Abstract
There is a link between visceral white adipose tissue (WAT) and the metabolic syndrome in humans, with health improvements produced with small visceral WAT reduction. By contrast, subcutaneous WAT provides a site for lipid storage that is rather innocuous relative to ectopic lipid storage in muscle or liver. The sympathetic nervous system (SNS) is the principal initiator for lipolysis in WAT by mammals. Nothing is known, however, about the central origins of the SNS circuitry innervating the only true visceral WAT in rodents, mesenteric WAT (MWAT), which drains into the hepatic portal vein. We tested whether the central sympathetic circuits to subcutaneous [inguinal WAT (IWAT)] and visceral WAT (MWAT) are separate or shared and whether they possess differential sympathetic drives with food deprivation in Siberian hamsters. Using two isogenic strains of pseudorabies virus, a retrograde transneuronal viral tract tracer within the same hamsters, we found some overlap (∼20–55% doubly infected neurons) between the two circuitries across the neural axis with lesser overlap proximal to the depots (spinal cord and sympathetic chain) and with more neurons involved in the innervation of IWAT than MWAT in some brain regions. Food deprivation triggered a greater sympathetic drive to subcutaneous (IWAT) than visceral (MWAT) depots. Collectively, we demonstrated both shared and separate populations of brain, spinal cord, and sympathetic chain neurons ultimately project to a subcutaneous WAT depot (IWAT) and the only visceral WAT depot in rodents (MWAT). In addition, the lipolytic stimulus of food deprivation only increased SNS drive to subcutaneous fat (IWAT).
Keywords: pseudorabies virus, norepinephrine turnover, food deprivation, Siberian hamsters, lipolysis
understanding the role of the central control of body fat is important given that obesity is at epidemic proportions, with serious secondary health consequences, such as Type II diabetes, cardiovascular disease, some cancers, and dementias, as well as being a significant economic burden (28, 43, 44, 66). White adipose tissue (WAT) stores energy in the form of triacylglycerol for mobilization during times of increased energetic demand. By contrast, when there is a surfeit of calories relative to energy expenditure, lipid is stored, but depending upon the storage location of the WAT depot, the excess lipid stores can be detrimental. The culprit most often pointed to as being detrimental to health is visceral WAT, which is thought to contribute to comorbidities previously mentioned above (for review, see Refs. 10, 24, 32). One hypothesis suggests that visceral WAT secretes proinflammatory cytokines, such as IL-6, that contribute to insulin resistance (33, 40), whereas another hypothesis, the “hepatic-portal theory”, proposes that the visceral WAT has a high lipolytic rate, resulting in large amounts of free fatty acids (FFAs) being transported to the liver through the portal vein and ultimately resulting in liver insulin resistance, although visceral WAT as the source of FFAs for this negative response has been questioned (e.g., Ref. 32).
Larger subcutaneous thigh WAT is seen as a potentially beneficial WAT depot because it is associated with the more favorable glucose and lipid profile in humans (51) and provides a potentially enormous location for lipid storage without the more direct organ-associated aspects of visceral WAT (29) or ectopic deposition of lipid in key organs involved in energy homeostasis (e.g., liver and muscle). Subcutaneous WAT also is associated with reductions in plasma insulin concentrations and hepatic steatosis as seen in improved muscle insulin sensitivity of lipodystrophic animal models, which are characterized by the small amount or the lack of body fat (46). Despite a deficit of body fat, some of the same metabolic characteristics associated with obesity are seen in lipodystrophic models because lipids are ectopically deposited in sites where they are typically not stored, thus being the hypothesized problem in the lipodystrophic disorders (46).
The central nervous system is now clearly recognized as a participator in the control of peripheral lipid storage and as an activator of the sympathetic nervous system (SNS) innervation of WAT. The SNS initiates lipolysis in humans and other mammals via norepinephrine (NE) release from its postganglionic nerve terminals (for review, see Refs. 6 and 7). Researchers using traditional monosynaptic neuronal and transneuronal viral tract tracers injected into WAT have described the sympathetic postganglionic innervation of WAT from the sympathetic chain (64), as well as the origins of the central circuits ultimately terminating in WAT depots, using Siberian hamsters, domestic swine, and laboratory rats (1, 4, 19, 20, 47, 52, 54, 56). More importantly, in terms of function, destruction of the sympathetic nerves innervating WAT by either chemical or surgical denervation significantly blocks lipid mobilization in response to several lipolytic stimuli, including food deprivation, short photoperiods, and estradiol replacement in ovariectomized obese rats (9, 14, 21, 62, 65).
Of the WAT depots in rodents, the two that represent visceral and subcutaneous WAT that have analogous counterparts in humans are mesenteric WAT (MWAT) and inguinal WAT (IWAT), respectively (60). MWAT is the only true visceral WAT in rodents, if the definition of visceral WAT is that it drains into the hepatic portal vein, because although epididymal WAT (EWAT) and retroperitoneal WAT (RWAT) are intra-abdominal WAT depots, they drain systemically through the inferior vena cava (16). In addition, the often cited EWAT as visceral WAT does not exist in humans (60) (Kral JG, personal communication). The rodent literature, however, is rife with supposed manipulations involving “visceral fat,” which was actually intra-abdominal WAT (e.g., RWAT, EWAT).
In our previous studies testing for changes in the sympathetic drive associated with energy challenges, as measured by a direct neurochemical assessment, norepinephrine turnover (NETO), we found different sympathetic drives across various WAT depots for each stimulus that we tested (i.e., food deprivation, cold exposure, glucoprivation, central melanocortin receptor agonism, and short photoperiod exposure) in Siberian hamsters (12, 13). To date, the central circuits defining the SNS outflow to MWAT have not been examined, nor has there been a functional in vivo test of sympathetic drive (i.e., NETO) to this WAT depot. In an attempt to understand human WAT depots functioning using nonhuman rodent animal models, it is important to test whether MWAT and IWAT have similar or separate central SNS outflow circuitries such that, potentially, a therapy could be designed to mobilize lipids from visceral WAT depots while relatively sparing that of subcutaneous WAT depots given that decreases in visceral WAT of as little as 5% has health benefits (16, 41) and the perhaps beneficial aspects of subcutaneous WAT.
Therefore, the purpose of the present investigation was to define the central SNS outflow circuits to MWAT and IWAT and to functionally test the sympathetic drive to these two depots in response to a lipolytic challenge (i.e., food deprivation). We used Siberian hamsters, our model of naturally occurring obesity that we have employed previously to define the sympathetic outflow to various WAT depots (4, 64), and sensory inflow from WAT depots to the brain (38, 55), in addition to assessing SNS drive using NETO with various energy challenges (12, 13). To define the separate/shared central SNS outflow circuitry, we used isogenic strains of pseudorabies virus (PRV) that have distinct reporters—PRV 152 [green fluorescent protein (GFP); Ref. 50] and PRV 614 [monomeric red fluorescent protein (mRFP); Ref. 5], each injected into one of the two WAT depots. Single- and double-labeled (infected) neurons were quantified across the neuroaxis, as well as in the spinal cord and the sympathetic chain. The functional test of differential SNS drive was accomplished by food-depriving hamsters for 16 h with ad libitum-fed hamsters as a control and measuring NETO.
MATERIALS AND METHODS
Animals.
Housing and all experimental procedures were approved by the Georgia State University Institutional Animal Care and Use Committee, in accordance with Public Health Service and United States Department of Agriculture guidelines. Adult male Siberian hamsters ∼3.5 mo old (Phodopus sungorus) were used. All hamsters (n = 41) were kept in individual cages in a vivarium under conditions of controlled lighting (16:8-h light-dark cycle) and temperature (21 ± 2°C) with ad libitum access to pelleted chow (LabDiet Rodent Chow 5001, St. Louis, MO), unless noted otherwise, and tap water.
Experiment 1: Viral Tract Tracing the SNS of MWAT and IWAT
Preliminary PRV injections tests.
It was necessary to verify that the two isogenic PRV strains infected neurons at a similar rate and that one was not more virulent than the other. As a control to ensure that they were functionally equivalent, a 1:1 mixture of PRV 152 (3 × 108 pfu/ml; generous gift by Lynn Enquist of Princeton University, Princeton, NJ) and PRV 614 (2.2 × 108 pfu/ml; Ref. 5) was injected into either MWAT (three injections) or IWAT (10 injections) at a volume of 150 nl/locus. There was relatively similar single and double labeling of the two PRV strains across the neuroaxis of these hamsters, thereby demonstrating functional equivalence of these doses of PRV 152 and PRV 614 (Nguyen NL and Bartness TJ, unpublished observations). We have previously demonstrated the specificity of PRV to retrogradely label only sympathetic neurons innervating WAT by chemically denervating the sympathetic nerves to WAT and then injecting PRV into the tissue, yielding no infected neurons across the neuroaxis, also suggesting specificity and no parasympathetic innervation of this tissue (26).
PRV injections.
