Abstract
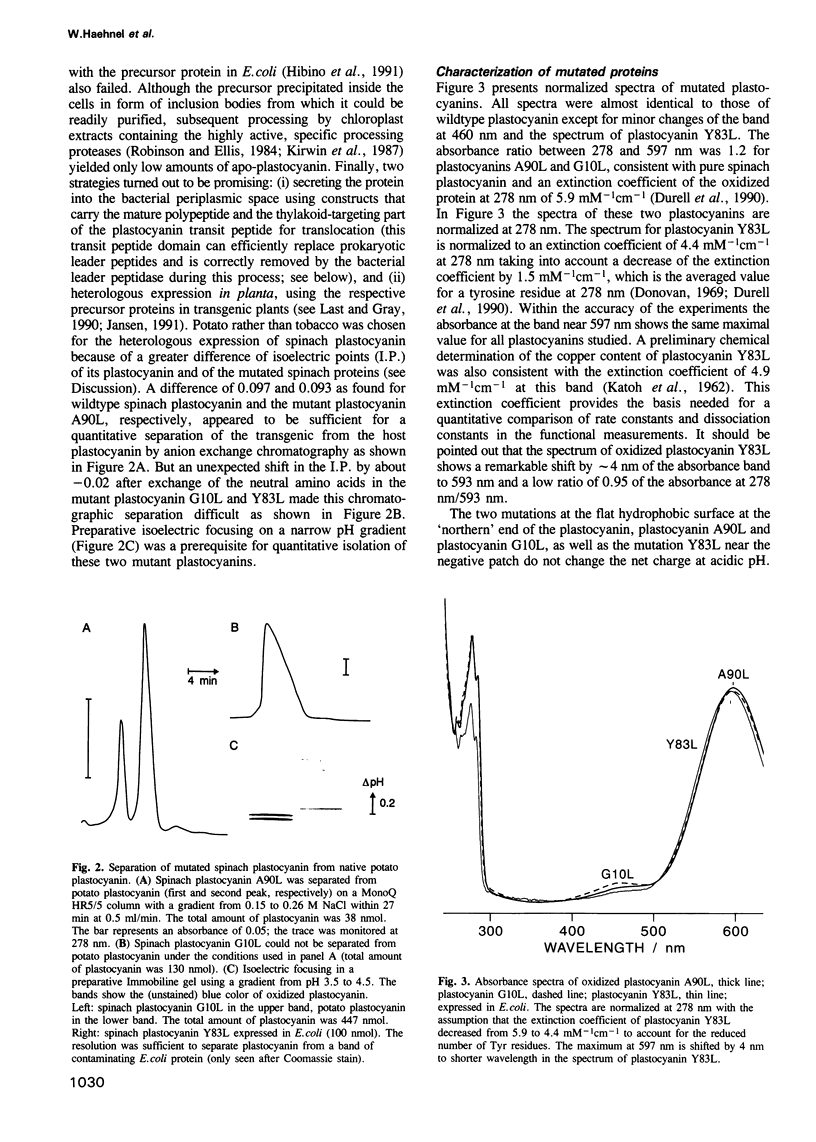
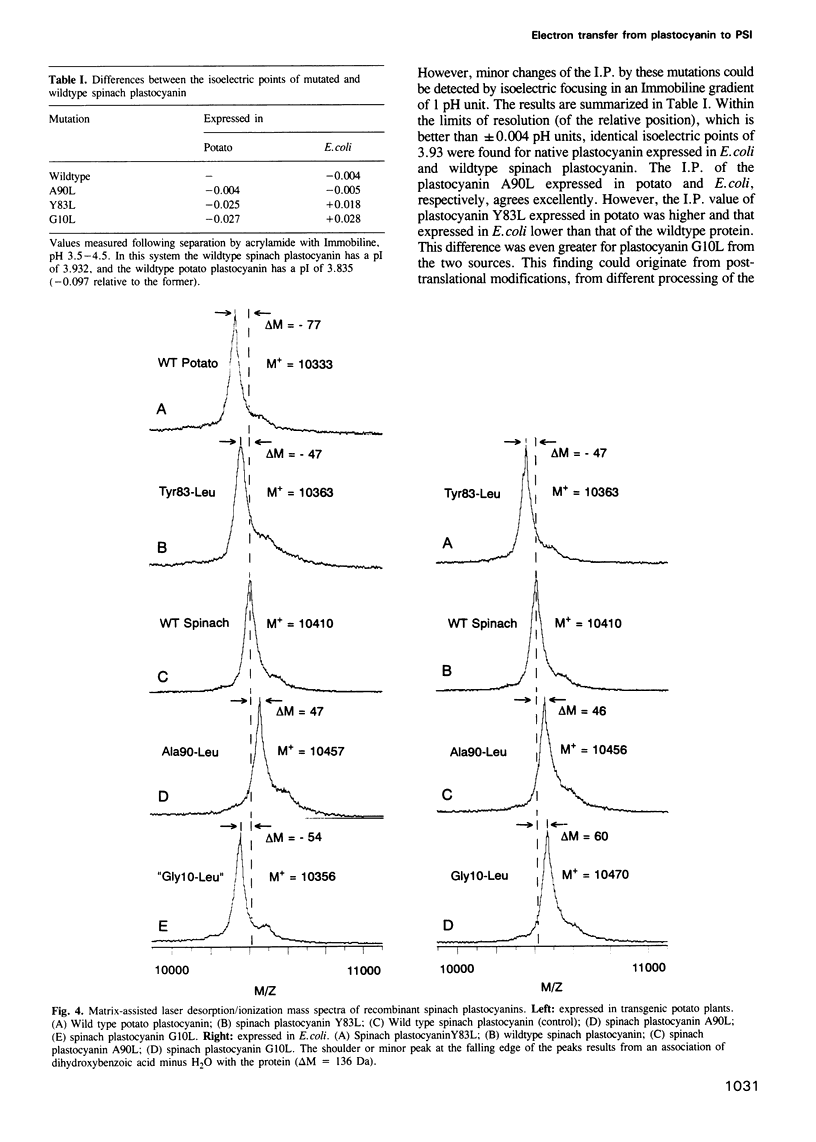
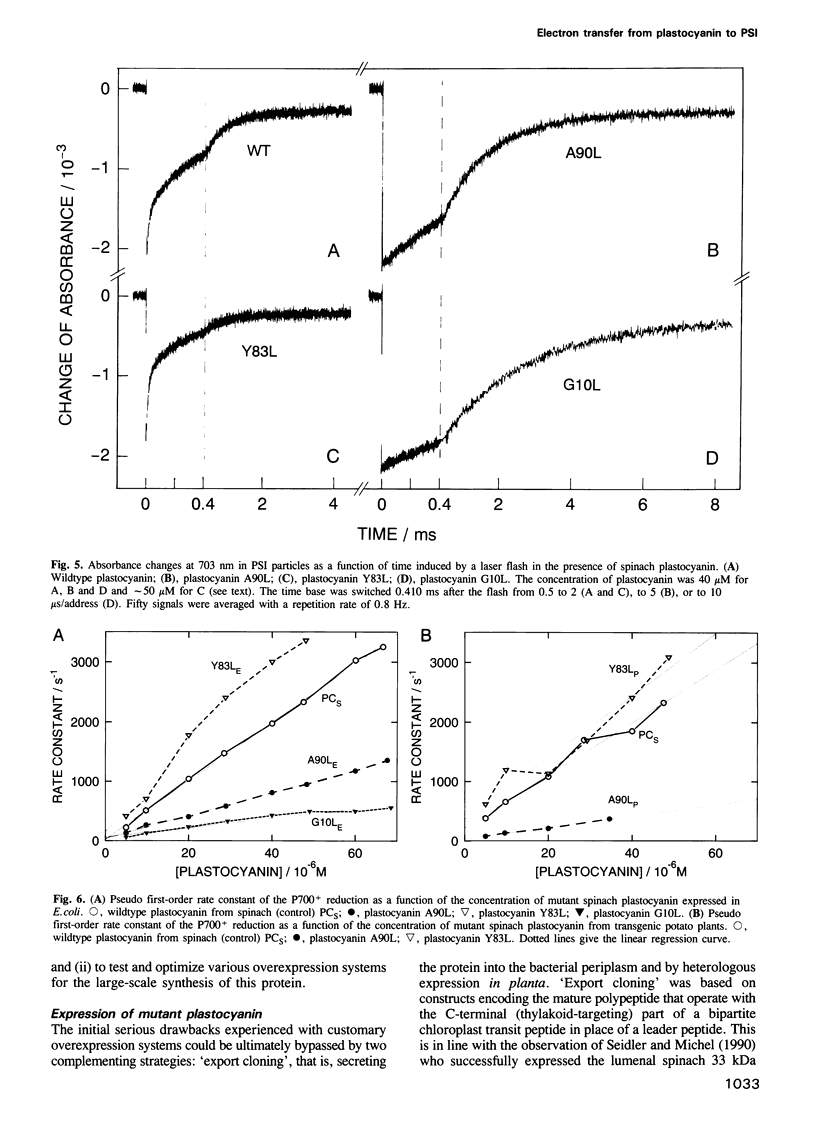
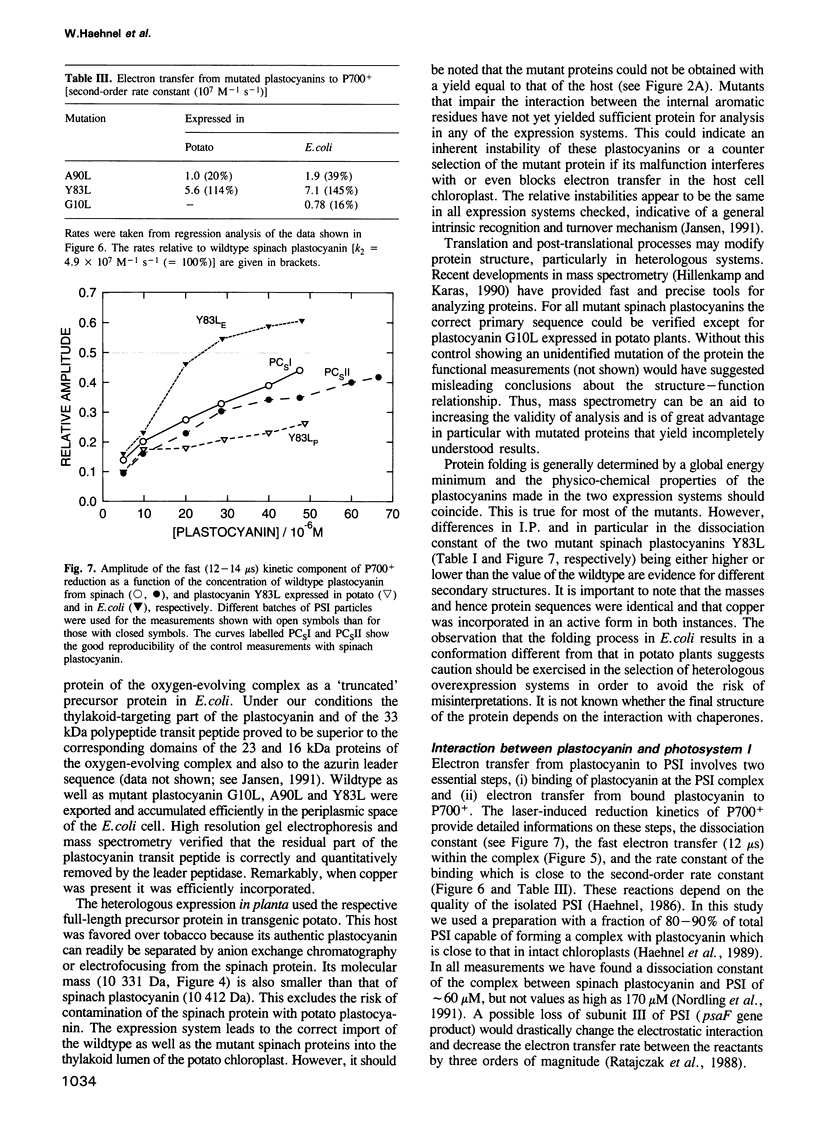
Mutant plastocyanins with Leu at position 10, 90 or 83 (Gly, Ala and Tyr respectively in wildtype) were constructed by site-specific mutagenesis of the spinach gene, and expressed in transgenic potato plants under the control of the authentic plastocyanin promoter, as well as in Escherichia coli as truncated precursor intermediates carrying the C-terminal 22 amino acid residues of the transit peptide, i.e. the thylakoid-targeting domain that acts as a bacterial export signal. The identity of the purified plastocyanins was verified by matrix-assisted laser desorption/ionization mass spectrometry. The formation of a complex between authentic or mutant spinach plastocyanin and isolated photosystem I and the electron transfer has been studied from the biphasic reduction kinetics of P700+ after excitation with laser flashes. The formation of the complex was abolished by the bulky hydrophobic group of Leu at the respective position of G10 or A90 which are part of the conserved flat hydrophobic surface around the copper ligand H87. The rate of electron transfer decreased by both mutations to < 20% of that found with wildtype plastocyanin. We conclude that the conserved flat surface of plastocyanin represents one of two crucial structural elements for both the docking at photosystem I and the efficient electron transfer via H87 to P700+. The Y83L mutant exhibited faster electron transfer to P700+ than did authentic plastocyanin. This proves that Y83 is not involved in electron transfer to P700 and suggests that electron transfer from cytochrome f and to P700 follows different routes in the plastocyanin molecule. Plastocyanin (Y83L) expressed in either E. coli or potato exhibited different isoelectric points and binding constants to photosystem I indicative of differences in the folding of the protein. The structure of the binding site at photosystem I and the mechanism of electron transfer are discussed.