Abstract
Purpose
Peripheral quantitative computed tomography (pQCT) is an essential tool for assessing bone parameters of the limbs, but subject movement and its impact on image quality remains a challenge to manage. The current approach to determine image viability is by visual inspection, but pQCT lacks a quantitative evaluation. Therefore, the aims of this study were to (1) examine the reliability of a qualitative visual inspection scale, and (2) establish a quantitative motion assessment methodology.
Methods
Scans were performed on 506 healthy girls (9–13yr) at diaphyseal regions of the femur and tibia. Scans were rated for movement independently by three technicians using a linear, nominal scale. Quantitatively, a ratio of movement to limb size (%Move) provided a measure of movement artifact. A repeat-scan subsample (n=46) was examined to determine %Move’s impact on bone parameters.
Results
Agreement between measurers was strong (ICC = .732 for tibia, .812 for femur), but greater variability was observed in scans rated 3 or 4, the delineation between repeat or no repeat. The quantitative approach found ≥95% of subjects had %Move <25%. Comparison of initial and repeat scans by groups above and below 25% initial movement, showed significant differences in the >25% grouping.
Conclusions
A pQCT visual inspection scale can be a reliable metric of image quality but technicians may periodically mischaracterize subject motion. The presented quantitative methodology yields more consistent movement assessment and could unify procedure across laboratories. Data suggest a delineation of 25% movement for determining whether a diaphyseal scan is viable or requires repeat.
Keywords: Peripheral quantitative computed tomography (pQCT), Image quality, Subject motion, Quantitative procedure, Qualitative procedure, Movement artifact
Introduction
Peripheral quantitative computed tomography (pQCT) continues to gain wider acceptance as a research standard for assessing bone properties and more recently soft tissue composition. Its ability to measure volumetric bone mineral density (vBMD) is an improvement over predecessors such as dual-energy X-ray absorptiometry (DXA) where measurements of areal BMD are confounded by size (changes in tissue depth) [1]. In addition to vBMD, pQCT provides measures of bone geometry, which in combination with vBMD can be used to calculate indices of bone strength, and delineation of cortical from trabecular bone, all previously unattainable at an equivalently low radiation exposure. The ability to distinguish between trabecular and cortical bone is an important advantage as these compartments have been shown to respond differently to stimuli such as hormonal changes, mechanical forces, and disease-related stresses [2]. pQCT has also been shown to be useful for measuring soft tissue components, including various muscle and fat indices, at the diaphyseal regions of the limbs [3–5]. Given the established relationships between muscle and bone [6,7], the interest in the effect of fat mass on bone [8,9], and more recently, the relationship of muscle quality to bone [10], pQCT’s ability to simultaneously examine both bone and soft tissue at a low radiation dose is particularly valuable.
Despite these advantages, pQCT also has limitations [2,11,12]. For example, partial volume effects have been shown to result in underestimation of cortical vBMD [13,2,14–16], although algorithms have been established to compensate for this limitation [17]. In addition, longitudinal measurements of children may be complicated by developing growth plates in long bones, which make consistently locating reference lines more difficult. pQCT is also limited by varying methodologies and a lack of evidence on which to determine a basis for any single method [16]. Measurement sites have varied among studies because of their varied response to the intervention type so attempts to compile findings yield mottled results, particularly given that the bone architecture of the arm and leg are only moderately correlated [18]. However, with greater familiarity and more widespread use, it is expected that pQCT methodologies will become more uniform, resolving many of these issues.
One concern, beyond the methodological parameters, that presents a persistent challenge for pQCT-based measures is subject motion and the associated movement artifacts present in the scan image. Subject movements during image acquisition can be as subtle as muscle twitches, as obvious as a cough/sneeze, or simply the result of a fidgety patient, particularly evident in children. Because these movements are unforeseeable, technicians cannot plan in advance for them and they become an unavoidable component of pQCT. Such motion can severely degrade the quality of the image and result in either unusable scans or require rescanning to obtain images of acceptable quality. To avoid missing data, it is important for a technician to be able to know when to perform a repeat scan, and the investigator requires objective criteria for deciding whether a scan can be included in data analysis.
The current approach for determining if too much movement is present in a scan is by visual inspection, where scans are qualitatively examined to determine if any motion artifacts are present in the image and to what degree they impact the image quality. Many laboratories, including our own, use a rating scale to determine the level of motion in the image by examining the amount of streaking and/or breaks in the cortical shell. Such scales are established to allow the technician to rate the movement in a scan and determine whether the scan is usable so that, if necessary, a repeat acquisition scan can be performed. While this is a widely used approach for movement assessment, it is subjective and can result in varied gradations for a single rating. To date no such scales have been validated for standard pQCT. However, studies with high resolution pQCT, a similar technology, have presented mixed evidence on the effectiveness of these types of scales [19–21].
Given the possibility of measurer error with this technique, it follows that a quantitative measure of movement could provide an improved and more objective assessment of image quality, one that could be uniformly used throughout the field. Researchers using the high resolution-pQCT considered this possibility and studies indicate that a quantitative scale provides a more consistent means of determining if a repeat scan should be performed [20,22,19]. It stands to reason that for standard pQCT, instituting a quantitative procedure for managing subject motion could not only help minimize data loss, but also potentially eliminate unnecessary repeat scan acquisition and the associated additional radiation exposure. To this end, the aims of the present study were two-fold: (1) to examine the reliability of an existing visual inspection rating scale, and (2) establish a quantitative motion assessment methodology.