Hamsters (n = 6) were single housed 1 wk before PRV injections, which were performed according to Biosafety Level II conditions. They were anesthetized with 2–3% isoflurane (Baxter Healthcare, Deerfield, IL), the fur around the haunch of their right leg was shaved, and the skin was alternatively wiped with povidone iodine (Ricca Chemical, Arlington, TX) and alcohol, and then with povidone iodine last. The hamsters were placed in lateral recumbency, and a subcutaneous incision was made to reveal IWAT. Using a 1.0-μl syringe, we injected 150 nl of PRV 152 (3 × 108 pfu/ml) at each of 10 loci across IWAT to evenly distribute the virus. The syringe was held in place for 1 min after each injection to prevent reflux up the outside of the needle and to allow time for the virus to disperse at each locus. The skin was closed with sterile wound clips (Stoelting, Wood Dale, IL) and nitrofurozone powder (nfz Puffer, Hess and Clark, Lexington, KY) was applied to minimize infection. The hamsters were then transferred to clean biohazard cages. All hamsters received subcutaneous injections of ketofen (5 mg/kg; Fort Dodge Animal Health, Fort Dodge, IA), an analgesic, for 3 days after virus injections and apple slices to supply readily consumed calories and water. We previously tested the transit times of PRV 152 and PRV 614 to reach the brain from the two WAT depots and concluded that 24 h after PRV 152 injections into IWAT was the optimal time point to inject PRV 614 into MWAT (Nguyen NT and Bartness TJ, unpublished observations). Therefore, the same hamsters were reanesthetized, shaved on the ventral side, and placed supine. A horizontal incision was made through the skin and the peritoneum to expose the abdominal cavity. The intestines were gently pulled out onto an isotonic saline-soaked sterile surgical drape to reveal MWAT and to prevent desiccation. Using a 1.0-μl syringe, l50 nl of PRV 614 (2.2 × 108 pfu/ml) was injected at each of three loci to distribute the virus evenly across MWAT. The peritoneum and skin were closed with sterile sutures (Ethicon, Johnson & Johnson, Somerville, NJ) and sterile wound clips, respectively. Nitrofurozone powder was applied, and the hamsters continued to receive ketofen and apple slices as a part of postoperational care. As an additional control for WAT depot, specific differences in viral uptake, in another group of hamsters, the WAT depots into which the viruses were injected were reversed (i.e., PRV 614 and PRV 152 were injected into IWAT and MWAT, respectively).
Histology.
The hamsters were euthanized by overdose with an intraperitoneal injection of pentobarbital sodium (300 mg/kg) 6 days after virus injections into IWAT, and transcardial perfusion was performed with heparinized 0.02% saline (75 ml), followed by 4% paraformaldehyde (150 ml) solution. The brains were removed and placed in the same fixative overnight and then immersed in a cryoprotectant solution of 30% sucrose. The brains were sectioned at 30 μm on a freezing-stage sliding microtome, and every 4th brain section was processed for double-fluorescent immunohistochemistry (IHC) against GFP and mRFP. The ipsilateral and contralateral sympathetic ganglia associated with vertebral segment thoracic 5 (T5) to lumbar 3 (L3) level of the spinal cord, as well as the spinal cords themselves were harvested and sliced at 16 μm and 40 μm, respectively, also on a freezing-stage sliding microtome. The sympathetic ganglia were collected onto slides (Superfrost Plus, VWR International, West Chester, PA) in a series of three. Every 4th ganglia and spinal cord sections were processed for double-fluorescent IHC against GFP and mRFP similar to the brain sections.
Immunohistochemistry.
We tested the primary and secondary antibodies against GFP and mRFP with positive and negative controls to ensure specificity and no cross-reactivity of the antibodies before performing the double-fluorescent IHC. Then, the brain and spinal cord sections were incubated in 0.1 M PBS (pH 7.4) × 3 rinses for 5 min each, followed by 20% normal goat serum (NGS; Vector Laboratories, Burlingame, CA) in 0.4% Triton X-100 for 30 min. The sections were incubated in a cocktail of primary antibodies consisting of mouse anti-GFP (1:500; Abcam, Cambridge, MA) and rabbit anti-RFP (1:2,000; Rockland Immunochemicals, Gilbertsville, PA) in 0.1 M PBS with 2% NGS and 0.4% Triton X-100 for 1 day at room temperature. They then were rinsed with 0.1 M PBS × 3 for 5 min each, and the sections were subsequently incubated in a cocktail of secondary antibodies consisting of goat anti-mouse Alexa Fluor 488 (1:500; Jackson ImmunoResearch, West Grove, PA) and goat anti-rabbit CY3 (1:800; Jackson ImmunoResearch) in 0.1 M PBS with 2% NGS and 0.4% Triton X-100 for 2 h at room temperature. The sections were mounted onto gelatin-subbed slides and cover slipped with ProLong Gold Antifade reagent (Life Technologies, Grand Island, NY). The double-fluorescent IHC for the sympathetic ganglia followed the same protocol as the free-floating brain sections and spinal cords, except the primary antibody concentrations of mouse anti-GFP and rabbit anti-RFP were increased to 1:400 and 1:600, respectively, and the incubation times increased to 2 days because the sympathetic ganglia sections were slide-mounted. The concentrations of the secondary antibodies of goat anti-mouse Alexa Fluor 488 and goat anti-rabbit CY3 were also increased to 1:350 and 1:550, respectively.
Experiment 2: Tests of the SNS Drive to MWAT and to IWAT after 16 h of Food Deprivation
Hamsters (n = 35) used for NETO were handled daily to adapt them to the handling associated with the test, and their body mass was monitored for 2 wk. Then, they were matched for absolute body mass and percent body mass change and were divided into experimental groups. The food deprivation length used was based on a robust increase in NETO by Siberian hamsters previously in our laboratory (13). Hamsters were divided into two groups: 1) ad libitum-fed (n = 17) and 2) food-deprived for 16 h (n = 18). On the day of the food deprivation, all hamsters had food removed from their cheek pouches, and body weight was measured, after which hamsters were transferred into clean cages. Food was removed for the food-deprived groups, but tap water was freely available. At the end of the 16-h food deprivation, body mass was measured for all hamsters.
NETO and tissue preparation.
Before tissue harvesting, hamsters were transferred to the laboratory and were acclimated for 2 h in a quiet environment to prevent unnecessary stress that can affect catecholamine release. Ad libitum-fed (n = 10) and food-deprived (n = 10) hamsters were terminated by rapid decapitation to obtain baseline values of NE for between group calculation of NETO, as we have done previously (12, 13, 48, 64). In brief, the interscapular brown adipose tissue (IBAT), IWAT, EWAT, RWAT, and MWAT were quickly harvested, weighed, frozen in liquid nitrogen, and stored in −80°C until NE extraction. NETO was assayed using the α-methyl-p-tyrosine (α-MPT; M3281, Sigma-Aldrich, St. Louis, MO) method during the last 4 h of the food deprivation. Alpha-MPT is an active competitive inhibitor for tyrosine hydroxylase, thereby preventing the synthesis of catecholamines (57). The remaining hamsters were given intraperitoneal injections of α-MPT (250 mg α-MPT/kg ip) 4 h before the completion of the food deprivation, and a supplemental dose (125 mg/kg) was given 2 h after the initial dose to ensure the inhibition of catecholamine synthesis. The same procedures for termination and WAT depots harvesting were carried out for these hamsters. The adipose tissues were processed and extracted for NE with dihydroxybenzylamine as an internal control to ensure extraction efficiency. The NE content was measured following our methods (for review, see Ref. 61) and modifications (64) of the method of Mefford (35).
Quantification analyses.
PRV-labeled neurons were considered positive based on cell size, shape, and fluorescent intensity. They were analyzed and quantified using an Olympus BX41 microscope. Images were acquired at ×4 and ×10 magnifications using an Olympus DP73 camera and were adjusted for brightness, contrast, sharpness, and overlaying of double-labeled neurons using Adobe Photoshop CS5 (Adobe Systems, San Jose, CA). A mouse brain atlas (42) was used as a reference to identify brain sites, because no commercially available Siberian hamster brain atlas exists, and the size and shape of most hamster brain areas are comparable to that of mouse rather than the Syrian hamster. Absolute values of positively labeled neurons in the brains were collapsed across each bilateral nucleus/region within each hamster and converted into total percentage of PRV-labeled neurons and then averaged across the number of hamsters. The percentages of double-labeled (infected) neurons are represented as the mean percentage of total PRV single- and double-labeled neurons. There was bilateral labeling from MWAT within the spinal cords and sympathetic chains, whereas IWAT had largely ipsilateral labeling with some trivial labeling on the contralateral side. Therefore, in the spinal cord and sympathetic chains, we quantified the labeling from the ipsilateral side of PRV injections into IWAT and also that same ipsilateral side for MWAT, presenting the data as total percentage of PRV-labeled neurons.
Statistical analyses.
All statistical analyses were carried out using NCSS (version 2007, Kaysville, UT). A one-way ANOVA with Duncan's new multiple-range post hoc test was used to compare the percentage of double-labeled neurons to single-labeled neurons either projecting to MWAT or to IWAT. The same analyses were performed for the quantifications of labeled neurons in the spinal cords and sympathetic chains. The Student's t-tests were performed comparing NETO values of ad libitum-fed and food-deprived hamsters. Exact probabilities and test values were omitted for simplicity and clarity of presentation, and statistical significance was considered if P < 0.05.
