Abstract
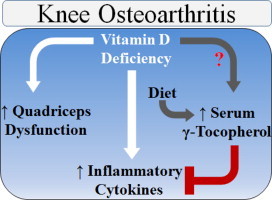
Knee osteoarthritis (OA) is a degenerative joint condition and a leading cause of physical disability in the United States. Quadriceps weakness and inflammatory cytokines contribute to the pathogenesis of knee OA, and both of which, increase with vitamin D deficiency. Other micronutrients, such as vitamins C and E and β-carotene, modulate inflammatory cytokines and decrease during inflammation. The purpose of this study was to test the hypothesis that vitamin D deficiency associates with quadriceps weakness, an increase in serum cytokines, and a decrease in circulating micronutrients in subjects with knee OA. Subjects (age, 48±1 y; serum 25(OH)D, 25.8±1.1 ng/mL) with knee OA were categorized as vitamin D deficient (n=17; serum 25(OH)D≤20 ng/mL), insufficient (n=21; serum 25(OH)D 20–29 ng/mL), or sufficient (n=18; serum 25(OH)D≥30 ng/mL). Single-leg strength (concentric knee extension–flexion contraction cycles at 60 °/s) and blood cytokine, carotene (α and β), ascorbic acid, and tocopherol (α and γ) concentrations were measured. Quadriceps peak torque, average power, total work, and deceleration were significantly (all p<0.05) impaired with vitamin D deficiency. Serum γ-tocopherol concentrations were significantly (p<0.05) increased with vitamin D deficiency. In the vitamin D sufficient group, γ-tocopherol inversely correlated (r=−0.47, p<0.05) with TNF-α, suggesting a pro-inflammatory increase with a γ-tocopherol decrease despite a sufficient serum 25(OH)D concentration. We conclude that vitamin D deficiency is detrimental to quadriceps function, and in subjects with vitamin D sufficiency, γ-tocopherol could have an important anti-inflammatory role in a pathophysiological condition mediated by inflammation.
Keywords: Vitamin D, Cytokines, Muscle strength, Vitamin E
Graphical abstract
Highlights
-
•
We investigated the vitamin D association with mediators of knee osteoarthritis.
-
•
Vitamin D deficiency associated with quadriceps dysfunction.
-
•
Vitamin D deficiency was not associated with serum cytokines.
-
•
Vitamin D deficiency associated with increased plasma γ-tocopherol concentrations.
-
•
γ-Tocopherol inversely correlated with TNF-α in vitamin D sufficient subjects.
Introduction
Knee osteoarthritis (OA) is a degenerative joint condition and a leading cause of physical disability in the United States, especially with advancing age. With the aging population growing, there is an increasing demand to improve the quality life and daily function in people suffering with knee OA. A compelling body of literature is revealing an increase in cartilage loss and knee OA with vitamin D deficiency (i.e., serum 25-hydroxyvitamin D (25(OH)D)<20 ng/mL) [1], [2]. This is an area of uncertainty, however, as conflicting reports suggest that vitamin D deficiency does not associate with knee OA [3], [4], [5], [6], [7]. Despite this ambiguity, vitamin D is an attractive therapeutic alternative because it modulates the mechanical (i.e., quadriceps weakness) [8] and biochemical (i.e., inflammatory cytokines) [9] factors believed to contribute to the pathogenesis of knee OA.
Quadricep weakness is an early symptom of OA that precedes and mediates cartilage loss by minimizing the shock absorption across the knee joint [10], [11]. In vitamin D deficient elderly, physical function is impaired and quadriceps weakness is apparent [12], [13], [14], [15], [16], [17]. The culprit(s) contributing to these impairments are unknown in humans, but evidence suggests that decrements in phosphate metabolism, calcium handling and transport, or cytoskeletal protein expression could be the underlying mechanisms mediating muscular weakness with low vitamin D [18], [19]. The influence of vitamin D on skeletal muscle function is illustrated further by reports demonstrating that supplemental vitamin D (i.e., with 25(OH)D or ergocalciferol) attenuates the age-related decrease or improves lower extremity function and muscle strength in elderly [20], [21]. In contrast, the impact of vitamin D on physical function or muscle strength in elderly is questioned as conflicting reports exist [22], [23]. Consistent with data suggesting that vitamin D is not influential on physical function in elderly, results in subjects with knee OA imply that an increase in serum 25(OH)D does not attenuate the decline in physical function [5], [24]. However, it is unknown if vitamin D deficiency associates with quadriceps weakness in subjects with knee OA.
Although caution is recommended in its interpretation as an exclusive indicator of knee OA [25], an increase in serum C-reactive protein (CRP) is apparent in subjects with knee OA [26]. Additionally, serum CRP and inflammatory cytokines (e.g., interleukin (IL)-6) predict cartilage loss [27], [28] and knee OA [29]. These systemic findings reflect the local production of pro-inflammatory cytokines, such as tumor necrosis factor (TNF)-α and IL-1β, that induce cartilage catabolism and knee OA [30], [31]. In isolated immune cells, vitamin D (1,25-dihydroxyvitamin D or increase in serum 25(OH)D) decreases pro-inflammatory cytokine production [32], [33]. The cytokine-modulating property of vitamin D is related to its interaction with the cell surface or nuclear vitamin D receptors (VDR). The nuclear VDR heterodimerizes with the retinoic X receptor (RXR) upon vitamin D (1,25-dihyroxyvitamin D) binding, forming a complex that attaches to a vitamin D response element that regulates cytokine gene expression see [34] for review. In addition to the VDR–RXR complex, vitamin D regulates cytokine expression by modulating the expression and activity of nuclear factor-κB (NF-κB) and nuclear factor of activated T cells see [34] for review. Upon binding to the cell surface receptor, vitamin D induces rapid and non-genomic cytokine responses through mitogen-activated protein kinase, protein kinase C, cGMP, and phosphoinositide metabolism signaling pathways, [35]. Consistent with its cytokine-modulating influence in isolated cell studies, low serum 25(OH)D concentrations associate with an increase in pro-inflammatory cytokines across a range of physiological and pathophysiological conditions in humans [36], [37], [38], [39]. There is, however, paucity in the data regarding the influence of vitamin D deficiency on inflammatory cytokines is subjects with knee OA.
It is also unknown if vitamin D deficiency is accompanied by a decrease in other micronutrients that modulate inflammatory cytokines. Vitamins C and E (α- or γ-tocopherol alone or in combination) are potent dietary antioxidants that decrease cytokine (such as IL-6 and TNF-α) levels in the blood [40] and inhibit cytokine production from isolated immune cells [41], [42], [43], [44], [45]. Conversely, β-carotene increases circulating TNF-α concentrations [46] and production from monocytes [47], [48]. In addition to their cytokine modulating property, an emerging hypothesis is that inflammation confounds the interpretation of circulating micronutrient levels [49], [50]. Support of this hypothesis is provided by data suggesting vitamin E and β-carotene decrease in the circulation after a hip fracture and during inflammation [51]. Reports from our lab [52], [53], [54] and others [55], [56] also suggest a circulating decrease in vitamins C (ascorbic acid) and D (25(OH)D) during the acute inflammatory phase following minor and major orthopedic surgery in humans.
Analyzing quadricep strength and inflammatory cytokines in subjects with knee OA and with contrasting serum 25(OH)D concentrations could identify the role of vitamin D status on mechanical and biochemical mediators of this degenerative joint condition. Inflammation contributes to this degenerative joint, and if inflammation decreases micronutrients in the blood, it is foreseeable that vitamins C and E and carotenoids decrease with vitamin D deficiency. Thus, the purpose of this study was to identify quadricep strength, inflammatory cytokines, and micronutrients in subjects with knee OA and with contrasting serum 25(OH)D concentrations. We hypothesized that vitamin D deficiency associates with quadriceps weakness, an increase in serum cytokine, and a decrease in circulating micronutrient in subjects with knee OA.
Material and methods
To address the proposed hypothesis, potential subjects who were modestly active (i.e., minimum level of 30 min of continuous activity at least 3 times per week) were recruited from the practice of orthopedic surgeons (RHT, GLR, and NGM) at The Orthopedic Specialty Hospital (Murray, UT USA). Subjects were initially screened for unilateral knee symptoms of knee OA, such as self-reported knee pain and muscular (i.e., quadriceps or hamstring) weakness. During screening, subjects were excluded from participation if: they were taking a daily dietary supplement during the previous year, currently using anti-inflammatory medications or other prescribed or recommended medications, suffered a lower leg injury during the previous year that required the use of crutches, tobacco use, or under physician guided treatment for known disease. We limited our study population to 60 years of age or younger as the association between serum 25(OH)D concentrations and knee OA is apparent in this demographic [2]. Subjects were informed of and provided written and verbal consent to the experimental protocol and procedures. The Urban Central Region Institutional Review Board at Intermountain Healthcare (Salt Lake City, UT USA) approved this study. Data was collected between December 2011 and October 2012 in Murray, UT (latitude 40 °N).
