Abstract
It is unknown whether cardiomyocyte hypertrophy and the transition to fatty acid oxidation as the main source of energy after birth is dependent on the maturation of the cardiomyocytes' metabolic system, or on the limitation of substrate availability before birth. This study aimed to investigate whether intrafetal administration of a peroxisome proliferator-activated receptor-γ (PPAR-γ) agonist, rosiglitazone, during late gestation can stimulate the expression of factors regulating cardiac growth and metabolism in preparation for birth, and the consequences of cardiac contractility in the fetal sheep at ∼140 days gestation. The mRNA expression and protein abundance of key factors regulating growth and metabolism were quantified using quantitative RT-PCR and Western blot analysis, respectively. Cardiac contractility was determined by measuring the Ca2+ sensitivity and maximum Ca2+-activated force of skinned cardiomyocyte bundles. Rosiglitazone-treated fetuses had a lower cardiac abundance of insulin-signaling molecules, including insulin receptor-β, insulin receptor substrate-1 (IRS-1), phospho-IRS-1 (Tyr-895), phosphatidylinositol 3-kinase (PI3K) regulatory subunit p85, PI3K catalytic subunit p110α, phospho-3-phosphoinositide-dependent protein kinase 1 (Ser-241), protein kinase B (Akt-1), phospho-Akt (Ser-273), PKCζ, phospho-PKCζ(Thr-410), Akt substrate 160 kDa (AS160), phospho-AS160 (Thr-642), and glucose transporter type-4. Additionally, cardiac abundance of regulators of fatty acid β-oxidation, including adiponectin receptor 1, AMPKα, phospho-AMPKα (Thr-172), phospho-acetyl CoA carboxylase (Ser-79), carnitine palmitoyltransferase-1, and PGC-1α was lower in the rosiglitazone-treated group. Rosiglitazone administration also resulted in a decrease in cardiomyocyte size. Rosiglitazone administration in the late-gestation sheep fetus resulted in a decreased abundance of factors regulating cardiac glucose uptake, fatty acid β-oxidation, and cardiomyocyte size. These findings suggest that activation of PPAR-γ using rosiglitazone does not promote the maturation of cardiomyocytes; rather, it may decrease cardiac metabolism and compromise cardiac health later in life.
Keywords: programming, insulin, fatty acid, glucose transporter, adiponectin, mononucleated, binucleated, contractility, fetus, pregnancy
early growth of the heart is associated with proliferation of mononucleated cardiomyocytes. In midgestation, these mononucleated cells become binucleated cardiomyocytes, which contribute to increasing cardiac mass by hypertrophy (5, 17). In the human and sheep, the endowment of cardiomyocytes present in the adult heart is largely determined before birth (42). In the fetal heart, lactate and glucose are the main sources of energy, while after birth, there is a switch to fatty acid β-oxidation (9, 21). It is not known whether the dominance of glucose as the main fuel source in the fetal cardiomyocyte is a consequence of the relatively limited availability of fatty acids in the fetal circulation or rather as a consequence of the immaturity of key enzyme systems present within the fetal cardiomyocyte. It is also unclear whether the maturation of cardiomyocytes is linked to cardiac metabolism; however, several factors are known to have an impact on cardiac maturation and metabolism. For example, glucocorticoids are essential in the maturation of key fetal organ systems, including the lung, gut, and heart in late gestation (10). In rats, glucocorticoid infusion increases the abundance of the transcription factor peroxisome proliferator-activated receptor gamma (PPAR-γ), leading to increased ATP production (26). Additionally, PPAR-γ may regulate cardiac insulin signaling, as it has been shown that cardiac specific PPAR-γ knockout mice have decreased phosphorylation of protein kinase B (Akt), which is a key insulin signaling molecule (8). Rosiglitazone, a PPAR-γ agonist, increases plasma adiponectin concentration, which is a key regulator of cardiac fatty acid β-oxidation (1). Rosiglitazone also upregulates adiponectin mRNA expression in perirenal fat in sheep (29) and increases cardiac adiponectin and adiponectin receptor 1 (AdipoR1) in cultured cardiomyocytes from adult rats and mice (7, 41). Furthermore, rosiglitazone administration in adult rats induces cardiac hypertrophy (8). Thus, one possibility is that an upregulation of PPAR-γ in late gestation may induce changes in factors that regulate insulin-dependent cardiac glucose uptake, fatty acid β-oxidation, and cardiac hypertrophy in fetal cardiomyocytes in preparation for the transition to extrauterine life.
Cardiac glucose uptake in the fetus is maintained through the activity of the insulin-independent glucose transporter type-1 (GLUT-1) (13). In postnatal life, however, cardiac glucose uptake is regulated by the insulin-dependent (GLUT-4) through the activation of the insulin receptor (IR), insulin receptor substrate-1 (IRS-1), phosphatidylinositol 3-kinase (PI3K), 3-phosphoinositide-dependent protein kinase 1 (PDPK-1), and/or Akt. Activation of PDPK-1 results in the phosphorylation and activation of the atypical PKCζ, while phosphorylation of Akt results in the phosphorylation and activation of the Akt substrate 160 kDa (AS160). Phosphorylated PKCζ and AS160 each play a major role in the translocation of the GLUT-4 to the plasma membrane to facilitate glucose uptake (38).
Cardiac fatty acid uptake is facilitated by fatty acid translocase (FAT/CD36) and fatty acid transport protein 1 (FATP1) (36). Fatty acid oxidation, however, is regulated by the activation of AdipoR1 by adiponectin binding, leading to the phosphorylation, and, hence, activation of AMPK, which, in turn, phosphorylates acetyl CoA carboxylase (ACC), resulting in its inhibition (32, 34). ACC catalyzes the production of malonyl CoA, which inhibits the action of carnitine palmitoyltransferase-1 (CPT-1) in facilitating fatty acid transport into the mitochondria (20). Fatty acid β-oxidation in the heart is also regulated by PGC-1α and peroxisome proliferator-activated receptor alpha (PPAR-α), which stimulate mitochondrial biogenesis and fatty acid β-oxidation by increasing the transcription of regulators such as CPT-1 (39). Pyruvate dehydrogenase kinase-4 (PDK-4) also plays a role in promoting cardiac fatty acid β-oxidation by inhibiting glucose oxidation through inhibition of the pyruvate dehydrogenase complex (37).
Insulin-like growth factor-1 (IGF-1) and IGF-2, which act through the IGF-1 receptor (IGF-1R), play an important role in cell growth and metabolism through activation of downstream signaling pathways (6). IGF-2 receptor (IGF-2R) is a clearance receptor, which functions to degrade IGF-2, therefore limiting its action on IGF-1R in normally grown fetuses (18). However, recent studies have shown that activation of IGF-2R signaling leads to pathological cardiac hypertrophy during late gestation in the sheep fetus (40), indicated by increased expression of the marker of hypertrophy, atrial natriuretic peptide (ANP) (30). Additionally, IGF-1 also regulates proliferation through the activation of the cyclin-dependent kinase 4 (CDK-4) and cyclin D1 complex, which is inhibited by the CDK inhibitor, p27 (24). The expression of CDK-4 is stimulated by the transcription factor c-myc (15).
