Abstract
Previous studies on Iberian × Landrace (IBMAP) pig intercrosses have enabled the identification of several quantitative trait locus (QTL) regions related to growth and fatness traits; however, the genetic variation underlying those QTLs are still unknown. These traits are not only relevant because of their impact on economically important production traits, but also because pig constitutes a widely studied animal model for human obesity and obesity-related diseases. The hypothalamus is the main gland regulating growth, food intake, and fat accumulation. Therefore, the aim of this work was to identify genes and/or gene transcripts involved in the determination of growth and fatness in pig by a comparison of the whole hypothalamic transcriptome (RNA-Seq) in two groups of phenotypically divergent IBMAP pigs. Around 16,000 of the ∼25.010 annotated genes were expressed in these hypothalamic samples, with most of them showing intermediate expression levels. Functional analyses supported the key role of the hypothalamus in the regulation of growth, fat accumulation, and energy expenditure. Moreover, 58,927 potentially new isoforms were detected. More than 250 differentially expressed genes and novel transcript isoforms were identified between the two groups of pigs. Twenty-one DE genes/transcripts that colocalized in previously identified QTL regions and/or whose biological functions are related to the traits of interest were explored in more detail. Additionally, the transcription factors potentially regulating these genes and the subjacent networks and pathways were also analyzed. This study allows us to propose strong candidate genes for growth and fatness based on expression patterns, genomic location, and network interactions.
Keywords: RNA-Seq, porcine, hypothalamus, growth, fatness
growth and fatness are some of the most economically important traits for meat production in livestock. Phenotypic traits such as average daily gain, backfat thickness, and carcass composition are closely related to the efficiency of pig production. Both growth and fatness are the outcome of the interactions between many genetic and environmental factors. Understanding the genetic pathways regulating these traits may provide new tools that will help to modulate animal growth and efficiency. Moreover, the pig is also being used as a biomedical model for studying human obesity, energy metabolism, and diabetes (58). In particular, the high similarity in metabolic and digestive features as well as other anatomical and physiological characteristics between human and pig makes the pig a favorable animal model compared with rodents, especially when translating results to the human. Despite extensive research aimed at identifying the molecular basis of obesity and related diseases, the underlying physiogenetic mechanisms have not been clearly identified (58). Furthermore, the use of pigs as a model offers the possibility of mating phenotypically divergent animals, creating populations segregating the traits of interest and subsequently intercrossing the offspring (30). Hence, studying the genetic basis of growth and fatness in the pigs is of interest from both an animal production and biomedical point of view.
Even though the regulation of feeding behavior is very complex and different brain areas are involved, the hypothalamus has been shown to be the main feeding regulatory center, responsible for food intake, energy homeostasis, and body weight (20). The hypothalamus is crucial for the detection of nutrient levels, and, in response to different brain and peripheral signals from the gut and adipose tissue, it modulates food intake and energy expenditure (57). The hypothalamus consists of several interconnected nuclei, including the arcuate nucleus (ARC), the paraventricular nucleus (PVN), the lateral hypothalamic area (LHA), the ventromedial nucleus (VMH), and the dorsomedial nucleus (DMH) (20, 57). The ARC is the key nucleus, strategically positioned to integrate a number of peripheral signals controlling food intake (57). The signal from ARC projects to secondary neurons in the PVN, LHA, VMH, and DMH that are organized into a complex network of orexigenic and anorexigenic neuropeptides that responds to meal-satiety signals for long-term regulation of energy homeostasis (20).
Several quantitative trait locus (QTL) regions have been identified in pig associated with growth and fatness (Pig QTLdb; 21), but there are few examples where the genetic variation responsible for the QTL effects have been identified (37, 63). We performed high-throughput RNA sequencing (RNA-Seq) of hypothalamic tissue to identify the genes responsible for these QTL effects. RNA-Seq technology is a powerful approach that allows a genome-wide transcriptome characterization and identification of the full set of transcripts expressed. RNA-Seq provides enormously detailed insight in transcript expression and in the discovery of new transcripts and gene polymorphisms in a single assay. In the last years, several studies have employed RNA-Seq to explore the porcine transcriptome of different tissues such as liver (3, 17, 26, 52), gonads (9, 17), endometrium (55), traqueobronchial lymph nodes (38), longissimus dorsi muscle, and abdominal fat (3). Previous pig hypothalamic transcriptome analyses have been conducted using microarrays (1, 11, 48, 69) or expressed sequence tags (ESTs) (15) focusing on sex or breed characterization. However, no hypothalamic RNA-Seq transcriptome studies have been carried out in pig so far. To our knowledge the only hypothalamic RNA-Seq transcriptome study performed in a livestock species has been conducted in cattle and focused on fertility-related traits (12).
The Iberian × Landrace (IBMAP) experimental population was specifically developed to identify QTLs for growth, fatness, and body composition traits, because of the large phenotypic divergence of both parental breeds in relation to these traits (47). Several studies based on this population have allowed the detection of QTL regions for the traits of interest (10, 65). Significant QTL regions associated with growth and fatness have been identified on porcine chromosomes (SSC) 2, 4, 5, 6, 14, and 17. Moreover, some candidate genes, such as LEPR, FABP4, or FABP5 have been analyzed reporting relevant associations of polymorphisms of these genes (LEPRc.1986C>T, FABP4:g.2634_2635insC, FABP4:g.6252C>T, and FABP5;g.3000T>G) with the traits of interest (8, 46). However, the identification and confirmation of the causal mutations underlying those QTLs have not been successfully solved yet, and further information is needed to understand the complex genetic basis of these traits.
In the present study, RNA-Seq technology has been employed to compare the transcriptome of hypothalamic samples of divergent pigs for growth and fatness traits from the IBMAP population, with the aim of identifying genes related to these traits taking into account previous reported results.
MATERIALS AND METHODS
Animal Material, RNA Isolation, and Sequencing
Animal manipulations were performed according to the Spanish Policy for Animal Protection RD1201/05, which meets the European Union Directive 86/609 about the protection of animals used in experimentation. Research protocols were approved by Animal Care and Use Committee of the Institut de Recerca I Tecnologies Agroalimentaries. The animals used in the present study grew in an experimental farm under good conditions and were fed according to their needs. Electric stunning was used to ameliorate the suffering of the animals before death. The animal material used in the present study is derived from a backcross (BC) generated from the IBMAP experimental population (45). In brief, three Iberian boars were mated with 30 Landrace sows (F0) to produce 70 F1 animals. To generate the BC, five F1 boars were mated with 25 Landrace sows, and 187 BC animals were obtained. All pigs were grown in an experimental farm under standard conditions. The animals were slaughtered at an approximate age of 175 days. Phenotypic traits related to growth, fatness, and body composition were measured in all BC animals (Table 1).
Table 1.
Phenotypic traits recorded from the backcrossed animals of the IBMAP population
| Whole Population |
Males |
Females |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Description | n | Mean | SD | n | Mean | SD | n | Mean | SD |
| Average daily gain, kg/day | 162 | 0.728 | 0.11 | 74 | 0.77 | 0.11 | 89 | 0.69 | 0.10 |
| Backfat thickness at around 90 kg, mm | 162 | 14.82 | 1.93 | 74 | 14.38 | 1.66 | 89 | 15.19 | 2.07 |
| Percentage of C18:2 in backfat | 157 | 14.22 | 1.76 | 74 | 14.73 | 1.84 | 89 | 13.81 | 1.59 |
| Percentage of C18:2 in intramuscular fat | 144 | 10.36 | 2.36 | 74 | 11.37 | 2.43 | 89 | 9.53 | 1.95 |
IBMAP, Iberian × Landrace.
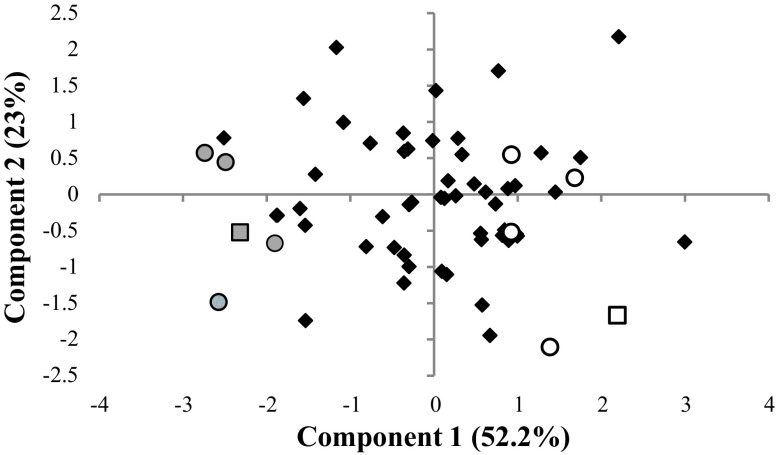
A principal component analysis (PCA) of the BC animals was performed according to four indicators for growth and fatness traits: average daily gain (kg/day), backfat thickness at 90 kg (mm), and percentages of C18:2 in backfat and intramuscular fat. Note that the C18:2 content of fat tissue is positively correlated to pig lean growth (67). These data were used to select the most extreme pigs. The selection was limited to males and the same slaughter batch, to avoid sex and batch effects. The 10 male pigs from the same slaughter batch with the most extreme phenotypes, according to the first principal component, were selected for this study and arranged into two groups (Fig. 1). The five males showing the highest values of growth and fatness indicators were included in the High (H) group, and the five males showing the lowest values for these traits were included in the Low (L) group (Fig. 1). The mean values of the four indicators in the H and L groups were, respectively, 0.92–0.74 kg/day of average daily gain, 16.2–11.6 mm of backfat thickness, 12.6–16.7% of C18:2 in backfat, and 8.1–11.9% of C18:2 in intramuscular fat.
Fig. 1.