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- A simple and general method for transferring genes into plants. Science. 1985 Mar 8;227(4691):1229–1231. doi: 10.1126/science.227.4691.1229. [DOI] [PubMed] [Google Scholar]
- Ahlquist P., Janda M. cDNA cloning and in vitro transcription of the complete brome mosaic virus genome. Mol Cell Biol. 1984 Dec;4(12):2876–2882. doi: 10.1128/mcb.4.12.2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann E., Brosius J., Ptashne M. Vectors bearing a hybrid trp-lac promoter useful for regulated expression of cloned genes in Escherichia coli. Gene. 1983 Nov;25(2-3):167–178. doi: 10.1016/0378-1119(83)90222-6. [DOI] [PubMed] [Google Scholar]
- Anderson G. P., Sanderson D. G., Lee C. H., Durell S., Anderson L. B., Gross E. L. The effect of ethylenediamine chemical modification of plastocyanin on the rate of cytochrome f oxidation and P-700+ reduction. Biochim Biophys Acta. 1987 Dec 17;894(3):386–398. doi: 10.1016/0005-2728(87)90117-4. [DOI] [PubMed] [Google Scholar]
- Augustin M. A., Chapman S. K., Davies D. M., Sykes A. G., Speck S. H., Margoliash E. Interaction of cytochrome c with the blue copper proteins, plastocyanin and azurin. J Biol Chem. 1983 May 25;258(10):6405–6409. [PubMed] [Google Scholar]
- Bevan M. Binary Agrobacterium vectors for plant transformation. Nucleic Acids Res. 1984 Nov 26;12(22):8711–8721. doi: 10.1093/nar/12.22.8711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bujard H., Gentz R., Lanzer M., Stueber D., Mueller M., Ibrahimi I., Haeuptle M. T., Dobberstein B. A T5 promoter-based transcription-translation system for the analysis of proteins in vitro and in vivo. Methods Enzymol. 1987;155:416–433. doi: 10.1016/0076-6879(87)55028-5. [DOI] [PubMed] [Google Scholar]