Study protocol
Following screening and enrollment, each subject reported to the Physiology Research Laboratory at The Orthopedic Specialty Hospital. At this visit, we obtained fasting blood samples and administered the pain (0–20; 0, no pain; 20, extreme pain) and physical function (0–68; 0, no physical impairment; 68, extreme physical impairment) subsections of the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) questionnaire [57]. We also completed single-leg strength testing and X-ray imaging. Subjects were excluded from analysis if: (1) the WOMAC pain score was<2 on any of the five questions in its subsection, (2) there was no muscular weakness (i.e., deficit in peak isokinetic knee-extension or flexion strength) in the symptomatic leg, or (3) a Kellgren‐Lawrence grade<2 was scored in the symptomatic knee (Fig. 1). Subjects were instructed to refrain from using non-steroidal anti-inflammatory drugs (NSAIDs), including aspirin, ibuprofen, and naproxen sodium, at least 3 days prior to data collection.
Fig. 1.
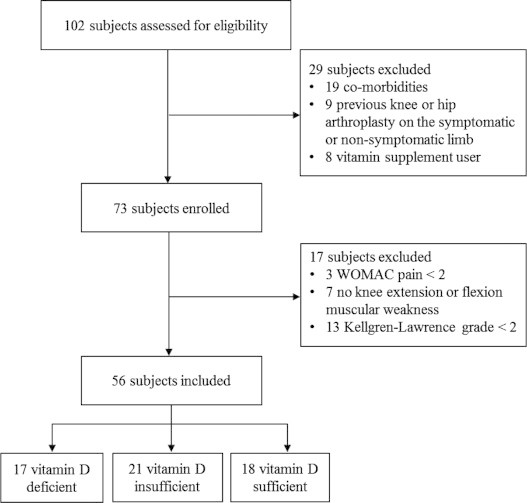
Eligibility screening, study enrollment, and subject inclusion flow chart.
Single-leg strength testing
Peak isokinetic strength was performed unilaterally on a Biodex S4 (Shirley, NY USA). Testing was performed on the leg with reportedly no symptoms of OA first. The rationale for performing the non-symptomatic knee first was to allow subjects to experience the testing protocol on a healthy knee prior to testing the symptomatic knee. Several practice repetitions were performed on each leg prior to testing for warm-up and to become familiar with the testing protocol and procedure. Once comfortable with the testing protocol and procedure, each subject performed 6 concentric knee extension–flexion contractions at 60° per second through a full range of motion (90° of knee flexion to full extension). Subjects were instructed and verbally encouraged to perform each contraction with maximal effort during the isokinetic testing.
Radiography
X-ray images were taken of each knee in the anterior-posterior view at 45° of knee flexion. Radiographs of the knee joint space narrowing (mm) between the symptomatic and non-symptomatic knee were viewed and analyzed using ImageJ software from the National Institutes of Health. Severity of knee OA was classified according to the scoring criteria established by Kellgren and Lawrence [58]. The Kellgren–Lawrence [58] grading was as the following: 0, no osteophytes or joint-space narrowing; 1, questionable osteophyte indicating possible OA; 2, definite osteophyte, no joint-narrowing indicating mild OA; 3, ≤50% joint-space narrowing indicating moderate OA; and 4, >50% joint-space narrowing indicating severe OA.
Analytical blood chemistry procedures
Circulating vitamins
Serum 25(OH)D concentrations (ng/mL) were determined using a chemiluminescent immunoassay (ARUP Laboratories, Salt Lake City, UT USA). Patients were classified as vitamin D deficient, insufficient, or sufficient if they had a serum 25(OH)D concentration ≤20, between 21 and 29, or ≥30 ng/mL, respectively. Serum 25(OH)D concentrations were not known during data collection.
The heparinized plasma samples mixed with oxalic acid were analyzed for ascorbic acid concentrations (mg/dL) using spectrophotometry (ARUP Laboratories). Serum tocopherol (α and γ) concentrations (mg/L) were analyzed with high performance liquid chromatography (HPLC; ARUP Laboratories). Serum α- and β-carotenes, lutein, and zeaxanthin concentrations (µg/L) were quantitated using HPLC (ARUP Laboratories).
Serum cytokine and CRP concentrations
The multiplex technology of Luminex (MAGPix; Austin, TX USA) was used to analyze inflammatory cytokine concentrations (pg/mL) with high-sensitivity. Serum high-sensitivity CRP concentrations (mg/L) were quantitated using immunoturbidimetry assay (ARUP Laboratories).
Clinical chemistries
Serum rheumatoid factor concentrations (IU/mL) were quantitated using an immunoturbidimetry assay and uric acid concentrations (mg/dL) were determined using a quantitative spectrophotometry assay (ARUP Laboratories). Serum parathyroid hormone intact (iPTH; pg/mL) with calcium (mg/dL) concentrations were measured using an electrochemiluminescent immunoassay (ARUP Laboratories).
Power calculation and statistical analysis
No previous study has investigated the role of vitamin D status on quadricep strength in subjects with knee OA. Based on pilot data from our lab, the power analysis indicated that in order to detect a 6% difference in peak torque between vitamin D status groups with a statistical power of 80% at the two-sided α=0.05 level, at least 17 subjects were needed per group. Considering the stringent inclusion criteria of radiographic evidence, pain, and muscular weakness, and since the aforementioned symptoms can occur independently from each other [59], [60], [61], [62], [63], [64], [65], we estimated screening 102 subjects to achieve the targeted accrual in each group (n=17/group).
Data were checked for normality with a Shapiro–Wilk test prior to statistical analysis. In order to achieve normality, rank (GM-CSF, IFN-γ, IL-5, IL-6, IL-7, IL-8, TNF-α, CRP, α-carotene, β-carotene, lutein, and zeaxanthin) or log (IL-12, IL-13, IL-1β, IL-2, and IL-4) transformations were performed prior to statistical analyses. Statistical significance of subject characteristics (age, height, body mass, BMI, serum 25(OH)D, rheumatoid factor, iPTH, and calcium) were assessed with a one-analysis of variance (ANOVA) followed by a Tukey׳s post-hoc test when appropriate. Statistical significance of the cytokine, micronutrient, and WOMAC data between vitamin D status groups were assessed with a one-way (vitamin D status) analysis of covariance (ANCOVA) followed by a Bonferroni post-hoc on multiple pairwise comparisons when appropriate. Statistical significance of leg strength data was assessed with a two-way (vitamin D status and leg) ANCOVA followed by a Bonferroni post-hoc test on multiple pairwise comparisons when appropriate. Due to the sparse fitting (i.e., frequency <5) in some of the cells, the association between vitamin D status and the Kellgren–Lawrence grade were assessed with a Goodman–Kruskal׳s gamma test. Relationships between variables were examined with a Pearson Product Moment Linear correlation. Gender, age, Kellgren–Lawrence grade, and body mass index (BMI) served as a statistical covariates. Body mass was not included as a covariate due to multicollinearity with BMI. Significance was set at p<0.05. All statistical analyses were performed with SYSTAT (version 13.1, Chicago, IL USA). Data presented as mean±SEM unless otherwise noted.
Results
Subject characteristics
Following eligibility screening, 73 subjects were enrolled and assessed for study inclusion (Fig. 1). Of the 73 enrolled, 56 subjects (age, 48±1 y; height, 168±1 cm; body mass, 94.1±2.8 kg; BMI, 33.0±0.9 kg/m2; serum 25(OH)D, 25.8±1.1 ng/mL) met the subject inclusion criteria.
Age, height, plasma rheumatoid factor, plasma calcium, and WOMAC pain and physical function scores were not significantly different between vitamin D groups (Table 1). However, serum 25(OH)D concentrations inversely correlated with pain (n=56, r=−0.27, p<0.05) and physical function (n=56, r=−0.28, p<0.05) (Fig. 2A and B), suggesting decreases in self-reported knee pain and physical function with an increase in serum 25(OH)D concentrations. Body mass, BMI, plasma iPTH (Table 1), and plasma uric acid (Fig. 3A) were significantly (all p<0.05) increased in the vitamin D deficient group. Approximately 9% of the subjects displayed a Kellgren–Lawrence grade of 2, while 68% and 23% of the subjects had a grade 3 or 4 score, respectively (Table 1). Importantly, the Kellgren–Lawrence scores were not significantly different between groups, suggesting that the severity of knee osteoarthritis was similar between groups.