Graphical representation of the 1st and 2nd principal components of the principal component analysis summarizing the phenotypical variation of the 4 traits related to growth and fatness. Animals assigned to the High (H) group are highlighted with white circles, and those to the Low (L) group with gray circles. The rest of the samples are represented by black diamonds. Samples excluded for further analyses are represented as squares.
Hypothalamic samples of the 10 selected animals were collected at slaughter, immediately frozen in liquid nitrogen and stored at −80°C until analyzed. Total RNA was extracted using the RiboPure of High Quality total RNA kit (Ambion, Austin, TX) following the manufacturer's recommendations and quantified using a NanoDrop-100 spectrophotometer (NanoDrop Technologies, Wilmington, DE). The integrity of the RNA was assessed with an Agilent 2100 Bioanalyzer device (Agilent Technologies, Santa Clara, CA). The RNA integrity value of the samples ranged between 7.1 and 8.1. Paired-end libraries with fragments of 300 bp were prepared using the TruSeq RNA Sample Prep Kit v2 (Illumina, San Diego CA) for each sample. Multiplex sequencing of the libraries was performed on a Illumina Hi-Seq 2000 (Fasteris, Plan-les-Ouates, Switzerland) with three samples per lane, according to the manufacturer's instructions at Centro Nacional de Análisis Genómico, generating paired-end reads of 75 bp. The raw sequence data have been deposited in the Gene Expression Omnibus (GEO) expression database under the accession number: GSE51968.
Mapping and Assembly
Quality of the raw sequencing data was accessed with FastQC (http://www.bioinformatics.babraham.ac.uk/projects/fastqc/). Trim Galore (http://www.bioinformatics.babraham.ac.uk/projects/trim_galore/) was used to quality trim the data with default settings and to remove the sequencing adaptors and poly A and T tails (stringency of 6 bp, -s 6) keeping only paired-end reads where both pairs were longer than 40 bp. Filtered reads were mapped against the pig reference genome (Sscrofa10.2) (16) using TopHat v.2.0.5 (60) with Bowtie2 (v.2.0.0.7) applying default settings except that reads were first aligned to the ENSEMBL (10.2.68) transcriptome annotation (-G option), and the distance between both pairs was set to 50 bp (inner-mean distance) and the standard deviation at 150 bp.
Transcripts were assembled and quantified in FPKM (fragments per kilobase of transcript per million mapped fragments) by Cufflinks v2.0.2 (61). The normalized expression values have been deposited in the GEO expression database under the accession number: GSE51968.1
Additionally, the CummeRbund Bioconductor R package (13) was employed to analyze Cuffdiff (see below) output and determine the clustering of the samples according to the expression data, to assess the consistence of the groups that are being compared. Two of the samples, one from each group, were discarded from the study because their clustering deviate largely from the expected clustering, probably due to sampling or RNA processing problems.
Identification of Novel Isoforms
Novel expressed isoforms were extracted by the Cuffcompare tool from Cufflinks. Cuffcompare was run with the ENSEMBL (10.2.68) transcriptome annotation as a reference for assessing the accuracy of the predicted Cufflinks mRNAs or gene models and reducing the set of reference transcripts to only those found to overlap any of the input loci. With these options, Cuffcompare compares Cufflinks transfrags with a reference annotation and classifies them in different class codes, such as novel isoforms, intergenic, or intronic transcripts. Isoforms are only considered novel when a transfrag shared at least one splice junction with a reference transcript.
Expression Quantification and Differential Expression Analysis
After the assembly phase, the Cuffdiff tool (61) from Cufflinks was used to calculate expression values and to perform the differential expression analysis of the annotated genes and the newly predicted isoforms detected between H and L groups. Cuffdiff was run using the bias correction (-b option) to improve accuracy of transcript abundance estimates, and the “rescue method” for multireads (-u option) to more accurately weight reads mapping to multiple locations in the genome. The remaining parameters were set as default.
The genes and new isoforms were filtered according to two criteria: 1) a minimum mean group expression >0.5 FPKM, 2) a fold change of the expression differences between H and L groups ≥1.5. The widely used fold change ≥1.5 has been set for data filtering as previous studies reported that this threshold improves the results by eliminating background noise (5). The R package q value (59) was employed to calculate the false discovery rate (FDR). Finally, those genes with a P value and a q value ≤ 0.05 were considered as differentially expressed (DE). Additionally, those novel isoforms with a P value ≤ 0.05 (equivalent to a q value ≤ 0.25) were considered as DE.
RNA-Seq Analysis Using the CLC Genomics Workbench Software
The whole RNA-Seq data analysis was also performed with the CLC Genomics Workbench using the same parameters as in the Tophat-Cufflinks analysis, to identify the overlapping between both methodologies. The strategies for filtering, mapping, transcript assembly, and quantification of this software are based on Mortazavi et al. (42). Mapping parameters were set as default, and the minimum and the maximum distance between pairs were defined as in the TopHat analysis. We excluded the broken pairs when counting the mapped reads to generate expression values. Expression data were normalized (RPKM values) by quantiles. The differential gene expression was analyzed by a t-test, where Gaussian distributions and homogeneous variance were assumed. Expression differences detected by both methodologies were considered the most consistent.
Validation of Differentially Expressed Genes and Novel Isoforms by quantitative PCR and Sequencing
To evaluate the reliability and reproducibility of the results obtained in the RNA-Seq analyses, we used complementary molecular analyses to validate several new isoforms and gene expression measures. Potentially novel isoforms identified by Cuffcompare were validated by cDNA sequencing. Among the DE novel isoforms, the ones identified for the PLAC8 and KIAA1462 genes were selected for validation. One primer pair was designed covering exons 1 to 3 of the PLAC8 gene (reference sequence: ENSSSCT0000010120) and another one between exons 2 and 3 (reference sequence: ENSSSCG00000011020) of the KIAA1462 gene (Table 2 for primer sequences). Different sizes of the PCR products allow for distinguishing the different isoforms. The PCR reactions were performed in a final volume of 25 μl, containing 2.5 μl of cDNA, 1 unit of Taq polymerase (Biotools), specific buffer, 2.5 mM of dNTPs, and 0.5 μM of each primer. Thermocycling was carried out under the following conditions: 94°C for 5 min, 40 cycles of 94°C for 45 s, 60°C for 45 s, and 72°C for 45 s, with a final extension of 72°C for 10 min. The PCR reactions were carried out in a GeneAmp PCR System 9700 (Applied Biosystems, Warrington, UK). The PCR products were purified with the GFX PCR DNA purification kit (GE Healthcare) according to the manufacturers' protocol. PCR products were sequenced with both forward and reverse primers using the 3100 BigDye Terminator v3.1 Matrix Standard in a 3730 DNA Analyzer (Applied Biosystems). The obtained sequences were edited and aligned with the EditSeq and MegAlign packages of the WinStar software for the comparison between the novel and the annotated isoforms in Ensembl.
Table 2.
Primer pairs, reference sequences according to which they have been designed, size of the amplified fragment, and PCR efficiency
| Transcript | Primer Sequence (5′ to 3′) | Reference Sequence | Size, bp | Eff., % |
|---|---|---|---|---|
| New isoform detection and quantification | ||||
| PLAC8 | Fw:GACACAGCCTGCCCAGAACCTC/Rv:AGACGCCGCAGTCGCTGAA | ENSSSCT00000010120 | 174 | 92 |
| KIAA1462 | Fw:GCGCCTGCCAGCCCGAGTC/Rv:GGCACTGTCCCTTTCGCCTACAA | ENSSSCG00000011020 | 150 | 72 |
| Reference gene quantification | ||||
| ACTB | Fw:TCTGGCACCACACCTTCT/Rv:GATCTGGGTCATCTTCTCAC | Erkens et al., 2006 | 114 | 83 |
| B2M | Fw:TTCACACCGCTCCAGTAG/Rv:CCAGATACATAGCAGTTCAGG | Kuijk et al., 2007 | 166 | 70 |
| TOP2B | Fw:AACTGGATGATGCTAATGATGCT/Rv:TGGAAAAACTCCGTATCTGTCTC | Erkens et al., 2006 | 137 | 74 |
| GADPH | Fw:TCGGAGTGAACGGATTTG/Rv:CCTGGAAGATGGTGATGG | Kuijk et al., 2007 | 219 | 87 |
| Differentially expressed gene quantification | ||||
| PRCP | Fw:GGTGCCCACTCATTCAAAGATTCC/Rv:CTGCAAGCATGCCACCGTAAGAG | ENSSSCG00000014889 | 163 | 89 |
| ACTA2 | Fw:AGAACACGGCATCATCACCAACTG/Rv:CACCGCCTGAATAGCCACATACAT | NM_001164650.1 | 205 | 81 |
| ADAMTS4 | Fw:CCGCACCCGCTTCCGTTCC/Rv:TGTAGCGAGGCACCCAGTCCAT | ENSSSCG00000006359 | 146 | 84 |
| VAMP8 | Fw:CCTGGCCCGGGGAGAAAACTTG/Rv:CCACCAGAACTTCCGAGCCACCTT | ENSSSCG00000022820 | 112 | 93 |
| IRF1 | Fw:ATCGGGCAGGACTTGGACATTGAA/Rv:TTCCCCTCCTCGTCCTCATCTGTT | NM_001097412.1 | 191 | 88 |
| BTG2 | Fw:AGGTTTTCAGCGGGGCTCTCC/Rv:CTCCCCGATGCGATAGGACACTT | NM_001097505.2 | 239 | 91 |
| FA2H | Fw:GGGCCTCTTCGTGCTGGGGATGCT/Rv:GGGGGAAGACCAGGCGGGACTCGT | XM_003126868.3 | 173 | 84 |
Eff., PCR efficiency.