We hypothesize that activation of PPAR-γ with intrafetal rosiglitazone infusion will stimulate cardiac insulin-dependent glucose uptake and fatty acid β-oxidation, thus stimulating cardiac maturation and growth. In this study, we have, therefore, determined the effect of PPAR-γ activation using rosiglitazone infusion to the sheep fetus for ∼16 days in late gestation on the mRNA expression and protein abundance of factors regulating cardiac glucose uptake, fatty acid β-oxidation, cardiomyocyte proliferation, and hypertrophy, as well as cardiomyocyte parameters in late gestation at ∼140 days gestation. We have also determined both Ca2+ sensitivity and maximum Ca2+-activated force in small bundles of chemically skinned cardiac muscle, as an indication of cardiac function.
MATERIALS AND METHODS
Animals, Surgery, and Rosiglitazone Administration
All procedures were approved by the Institute for Medical and Veterinary Science Animal Ethics Committee.
Pregnancies were confirmed in 14 adult Merino ewes by ultrasound scanning in early gestation. Surgery was performed between 123 and 126 days gestation using aseptic techniques. General anesthesia was induced by intravenous injection of sodium thiopentone (1.25 g pentothal; Rhone Merieux, Pinkenba, Queensland, Australia) and maintained with 1.5–2.5% isoflurane (Fluothane; Imperial Chemical Industries, Melbourne, Victoria, Australia) in oxygen.
Ethanol was diluted in water to make a sterile 15% ethanol (vol/vol) solution. Rosiglitazone (30 mg, generously donated by GlaxoSmithKline, Brentford, UK) was dissolved in sterile 15% ethanol (15 mg/ml) and then injected into a 2-ml Alzet osmotic pump (Durect, Cupertino, CA) under sterile conditions. Rosiglitazone was administered directly to the fetus with Alzet osmotic pumps, which were inserted subcutaneously over the scapula at surgery, as previously described (29). Fetuses assigned to the control group (vehicle) also had Alzet osmotic pumps inserted containing 15% ethanol. The solution was released from the osmotic pumps at an average rate of 60 μl/day for both rosiglitazone and control groups, according to the manufacturer's specifications for estimated flow rate of the pumps (Durect). On the basis of this flow rate and the amount of drug initially loaded into each pump, this regimen delivered a dose of ∼3.6 mg·fetus−1·day−1 of rosiglitazone. This resulted in a plasma concentration of ∼25 ng/ml across the infusion period (3) and was sufficient to activate PPAR-γ target genes in adipose tissue, liver, and skeletal muscle (29). Further, we have reported previously that this regime resulted in accumulation of rosiglitazone in the fetus throughout the infusion period (3).
Blood Sampling, Postmortem, and Tissue Collection
Fetal arterial blood (0.5 ml) was collected daily from the time of surgery to postmortem for determination of fetal blood gases Po2 and Pco2 using an ABL 520 analyzer (Radiometer, Copenhagen, Denmark) (29).
Between 137 and 140 days gestation, ewes were humanely killed with an overdose of pentobarbitol sodium (Virbac, Peakhurst, NSW, Australia). Timing of tissue collection was determined to allow rosiglitazone infusion for 16 ± 1 days. Singleton and twin fetuses from the control (n = 12) and rosiglitazone-treated (n = 9) groups were delivered by hysterectomy and weighed. All organs were dissected and weighed, and samples of heart muscle (left ventricle) were snap frozen in liquid nitrogen and stored at −80°C. The remainder of the heart was perfused through the aorta with heparin and saturated potassium chloride to prevent blood clotting and to arrest the heart in diastole. Cardiomyocytes were enzymatically isolated from the heart, as previously described (27) and fixed in 1% paraformaldehyde (Table 1) and stored until determination of the percentage of mononucleated cardiomyocytes and cardiomyocyte size.
Table 1.
Number of animals from each treatment group used in each set of analyses
| Measurements | Control (n = 12) | Rosiglitazone (n = 9) |
|---|---|---|
| Cardiomyocyte measures | 7 males = 4, females = 3 | 5 males = 3, females = 2 |
| mRNA expression | 5 males = 5, females = 0 | 7 males = 5, females = 2 |
| Protein abundance | 5 males = 5, females = 0 | 7 males = 5, females = 2 |
| Contractility (Ca2+-activated force) | 7 males = 3, females = 4 | 7 males = 5, females = 2 |
| Contractility (Ca2+ sensitivity) | 10 males = 6, females = 4 | 7 males = 4, females = 3 |
Quantitative Real-Time RT-PCR
RNA was extracted from ∼50 mg of left ventricle tissue using TRIzol reagent (Invitrogen) (Table 1). RNA was purified using the RNeasy mini kit (Qiagen). cDNA was synthesized using the purified RNA and Superscript 3 reverse transcriptase (Invitrogen) with random hexamers. The expression of mRNA transcripts of glucose transporters (GLUT-1 and GLUT-4), cardiac lipid metabolism factors (adiponectin, AdipoR1, AdipoR2, CD36, FATP, PPAR-α, PGC-1α, and PDK-4), cardiac growth factors (IGF-1, IGF-2, IGF-1R, and IGF-2R), proliferative factors (p27, cyclin D1, CDK-4, and c-myc), cardiac hypertrophy markers (ANP), and the housekeeping genes hypoxanthine phosphoribosyltransferase 1 (HPRT), phosphoglycerate kinase 1, and GAPDH (33) was measured by quantitative real-time reverse transcription-PCR (qRT-PCR) using the SYBR Green system in an ABI Prism 7500 sequence detection system (Applied Biosystems, Foster City, CA). Normalized expression of the target genes was calculated using DataAssist Software v3.0 (Applied Biosystems) (14).
Primer sequences were validated for use in sheep in this (Table 2) or in prior studies (23, 28, 29). Each amplicon was sequenced to ensure the authenticity of the DNA product, and a dissociation melt curve analysis was performed after each run to demonstrate amplicon homogeneity. Each qRT-PCR reaction well contained 5 μl SYBR Green Master Mix (Applied Biosystems), 2 μl primer (forward and reverse), 2 μl molecular grade H2O, and 1 μl of cDNA (50 ng/μl). The cycling conditions consisted of 40 cycles of 95°C for 15 min and 60°C for 1 min.
Table 2.