Table 1.
Subject characteristics and clinical chemistries.
| Vitamin D status |
||||
|---|---|---|---|---|
| Deficient | Insufficient | Sufficient | p-value | |
| n (males:females) | 17 (9:8) | 21 (9:12) | 18 (7:11) | |
| Age (y) | 51±3 | 48±2 | 47±3 | 0.536 |
| Height (cm) | 167±2 | 170±2 | 168±2 | 0.487 |
| Body mass (kg) | 100.6±5.4 | 99.7±4.6 | 81.4±3.3a,b | 0.006 |
| BMI (kg/m2) | 36.0±1.8 | 34.2±1.3 | 28.6±0.8a,b | 0.001 |
| Serum 25(OH)D (ng/ml) | 16.3±0.7 | 24.7±0.6a | 36.0±0.8a,b | <0.001 |
| Rheumatoid factor (IU/ml) | 9.18±0.46 | 8.95±0.44 | 8.83±0.45 | 0.599 |
| iPTH (pg/mL) | 50.1±3.8 | 44.1±3.6 | 33.6±2.2a,c | 0.004 |
| Calcium (mg/dL) | 9.35±0.13 | 9.29±0.1 | 9.49±0.10 | 0.632 |
| WOMAC pain (0–20) | 9.47±1.03 | 7.60±0.63 | 6.69±0.82 | 0.320 |
| WOMAC function (0–68) | 30.1±3.4 | 24.7±2.4 | 19.7±2.5 | 0.461 |
| Kellgren–Lawrence grade | 0.468 | |||
| 2 (n) | 1 | 4 | 0 | |
| 3 (n) | 14 | 9 | 15 | |
| 4 (n) | 2 | 8 | 3 | |
Data presented as mean±SEM unless otherwise noted.
All subjects were Caucasian.
p<0.05 vs. deficient.
p<0.05 vs. insufficient.
p=0.07 vs. insufficient.
Fig. 2.
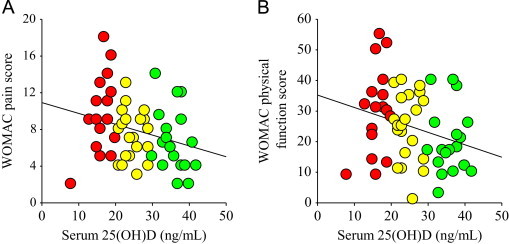
Serum 25(OH)D concentrations correlated with pain and physical function. Serum 25(OH)D concentrations (ng/mL) inversely correlated with pain (n=56, r=−0.27, p<0.05) and physical function (n=56, r=−0.28, p<0.05). Vitamin D deficient, red circles; vitamin D insufficient, yellow circles; vitamin D sufficient, green circles.
Fig. 3.
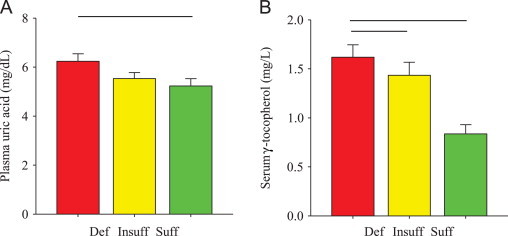
Plasma uric acid and serum γ-tocopherol concentrations. (A) Plasma uric acid concentrations (mg/dL) were significantly (bar, p<0.05) increased in the vitamin D deficient group compared to the vitamin D sufficient group. (B) Serum γ-tocopherol concentrations (mg/L) were significantly (bars, p<0.05) increased in the vitamin D deficient group compared to those in the insufficient and sufficient groups. Def, vitamin D deficient (red); Insuff, vitamin D insufficient (yellow); Suff, vitamin D sufficient (green). Data presented as mean±SEM.
Circulating chemistries
Despite trends, circulating α-carotene, β-carotene, lutein, zeaxanthin, ascorbic acid, and α-tocopherol were not significantly different between groups after adjusting for BMI and other covariates (Table 2). In contrast, serum γ-tocopherol concentrations were significantly (p<0.05) increased (~94% vs. vitamin D sufficient and ~13% vs. vitamin D insufficient) in the vitamin D deficient group (Fig. 3B). In the vitamin D sufficient group, serum γ-tocopherol inversely correlated (r=−0.47, p<0.05) with serum TNF-α (Table 3), suggesting a pro-inflammatory increase with a γ-tocopherol decrease despite a sufficient serum 25(OH)D concentration. Circulating cytokine and CRP concentrations were not significantly different between groups (Table 2).
Table 2.
Circulating vitamin and cytokine concentrations.
| Vitamin D status |
||||
|---|---|---|---|---|
| Deficient | Insufficient | Sufficient | p-value | |
| α-Carotene (μg/L) | 47.5±7.1 | 55.8±7.2 | 87.3±17.1 | 0.646 |
| β-Carotene (μg/L) | 99.9±10.8 | 169.1±28.6 | 271.0±49.6 | 0.122 |
| Lutein (μg/L) | 102±15 | 119±14 | 138±16 | 0.832 |
| Zeaxanthin (μg/L) | 25.9±3.6 | 29.5±3.0 | 29.5±2.8 | 0.535 |
| Ascorbic acid (mg/dl) | 0.89±0.12 | 1.20±0.11 | 1.13±0.09 | 0.176 |
| α-Tocopherol (mg/L) | 11.3±0.8 | 11.2±0.6 | 11.4±0.7 | 0.941 |
| GM-CSF (pg/mL) | 1.32±0.34 | 4.30±1.75 | 2.74±1.00 | 0.864 |
| IFN-γ (pg/mL) | 10.4±2.53 | 18.9±4.27 | 16.3±6.59 | 0.216 |
| TNF-α (pg/mL) | 5.76±0.71 | 5.43±0.53 | 5.28±1.07 | 0.660 |
| IL-1β (pg/mL) | 0.66±0.22 | 0.95±0.30 | 1.16±0.45 | 0.526 |
| IL-2 (pg/mL) | 1.31±0.44 | 2.20±0.64 | 2.54±0.86 | 0.423 |
| IL-4 (pg/mL) | 16.4±5.63 | 30.9±11.5 | 25.5±11.7 | 0.920 |
| IL-5 (pg/mL) | 0.14±0.05 | 0.79±0.27 | 0.38±0.16 | 0.269 |
| IL-6 (pg/mL) | 1.52±0.15 | 2.83±0.58 | 1.50±0.25 | 0.185 |
| IL-7 (pg/mL) | 6.80±1.04 | 7.44±1.11 | 8.70±1.54 | 0.694 |
| IL-8 (pg/mL) | 6.70±0.53 | 8.37±1.15 | 5.75±0.57 | 0.536 |
| IL-10 (pg/mL) | 19.8±3.10 | 42.1±16.1 | 25.9±4.25 | 0.249 |
| IL-12 (pg/mL) | 1.64±0.69 | 7.35±4.40 | 5.22±3.20 | 0.248 |
| IL-13 (pg/mL) | 10.9±2.95 | 14.9±4.13 | 10.6±3.74 | 0.635 |
| CRP (mg/L) | 3.38±0.72 | 4.61±1.31 | 2.32±0.57 | 0.365 |
Data presented as mean±SEM.
Table 3.
Serum γ-T and cytokine correlation coefficients.
| Vitamin D status |
||||
|---|---|---|---|---|
| Deficient |
Insufficient |
Sufficient |
Group |
|
| γ-T | γ-T | γ-T | γ-T | |
| GM-CSF | 0.04 | 0.13 | −0.21 | 0.01 |
| IFN-γ | −0.12 | 0.02 | −0.31 | −0.04 |
| TNF-α | −0.15 | 0.00 | −0.47† | −0.03 |
| IL-1β | 0.03 | 0.19 | −0.22 | 0.00 |
| IL-2 | −0.07 | 0.15 | −0.15 | −0.05 |
| IL-4 | −0.18 | 0.23 | −0.21 | 0.02 |
| IL-5 | 0.22 | −0.14 | −0.28 | −0.09 |
| IL-6 | 0.35 | −0.06 | −0.20 | 0.08 |
| IL-7 | −0.18 | 0.15 | −0.06 | −0.04 |
| IL-8 | 0.10 | 0.16 | −0.25 | 0.15 |
| IL-10 | 0.35 | 0.06 | −0.10 | 0.04 |
| IL-12 | 0.15 | 0.23 | −0.29 | 0.06 |
| IL-13 | −0.29 | 0.28 | −0.05 | 0.08 |
γ-T, γ-tocopherol.