Materials and Methods
Study Subjects
Cross-sectional data were analyzed for 506 healthy girls aged 9 to 13 years who were participants in the Jump-In: Building Better Bones study. The details of the Jump-In study have been published elsewhere [23,24]. The University of Arizona’s Human Subjects Institutional Review Board approved the study and all participants gave written informed assent and parental consent before participation in the study. The study was conducted in accordance with the Helsinki Declaration.
Anthropometry
Standing height and weight measurements were obtained with subjects wearing light-weight clothing and no shoes. Height was measured at maximal inhalation to the nearest 0.1 cm using a Schorr measuring board (Schorr Products, Olney, MD). Weight was measured on a calibrated digital scale (model 880; SECA, Hamburg, Germany), accurate to 0.1 kg. The average of two measurements for both height and weight were used as the criterion measurements. BMI was calculated as weight (kg) divided by height squared (m2).
pQCT
Bone cross-sectional area (CSA), vBMD, circumference, and strength-strain index (SSI) and regional soft tissue composition, including fat, muscle and skin CSA and muscle density, were assessed at the 20% femur (thigh) and 66% tibia (calf) sites relative to the respective distal growth plates of the non-dominant limb using pQCT (XCT 3000, Stratec Medizintechnik GmbH, Pforzheim, Germany, Division of Orthometrix; White Plains, NY, USA). Scout scans were performed to locate the distal growth plates, with the scanner programmed to find the site of interest based on relevant bone lengths. Slice thicknesses were 2.3 mm, and voxel sizes were set at 0.4 mm. Scanner speed was set at 25 mm/s. Technicians were trained for pQCT data acquisition and analyses following guidelines provided by Bone Diagnostic, Inc. (Fort Atkinson, WI, USA). Edge detection and threshold techniques were used to obtain tibia and femur circumferences and to separate tissues (i.e., adipose, muscle, and bone) based on attenuation characteristics, which are directly related to tissue composition and density [25,26]. The pQCT scanner records density on a scale where the approximate density value of fat is 0 mg/cm3, muscle 80 mg/cm3, and cortical bone 1400 mg/cm3. Hence, tissue separation thresholds were created at 40 mg/cm3 to separate fat from muscle and at 150 mg/cm3 to separate muscle from bone. Due to the relatively close density values of fat and muscle, image filters were applied so contour algorithms can better detect the edges of each item of interest. A single investigator performed all scan analyses.
Qualitative Assessment of Movement
Subject motion presents itself in images as streaking and/or broken cortical bone shells. Determining the viability of a pQCT image, or if a repeat acquisition is required, was completed by rating the level of movement. Each scan was visually inspected and rated independently by three technicians whose experience with pQCT ranged from 1–5 yrs using a linear, ordinal scale of 1 to 5 to assess the level of motion artifact present. A score of 1 represents a scan with no movement and 5 represents extreme movement such that significant image streaking and disruption of the cortical shell was present. Representative images for each rating on the scale (Figure 1) were posted for the technician’s reference next to the analysis work station. Images graded 4 or 5 are deemed to have unacceptable motion artifact for bone and soft tissue analysis and require rescanning or exclusion from analysis. For images graded 1–3, rescanning was not recommended because motion at this level was not predicted to impact analysis quality based on prior analysis experience.
Figure 1.
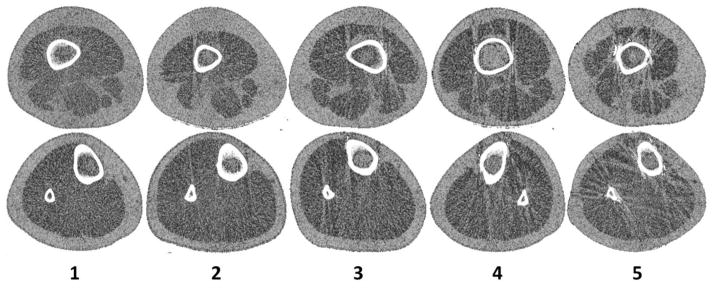
Visual inspection rating scale for femur (upper row) and tibia (lower row). Each score reflects the level of movement: 1 – none, very minimal; 2 – minimal; 3 – moderate; 4 – severe; 5 – extreme.
