Abstract
Intestinal nutrient infusions result in variable decreases in food intake and body weight based on the nutrient type and the specific intestinal infusion site. We previously found that intrajejunal infusions of a fatty acid and glucose, but not casein hydrolysate, decreases food intake and body weight in lean chow-fed laboratory rats. To test whether obese, high fat-fed animals would show similar decreases in food intake and body weight in response to intrajejunal infusions of the same nutrients, equal kilocalorie loads of these nutrients (11.4 kcal) or vehicle were infused into the jejunum of obese, high fat-fed male Sprague-Dawley rats over 7 h/day for 5 consecutive days. Food intake was continuously monitored, and body weight was measured daily. After the infusion on the final day, rats were killed and plasma was collected. Similar to lean chow-fed rats, intrajejunal infusions of linoleic acid (LA) and glucose (Glu), but not casein hydrolysate (Cas), suppressed food intake with no compensatory increase in food intake after the infusion period. In contrast to lean chow-fed rats, only the LA, and not the Glu or Cas, produced decreases in body weight in the obese high fat-fed rat. There also were no differences in plasma glucagon-like peptide-1 levels in any of the nutrient infusion groups compared with saline infusion. These results suggest that there is a differential response to the same nutrients in lean vs. obese animals.
Keywords: intestinal infusion, food intake, glucagon-like peptide-1, obese
every day, we are eating a variety of foods and our body needs to be able to assess what we are eating. Oral, gastric, intestinal, and postabsorptive receptors sense the quantity and quality of ingested food to control overall intake and to direct the nutrients to the appropriate target tissues and metabolic pathways. Even though the sight, smell, and taste of our food can provide signals as to the macronutrient composition of our diet, the importance of the gastrointestinal tract (GI) in nutrient sensing has been suggested from the success of bariatric surgery in decreasing appetite and improving glucose homeostasis in patients that have undergone different GI surgical procedures (26, 34). It appears that manipulating the nutrient flow within the GI tract is able to change how our body responds to the food that we eat. Roux-en-Y gastric bypass (RYGB), a surgical procedure that combines gastric restriction and upper intestinal bypass, results in changes in satiety hormone levels and sustained decreases in food intake and body weight (21, 37). When RYGB patients ingest food, nutrients bypass most of the stomach and the duodenum to enter directly into the jejunum. This redistribution of nutrient sensing within the GI tract, with more undigested nutrients reaching lower in the intestine, may then accentuate the secretion of the distal intestinal satiety peptides, glucagon-like peptide 1 (GLP-1) and peptide YY (PYY), from enteroendocrine cells of the mucosal epithelium (21). Both peptides decrease food intake (27) and GLP-1 is also considered an antidiabetic agent because it stimulates insulin secretion, inhibits glucagon secretion, and delays gastric emptying (22). Thus, it is possible that direct nutrient stimulation of the release of distal intestinal satiety peptides may positively alter energy processing and be a contributing mechanism to the success of RYGB.
It is relatively well established that obesity and/or high-fat diet are associated with a diminished response to satiation signals, such as nutrients or satiety peptides. Obese individuals, unlike lean individuals, do not reduce food intake after a high-fat test meal (4), have an attenuated postprandial GLP-1 response (1, 32, 38), and do not produce the same increases in motility and satiety peptides (i.e., CCK and PYY) after intraduodenal infusions of oleic acid (35). This diminished response to dietary fat is also reported after both humans and other animals are chronically maintained on a high-fat diet (24). In laboratory rats, there appears to be an interaction of obesity and high-fat feeding that leads to an attenuation of satiety responses. Diet-induced obese (DIO) rats have decreased circulating GLP-1 levels compared with lean, low fat-fed rats (40). When rats are maintained on a high-energy/high-fat diet, only the obese-prone (OP) rats and not the obese-resistant (OR) rats exhibit an attenuated suppression of food intake and reduced GLP-1 protein expression after gastric infusions of lipids (16). OP high-energy/high-fat fed rats also have significantly lower plasma GLP-1 levels, decreased protein levels of GLP-1 in the intestinal epithelium, and a reduced number of L cells in the distal ileum compared with OR rats fed a high-energy/high-fat diet or OP rats fed a chow diet (15). Overall, these data support the idea that obesity and/or high-fat diet can lead to a decrease in nutrient-driven satiety responses. Because individuals undergoing the RYGB procedure are obese, nutrient-driven increases in satiety peptides from the distal intestine may not be the major mechanism by which there are decreases in food intake and body weight soon after surgery. Identifying the relative sensitivity of obese individuals to direct delivery of nutrients into the distal intestine may reveal the degree to which nutrient stimulation of satiety signals is a contributing mechanism to the success of RYGB. In experiment 1, we tested whether jejunal infusions of the major classes of macronutrients [linoleic acid (LA), glucose (Glu), and casein hydrolysate (Cas)] would produce a decrease in food intake, body weight, and increase satiety peptides in obese rats and compare this with the results we have previously documented in lean rats (14).
Many peptides follow a biological rhythm of peaks and nadirs throughout the day-night cycle [e.g., leptin (36), ghrelin (9), and cortisol (10)], and these rhythms are altered in obese vs. lean individuals (17). These changes are evident as a shift in the rhythm of expression/release or a decrease in the amplitude of expression/release at a particular time point (17). There is even a temporal profile for GLP-1 receptor-mediated hypophagia in rats that is altered by high-fat diet-induced obesity (28). On the basis of these data and the results from experiment 1, we investigated whether there are nutrient-driven changes in the rhythm of GLP-1 in lean vs. obese rats before, during, and after intrajejunal infusion of LA in experiment 2. Results from both experiments may help in understanding differences in lean vs. obese individuals in nutrient sensing.
MATERIALS AND METHODS
Animals
Male Sprague-Dawley rats (Charles River) were used for both experiments. A total of 48 rats (n = 16 per LA, Glu, or Cas experiment with n = 8 nutrient infused and n = 8 saline infused) were included in experiment 1, and 32 rats (n = 16 per lean/chow fed or obese/high-fat fed conditions) were included in experiment 2. In experiment 1, each of the macronutrient infusion and corresponding saline control animals were run separately. In experiment 2, the lean/chow-fed and obese/high-fat fed animals were run together. Animals with an initial weight range of 300–325 g were individually housed and maintained on a 12:12-h light-dark cycle (lights off at 1000). In experiment 1, all rats received ad libitum high-fat diet (Research Diets D12492: 5.24 kcal/g) unless otherwise specified. In experiment 2, half of the rats received ad libitum high-fat diet (Research Diets D12492: 5.24 kcal/g), and half received standard laboratory chow (Global Diet-2018, Harlan Teklad: 3.3 kcal/g) unless otherwise specified. In our experience, it has repeatedly taken 5 wk to see a significant increase in body weight between male Sprague-Dawley rats on a high-fat diet and those on a chow diet, including in experiment 2 in the present study. Thus, 5 wk on the high-fat diet was the used as the time point when nutrient infusions were begun in experiment 1 and experiment 2. Water was available at all times during the experiment. All procedures were approved by the Institutional Animal Care and Use Committee of the Johns Hopkins University.