Primer sequences for qRT-PCR
| Gene Name | Sequence | Accession No. |
|---|---|---|
| HPRT1 | F: 5′ GCTGAGGATTTGGAGAAGGTGT 3′ | NM_001034035.1 |
| R: 5′ GGCCACCCATCTCCTTCAT 3′ | ||
| PGK1 | F: 5′ ACTCCTTGCAGCCAGTTGCT 3′ | NM_001034299 |
| R: 5′ AGCACAAGCCTTCTCCACTTCT 3′ | ||
| GAPDH | F: 5′ CCTGGAGAAACCTGCCAAGT 3′ | DQ152956.1 |
| R: 5′ GCCAAATTCATTGTCGTACCA 3′ | ||
| p27 | F: 5′ AAACCCAGAGGACACGCATTTGGT 3′ | NM_001100346.1 |
| R: 5′ TTTGAGGAGAGGAATCATCTGCGG 3′ | ||
| Cyclin D1 | F: 5′ GCCGAGAAGCTGTGCATTTAC 3′ | NM_001046273.1 |
| R: 5′ CCAGGACCAGCTCCATGTG 3′ | ||
| CDK-4 | F: 5′ AGGCTTGCCAGTGGAGACCATAAA 3′ | NM_001037594.1 |
| R: 5′ GGTGAACGATGCAGTTGGCATGAA 3′ | ||
| c-myc | F: 5′ CTACAGATGCCCACAATCTGCACT 3′ | NM_001174109.1 |
| R: 5′ TGGTATGGTTTCATCTGGGAAGGC 3′ | ||
| ANP | F: 5′ ATCACCACGAGCTTCCTCCTCTTT 3′ | NM_001160027.1 |
| R: 5′ ATACTTGTGAGGGCACAGCCTCAT 3′ | ||
| AdipoR1 | F: 5′ ACACTCCCTGGGCAATAAACTCCA 3′ | BC102259 |
| R: 5′ TTCTGAAGTCCCAGTCCATCGCTT 3′ | ||
| AdipoR2 | F: 5′ TCTCATGGCTGTTCCACACAGTCT 3′ | BC110019 |
| R: 5′ AGCAAGGTTGCGGGTTACAGTAGA 3′ | ||
| CD36 | F: 5′ TGGTGTGCTAGACATTGGCAAATG 3′ | BC103112.1 |
| R: 5′ TGTTGACCTGCAGCCGTTTTGC 3′ | ||
| FATP1 | F: 5′ AGCCTGGTCAAGTTCTGTTCTGGA 3′ | NM_001033625.2 |
| R: 5′ AGAAGAGTCGATCATCCATGCCCT 3′ | ||
| PDK-4 | F: 5′ GCACCAACGCCTGTGATGGATAAT 3′ | NM_001101883.1 |
| R: 5′ AGCATCAGTTCCGTATCCTGGCAA 3′ |
Quantification of Protein Abundance
The protein abundance of factors regulating cardiomyocyte proliferation and hypertrophy, glucose and fatty acid metabolism, and cardiac contractility were determined using Western blot analysis (31). Briefly, left ventricle samples (∼50 mg) (Table 1) were sonicated in 800 μl lysis buffer (50 mM Tris·HCl pH 8.0, 150 mM NaCl, 1% NP-40, 1 mM Na3VO4, 30 mM NaF, 10 mM Na4P2O7, 10 mM EDTA, and 1 protease inhibitor tablet) and centrifuged at 12,000 g at 4°C for 15 min to remove insoluble material. Protein content of the clarified extracts was quantified using microbicinchoninic acid protein assay. Prior to Western blot analysis, samples (10 μg protein) were subjected to SDS-PAGE and stained with Coomassie blue reagent (Thermo Fisher Scientific, Rockford, IL) to ensure equal loading of the proteins. Equal volumes and concentrations of protein were subjected to SDS-PAGE. The proteins were transferred onto a PolyScreen polyvinylidene difluoride hybridization transfer membrane (PerkinElmer, Waltham, MA) using a semi-dry blotter (Hoefer, Holliston, CA). The membranes were blocked with 5% BSA in TBS with 1% Tween-20 at room temperature for 1 h and then incubated overnight with primary antibody against IRβ, PKCζ, GLUT-1, PPAR-α, CPT-1 (Santa Cruz Biotechnology, Santa Cruz, CA), IGF-1R, phospho-IRS-1 (Tyr-895), p110α, Akt1, Akt2, total phospho-Akt (Ser-473), PDPK-1, phospho-PDPK1 (Ser-241), phospho-PKCζ (Thr-410), AS160, phospho-AS160 (Thr-642), total AMPK, total phospho-AMPK (Thr-172), PGC-1α, ACC, phospho-ACC (Ser-79) (Cell Signaling, Danvers, MA), IRS-1, p85 (Merck Millipore, Billerica, MA), AdipoR1 (Epitomics, Burlingame, CA), GLUT-4, PDK-4, ANP (Abcam, Cambridge, UK), and IGF-2R (BD Transduction Laboratories, San Jose, CA). Membranes were washed and bound antibody was detected using anti-rabbit or anti-mouse (Cell Signaling) horseradish peroxidase-conjugated secondary IgG antibodies at room temperature for 1 h. Enhanced chemiluminescence reagents SuperSignal West Pico Chemiluminescent Substrate (Thermo Fisher Scientific) and ImageQuant LAS 4000 (GE Healthcare, Rydalmere, NSW, Australia) was used to detect the protein:antibody complexes. AlphaEaseFC (Alpha Innotech, Santa Clara, CA) was utilized to quantify the optical density of the specific bands of the target proteins (40).
Determination of Proportion of Mononucleated Cardiomyocytes and Cardiomyocyte Size
Cardiomyocytes were stained with methylene blue (ProSiTech, Thuringowa, Queensland, Australia) and examined using an Olympus VANOX-T microscope (Olympus Optical, Tokyo, Japan). The relative proportion of mononucleated and binucleated cardiomyocytes was determined by counting a total of 300 cardiomyocytes. To determine cardiomyocyte size, the length and width of 50 mononucleated and 50 binucleated cardiomyocytes were assessed using AnalySIS software (Software Imaging System, Gulfview Heights, South Australia, Australia) (40).
Cardiac Contractility Studies
Bundle isolation.
Under a dissecting microscope, small bundles of cardiomyocytes (Table 1) of ∼300-μm diameter were isolated from the left ventricle and then attached between a force transducer (AE801 Memscap, Skoppun, Norway) and stationary pin with fine suture silk. The bundle was then briefly immersed in a high-EGTA physiological solution (solution 1; see Force calcium relationship). We have shown in a previous study that stretching bundles by 130% of the resting length resulted in the production of optimum force to Ca2+ activation consistent with the approach of other studies (35). In this study, the bundle was, therefore, stretched by 120% of its slack length to produce ∼90% of optimum maximum Ca2+-activated force. Bundles were then chemically skinned in solution 1 containing 2% Triton X-100 for 30 min (35) (see Force-calcium relationship). The output of the transducer was acquired and digitized by a PowerLab/8Sp (ADInstruments, Castle Hill, New South Wales, Australia) data acquisition system, and the subsequent force responses were recorded onto both a paper chart recorder (Kipp Zonnen, Bohemia, NY) and computer using PowerLab Chart v4.1 computer software (ADInstruments).
Force-calcium relationship.
The standard composition of the skinned fiber solutions used were (in mM) Solution 1: 90 HEPES, 50 EGTA, 10.3 total Mg2+, 8 total ATP, and 10 creatine phosphate (CP); Solution 2: 90 HEPES, 50 EGTA, 48.5 total Ca2+, 8.12 total Mg2+, 8 total ATP, and 10 CP; and Solution 3: 90 HEPES, 0.05 EGTA, 50 1,6-diaminohexane-N,N,N_,N_-tetraacetic acid (HDTA2−), 8.6 total Mg2+, 8 total ATP, and 10 CP. All solutions contained (in mM): 126 K+, 36 Na+, 1 azide, 1 free Mg2+, and the pH and osmolality were 7.10 ± 0.01 and 295 mmol/kg, respectively.