RESULTS
Experiment 1: Viral Tract Tracing the SNS of MWAT and IWAT
The PRV-injected hamsters showed signs of viral infection, such as unkempt coats, but no overwhelming symptoms of illness before termination. We compared the sympathetic innervations of MWAT and IWAT at the levels of the sympathetic chain, spinal cord, and brain.
Sympathetic chain.
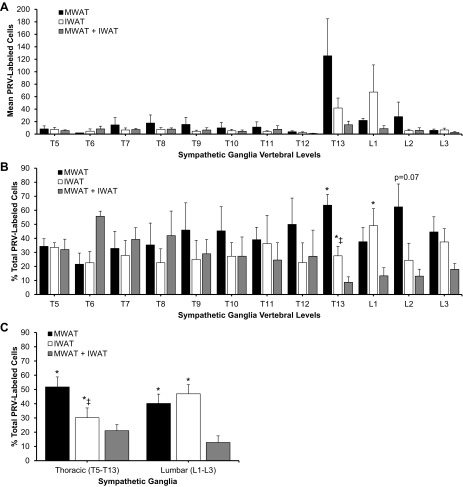
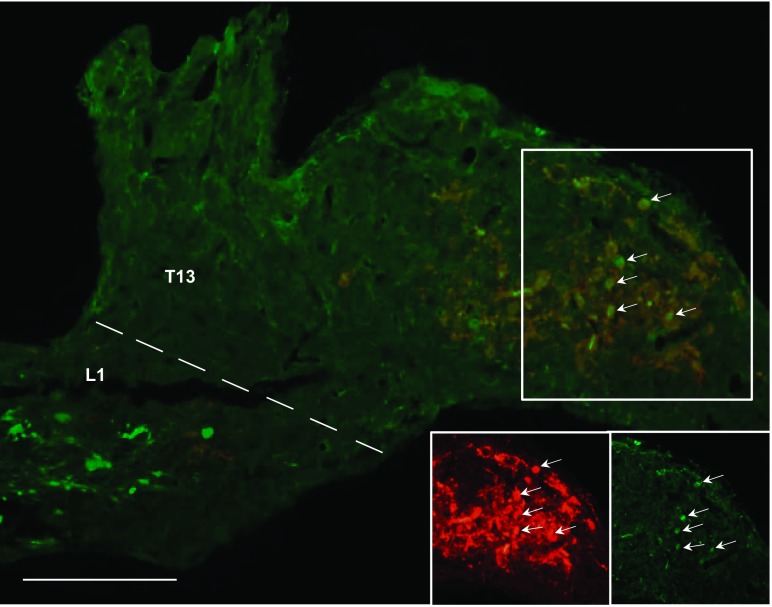
Labeled neurons projecting to MWAT and IWAT that were single-labeled, as well as those that were double-labeled projecting to both WAT depots were found in the sympathetic ganglia associated with the T5 to L3 spinal levels (Fig. 1A). The majority of single-labeled neurons that projected to either MWAT or IWAT were distributed at T13, L1, and L2 (Fig. 1A). The mean absolute number of PRV-labeled neurons projecting to either WAT depot did not reach statistical significance (Fig. 1A); however, when expressed as the percentage of the total number of PRV-labeled neurons from T5 to L3, there were statistically significantly more PRV-infected neurons projecting to MWAT at T13 compared with IWAT (P < 0.05; Fig. 1B). The regional specificity of single-labeled PRV labeling in the sympathetic ganglia was most notably seen at T13-L1 (P < 0.05; Fig. 1B; Fig. 2). These ganglia are physically fused (64), but they can be easily discriminated because T13 is round and similar in shape to the other thoracic sympathetic ganglia, whereas L1 is long and cylindrically shaped, similar to the other lumbar sympathetic ganglia (Fig. 2). Unlike the other sympathetic ganglia that had relatively similar percentages of both single- and double-labeled neurons (∼30–50%), T13 had a statistically significantly lower percentage of double-labeled neurons (∼10%) compared with the single-labeled neurons innervating MWAT or IWAT, while L1 only had lower percentage of double-labeled neurons when compared with single-labeled neurons projecting to IWAT (P < 0.05; Fig. 1B). When the data were collapsed across the thoracic sympathetic ganglia (T5–T13), there was a significantly greater percentage of labeled neurons projecting to MWAT than to IWAT (P < 0.05; Fig. 1C), but a similar analysis for the data collapsed across the lumbar sympathetic ganglia (L1-L3) revealed no such difference (Fig. 1C). The double-labeled neurons projecting to both MWAT and IWAT were statistically significantly lower compared with the single-labeled neurons when the thoracic and lumbar sympathetic ganglia data were collapsed (P < 0.05; Fig. 1C). When all of the sympathetic ganglia (T5-L3) were collapsed across all hamsters, the single-labeled neurons projecting to MWAT and IWAT were statistically greater than the double-labeled neurons, and MWAT had an overall greater percentage of single-labeled neurons compared with IWAT (data not shown).
Fig. 1.
Sympathetic innervations of mesenteric white adipose tissue (MWAT) and inguinal white adipose tissue (IWAT) at the T5-L3 sympathetic chain levels. A: distribution of pseudorabies virus (PRV)-labeled cells across vertebral levels. B: total percentage of PRV-labeled cells. C: total percentage of PRV-labeled cells in collapsed thoracic (T5-T13) and lumbar (L1-L3) sympathetic ganglia. ‡P < 0.05 vs. MWAT; *P < 0.05 vs. MWAT + IWAT.
Fig. 2.
Photomicrographs illustrating isogenic strains of PRV labeling the sympathetic innervation of MWAT (red), of IWAT (green), and of both WAT depots (yellow; white arrows) at the vertebral level of T13-L1. Scale bar = 100 μm.
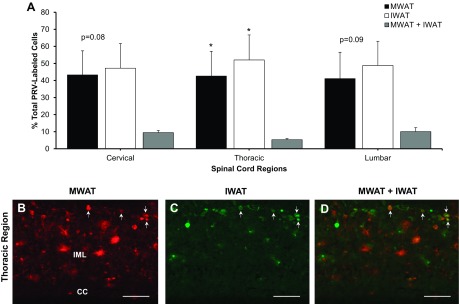
Spinal cord.
PRV 152 and PRV 614 labeled the spinal cord as the viruses moved transneuronally and retrogradely toward the brain. There was positive labeling across the cervical, thoracic, and lumbar portions of the spinal cord (Fig. 3A). The intermediolateral (IML) nucleus of the spinal cord, home of the sympathetic preganglionic neurons, had few doubly infected cells, and this sparse double-labeling was similar to the labeling observed in the sympathetic ganglia (Fig. 3, B, C, and D). The percentage of double-labeled neurons was significantly lower compared with that of singe-labeled neurons projecting to either MWAT or IWAT (P < 0.05; Fig. 3A).
Fig. 3.
Distribution of PRV-labeled cells in the spinal cord. A: quantification of positively labeled PRV cells across the cervical, thoracic, and lumbar regions. B–D: photomicrographs of sympathetic neurons projecting to MWAT (red), to IWAT (green), and to both WAT depots (yellow; white arrows) at the thoracic region of the spinal cord. *P < 0.05 vs. MWAT + IWAT. CC, central canal; IML, intermediolateral nucleus. Scale bar = 100 μm.
Brain.
There was bilateral infection in the hindbrain, midbrain, and forebrain as a result of the PRVs infecting high-order neurons retrogradely from MWAT and IWAT. Overall, this general pattern of infection was similar to previous PRV labeling of EWAT (4, 54, 64), RWAT (1) and IWAT (4, 54) in earlier studies. The percentages of neurons ultimately projecting to MWAT and IWAT were quite similar to each other, with the exception of some nuclei in the hindbrain, midbrain, and forebrain (see Supplemental Table S1).