Jejunal Cannulations
Two days prior to surgery, rats were switched from a high-fat diet or standard chow diet to liquid Ensure (Abbott Laboratories, Abbott Park, IL). For cannula implantations, rats were anesthetized with an intraperitoneal injection of a mixture of ketamine (100 mg/kg) and xylazine (20 mg/kg) at a dose of 1 ml/kg. A laparotomy incision along the ventral midline was made to expose the gastrointestinal tract. A polyurethane catheter (microrenathane 065; Braintree Scientific, Braintree, MA) was inserted into the jejunum. The other end of the catheter was threaded through an opening in the abdominal wall and then passed subcutaneously to an exit on the dorsal surface of the neck. The animals recovered from surgery in their home cage for 5–7 days. They received 2 days of Ensure liquid diet after surgery followed by chow or high-fat diet. Cannula placement and viability were assessed after death by ensuring that the cannula insertion was 50 cm from ileocecal junction and that saline infused into tubing resulted in fluid properly entering the jejunum and traveling toward the distal part of the intestine.
Feeding Tests
After recovery from surgery, the rats were transferred and housed in AccuDiet food intake-monitoring cages (13 in. by 9 in.; Accuscan Instruments, Columbus, OH). A powdered form of the high-fat diet (Research Diets D12492: 5.24 kcal/g) or chow (Global Diet-2018C, Harlan Teklad: 3.3 kcal/g) and water were available ad libitum. Microrenathane tubing (∼5 ft in length; MRE-065; Braintree Scientific, Braintree, MA) was connected to the exteriorized catheter of the animal on one end and to syringes on a multisyringe pump at the other end. Rats were able to freely move within the chamber and access the food cups. All rats received jejunal infusions of 0.9% saline [at a range of 0.2 ml/h to 1.73 ml/h depending on the specific nutrient infused (see below) for 7 h beginning at lights out] for 3 days to allow the rats to habituate to the test chamber and infusion cycle. Food intake was continuously monitored by the AccuDiet system for 22 h (2 h with no food access to collect data and to prepare for the next infusion cycle). After the habituation period, the animals were divided into two groups (saline or specific nutrient infusion) based on average body weight and food intake. Half of the rats in each group received jejunal infusions of either 0.9% saline or a nutrient infusion containing a total caloric load of 11.4 kcal for 7 h beginning at lights out. These parameters were chosen on the basis of prior research showing a reduction in food intake over a multiday infusion of an equal caloric load and duration of infusion of linoleic and oleic acid in lean rats (8). Additionally, stopping the infusion with 5 h left in the dark cycle allows us to test whether there could be a compensatory increase in intake after the infusion, while the animals are still in the dark cycle, the period during which the majority of food intake occurs in rats. Because the total load appears to be more effective than the volume of infusion in decreasing food intake (8), the caloric total was held constant across the three infusates used in the present study. In experiment 1, three different nutrients were used and infused into the jejunum for five consecutive days. Caloric concentrations of the solutions differed such that the volumes infused were LA (0.2 ml/h; Sigma-Aldrich, St. Louis, MO), Cas (1.62 ml/h; MP Biomedicals, Solon, OH), or Glu (1.73 ml/h; Sigma-Aldrich) to deliver the total load of 11.4 kcal. In experiment 2, half of the animals received saline, while the other half received LA at the same volume as for experiment 1, and the infusions were administered for 10 consecutive days. For both experiments, food intake was continuously monitored, as described above. Using custom-designed software, food intake data were analyzed to determine meal patterns. Initiation of a “meal” was defined as ≥200 mg food consumed. The end of each meal was registered when there was >10 min following the end of the last meal.
Plasma Hormone Assays
In experiment 1, rats were decapitated after the last 7-h infusion day, and trunk blood was collected. In experiment 2, four blood samples were collected from each animal at 1000, 1200, 1700, and 2000. Approximately two-hundred microliters of blood was collected via a small tail nick at each time point. Blood was collected from each animal in a random design, so that different animals were sampled on a given day with no more than two blood samples each day with 2 days in between blood sampling days for a given animal. For each experiment, blood from each rat was collected into an EDTA-coated tube and maintained on ice until centrifuged at 3,000 rpm for 10 min. Prior to centrifugation, blood for GLP-1 measurements was treated with 10 μl of DPP-IV inhibitor per milliliter of blood, and blood for PYY was treated with both DPP-IV and 500 KIU of aprotinin. The plasma samples were stored at −80°C until ready to process. The plasma samples from experiment 1 were run in a single assay to determine PYY or GLP-1 to control for interassay variability between the separate macronutrient infusion experiments. A standard radioimmunoassay kit (Millipore, St. Charles, MO) was used to determine plasma PYY (1–36 and 3–36) and was processed according to the manufacturers' protocol. Plasma GLP-1 (active) was determined using an ELISA kit (Millipore) and was processed according to the manufacturers' instructions.
Data Analysis
Statistical analysis was only run on data presented within each graph. In experiment 1, food intake and body weight measures were analyzed using separate two-way repeated-measures analyses of variance (ANOVA; 2 × 4; infusate × days) for each nutrient/saline infusate pair with infusate as the between-subject factor and days as the within-subject factor using Number Crunching Statistical Software (NCSS v 2000; Kaysville, UT). Plasma peptide levels were analyzed using a one-way ANOVA for each nutrient/saline infusate pair with infusate as a between-subject factor. In experiment 2, body weight was analyzed using a two-way repeated-measures ANOVA (2 × 9; infusate × days). Food intake measures during infusion, after infusion, and for the dark and light cycles were analyzed using a separate two-way ANOVA (2 × 2; diet × infusate). Plasma GLP-1 levels were analyzed for each diet condition separately using a two-way repeated-measures ANOVA with infusate as the between-subject factor and time as the within-subject factor. The Newman-Keuls method was utilized to test the significance of multiple comparisons of group means when appropriate. Differences among groups were considered statistically significant if P < 0.05.
RESULTS
Experiment 1
Food intake.