Group, all subjects combined.
p<0.05.
Isokinetic leg strength data
Concentric-knee extension peak torque, average power, and total work were significantly (all p<0.05) decreased and concentric-knee extension deceleration was significantly (p<0.05) slower in the vitamin D deficient group (Figs. 4A–D). Concentric-knee flexion deceleration was significantly slower in the symptomatic compared to the non-symptomatic leg, but differences between vitamin D groups were not significant (Table 4). Concentric-knee flexion torque, power, work, and acceleration were not significantly different between vitamin D groups.
Fig. 4.
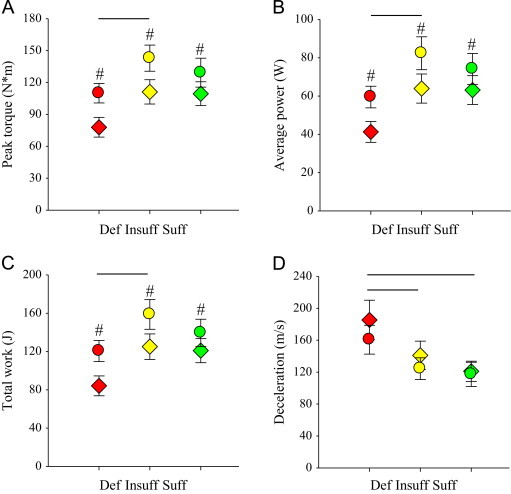
Isokinetic-concentric knee extension peak torque, average power, total work, and deceleration in the symptomatic and non-symptomatic legs. (A) Peak torque (Nm) was significantly decreased in the symptomatic leg (#p<0.05 vs. non-symptomatic leg) and in the vitamin D deficient group (bar, p<0.05). (B) Average power (W) was significantly decreased in the symptomatic leg (#p<0.05 vs. non-symptomatic leg) and in the vitamin D deficient group (bar, p<0.05). (C) Total work (J) was significantly decreased in the symptomatic leg (#p<0.05 vs. non-symptomatic leg) and in the vitamin D deficient group (bar, p<0.05). (D) Deceleration (m/s) was significantly slower in the vitamin D deficient group compared to that in the vitamin D insufficient and sufficient groups (bars, p<0.05). Def, vitamin D deficient (red); Insuff, vitamin D insufficient (yellow); Suff, vitamin D sufficient (green); Symptomatic leg (colored diamonds, ♢); Non-symptomatic leg (colored circles, ○). Figure legend provided in ‘A’. Data presented as mean±SEM.
Table 4.
Symptomatic and non-symptomatic isokinetic leg strength data.
| Vitamin D status |
Vitamin D status main effect p-value | |||
|---|---|---|---|---|
| Deficient | Insufficient | Sufficient | ||
| Concentric knee extension (60 °/s) | ||||
| Symptomatic acceleration (m/s) | 62.4±7.0 | 56.3±5.7 | 52.8±4.4 | 0.071 |
| Non-symptomatic acceleration (m/s) | 70.6±9.7 | 54.3±5.2 | 43.3±4.6 | |
| Leg main effect p-value | 0.835 | |||
| Concentric knee flexion (60 °/s) | ||||
| Symptomatic peak torque (Nm) | 59.4±6.0 | 78.2±7.9 | 78.8±9.7 | 0.127 |
| Non-symptomatic peak torque (Nm) | 72.5±4.6 | 84.7±6.5 | 83.7±11.7 | |
| Leg main effect p-value | 0.224 | |||
| Sympomatic average power (W) | 35.6±4.2 | 46.0±5.6 | 48.6±5.9 | 0.800 |
| Non-symptomatic average power (W) | 87.1±43.8 | 55.5±4.7 | 51.7±5.6 | |
| Leg main effect p-value | 0.122 | |||
| Symptomatic total work (J) | 68.6±7.0 | 102.0±11.5 | 105.0±16.7 | 0.097 |
| Non-symptomatic total work (J) | 87.3±6.3 | 105.0±9.0 | 108.8±19.6 | |
| Leg main effect p-value | 0.414 | |||
| Symptomatic acceleration (m/s) | 85.6±12.2 | 86.4±14.2 | 71.1±4.7 | 0.791 |
| Non-symptomatic acceleration (m/s) | 62.4±4.7 | 58.6±4.3 | 52.8±3.0 | |
| Leg main effect p-value | 0.213 | |||
| Symptomatic deceleration (m/s) | 175±18 | 153±14 | 148±14 | 0.543 |
| Non-symptomatic deceleration (m/s) | 134±18 | 126±11 | 119±14 | |
| Leg main effect p-value | 0.009 | |||
Data presented as mean±SEM.
Discussion
Muscular weakness is a major impairment that hinders the quality of life and activities of daily living in those with and without knee OA. The major finding of the present investigation was that vitamin D deficiency associated with quadricep weakness, and that an increase in serum 25(OH)D correlated with a decrease in perceived physical dysfunction in subjects with knee OA. The present report also provides original data suggesting that vitamin D deficiency is not associated with an increase in inflammatory cytokines or a decrease in micronutrients that modulate inflammatory cytokines. However, the γ-tocopherol decrease with vitamin D sufficiency correlated with a TNF-α increase in subjects with knee OA, suggesting an anti-inflammatory role of vitamin E in a pathophysiological condition mediated by inflammation and in subjects with sufficient vitamin D.
Quadriceps weakness is a common and early symptom of knee OA that predicts and occurs before radiographic evidence of knee OA and knee pain [10], [11]. Although previous studies illustrate the association between low serum 25(OH)D concentrations and muscular weakness across a range of physiological and pathophysiological conditions in humans, this study provides unique data indicating that vitamin D deficiency associates with quadricep weakness in subjects with knee OA. Additionally, vitamin D deficiency associated with slower concentric-knee extension deceleration times, which implies a decrement in the eccentric control of the quadriceps with low serum 25(OH)D concentrations. As proposed elsewhere [8], [66], weakness and impaired eccentric control of the quadriceps is detrimental to the structural integrity of the knee joint by minimizing shock absorption. Therefore, altering serum 25(OH)D concentrations could be an attractive therapeutic approach to attenuate quadriceps weakness, which importantly, contributes to knee OA.
Perceived physical function is impaired in elderly with vitamin D deficiency. In knee OA subjects, studies investigating the association between vitamin D and physical function are scarce. Using Lequesne׳s indices to assess physical function in subjects with knee OA, Al-Jarallah et al. [5] reported that serum 25(OH)D concentrations did not associate with physical function. In an intervention study, supplemental vitamin D (2000 IU/d for 2 y) increased serum 25(OH)D concentrations without improving physical function, such as standing from a chair or a 20-m walk, in subjects with knee OA [24]. Consistent with those results, perceived physical function was not significantly different between patients with contrasting serum 25(OH)D concentrations in the present investigation. However, serum 25(OH)D concentrations inversely correlated with physical dysfunction, suggesting an improvement in physical function with an increase in serum 25(OH)D concentrations. The discrepancy between the group comparisons and correlative findings regarding the association between vitamin D and physical function possibly suggest a narrow range in serum 25(OH)D concentrations or that the demarcation between vitamin D status levels were inappropriately defined to delineate a significant difference in self-reported physical function. In agreement with the latter postulate, Houston et al. [12] reported that the serum 25(OH)D concentration for improved physical function (28–32 ng/mL) was greater than that for muscular strength (22–28 ng/mL) in elderly.
Despite vitamin D deficiency, circulating cytokine concentrations were similar between vitamin D groups. In a population whose degenerative joint condition is mediated in part by pro-inflammatory cytokines, it appears that low vitamin D does not increase inflammatory cytokines in the blood. This finding, however, conflicts with results showing a circulating increase of pro-inflammatory cytokines in young reportedly healthy adults [36], obese adults [37], chronic heart failure patients [38], and type 2 diabetics [39] with low serum 25(OH)D concentrations. Further, a pro-inflammatory cytokine increase in the blood associates with muscular weakness and impaired physical mobility in elderly [67], [68], [69], [70], the development of knee OA [28], [71], and muscular weakness in patients with knee OA [9]. Based on those findings, we anticipated vitamin D deficiency to associate with muscular weakness and a cytokine increase in the blood. In contrast, the data here suggest that vitamin D deficiency associates with muscular weakness but not serum cytokines in subjects with knee OA.