All bundles were chemically skinned in solution 1 containing 2% Triton-X 100 for 30 min. This procedure destroys all membranes, leaving only the contractile apparatus intact. Skinned bundles were then washed in fresh solution 1 for 5 min and then equilibrated in a weakly buffered (2 mM) EGTA solution by combining proportions of solutions 1 and 3. The force-pCa relationship was then determined by activating each bundle in solutions of increasing free Ca2+, created by combining solutions 1 and 2 in various ratios (pCa = log10[Ca2+]; 7.3 to 5.5); the precise pCa in each activation ratio was subsequently measured by using an Orion Ca2+-sensitive electrode. Bundles were maximally activated by exposure to solution 2 (pCa ∼4.5). The maximum Ca2+-activated force responses in bundles were normalized to the cross-sectional area of the bundle (mN/mm2) for comparison. Cross-sectional area was determined by the equation area πr2, assuming the muscle bundle had a cylindrical form and taking the average diameter across the fiber bundle. Submaximal force relative to the maximum Ca2+-activated force was used in determination of the force-pCa relationship. For each fiber bundle, the relative force produced for each free [Ca2+] was plotted by use of GraphPad Prism v4.01 (GraphPad Software, San Diego, CA) and a sigmoidal dose-response curve [Hill equation: Y = min + (max − min)]/{1 + 10[(LogEC50 − X) × n)]} was fitted. Parameters Max (pCa 4.5) and Min (pCa 7.0) of the fitted curve were set to 100 and 0%, respectively. From each resulting curve, the pCa required to produce 50% (pCa50) of maximum Ca2+-activated force and the Hill coefficient (n) were measured and averaged as reported in previous studies (35).
Statistical Analyses
All data are presented as means ± SE. Two-way ANOVA was performed using the Statistical Package for the Social Sciences Software (SPSS, Chicago, IL), and showed no effect of fetal number; thus, data from singletons and twins were combined, and Student's unpaired t-tests were used to determine the effects of rosiglitazone compared with controls on cardiac mRNA expression and protein abundance and to compare contractility parameters. A probability level of 5% (P < 0.05) was considered significant.
RESULTS
There was no effect of rosiglitazone administration on fetal weight at ∼140-day gestation (control: 4.65 ± 0.15 kg; rosiglitazone: 4.83 ± 0.17 kg). There was also no effect of rosiglitazone administration on mean fetal arterial Po2 (control: 22.5 ± 0.6 mmHg; rosiglitazone: 21.5 ± 1.0 mmHg) and Pco2 (control: 49.9 ± 0.7 mmHg; rosiglitazone: 49.5 ± 0.5 mmHg) in late gestation.
Impact of Rosiglitazone on the mRNA Expression and Protein Abundance of Factors Regulating Cardiac Glucose Uptake in Late Gestation
Rosiglitazone administration during late gestation decreased the cardiac protein abundance of IRβ (P < 0.05), IRS-1 (P < 0.05), phospho-IRS-1 (Tyr-895) (P < 0.05), PI3K (p85) (P < 0.05), PI3K (p110α) (P < 0.05), phospho-PDPK-1 (Ser-241) (P < 0.05), Akt1 (P < 0.05), phospho-Akt (Ser-273) (P < 0.001), PKCζ (P < 0.05), phospho-PKCζ (Thr-410) (P < 0.01), AS160 (P < 0.05), phospho-AS160 (Thr-642) (P < 0.05), and GLUT-4 (P < 0.01) (Table 3). The cardiac abundance of GLUT-1, however, was increased (P < 0.05) in rosiglitazone-treated fetuses compared with controls (Table 3). The protein abundance of PDPK-1 (Table 3) and mRNA expression of GLUT-1 and GLUT-4 were not different in rosiglitazone-treated fetuses compared with controls (Table 3).
Table 3.
Impact of rosiglitazone on the mRNA expression and protein abundance of factors regulating glucose uptake in heart muscle in late gestation
| Control | Rosiglitazone | |
|---|---|---|
| Gene expression (MNE) | ||
| GLUT-1 | 0.050 ± 0.002 | 0.051 ± 0.006 |
| GLUT-4 | 0.14 ± 0.02 | 0.13 ± 0.01 |
| Protein abundance, AU × 102 | ||
| IRβ | 848 ± 91 | 572 ± 44* |
| IRS-1 | 384 ± 63 | 239 ± 23* |
| phospho-IRS-1 (Tyr895) | 1,018 ± 57 | 860 ± 88* |
| PI3K (p85) | 199 ± 9 | 158 ± 11* |
| PI3K (p110α) | 456 ± 9 | 355 ± 32* |
| PDPK-1 | 628 ± 93 | 475 ± 65 |
| phospho-PDPK-1 (Ser241) | 301 ± 29 | 207 ± 23* |
| Akt1 | 253 ± 25 | 169 ± 21* |
| phospho-Akt (Ser273) | 1,818 ± 228 | 234 ± 55*** |
| PKCζ | 1,004 ± 101 | 696 ± 72* |
| phospho-PKCζ (Thr410) | 952 ± 106 | 337 ± 71** |
| AS160 | 216 ± 38 | 105 ± 19* |
| phospho-AS160 (Thr642) | 253 ± 9 | 76 ± 30** |
| GLUT-4 | 245 ± 17 | 185 ± 18* |
| GLUT-1 | 50 ± 8 | 76 ± 6* |
Data are presented as means ± SE. MNE, mean normalized expression; AU, arbitrary units.
P < 0.05,
P < 0.01,
P < 0.001.