- Chait B. T., Kent S. B. Weighing naked proteins: practical, high-accuracy mass measurement of peptides and proteins. Science. 1992 Sep 25;257(5078):1885–1894. doi: 10.1126/science.1411504. [DOI] [PubMed] [Google Scholar]
- Clausmeyer S., Klösgen R. B., Herrmann R. G. Protein import into chloroplasts. The hydrophilic lumenal proteins exhibit unexpected import and sorting specificities in spite of structurally conserved transit peptides. J Biol Chem. 1993 Jul 5;268(19):13869–13876. [PubMed] [Google Scholar]
- Covey T. R., Huang E. C., Henion J. D. Structural characterization of protein tryptic peptides via liquid chromatography/mass spectrometry and collision-induced dissociation of their doubly charged molecular ions. Anal Chem. 1991 Jul 1;63(13):1193–1200. doi: 10.1021/ac00013a003. [DOI] [PubMed] [Google Scholar]
- Doerfler W. Expression of the Autographa californica nuclear polyhedrosis virus genome in insect cells: homologous viral and heterologous vertebrate genes--the baculovirus vector system. Curr Top Microbiol Immunol. 1986;131:51–68. doi: 10.1007/978-3-642-71589-1_4. [DOI] [PubMed] [Google Scholar]
- Durell S. R., Lee C. H., Ross R. T., Gross E. L. Factor analysis of the near-ultraviolet absorption spectrum of plastocyanin using bilinear, trilinear, and quadrilinear models. Arch Biochem Biophys. 1990 Apr;278(1):148–160. doi: 10.1016/0003-9861(90)90243-r. [DOI] [PubMed] [Google Scholar]
- Figurski D. H., Helinski D. R. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz H. J., Hohlmaier J., Kramer W., Ohmayer A., Wippler J. Oligonucleotide-directed construction of mutations: a gapped duplex DNA procedure without enzymatic reactions in vitro. Nucleic Acids Res. 1988 Jul 25;16(14B):6987–6999. doi: 10.1093/nar/16.14.6987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guss J. M., Freeman H. C. Structure of oxidized poplar plastocyanin at 1.6 A resolution. J Mol Biol. 1983 Sep 15;169(2):521–563. doi: 10.1016/s0022-2836(83)80064-3. [DOI] [PubMed] [Google Scholar]