Quantitative Analysis for Percent Movement
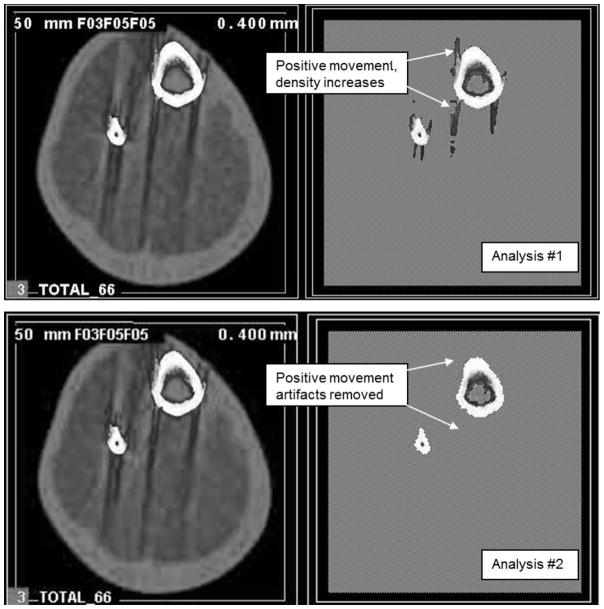
Subject movement during a scan results in movement artifacts along the edges of the bone and into soft tissue along the bone edge, where the higher bone density is shifted into the muscle area. Consequently, this analysis needs to include the whole limb so an irregular region of interest (ROI) is drawn accordingly to avoid the potential exclusion of some movement artifacts that would be excluded with the bone ROI alone. The movement can be quantitatively represented by both a positive and a negative density change in the muscle, hereafter referred to as positive and negative movement, respectively. The calculation of positive movement provides an indirect measure of movement artifact. To obtain this value, images were examined using a series of two analyses. In Analysis #1, images were filtered using contour mode 3 (−101 mg/cm3 threshold) and peel mode 2 (40 mg/cm3 threshold) to separate adipose (<40 mg/cm3) and muscle/bone (>40 mg/cm3), respectively, and then further filtered with muscle smooth 3, manufacturer image filter (F03F05F05), which is a combination of a 3×3 kernel filter and consecutive 5×5 kernel filters that enhanced the delineation between muscle and subcutaneous fat. Cortical bone density analysis mode 4 (149 mg/cm3 threshold, 40 mg/cm3 inner threshold) was then used to find all bone area plus any positive movement, concurrently removing marrow. Analysis #2 filtered images using contour mode 31 (40 mg/cm3 threshold) to separate subcutaneous fat from muscle, and peel mode 2 (40 mg/cm3 threshold) with an image filter (F03F05F05) to find marrow and any negative movement artifact area. Cortical bone density analysis mode 4 using a 710 mg/cm3 threshold separated total bone from all other tissues, while a 40 mg/cm3 inner threshold removed marrow. The difference between cortical area from Analysis #1 and cortical area from Analysis #2 yielded a value for positive movement (Figure 2). Positive movement divided by the cortical area from Analysis #2 generated a movement percentage (%Move). Positive movement density normally falls between the standard 710 threshold and 150 threshold. It is the difference between these two analysis thresholds that produces a positive movement area/percentage result. Because beam hardening artifact can look much like positive movement artifact, it is important to distinguish them. If negative movement area is close to zero, beam hardening would be indicated. However, because beam hardening is caused by excessive X-ray scatter along a dense long bone edge it is more commonly observed at metaphyseal sites where the majority of the image is bone. This should not generally be an issue at the diaphyseal site (and was not in this study) where the bone mass to soft tissue ratio is relatively small and the cortical shell is not as thick resulting in less scatter.
Figure 2.
Analyses for quantitative assessment of movement. Analysis #1 is used find all bone area plus any positive movement. Analysis #2 finds only the bone. The difference in area between these two analyses will measure the movement in the scan.
Repeat Scan Subsample
Repeat scans were performed for subjects rating 4 or 5 on the visual inspection scale. This subsample provided the opportunity to examine the effect of the level of movement on the accuracy of results. Because repeat scans can also have significant movement, only scans with little movement (rated 1 or 2) upon repeat were included in the subsample (femur, n=22; tibia, n=24).
Statistical Analysis
Descriptive statistics were calculated for the entire sample. The intra-class correlation coefficient (ICC) was used to assess the consistency, or conformity, of measurements made by the multiple raters [27]. Specifically, a two-way mixed, single measure form, ICC (3,1), was used. Pearson’s product moment correlations were calculated and linear regression analyses were performed to evaluate the relationship between the calculated %Move and the average of the three raters’ movement ranking. Paired sample t-tests were performed to check for group differences between initial scans and repeat scans in the subsample, grouping by less than 25% movement and greater than 25% movement. The selection of 25% movement was made based on post hoc analysis examining 95% confidence intervals. Technician precision, or relative technical error of measurement (%), for all clean repeat scans was calculated for all bone and soft tissue variables. All data were analyzed using the Statistical Package for the Social Sciences for Windows, Version 20.0 (SPSS, Chicago, IL, USA).
Results
Descriptives
Table 1 presents the means, SDs, and ranges of physical characteristics of the sample. Our sample was 69% non-Hispanic white (n=347), 21% Hispanic (n=107), 6% Asian American (n=31), 3% African American (n=14), and 1% Other (n=7). Based on BMI classifications suggested by the NIH [28], 2.9% were underweight, 74.3% were within the healthy weight range, 15.0% were overweight, and 7.7% were obese. Tibia and femur circumferences ranged from 210–410mm and 220–520mm, respectively, and all fell well below the instrument’s maximum circumference limit of approximately 780mm.
Table 1.
Descriptive characteristics of total sample (n=506)
| Mean ± SD† | Range | |
|---|---|---|
| Age (y) | 10.7 ± 1.1 | 9 – 13 |
| Height (cm) | 144.5 ± 9.7 | 120.3 – 171.9 |
| Weight (kg) | 39.2 ± 10.5 | 19.3 – 84.8 |
| BMI (kg/m2) | 18.5 ± 3.3 | 12.4 – 32.1 |
| BMI Percentile | 56.4 ±29.4 | 0.1 – 99.0 |
| Total Body %Fat | 27.8 ± 8.5 | 8.5 – 50.9 |
| Tibia Circumference (cm) | 28.6 ± 3.2 | 21.6 – 41.1 |
| Femur Circumference (cm) | 33.3 ± 4.5 | 22.1 – 51.8 |
Standard deviation
Qualitative Assessment/Visual Inspection
A mean movement score was determined for each subject by averaging the rating of the three technicians. This average showed a mean rating of 1.75 (SEE=.045) for the femur and 1.67 (SEE=.036) for the tibia. Over half of the scans showed little or no movement (i.e. average rating <1.7) (femur=56%, tibia=53%). A score of 4.00 for the femur and 3.33 for the tibia represented the 95th percentile. Figure 2 summarizes the distribution of the movement rating score for each rater within the 95% confidence interval and shows the variability of the visual inspection method. With the exception of rater 2 for the motion rating of 5 at the tibia, the greatest variability was observed in scans rated 3 or 4, the delineation between repeat or no repeat. Agreement between the three measurers was strong, ICC = .732 for tibia and .812 for femur.