Similar to vitamin D, other dietary micronutrients (e.g., β-carotene, ascorbic acid, and α- and γ-tocopherol׳s) modulate inflammatory cytokines. Surprisingly, no study has reported previously the level of other micronutrients concomitantly with serum 25(OH)D when investigating the role of vitamin D status on inflammatory cytokines. Although α- and β-carotene׳s, lutein, zeaxanthin, ascorbic acid, and α-tocopherol were not significantly different between groups, serum γ-tocopherol concentrations were significantly increased with vitamin D deficiency. The explanation for the γ-tocopherol increase with vitamin D deficiency is unknown and awaits future resolve, but it is plausible that the serum γ-tocopherol difference between groups is related to disparate dietary habits as γ-tocopherol is the most abundant form of vitamin E in the American diet [72], [73].
As mentioned previously, pro-inflammatory cytokines are increased in various pathophysiological conditions with low vitamin D [38], [39], and similar to vitamin D, γ-tocopherol is a potent anti-inflammatory micronutrient [74]. γ-Tocopherol inhibits cytokine production by decreasing 5-lipoxygenase, cyclooxygenase-2, and NF-κB activation and impairing jun terminal kinase and extracellular signal-regulated kinases 1/2 phosphorylation [44], [75], [76], [77]. Based on the cytokine modulating properties of both vitamin D and E, it is plausible that contrasting or divergent micronutrient levels (e.g., high vitamin D and low vitamin E) could influence cytokine levels in the blood. Indeed, serum TNF-α inversely correlated with γ-tocopherol in the vitamin D sufficient group with low serum γ-tocopherol (Table 3). This finding indicates that a decrease in γ-tocopherol could increase a pro-inflammatory cytokine despite a sufficient serum 25(OH)D concentration in subjects with knee OA. Given the similar cytokine, carotenoid, ascorbic acid, and α-tocopherol concentrations between groups, we speculate that the anti-inflammatory property of γ-tocopherol is balancing the serum cytokine concentrations in the vitamin D deficient group compared to those with insufficient and sufficient vitamin D. This speculation, however, warrants future investigation. Results from future studies could advance our knowledge pertaining to the anti-inflammatory role of vitamin E in vitamin D deficient patients suffering from knee OA, a degenerative joint condition mediated in part by pro-inflammatory cytokines, including but not limited to TNF-α [78].
Plasma uric acid increases with the prevalence of knee arthritis [79], and in this study, plasma uric acid concentrations increased in knee OA subjects with vitamin D deficiency. A chronic increase in plasma uric acid is a risk factor for cardiovascular disease, gout, renal failure, and metabolic syndrome. However, an acute increase of uric acid might possess an antioxidant property. Antioxidants prevent the reactive oxygen species-mediated activation of intracellular redox-sensitive signaling pathways that induce cytokine expression. The antioxidant property of uric acid could explain the similar cytokine concentrations between vitamin D groups. Unfortunately, due to the cross-sectional design of this study, it is unclear if the increase in uric acid with vitamin D deficiency was acute or chronic.
The explanation for an increase in uric acid is lower excretion, increase synthesis, or both. Serum uric acid concentrations also increase with serum PTH concentrations [80], [81], and vitamin D deficiency or insufficiency increases PTH concentrations [82]. In the vitamin D deficient group here, the increase in plasma uric acid occurred in parallel with an increase in plasma iPTH. It is plausible, therefore, that the increase in iPTH concentrations with vitamin D deficiency is stimulating an increase in uric acid. However, it is reasonable that other factors are contributing to the uric acid increase with vitamin D deficiency as well.
A limitation of this study is the lack of dietary data that could reveal the impact of micronutrient intake on blood 25(OH)D and other micronutrient concentrations. Along these lines, there were apparent decreases in α- and β-carotene׳s, lutein, and ascorbic acid with vitamin D deficiency. These decreases, however, were not significant after adjusting for BMI and other covariates. In addition, it is plausible that the contrasting serum 25(OH)D concentrations could be the product of differences in lifestyle habits. Future studies are encouraged to include dietary, lifestyle, and other circulating micronutrient analyses when investigating the role of vitamin D on inflammatory cytokines and skeletal muscle strength in conditions associated with systemic inflammation. Another limitation of this study includes the selected age range in subjects with modest activity levels. Thus, caution is recommended when extrapolating these findings to other populations. This study also consisted of a cross-sectional design that precludes potential inference on causality. Finally, this was a small sample size study, but in addition to its original findings, these data are valuable in generating new hypotheses.
In summary, muscular weakness and cytokines alter the mechanical stability and inflammatory environment in the knee, respectively, and contribute to the pathogenesis of OA. In the present investigation, vitamin D deficiency associated with quadriceps dysfunction but not inflammatory cytokines in subjects with knee OA. We propose that the increase in γ-tocopherol with vitamin D deficiency could abrogate a cytokine increase and consequentially result in similar cytokine levels between vitamin D groups. In support of this premise, a decrease in γ-tocopherol correlated with an increase in TNF-α in the vitamin D sufficient group. We conclude that vitamin D deficiency associates with quadriceps dysfunction and an increase in γ-tocopherol, and that γ-tocopherol could have an important anti-inflammatory role in subjects with vitamin D sufficiency.
Acknowledgments
We would like to thank all the subjects that participated in this study. This study was funded in part by USANA Health Sciences, Inc. (Salt Lake City, UT USA) (TB).
References
- 1.McAlindon T.E., Felson D.T., Zhang Y., Hannan M.T., Aliabadi P., Weissman B., Rush D., Wilson P.W., Jacques P. Relation of dietary intake and serum levels of vitamin D to progression of osteoarthritis of the knee among participants in the Framingham study. Ann. Intern. Med. 1996;125:353–359. doi: 10.7326/0003-4819-125-5-199609010-00001. [DOI] [PubMed] [Google Scholar]
- 2.Heidari B., Heidari P., Hajian-Tilaki K. Association between serum vitamin D deficiency and knee osteoarthritis. Int. Orthop. 2010;35:1627–1631. doi: 10.1007/s00264-010-1186-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muraki S., Dennison E., Jameson K., Boucher B.J., Akune T., Yoshimura N., Judge A., Arden N.K., Javaid K., Cooper C. Association of vitamin D status with knee pain and radiographic knee osteoarthritis. Osteoarthr. Cartil. 2011;19:1301–1306. doi: 10.1016/j.joca.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 4.Ding C., Cicuttini F., Parameswaran V., Burgess J., Quinn S., Jones G. Serum levels of vitamin D, sunlight exposure, and knee cartilage loss in older adults: the Tasmanian older adult cohort study. Arthritis Rheum. 2009;60:1381–1389. doi: 10.1002/art.24486. [DOI] [PubMed] [Google Scholar]