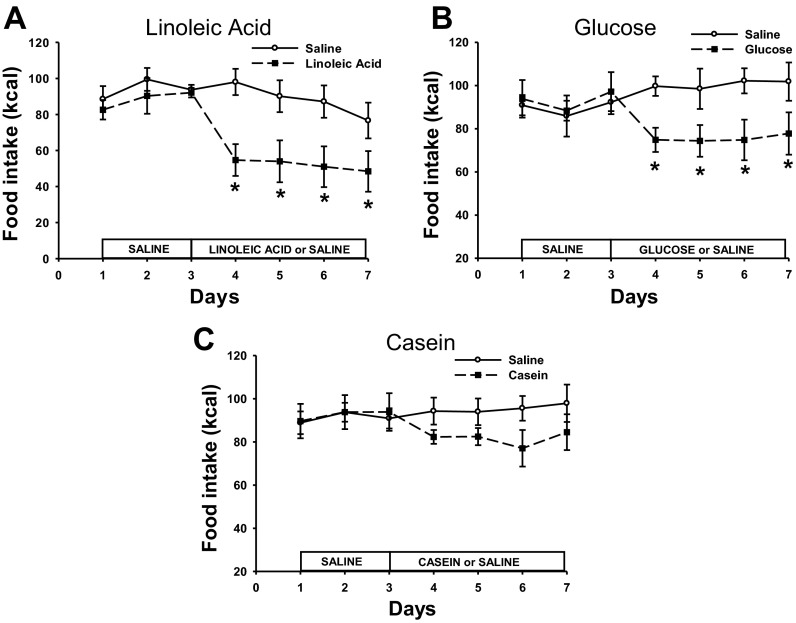
Total caloric intake was significantly decreased over the course of infusion days in animals that were infused with LA or Glu, with no change in total caloric intake after Cas infusions (P < 0.05; Fig. 1). Post hoc tests revealed that jejunal infusions of both LA and Glu significantly decreased the caloric intake on each of the infusion days compared with saline infusions (P < 0.05; Fig. 1, A and B). This suppression of food intake was significantly greater than the 11.4 kcal of LA infused because the significant decrease in food intake remains, even when the 11.4 kcal are added to the total caloric intake for the LA group for days 4–6 (data not shown). Glu also significantly decreased caloric intake beyond that of the infusate, but only for days 4 and 6 (data not shown).
Fig. 1.
Daily caloric intake of animals infused with linoleic acid (LA; A), glucose (Glu; B), casein hydrolysate (Cas; C) or saline for each treatment. ○ represents the caloric intake of animals infused with saline in each treatment, ● represents the caloric intake of animals infused with LA, Glu, or Cas. Values are expressed as means ± SE. *P < 0.05 compared with saline-infused animals.
The decrease in total daily caloric intake in the LA treatment was accounted for by decreases in meal size and meal number (P < 0.05; data not shown), whereas the decrease in the Glu-infused animals was mainly accounted for by a significant decrease in meal size across infusion days compared with animals infused with saline (P < 0.05; data not shown). Meal size and number were not affected in Cas-infused animals.
Body weight.
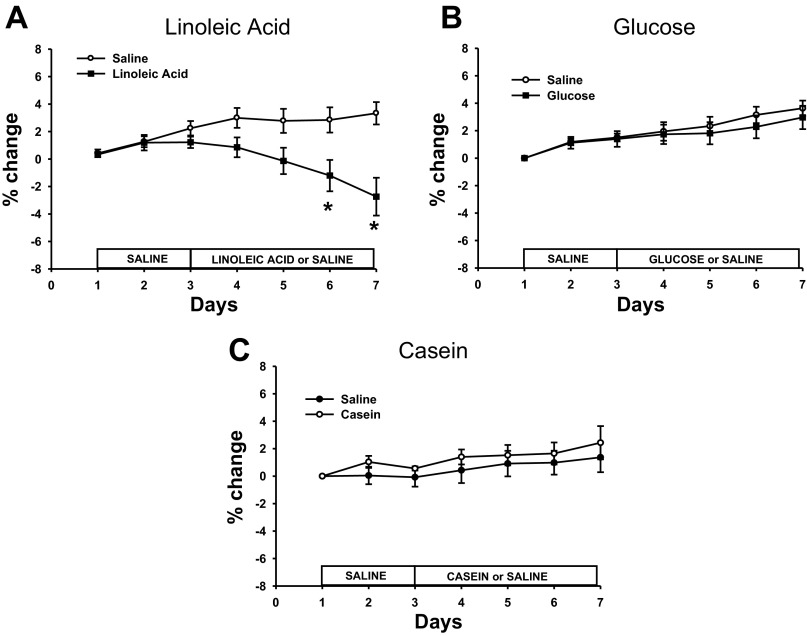
The original mean body weight for each of the macronutrient infusion groups at day 1 (see Fig. 2) was as follows: LA = 620 ± 11.5 g, Glu = 624 ± 13.7 g, Cas = 618 ± 15.3 g. LA infusions resulted in significant decreases in body weight compared with saline infusions (P < 0.05; Fig. 2A). Specifically, significant decreases in body weight on days 6 and 7 were seen for both the LA-treated animals compared with saline-infused animals (P < 0.05; Fig. 2A). There were no significant differences in body weight for the Glu- or Cas-infused animals compared with the saline-infused animals (Fig. 2, B and C).
Fig. 2.
% change in body weight in animals infused with LA (A), Glu (B), Cas (C), or saline for each treatment. Values are expressed as means ± SE. *P < 0.05 compared with saline-infused animals.
Plasma hormones.