In addition, to evaluate the reproducibility of the expression measures obtained, relative transcript quantification by RT-quantitative (q) PCR of seven DE genes (BTG2, ACTA2, PRCP, FA2H, ADAMTS4, VAMP8, and IRF1) and two DE novel isoforms (PLAC8 and KIAA1462) was performed in the eight animals. The reactions were performed in 384-well plates using the LightCycler480 Real-Time PCR System (Roche Diagnostic, Mannheim, Germany). Four widely used presumable constitutively expressed reference genes, GAPDH, B2M, TOP2B, and ACTB, were included in the plates to select the most suitable ones for expression data normalization. The GeNorm algorithm was used to identify the reference genes with the highest stability in this particular dataset. Real-time qPCR reactions were performed in a total volume of 20 μl containing 2.5 μl of cDNA (1/10 dilution), 10 μl of Roche LightCycler mix and 0.5 μl of the specific primer pairs. All primer pairs used are detailed in Table 2. Standard PCR on cDNA was carried out to verify amplicon sizes. Cycling conditions were 95°C for 10 min, followed by 45 cycles of 95°C (15 s) and 60°C (1 min), when the fluorescence was acquired. A dissociation curve to test PCR specificity was generated by one cycle at 95°C (15 s), followed by 60°C (20 s), and ramped up to 95°C with the fluorescence acquired during the increase to 0.01°C/s. All points and samples were run in triplicates as technical replicates and dissociation curves were analyzed for each individual replicate. Single peaks in the dissociation curves confirmed the specific amplification of the primer pairs and the absence of primer dimers. A nontemplate control, without cDNA, was included as negative control. PCR efficiency of each primer pair, shown in Table 2, was estimated by standard curve calculation using four points of cDNA serial dilutions (1, 1/2, 1/4, and 1/8) of a pool of the eight samples used. Data generated were analyzed with LightCycler 480 software (Roche) by the second derivative method (35). Mean crossing point (Cp) values were transformed to quantities by the comparative Cp method, setting the highest relative quantities for each gene to 1 (quantity = 10−ΔCp/slope). Data normalization was carried out with the reference genes GAPDH and ACTB, which showed the highest stability. The M stability value of GAPDH was 0.221 and for ACTB was 0.208, while TOP2B and B2M showed lower stabilities with M values of 0.242 and 0.260, respectively. Both selected reference genes showed efficiency values >90%. Moreover, the pairwise variation V2/3 value was 0.066, below the recommended 0.15 threshold (64), indicating that the use of these two reference genes would be suitable for normalizing this dataset.
Pearson correlations were calculated between RT-qPCR and RNA-Seq expression data for the nine tested transcripts.
Gene Functional Classification, Network and Pathway Analyses
A functional analysis of the genes expressed in hypothalamus was performed by analyzing pathways enrichment with the FatiGO browser from Babelomics 4.3 (http://babelomics.bioinfo.cipf.es) using the Reactome database and the human whole genome as the background. Similarly, a functional analysis of the differentially expressed genes between H and L groups was performed by analyzing GO enrichment with FatiGO using GO database. This software provides an adjusted P value according to the FDR procedure of Benjamini and Hochberg for the enrichment analyses. The functions and published literature of all relevant DE genes and isoforms have been analyzed with the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov/) and Ensembl (http://www.ensembl.org/) databases. In addition, Ingenuity Pathways Analysis (IPA) software (Ingenuity Systems, http://www.ingenuity.com) was employed to generate networks that allow us to identify the relationship among DE genes and between DE genes and other genes. Furthermore, the most significant biological functions associated with the genes in the network were also identified.
To identify potential regulatory elements of the most relevant genes identified, GeneCards database (http://www.genecards.org) was employed using the gene official names. From the GeneCards website, the regulatory elements were accessed through the SABiosciences Regulatory transcription factor binding sites application, which displays the most relevant transcription factor binding sites in the promoter of the query genes as predicted by SABiosciences' text mining application (http://www.sabiosciences.com) and the UCSC Genome Browser (http://genome.ucsc.edu/).
It should be noted that all these tools are based on the available information of different species and cell types and the relationship among genes and TF are not necessary conserved.
RESULTS AND DISCUSSION
Characterization of the Porcine Hypothalamic Transcriptome
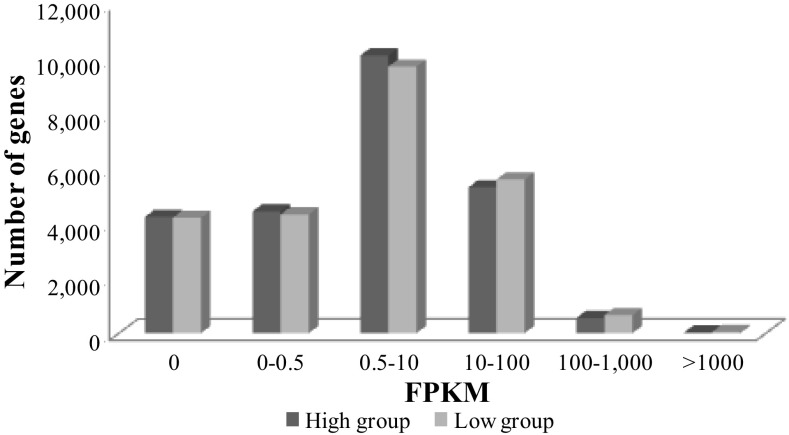
A total of 839 million paired-end reads were obtained from the transcriptome sequencing of the eight hypothalamic samples. After trimming and filtering according to quality parameters, we reduced this number to 1,026 million reads (Table 3). The TopHat pipeline mapped ∼83% of those reads against the pig reference genome (build 10.2). The distribution of the gene expression values obtained with Cuffdiff for the 25,010 genes annotated in the pig genome is represented in Fig. 2. Only 4,762 of the annotated genes showed no expression in this dataset, while 16,024 genes were expressed in these samples with a mean expression value >0.5 FPKM in both groups of animals. The number of expressed genes in this tissue was higher than found in other porcine tissues in previous studies. A total of 12,816 annotated genes were expressed in gonads (9), and ∼11,000 genes in liver (26, 52) with a mean expression value higher than zero in both cases. This result is in agreement with studies in mouse and human that have reported higher levels of RNA editing, associated with an increase in transcript diversity in brain compared with other pig tissues (7, 34). However, these differences could also be due to the use of a different annotation of the pig genome, the higher sequencing depth obtained in the present study, and the different filtering criteria set up in the different studies. Most of the genes showed a low or intermediate expression level between 0.5 and 100 FPKMs, and only a small proportion showed very high expression levels >1,000 FPKMs, in agreement with the results obtained in other tissues (9, 52).
Table 3.
Total number of reads, filtered reads, and percentage of mapped reads per sample
| Sample | Total Reads, n | Filtered Reads, n | Mapped Reads, % |
|---|---|---|---|
| High_1 | 100,805,876 | 98,564,915 | 58.00 |
| High_2 | 100,775,172 | 97,676,522 | 56.84 |
| High_3 | 133,564,350 | 131,213,812 | 60.88 |
| High_4* | 103,254,478 | 98,162,348 | 60.27 |
| High_5 | 98,490,020 | 95,578,205 | 55.55 |
| Low_1* | 116,030,772 | 112,047,709 | 61.94 |
| Low_2 | 106,603,392 | 103,399,969 | 60.04 |
| Low_3 | 105,426,048 | 102,701,020 | 62.10 |
| Low_4 | 106,184,416 | 103,190,054 | 62.96 |
| Low_5 | 87,573,458 | 84,170,362 | 57.68 |
Samples not included in further analyses.
Fig. 2.
Gene expression distribution of the 25,010 genes annotated in the pig genome in fragments per kilobase of transcript per million mapped fragments (FPKMs) normalized values for the H (black) and L (gray) groups.
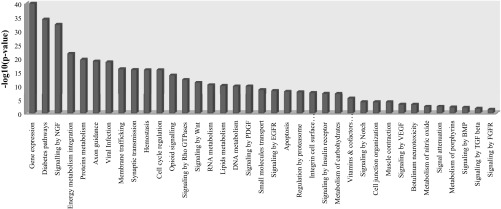
The functional enrichment analysis performed with Babelomics against the Reactome database revealed that genes expressed in the hypothalamus are involved in a wide range of functions (Fig. 3). As expected, among the strongest enriched pathways are several specific functions of the central nervous system such as axon guidance, nerve growth factor, and synaptic transmission. In addition, it is worth mentioning that 35% of the enriched pathways identified are related to signaling pathways, and therefore different types of growth factors signaling (platelet, epidermal, vascular, bone, and fibroblast growth factors) are overrepresented, supporting the importance of the hypothalamus in signaling, growth, and developmental processes. Also, diabetes and signaling by insulin receptor appeared as the second most enriched pathway, showing the high importance of the hypothalamus in glucose and insulin metabolism and signaling. These results, together with the enrichment in lipid metabolism signaling processes and energy metabolism integration, show the key role of this tissue for the traits of interest of the present study, supporting the decision to focus on the hypothalamus to investigate genes regulating growth and fatness. Moreover, previous studies based on the animal material used in the present study (IBMAP cross) have shown the relevance of the hypothalamic LEPR gene expression in growth and fatness (46, 50). An exonic polymorphism of this gene, LEPRc.1987C<T, is highly associated with growth, fatness, and body composition (46). Differential hypothalamic expression of two different LEPR isoforms was found linked to this single nucleotide polymorphism (SNP) (50). Unfortunately, despite the obvious importance of this gene, it could not be analyzed in the present study because LEPR is incompletely annotated in the current genome annotation.
Fig. 3.
Reactome pathways enriched in the set of genes expressed in the hypothalamus samples analyzed.