Quantitative Assessment
Mean %Move for the femur was 9.9±10.3% with a SEE of 0.46 and a range of 3.1% to 100%. A %Move of less than 25% in the femur captured 95% of subjects. The mean %Move for the tibia was slightly higher but less variable at 12.0±6.6% with a SEE of 0.30 and a range of 6.1% to 85.2%. A movement of less than 24% in the tibia included 95% of the subjects.
Qualitative/Quantitative Relationship
Linear regression analysis showed that the average movement rating and %Move for the tibia and femur were moderately correlated (r=0.70 for tibia; r=0.67 for femur) with a SEE’s of 4.7 and 7.7 for the tibia and femur, respectively. Figure 3 illustrates the relationship for both bone sites and includes reference lines at the 95% confidence intervals.
Figure 3.
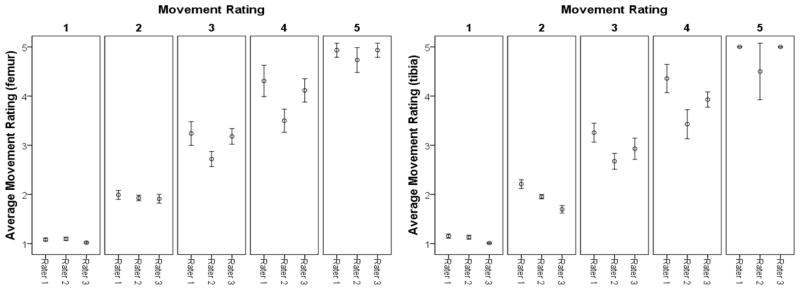
Error plots (95% confidence interval bars) illustrating the distribution of the movement rating score for each rater within the average movement rating.
Repeat Scan Subsample Quantitative Movement Comparison
Table 2 presents the mean differences of selected measurement variables at the tibia and femur for the subsample of repeat scans, which were only included if their %Move was less than that of the initial scan and less than 25% (tibia, n=24; femur, n=22). Comparison of initial tibia scans with <25% movement scans (movement = 16±4%) determined by quantitative assessment (n=13) with the paired repeat scans (movement = 11±2%) showed no significant differences (p>0.01) for all of the selected measurement variables. Eleven tibia scans with >25% movement (45±13%) compared to paired repeat scans (13±6%) showed significant differences (p<0.01) for all measures except muscle and skin CSA in the soft tissue, and cortical BMD and periosteal circumference in the bone. Initial femur scans with <25% movement (n=7; 14±4%) were found to not have any significant differences from repeat scans (9±5%) except in total and subcutaneous fat CSA and SSI. All variables were found to be significantly different between femur scans with >25% movement (n=15; 53±22%) compared to repeat scans (12±7%) except for muscle CSA. Relative technical error of measurement examining bone and soft tissue variables for clean initial and repeat scans showed the following variance for the tibia (n=11) and femur (n=7), respectively: total bone CSA, 6.5% and 4.9%; cortical CSA, 5.2% and 1.3%; cortical BMD, 2.1% and 0.8%; periosteal circumference, 7.6% and 0.6%, strength-strain index, 9.6% and 2.2%; muscle CSA, 8.6% and 2.0%; muscle density, 0.8% and 0.9%; total fat CSA, 6.3% and 10.1%; subcutaneous fat CSA, 7.4% and 11.7%; and skin CSA, 4.4% and 1.9%.
Table 2.
Mean values and mean differences (Δ) between initial and repeat scans for selected measures at the tibia and femur grouped by level of movement of initial scan.