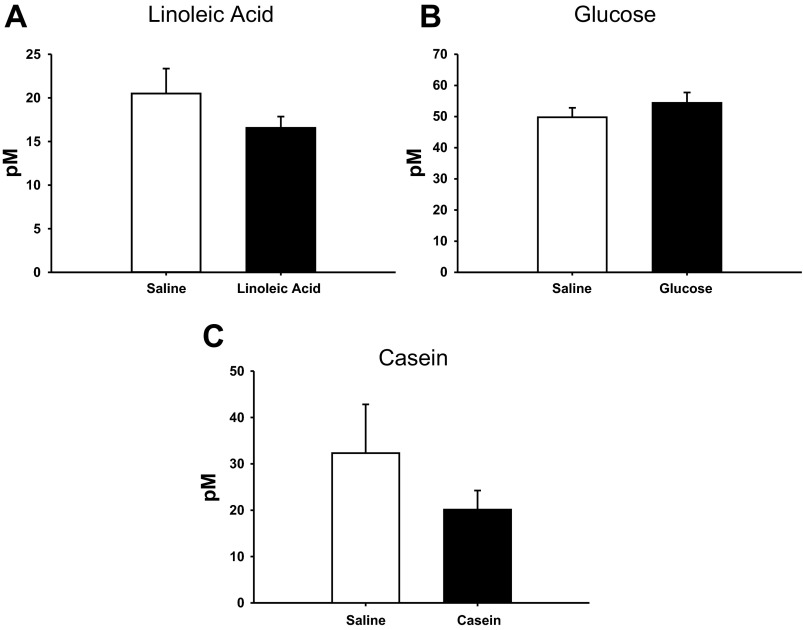
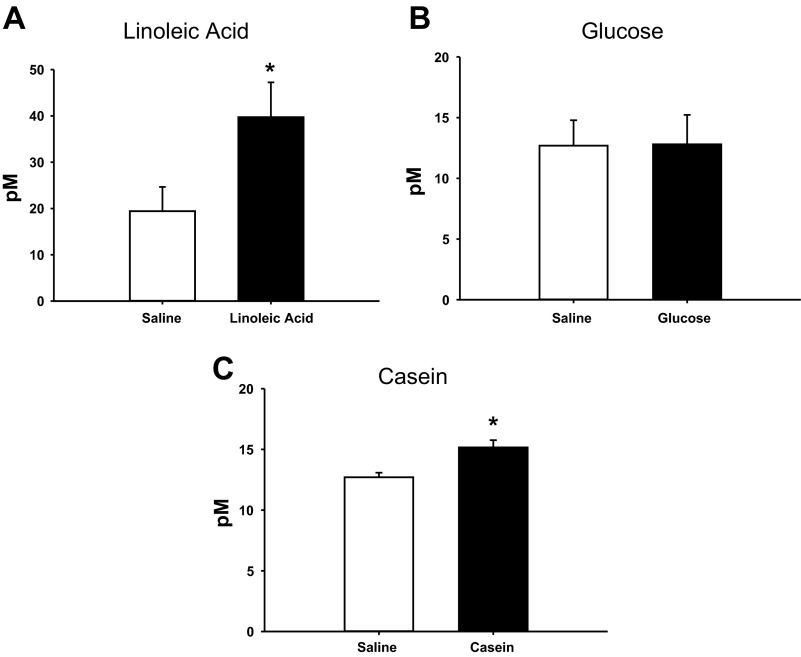
There were no significant differences in plasma GLP-1 levels between saline and nutrient infusions across the nutrient treatments (Fig. 3). Plasma PYY levels were significantly increased in the LA- and Cas-infused animals compared with the saline-infused controls in each treatment (P < 0.05; Figs. 4, A and C), with no effect of Glu infusion on PYY levels (Fig. 4B).
Fig. 3.
Plasma glucagon like peptide-1 (GLP-1) of animals infused with LA (A), Glu (B), Cas (C), or saline for each treatment. Values are expressed as means ± SE.
Fig. 4.
Plasma peptide YY (PYY) of animals infused with LA (A), Glu (B), Cas (C) or saline for each treatment. Values are expressed as means ± SE. *P < 0.05 compared with saline-infused animals.
Experiment 2
Food intake.
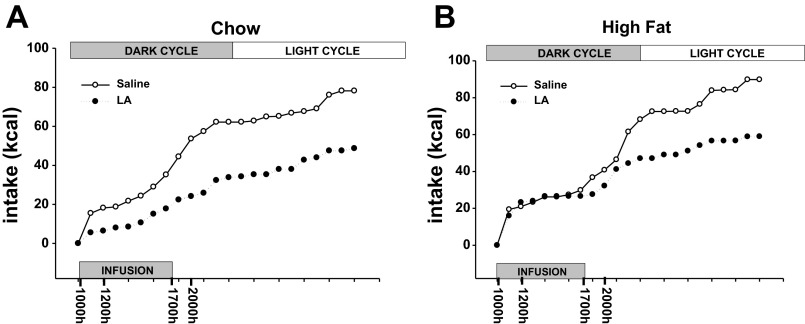
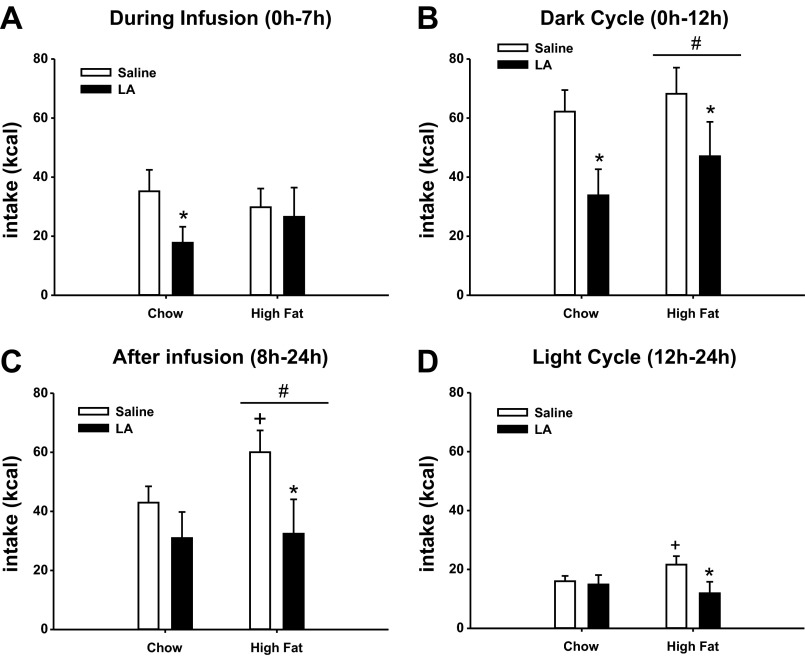
Total caloric intake was significantly decreased over the course of infusion days in animals that were infused with LA compared with saline in both the lean/chow-fed and obese/high-fat fed animals (data not shown). Figure 5 represents the cumulative hourly intake (averaged across days 4–12 of infusions) for the saline or LA-infused animals in the chow and high-fat groups. These data were further analyzed in bins of time including, food intake during infusion (0–7 h), after infusion (0–24 h), during the dark cycle (0–12 h), and during the light cycle (12–24 h) in each group of animals (Fig. 6). During the infusion period, LA significantly decreased food intake compared with saline only in the chow-fed animals (P < 0.05; Fig. 6A). After the infusion period, though, the animals that had received LA showed a significant decrease in intake only in the high fat-fed group (P < 0.05; Fig. 6C). LA infusion caused a significant decrease in intake compared with saline infusion in both the chow and high-fat groups in the dark cycle (P < 0.05; Fig. 6C), but only in the high-fat group during the light cycle (P < 0.05; Fig. 6D).