A total of 268,608 different transcripts were identified by Cufflinks in the eight hypothalamic samples. The classification of these transcripts performed with Cuffcompare is summarized in Table 4. A high percentage of the transcripts (27.8%) was annotated as intergenic transcripts, in agreement with previous studies in different porcine tissues: 36.1% of intergenic transcripts found in gonads (with the previous build 9 of the pig genome reference; 9) and 20.2% in liver (using the pig genome version 9.61 as reference; 52). There is also a large number of transcripts falling entirely within a reference intron (15.3%) that could indicate intron retention events, incorrect annotation of exons, and even errors or missing prediction of isoforms. The latter would be in agreement with the high number of potentially novel isoforms identified. A total of 58,927 potentially new isoforms was predicted by Cuffcompare, sharing at least one splice junction with a reference transcript and representing 21.9% of the total transcripts detected. All these data probably indicate the incompleteness of the available porcine genome annotation, especially regarding noncoding RNA genes, which could explain the large number of novel intergenic and intronic transcripts.
Table 4.
Classification of the transcripts identified in the hypothalamic samples in relation to the Ensembl annotated pig genes
| Cuffcompare Class | Transcripts, n | Transcripts, % |
|---|---|---|
| Complete match of intron chain | 8,702 | 3.2 |
| Multiple classifications | 33,965 | 12.6 |
| Contained in the reference | 22,923 | 8.5 |
| Possible pre-mRNA fragment | 9,122 | 3.4 |
| Transcript falling within a reference intron | 41,008 | 15.3 |
| Potentially novel isoforms | 58,927 | 21.9 |
| Generic overlap with a reference transcript | 8,196 | 3.0 |
| Possible polymerase run-on fragment | 7,763 | 2.9 |
| Intergenic transcript | 74,845 | 27.8 |
| Exonic overlap on the opposite strand | 3,157 | 1.1 |
| TOTAL | 268,608 | 100 |
Differential Expression Analysis
DE annotated genes.
The number of paired-end reads obtained before and after trimming were similar in both groups, ∼400 million reads for both H and L groups (Table 3). Similar percentages of mapped reads were also observed, 83.2% in the H group and 83.4% in the L group (Table 3). In addition, as shown in Fig. 2, global gene expression levels were equally distributed in both groups. Taking these into account, one can probably attribute a larger part of the differences in gene expression observed between both groups to the divergence of the two backcrossed breeds for growth and fatness traits. While the Landrace breed is characterized by a lean and fast growth, the Iberian breed shows a higher food intake, higher proportion of fat, and slower growth. The differential expression analysis of annotated genes performed with Cuffdiff revealed a total of 210 DE genes between H and L groups, according to the employed cut-off (mean expression ≥ 0.5 FPKM, fold change ≥ 1.5 and P value and q value ≤ 0.05). Among them, 17 genes showed highest expression in the H group, and the remaining 193 DE genes showed highest expression in the L group. The fold change of these DE genes ranged from 1.65 to 18.19. The GO enrichment analysis performed by FatiGO (Babelomics) on the 210 DE genes indicated that several GO molecular functions related to the traits of interest are overrepresented: peptidase activity (adjusted P value = 1.1 × 10−2), growth factor binding (adjusted P value = 5.0 × 10−3), and insulin-like growth factor binding (adjusted P value = 4.9 × 10−2). Similarly, among the GO biological processes enriched in the DE genes, several processes related to growth and fatness traits could be identified: skeletal system development (adjusted P value = 1.8 × 10−6), tissue development (adjusted P value = 9.7 × 10−4), response to hormone stimulus (adjusted P value = 4.7 × 10−8), transforming growth factor beta receptor signaling pathway (adjusted P value = 1.5 × 10−5), and response to nutrient levels (adjusted P value = 0.024). A selection of candidate genes was performed fitting two criteria: colocalization with previous QTLs identified in the same animal material and (/or) overlap with an independent differential expression analyses conducted with CLC Genomics software.
The main aim of the current study was to identify genes potentially causal of growth and fatness phenotypic differences. Therefore and taking into account previous reported results in the same animal material, we used two main criteria to highly genes and gene networks. Two criteria were employed to select the most interesting DE genes 1) being DE by the TopHat-Cuffdiff analysis and located within a QTL region associated to the traits of interest and 2) being DE by both analysis methodologies (TopHat-Cuffdiff and CLC Genomics).
DE GENES LOCATED WITHIN PREVIOUSLY DESCRIBED QTL REGIONS FOR GROWTH AND FATNESS.
Assuming that some of the QTL regions previously detected for growth and fatness (10) could carry gene expression regulatory mutations, the analysis was focused on identifying differentially expressed genes located within those regions that could explain the reported QTL effects.
In total 15 of the 210 DE genes were located close (within 8 Mb from QTL confidence interval) or within these QTL regions (Table 5), highlighting relevant candidate genes. A deeper analysis of these 15 DE genes revealed that six of them are functionally linked to the traits of interest (IRF1, ADAMTS4, FA2H, EGR-1, PMCH, and MFAP5; Table 5). The IRF1 codes for a transcription factor (TF) involved in growth hormone regulation (22), the ADAMTS4 gene is associated to an increase in beef marbling (33), the FA2H codes for a regulator of adipocyte differentiation and lipogenesis controlling body weight (19), and the TF EGR-1 gene product is involved in the interaction between leptin and cholecystokinin hormones, which play important roles in regulating food intake, meal size, and duration (31, 32). Moreover, a direct regulation of systemic cholesterol levels via regulation of cholesterol synthesis in the liver has also been reported for the EGR-1 gene (14). PMCH, described as a hypothalamus-specific gene (49), codes for the melanin-concentrating hormone (MCH) precursor, a key regulator of food intake and metabolism (43). Finally, MFAP5 gene expression has been correlated to changes in the amount of body fat, adiponectin, and leptin levels, as well as to the expression of several genes associated with adipose tissue development and differentiation such as PPARG, CCND2, and ADAM12 (62).
Table 5.
DE genes matching QTL regions previously detected for the traits of interest in the same animal material (8) and their respective position
| Chr. | DE Genes | Position, Mb | QTL CI, Mb | Associated Trait |
|---|---|---|---|---|
| 2 | IRF1* | 140 | 148–158 | mean weight of shoulders, weight of bone-in loins |
| EGR-1* | 146 | |||
| 4 | ADAMTS4* | 97 | 68–85 | mean weight of shoulders, weight of bone-in loins |
| C8ORF46 | 74 | |||
| SELE | 88 | |||
| 4 | HFM1 | 137 | 129–134 | backfat thickness at 75 kg |
| 5 | SLC6A13 | 69 | 69–83 | backfat thickness at slaughter |
| MFAP5* | 65 | |||
| PMCH* | 85 | |||
| 6 | FA2H* | 128 | 134–145 | backfat thickness at slaughter |
| 14 | CPXM2 | 145 | 149–153 | backfat thickness at 75 kg, backfat thickness at slaughter |
| 17 | MYL9 | 45 | 39–42 | backfat thickness at 75 kg |
| TGM2 | 46 | |||
| PROCR | 43 | |||
| CPXM1 | 37 |
Differentially expressed (DE) genes with biological functions related to the traits of interest. QTL, quantitative trait locus.
Chr, chromosome; CI, confidence interval.
DE GENES DETECTED BY TOPHAT-CUFFDIFF AND CLC GENOMICS.
To focus on those genes with the highest evidence of being DE between both groups, an additional RNA-Seq data analysis was conducted with CLC Genomics Workbench software using the same parameters and reference genome as in the Tophat-Cufflinks analysis. There are several differences in the methodology used by both analyses (CLC user manual, http://www.clcsupport.com/clcgenomicsworkbench/current/; Cufflinks user manual, http://cufflinks.cbcb.umd.edu/howitworks.html). The CLC Genomics performs normalization by quantiles of the RPKM expression values, and the Cufflinks uses the FPKM normalized values. Besides, the Cufflinks pipeline incorporates some options such as the multimapped read correction and the sequence bias correction that improve the estimation of the abundances. However, the greatest differences are in the approaches used to identify DE genes. The CLC Genomics assumes a Gaussian distribution of the data, whereas Cuffdiff “learns” the distribution from the input data, providing a most accurate estimation. Additionally, the CLC Genomics performs a classic test based on the means and variances within groups and then calculates a t-test assuming homogeneous variances and calculates a two-sided P value, while the Cuffdiff is based in the log-fold-change, estimates the variance in the log-fold-change using simulated draws of the model of variance in expression for each group, and calculates an empirical P value based on these simulations. It is notable that the correlations between expression measures obtained by both RNA-Seq analysis methodologies, TopHat-Cufflinks and CLC Genomics, were high and significant (the mean correlation was 0.88, P value < 0.01). Moreover, the concordance correlation coefficient (CCC) (39) that provides a global indication of the reproducibility of gene expression studies was 0.98, indicating an almost perfect agreement between both RNA-Seq analysis methodologies. Even though the concordance in gene expression measures between both methodologies is high, as mentioned above, the strategies to analyze the differential expression are different. Those genes that appeared as DE in both analyses were considered the most consistent. A total of 384 DE genes were detected by the CLC Genomics and 210 by the TopHat-Cuffdiff analyses, and 56 of these annotated genes were identified as DE by both approaches. Among them, 13 were considered as most interesting as they have previously been associated with growth and fatness traits in pig or other mammalian species (PRCP, FA2H, ALDH2, IRF1, ANGPTL2, VAMP8, PLAC8, JUNB, TNFAIP3, BMP5, BTG2, ACTA2, and ADAMTS4). Nine of them have known biological functions directly related to these traits: PRCP, FA2H, ALDH2, IRF1, ANGPTL2, VAMP8, PLAC8, JUNB, and TNFAIP3, and two, IRF1 and FA2H genes, are located within the QTL regions discussed above. The PRCP gene codes for a prolylcarboxipeptidase, a hypothalamic regulator of food intake and energy homeostasis in rodents (24, 66). The JUNB gene product has been described as a growth-inhibiting protein, and JUNB-deficient mice showed a retarded growth and a reduction of adipose tissue (51). The PLAC8 gene codes for an important regulator of lipogenesis and adipocyte differentiation, being involved in the control of body weight (25). The ALDH2 is a downstream target of PPARG signaling (44), and ANGPTL2 and VAMP8 genes are involved in insulin sensitivity and glucose metabolism (27, 29, 70). Finally, the TNFAIP3 gene has been related to lipid and fatty acid metabolism in mice (6). For the remaining four genes (BMP5, BTG2, ACTA2, and ADAMTS4), even though they do not have a known biological function directly related to growth and fatness, they have been previously linked to these traits through association analyses. The gene ADAMTS4 mapped within a previously described QTL region, and its association to the traits of interest was discussed above (33). The BMP5 is located within a major QTL region identified in pig affecting carcass fat content, and a SNP within this gene has been associated with fatness (56). Polymorphisms in the porcine BTG2 and ACTA2 genes have been previously associated to fat content (23, 40).