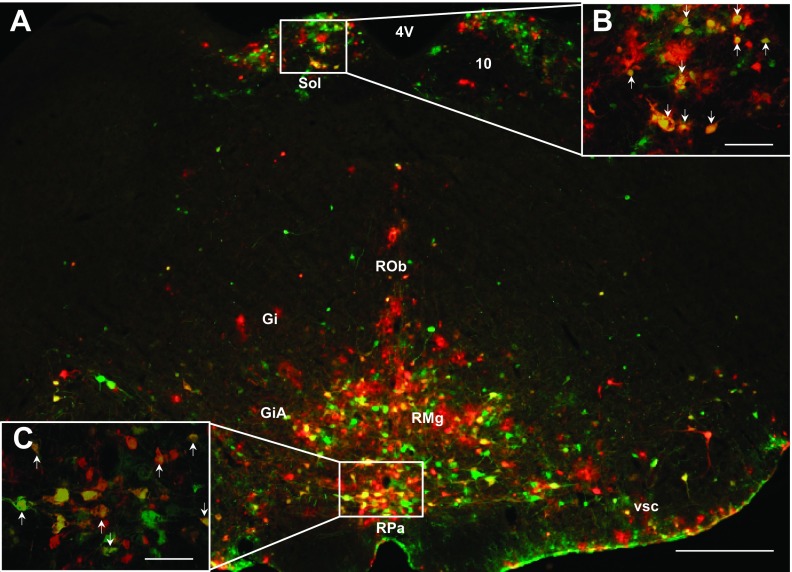
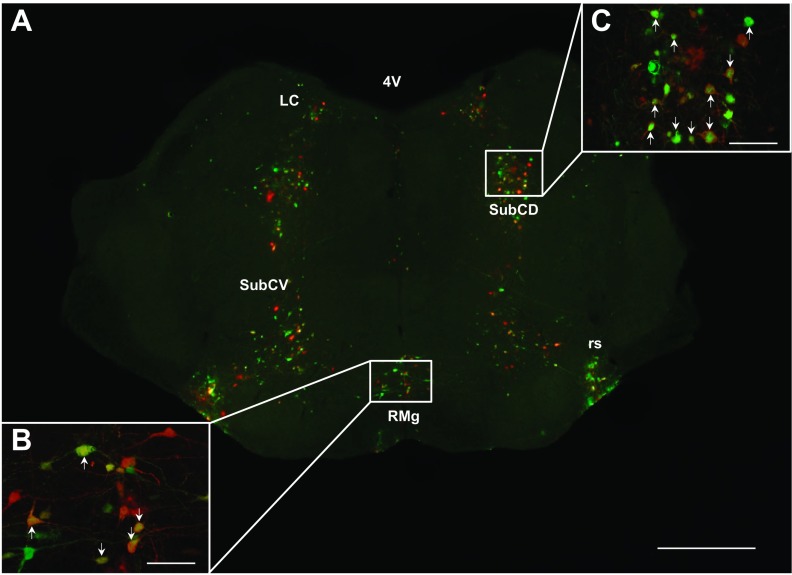
For some of the hindbrain regions, such as the ambiguus nucleus (Amb), area postrema (AP), dorsal paragigantocellular nucleus (DPGi), linear nucleus of the medulla (Li), peripyramidal nucleus (Ppy), dorsal raphe nucleus (DR), caudal/rostral linear nucleus of the raphe (CLi/RLi), lateral/parvicellular part of reticular nucleus (LRt/-PC), nucleus of the solitary tract (Sol; Fig. 4, A and B), and ventral spinocerebellar tract (vsc; Fig. 4A), they had significantly more labeled sympathetic circuit neurons projecting to IWAT than to MWAT (P < 0.05; see Supplemental Table S1). There were exceptions, however, such as the hypoglossal nucleus, A5 region, facial regions, gigantocellular areas (Fig. 4A), parabrachial areas, the dorsal and ventral parts of the subcoeruleus nucleus, (Fig. 5, A and B), locus coeruleus (Fig. 5A), raphe magnus nucleus (RMg; Fig. 4, A; Fig. 5, A and B), raphe obscurus nucleus (Fig. 4A), raphe pallidus (RPa; Fig. 4, A and B), rubrospinal tract (Fig. 5A), and trigeminal areas, where there were similar percentages of single-labeled neurons projecting to MWAT and to IWAT that did not reach statistical significance. For the other remaining quantified hindbrain regions though, statistical significance was not reached, and there was a tendency for IWAT to have a greater percentage of single-labeled neurons than MWAT. It appears that in the hindbrain, IWAT (∼30–45%; subcutaneous WAT) has a greater percentage of sympathetic circuit neurons than MWAT (∼20–35%; visceral WAT) with ∼20–40% of double-labeled neurons projecting to both WAT depots.
Fig. 4.
A: low-magnification (×4) photomicrograph illustrating PRV labeling in the hindbrain. High-magnification (×10) photomicrograph illustrating PRV labeling in the nucleus of the solitary tract (Sol; B) and raphe pallidus (RPa; C). A–C: sympathetic neurons projecting to MWAT (red), to IWAT (green), and to both WAT depots (yellow; white arrows). 4V, fourth ventricle; 10, dorsal motor nucleus of the vagus; Gi, gigantocellular reticular nucleus; GiA, gigantocellular reticular nucleus, alpha part; RMg, raphe magnus nucleus; ROb, raphe obscurus nucleus; RPa, raphe pallidus nucleus; Sol, nucleus of the solitary tract; vsc, ventral spinocerebellar tract. A: scale bar = 500 μm. B and C: scale bar = 200 μm.
Fig. 5.
A: low-magnification (×4) photomicrograph illustrating PRV labeling in the midbrain. High-magnification (×10) photomicrograph illustrating PRV labeling in the RMg (B) and SubCD (C). A–C: sympathetic neurons projecting to MWAT (red) to IWAT (green) and to both WAT depots (yellow; white arrows). 4V, fourth ventricle; LC, locus coeruleus; RMg, raphe magnus nucleus; rs, rubrospinal tract; SubCD, subcoeruleus nucleus, dorsal part; SubCV, subcoeruleus nucleus, ventral part. A: scale bar = 500 μm. B and C: scale bar = 200 μm.
Nuclei within the midbrain had ∼20–50% of double-labeled neurons (see Supplemental Table S1). PRV labeling of neurons ultimately projecting to IWAT were statistically greater in the posterior commissure, cuneiform nucleus (CnF), Edinger-Westphal nucleus (EW), medial longitudinal fasciculus (mlf), dorsolateral periaqueductal gray (DLPAG), dorsomedial periaqueductal gray (DMPAG), lateral periaqueductal gray (LPAG), ventral lateral periaqueductal gray (VLPAG), rubral areas, laterodorsal tegmental nucleus ventral part (LDTg/-V), and pedunculopontine tegmental nucleus (PPTg) than neurons that comprise the circuit ultimately innervating MWAT (P < 0.05; see Supplemental Table S1). Similar to the hindbrain, there also were regions where we observed no statistical difference between the numbers of single- and double-labeled neurons.
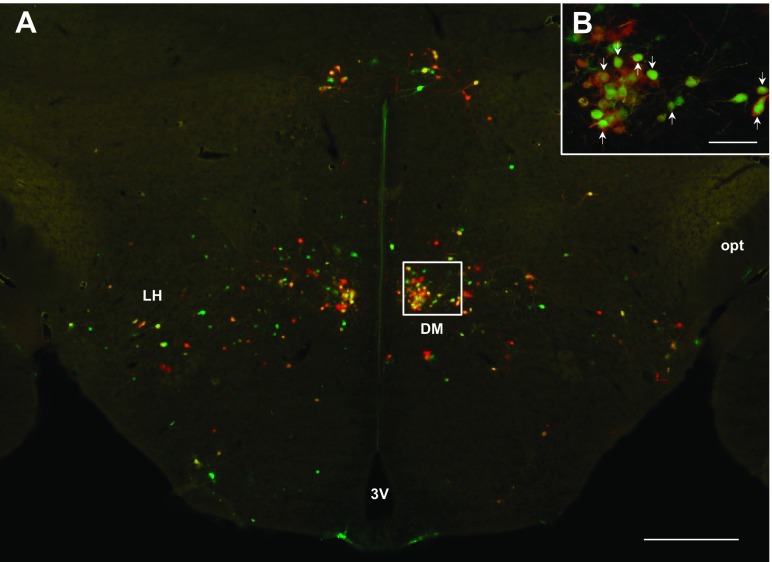
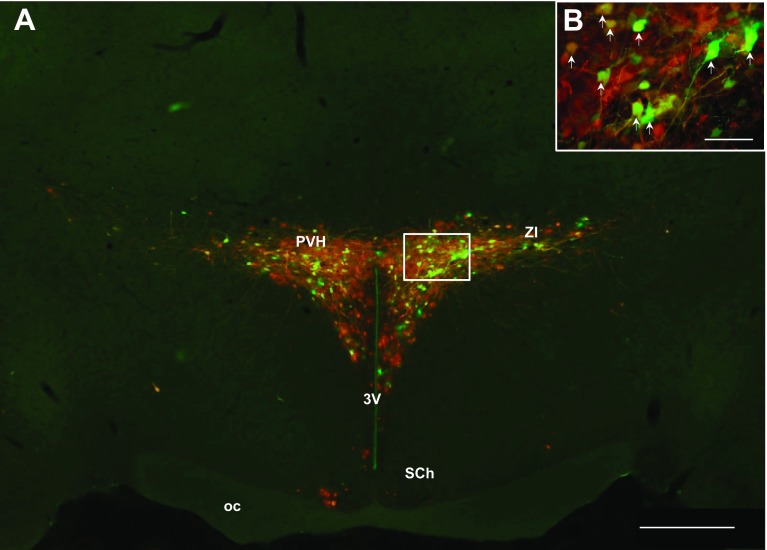
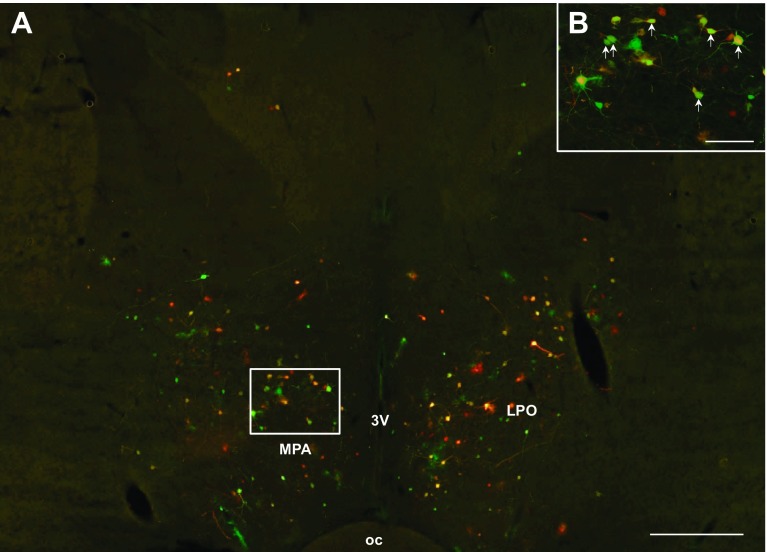
Heavy PRV labeling from MWAT and IWAT was seen in the forebrain. There was not a significant difference between the percentage of neurons comprising the circuits ultimately innervating MWAT and IWAT in the large majority of forebrain regions, such as the dorsomedial hypothalamic nucleus (Fig. 6, A and B), subzona incerta, posterior hypothalamic area, the majority of the subnuclei of the preoptic area, the paraventricular nucleus of the hypothalamus (PVH; Fig. 7, A and B), the suprachiasmatic nucleus, and thalamic regions. There were, however, a statistically significantly greater percentage of neurons ultimately projecting to IWAT than to MWAT in only a few areas, such as the medial preoptic area (MPA; Fig. 8, A and B), and lateral hypothalamic area proper (LH proper; P < 0.05; see Supplemental Table S1). We only found differences in these two areas (LH proper and MPA) within the forebrain, whereas the midbrain and hindbrain had many brain regions that showed differences between the innervation to MWAT and IWAT. The percentage of double-labeled neurons was ∼20–40% in forebrain brain regions, whereas the percentage of single-labeled neurons was similar (∼20–45%; see Supplemental Table S1). Thus, we did not detect many distinct differences between the circuits ultimately innervating MWAT (visceral WAT) and IWAT (subcutaneous WAT) in the forebrain compared with more numerous differences in the midbrain and hindbrain.
