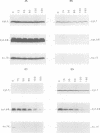
As an approach to the study of the biogenesis of the cytochrome b6/f complex, we characterized the behaviour of its constitutive subunits in mutant strains of Chlamydomonas reinhardtii bearing well-defined mutations. To this end, we have constructed three deletion mutant strains, each lacking one of the major chloroplast pet genes: the delta petA, delta petB and delta petD strains were unable to synthesize cyt f, cyt b6 and subunit IV (suIV) respectively. Western blotting analysis, pulse-labelling and pulse-chase experiments allowed us to compare the cellular accumulation, the rates of synthesis and the turnover of the cyt b6/f subunits remaining in the various strains. We show that the rates of synthesis of cyt b6 and suIV are independent of the presence of the other subunits of the complex but that their stabilization in the thylakoid membranes is a concerted process, with a marked dependence of suIV stability on the presence of cyt b6. In contrast, mature cyt f was stable in the absence of either suIV or cyt b6 but its rate of synthesis was severely decreased in these conditions. We conclude that the stoichiometric accumulation of the chloroplast-encoded subunits of the cyt b6/f complex results from two regulation processes: a post-translational regulation leading to the proteolytic disposal of unassembled cyt b6 and suIV and a co-translational (or early post-translational) regulation which ensures the production of cyt f next to its site of assembly.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blount P., Merlie J. P. Mutational analysis of muscle nicotinic acetylcholine receptor subunit assembly. J Cell Biol. 1990 Dec;111(6 Pt 1):2613–2622. doi: 10.1083/jcb.111.6.2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulay F., Doms R. W., Webster R. G., Helenius A. Posttranslational oligomerization and cooperative acid activation of mixed influenza hemagglutinin trimers. J Cell Biol. 1988 Mar;106(3):629–639. doi: 10.1083/jcb.106.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boynton J. E., Gillham N. W., Harris E. H., Hosler J. P., Johnson A. M., Jones A. R., Randolph-Anderson B. L., Robertson D., Klein T. M., Shark K. B. Chloroplast transformation in Chlamydomonas with high velocity microprojectiles. Science. 1988 Jun 10;240(4858):1534–1538. doi: 10.1126/science.2897716. [DOI] [PubMed] [Google Scholar]
- Bruce B. D., Malkin R. Biosynthesis of the chloroplast cytochrome b6f complex: studies in a photosynthetic mutant of Lemna. Plant Cell. 1991 Feb;3(2):203–212. doi: 10.1105/tpc.3.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulté L., Wollman F. A. Evidence for a selective destabilization of an integral membrane protein, the cytochrome b6/f complex, during gametogenesis in Chlamydomonas reinhardtii. Eur J Biochem. 1992 Feb 15;204(1):327–336. doi: 10.1111/j.1432-1033.1992.tb16641.x. [DOI] [PubMed] [Google Scholar]
- Büschlen S., Choquet Y., Kuras R., Wollman F. A. Nucleotide sequences of the continuous and separated petA, petB and petD chloroplast genes in Chlamydomonas reinhardtii. FEBS Lett. 1991 Jun 24;284(2):257–262. doi: 10.1016/0014-5793(91)80698-3. [DOI] [PubMed] [Google Scholar]
- Chen X., Kindle K., Stern D. Initiation codon mutations in the Chlamydomonas chloroplast petD gene result in temperature-sensitive photosynthetic growth. EMBO J. 1993 Sep;12(9):3627–3635. doi: 10.1002/j.1460-2075.1993.tb06036.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua N. H., Bennoun P. Thylakoid membrane polypeptides of Chlamydomonas reinhardtii: wild-type and mutant strains deficient in photosystem II reaction center. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2175–2179. doi: 10.1073/pnas.72.6.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper C. E., Nicholls P., Freedman J. A. Cytochrome c oxidase: structure, function, and membrane topology of the polypeptide subunits. Biochem Cell Biol. 1991 Sep;69(9):586–607. doi: 10.1139/o91-089. [DOI] [PubMed] [Google Scholar]
- Crivellone M. D., Wu M. A., Tzagoloff A. Assembly of the mitochondrial membrane system. Analysis of structural mutants of the yeast coenzyme QH2-cytochrome c reductase complex. J Biol Chem. 1988 Oct 5;263(28):14323–14333. [PubMed] [Google Scholar]