Identification of new isoforms DE.
The differential expression analysis performed with the 58,927 new isoforms revealed a total of 50 DE isoforms between H and L groups. All of them showed a higher expression in the L group compared with the H group. These new isoforms are characterized by containing extra exons, intron retention events, or exon skipping. The biological annotation showed that seven of them are splice forms of genes related to growth and fatness (AEBP1, SETDB1, CD44, SLC44A1, PLAC8, BMP5, and KIAA1462). The AEBP1 gene codes for the adipocyte enhancer-binding protein that modulates adiposity and energy homeostasis (53), the CD44 seems to participate in the insulin resistance mechanism associated with obesity (28), and the PLAC8 gene is a regulator of brown fat tissue adipocyte differentiation (25). The product of the gene SLC44A1 is a choline transporter involved in lipid metabolism (36), and the SETDB1 gene codes for a histone methyltransferase part of a repressor complex of PPARG that determines the differentiation of stem cells into adipocytes (18). Although the remaining two genes, BMP5 and KIAA1462, have no known function related to the traits of interest, they are located within QTL regions and contain SNPs previously associated with fat content and deposition, respectively (56, 68).
The existence of new PLAC8 and KIAA1462 isoforms has been validated by cDNA sequencing. For the PLAC8 gene, an isoform lacking exon 2 was confirmed. For the KIAA1462 gene, a transcript containing the intronic region between exons 3 and 4 was also confirmed. The validation of these two novel isoforms supports the reliability of the strategy used for new isoform identification. It should be noted that the applied correction for multiple testing in the new isoform expression analyses was less stringent (q value < 0.25), and probably a higher number of false positives are included. In any case, validation for the remaining new isoforms would be required before further studies.
qPCR Validation of Differential Expression Analyses
Among the 56 DE genes identified both by Cuffdiff and CLC Genomics, seven were selected to be validated by qPCR. They included three DE genes located within previously detected QTL regions for growth and fatness (FA2H, IRF1, and ADAMTS4) and four genes identified by both RNA-Seq analyses, which previously have been associated to the traits of interest (PRCP, VAMP8, BTG2, and ACTA2). The expression values of the two new DE isoforms validated by sequencing (PLAC8 and KIAA1462) were also selected for qPCR validation.
The expression values of the nine transcripts were measured by qPCR and normalized with GAPDH and ACTB as reference. The correlations of the expression values obtained with qPCR, TopHat-Cufflinks, and CLC Genomics are shown in Table 6. The correlations obtained between both RNA-Seq analyses and qPCR were highly significant, ranging from 0.67 (PRCP gene) to 0.98 (PLAC8 new isoform). The average correlation between TopHat-Cufflinks and qPCR was 0.86, which is in agreement with the results from Roberts et al. (54), who reported a correlation of 0.81 between Cufflinks and qPCR expression data. Several limitations make it impossible to calculate the CCC between qPCR and RNA-Seq: the limited number of validated genes, the nonrandom selection of these genes, and the intrinsic differences in the expression measures of both types of methodologies (i.e., while all different transcripts of a gene are being measured in the RNA-Seq analyses, only those covered by the primers designed are measured by qPCR).
Table 6.
Pearson correlation between the expression values obtained from qPCR, TopHat-Cufflinks, and CLC Genomics for nine transcripts/genes
| Gene | qPCR-TC | P Value | qPCR-CLC | P Value |
|---|---|---|---|---|
| Annotated genes | ||||
| PRCP | 0.69 | 2 × 10−2 | 0.67 | 3 × 10−2 |
| FA2H | 0.74 | 1 × 10−2 | 0.72 | 2 × 10−2 |
| IRF1 | 0.92 | 4 × 10−4 | 0.91 | 6 × 10−4 |
| VAMP8 | 0.88 | 1 × 10−3 | 0.88 | 1 × 10−3 |
| BTG2 | 0.94 | 1 × 10−4 | 0.92 | 4 × 10−4 |
| ACTA2 | 0.97 | 1 × 10−5 | 0.96 | 5 × 10−5 |
| ADAMTS4 | 0.80 | 7 × 10−3 | 0.79 | 8 × 10−3 |
| New isoforms | ||||
| PLAC8 | 0.98 | 3 × 10−6 | ||
| KIAA1462 | 0.84 | 4 × 10−3 | ||
qPCR, quantitative PCR; TC, TopHat-Cufflinks.
TF Related to Growth and Fatness Traits
Several genes related to growth and fatness traits have been highlighted through the different approaches used (Table 7). A search for potential regulatory elements on this set of genes was conducted with GeneCards database, the SABiosciences' Text Mining Application, and the UCSC Genome Browser. A total of 116 potential TF regulators were found. Among them, TFs located within the QTL regions discussed above were selected as powerful TF candidate genes. Six TFs are located within a QTL region: IRF1 (QTL SSC2), EGR-1 (QTL SSC2), PBX1a (QTL SSC4), POU2F1 (QTL SSC4), NR3C1 (QTL SSC2), and NF-Yb (QTL SSC5). Both IRF1 and EGR-1 are considered highly relevant as they also appeared DE between H and L groups. Both are TFs regulating a set of relevant DE genes (BTG2, ACTA2, JUNB, and CD44) and are located within QTL regions associated with the traits of interest (SSC2: 148–152 Mb). Moreover, there is a high correlation between the expression of IRF1 and JUNB (0.98), IRF1 and BTG2 (0.98), and EGR-1 and JUNB (0.93). Remarkably, EGR-1 is a potential regulator of IRF1, and their expression levels are highly correlated (0.91). Additionally, the NR3C1, a potential regulator of SLC12A2, SELE, CPXM1, ACTA2, and ALDH2, also maps in the interval of the same QTL region on SSC2, and it codes for a glucocorticoid receptor associated with obesity and eating disorders in humans (2). This gene also seems to be regulated by EGR-1 and IRF1. Similarly, two of the identified TFs are located in the interval of SSC4 QTL (68–85 Mb), PBX1a and POU2F1. PBX1a regulates MYL9 and KIAA1462 DE transcripts and has been proposed as an adipocyte development regulator at multiple levels. It promotes the generation of adipocyte progenitors during embryogenesis, while favoring adipocyte progenitors proliferation and commitment to the adipocyte lineage in postnatal life (41). POU2F1 gene regulates the expression of ANO6 DE gene and has been previously associated with lipoprotein lipase transcriptional regulation involved in triglyceride hydrolysis and free fatty acid release (4). The final relevant TF is NF-Yb, located within the QTL region on SSC5 (69–83 Mb). It potentially controls the expression of PRCP DE gene, which has an important role in the regulation of food intake and energy homeostasis (24). The fact that some of these TFs are not DE conditional on the analyzed groups does not necessary mean that they are not related to these traits, as it is known that small expression variations of TFs could have great impact on its target gene's expression.
Table 7.
Relevant differentially expressed genes identified from the comparison between low and high groups
| Ensembl ID | Gene ID | Expression Ratio | q Value |
|---|---|---|---|
| ENSSSCG00000010447 | ACTA2 | 3.15 | 3.6 × 10−4 |
| ENSSSCG00000006359 | ADAMTS4 | 1.72 | 4.9 × 10−4 |
| ENSSSCG00000016754 | AEBP1 | 4.34 | 3.0 × 10−1 |
| ENSSSCG00000009889 | ALDH2 | 1.79 | 3.8 × 10−2 |
| ENSSSCG00000005608 | ANGPTL2 | 2.48 | 1.2 × 10−2 |
| ENSSSCG00000001478 | BMP5 | 2.31 | 4.7 × 10−2 |
| ENSSSCG00000028322 | BTG2 | 3.21 | 9.0 × 10−5 |
| ENSSSCG00000013297 | CD44 | 4.87 | 3.1 × 10−1 |
| ENSSSCG00000014336 | EGR-1 | 1.9 | 3.7 × 10−2 |
| ENSSSCG00000002718 | FA2H | 1.79 | 4.0 × 10−2 |
| ENSSSCG00000014277 | IRF1 | 1.98 | 3.1 × 10−2 |
| ENSSSCG00000013735 | JUNB | 3.28 | 2.3 × 10−3 |
| ENSSSCG00000011020 | KIAA1462 | 1.9 | 3.1 × 10−1 |
| ENSSSCG00000000666 | MFAP5 | 3.77 | 4.8 × 10−2 |
| ENSSSCG00000009240 | PLAC8 | 2.93 | 3.1 × 10−1 |
| ENSSSCG00000000858 | PMCH | 1.83 | 4.6 × 10−2 |
| ENSSSCG00000014899 | PRCP | 1.8 | 4.1 × 10−2 |
| ENSSSCG00000006645 | SETDB1 | 2.79 | 3.1 × 10−1 |
| ENSSSCG00000014255 | SLC12A2 | 3.17 | 3.1 × 10−1 |
| ENSSSCG00000005425 | SLC44A1 | 2.01 | 3.1 × 10−1 |
| ENSSSCG00000022820 | VAMP8 | 1.81 | 4.7 × 10−2 |
The expression ratio is measured as low/high.