Fig. 6.
A: low-magnification (×4) photomicrograph illustrating PRV labeling in the dorsomedial hypothalamus (DMH). High-magnification (×10) photomicrograph illustrating PRV labeling in the DMH (B). A and B: sympathetic neurons projecting to MWAT (red), IWAT (green), and both WAT depots (yellow; white arrows). 3V, third ventricle; DM, dorsomedial hypothalamic nucleus; LH, lateral hypothalamic area; opt, optic tract. A: scale bar = 500 μm. B: scale bar = 200 μm.
Fig. 7.
A: low magnification (×4) photomicrograph illustrating PRV labeling in the PVH. High-magnification (×10) photomicrograph of PRV labeling in the PVH (B). A and B: sympathetic neurons projecting to MWAT (red) to IWAT (green) and to both WAT depots (yellow; white arrows). 3V, third ventricle; oc, optic chiasm; PVH, paraventricular nucleus of the hypothalamus; SCh, suprachiasmatic nucleus; ZI, zona incerta. A: scale bar = 500 μm. B: scale bar = 200 μm.
Fig. 8.
A: low-magnification (×4) photomicrograph illustrating PRV labeling in the preoptic area (POA). High-magnification (×10) photomicrograph of PRV labeling in the medial preoptic area (MPA), a subregion of the POA (B). A and B: sympathetic neurons projecting to MWAT (red) to IWAT (green) and to both WAT depots (yellow; white arrows). 3V, third ventricle; LPO, lateral preoptic area; MPA, medial preoptic area; oc, optic chiasm. A: scale bar = 500 μm. B: scale bar = 200 μm.
Experiment 2: Tests of the SNS Drive to MWAT and to IWAT After 16-h Food Deprivation
Utilizing the NETO tests, we compared the sympathetic drives of MWAT and IWAT, as well as other WAT depots in response to standard energy challenge that stimulates the SNS drive to WAT-food deprivation (13, 36).
Body mass.
The mean absolute body mass from pre- to post-food deprivation was statistically significantly decreased after the 16-h food deprivation (P < 0.05; Table 1), but not surprisingly, for the ad libitum-fed group. When the change in body mass was examined, the average change in body mass from pre- to post-food deprivation was significantly decreased compared with that of the corresponding ad libitum-fed groups (P < 0.05; Table 1).
Table 1.
Body weight changes before and after 16-h food deprivation
| Condition | Initial Body Mass, g | Final Body Mass, g | Δ Body Mass, g |
|---|---|---|---|
| Ad libitum-Fed | 39.30 ± 1.00 | 39.29 ± 1.01 | −0.01 ± 0.22 |
| 16-h Food Deprivation | 38.91 ± 0.97 | 36.82 ± 0.85* | −2.10 ± 0.18* |
Data are presented as means ± SE.
P < 0.05 vs. ad libitum-fed.
NETO.
MWAT in the 16-h food deprivation group often had negative NETO values because the basal levels of NETO (0 h) were lower than at the time of NETO testing (4 h), which contradicts physiological reality. Therefore, for those cases of negative NETO values, the values were set to zero, as we have done previously (12, 13, 37, 39, 48, 64). This can happen in some samples by chance when basal levels of NE are initially low due to low sympathetic drive (12, 13, 39).
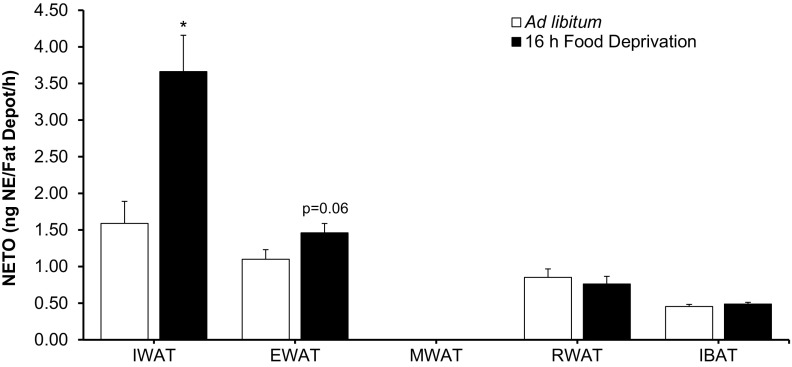
We included IBAT as a negative control for the NETO tests, and we expected that the food-deprived hamsters would decrease their IBAT utilization when energy is not available (i.e., food deprivation) given that BAT thermogenesis is energetically costly, as we have seen previously (e.g., Ref. 13) and, therefore, have low NETO. This also was the case here. Food deprivation of 16 h produced differential NETO that was WAT depot-specific. Specifically, IWAT NETO from food-deprived hamsters was significantly greater than IWAT from ad libitum-fed hamsters, whereas MWAT had negligible NETO for both the ad libitum-fed and the 16-h food-deprived groups. We previously found a small, but significant, EWAT NETO increase with food deprivation (13), whereas, here, there was a tendency (P = 0.06) for a similar small increase, but not a difference between RWAT and IBAT NETO between ad libitum-fed and food-deprived animals (Fig. 9).
Fig. 9.
Values are expressed as means ± SE. Norepinephrine turnover (NETO) expressed per fat depot in Siberian hamsters subjected to 16-h food deprivation. *P < 0.05 vs. ad libitum-fed.
DISCUSSION
In the present study, we used isogenic strains of PRV that have a distinct fluorescent reporter of either GFP or mRFP, revealing for the first time both separate and shared SNS outflow circuitries between true visceral WAT (MWAT) and subcutaneous WAT (IWAT) in a rodent species and, to our knowledge, any species. The labeled central outflow circuits to MWAT and IWAT from the present study are reminiscent of the general overall pattern of viral infections labeling the SNS outflow from the brain to WAT seen previously for IWAT (4, 4, 54, 64), EWAT (4, 64), and RWAT (1) using PRV.