- Guss J. M., Harrowell P. R., Murata M., Norris V. A., Freeman H. C. Crystal structure analyses of reduced (CuI) poplar plastocyanin at six pH values. J Mol Biol. 1986 Nov 20;192(2):361–387. doi: 10.1016/0022-2836(86)90371-2. [DOI] [PubMed] [Google Scholar]
- Haehnel W., Pröpper A., Krause H. Evidence for complexed plastocyanin as the immediate electron donor of P-700. Biochim Biophys Acta. 1980 Dec 3;593(2):384–399. doi: 10.1016/0005-2728(80)90075-4. [DOI] [PubMed] [Google Scholar]
- Haehnel W., Ratajczak R., Robenek H. Lateral distribution and diffusion of plastocyanin in chloroplast thylakoids. J Cell Biol. 1989 Apr;108(4):1397–1405. doi: 10.1083/jcb.108.4.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S., Modi S., Bendall D. S., Gray J. C. The surface-exposed tyrosine residue Tyr83 of pea plastocyanin is involved in both binding and electron transfer reactions with cytochrome f. EMBO J. 1991 Dec;10(13):4011–4016. doi: 10.1002/j.1460-2075.1991.tb04976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibino T., Douwe de Boer A., Weisbeek P. J., Takabe T. Reconstitution of mature plastocyanin from precursor apo-plastocyanin expressed in Escherichia coli. Biochim Biophys Acta. 1991 Jun 17;1058(2):107–112. doi: 10.1016/s0005-2728(05)80226-9. [DOI] [PubMed] [Google Scholar]
- Hillenkamp F., Karas M. Mass spectrometry of peptides and proteins by matrix-assisted ultraviolet laser desorption/ionization. Methods Enzymol. 1990;193:280–295. doi: 10.1016/0076-6879(90)93420-p. [DOI] [PubMed] [Google Scholar]
- Johnston M., Davis R. W. Sequences that regulate the divergent GAL1-GAL10 promoter in Saccharomyces cerevisiae. Mol Cell Biol. 1984 Aug;4(8):1440–1448. doi: 10.1128/mcb.4.8.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATOH S., SHIRATORI I., TAKAMIYA A. Purification and some properties of spinach plastocyanin. J Biochem. 1962 Jan;51:32–40. doi: 10.1093/oxfordjournals.jbchem.a127497. [DOI] [PubMed] [Google Scholar]