Functional Network Analysis
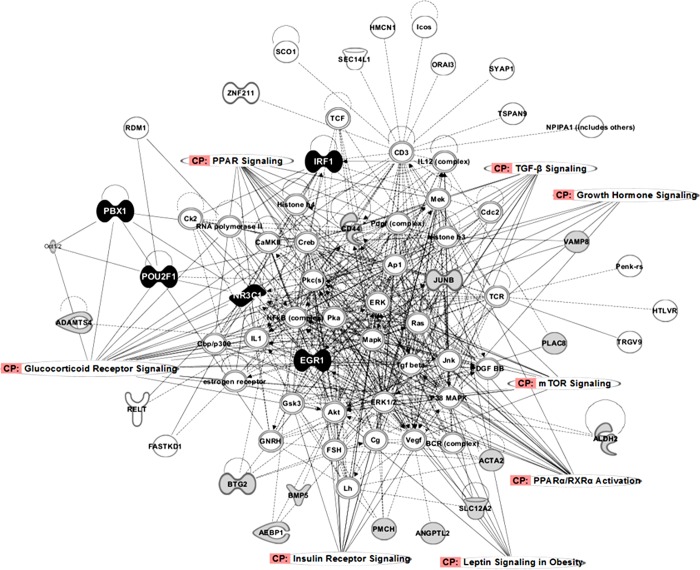
The IPA software was used to build a network with the most relevant DE genes and the TFs located in QTL regions and therefore potentially regulating DE genes. All these genes matched within four different networks (Table 8). Networks 1 and 2 were the most informative according to their scores, the number of discussed genes, and the associated functions. For further insights, networks 1 and 2 were merged, and the resulting network is presented in Fig. 4. In addition, the relevant canonical pathways related to the traits of interest, such as growth hormone, leptin, and PPAR signaling, were also included in the network. The overview of the network shows the high number of gene connections and central location of some genes, indicating the important role of the abovementioned TFs in these processes, especially for EGR-1, NR3C1, and IRF1. The NR3C1 TF is linked with all the TFs of the network, IRF1, PBX1, EGR-1, and POU2F1, highlighting a key regulatory role of EGR-1 in growth and fatness. Finally, even though JUNB is not located in a QTL region, the high number of connections of this gene and its central location in the network point out a relevant role of this TF in these processes.
Table 8.
Networks built with the relevant DE genes and TFs by IPA software
| ID | Molecules in Network | Score | Focus Molecules | Top Functions |
|---|---|---|---|---|
| 1 | ACTA2, AEBP1, ALDH2, ANGPTL2, Ap1, BRC, BTG2, CaMKII, Cbp/p300, CD3, CD44, CD82, Cdc2, Cg, Creb, EGR1, ERK, ERK1/2, FSH, GNRH, HIST3H3, IL1, JUNB, Lh, Mapk, Mek, P38 MAPK, Pdgf, PDGF BB, PMCH, SLC12A2, TCF, Tgf β/, VAMP8, Vegf | 28 | 11 | cancer, neurological disease, endocrine system disorders |
| 2 | Akt, ALOX12/15, AMIGO2, Atf, BMP5, C8orf4, CARD14, Ck2, ESR1, FASTKD1, FGD4, PTK2, HIS4, IL12, IP6K3, IRF1, Jnk, NFkB, NR3C1, Oc1/2, Orm, PBX1, Pka, Pkc, Ras PLAC8, POU2F1, RDM1, RELT, POLR2A, Taok2, TCR, Tnfrsf22/Tnfrsf23, vitK1, ZNF675 | 12 | 6 | cellular development, growth and proliferation, organ morphology |
| 3 | ADAMTS4, AKAP17A, ANO2, CDS2, CEP57L1, CHPT1, CHUK, CTC1, FAM184B, FAM49B, FAM92A1, FLYWCH1, KBTBD8, L2HGDH, METTL9, METTL2B, MFAP5, NFYB, PRCP, PXMP4, RIMBP2, SETDB1, SLC44A1, SNX10, TM7SF3, TMEM39A, TTC39B, TYW1, UBC, UBE3B, ZNF468, ZNF484, ZNF610, ZNF611, ZNF765 | 12 | 6 | cardiovascular disease, connective tissue disorders, developmental disorder |
| 4 | C18-ceramide, cholesterol, DC16-ceramide, Epgn, FA2H, fatty acid, lipid, miR-124-3p, SBDS, UBQLN4 | 2 | 1 | ophthalmic disease, hair, skin and organ development |
Transcription factors (TFs) are shown in boldface.
IPA, Ingenuity Pathway Analysis.
Fig. 4.
Graphical representation of merged networks 1 and 2 generated by the Ingenuity Pathways Analysis software. The relevant differentially expressed genes are colored in gray, transcription factors in black, and the remaining genes added to build the network in white. Overrepresented canonical pathways related to the traits of interest were also overlaid on the network.
Conclusions
The undertaken RNA-Seq approach has enabled us to explore the hypothalamic transcriptome of pigs divergent for growth and fatness traits. The transcriptome profile of the hypothalamus confirms its important role in the regulation of growth, fat accumulation, and energy expenditure. The differential expression analyses performed with TopHat-Cufflinks revealed a total of 210 DE genes between H and L groups. Among them, 15 are located within QTL regions previously identified for these traits. An additional analysis conducted with CLC Genomics software supported the results obtained for 56 DE genes, of which 13 were biologically related to growth and fatness and three mapped within QTL regions. Finally, identification of new isoforms allowed us to identify a total of 50 DE novel isoforms, seven of them related to the traits of interest. In summary, a set of 21 relevant DE genes could be highlighted. Additionally, six TFs were identified as potential regulators of the 21 DE genes. Among them, EGR-1, IRF1, and NR3C1 are the strongest candidate TF genes for growth and fatness according to their expression pattern, genomic location within QTL regions, and network interactions. Overall, the results of the present study contribute to the knowledge of relevant routes for growth and fatness traits, relevant not only for pig production, but also in relation to human obesity and related diseases. The next step in the search for the causal genetic variation would be the analysis of polymorphisms in the promoter regions of the highlighted genes and even in the TFs potentially responsible for the expression differences found in the current study.
GRANTS
This work was funded by Ministerio de Ciencia e Innovación (MICINN) project AGL2011-29821-C02. D. Pérez-Montarelo was funded by a Formación de Personal Investigador (FPI) PhD grant from the Spanish Ministerio de Ciencia e Innovación (BES-2009-025417). O. Madsen and M. A. M. Groenen were supported by European Research Council Grant ERC-2009-AdG:249894.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: D.P.-M. and E.A. performed experiments; D.P.-M., O.M., M.A.M.G., and A.I.F. analyzed data; D.P.-M., O.M., and A.I.F. interpreted results of experiments; D.P.-M. prepared figures; D.P.-M. and A.I.F. drafted manuscript; D.P.-M., O.M., E.A., M.R., J.M.F., J.L.N., M.A.M.G., and A.I.F. approved final version of manuscript; O.M., E.A., M.R., J.M.F., J.L.N., M.A.M.G., and A.I.F. edited and revised manuscript; M.R., J.M.F., J.L.N., and A.I.F. conception and design of research.
Supplementary Material
ACKNOWLEDGMENTS
We thank Rita Benítez, Yolanda Nuñez, and Fabián García for technical support. Moreover, we acknowledge Luis Silió and Noelia Ibáñez-Escriche for help in the revision of the manuscript.
Footnotes
The online version of this article contains supplemental material.