We performed extensive histological analyses across the neuroaxis. We previously demonstrated the distribution of the postganglionic SNS innervation of IWAT and EWAT (64) using a fluorescent monosynaptic retrograde tract tracer (FluoroGold) and demonstrated largely differential innervation of this subcutaneous and intra-abdominal WAT depot, respectively (64). Here, we found largely separate SNS innervation between MWAT and IWAT with a greater percentage of single-labeled neurons to IWAT compared with MWAT and ∼20–55% of shared neurons between the two WAT depots within the brain. Such separation of the SNS outflow from brain to WAT depots, such as these, allows for the relative unique patterns of sympathetic drive to WAT depots with various lipolytic challenges (for review, see Refs. 6 and 7). This peripheral delineation of innervation between MWAT and IWAT makes it plausible to support the contribution of MWAT to hepatic insulin resistance, and overall, the metabolic syndrome with IWAT contributing as a relatively safe repository of excess lipid energy (25, 46). We found a higher percentage of single-labeled neurons from the T5-T13 for MWAT than IWAT when we collapsed the labeled cells for each sympathetic ganglia from the thoracic (T5-T13) and lumbar (L1-L3) sympathetic ganglia, suggesting that MWAT has a greater thoracic SNS innervation as well, perhaps, contributing to a higher level of basal lipolysis and, as a result, increases in FFA release to the hepatic portal vein (2). As noted, however, this apparent increase in postganglionic sympathetic innervation of MWAT did not translate into a greater NETO with food deprivation to this depot relative to the other WAT pads. The differences between innervation of MWAT and IWAT in the IML of the spinal cord were not observed, suggesting that differential SNS drive to MWAT and WAT may originate from the sympathetic chain (∼60% of single-labeled neurons for each WAT depot).
The arrangement of preganglionic neurons of the IML innervating several postganglionic neurons within the sympathetic chain before that final projection to the target tissue permits a single sympathetic neuron within the IML nucleus of the spinal cord to synapse on many excitatory or inhibitory postganglionic neurons or in other cases to have a single or few projections to postganglionic neurons (30). Therefore, this aspect of the SNS allows for both convergence and divergence for target tissue innervation, resulting in possible coordination of sympathetic outflow to multiple WAT depots, as well as control of outflow to individual WAT depots. This divergence is perhaps exemplified not only by the increased NETO to IWAT vs. MWAT in the present study, but also by the unique patterns of sympathetic drive (NETO) across WAT and BAT pads for each lipolytic stimulus [food deprivation (13), short-day photoperiod (64), central melanocortin 4-receptor agonism (12), food deprivation (13), cold exposure (13), and glucoprivation (13)]. Thus, it is not surprising that there are low percentages of double-labeled neurons in the sympathetic chain (∼10–30%), because if they were plentiful or completely overlapping, then such nearly unique patterns of sympathetic drive for each stimulus would be rare or not even possible.
More centrally across the brain, we found an overlap (∼20–55% of doubly infected neurons) between the two SNS outflow circuitries to MWAT and to IWAT. IWAT had significantly more single-labeled neurons than MWAT for several hindbrain regions: Amb, AP, DPGi, Li, Ppy, prepositus nucleus, DR, CLi/RLi, LRt/-PC, Sol, vestibular areas, and vsc; some midbrain regions: posterior commissure, CnF, EW, mlf, DLPAG, DMPAG, LPAG, VLPAG, rubral areas, LDTg/-V, and PPTg; and a few forebrain sites: LH proper and MPA. The remaining brain regions had roughly equal percentages of single-labeled neurons across the brain for the outflow circuitries to IWAT and to MWAT. The regions that are typically known to be involved in energy metabolism and of sympathetic outflow were labeled by both PRVs as seen in our earlier work (4, 11, 54) and always include the original five areas noted by Strack et al. (59): the PVH, A5 noradrenergic cell group, caudal raphe region (including the Sol, raphe pallidus, RMg, and RPa,), rostral ventrolateral medulla, and ventromedial medulla. Although there are these distinct differences across these brain regions, many of the brain areas ultimately innervating these two WAT depots were more similar than different.
There are caveats with utilization of two isogenic viral tract tracers (PRVs), including but not limited to the issue of one PRV infecting a neuron first and preventing the second PRV from doing so—the so-called “principle of exclusion” in virology, as well as the virulence of one PRV dominating the infection if they do reach the neurons at approximately the same time (for review, see Refs. 15, 53). We have decreased the likelihood of these problems by conducting preliminary studies; however, as discussed in materials and methods. It should be noted that it is not possible to design the timing of the injections from two peripheral sites to ensure nearly simultaneous reaching of the two viruses for every area of interest in the brain. Because of these potential difficulties, even though we attempted to minimize them, it is always assumed that the percentage of double-labeled neurons may actually be much greater (53).
We functionally tested this differential (but also shared) SNS innervation of IWAT and MWAT depots through the energetic challenge of food deprivation (16 h), which we previously significantly increased IWAT and EWAT NETO (the latter, a small but significant increase) with food deprivation, but no change for RWAT or IBAT (13). Here, IWAT NETO more than doubled, and EWAT NETO showed a small but nonsignificant increase (P = 0.06), with no change in NETO for RWAT or IBAT, as seen previously (13). This is the first time that we assayed MWAT NETO. The finding of differential sympathetic drives to MWAT and to IWAT in this study may have, as its neuroanatomical basis, some of the differences we found in the PRV-induced labeling of the sympathetic outflow circuits from the brain to these WAT depots across the neuroaxis. At most, only ∼20–55% of PRV-infected neurons were doubly labeled, indicating participation in the SNS outflow to both depots, and much less when approaching the final common pathways in the IML (∼10%) and sympathetic chain (∼10–30%). Human visceral WAT has higher lipolytic rates than subcutaneous WAT (see perspectives and significance directly below), but unlike humans, MWAT is a relatively small intra-abdominal WAT depot in rodents in general (18) and in our hamsters specifically. In humans, there are clear distinctions between substances produced and possible contributions of MWAT vs. the normally greater mass of omental WAT (60). Omental WAT is absent in rodents or perhaps may be present as an almost imperceptible white “spot” or “stripe” on the stomach fundus of laboratory rats, mice, and Syrian and Siberian hamsters (Bartness TJ, Harris RS, and Scherer P, independent unpublished observations). IWAT, given its larger capacity to store lipid and its nonassociation with an organ that can have local physiological functions (17), in principle, is in a better position to supply lipid energy fuels with food deprivation than other WAT pads. Indeed, with the exception of short winter-like photoperiod exposure in Siberian hamsters, in which NETO is greater in EWAT than IWAT (64), NETO in IWAT is increased more than in any of the other WAT pads with cold exposure (13), food deprivation [shown previously (13) and here], glucoprivation (13), and central melanocortin receptor agonism (12).
Here, we have demonstrated that MWAT (visceral WAT) and IWAT (subcutaneous WAT) have relatively separate SNS circuitries in the periphery with moderately shared but more substantial separate circuitries across the brain. We demonstrated that subcutaneous WAT (IWAT) has a greater sympathetic drive with food deprivation when compared with visceral WAT (MWAT).
Perspectives and Significance
Several studies demonstrated that visceral WAT has a higher rate of basal (i.e., non-SNS/NE-stimulated lipolysis) lipolysis and that, in general, human visceral white adipocytes have higher basal lipolytic rates than do subcutaneous adipocytes (e.g., Refs. 45 and 63, but compare Ref. 3). The role of adrenoceptor stimulation in basal lipolysis also may be somewhat independent of β-adrenoceptor stimulation, with factors affecting adipose triglyceride lipase (also known as desnutrin) being more important for basal lipolysis (27). Clearly, however, as we have shown here and previously, there is sympathetic drive occurring in the in vivo nonenergetically challenged situation that also should be important for basal lipolysis via the adenylyl cyclase/cAMP/PKA phosphorylation/hormone-sensitive lipase and perilipin A phosphorylation intracellular signaling pathway (8).
Regarding SNS/NE-stimulated lipolysis, however, the data are mixed, with subcutaneous WAT having greater stimulated lipolysis than MWAT in vitro (63). In vivo, using phosphorylated HSL and perilipin A as markers of lipolysis in WAT pads, central melanocortin 4-receptor-stimulated lipolysis that increases SNS drive (NETO) to subcutaneous WAT (IWAT and dorsosubcutaneous WAT), but not intra-abdominal WAT (EWAT, RWAT; see Ref. 12), only increases phosphorylated HSL and phosphorylated perilipin A in these subcutaneous WAT pads with increases in NETO, but not the intra-abdominal ones (49), demonstrating the in vivo physiological coupling of increases in SNS drive to lipolysis. Thus, it is difficult to unequivocally unify in vitro tests of β-adrenoceptor-stimulated lipolysis to the in vivo physiological condition.