- 5.Al-Jarallah K.F., Shehab D., Al-Awadhi A., Nahar I., Haider M.Z., Moussa M.A. Are 25(OH)D levels related to the severity of knee osteoarthritis and function? Med. Princ. Pract. 2011;21:74–78. doi: 10.1159/000330025. [DOI] [PubMed] [Google Scholar]
- 6.Felson D.T., Niu J., Clancy M., Aliabadi P., Sack B., Guermazi A., Hunter D.J., Amin S., Rogers G., Booth S.L. Low levels of vitamin D and worsening of knee osteoarthritis: results of two longitudinal studies. Arthritis Rheum. 2007;56:129–136. doi: 10.1002/art.22292. [DOI] [PubMed] [Google Scholar]
- 7.Konstari S., Paananen M., Heliovaara M., Knekt P., Marniemi J., Impivaara O., Arokoski J., Karppinen J. Association of 25-hydroxyvitamin D with the incidence of knee and hip osteoarthritis: a 22-year follow-up study. Scand. J Rheumatol. 2011;41:124–131. doi: 10.3109/03009742.2011.617314. [DOI] [PubMed] [Google Scholar]
- 8.Hurley M.V., Scott D.L. Improvements in quadriceps sensorimotor function and disability of patients with knee osteoarthritis following a clinically practicable exercise regime. Br. J. Rheumatol. 1998;37:1181–1187. doi: 10.1093/rheumatology/37.11.1181. [DOI] [PubMed] [Google Scholar]
- 9.Santos M.L., Gomes W.F., Pereira D.S., Oliveira D.M., Dias J.M., Ferrioli E., Pereira L.S. Muscle strength, muscle balance, physical function and plasma interleukin-6 (IL-6) levels in elderly women with knee osteoarthritis (OA) Arch. Gerontol. Geriatr. 2011;52:322–326. doi: 10.1016/j.archger.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 10.Slemenda C., Brandt K.D., Heilman D.K., Mazzuca S., Braunstein E.M., Katz B.P., Wolinsky F.D. Quadriceps weakness and osteoarthritis of the knee. Ann. Intern. Med. 1997;127:97–104. doi: 10.7326/0003-4819-127-2-199707150-00001. [DOI] [PubMed] [Google Scholar]
- 11.Fisher N.M., Pendergast D.R., Gresham G.E., Calkins E. Muscle rehabilitation: its effect on muscular and functional performance of patients with knee osteoarthritis. Arch. Phys. Med. Rehabil. 1991;72:367–374. [PubMed] [Google Scholar]
- 12.Houston D.K., Tooze J.A., Neiberg R.H., Hausman D.B., Johnson M.A., Cauley J.A., Bauer D.C., Cawthon P.M., Shea M.K., Schwartz G.G., Williamson J.D., Tylavsky F.A., Visser M., Simonsick E.M., Harris T.B., Kritchevsky S.B. 25-hydroxyvitamin D status and change in physical performance and strength in older adults: the health, aging, and body composition study. Am. J. Epidemiol. 2012;176:1025–1034. doi: 10.1093/aje/kws147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bischoff-Ferrari H.A., Dietrich T., Orav E.J., Hu F.B., Zhang Y., Karlson E.W., Dawson-Hughes B. Higher 25-hydroxyvitamin D concentrations are associated with better lower-extremity function in both active and inactive persons aged>or=60 y. Am. J. Clin. Nutr. 2004;80:752–758. doi: 10.1093/ajcn/80.3.752. [DOI] [PubMed] [Google Scholar]
- 14.Mastaglia S.R., Seijo M., Muzio D., Somoza J., Nunez M., Oliveri B. Effect of vitamin D nutritional status on muscle function and strength in healthy women aged over sixty-five years. J. Nutr. Health Aging. 2011;15:349–354. doi: 10.1007/s12603-010-0287-3. [DOI] [PubMed] [Google Scholar]
- 15.Wicherts I.S., van Schoor N.M., Boeke A.J., Visser M., Deeg D.J., Smit J., Knol D.L., Lips P. Vitamin D status predicts physical performance and its decline in older persons. J. Clin. Endocrinol. Metab. 2007;92:2058–2065. doi: 10.1210/jc.2006-1525. [DOI] [PubMed] [Google Scholar]
- 16.Visser M., Deeg D.J., Lips P. Low vitamin D and high parathyroid hormone levels as determinants of loss of muscle strength and muscle mass (sarcopenia): the longitudinal aging study Amsterdam. J. Clin. Endocrinol. Metab. 2003;88:5766–5772. doi: 10.1210/jc.2003-030604. [DOI] [PubMed] [Google Scholar]
- 17.Dhesi J.K., Bearne L.M., Moniz C., Hurley M.V., Jackson S.H., Swift C.G., Allain T.J. Neuromuscular and psychomotor function in elderly subjects who fall and the relationship with vitamin D status. J. Bone Miner. Res. 2002;17:891–897. doi: 10.1359/jbmr.2002.17.5.891. [DOI] [PubMed] [Google Scholar]
- 18.Ceglia L. Vitamin D and skeletal muscle tissue and function. Mol. Asp. Med. 2008;29:407–414. doi: 10.1016/j.mam.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 19.Boland R.L. VDR activation of intracellular signaling pathways in skeletal muscle. Mol. Cell Endocrinol. 2011;347:11–16. doi: 10.1016/j.mce.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 20.Bischoff-Ferrari H.A., Dawson-Hughes B., Stocklin E., Sidelnikov E., Willett W.C., Orav E.J., Stahelin H.B., Wolfram S., Jetter A., Schwager J., Henschkowski J., von E.A., Egli A. Oral supplementation with 25(OH)D(3) versus vitamin D(3): effects on 25(OH)D levels, lower extremity function, blood pressure and markers of innate immunity. J. Bone Miner. Res. 2011 doi: 10.1002/jbmr.551. [DOI] [PubMed] [Google Scholar]
- 21.Sato Y., Iwamoto J., Kanoko T., Satoh K. Low-dose vitamin D prevents muscular atrophy and reduces falls and hip fractures in women after stroke: a randomized controlled trial. Cerebrovasc. Dis. 2005;20:187–192. doi: 10.1159/000087203. [DOI] [PubMed] [Google Scholar]
- 22.Annweiler C., Schott-Petelaz A.M., Berrut G., Kressig R.W., Bridenbaugh S., Herrmann F.R., Beauchet O. Vitamin D deficiency-related quadriceps weakness: results of the epidemiologie De l׳Osteoporose cohort. J. Am. Geriatr. Soc. 2009;57:368–369. doi: 10.1111/j.1532-5415.2009.02118.x. [DOI] [PubMed] [Google Scholar]
- 23.Kenny A.M., Biskup B., Robbins B., Marcella G., Burleson J.A. Effects of vitamin D supplementation on strength, physical function, and health perception in older, community-dwelling men. J. Am. Geriatr. Soc. 2003;51:1762–1767. doi: 10.1046/j.1532-5415.2003.51561.x. [DOI] [PubMed] [Google Scholar]
- 24.McAlindon T., LaValley M., Schneider E., Nuite M., Lee J.Y., Price L.L., Lo G., Dawson-Hughes B. Effect of vitamin D supplementation on progression of knee pain and cartilage volume loss in patients with symptomatic osteoarthritis: a randomized controlled trial. J. Am. Med. Assoc. 2013;309:155–162. doi: 10.1001/jama.2012.164487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sowers M., Jannausch M., Stein E., Jamadar D., Hochberg M., Lachance L. C-reactive protein as a biomarker of emergent osteoarthritis. Osteoarthr. Cartil. 2002;10:595–601. doi: 10.1053/joca.2002.0800. [DOI] [PubMed] [Google Scholar]
- 26.Spector T.D., Hart D.J., Nandra D., Doyle D.V., Mackillop N., Gallimore J.R., Pepys M.B. Low-level increases in serum C-reactive protein are present in early osteoarthritis of the knee and predict progressive disease. Arthritis Rheum. 1997;40:723–727. doi: 10.1002/art.1780400419. [DOI] [PubMed] [Google Scholar]
- 27.Pelletier J.P., Raynauld J.P., Caron J., Mineau F., Abram F., Dorais M., Haraoui B., Choquette D., Martel-Pelletier J. Decrease in serum level of matrix metalloproteinases is predictive of the disease-modifying effect of osteoarthritis drugs assessed by quantitative MRI in patients with knee osteoarthritis. Ann. Rheum. Dis. 2010;69:2095–2101. doi: 10.1136/ard.2009.122002. [DOI] [PubMed] [Google Scholar]
- 28.Stannus O., Jones G., Cicuttini F., Parameswaran V., Quinn S., Burgess J., Ding C. Circulating levels of IL-6 and TNF-alpha are associated with knee radiographic osteoarthritis and knee cartilage loss in older adults. Osteoarthr. Cartil. 2010;18:1441–1447. doi: 10.1016/j.joca.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 29.Livshits G., Zhai G., Hart D.J., Kato B.S., Wang H., Williams F.M., Spector T.D. Interleukin-6 is a significant predictor of radiographic knee osteoarthritis: the Chingford study. Arthritis Rheum. 2009;60:2037–2045. doi: 10.1002/art.24598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith M.D., Triantafillou S., Parker A., Youssef P.P., Coleman M. Synovial membrane inflammation and cytokine production in patients with early osteoarthritis. J. Rheumatol. 1997;24:365–371. [PubMed] [Google Scholar]