REFERENCES
- 1.Barb CR, Hausman GJ, Rekaya R, Lents CA, Lkhagvadorj S, Qu L, Cai W, Couture OP, Anderson LL, Dekkers JC, Tuggle CK. Gene expression in hypothalamus, liver and adipose tissues and food intake response to melanocortin-4 receptor (MC4R) agonist in pigs expressing MC4R mutations. Physiol Genomics 41: 254–268, 2010. [DOI] [PubMed] [Google Scholar]
- 2.Cellini E, Castellini G, Ricca V, Bagnoli S, Tedde A, Rotella CM, Faravelli C, Sorbi S, Nacmias B. Glucocorticoid receptor gene polymorphisms in Italian patients with eating disorders and obesity. Psychiatr Genet 20: 282–288, 2010. [DOI] [PubMed] [Google Scholar]
- 3.Chen C, Ai H, Ren J, Li W, Li P, Qiao R, Ouyang J, Yang M, Ma J, Huang L. A global view of porcine transcriptome in three tissues from a full-sib pair with extreme phenotypes in growth and fat deposition by paired-end RNA sequencing. BMC Genomics 12: 448, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Currie RA, Eckel RH. Characterization of a high affinity octamer transcription factor binding site in the human lipoprotein lipase promoter. Arch Biochem Biophys 298: 630–639, 1992. [DOI] [PubMed] [Google Scholar]
- 5.Dalman MR, Deeter A, Nimishakavi G, Duan ZH. Fold change and p-value cutoffs significantly alter microarray interpretations. BMC Bioinformatics 13, Suppl 2: S11, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Damrauer SM, Studer P, da Silva CG, Longo CR, Ramsey HE, Csizmadia E, Shrikhande GV, Scali ST, Libermann TA, Bhasin MK, Ferran C. A20 modulates lipid metabolism and energy production to promote liver regeneration. PLoS One 6: e17715, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Danecek P, Nellåker C, McIntyre RE, Buendia-Buendia JE, Bumpstead S, Ponting CP, Flint J, Durbin R, Keane TM, Adams DJ. High levels of RNA-editing site conservation amongst 15 laboratory mouse strains. Genome Biol 13: 26, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7a.Erkens T, Van Poucke M, Vandesompele J, Goossens K, Van Zeveren A, Peelman LJ. Development of a new set of reference genes for normalization of real-time RT-PCR data of porcine backfat and longissimus dorsi muscle, and evaluation with PPARGC1A. BMC Biotechnol 6: 41, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Estellé J, Pérez-Enciso M, Mercadé A, Varona L, Alves E, Sánchez A, Folch JM. Characterization of the porcine FABP5 gene and its association with the FAT1 QTL in an Iberian by Landrace cross. Anim Genet 37: 589–591, 2006. [DOI] [PubMed] [Google Scholar]
- 9.Esteve-Codina A, Kofler R, Palmieri N, Bussotti G, Notredame C, Pérez-Enciso M. Exploring the gonad transcriptome of two extreme male pigs with RNA-seq. BMC Genomics 12: 552, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fernández AI, Pérez-Montarelo D, Barragán C, Ramayo-Caldas Y, Ibáñez-Escriche N, Castelló A, Noguera JL, Silió L, Folch JM, Rodríguez MC. Genome-wide linkage analysis of QTL for growth and body composition employing the PorcineSNP60 BeadChip. BMC Genet 13: 41, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferraz AL, Ojeda A, López-Béjar M, Fernandes LT, Castelló A, Folch JM, Pérez-Enciso M. Transcriptome architecture across tissues in the pig. BMC Genomics 16: 173, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fortes MR, Snelling WM, Reverter A, Nagaraj SH, Lehnert SA, Hawken RJ, DeAtley KL, Peters SO, Silver GA, Rincon G, Medrano JF, Islas-Trejo A, Thomas MG. Gene network analyses of first service conception in Brangus heifers: use of genome and trait associations, hypothalamic-transcriptome information, and transcription factors. J Anim Sci 90: 2894–2906, 2012. [DOI] [PubMed] [Google Scholar]
- 13.Goff L, Trapnell C, Kelley D. CummeRbund: analysis, exploration, manipulation, and visualization of Cufflinks high-throughput sequencing data. Bioconductor, R package version 2.0.0, 2012. [Google Scholar]
- 14.Gokey NG, Lopez-Anido C, Gillian-Daniel AL, Svaren J. Early growth response 1 (Egr1) regulates cholesterol biosynthetic gene expression. J Biol Chem 286: 29501–29510, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gorodkin J, Cirera S, Hedegaard J, Gilchrist MJ, Panitz F, Jørgensen C, Scheibye-Knudsen K, Arvin T, Lumholdt S, Sawera M, Green T, Nielsen BJ, Havgaard JH, Rosenkilde C, Wang J, Li H, Li R, Liu B, Hu S, Dong W, Li W, Yu J, Wang J, Staefeldt HH, Wernersson R, Madsen LB, Thomsen B, Hornshøj H, Bujie Z, Wang X, Wang X, Bolund L, Brunak S, Yang H, Bendixen C, Fredholm M. Porcine transcriptome analysis based on 97 non-normalized cDNA libraries and assembly of 1,021,891 expressed sequence tags. Genome Biol 8: R45, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Groenen MA, Archibald AL, Uenishi H, Tuggle CK, Takeuchi Y, Rothschild MF, Rogel-Gaillard C, Park C, Milan D, Megens HJ, Li S, Larkin DM, Kim H, Frantz LA, Caccamo M, Ahn H, Aken BL, Anselmo A, Anthon C, Auvil L, Badaoui B, Beattie CW, Bendixen C, Berman D, Blecha F, Blomberg J, Bolund L, Bosse M, Botti S, Bujie Z, Bystrom M, Capitanu B, Carvalho-Silva D, Chardon P, Chen C, Cheng R, Choi SH, Chow W, Clark RC, Clee C, Crooijmans RP, Dawson HD, Dehais P, De Sapio F, Dibbits B, Drou N, Du ZQ, Eversole K, Fadista J, Fairley S, Faraut T, Faulkner GJ, Fowler KE, Fredholm M, Fritz E, Gilbert JG, Giuffra E, Gorodkin J, Griffin DK, Harrow JL, Hayward A, Howe K, Hu ZL, Humphray SJ, Hunt T, Hornshøj H, Jeon JT, Jern P, Jones M, Jurka J, Kanamori H, Kapetanovic R, Kim J, Kim JH, Kim KW, Kim TH, Larson G, Lee K, Lee KT, Leggett R, Lewin HA, Li Y, Liu W, Loveland JE, Lu Y, Lunney JK, Ma J, Madsen O, Mann K, Matthews L, McLaren S, Morozumi T, Murtaugh MP, Narayan J, Nguyen DT, Ni P, Oh SJ, Onteru S, Panitz F, Park EW, Park HS, Pascal G, Paudel Y, Perez-Enciso M, Ramirez-Gonzalez R, Reecy JM, Rodriguez-Zas S, Rohrer GA, Rund L, Sang Y, Schachtschneider K, Schraiber JG, Schwartz J, Scobie L, Scott C, Searle S, Servin B, Southey BR, Sperber G, Stadler P, Sweedler JV, Tafer H, Thomsen B, Wali R, Wang J, Wang J, White S, Xu X, Yerle M, Zhang G, Zhang J, Zhang J, Zhao S, Rogers J, Churcher C, Schook LB. Analyses of pig genomes provide insight into porcine demography and evolution. Nature 491: 393–398, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gunawan A, Sahadevan S, Neuhoff C, Große-Brinkhaus C, Gad A, Frieden L, Tesfaye D, Tholen E, Looft C, Uddin MJ, Schellander K, Cinar MU. RNA deep sequencing reveals novel candidate genes and polymorphisms in boar testis and liver tissues with divergent androstenone levels. PLoS One 16: e63259, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Günther T, Schüle R. Fat or bone? A non-canonical decision. Nat Cell Biol 9: 1229–1231, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Guo L, Zhou D, Pryse KM, Okunade AL, Su X. Fatty acid 2-hydroxylase mediates diffusional mobility of Raft-associated lipids, GLUT4 level, and lipogenesis in 3T3–L1 adipocytes. J Biol Chem 285: 25438–25447, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hillebrand JJ, de Wied D, Adan RA. Neuropeptides, food intake and body weight regulation: a hypothalamic focus. Peptides 23: 2283–2306, 2002. [DOI] [PubMed] [Google Scholar]
- 21.Hu ZH, Park CA, Wu XL, Reecy JM. Animal QTLdb: an improved database tool for livestock animal QTL/association data dissemination in the post-genome era. Nucleic Acids Res 41: D871–D879, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu J, Lou D, Carow B, Winerdal ME, Rottenberg M, Wikström AC, Norstedt G, Winqvist O. LPS regulates SOCS2 transcription in a type I interferon dependent autocrine-paracrine loop. PLoS One 7: e30166, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang JS, Xiong YZ, Deng CY. Cloning and expression analysis of porcine ACTA2 gene and its association with production traits. Yi Chuan 31: 489–494, 2009. [DOI] [PubMed] [Google Scholar]
- 24.Jeong JK, Szabo G, Kelly K, Diano S. Prolyl carboxypeptidase regulates energy expenditure and the thyroid axis. Endocrinology 153: 683–689, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jimenez-Preitner M, Berney X, Uldry M, Vitali A, Cinti S, Ledford JG, Thorens B. Plac8 is an inducer of C/EBPβ required for brown fat differentiation, thermoregulation, and control of body weight. Cell Metab 14: 658–670, 2011. [DOI] [PubMed] [Google Scholar]
- 26.Jung WY, Kwon SG, Son M, Cho ES, Lee Y, Kim JH, Kim BW, Park da H, Hwang JH, Kim TW, Park HC, Park BY, Choi JS, Cho KK, Chung KH, Song YM, Kim IS, Jin SK, Kim DH, Lee SW, Lee KW, Bang WY, Kim CW. RNA-Seq approach for genetic improvement of meat quality in pig and evolutionary insight into the substrate specificity of animal carbonyl reductases. PLoS One 7: e42198, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kadomatsu T, Tabata M, Oike Y. Angiopoietin-like proteins: emerging targets for treatment of obesity and related metabolic diseases. FEBS J 278: 559–564, 2011. [DOI] [PubMed] [Google Scholar]
- 28.Kang HS, Liao G, DeGraff LM, Gerrish K, Bortner CD, Garantziotis S, Jetten AM. CD44 plays a critical role in regulating diet-induced adipose inflammation, hepatic steatosis, and insulin resistance. PLoS One 8: e58417, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kitazawa M, Nagano M, Masumoto KH, Shigeyoshi Y, Natsume T, Hashimoto S. Angiopoietin-like 2, a circadian gene, improves type 2 diabetes through potentiation of insulin sensitivity in mice adipocytes. Endocrinology 152: 2558–2567, 2011. [DOI] [PubMed] [Google Scholar]