Fig. 5.
Cumulative hourly food intake for animals infused with LA or saline in the chow (A) or high-fat (B) condition. Values indicate the average intake at each hour across the days of the infusion. The times on the x-axis denote the blood-sampling times.
Fig. 6.
Food intake for animals infused with LA or saline in the lean, chow-fed, and high-fat fed condition during the infusion (A), during the dark period (B), after the infusion (C), and during the light period (D). Mean values for each group are represented. Values are expressed as means ± SE. *P < 0.05 compared with saline-infused animals within each diet condition. +P < 0.05 compared with chow-fed, saline-infused animals. #P < 0.05 compared with chow-fed animals.
Body weight.
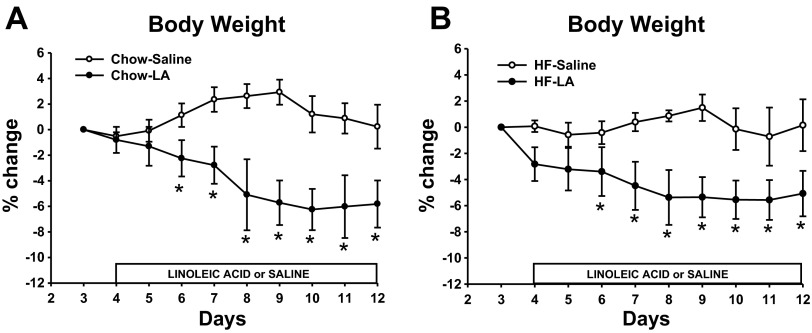
The body weight of the animals fed a high-fat diet was significantly greater than the animals on a chow diet on the last day of saline infusion during the habituation period on day 3 (see Fig. 7; high-fat diet = 619 ± 24.6 g compared with chow = 463 ± 16.5 g). LA infusions resulted in significant decreases in % change of body weight compared with saline infusions in both the lean and obese animals (P < 0.05; Figs. 7, A and B). In the chow-fed animals, LA infusions resulted in a 6% decrease from the original average 463 g for the group, whereas the saline infusion resulted in no change in body weight. In the high-fat fed animals, LA infusions resulted in a 5% decrease from the original average 619 g for the group.
Fig. 7.
% change in body weight in animals infused with LA or saline in the lean, chow-fed condition (A) or obese, high-fat fed condition (B). Values are expressed as means ± SE. *P < 0.05 compared with saline-infused animals.
Plasma hormones.
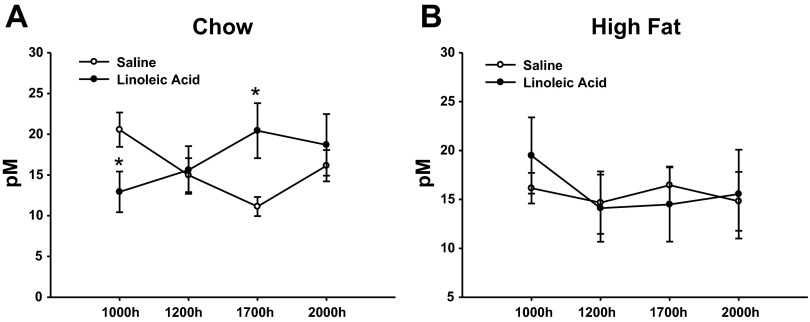
When the plasma GLP-1 levels were averaged across the four time points assessed, there were no overall differences between the LA and saline-infused animals in either the lean or obese conditions (Fig. 8, A and B). However, jejunal infusions of LA produced a change in the rhythm of GLP-1 across the times assessed in the lean animals (Fig. 8A). There was a decrease in the GLP-1 levels at the 1000 time point (just prior to beginning the 7-h infusion) and an increase at the 1700 time point (at the end of 7-h infusion) when the LA-infused lean animals are compared with the lean saline-infused animals (P < 0.05; Fig. 8A). There were no time-related differences in the obese rats.
Fig. 8.
Plasma GLP-1 of animals infused with LA or saline in the lean, chow-fed condition (A) or obese, high-fat fed condition (B). Values are expressed as means ± SE. *P < 0.05 compared with saline-infused animals.
DISCUSSION
The aim of these experiments was to investigate whether obese rats would show similar changes in food intake, body weight, and plasma satiety signals in response to intrajejunal infusions of specific nutrients to those that were previously seen in lean rats (14). The major findings were 1) intrajejunal infusions of LA and Glu, but not Cas, suppressed food intake compared with saline-infused animals, 2) plasma GLP-1 levels were not altered in any of the infusion treatments in the obese rats, 3) plasma levels of PYY were increased in the LA- and Cas-infused obese animals, and 4) the rhythm of plasma levels of GLP-1 in lean animals is lost in obese animals.
When the nutrient-driven responses in the obese animals in the present study are compared with previously published responses in lean animals using the same methods (14), we see that jejunal fatty acid and Glu infusions inhibit food intake in both lean and obese animals, with the same lack of response after Cas infusions. LA also is able to decrease body weight in both obese and lean animals. In contrast, Glu infusions decrease body weight only in the lean animals. This may be due to the extent of the decrease in food intake induced by the Glu infusions. In lean animals, Glu infusions are able to decrease food intake ∼2–3 times beyond the calories of the infusate, a value much greater than in what we observed in the obese animals. The satiety peptide levels did not always correlate with decreases in food intake in lean or obese animals. LA and Cas infusions resulted in increases in plasma PYY levels in both lean and obese animals, but only intrajejunal LA produced decreases in food intake. Even though Glu produced a decrease in food intake in lean and obese animals, PYY levels were not increased in either nutrient infusion treatment. Thus, plasma PYY levels do not correlate with decreases in food intake using our methods. In contrast to the similar effects of nutrients on PYY in lean and obese animals, there was a differential GLP-1 response between the two groups. In lean animals, GLP-1 levels were increased after LA and Glu infusions, the two treatments that also decreased food intake. Even though these two nutrients produced a decrease in food intake in the obese animals, there was no increase in GLP-1 observed. Even after measuring plasma GLP-1 levels at multiple times before, during, and after the nutrient infusion in experiment 2, there still was no increase observed at any time point measured. Taken together, we find that obese and lean animals exhibit the same food intake responses to intrajejunal infusions of nutrients, have the same nutrient-driven PYY response, but a different GLP-1 response.