Impact of Rosiglitazone on the mRNA Expression and Protein Abundance of Factors Regulating Cardiac Fatty Acid β-Oxidation in Late Gestation
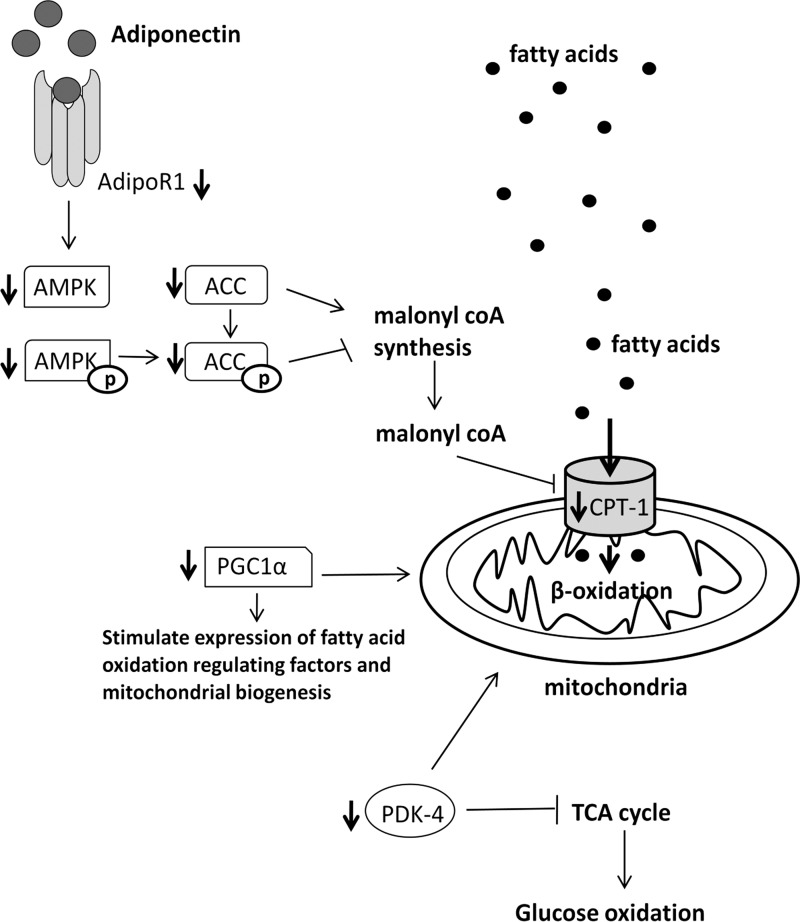
The cardiac protein abundance of AdipoR1 (P < 0.01), AMPK (P < 0.05), phospho-AMPK (Thr-172) (P < 0.05), ACC (P < 0.01), phospho-ACC (Ser-79) (P < 0.05), CPT-1 (P < 0.05), PDK-4 (P < 0.05), and PGC-1α (P < 0.05) (Table 4) was decreased in rosiglitazone-treated fetuses compared with controls. There were no differences, however, in the mRNA expression of cardiac PPAR-γ, adiponectin, AdipoR1, AdipoR2, CD36, FATP1, PPAR-α, and PGC-1α between groups (Table 4).
Table 4.
Impact of rosiglitazone on the mRNA expression and protein abundance of factors regulating lipid metabolism in heart muscle in late gestation
| Control | Rosiglitazone | |
|---|---|---|
| Gene expression (MNE) | ||
| PPARγ | 0.016 ± 0.003 | 0.015 ± 0.002 |
| Adiponectin | 0.005 ± 0.001 | 0.004 ± 0.001 |
| AdipoR1 | 0.32 ± 0.10 | 0.20 ± 0.02 |
| AdipoR2 | 1.40 ± 0.51 | 0.75 ± 0.13 |
| CD36 | 4.52 ± 0.46 | 4.32 ± 0.46 |
| FATP1 | 0.13 ± 0.02 | 0.13 ± 0.02 |
| PPARα | 0.22 ± 0.06 | 0.18 ± 0.03 |
| PGC1α | 0.81 ± 0.19 | 0.63 ± 0.06 |
| Protein abundance (AU × 102) | ||
| AdipoR1 | 477 ± 89 | 191 ± 16** |
| AMPK | 570 ± 35 | 445 ± 31* |
| phospho-AMPK (Thr-172) | 432 ± 88 | 193 ± 42* |
| ACC | 279 ± 31 | 172 ± 16** |
| phospho-ACC (Ser-79) | 310 ± 54 | 193 ± 23* |
| CPT-1 | 99 ± 12 | 59 ± 10* |
| PDK-4 | 157 ± 38 | 66 ± 13* |
| PGC1α | 304 ± 45 | 112 ± 41* |
Data are presented as means ± SE.
P < 0.05,
P < 0.01.
Impact of Rosiglitazone on the mRNA Expression and Protein Abundance of Factors Regulating Cardiac Proliferation and Hypertrophy and Cardiac Parameters in Late Gestation
There was no effect of rosiglitazone on the mRNA expression of cardiac IGF-1, IGF-2, IGF-1R, IGF-2R, c-myc, CDK-4, cyclin D1, p27, and ANP (Table 5). There was also no difference in the protein abundance of IGF-1R, IGF-2R, and ANP in the rosiglitazone-treated fetuses compared with controls (Table 5). There was, however, a decrease in the absolute length of the mononucleated (P < 0.05) and binucleated (P < 0.05) cardiomyocytes (Table 6). The absolute and relative heart weight and absolute width of the mononucleated and binucleated cardiomyocytes, as well as the percentage of mononucleated cardiomyocytes, were not changed in rosiglitazone-treated fetuses compared with controls (Table 6).
Table 5.
Impact of rosiglitazone on the mRNA expression and protein abundance of factors regulating proliferation and hypertrophy and markers of hypertrophy in heart muscle in late gestation
| Control | Rosiglitazone | |
|---|---|---|
| Gene expression (MNE) | ||
| IGF-1 | 0.12 ± 0.01 | 0.09 ± 0.02 |
| IGF-2 | 12.0 ± 1.4 | 13.3 ± 1.6 |
| IGF-1R | 0.58 ± 0.05 | 0.57 ± 0.04 |
| IGF-2R | 1.8 ± 0.1 | 1.8 ± 0.2 |
| p27 | 0.31 ± 0.06 | 0.30 ± 0.02 |
| Cyclin D1 | 0.021 ± 0.003 | 0.018 ± 0.003 |
| CDK-4 | 0.18 ± 0.04 | 0.16 ± 0.03 |
| c-myc | 0.23 ± 0.03 | 0.23 ± 0.03 |
| ANP | 0.32 ± 0.09 | 0.27 ± 0.07 |
| Protein abundance, AU × 102 | ||
| IGF-1R | 540 ± 74 | 377 ± 48 |
| IGF-2R | 529 ± 14 | 565 ± 76 |
| ANP | 167 ± 18 | 161 ± 10 |
Data are presented as means ± SE.
Table 6.
Impact of rosiglitazone on heart and cardiomyocyte growth in heart muscle in late gestation
| Heart and Cardiomyocyte Measures | Control | Rosiglitazone |
|---|---|---|
| Absolute heart weight, g/kg | 32.9 ± 1.4 | 34.0 ± 1.9 |
| Relative heart weight, g/kg | 7.1 ± 0.3 | 6.9 ± 0.2 |
| Percentage of mononucleated cardiomyocytes, % | 50.5 ± 1.4 | 54.1 ± 4.0 |
| Mononucleated cardiomyocyte length, mm | 60.3 ± 1.7 | 53.2 ± 1.0* |
| Mononucleated cardiomyocyte width, mm | 10.0 ± 0.6 | 10.8 ± 0.4 |
| Binucleated cardiomyocyte length, mm | 77.7 ± 2.3 | 68.0 ± 1.2* |
| Binucleatedcardiomyocyte width, mm | 10.8 ± 0.6 | 11.6 ± 0.4 |
Data are presented as means ± SE.
P < 0.05.