- 31.Pearle A.D., Scanzello C.R., George S., Mandl L.A., DiCarlo E.F., Peterson M., Sculco T.P., Crow M.K. Elevated high-sensitivity C-reactive protein levels are associated with local inflammatory findings in patients with osteoarthritis. Osteoarthr. Cartil. 2007;15:516–523. doi: 10.1016/j.joca.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 32.Devaraj S., Yun J.M., Duncan-Staley C.R., Jialal I. Low vitamin D levels correlate with the proinflammatory state in type 1 diabetic subjects with and without microvascular complications. Am. J. Clin. Pathol. 2011;135:429–433. doi: 10.1309/AJCPJGZQX42BIAXL. [DOI] [PubMed] [Google Scholar]
- 33.Khoo A.L., Chai L.Y., Koenen H.J., Sweep F.C., Joosten I., Netea M.G., van der Ven A.J. Regulation of cytokine responses by seasonality of vitamin D status in healthy individuals. Clin. Exp. Immunol. 2011;164:72–79. doi: 10.1111/j.1365-2249.2010.04315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barker T. In: Nutrition & Physical Activity in Inflammatory Diseases. Garg M.L., Wood L.G., editors. CABI; Boston: 2013. Vitamin D and inflammation; pp. 75–86. [Google Scholar]
- 35.Dusso A.S., Brown A.J., Slatopolsky E., Vitamin D. Am. J. Physiol. Ren. Physiol. 2005;289:F8–28. doi: 10.1152/ajprenal.00336.2004. [DOI] [PubMed] [Google Scholar]
- 36.Barker T., Martins T.B., Hill H.R., Kjeldsberg C.R., Dixon B.M., Schneider E.D., Henriksen V.T., Weaver L.K. Circulating pro-inflammatory cytokines are elevated and peak power output correlates with 25-hydroxyvitamin D in vitamin D insufficient adults. Eur. J. Appl. Physiol. 2013;113:1523–1534. doi: 10.1007/s00421-012-2582-7. [DOI] [PubMed] [Google Scholar]
- 37.Bellia A., Garcovich C., D׳Adamo M., Lombardo M., Tesauro M., Donadel G., Gentileschi P., Lauro D., Federici M., Lauro R., Sbraccia P. Serum 25-hydroxyvitamin D levels are inversely associated with systemic inflammation in severe obese subjects. Intern. Emerg. Med. 2013;8:33–40. doi: 10.1007/s11739-011-0559-x. [DOI] [PubMed] [Google Scholar]
- 38.Milovanovic M., Pesic G., Nikolic V., Jevtovic-Stoimenov T., Vasic K., Jovic Z., Deljanin-Ilic M., Pesic S. Vitamin D deficiency is associated with increased IL-17 and TNFalpha levels in patients with chronic heart failure. Arq. Bras. Cardiol. 2012;98:259–265. doi: 10.1590/s0066-782x2012005000019. [DOI] [PubMed] [Google Scholar]
- 39.Shab-Bidar S., Neyestani T.R., Djazayery A., Eshraghian M.R., Houshiarrad A., Kalayi A., Shariatzadeh N., Khalaji N., Gharavi A. Improvement of vitamin D status resulted in amelioration of biomarkers of systemic inflammation in the subjects with type 2 diabetes. Diabetes Metab. Res. Rev. 2012;28:424–430. doi: 10.1002/dmrr.2290. [DOI] [PubMed] [Google Scholar]
- 40.Mah E., Pei R., Guo Y., Ballard K.D., Barker T., Rogers V.E., Parker B.A., Taylor A.W., Traber M.G., Volek J.S., Bruno R.S. Gamma-tocopherol-rich supplementation additively improves vascular endothelial function during smoking cessation. Free Radic. Biol. Med. 2013;65C:1291–1299. doi: 10.1016/j.freeradbiomed.2013.09.016. [DOI] [PubMed] [Google Scholar]
- 41.Devaraj S., Leonard S., Traber M.G., Jialal I. Gamma-tocopherol supplementation alone and in combination with alpha-tocopherol alters biomarkers of oxidative stress and inflammation in subjects with metabolic syndrome. Free Radic. Biol. Med. 2008;44:1203–1208. doi: 10.1016/j.freeradbiomed.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Devaraj S., Jialal I. Low-density lipoprotein postsecretory modification, monocyte function, and circulating adhesion molecules in type 2 diabetic patients with and without macrovascular complications: the effect of {alpha}-tocopherol supplementation. Circulation. 2000;102:191–196. doi: 10.1161/01.cir.102.2.191. [DOI] [PubMed] [Google Scholar]
- 43.England A., Valdes A.M., Slater-Jefferies J.L., Gill R., Howell W.M., Calder P.C., Grimble R.F. Variants in the genes encoding TNF-alpha, IL-10, and GSTP1 influence the effect of alpha-tocopherol on inflammatory cell responses in healthy men. Am. J. Clin. Nutr. 2012;95:1461–1467. doi: 10.3945/ajcn.111.012781. [DOI] [PubMed] [Google Scholar]
- 44.Wiser J., Alexis N.E., Jiang Q., Wu W., Robinette C., Roubey R., Peden D.B. In vivo gamma-tocopherol supplementation decreases systemic oxidative stress and cytokine responses of human monocytes in normal and asthmatic subjects. Free Radic. Biol. Med. 2008;45:40–49. doi: 10.1016/j.freeradbiomed.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hartel C., Strunk T., Bucsky P., Schultz C. Effects of vitamin C on intracytoplasmic cytokine production in human whole blood monocytes and lymphocytes. Cytokine. 2004;27:101–106. doi: 10.1016/j.cyto.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 46.Prabhala R.H., Braune L.M., Garewal H.S., Watson R.R. Influence of beta-carotene on immune functions. Ann. N. Y. Acad. Sci. 1993;691:262–263. doi: 10.1111/j.1749-6632.1993.tb26189.x. [DOI] [PubMed] [Google Scholar]
- 47.Hughes D.A., Wright A.J., Finglas P.M., Peerless A.C., Bailey A.L., Astley S.B., Pinder A.C., Southon S. The effect of beta-carotene supplementation on the immune function of blood monocytes from healthy male nonsmokers. J. Lab. Clin. Med. 1997;129:309–317. doi: 10.1016/s0022-2143(97)90179-7. [DOI] [PubMed] [Google Scholar]
- 48.Hughes D.A., Finglas P.M., Wright A.J., Peerless A.C., Bailey A.L., Astley S.B., Southon S. Dietary beta-carotene supplementation modulates the production of tumour necrosis factor-alpha by human monocytes. Biochem. Soc. Trans. 1996;24:387S. doi: 10.1042/bst024387s. [DOI] [PubMed] [Google Scholar]
- 49.Duncan A., Talwar D., McMillan D.C., Stefanowicz F., O׳Reilly D.S. Quantitative data on the magnitude of the systemic inflammatory response and its effect on micronutrient status based on plasma measurements. Am. J. Clin. Nutr. 2012;95:64–71. doi: 10.3945/ajcn.111.023812. [DOI] [PubMed] [Google Scholar]
- 50.Erlinger T.P., Guallar E., Miller E.R., I.I.I., Stolzenberg-Solomon R., Appel L.J. Relationship between systemic markers of inflammation and serum beta-carotene levels. Arch. Intern. Med. 2001;161:1903–1908. doi: 10.1001/archinte.161.15.1903. [DOI] [PubMed] [Google Scholar]
- 51.D׳Adamo C.R., Miller R.R., Shardell M.D., Orwig D.L., Hochberg M.C., Ferrucci L., Semba R.D., Yu-Yahiro J.A., Magaziner J., Hicks G.E. Higher serum concentrations of dietary antioxidants are associated with lower levels of inflammatory biomarkers during the year after hip fracture. Clin. Nutr. 2012;31:659–665. doi: 10.1016/j.clnu.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barker T., Martins T.B., Kjeldsberg C.R., Trawick R.H., Hill H.R. Circulating interferon-gamma correlates with 1,25(OH)D and the 1,25(OH)D-to-25(OH)D ratio. Cytokine. 2012;60:23–26. doi: 10.1016/j.cyto.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 53.Barker T., Leonard S.W., Trawick R.H., Martins T.B., Kjeldsberg C.R., Hill H.R., Traber M.G. Modulation of inflammation by vitamin E and C supplementation prior to anterior cruciate ligament surgery. Free. Radic. Biol. Med. 2009;46:599–606. doi: 10.1016/j.freeradbiomed.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 54.Henriksen V.T., Rogers V.E., Rasmussen G.L., Trawick R.H., Momberger N.G., Aguirre D., Barker T. Pro-inflammatory cytokines mediate the decrease in serum 25(OH)D concentrations after total knee arthroplasty? Med. Hypotheses. 2014;82:134–137. doi: 10.1016/j.mehy.2013.11.020. [DOI] [PubMed] [Google Scholar]