Both obese and lean animals reduced their food intake in response to intrajejunal infusions of LA and Glu, but only the lean animals showed an increase in plasma GLP-1 levels at the end of the infusion cycle (14). Moreover, blocking the action of the GLP-1 using the GLP-1 receptor antagonist exendin-9 completely attenuates the decrease in food intake after jejunal infusions of LA in lean animals (11). These data suggest a variety of conclusions. First, we know that exendin-9 can have a long-lasting effect on the GLP-1 receptors and, thus, stimulate a greater population of receptors (39). This suggests that exendin-9 may be able to modulate central receptors, while the endogenous intestinally derived GLP-1 may be degraded before it is able to reach the brain. Second, the plasma level of GLP-1 may not be a proper measure of nutrient-driven changes in GLP-1 to affect food intake. Data support a role for local GLP-1 receptors within the intestinal wall in decreasing appetite (for a review, see Ref. 27). LA and Glu may actually increase the release of GLP-1 locally, which can then bind onto receptors located on enteric and sensory neurons to have an effect on food intake (2, 29). This local effect of GLP-1 within the intestine may explain why there is a LA- and Glu-driven decrease in food intake without a correlated increase in plasma GLP-1 levels in the obese animals. It also may explain one mechanism by which the GLP-1R antagonist was able to block this decrease in food intake, by binding onto GLP-1R located within the intestinal wall. Similar effects with CCK, another intestinally derived satiety peptide, have been found. A CCK-A receptor antagonist blocks the feeding suppression due to glucose intestinal infusions even when there is no increase in plasma CCK levels (7). A third explanation could be that local GLP-1 is increased in our obese animals but occurs along with an increase in dipeptidyl peptidase IV (DPP-IV), the protease inhibitor known to quickly degrade GLP-1. DPP-IV is produced within the intestine and is increased in rats fed a high-fat diet compared with a standard rodent chow [albeit, these diets differed from those used in the present study (41)] and in obese humans (25). Higher levels of intestinal DPP-IV may then be able to degrade GLP-1 before it reaches systemic circulation and can be measured through our blood sampling. Carr et al. (6), though, suggest that an elevation in plasma DPP-IV activity in obese humans is not the cause of the lower levels of GLP-1 in obese vs. lean individuals. After a mixed meal or oral glucose, plasma levels of total GLP-1 and active GLP-1 are equally reduced in obese subjects. Thus, increased DDP-IV activity in obese individuals would not be the cause. Carr et al. (6) suggest that it is more likely that lower GLP-1 levels reflect a reduction in secretion from the intestine. Even with some of these possible explanations, it still is not clear whether the jejunal nutrients were able to alter behavior in obese animals through gut peptide signaling or through other mechanisms.
Data from humans do not clearly define the relative role of satiety peptides controlling food intake in obese vs. lean individuals but suggest a negative correlation with obesity. Basal GLP-1 concentrations and postprandial GLP-1 release seem to be attenuated in obese compared with lean adults (1, 32). PYY levels also are lower in obese individuals compared with lean (20, 23, 42). In obese children, GLP-1 fasting levels are significantly lower than in lean children and a negative correlation between GLP-1 and body mass index and waist circumference is evident (3). As far as evidence for nutrient-driven changes in satiety hormones in obese individuals, Gibbons et al. (18) found that obese men and women show increases in GLP-1 and PYY after a meal. A high-fat meal will increase GLP-1 and PYY levels to a greater extent than a high-carbohydrate meal. In addition, a rise in GLP-1 after the morning of a high-fat or high-carbohydrate meal is associated with lower-energy intake at a lunch meal. In contrast, PYY was not correlated with the amount of food eaten at the lunch meal. When the effect of weight loss is investigated, weight loss appears to increase postprandial GLP-1 concentrations to a level that is between that of lean and obese individuals (38). Another study, though, showed that postprandial GLP-1 concentrations were significantly lower after weight loss compared with before weight loss levels, even though ratings of satiety were increased and hunger scores decreased (1). After RYGB, patients exhibited a marked increase in postprandial GLP-1 levels as early as 1 wk after surgery, and this marked increase in GLP-1 levels remained increased at 3 mo and 1 yr after surgery (30). There are obvious confounds with oral ingestion vs. the method of direct infusion of nutrients into the intestine utilized in the present study. Taken together, though, these data indicate that there are significant differences between lean and obese individuals with respect to hormone release, and that the gut may respond differently to ingested nutrients in obese subjects, compared with lean subjects.
Adam et al. (1) had suggested that the effect of GLP-1 to decrease appetite may be driven by the change in GLP-1 levels across time. In experiment 2, we see rhythmic changes in GLP-1 during the dark phase of the lean animals, but only a steady GLP-1 level in the obese animals. In addition, the rhythm of plasma GLP-1 is altered in lean animals in response to jejunal infusions of LA. This nutrient-driven change in GLP-1 peaks and nadirs is not apparent in the obese animals. The plateau in GLP-1 rhythm in the obese animals and the shift in rhythm produced by the additional intrajejunal LA infusion were only evident when multiple blood samples were taken. Thus, the variability of results across studies investigating satiety peptide levels may be explained because many studies only sample blood at one time point in a given day. However, this may not be as relevant as seeing whether there is a change in GLP-1 levels before and after a manipulation.
The present results do not differentiate between the effects of obesity and high-fat diet on nutrient-driven changes in ingestive behaviors and satiety signals. One method utilized to try to understand the effect of each of these factors separately is to use two strains of rats that differ in their susceptibility to diet-induced obesity, termed obese-resistant (OR) and obese-prone (OP). Both gastric and duodenal infusion of fats (i.e., Intralipid or sodium linoleate) decreases intake of a high-fat diet to a greater extent in the OR than the OP rats (16, 19). OP, compared with OR, high-energy/high-fat fed rats also have significantly lower plasma GLP-1 levels, decreased protein levels of GLP-1 in the intestinal epithelium, and a reduced number of L cells in the distal ileum (15). Moreover, peripheral administration of a GLP-1 agonist, exendin 4, suppresses high-fat intake to a greater extent in OR than OP rat, indicating greater GLP-1 sensitivity in the OR animals (31). These data suggest that obesity, and not diet, may drive differences in nutrient-driven satiating mechanisms. The caveat with these results is that OP rats eat significantly more of the high-energy/high-fat diet than the OR. Moreover, the decrease in L cell number, GLP-1 protein expression, and plasma GLP-1 levels in OP rats does not occur when OP rats are fed a chow diet (15). Thus, there appears to be an interaction of obesity with the amount of food or type of food in determining sensitivity to nutrients and satiety mechanisms.