Impact of Rosiglitazone on Cardiac Contractility Parameters in Late Gestation
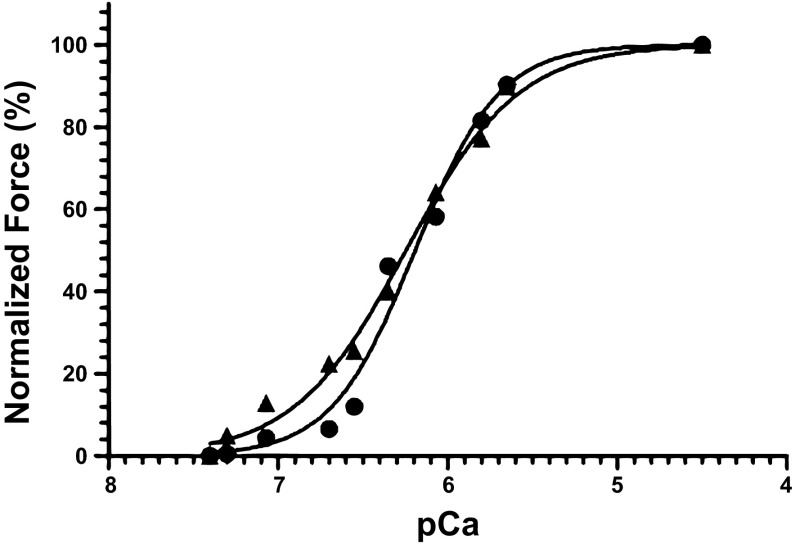
There was no difference in the Ca2+ sensitivity of the contractile apparatus (Fig. 1) and maximum Ca2+-activated force between control and rosiglitazone groups (Table 7).
Fig. 1.
Representative traces of the force-pCa relationship from chemically skinned bundles of fetal cardiomyocytes from the left ventricles of control (▲) and rosiglitazone-treated (●) animals. The pCa and Hill coefficients for control fetuses were 6.19 and 1.80, respectively, and for rosiglitazone-treated fetuses, they were 6.24 and 1.31, respectively.
Table 7.
Impact of rosiglitazone on the contractile apparatus of small bundles of fetal sheep heart tissue
| pCa50 | Hill Coefficient | Force/Cross-Sectional Area, mN/mm2 | |
|---|---|---|---|
| Control | 6.07 ± 08 | 1.92 ± 0.17 | 7.10 ± 1.57 |
| Rosiglitazone | 6.12 ± 0.1 | 1.50 ± 0.14 | 5.60 ± 0.90 |
Data are presented as means ± SE.
DISCUSSION
In this study, we aimed to determine whether activation of PPAR-γ with intrafetal rosiglitazone infusion could stimulate cardiac insulin-dependent glucose uptake and fatty acid β-oxidation. Interestingly, we have shown that rosiglitazone administration during late gestation resulted in decreased protein abundance of key insulin-signaling molecules (Fig. 2), which may lead to a decrease in cardiac glucose uptake in postnatal life. This finding is in contrast to the known effect of rosiglitazone in improving whole body insulin sensitivity and glucose uptake in the heart and skeletal muscle in adult humans and mice with Type 2 diabetes (12, 19, 25). In addition, rosiglitazone-treated fetuses also had a decrease in the protein abundance of key regulators of cardiac fatty acid β-oxidation (Fig. 3), which may have a detrimental effect in postnatal life, as the cardiomyocytes are more reliant on fatty acid β-oxidation to produce energy. This finding is in contrast to studies in nondiabetic adults in humans, rats, and mice, whereby rosiglitazone increased cardiac adiponectin and AdipoR1 expression (1, 7, 41). However, rosiglitazone resulted in similar decrease in the mRNA expression of AdipoR1 and protein abundance of GLUT-4 and phospho-AMPK (Thr-172) in diabetic rats treated with 3 mg·kg−1·day−1 of rosiglitazone compared with untreated diabetic rats (11). Our findings showed that rosiglitazone administration in late-gestation fetuses resulted in a different effect than in adults, but similar to when administered to adult diabetic rats. It is interesting that we found a decrease in the abundance of the insulin signaling and fatty acid β-oxidation molecules in this study despite no change in the maternal and fetal glucose and free fatty acid concentration in this cohort of animals (29). We have previously shown that intrafetal infusion of rosiglitazone resulted in decreased plasma insulin concentrations in late gestation (29). It is, therefore, possible that this resulted in the observed decrease in the abundance of the insulin-signaling factors, as an adaptation to the decreased stimulus. We speculate that the decrease in the abundance of the cardiac regulators of fatty acid β-oxidation may be a consequence of limited availability of fatty acids in utero and/or as a negative response to the increased adiponectin expression in the fetal perirenal adipose tissue, which is the main source of plasma adiponectin (29).
Fig. 2.
Summary diagram of the impact of rosiglitazone administration on protein abundance of factors regulating cardiac glucose uptake in late-gestation sheep fetus.
Fig. 3.
Summary diagram of the impact of rosiglitazone administration on protein abundance of factors regulating cardiac lipid metabolism in late-gestation sheep fetus.
We have also shown that rosiglitazone administration did not change the Ca2+ sensitivity of the contractile apparatus and maximum Ca2+-activated force. There was, however, increased cardiac GLUT-1 protein abundance in rosiglitazone-treated fetuses. This finding shows that the decrease in the abundance of insulin signaling and fatty acid β-oxidation molecules may not affect cardiac function in late-gestation fetuses, which is consistent with the knowledge that fetal cardiomyocytes are dependent on glycolysis (21) from glucose uptake facilitated by GLUT-1. Interestingly, rosiglitazone-treated fetuses had reduced absolute mononucleated and binucleated cardiomyocyte length, in the absence of any differences in absolute or relative heart weight. This finding is in contrast to a study in adult rats administered with rosiglitazone, which resulted in cardiac hypertrophy (8), but consistent with reports of the antihypertrophic effect of PPAR-γ in PPAR-γ knockout mice (22). Furthermore, rosiglitazone and pioglitazone interact with numerous “off-target” proteins involved in lipid and glucose metabolism (16). Additionally, administration of thiazolidinediones (TZDs) in adult mice limits cardiac lipid accumulation following a high-fat diet, but with a decrease in PPAR-γ expression and other factors regulating cardiac fatty acid β-oxidation (2). This finding leads the authors to speculate that TZDs may exert this effect through a cardiac PPAR-γ-independent mechanism. Findings from this and other studies (2, 16) and the opposing effect between the impact of rosiglitazone administration and PPAR-γ knockout on hypertrophy in adult rats and mice (8, 22), therefore, raise the possibility that the effect of rosiglitazone on cardiomyocyte growth and metabolism may be a consequence of indirect binding or the “off-target” effects of rosiglitazone. Furthermore, these findings raise concerns regarding the specificity of TZDs, such as rosiglitazone, as a PPAR-γ-“specific” agonist.