- 30.Kogelman LJ, Kadarmideen HN, Mark T, Karlskov-Mortensen P, Bruun CS, Cirera S, Jacobsen MJ, Jørgensen CB, Fredholm M. An f2 pig resource population as a model for genetic studies of obesity and obesity-related diseases in humans: design and genetic parameters. Front Genet 4: 29, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30a.Kuijk EW, du Puy L, van Tol HT, Haagsman HP, Colenbrander B, Roelen BA. Validation of reference genes for quantitative RT-PCR studies in porcine oocytes and preimplantation embryos. BMC Dev Biol 7: 58, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Lartigue G, Lur G, Dimaline R, Varro A, Raybould H, Dockray GJ. EGR1 is a target for cooperative interactions between cholecystokinin and leptin, and inhibition by ghrelin, in vagal afferent neurons. Endocrinology 151: 3589–3599, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Lartigue G, Barbier de la Serre C, Espero E, Lee J, Raybould HE. Leptin resistance in vagal afferent neurons inhibits cholecystokinin signaling and satiation in diet induced obese rats. PLoS One 7: e32967, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee SH, Gondro C, van der Werf J, Kim NK, Lim DJ, Park EW, Oh SJ, Gibson JP, Thompson JM. Use of a bovine genome array to identify new biological pathways for beef marbling in Hanwoo (Korean Cattle). BMC Genomics 11: 623, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li JB, Levanon EY, Yoon JK, Aach J, Xie B, Leproust E, Zhang K, Gao Y, Church GM. Genome-wide identification of human RNA editing sites by parallel DNA capturing and sequencing. Science 324: 1210–1213, 2009. [DOI] [PubMed] [Google Scholar]
- 35.Luu-The V, Paquet N, Calvo E, Cumps J. Improved real-time RT-PCR method for high-throughput measurements using second derivative calculation and double correction. Biotechniques 38: 287–293, 2005. [DOI] [PubMed] [Google Scholar]
- 36.Michel V, Singh RK, Bakovic M. The impact of choline availability on muscle lipid metabolism. Food Funct 2: 53–62, 2011. [DOI] [PubMed] [Google Scholar]
- 37.Milan D, Jeon JT, Looft C, Amarger V, Robic A, Thelander M, Rogel-Gaillard C, Paul S, Iannuccelli N, Rask L, Ronne H, Lundström K, Reinsch N, Gellin J, Kalm E, Roy PL, Chardon P, Andersson L. A mutation in PRKAG3 associated with excess glycogen content in pig skeletal muscle. Science 19: 1248–1251, 2000. [DOI] [PubMed] [Google Scholar]
- 38.Miller LC, Fleming D, Arbogast A, Bayles DO, Guo B, Lager KM, Henningson JN, Schlink SN, Yang HC, Faaberg KS, Kehrli ME., Jr. Analysis of the swine tracheobronchial lymph node transcriptomic response to infection with a Chinese highly pathogenic strain of porcine reproductive and respiratory syndrome virus. BMC Vet Res 8: 208, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miron M, Woody OZ, Marcil A, Murie C, Sladek R, Nadon R. A methodology for global validation of microarray experiments. BMC Bioinformatics 7: 333, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mo XY, Lan J, Jiao QZ, Xiong YZ, Zuo B, Li FE, Xu DQ, Lei MG. Molecular characterization, expression pattern and association analysis of the porcine BTG2 gene. Mol Biol Rep 38: 4389–4396, 2011. [DOI] [PubMed] [Google Scholar]
- 41.Monteiro MC, Sanyal M, Cleary ML, Sengenès C, Bouloumié A, Dani C, Billon N. PBX1: a novel stage-specific regulator of adipocyte development. Stem Cells 29: 1837–1848, 2011. [DOI] [PubMed] [Google Scholar]
- 42.Mortazavi A, Williams BA, McCue K, Schaeffer L, Wold B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat Meth 5: 621–628, 2008. [DOI] [PubMed] [Google Scholar]
- 43.Mul JD, la Fleur SE, Toonen PW, Afrasiab-Middelman A, Binnekade R, Schetters D, Verheij MM, Sears RM, Homberg JR, Schoffelmeer AN, Adan RA, DiLeone RJ, De Vries TJ, Cuppen E. Chronic loss of melanin-concentrating hormone affects motivational aspects of feeding in the rat. PLoS One 6: e19600, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Okuno M, Arimoto E, Nishizuka M, Nishihara T, Imagawa M. Isolation of up- or down-regulated genes in PPARgamma-expressing NIH-3T3 cells during differentiation into adipocytes. FEBS Lett 519: 108–112, 2002. [DOI] [PubMed] [Google Scholar]
- 45.Ovilo C, Pérez-Enciso M, Barragán C, Clop A, Rodríquez C, Oliver MA, Toro MA, Noruera JL. A QTL for intramuscular fat and backfat thickness is located on porcine chromosome 6. Mamm Genome 11: 344–346, 2000. [DOI] [PubMed] [Google Scholar]
- 46.Ovilo C, Fernández A, Fernández AI, Folch JM, Varona L, Benítez R, Nuñez Y, Rodríguez C, Silió L. Hypothalamic expression of porcine leptin receptor (LEPR), neuropeptide Y (NPY), and cocaine- and amphetamine-regulated transcript (CART) genes is influenced by LEPR genotype. Mamm Genome 21: 583–591, 2010. [DOI] [PubMed] [Google Scholar]
- 47.Pérez-Enciso M, Varona L. Quantitative trait loci mapping in F(2) crosses between outbred lines. Genetics 155: 391–405, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pérez-Enciso M, Ferraz AL, Ojeda A, López-Béjar M. Impact of breed and sex on porcine endocrine transcriptome: a Bayesian biometrical analysis. BMC Genomics 10: 89, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pérez-Montarelo D, Hudson NJ, Fernández AI, Ramayo-Caldas Y, Dalrymple BP, Reverter A. Porcine tissue-specific regulatory networks derived from meta-analysis of the transcriptome. PLoS One 7: e46159, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pérez-Montarelo D, Fernández A, Barragán C, Noguera JL, Folch JM, Rodríguez MC, Ovilo C, Silió L, Fernández AI. Transcriptional characterization of porcine leptin and leptin receptor genes. PLoS One 8: e66398, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pinent M, Prokesch A, Hackl H, Voshol PJ, Klatzer A, Walenta E, Panzenboeck U, Kenner L, Trajanoski Z, Hoefler G, Bogner-Strauss JG. Adipose triglyceride lipase and hormone-sensitive lipase are involved in fat loss in JunB-deficient mice. Endocrinology 152: 2678–2689, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ramayo-Caldas Y, Mach N, Esteve-Codina A, Corominas J, Castelló A, Ballester M, Estellé J, Ibáñez-Escriche N, Fernández AI, Pérez-Enciso M, Folch JM. Liver transcriptome profile in pigs with extreme phenotypes of intramuscular fatty acid composition. BMC Genomics 13: 547, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ro HS, Zhang L, Majdalawieh A, Kim SW, Wu X, Lyons PJ, Webber C, Ma H, Reidy SP, Boudreau A, Miller JR, Mitchell P, McLeod RS. Adipocyte enhancer-binding protein 1 modulates adiposity and energy homeostasis. Obesity (Silver Spring) 15: 288–302, 2007. [DOI] [PubMed] [Google Scholar]
- 54.Roberts A, Trapnell C, Donaghey J, Rinn JL, Pachter L. Improving RNA-Seq expression estimates by correcting for fragment bias. Genome Biol 12: R22, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Samborski A, Graf A, Krebs S, Kessler B, Bauersachs S. Deep sequencing of the porcine endometrial transcriptome on day 14 of pregnancy. Biol Reprod 88: 84, 2013. [DOI] [PubMed] [Google Scholar]
- 56.Shao GC, Luo LF, Jiang SW, Deng CY, Xiong YZ, Li FE. A C/T mutation in microRNA target sites in BMP5 gene is potentially associated with fatness in pigs. Meat Sci 87: 299–303, 2011. [DOI] [PubMed] [Google Scholar]
- 57.Simpson KA, Martin NM, Bloom SR. Hypothalamic regulation of food intake and clinical therapeutic applications. Arq Bras Endocrinol Metabol 53: 120–128, 2009. [DOI] [PubMed] [Google Scholar]
- 58.Spurlock ME, Gabler NK. The development of porcine models of obesity and the metabolic syndrome. J Nutr 138: 397–402, 2008. [DOI] [PubMed] [Google Scholar]
- 59.Storey JD, Tibshirani R. Statistical significance for genomewide studies. Proc Natl Acad Sci USA 100: 9440–9445, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Trapnell C, Pachter L, Salzberg SL. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics 25: 1105–1511, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Trapnell C, Roberts A, Goff L, Pertea G, Kim D, Kelley DR, Pimentel H, Salzberg SL, Rinn JL, Pachter L. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat Protoc 7: 562–578, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vaittinen M, Kolehmainen M, Schwab U, Uusitupa M, Pulkkinen L. Microfibrillar-associated protein 5 is linked with markers of obesity-related extracellular matrix remodeling and inflammation. Nutr Diabetes 1: e15, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Van Laere AS, Nguyen M, Braunschweig M, Nezer C, Collette C, Moreau L, Archibald AL, Haley CS, Buys N, Tally M, Andersson G, Georges M, Andersson L. A regulatory mutation in IGF2 causes a major QTL effect on muscle growth in the pig. Nature 425: 832–836, 2003. [DOI] [PubMed] [Google Scholar]
- 64.Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3: RESEARCH0034, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Varona L, Ovilo C, Clop A, Noguera JL, Pérez-Enciso M, Coll A, Folch JM, Barragán C, Toro MA, Babot D, Sánchez A. QTL mapping for growth and carcass traits in an Iberian by Landrace pig intercross: additive, dominant and epistatic effects. Genet Res 80: 145–154, 2002. [DOI] [PubMed] [Google Scholar]
- 66.Wallingford N, Perroud B, Gao Q, Coppola A, Gyengesi E, Liu ZW, Gao XB, Diament A, Haus KA, Shariat-Madar Z, Mahdi F, Wardlaw SL, Schmaier AH, Warden CH, Diano S. Prolylcarboxypeptidase regulates food intake by inactivating alpha-MSH in rodents. J Clin Invest 119: 2291–2303, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wood JD, Enser M, Fisher AV, Nute GR, Sheard PR, Richardson RI, Hughes SI, Whittington FM. Fat deposition, fatty acid composition and meat quality: a review. Wood Meat Sci 78: 343–358, 2008. [DOI] [PubMed] [Google Scholar]
- 68.Xiao Q, Wu XL, Michal JJ, Reeves JJ, Busboom JR, Thorgaard GH, Jiang Z. A novel nuclear-encoded mitochondrial poly(A) polymerase PAPD1 is a potential candidate gene for the extreme obesity related phenotypes in mammals. Int J Biol Sci 2: 171–178, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang B, Saco Y, Pérez-Enciso M. Association between plasma metabolites and gene expression profiles in five porcine endocrine tissues. Genet Sel Evol 43: 28, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zong H, Wang CC, Vaitheesvaran B, Kurland IJ, Hong W, Pessin JE. Enhanced energy expenditure, glucose utilization, and insulin sensitivity in VAMP8 null mice. Diabetes 60: 30–38, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.