We have examined the effect of jejunal infusion of nutrients in DIO animals as a model of the effect of greater nutrient stimulation in the distal intestine of obese patients that have undergone RYGB. Both the lean and obese animals in our studies decrease food intake after jejunal LA and Glu infusions and decrease body weight after LA, but there is a differential GLP-1 response. This suggests that the decrease in food intake and body weight does not require systemic increases in GLP-1. The increase in GLP-1 levels seen only in our lean animals may be mediated by a nonnutrient-driven mechanism. Indirect GLP-1 stimulation has been documented (13, 33) and can occur through a neuroendocrine loop or through the enteric nervous system (for a review, see Ref. 12). Obesity is known to induce neuroplasticity in autonomic nerves and, thus, our obese rats may have decreased neural mediated GLP-1 secretion. RYGB in DIO rats improves vagal efferent nerve responsiveness to satiety signals (5). This outlines an additional mechanism beyond direct nutrient stimulation by which RYGB may improve satiation.
Perspectives and Significance
Even with the many unknown factors and discrepancies among studies, it is clear that there are differences in satiety hormone release and downstream behavioral responses in lean and obese individuals. Greater nutrient delivery to the intestine that occurs with overeating or factors secondary to obesity may alter the morphology and function of intestinal cells. Alterations in the gastrointestinal tract may then lead to greater physiological alterations beyond the digestion and absorption of food. The neural and hormonal connections between the cells of the GI tract and the nervous system play an integral role in affecting metabolism and appetite. If we can understand how nutrients interact with the GI tract to alter cellular morphology and function, we may be able to target segments or cell types along the GI tract through pharmacological manipulation to produce beneficial effects on energy homeostasis.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: M.J.D. and T.H.M. conception and design of research; M.J.D. and A.A.M. performed experiments; M.J.D. and A.A.M. analyzed data; M.J.D. and T.H.M. interpreted results of experiments; M.J.D. prepared figures; M.J.D. drafted manuscript; M.J.D., A.A.M., and T.H.M. edited and revised manuscript; M.J.D., A.A.M., and T.H.M. approved final version of manuscript.
GRANTS
This work was supported by National Institutes of Health Grants DK-19302 (to T. H. Moran) and DK-092126 (to M. J. Dailey).
REFERENCES
- 1.Adam TCMT, Jocken JJ, Westerterp-Plantenga MSM. Decreased glucagon-like peptide 1 release after weight loss in overweight/obese subjects. Obesity (Silver Spring) 13: 710–716, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Amato A, Cinci L, Rotondo A, Serio R, Faussone-Pellegrini MS, Vannucchi MG, Mulè F. Peripheral motor action of glucagon-like peptide-1 through enteric neuronal receptors. Neurogastroenterol Motil 22: 664-e203, 2010 [DOI] [PubMed] [Google Scholar]
- 3.Bascietto C, Giannini C, D'Adamo E, de Giorgis T, Chiarelli F, Mohn A. Implications of gastrointestinal hormones in the pathogenesis of obesity in prepubertal children. J Pediatr Endocrinol Metab 25: 255–260, 2012 [DOI] [PubMed] [Google Scholar]
- 4.Brennan IM, Luscombe-Marsh ND, Seimon RV, Otto B, Horowitz M, Wishart JM, Feinle-Bisset C. Effects of fat, protein, and carbohydrate and protein load on appetite, plasma cholecystokinin, peptide YY, and ghrelin, and energy intake in lean and obese men. Am J Physiol Gastrointest Liver Physiol 303: G129–G140, 2012 [DOI] [PubMed] [Google Scholar]
- 5.Browning KN, Fortna SR, Hajnal A. Roux-en-Y gastric bypass reverses the effects of diet-induced obesity to inhibit the responsiveness of central vagal motoneurones. J Physiol 591: 2357–2372, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carr RD, Larsen MO, Jelic K, Lindgren O, Vikman J, Holst JJ, Deacon CF, Ahren B. Secretion and dipeptidyl peptidase-4-mediated metabolism of incretin hormones after a mixed meal or glucose ingestion in obese compared to lean, nondiabetic men. J Clin Endocrinol Metab 95: 872–878, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Covasa M, Ritter RC. Attenuated satiation response to intestinal nutrients in rats that do not express CCK-A receptors. Peptides 22: 1339–1348, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Cox JE, Tyler WJ, Randich A, Kelm GR, Bharaj SS, Jandacek RJ, Meller ST. Suppression of food intake, body weight, and body fat by jejunal fatty acid infusions. Am J Physiol Regul Integr Comp Physiol 278: R604–R610, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes 50: 1714–1719, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Czeisler CA, Klerman EB. Circadian and sleep-dependent regulation of hormone release in humans. Recent Prog Horm Res 54: 97–130- discussion 130–2, 1999 [PubMed] [Google Scholar]
- 11.Dailey MJ, Moghadam AA, Moran TH. Jejunal linoleic acid infusions require GLP-1 receptor signaling to inhibit food intake: implications for the effectiveness of Roux-en-Y gastric bypass. Am J Physiol Endocrinol Metab 301: E1184–E1190, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dailey MJ, Moran TH. Glucagon-like peptide 1 and appetite. Trends Endocrinol Metab 24: 85–91, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dailey MJ, Stingl KC, Moran TH. Disassociation between preprandial gut peptide release and food-anticipatory activity. Endocrinology 153: 132–142, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dailey MJ, Tamashiro KLK, Terrillion CE, Moran TH. Nutrient specific feeding and endocrine effects of jejunal infusions. Obesity (Silver Spring) 18: 904–910, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Duca FA, Sakar Y, Covasa M. The modulatory role of high fat feeding on gastrointestinal signals in obesity. J Nutr Biochem 24: 1663–1677, 2013 [DOI] [PubMed] [Google Scholar]
- 16.Duca FAF, Swartz TDT, Sakar YY, Covasa MM. Decreased intestinal nutrient response in diet-induced obese rats: role of gut peptides and nutrient receptors. Int J Obes Relat Metab Disord 37: 375–381, 2013 [DOI] [PubMed] [Google Scholar]
- 17.Froy O. Metabolism and circadian rhythms—implications for obesity. Endocr Rev 31: 1–24, 2010 [DOI] [PubMed] [Google Scholar]