In addition, a decrease in cardiomyocyte length in the absence of a reduction in absolute or relative heart weight may suggest an increase in the number of cardiomyocytes in the heart. This hypothesis is consistent with the increase in GLUT-1 abundance, which may result in increased substrate availability for glycolysis, which is the major source of energy for proliferating cardiomyocytes (21). However, we were not able to measure cardiomyocyte number in this cohort because the tissue was not collected appropriately for nonbiased assessment of this parameter (4). We also cannot exclude the possibility that there were differences in the cardiac response to rosiglitazone exposure between males and females, and as such, the small differences in the relative number of males and females between the control and rosiglitazone-treated groups used in the protein quantification assay, and the contractility assays needs to be considered when interpreting the results. It is possible, therefore, that the decrease in the abundance of the insulin signaling and fatty acid β-oxidation molecules found in this study is only applicable for males, and the lack of change in the contractility (Ca2+ activated force) study may be due to the male dominance in the rosiglitazone-treated group. Furthermore, it is worth noting that there was a variation of 3 days in the gestational age at which rosiglitazone exposure commenced. It is possible that this may have had an impact on the response of the cardiomyocytes to rosiglitazone treatment, although we have previously shown that there was no difference in the percentage of mononucleated cardiomyocytes present in the fetal heart between 132- and 134-day and 137- and 141-day gestation (27).
Perspectives and Significance
Rosiglitazone administration during late gestation resulted in decreased abundance of cardiac insulin signaling molecules and regulators of fatty acid β-oxidation, as well as a decrease in cardiomyocyte size, with no effect on measures of cardiac contractility. These findings suggest that stimulation of PPAR-γ using rosiglitazone in late gestation is not adequate to stimulate cardiac insulin-dependent glucose uptake and fatty acid β-oxidation, but it may result in adverse effects for cardiac health in later life. However, it is important to note that findings from this and other studies (2, 8, 16, 22) also suggest that rosiglitazone and other TZDs may not specifically act as PPAR-γ agonists and that the potential adverse cardiometabolic effects may not necessarily be due to the activation of cardiac PPAR-γ.
GRANTS
The animal component of this project was funded by an National Health and Medical Research Council Project Grant (to I. C. McMillen and B. S. Muhlhausler). The molecular analysis component of this project and J. L. Morrison were funded by a South Australian Cardiovascular Research Network Fellowship (CR10A4988).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: S.L., I.C.M., B.S.M., and J.L.M. conception and design of research; S.L., M.H., G.S.P., and S.L.D. performed experiments; S.L., M.H., I.C.M., B.S.M., G.S.P., S.L.D., K.C.W., and J.L.M. analyzed data; S.L., M.H., I.C.M., B.S.M., G.S.P., K.C.W., K.J.B., and J.L.M. interpreted results of experiments; S.L., M.H., G.S.P., and S.L.D. prepared figures; S.L., M.H., and K.C.W. drafted manuscript; S.L., M.H., I.C.M., B.S.M., G.S.P., S.L.D., K.C.W., K.J.B., and J.L.M. edited and revised manuscript; S.L., I.C.M., B.S.M., G.S.P., S.L.D., K.C.W., K.J.B., and J.L.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We are grateful to Melissa Walker for her expert assistance during sheep surgery and the conduct of the protocols using the pregnant ewes in this study. We also thank Darran Tosh for his assistance with the quantitative real-time RT-PCR.
REFERENCES
- 1.Aquilante C, Kosmiski L, Zineh I, Rome L, Knutsen S. Pharmacodynamic effects of rosiglitazone in nondiabetic patients with metabolic syndrome. Pharmacotherapy 30: 236–247, 2010 [DOI] [PubMed] [Google Scholar]
- 2.Barbieri M, Di Filippo C, Esposito A, Marfella R, Rizzo MR, D'Amico M, Ferraraccio F, Di Ronza C, Duan SZ, Mortensen RM, Rossi F, Paolisso G. Effects of PPARs agonists on cardiac metabolism in littermate and cardiomyocyte-specific PPAR-γ-knockout (CM-PGKO) mice. PLoS One 7: e35999, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bazargan M, Davey AK, Muhlhausler BS, Morrison JL, McMillen IC, Foster DJ. Simple HPLC method for determination of rosiglitazone in sheep plasma and amniotic fluid and its application in a pregnant sheep model. J Pharm Biomed Anal 55: 360–365, 2011 [DOI] [PubMed] [Google Scholar]
- 4.Brüel A, Nyengaard JR. Design-based stereological estimation of the total number of cardiac myocytes in histological sections. Basic Res Cardiol 100: 311–319, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Burrell JH, Boyn AM, Kumarasamy V, Hsieh A, Head SI, Lumbers ER. Growth and maturation of cardiac myocytes in fetal sheep in the second half of gestation. Anat Rec A Discov Mol Cell Evol Biol 274A: 952–961, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Cohick WS, Clemmons DR. The insulin-like growth factors. Annu Rev Physiol 55: 131–153, 1993 [DOI] [PubMed] [Google Scholar]
- 7.Ding G, Qin Q, He N, Francis-David S, Hou J, Liu J, Ricks E, Yang Q. Adiponectin and its receptors are expressed in adult ventricular cardiomyocytes and upregulated by activation of peroxisome proliferator-activated receptor γ. J Mol Cell Cardiol 43: 73–84, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duan S, Ivashchenko C, Russell M, Milstone D, Mortensen R. Cardiomyocyte-specific knockout and agonist of peroxisome proliferator-activated receptor-γ both induce cardiac hypertrophy in mice. Circ Res 97: 372–379, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Fisher DJ, Heymann MA, Rudolph AM. Myocardial oxygen and carbohydrate consumption in fetal lambs in utero and in adult sheep. Am J Physiol Heart Circ Physiol 238: H399–H405, 1980 [DOI] [PubMed] [Google Scholar]
- 10.Gluckman PD, Sizonenko SV, Bassett NS. The transition from fetus to neonate—an endocrine perspective. Acta Paediatr Suppl 428: 7–11, 1999 [DOI] [PubMed] [Google Scholar]
- 11.Guo Z, Qin Z, Zhang R, Li J, Yin Y. Effect of rosiglitazone on the expression of cardiac adiponectin receptors and NADPH oxidase in type 2 diabetic rats. Eur J Pharmacol 685: 116–125, 2012 [DOI] [PubMed] [Google Scholar]
- 12.Hällsten K, Virtanen KA, Lönnqvist F, Janatuinen T, Turiceanu M, Rönnemaa T, Viikari J, Lehtimäki T, Knuuti J, Nuutila P. Enhancement of insulin-stimulated myocardial glucose uptake in patients with Type 2 diabetes treated with rosiglitazone. Diabet Med 21: 1280–1287, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Hay WWJ. Placental transport of nutrients to the fetus. Horm Res 42: 215–222, 1994 [DOI] [PubMed] [Google Scholar]