- 55.Reid D., Toole B.J., Knox S., Talwar D., Harten J., St, J.O., Blackwell S., Kinsella J., McMillan D.C., Wallace A.M. The relation between acute changes in the systemic inflammatory response and plasma 25-hydroxyvitamin D concentrations after elective knee arthroplasty. Am. J. Clin. Nutr. 2011;93:1006–1011. doi: 10.3945/ajcn.110.008490. [DOI] [PubMed] [Google Scholar]
- 56.Louw J.A., Werbeck A., Louw M.E., Kotze T.J., Cooper R., Labadarios D. Blood vitamin concentrations during the acute-phase response. Crit. Care Med. 1992;20:934–941. doi: 10.1097/00003246-199207000-00007. [DOI] [PubMed] [Google Scholar]
- 57.Bellamy N., Buchanan W.W., Goldsmith C.H., Campbell J., Stitt L.W. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J. Rheumatol. 1988;15:1833–1840. [PubMed] [Google Scholar]
- 58.Kellgren J.H., Lawrence J.S. Radiological assessment of osteo-arthrosis. Ann. Rheum. Dis. 1957;16:494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Barker K., Lamb S.E., Toye F., Jackson S., Barrington S. Association between radiographic joint space narrowing, function, pain and muscle power in severe osteoarthritis of the knee. Clin. Rehabil. 2004;18:793–800. doi: 10.1191/0269215504cr754oa. [DOI] [PubMed] [Google Scholar]
- 60.Segal N.A., Torner J.C., Felson D.T., Niu J., Sharma L., Lewis C.E., Nevitt M. Knee extensor strength does not protect against incident knee symptoms at 30 months in the multicenter knee osteoarthritis (MOST) cohort. PM R. 2009;1:459–465. doi: 10.1016/j.pmrj.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hall M.C., Mockett S.P., Doherty M. Relative impact of radiographic osteoarthritis and pain on quadriceps strength, proprioception, static postural sway and lower limb function. Ann. Rheum. Dis. 2006;65:865–870. doi: 10.1136/ard.2005.043653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Creamer P., Lethbridge-Cejku M., Hochberg M.C. Factors associated with functional impairment in symptomatic knee osteoarthritis. Rheumatology (Oxford) 2000;39:490–496. doi: 10.1093/rheumatology/39.5.490. [DOI] [PubMed] [Google Scholar]
- 63.McAlindon T.E., Cooper C., Kirwan J.R., Dieppe P.A. Determinants of disability in osteoarthritis of the knee. Ann. Rheum. Dis. 1993;52:258–262. doi: 10.1136/ard.52.4.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Steultjens M.P., Dekker J., van Baar M.E., Oostendorp R.A., Bijlsma J.W. Muscle strength, pain and disability in patients with osteoarthritis. Clin. Rehabil. 2001;15:331–341. doi: 10.1191/026921501673178408. [DOI] [PubMed] [Google Scholar]
- 65.Bruyere O., Honore A., Rovati L.C., Giacovelli G., Henrotin Y.E., Seidel L., Reginster J.Y. Radiologic features poorly predict clinical outcomes in knee osteoarthritis. Scand. J. Rheumatol. 2002;31:13–16. doi: 10.1080/030097402317255309. [DOI] [PubMed] [Google Scholar]
- 66.Radin E.L., Rose R.M. Role of subchondral bone in the initiation and progression of cartilage damage. Clin. Orthop. Relat. Res. 1986:34–40. [PubMed] [Google Scholar]
- 67.Penninx B.W., Abbas H., Ambrosius W., Nicklas B.J., Davis C., Messier S.P., Pahor M. Inflammatory markers and physical function among older adults with knee osteoarthritis. J. Rheumatol. 2004;31:2027–2031. [PubMed] [Google Scholar]
- 68.Visser M., Pahor M., Taaffe D.R., Goodpaster B.H., Simonsick E.M., Newman A.B., Nevitt M., Harris T.B. Relationship of interleukin-6 and tumor necrosis factor-alpha with muscle mass and muscle strength in elderly men and women: the Health ABC Study. J. Gerontol. A Biol. Sci. Med. Sci. 2002;57:M326–M332. doi: 10.1093/gerona/57.5.m326. [DOI] [PubMed] [Google Scholar]
- 69.Schaap L.A., Pluijm S.M., Deeg D.J., Visser M. Inflammatory markers and loss of muscle mass (sarcopenia) and strength. Am. J. Med. 2006;119 doi: 10.1016/j.amjmed.2005.10.049. (e9–e17) [DOI] [PubMed] [Google Scholar]
- 70.Cesari M., Penninx B.W., Pahor M., Lauretani F., Corsi A.M., Rhys W.G., Guralnik J.M., Ferrucci L. Inflammatory markers and physical performance in older persons: the InCHIANTI study. J. Gerontol. A Biol. Sci. Med. Sci. 2004;59:242–248. doi: 10.1093/gerona/59.3.m242. [DOI] [PubMed] [Google Scholar]
- 71.Stannus O.P., Jones G., Blizzard L., Cicuttini F.M., Ding C. Associations between serum levels of inflammatory markers and change in knee pain over 5 years in older adults: a prospective cohort study. Ann. Rheum. Dis. 2013;72:535–540. doi: 10.1136/annrheumdis-2011-201047. [DOI] [PubMed] [Google Scholar]
- 72.McLaughlin P.J., Weihrauch J.L. Vitamin E content of foods. J. Am. Diet Assoc. 1979;75:647–665. [PubMed] [Google Scholar]
- 73.Lehmann J., Martin H.L., Lashley E.L., Marshall M.W., Judd J.T. Vitamin E in foods from high and low linoleic acid diets. J. Am. Diet Assoc. 1986;86:1208–1216. [PubMed] [Google Scholar]
- 74.Barker T. In: Nutrition & Physical Activity in Inflammatory Diseases. Garg M.L., Wood L.G., editors. CABI; Boston: 2013. Vitamin E and inflammation; pp. 87–98. [Google Scholar]
- 75.Jiang Q., Gamma-tocopherol Ames B.N. but not alpha-tocopherol, decreases proinflammatory eicosanoids and inflammation damage in rats. J. Federation Am. Soc. Exp. Biol. 2003;17:816–822. doi: 10.1096/fj.02-0877com. [DOI] [PubMed] [Google Scholar]
- 76.Jiang Q., Elson-Schwab I., Courtemanche C., Ames B.N. gamma-tocopherol and its major metabolite, in contrast to alpha-tocopherol, inhibit cyclooxygenase activity in macrophages and epithelial cells. Proc. Natl. Acad. Sci. U. S. A. 2000;97:11494–11499. doi: 10.1073/pnas.200357097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jiang Z., Yin X., Jiang Q. Natural forms of vitamin E and 13'-carboxychromanol, a long-chain vitamin E metabolite, inhibit leukotriene generation from stimulated neutrophils by blocking calcium influx and suppressing 5-lipoxygenase activity, respectively. J. Immunol. 2011;186:1173–1179. doi: 10.4049/jimmunol.1002342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Joosten L.A., Helsen M.M., van de Loo F.A., van den Berg W.B. Anticytokine treatment of established type II collagen-induced arthritis in DBA/1 mice. A comparative study using anti-TNF alpha, anti-IL-1 alpha/beta, and IL-1Ra. Arthritis Rheum. 1996;39:797–809. doi: 10.1002/art.1780390513. [DOI] [PubMed] [Google Scholar]
- 79.Ruggiero C., Cherubini A., Guralnik J., Semba R.D., Maggio M., Ling S.M., Lauretani F., Bandinelli S., Senin U., Ferrucci L. The interplay between uric acid and antioxidants in relation to physical function in older persons. J. Am. Geriatr. Soc. 2007;55:1206–1215. doi: 10.1111/j.1532-5415.2007.01260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dalbeth N., Horne A., Gamble G.D., Ames R., Mason B., McQueen F.M., Bolland M.J., Grey A., Reid I.R. The effect of calcium supplementation on serum urate: analysis of a randomized controlled trial. Rheumatology (Oxford) 2009;48:195–197. doi: 10.1093/rheumatology/ken416. [DOI] [PubMed] [Google Scholar]
- 81.Hui J.Y., Choi J.W., Mount D.B., Zhu Y., Zhang Y., Choi H.K. The independent association between parathyroid hormone levels and hyperuricemia: a national population study. Arthritis Res. Ther. 2012;14:R56. doi: 10.1186/ar3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chapuy M.C., Preziosi P., Maamer M., Arnaud S., Galan P., Hercberg S., Meunier P.J. Prevalence of vitamin D insufficiency in an adult normal population. Osteoporos. Int. 1997;7:439–443. doi: 10.1007/s001980050030. [DOI] [PubMed] [Google Scholar]