| pQCT-derived variables | Tibia
|
Femur
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <25% MovementA (n=13) | >25% MovementB (n=11) | <25% MovementA (n=7) | >25% MovementB (n=15) | |||||||||
| Initial | Repeat | Δ | Initial | Repeat | Δ | Initial | Repeat | Δ | Initial | Repeat | Δ | |
| Soft Tissue | ||||||||||||
| Muscle CSA (mm2) | 4130.7±1102.0 | 3994.3±924.1 | 136.4±493.4 | 4102.7±831.9 | 4077.6±801.4 | 25.1±43.4 | 3806.3±871.5 | 3770.9±802.2 | 35.4±106.4 | 3926.4±672.4 | 3891.1±682.7 | 35.3±231.9 |
| Muscle Density (mg/cm3) | 79.1±1.0 | 78.9±1.2 | 0.17±0.9 | 80.5±1.4 | 79.3±1.7 | 1.19±1.1* | 77.4±1.2 | 77.2±1.7 | 0.23±1.0 | 76.8±1.5 | 75.7±1.3 | 1.11±1.2* |
| Total Fat CSA (mm2) | 2428.0±1158.6 | 2408.4±1066.3 | 19.6±224.9 | 2006.9±1032.9 | 2465.1±1132.4 | 458.2±174.1* | 1591.7±360.8 | 1814.5±407.8 | 222.8±107.9* | 2093.2±1026.6 | 2644.9±895.4 | 551.7±573.0* |
| Sub Fat CSA (mm2) | 1837.6±300.6 | 1826.2±1039.7 | 11.4±198.0 | 1481.0±939.4 | 1864.0±1029.9 | 383.0±144.3* | 1191.9±268.7 | 1378.7±326.7 | 186.8±111.0* | 1790.9±979.6 | 2218.8±853.1 | 427.9±516.9* |
| Skin CSA (mm2) | 168.3±7.1 | 166.2±5.6 | 2.1±10.7 | 164.8±22.0 | 167.5±21.6 | 2.7±3.5 | 162.4±8.2 | 165.4±20.8 | 3.1±3.2 | 173.2±17.1 | 177.3±16.3 | 4.2±4.7* |
| Bone | ||||||||||||
| Total Bone CSA (mm2) | 434.5±60.1 | 409.5±64.1 | 25.0±30.5 | 526.8±84.5 | 421.0±81.1 | 105.8±25.7* | 437.6±102.8 | 414.8±114.8 | 22.8±20.2 | 514.9±108.4 | 394.5±77.0 | 120.3±70.8* |
| Cortical CSA (mm2) | 205.9±38.8 | 210.8±39.8 | 4.9±12.7 | 187.3±34.6 | 196.6±34.9 | 9.2±6.4* | 150.6±27.4 | 152.8±27.1 | 2.2±1.8 | 146.5±20.9 | 160.4±20.4 | 13.9±10.2* |
| Cortical BMD (mg/cm3) | 1040.3±28.4 | 1051.5±25.3 | 11.2±30.9 | 1022.4±29.0 | 1027.8±31.9 | 5.4±14.4 | 1056.3±10.4 | 1061.2±13.6 | 4.9±11.4 | 1039.0±18.3 | 1052.2±15.6 | 13.2±17.0* |
| Peri Circ (mm) | 71.2±5.6 | 69.5±7.0 | 1.7±7.7 | 70.4±5.5 | 70.9±5.8 | 0.52±0.88 | 74.9±8.9 | 74.9±9.2 | 0.59±0.02 | 68.4±6.9 | 73.0±6.1 | 4.6±4.5* |
| SSI (mm3) | 1239.8±317.9 | 1221.0±86.7 | 18.7±172.0 | 1110.4±239.6 | 1174.8±266.8 | 64.5±66.9* | 1159.1±427.6 | 1187.0±422.6 | 27.9±19.1* | 997.7±204.4 | 1146.4±221.5 | 148.7±107.0* |
| Movement CSA (mm2) | 58.7±16.7 | 40.1±8.2 | 18.6±13.6 | 159.0±38.6 | 49.4±21.7 | 109.6±31.8 | 53.7±16.7 | 33.1±18.7 | 20.5±16.6 | 182.1±98.3 | 33.9±14.4 | 148.2±94.6 |
| Movement Percentage (%) | 15.7±4.2 | 11.0±2.5 | 4.7±3.5 | 44.7±13.1 | 13.7±6.4 | 31.0±11.0 | 14.2±4.4 | 8.6±4.7 | 5.6±4.6 | 58.8±44.1 | 9.4±3.4 | 49.4±43.6 |
CSA – cross-sectional area (mm2); Sub Fat – subcutaneous fat; BMD – bone mineral density (mg/cm3); Peri Circ – periosteal circumference (mm); SSI – strength-strain index (mm3)
Comparison of initial scans with <25% movement as determined by quantitative assessment with the paired repeat scans, where movement was less than 25%
Comparison of initial scans with >25% movement as determined by quantitative assessment with the paired repeat scans, where movement was less than 25%
t-test, statistically significant difference (p < 0.01)
Discussion
As the use of pQCT increases, there is greater need for standardization of its methodology. This study aimed to develop a method for standardization of one such component, the management of image quality in the presence of subject motion. The greater the movement by the subject the more severely degraded the pQCT image, but defining exactly how much movement is too much can vary between laboratories and even between raters within a laboratory. We present a method that provides a quantitative estimate of subject motion artifact for use in determining the validity of pQCT scan images of the diaphyseal bone. In addition, a careful evaluation of a commonly used methodology was performed to study its consistency and value as a qualitative metric.
Because of the subjective nature of the current prevailing procedure, where scans are visually inspected and rated, it is inherently predisposed to discordant interpretations. However, agreement between raters in this study was good (ICC=.732 for tibia and .812 for femur), indicating that the grading procedure can be considered useful [29]. These results suggest that a simple description of the grading scale along with sample rated images can produce consistent results and be considered an adequate procedure for image quality assessment. Consequently, it could be speculated that its use to date by trained technicians has yielded reliable results and minimized loss of data.
Nevertheless, the increased variability present at higher ratings shows that even with a trained technician, there will be instances in which visual inspection mischaracterizes movement. Understandably, when raters evaluate images with less movement (i.e. rated 1 or 2) consistent judging occurs more easily (Figure 2). In contrast, images with moderate to severe movement (scores of 3 and 4) are rated more variably, and although these images are less common, this issue is concerning given that this is the range where the technician must decide if rescanning is required. Although the qualitative and quantitative methods were highly correlated, classification errors are inherent in the visual inspection method. As shown in Figure 3, ratings falling in the upper left quadrant and lower right quadrant would have been incorrectly classified based on the quantitative approach.