- 18.Gibbons C, Caudwell P, Finlayson G, Webb DL, Hellström PM, Näslund E, Blundell JE. Comparison of postprandial profiles of ghrelin, active GLP-1, and total PYY to meals varying in fat and carbohydrate and their association with hunger and the phases of satiety. J Clin Endocrinol Metab 98: E847–E855, 2013 [DOI] [PubMed] [Google Scholar]
- 19.Greenberg D, McCaffery J, Potack JZ, Bray GA, York DA. Differential satiating effects of fats in the small intestine of obesity-resistant and obesity-prone rats. Physiol Behav 66: 621–626, 1999 [DOI] [PubMed] [Google Scholar]
- 20.Guo Y, Ma L, Enriori PJ, Koska J, Franks PW, Brookshire T, Cowley MA, Salbe AD, DelParigi A, Tataranni PA. Physiological evidence for the involvement of peptide YY in the regulation of energy homeostasis in humans. Obesity (Silver Spring) 14: 1562–1570, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Hage MP, Safadi B, Salti I, Nasrallah M. Role of gut-related peptides and other hormones in the amelioration of type 2 diabetes after Roux-en-Y gastric bypass surgery. ISRN Endocrinol 2012: 504756–504756, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holst JJ. Enteroglucagon. Annu Rev Physiol 59: 257–271, 1997 [DOI] [PubMed] [Google Scholar]
- 23.Le Roux CW, Batterham RL, Aylwin SJB, Patterson M, Borg CM, Wynne KJ, Kent A, Vincent RP, Gardiner J, Ghatei MA, Bloom SR. Attenuated peptide YY release in obese subjects is associated with reduced satiety. Endocrinology 147: 3–8, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Little TJ, Horowitz M, Feinle-Bisset C. Modulation by high-fat diets of gastrointestinal function and hormones associated with the regulation of energy intake: implications for the pathophysiology of obesity. Am J Clin Nutr 86: 531–541, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Lugari R, Dei Cas A, Ugolotti D, Barilli AL, Camellini C, Ganzerla GC, Luciani A, Salerni B, Mittenperger F, Nodari S, Gnudi A, Zandomeneghi R. Glucagon-like peptide 1 (GLP-1) secretion and plasma dipeptidyl peptidase IV (DPP-IV) activity in morbidly obese patients undergoing biliopancreatic diversion. Horm Metab Res 36: 111–115, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Maggard-Gibbons M, Maglione M, Livhits M, Ewing B, Maher AR, Hu J, Li Z, Shekelle PG. Bariatric surgery for weight loss and glycemic control in nonmorbidly obese adults with diabetes: a systematic review. JAMA 309: 2250–2261, 2013 [DOI] [PubMed] [Google Scholar]
- 27.Moran TH, Dailey MJ. Intestinal feedback signaling and satiety. Physiol Behav 105: 77–81, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mul JD, Begg DP, Barrera JG, Li B, Matter EK, D'Alessio DA, Woods SC, Seeley RJ, Sandoval DA. High-fat diet changes the temporal profile of GLP-1 receptor-mediated hypophagia in rats. Am J Physiol Regul Integr Comp Physiol 305: R68–R77, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakagawa A, Satake H, Nakabayashi H, Nishizawa M, Furuya K, Nakano S, Kigoshi T, Nakayama K, Uchida K. Receptor gene expression of glucagon-like peptide-1, but not glucose-dependent insulinotropic polypeptide, in rat nodose ganglion cells. Auton Neurosci 110: 36–43, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Peterli R, Steinert RE, Woelnerhanssen B, Peters T, Christoffel-Courtin C, Gass M, Kern B, Fluee von M, Beglinger C. Metabolic and hormonal changes after laparoscopic Roux-en-Y gastric bypass and sleeve gastrectomy: a randomized, prospective trial. Obes Surg 22: 740–748, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Primeaux SD, Barnes MJ, Braymer HD, Bray GA. Sensitivity to the satiating effects of exendin 4 is decreased in obesity-prone Osborne-Mendel rats compared to obesity-resistant S5B/Pl rats. Int J Obes (Lond) 34: 1427–1433, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ranganath LRL, Beety JMJ, Morgan LML, Wright JWJ, Howland RR, Marks VV. Attenuated GLP-1 secretion in obesity: cause or consequence? Gut 38: 916–919, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rocca AS, Brubaker PL. Role of the vagus nerve in mediating proximal nutrient-induced glucagon-like peptide-1 secretion. Endocrinology 140: 1687–1694, 1999 [DOI] [PubMed] [Google Scholar]
- 34.Scott WR, Batterham RL. Roux-en-Y gastric bypass and laparoscopic sleeve gastrectomy: understanding weight loss and improvements in type 2 diabetes after bariatric surgery. Am J Physiol Regul Integr Comp Physiol 301: R15–R27, 2011 [DOI] [PubMed] [Google Scholar]
- 35.Stewart JE, Seimon RV, Otto B, Keast RSJ, Clifton PM, Feinle-Bisset C. Marked differences in gustatory and gastrointestinal sensitivity to oleic acid between lean and obese men. Am J Clin Nutr 93: 703–711, 2011 [DOI] [PubMed] [Google Scholar]
- 36.van Aggel-Leijssen DP, Van Baak MA, Tenenbaum R, Campfield LA, Saris WH. Regulation of average 24 h human plasma leptin level; the influence of exercise and physiological changes in energy balance. Int J Obes Relat Metab Disord 23: 151–158, 1999 [DOI] [PubMed] [Google Scholar]
- 37.Vella AA. Enteroendocrine secretion after Roux-en-Y gastric bypass: is it important? Neurogastroenterol Motil 25: 1–3, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Verdich C, Toubro S, Buemann B, Lysgård Madsen J, Juul Holst J, Astrup A. The role of postprandial releases of insulin and incretin hormones in meal-induced satiety—effect of obesity and weight reduction. Int J Obes Relat Metab Disord 25: 1206–1214, 2001 [DOI] [PubMed] [Google Scholar]
- 39.Wang Z, Wang RM, Owji AA, Smith DM, Ghatei MA, Bloom SR. Glucagon-like peptide-1 is a physiological incretin in rat. J Clin Invest 95: 417–421, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Williams DL, Hyvarinen N, Lilly N, Kay K, Dossat A, Parise E, Torregrossa AM. Maintenance on a high-fat diet impairs the anorexic response to glucagon-like-peptide-1 receptor activation. Physiol Behav 103: 557–564, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang J, Campitelli J, Hu G, Lin Y, Luo J, Xue C. Increase in DPP-IV in the intestine, liver and kidney of the rat treated with high-fat diet and streptozotocin. Life Sci 81: 272–279, 2007 [DOI] [PubMed] [Google Scholar]
- 42.Zwirska-Korczala K, Konturek SJ, Sodowski M, Wylezol M, Kuka D, Sowa P, Adamczyk-Sowa M, Kukla M, Berdowska A, Rehfeld JF, Bielanski W, Brzozowski T. Basal and postprandial plasma levels of PYY, ghrelin, cholecystokinin, gastrin and insulin in women with moderate and morbid obesity and metabolic syndrome. J Physiol Pharmacol 58 Suppl 1: 13–35, 2007 [PubMed] [Google Scholar]