- 14.Hellemans J, Mortier G, De Paepe A, Speleman F, Vandesompele J. qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol 8: R19, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hermeking H, Rago C, Schuhmacher M, Li Q, Barrett JF, Obaya AJ, O'Connell BC, Mateyak MK, Tam W, Kohlhuber F, Dang CV, Sedivy JM, Eick D, Vogelstein B, Kinzler KW. Identification of CDK4 as a target of c-MYC. Proc Natl Acad Sci USA 97: 2229–2234, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoffmann BR, El-Mansy MF, Sem DS, Greene AS. Chemical proteomics-based analysis of off-target binding profiles for rosiglitazone and pioglitazone: clues for assessing potential for cardiotoxicity. J Med Chem 55: 8260–8271, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jonker SS, Zhang L, Louey S, Giraud GD, Thornburg KL, Faber JJ. Myocyte enlargement, differentiation, and proliferation kinetics in the fetal sheep heart. J Appl Physiol 102: 1130–1142, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Kornfeld S. Structure and function of the mannose 6-phosphate/insulin-like growth factor II receptors. Annu Rev Biochem 61: 307–330, 1992 [DOI] [PubMed] [Google Scholar]
- 19.Lautamäki R, Airaksinen KE, Seppänen M, Toikka J, Luotolahti M, Ball E, Borra R, Härkönen R, Iozzo P, Stewart M, Knuuti J, Nuutila P. Rosiglitazone improves myocardial glucose uptake in patients with type 2 diabetes and coronary artery disease: a 16-week randomized, double-blind, placebo-controlled study. Diabetes 54: 2787–2794, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Lopaschuk GD, Gamble J. The 1993 Merck Frosst Award. Acetyl-CoA carboxylase: an important regulator of fatty acid oxidation in the heart. Can J Physiol Pharmacol 72: 1101–1109, 1994 [DOI] [PubMed] [Google Scholar]
- 21.Lopaschuk GD, Jaswal JS. Energy metabolic phenotype of the cardiomyocyte during development, differentiation, and postnatal maturation. J Cardiovasc Pharmacol 56: 130–140, 2010 [DOI] [PubMed] [Google Scholar]
- 22.Luo J, Wu S, Liu J, Li Y, Yang H, Kim T, Zhelyabovska O, Ding G, Zhou Y, Yang Y, Yang Q. Conditional PPARγ knockout from cardiomyocytes of adult mice impairs myocardial fatty acid utilization and cardiac function. Am J Transl Res 3: 61–72, 2010 [PMC free article] [PubMed] [Google Scholar]
- 23.MacLaughlin SM, Walker SK, Kleemann DO, Sibbons JP, Tosh DN, Gentili S, Coulter CL, McMillen IC. Impact of periconceptional undernutrition on adrenal growth and adrenal insulin-like growth factor and steroidogenic enzyme expression in the sheep fetus during early pregnancy. Endocrinology 148: 1911–1920, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Mairet-Coello G, Tury A, DiCicco-Bloom E. Insulin-like growth factor-1 promotes G(1)/S cell cycle progression through bidirectional regulation of cyclins and cyclin-dependent kinase inhibitors via the phosphatidylinositol 3-kinase/Akt pathway in developing rat cerebral cortex. J Neurosci 29: 775–788, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mayerson AB, Hundal RS, Dufour S, Lebon V, Befroy D, Cline GW, Enocksson S, Inzucchi SE, Shulman GI, Petersen KF. The effects of rosiglitazone on insulin sensitivity, lipolysis, and hepatic and skeletal muscle triglyceride content in patients with type 2 diabetes. Diabetes 51: 797–802, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mizuno M, Takeba Y, Matsumoto N, Tsuzuki Y, Asoh K, Takagi M, Kobayashi S, Yamamoto H. Antenatal glucocorticoid therapy accelerates ATP production with creatine kinase increase in the growth-enhanced fetal rat heart. Circ J 74: 171–180, 2010 [DOI] [PubMed] [Google Scholar]
- 27.Morrison JL, Botting KJ, Dyer JL, Williams SJ, Thornburg KL, McMillen IC. Restriction of placental function alters heart development in the sheep fetus. Am J Physiol Regul Integr Comp Physiol 293: R306–R313, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Muhlhausler BS, Duffield JA, Ozanne SE, Pilgrim C, Turner N, Morrison JL, McMillen IC. The transition from fetal growth restriction to accelerated postnatal growth: a potential role for insulin signalling in skeletal muscle. J Physiol 587: 4199–4211, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muhlhausler BS, Morrison JL, McMillen IC. Rosiglitazone increases the expression of peroxisome proliferator-activated receptor-gamma target genes in adipose tissue, liver, and skeletal muscle in the sheep fetus in late gestation. Endocrinology 150: 4287–4294, 2009 [DOI] [PubMed] [Google Scholar]
- 30.Nishikimi T, Maeda N, Matsuoka H. The role of natriuretic peptides in cardioprotection. Cardiovasc Res 69: 318–328, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Ozanne S, Jensen C, Tingey K, Martin-Gronert M, Grunnet L, Brons C, Storgaard H, Vaag A. Decreased protein levels of key insulin signalling molecules in adipose tissue from young men with a low birthweight—potential link to increased risk of diabetes? Diabetologia 49: 2993–2999, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Park SH, Gammon SR, Knippers JD, Paulsen SR, Rubink DS, Winder WW. Phosphorylation-activity relationships of AMPK and acetyl-CoA carboxylase in muscle. J Appl Physiol 92: 2475–2482, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Passmore M, Nataatmadja M, Fraser J. Selection of reference genes for normalisation of real-time RT-PCR in brain-stem death injury in Ovis aries. BMC Mol Biol 10: 72, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pineiro R, Iglesias MJ, Gallego R, Raghay K, Eiras S, Rubio J, Diequez C, Gualillo O, Gonzalez-Juanatey JR, Lago F. Adiponectin is synthesized and secreted by human and murine cardiomyocytes. FEBS Lett 579: 5163–5169, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Spencer TN, Botting KJ, Morrison JL, Posterino GS. Contractile and Ca2+-handling properties of the right ventricular papillary muscle in the late-gestation sheep fetus. J Appl Physiol 101: 728–733, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Stahl A, Gimeno RE, Tartaglia LA, Lodish HF. Fatty acid transport proteins: a current view of a growing family. Trends Endocrinol Metab 12: 266–273, 2001 [DOI] [PubMed] [Google Scholar]
- 37.Sugden MC, Holness MJ. Mechanisms underlying regulation of the expression and activities of the mammalian pyruvate dehydrogenase kinases. Arch Physiol Biochem 112: 139–149, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Taniguchi CM, Emanuelli B, Kahn CR. Critical nodes in signalling pathways: insights into insulin action. Nat Rev Mol Cell Biol 7: 85–96, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Vega RB, Huss JM, Kelly DP. The coactivator PGC-1 cooperates with peroxisome proliferator-activated receptor α in transcriptional control of nuclear genes encoding mitochondrial fatty acid oxidation enzymes. Mol Cell Biol 20: 1868–1876, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang KC, Brooks DA, Thornburg KL, Morrison JL. Activation of IGF-2R stimulates cardiomyocyte hypertrophy in the late-gestation sheep fetus. J Physiol 590: 5425–5437, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang Y, Lau W, Gao E, Tao L, Yuan Y, Li R, Wang X, Koch W, Ma X. Cardiomyocyte-derived adiponectin is biologically active in protecting against myocardial ischemia-reperfusion injury. Am J Physiol Endocrinol Metab 298: E663–E670, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Woodcock EA, Matkovich SJ. Cardiomyocytes structure, function and associated pathologies. Int J Biochem Cell Biol 37: 1746–1751, 2005 [DOI] [PubMed] [Google Scholar]