Leaving the decision of performing a repeat measurement to subjective assessment by the scanning technician could result in loss of data if investigators later determine that the movement was too great to yield viable information. It is common to find investigations using pQCT that mention this loss of data as a result of movement [30,31,3,32,33]. According to these criteria, 1.5% (n=15) of our sample should have been rescanned but were not. Conversely, rescanning subjects when a repeat is unnecessary is also undesirable, which occurred in 2.8% (n=28) of our sample. Although the radiation exposure with pQCT is minimal, unnecessary exposure must be avoided. It is the responsibility of the investigator to ensure that radiation exposure to the subject is kept “as low as reasonably achievable” (ALARA) [34], and therefore, an objective index for determining when to repeat a scan would support this goal.
A quantitative procedure will eliminate the subjectivity and potential misjudgments by the technician’s evaluation. Implementing the procedure outlined here, where the calculation of positive movement yields a movement percentage value, provides a consistent and reliable measure of the level of motion present in a pQCT image. Establishing a specific level of movement where rescanning would be required would provide greater uniformity in the field and eliminate the subjectivity associated with the visual grading technique. Although HR-pQCT has managed to do this [22,19,20], not only is the technology and hardware not comparable, but the HR-pQCT is limited to the metaphyseal bone sites whereas this pQCT movement analysis is designed for the diaphyseal bone. In our sample, most (95%) scans had a %Move of <25%, corresponding to a score between 3.3 (tibia) and 4.0 (femur) on the visual inspection scale. Based on these results, we examined the use of 25% movement as the delineation for determining whether a scan is usable or requires repeat scanning or subsequent exclusion from data analysis.
A subsample of participants had an initial scan judged to have more than desirable movement followed by a repeat scan that showed little movement. This subsample provided the opportunity to compare a group with >25% movement to a group with <25% movement. Our finding that indices examined in the <25% group were not significantly different from the repeat scan results, whereas the >25% group showed significant differences from their repeat counterpart, suggests that in the present sample 25% movement is a viable cut point for determining if a repeat scan is required or if the data should be included in analysis.
It is not clear if a movement delineation of 25% would be generalizable given the narrow age range of the study’s participants. Because the limb sizes of children and adolescents are smaller than those of an adult, it is possible that the use of the positive movement assessment alone may not be sufficient for individuals of all ages. Nonetheless, expressing positive movement as a percentage of total size would essentially equalize limb sizes and thus make the measure more broadly applicable. Furthermore, the present population is likely one of the more movement-prone, healthy populations (age 9–13yrs). Therefore, we would expect that the use of the 25% movement cutoff would result in <5% of a healthy adult sample requiring a rescan. The procedure is also translatable to all types of movement, including muscle twitches or tremors that can be present in the elderly or diseased, but the tolerance level for movement (25%) would require reexamination.
A limitation of this study was the inability to compare multiple scans, over a controlled range of movement, to repeat scans with zero movement. Although our subsample included a wide range of movement, sample size was relatively small for any given level of movement making it difficult to pinpoint an exact percentage. Future studies of soft tissue and bone phantoms of differing size, capable of simulating varying levels of movement would be ideal. It is also important to note that the procedure is limited to the diaphyseal bone regions because of the requirement to include soft tissue in the analysis, which is not sufficiently present at metaphyseal regions. Also, because we lack an existing, independent gold standard for assessing movement, another potential limitation is shown in Figure 4, which indicates that the quantitative method may fail to detect some scans that require rescanning according to the qualitative method.
Figure 4.
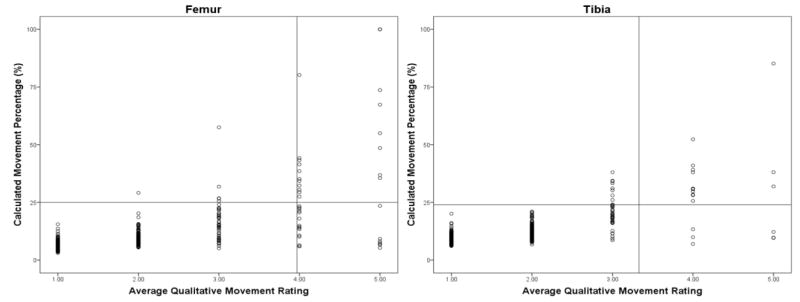
Relationship between the quantitative movement percentage and the technicians’ average movement rating. Vertical and horizontal reference lines represent 95% confidence intervals for respective variables.
These limitations notwithstanding, the presented procedure provides a feasible basis for the management of pQCT image quality in the presence of subject motion for the diaphyseal bone. Use of this quantitative approach would help standardize protocols for repeating scans across technicians and across laboratories. Furthermore, the application of the proposed quantitative approach would eliminate technician rating bias and reduce unnecessary radiation exposure to subjects as well as provide specific criteria for investigators to decide when data should be excluded. A delineation of 25% movement for determining whether a scan is viable or requires repeat is proposed for healthy populations but additional research is suggested to validate this level.
Acknowledgments
We appreciate the participation and support of principals, teachers, parents, and students from the schools in the Catalina Foothills and Marana School Districts. We also wish to thank the radiation technicians and all other members of the Jump-In study team for their contributions. The project described was supported by Award Number HD-050775 (SG) from the National Institute of Child Health and Human Development. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Child Health and Human Development or the National Institutes of Health. Grant support: National Institutes of Health grant no. HD050775.