- Kirwin P. M., Elderfield P. D., Robinson C. Transport of proteins into chloroplasts. Partial purification of a thylakoidal processing peptidase involved in plastocyanin biogenesis. J Biol Chem. 1987 Dec 5;262(34):16386–16390. [PubMed] [Google Scholar]
- Kramer W., Drutsa V., Jansen H. W., Kramer B., Pflugfelder M., Fritz H. J. The gapped duplex DNA approach to oligonucleotide-directed mutation construction. Nucleic Acids Res. 1984 Dec 21;12(24):9441–9456. doi: 10.1093/nar/12.24.9441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Last D. I., Gray J. C. Synthesis and accumulation of pea plastocyanin in transgenic tobacco plants. Plant Mol Biol. 1990 Feb;14(2):229–238. doi: 10.1007/BF00018563. [DOI] [PubMed] [Google Scholar]
- Modi S., Nordling M., Lundberg L. G., Hansson O., Bendall D. S. Reactivity of cytochromes c and f with mutant forms of spinach plastocyanin. Biochim Biophys Acta. 1992 Aug 28;1102(1):85–90. doi: 10.1016/0005-2728(92)90068-d. [DOI] [PubMed] [Google Scholar]
- Nordling M., Sigfridsson K., Young S., Lundberg L. G., Hansson O. Flash-photolysis studies of the electron transfer from genetically modified spinach plastocyanin to photosystem I. FEBS Lett. 1991 Oct 21;291(2):327–330. doi: 10.1016/0014-5793(91)81313-w. [DOI] [PubMed] [Google Scholar]
- Plesnicar M., Bendall D. S. The plastocyanin content of chloroplasts from some higher plants estimated by a sensitive enzymatic assay. Biochim Biophys Acta. 1970 Aug 4;216(1):192–199. doi: 10.1016/0005-2728(70)90170-2. [DOI] [PubMed] [Google Scholar]
- Robinson C., Ellis R. J. Transport of proteins into chloroplasts. Partial purification of a chloroplast protease involved in the processing of important precursor polypeptides. Eur J Biochem. 1984 Jul 16;142(2):337–342. doi: 10.1111/j.1432-1033.1984.tb08291.x. [DOI] [PubMed] [Google Scholar]
- Rosenberg A. H., Lade B. N., Chui D. S., Lin S. W., Dunn J. J., Studier F. W. Vectors for selective expression of cloned DNAs by T7 RNA polymerase. Gene. 1987;56(1):125–135. doi: 10.1016/0378-1119(87)90165-x. [DOI] [PubMed] [Google Scholar]