- Crofts A., Hacker B., Barquera B., Yun C. H., Gennis R. Structure and function of the bc-complex of Rhodobacter sphaeroides. Biochim Biophys Acta. 1992 Jul 17;1101(2):162–165. [PubMed] [Google Scholar]
- Davidson E., Ohnishi T., Tokito M., Daldal F. Rhodobacter capsulatus mutants lacking the Rieske FeS protein form a stable cytochrome bc1 subcomplex with an intact quinone reduction site. Biochemistry. 1992 Apr 7;31(13):3351–3358. doi: 10.1021/bi00128a007. [DOI] [PubMed] [Google Scholar]
- Dowhan W., Bibus C. R., Schatz G. The cytoplasmically-made subunit IV is necessary for assembly of cytochrome c oxidase in yeast. EMBO J. 1985 Jan;4(1):179–184. doi: 10.1002/j.1460-2075.1985.tb02334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drapier D., Girard-Bascou J., Wollman F. A. Evidence for Nuclear Control of the Expression of the atpA and atpB Chloroplast Genes in Chlamydomonas. Plant Cell. 1992 Mar;4(3):283–295. doi: 10.1105/tpc.4.3.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson J. M., Rahire M., Malnoë P., Girard-Bascou J., Pierre Y., Bennoun P., Rochaix J. D. Lack of the D2 protein in a Chlamydomonas reinhardtii psbD mutant affects photosystem II stability and D1 expression. EMBO J. 1986 Aug;5(8):1745–1754. doi: 10.1002/j.1460-2075.1986.tb04422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gething M. J., McCammon K., Sambrook J. Expression of wild-type and mutant forms of influenza hemagglutinin: the role of folding in intracellular transport. Cell. 1986 Sep 12;46(6):939–950. doi: 10.1016/0092-8674(86)90076-0. [DOI] [PubMed] [Google Scholar]
- Girard-Bascou J., Choquet Y., Schneider M., Delosme M., Dron M. Characterization of a chloroplast mutation in the psaA2 gene of Chlamydomonas reinhardtii. Curr Genet. 1987;12(7):489–495. doi: 10.1007/BF00419557. [DOI] [PubMed] [Google Scholar]
- Goldschmidt-Clermont M. Transgenic expression of aminoglycoside adenine transferase in the chloroplast: a selectable marker of site-directed transformation of chlamydomonas. Nucleic Acids Res. 1991 Aug 11;19(15):4083–4089. doi: 10.1093/nar/19.15.4083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurtley S. M., Bole D. G., Hoover-Litty H., Helenius A., Copeland C. S. Interactions of misfolded influenza virus hemagglutinin with binding protein (BiP). J Cell Biol. 1989 Jun;108(6):2117–2126. doi: 10.1083/jcb.108.6.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchka M. R., Mayfield S. P., Rochaix J. D. Nuclear mutations specifically affect the synthesis and/or degradation of the chloroplast-encoded D2 polypeptide of photosystem II in Chlamydomonas reinhardtii. EMBO J. 1988 Feb;7(2):319–324. doi: 10.1002/j.1460-2075.1988.tb02815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Lemaire C., Wollman F. A. The chloroplast ATP synthase in Chlamydomonas reinhardtii. II. Biochemical studies on its biogenesis using mutants defective in photophosphorylation. J Biol Chem. 1989 Jun 15;264(17):10235–10242. [PubMed] [Google Scholar]
- Michaelis U., Körte A., Rödel G. Association of cytochrome b translational activator proteins with the mitochondrial membrane: implications for cytochrome b expression in yeast. Mol Gen Genet. 1991 Nov;230(1-2):177–185. doi: 10.1007/BF00290666. [DOI] [PubMed] [Google Scholar]
- Roffey R. A., Golbeck J. H., Hille C. R., Sayre R. T. Photosynthetic electron transport in genetically altered photosystem II reaction centers of chloroplasts. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):9122–9126. doi: 10.1073/pnas.88.20.9122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuman H. A. The genetics of active transport in bacteria. Annu Rev Genet. 1987;21:155–177. doi: 10.1146/annurev.ge.21.120187.001103. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vitry C., Olive J., Drapier D., Recouvreur M., Wollman F. A. Posttranslational events leading to the assembly of photosystem II protein complex: a study using photosynthesis mutants from Chlamydomonas reinhardtii. J Cell Biol. 1989 Sep;109(3):991–1006. doi: 10.1083/jcb.109.3.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- di Rago J. P., Netter P., Slonimski P. P. Intragenic suppressors reveal long distance interactions between inactivating and reactivating amino acid replacements generating three-dimensional constraints in the structure of mitochondrial cytochrome b. J Biol Chem. 1990 Sep 15;265(26):15750–15757. [PubMed] [Google Scholar]