Footnotes
Disclosure Statement:
The authors have nothing to disclose.
Disclosures
No conflicts of interest, financial or otherwise, are declared by the author(s).
The authors have stated that they have no conflict of interest.
Contributor Information
Robert M. Blew, Email: rblew@email.arizona.edu.
Vinson R. Lee, Email: vinsonl@email.arizona.edu.
Joshua N. Farr, Email: farr.joshua@mayo.edu.
Daniel J. Schiferl, Email: bone@bonediagnostic.com.
Scott B. Going, Email: going@email.arizona.edu.
References
- 1.Bachrach LK. Bare-bones fact--children are not small adults. N Engl J Med. 2004;351 (9):924–926. doi: 10.1056/NEJMe048193. [DOI] [PubMed] [Google Scholar]
- 2.Binkley TL, Berry R, Specker BL. Methods for measurement of pediatric bone. Rev Endocr Metab Disord. 2008;9 (2):95–106. doi: 10.1007/s11154-008-9073-5. [DOI] [PubMed] [Google Scholar]
- 3.Ducher G, Daly RM, Hill B, Eser P, Naughton GA, Gravenmaker KJ, Seibel MJ, Javaid A, Telford RD, Bass SL. Relationship between indices of adiposity obtained by peripheral quantitative computed tomography and dual-energy X-ray absorptiometry in pre-pubertal children. Annals of Human Biology. 2009;36 (6):705–716. doi: 10.3109/03014460903055139. [DOI] [PubMed] [Google Scholar]
- 4.Fricke O, Sumnik Z, Remer T, Stabrey A, Tutlewski B, Schoenau E. Cross-sectional fat area at the forearm in children and adolescents. Horm Res. 2008;69 (3):160–164. doi: 10.1159/000112589. [DOI] [PubMed] [Google Scholar]
- 5.Sherk VD, Bemben MG, Palmer IJ, Bemben DA. Effects of filtering methods on muscle and fat cross-sectional area measurement by pQCT: a technical note. Physiol Meas. 2011;32 (12):N65–72. doi: 10.1088/0967-3334/32/12/N01. [DOI] [PubMed] [Google Scholar]
- 6.Schoenau E, Neu CM, Beck B, Manz F, Rauch F. Bone mineral content per muscle cross-sectional area as an index of the functional muscle-bone unit. Journal of Bone and Mineral Research. 2002;17 (6):1095–1101. doi: 10.1359/jbmr.2002.17.6.1095. [DOI] [PubMed] [Google Scholar]
- 7.Ashby RL, Adams JE, Roberts SA, Mughal MZ, Ward KA. The muscle-bone unit of peripheral and central skeletal sites in children and young adults. Osteoporos Int. 2011;22 (1):121–132. doi: 10.1007/s00198-010-1216-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reid IR. Relationships between fat and bone. Osteoporosis Int. 2008;19 (5):595–606. doi: 10.1007/s00198-007-0492-z. [DOI] [PubMed] [Google Scholar]
- 9.Laaksonen MML, Sievänen H, Tolonen S, Mikkilä V, Räsänen L, Viikari J, Lehtimäki T, Kähönen M, Raitakari OT Group TCRiYFS. Determinants of bone strength and fracture incidence in adult Finns: Cardiovascular Risk in Young Finns Study (the GENDI pQCT study) Arch Osteoporos. 2010;5:119–130. [Google Scholar]
- 10.Farr JN, Funk JL, Chen Z, Lisse JR, Blew RM, Lee VR, Laudermilk M, Lohman TG, Going SB. Skeletal Muscle Fat Content Is Inversely Associated With Bone Strength in Young Girls. Journal of Bone and Mineral Research. 2011;26 (9):2217–2225. doi: 10.1002/Jbmr.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zemel B, Bass S, Binkley T, Ducher G, Macdonald H, McKay H, Moyer-Mileur L, Shepherd J, Specker B, Ward K, Hans D. Peripheral Quantitative Computed Tomography in Children and Adolescents: The 2007 ISCD Pediatric Official Positions. Journal of Clinical Densitometry. 2008;11 (1):59–74. doi: 10.1016/j.jocd.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 12.Zemel BS. Quantitative Computed Tomography and Computed Tomography in Children. Curr Osteoporos Rep. 2011;9:284–290. doi: 10.1007/s11914-011-0076-x. [DOI] [PubMed] [Google Scholar]
- 13.Augat P, Gordon CL, Lang TF, Iida H, Genant HK. Accuracy of cortical and trabecular bone measurements with peripheral quantitative computed tomography (pQCT) Phys Med Biol. 1998;43 (10):2873–2883. doi: 10.1088/0031-9155/43/10/015. [DOI] [PubMed] [Google Scholar]
- 14.Schoenau E. Problems of bone analysis in childhood and adolescence. Pediatr Nephrol. 1998;12:420–429. doi: 10.1007/s004670050479. [DOI] [PubMed] [Google Scholar]
- 15.Schoenau E, Neu CM, Rauch F, Manz F. Gender-specific pubertal changes in volumetric cortical bone mineral density at the proximal radius. Bone. 2002;31 (1):110–113. doi: 10.1016/s8756-3282(02)00802-5. [DOI] [PubMed] [Google Scholar]
- 16.Zemel B, Bass S, Binkley T, Ducher G, Macdonald H, McKay H, Moyer-Mileur L, Shepherd J, Specker B, Ward K, Hans D. Peripheral quantitative computed tomography in children and adolescents: the 2007 ISCD Pediatric Official Positions. J Clin Densitom. 2008;11 (1):59–74. doi: 10.1016/j.jocd.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 17.Rittweger J, Michaelis I, Giehl M, Wusecke P, Felsenberg D. Adjusting for the partial volume effect in cortical bone analyses of pQCT images. J Musculoskelet Neuronal Interact. 2004;4 (4):436–441. [PubMed] [Google Scholar]