- Rother C., Jansen T., Tyagi A., Tittgen J., Herrmann R. G. Plastocyanin is encoded by an uninterrupted nuclear gene in spinach. Curr Genet. 1986;11(3):171–176. doi: 10.1007/BF00420603. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidler A., Michel H. Expression in Escherichia coli of the psbO gene encoding the 33 kd protein of the oxygen-evolving complex from spinach. EMBO J. 1990 Jun;9(6):1743–1748. doi: 10.1002/j.1460-2075.1990.tb08298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeekens S., Bauerle C., Hageman J., Keegstra K., Weisbeek P. The role of the transit peptide in the routing of precursors toward different chloroplast compartments. Cell. 1986 Aug 1;46(3):365–375. doi: 10.1016/0092-8674(86)90657-4. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Moffatt B. A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986 May 5;189(1):113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- Stueber D., Ibrahimi I., Cutler D., Dobberstein B., Bujard H. A novel in vitro transcription-translation system: accurate and efficient synthesis of single proteins from cloned DNA sequences. EMBO J. 1984 Dec 20;3(13):3143–3148. doi: 10.1002/j.1460-2075.1984.tb02271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiede D. M., Vashishta A. C., Gunner M. R. Electron-transfer kinetics and electrostatic properties of the Rhodobacter sphaeroides reaction center and soluble c-cytochromes. Biochemistry. 1993 May 4;32(17):4515–4531. doi: 10.1021/bi00068a006. [DOI] [PubMed] [Google Scholar]
- Wynn R. M., Malkin R. Interaction of plastocyanin with photosystem I: a chemical cross-linking study of the polypeptide that binds plastocyanin. Biochemistry. 1988 Aug 9;27(16):5863–5869. doi: 10.1021/bi00416a007. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Signal sequences. The limits of variation. J Mol Biol. 1985 Jul 5;184(1):99–105. doi: 10.1016/0022-2836(85)90046-4. [DOI] [PubMed] [Google Scholar]