The conventional wisdom for the involvement of lipolysis/FFAs in the metabolic syndrome is that it is the result of excess systemic FFAs coming from visceral WAT that would include mesenteric and omental WAT depots in humans that drain into the hepatic-portal vein (reviewed in Ref. 32). It is now clear, however, that upper body nonsplanchnic draining WAT instead of visceral hepatic-portal vein draining WAT is the main source of FFAs and, thus, the likely culprit for the adverse health consequences in humans (for review, see Refs. 31 and 32). Most nonhuman animal studies that purport to study the effects of or differences in visceral vs. subcutaneous WAT do not use hepatic-portal draining WAT, which, as noted above, is only MWAT in rodents. The negligible/undetectable sympathetic drive (NETO) to MWAT with food deprivation in the present studies casts some doubt as to whether, although visceral WAT by definition, this WAT depot is the human equivalent of visceral WAT and, therefore, even actual visceral WAT in rodents may not mimic the human condition. In humans, distinctions between the functions and pathophysiology of MWAT and omental WAT are emerging, with MWAT seen as having independent effects on the metabolic syndrome from omental WAT (34). For example, omental WAT has greater adrenoceptor-stimulated lipolysis than MWAT in vitro (23). Thus, for human and nonhuman animals, it seems evident that WAT depots should not be thought of as uniform in any sense of the word, including innervation and sympathetic drive, along with cytokine and other substance production (60). This begs for a more thorough understanding of why the depots are associated with nearby organs and whether this is just by chance, or as with EWAT and the testes in frogs, laboratory rats and mice, and Syrian hamsters, is necessary for functions of the adjacent organ, in this example, spermatogenesis (17, 22, 22, 58).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: T.J.B., N.L.N., J.R., and B.W.B. conception and design of research; T.J.B. and N.L.N. interpreted results of experiments; T.J.B., N.L.N., and B.W.B. edited and revised manuscript; T.J.B., N.L.N., J.R., and B.W.B. approved final version of manuscript; N.L.N., J.R., and B.W.B. performed experiments; N.L.N. analyzed data; N.L.N. prepared figures; N.L.N. drafted manuscript.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Yaakov G. Mitchell for assistance with virus injections, Keegan T. Murphy, and Danni Liu for assistance with tissue harvesting and Eleen Zarebidaki and Benjamin Blaschke with norepinephrine extractions. The authors also thank Dr. Vitaly Ryu for helpful discussions on the manuscript. This work was funded by National Institutes of Health DK-35254 to T. J. Bartness.
REFERENCES
- 1.Adler ES, Hollis JH, Clarke IJ, Grattan DR, Oldfield BJ. Neurochemical characterization and sexual dimorphism of projections from the brain to abdominal and subcutaneous white adipose tissue in the rat. J Neurosci 32: 15913–15921, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arner P. Insulin resistance in type 2 diabetes: role of fatty acids. Diabetes Metab Res Rev 18 Suppl 2: S5–S9, 2002. [DOI] [PubMed] [Google Scholar]
- 3.Arner P. Human fat cell lipolysis: biochemistry, regulation and clinical role. Best Pract Res Clin Endocrinol Metab 19: 471–482, 2005. [DOI] [PubMed] [Google Scholar]
- 4.Bamshad M, Aoki VT, Adkison MG, Warren WS, Bartness TJ. Central nervous system origins of the sympathetic nervous system outflow to white adipose tissue. Am J Physiol Regul Integr Comp Physiol 275: R291–R299, 1998. [DOI] [PubMed] [Google Scholar]
- 5.Banfield BW, Kaufman JD, Randall JA, Pickard GE. Development of pseudorabies virus strains expressing red fluorescent proteins: new tools for multisynaptic labeling applications. J Virol 77: 10106–10112, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartness TJ, Bamshad M. Innervation of mammalian white adipose tissue: Implications for the regulation of total body fat. Am J Physiol Regul Integr Comp Physiol 275: R1399–R1411, 1998. [DOI] [PubMed] [Google Scholar]
- 7.Bartness TJ, Shrestha YB, Vaughan CH, Schwartz GJ, Song CK. Sensory and sympathetic nervous system control of white adipose tissue lipolysis. Mol Cell Endocrinol 318: 34–43, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bezaire V, Langin D. Regulation of adipose tissue lipolysis revisited. Proc Nutr Soc 68: 350–360, 2009. [DOI] [PubMed] [Google Scholar]
- 9.Beznak ABL, Hasch Z. The effect of sympathectomy on the fatty deposit in connective tissue. Quart J Exptl Physiol 27: 1–15, 1937. [Google Scholar]
- 10.Bjorntorp P. Adipose tissue distribution and function. Int J Obesity 15: 67–81, 1991. [PubMed] [Google Scholar]
- 11.Bowers RR, Festuccia WTL, Song CK, Shi H, Migliorini RH, Bartness TJ. Sympathetic innervation of white adipose tissue and its regulation of fat cell number. Am J Physiol Regul Integr Comp Physiol 286: R1167–R1175, 2004. [DOI] [PubMed] [Google Scholar]
- 12.Brito MN, Brito NA, Baro DJ, Song CK, Bartness TJ. Differential activation of the sympathetic innervation of adipose tissues by melanocortin receptor stimulation. Endocrinology 148: 5339–53347, 2007. [DOI] [PubMed] [Google Scholar]
- 13.Brito NA, Brito MN, Bartness TJ. Differential sympathetic drive to adipose tissues after food deprivation, cold exposure or glucoprivation. Am J Physiol Regul Integr Comp Physiol 294: R1445–R1452, 2008. [DOI] [PubMed] [Google Scholar]
- 14.Cantu RC, Goodman HM. Effects of denervation and fasting on white adipose tissue. Am J Physiol 212: 207–212, 1967. [DOI] [PubMed] [Google Scholar]
- 15.Card JP. Practical considerations for the use of pseudorabies virus in transneuronal studies of neural circuitry. Neurosci Biobehav Rev 22: 685–694, 1998. [DOI] [PubMed] [Google Scholar]
- 16.Catalano KJ, Stefanovski D, Bergman RN. Critical role of the mesenteric depot versus other intra-abdominal adipose depots in the development of insulin resistance in young rats. Diabetes 59: 1416–1423, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chu Y, Huddleston GG, Clancy AN, Harris RB, Bartness TJ. Epididymal fat is necessary for spermatogenesis, but not testosterone production or copulatory behavior. Endocrinology 151: 5669–5779, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cinti S. The Adipose Organ. Milan, Italy: Editrice Kurtis, 1999. [Google Scholar]
- 19.Czaja K, Barb CR, Kraeling RR. Hypothalamic neurons innervating fat tissue in the pig express leptin receptor immunoreactivity. Neurosci Lett 425: 6–11, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Czaja K, Kraeling RR, Barb CR. Are hypothalamic neurons transsynaptically connected to porcine adipose tissue? Biochem Biophys Res Commun 311: 482–485, 2003. [DOI] [PubMed] [Google Scholar]
- 21.Demas GE, Bartness TJ. Direct innervation of white fat and adrenal medullary catecholamines mediate photoperiodic changes in body fat. Am J Physiol Regul Integr Comp Physiol 281: R1499–R1505, 2001. [DOI] [PubMed] [Google Scholar]
- 22.Faust IM, Johnson PR, Hirsch J. Noncompensation of adipose mass in partially lipectomized mice and rats. Am J Physiol 231: 538–544, 1976. [DOI] [PubMed] [Google Scholar]
- 23.Fried SK, Leibel RL, Edens NK, Kral JG. Lipolysis in intraabdominal adipose tissues of obese women and men. Obes Res 1: 443–448, 1993. [DOI] [PubMed] [Google Scholar]
- 24.Gasteyger C, Tremblay A. Metabolic impact of body fat distribution. J Endocrinol Invest 25: 876–883, 2002. [DOI] [PubMed] [Google Scholar]
- 25.Gavrilova O, Marcus-Samuels B, Graham D, Kim JK, Shulman GI, Castle AL, Vinson C, Eckhaus M, Reitman ML. Surgical implantation of adipose tissue reverses diabetes in lipoatrophic mice. J Clin Invest 105: 271–278, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giordano A, Song CK, Bowers RR, Ehlen JC, Frontini A, Cinti S, Bartness TJ. White adipose tissue lacks significant vagal innervation and immunohistochemical evidence of parasympathetic innervation. Am J Physiol Regul Integr Comp Physiol 291: R1243–R1255, 2006. [DOI] [PubMed] [Google Scholar]
- 27.Girousse A, Langin D. Adipocyte lipases and lipid droplet-associated proteins: insight from transgenic mouse models. Int J Obes (Lond) 36: 581–594, 2012. [DOI] [PubMed] [Google Scholar]
- 28.Goldstein LB, Bushnell CD, Adams RJ, Appel LJ, Braun LT, Chaturvedi S, Creager MA, Culebras A, Eckel RH, Hart RG, Hinchey JA, Howard VJ, Jauch EC, Levine SR, Meschia JF, Moore WS, Nixon JV, Pearson TA. Guidelines for the primary prevention of stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 42: 517–584, 2011. [DOI] [PubMed] [Google Scholar]