- 18.Liu DM, Burrows M, Egeli D, McKay H. Site Specificity of Bone Architecture Between the Distal Radius and Distal Tibia in Children and Adolescents: An HR-pQCT Study. Calcified Tissue Int. 2010;87 (4):314–323. doi: 10.1007/s00223-010-9405-9. [DOI] [PubMed] [Google Scholar]
- 19.Pauchard Y, Liphardt AM, Macdonald HM, Hanley DA, Boyd SK. Quality control for bone quality parameters affected by subject motion in high-resolution peripheral quantitative computed tomography. Bone. 2012;50 (6):1304–1310. doi: 10.1016/j.bone.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 20.Sode M, Burghardt AJ, Pialat JB, Link TM, Majumdar S. Quantitative characterization of subject motion in HR-pQCT images of the distal radius and tibia. Bone. 2011;48 (6):1291–1297. doi: 10.1016/j.bone.2011.03.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pialat JB, Burghardt AJ, Sode M, Link TM, Majumdar S. Visual grading of motion induced image degradation in high resolution peripheral computed tomography: impact of image quality on measures of bone density and micro-architecture. Bone. 2012;50 (1):111–118. doi: 10.1016/j.bone.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 22.Pauchard Y, Ayres FJ, Boyd SK. Automated quantification of three-dimensional subject motion to monitor image quality in high-resolution peripheral quantitative computed tomography. Phys Med Biol. 2011;56 (20):6523–6543. doi: 10.1088/0031-9155/56/20/001. [DOI] [PubMed] [Google Scholar]
- 23.Farr JN, Chen Z, Lisse JR, Lohman TG, Going SB. Relationship of total body fat mass to weight-bearing bone volumetric density, geometry, and strength in young girls. Bone. 2010;46 (4):977–984. doi: 10.1016/j.bone.2009.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farr JN, Tomas R, Chen Z, Lisse JR, Lohman TG, Going SB. Lower Trabecular Volumetric BMD at Metaphyseal Regions of Weight-Bearing Bones Is Associated With Prior Fracture in Young Girls. Journal of Bone and Mineral Research. 2011;26 (2):380–387. doi: 10.1002/Jbmr.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goodpaster BH, Kelley DE, Thaete FL, He J, Ross R. Skeletal muscle attenuation determined by computed tomography is associated with skeletal muscle lipid content. J Appl Physiol. 2000;89 (1):104–110. doi: 10.1152/jappl.2000.89.1.104. [DOI] [PubMed] [Google Scholar]
- 26.Kelley DE, Slasky BS, Janosky J. Skeletal muscle density: effects of obesity and non-insulin-dependent diabetes mellitus. Am J Clin Nutr. 1991;54 (3):509–515. doi: 10.1093/ajcn/54.3.509. [DOI] [PubMed] [Google Scholar]
- 27.Shrout P, Fleiss JL. Intraclass correlation: uses in assessing rater reliability. Psychological Bulletin. 1979;86 (2):420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- 28.Barlow SE. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: Summary report. Pediatrics. 2007;120 (Supplement December):S164–S192. doi: 10.1542/peds.2007-2329C. [DOI] [PubMed] [Google Scholar]
- 29.Nunnally JC. Psychometric theory. 2. Mcgraw-Hill College; 1978. [Google Scholar]
- 30.Ducher G, Hill BL, Angeli T, Bass SL, Eser P. Comparison of pQCT parameters between ulna and radius in retired elite gymnasts: the skeletal benefits associated with long-term gymnastics are bone- and site-specific. J Musculoskel Neuron. 2009;9 (4):247–255. [PubMed] [Google Scholar]
- 31.Cervinkaa T, Hyttinena J, Sievanen H. Enhanced bone structural analysis through pQCT image preprocessing. Medical Engineering & Physics. 2010;32:398–406. doi: 10.1016/j.medengphy.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 32.Fung EB, Vichinsky EP, Kwiatkowski JL, Huang J, Bachrach LK, Sawyer AJ, Zemel BS. Characterization of low bone mass in young patients with thalassemia by DXA, pQCT and markers of bone turnover. Bone. 2011;48 (6):1305–1312. doi: 10.1016/j.bone.2011.03.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wetzsteon RJ, Shults J, Zemel BS, Gupta PU, Burnham JM, Herskovitz RM, Howard KM, Leonard MB. Divergent effects of glucocorticoids on cortical and trabecular compartment BMD in childhood nephrotic syndrome. J Bone Miner Res. 2009;24 (3):503–513. doi: 10.1359/jbmr.081101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hendee WR, Edwards FM. ALARA and an integrated approach to radiation protection. Semin Nucl Med. 1986;16 (2):142–150. doi: 10.1016/s0001-2998(86)80027-7. [DOI] [PubMed] [Google Scholar]