- 29.Heilbronn L, Smith SR, Ravussin E. Failure of fat cell proliferation, mitochondrial function and fat oxidation results in ectopic fat storage, insulin resistance and type II diabetes mellitus. Int J Obes Relat Metab Disord 28 Suppl 4: S12–S21, 2004. [DOI] [PubMed] [Google Scholar]
- 30.Janig W, McLachlan EM. Characteristics of function-specific pathways in the sympathetic nervous system. TINS 15: 475–481, 1992. [DOI] [PubMed] [Google Scholar]
- 31.Jensen MD. Lipolysis: contribution from regional fat. Annu Rev Nutr 17: 127–139, 1997. [DOI] [PubMed] [Google Scholar]
- 32.Jensen MD. Is visceral fat involved in the pathogenesis of the metabolic syndrome? Human Model. Obesity (Silver Spring) 14 Suppl 1: 20S–24S, 2006. [DOI] [PubMed] [Google Scholar]
- 33.Li Y, Bujo H, Takahashi K, Shibasaki M, Zhu Y, Yoshida Y, Otsuka Y, Hashimoto N, Saito Y. Visceral fat: higher responsiveness of fat mass and gene expression to calorie restriction than subcutaneous fat. Exp Biol Med (Maywood) 228: 1118–1123, 2003. [DOI] [PubMed] [Google Scholar]
- 34.Liu KH, Chan YL, Chan WB, Chan JC, Chu CW. Mesenteric fat thickness is an independent determinant of metabolic syndrome and identifies subjects with increased carotid intima-media thickness. Diabetes Care 29: 379–384, 2006. [DOI] [PubMed] [Google Scholar]
- 35.Mefford IN. Application of high performance liquid chromatography with electrochemical detection to neurochemical analysis: measurement of catecholamines, serotonin and metabolites in rat brain. J Neurosci Methods 3: 207–224, 1981. [DOI] [PubMed] [Google Scholar]
- 36.Migliorini RH, Garofalo MAR, Kettelhut IC. Increased sympathetic activity in rat white adipose tissue during prolonged fasting. Am J Physiol Regul Integr Comp Physiol 272: R656–R661, 1997. [DOI] [PubMed] [Google Scholar]
- 37.Mul JD, O'Duibhir E, Shrestha YB, Koppen A, Vargovic P, Toonen PW, Zarebidaki E, Kvetnansky R, Kalkhoven E, Cuppen E, Bartness TJ. Pmch-deficiency in rats is associated with normal adipocyte differentiation and lower sympathetic adipose drive. PLoS One 8: e60214, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murphy KT, Schwartz GJ, Nguyen NL, Mendez JM, Ryu V, Bartness TJ. Leptin-sensitive sensory nerves innervate white fat. Am J Physiol Endocrinol Metab 304: E1338–E1347, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nautiyal KM, Dailey MJ, Brito NA, Brito MN, Harris RBS, Bartness TJ, Grill HJ. Energetic responses to cold temperatures in rats lacking forebrain-caudal brainstem connections. Am J Physiol Regul Integr Comp Physiol 295: R789–R798, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ohman MK, Shen Y, Obimba CI, Wright AP, Warnock M, Lawrence DA, Eitzman DT. Visceral adipose tissue inflammation accelerates atherosclerosis in apolipoprotein E-deficient mice. Circulation 117: 798–805, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pasanisi F, Contaldo F, de Simone G, Mancini M. Benefits of sustained moderate weight loss in obesity. Nutr Metab Cardiovasc Dis 11: 401–406, 2001. [PubMed] [Google Scholar]
- 42.Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. San Diego, CA: Academic, 2001. [Google Scholar]
- 43.Rao GH, Thethi I, Fareed J. Vascular disease: obesity and excess weight as modulators of risk. Expert Rev Cardiovasc Ther 9: 525–534, 2011. [DOI] [PubMed] [Google Scholar]
- 44.Reaven GM. Insulin resistance: the link between obesity and cardiovascular disease. Med Clin North Am 95: 875–892, 2011. [DOI] [PubMed] [Google Scholar]
- 45.Rebuffe-Scrive M, Andersson B, Olbe L, Bjorntorp P. Metabolism of adipose tissue in intraabdominal depots of nonobese men and women. Metabolism 38: 453–458, 1989. [DOI] [PubMed] [Google Scholar]
- 46.Reitman ML, Mason MM, Moitra J, Gavrilova O, Marcus-Samuels B, Eckhaus M, Vinson C. Transgenic mice lacking white fat: models for understanding human lipoatrophic diabetes. Ann NY Acad Sci 892: 289–296, 1999. [DOI] [PubMed] [Google Scholar]
- 47.Shi H, Bartness TJ. Neurochemical phenotype of sympathetic nervous system outflow from brain to white fat. Brain Res Bull 54: 375–385, 2001. [DOI] [PubMed] [Google Scholar]
- 48.Shi H, Bowers RR, Bartness TJ. Norepinephrine turnover in brown and white adipose tissue after partial lipectomy. Physiol Behav 81: 535–543, 2004. [DOI] [PubMed] [Google Scholar]
- 49.Shrestha YB, Vaughan CH, Smith BJ, Jr, Song CK, Baro DJ, Bartness TJ. Central melanocortin stimulation increases phosphorylated perilipin A and hormone-sensitive lipase in adipose tissues. Am J Physiol Regul Integr Comp Physiol 299: R140–R149, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smith BN, Banfield BW, Smeraski CA, Wilcox CL, Dudek FE, Enquist LW, Pickard GE. Pseudorabies virus expressing enhanced green fluorescent protein: A tool for in vitro electrophysiological analysis of transsynaptically labeled neurons in identified central nervous system circuits. Proc Natl Acad Sci USA 97: 9264–9269, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Snijder MB, Dekker JM, Visser M, Bouter LM, Stehouwer CD, Kostense PJ, Yudkin JS, Heine RJ, Nijpels G, Seidell JC. Associations of hip and thigh circumferences independent of waist circumference with the incidence of type 2 diabetes: the Hoorn Study. Am J Clin Nutr 77: 1192–1197, 2003. [DOI] [PubMed] [Google Scholar]
- 52.Song CK, Bartness TJ. CNS sympathetic outflow neurons to white fat that express melatonin receptors may mediate seasonal adiposity. Am J Physiol Regul Integr Comp Physiol 281: R666–R672, 2001. [DOI] [PubMed] [Google Scholar]
- 53.Song CK, Enquist LW, Bartness TJ. New developments in tracing neural circuits with herpesviruses. Virus Res 111: 235–249, 2005. [DOI] [PubMed] [Google Scholar]
- 54.Song CK, Jackson RM, Harris RB, Richard D, Bartness TJ. Melanocortin-4 receptor mRNA is expressed in sympathetic nervous system outflow neurons to white adipose tissue. Am J Physiol Regul Integr Comp Physiol 289: R1467–R1476, 2005. [DOI] [PubMed] [Google Scholar]
- 55.Song CK, Schwartz GJ, Bartness TJ. Anterograde transneuronal viral tract tracing reveals central sensory circuits from white adipose tissue. Am J Physiol Regul Integr Comp Physiol 296: R501–R511, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Song CK, Vaughan CH, Keen-Rhinehart E, Harris RB, Richard D, Bartness TJ. Melanocortin-4 receptor mRNA expressed in sympathetic outflow neurons to brown adipose tissue: Neuroanatomical and functional evidence. Am J Physiol Regul Integr Comp Physiol 295: R417–R428, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Spector S, Sjoerdsma A, Udenfriend S. Blockade of endogenous norepinephrine synthis by alpha-methyl-tyrosine, an inhibitor of tyrosine hydroxylase. J Pharmacol Exp Ther 147: 86–95, 1965. [PubMed] [Google Scholar]
- 58.Srinivasan V, Thombre DP, Lakshmanan S, Chakrabarty AS. Effect of removal of epididymal fat on spermatogenesis in albino rats. Indian J Exp Biol 24: 487–488, 1986. [PubMed] [Google Scholar]
- 59.Strack AM, Sawyer WB, Hughes JH, Platt KB, Loewy AD. A general pattern of CNS innervation of the sympathetic outflow demonstrated by transneuronal pseudorabies viral infections. Brain Res 491: 156–162, 1989. [DOI] [PubMed] [Google Scholar]
- 60.Tchkonia T, Thomou T, Zhu Y, Karagiannides I, Pothoulakis C, Jensen MD, Kirkland JL. Mechanisms and metabolic implications of regional differences among fat depots. Cell Metab 17: 644–656, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vaughan CH, Zarebidaki E, Ehlen JC, Bartness TJ. Analysis and measurement of the sympathetic and sensory innervation of white and brown adipose tissue. Methods Enzymol 537: 199–255, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wertheimer E. [Stoffwechselregulationen. I. Regulation des Fettstoffwechsels. Die zentrale Regulierung der Fettmobilisierung]. Pflugers Arch Ges Physiol 213: 262–298, 1926. [Google Scholar]
- 63.Yang YK, Chen M, Clements RH, Abrams GA, Aprahamian CJ, Harmon CM. Human mesenteric adipose tissue plays unique role versus subcutaneous and omental fat in obesity related diabetes. Cell Physiol Biochem 22: 531–538, 2008. [DOI] [PubMed] [Google Scholar]
- 64.Youngstrom TG, Bartness TJ. Catecholaminergic innervation of white adipose tissue in the Siberian hamster. Am J Physiol Regul Integr Comp Physiol 268: R744–R751, 1995. [DOI] [PubMed] [Google Scholar]
- 65.Youngstrom TG, Bartness TJ. White adipose tissue sympathetic nervous system denervation increases fat pad mass and fat cell number. Am J Physiol Regul Integr Comp Physiol 275: R1488–R1493, 1998. [DOI] [PubMed] [Google Scholar]
- 66.Zalesin KC, Franklin BA, Miller WM, Peterson ED, McCullough PA. Impact of obesity on cardiovascular disease. Med Clin North Am 95: 919–937, 2011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.