Abstract
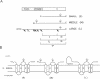
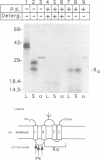
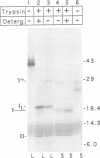
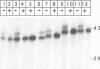
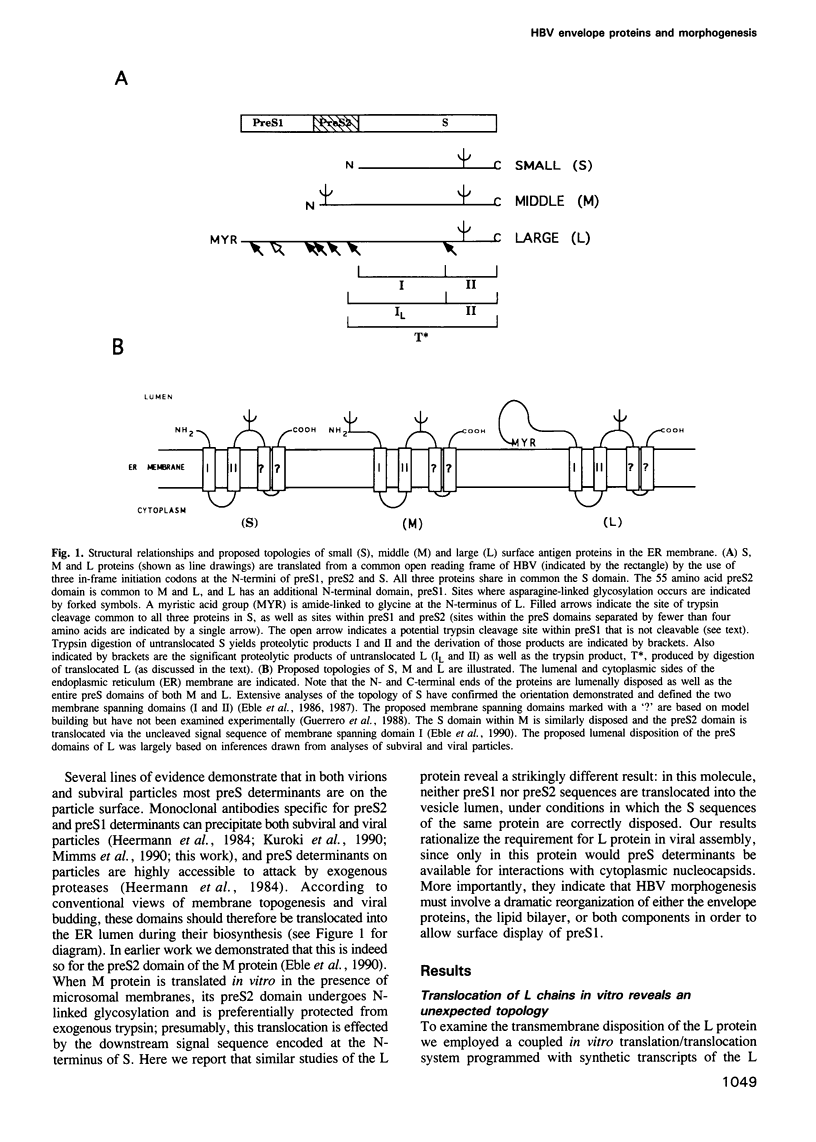
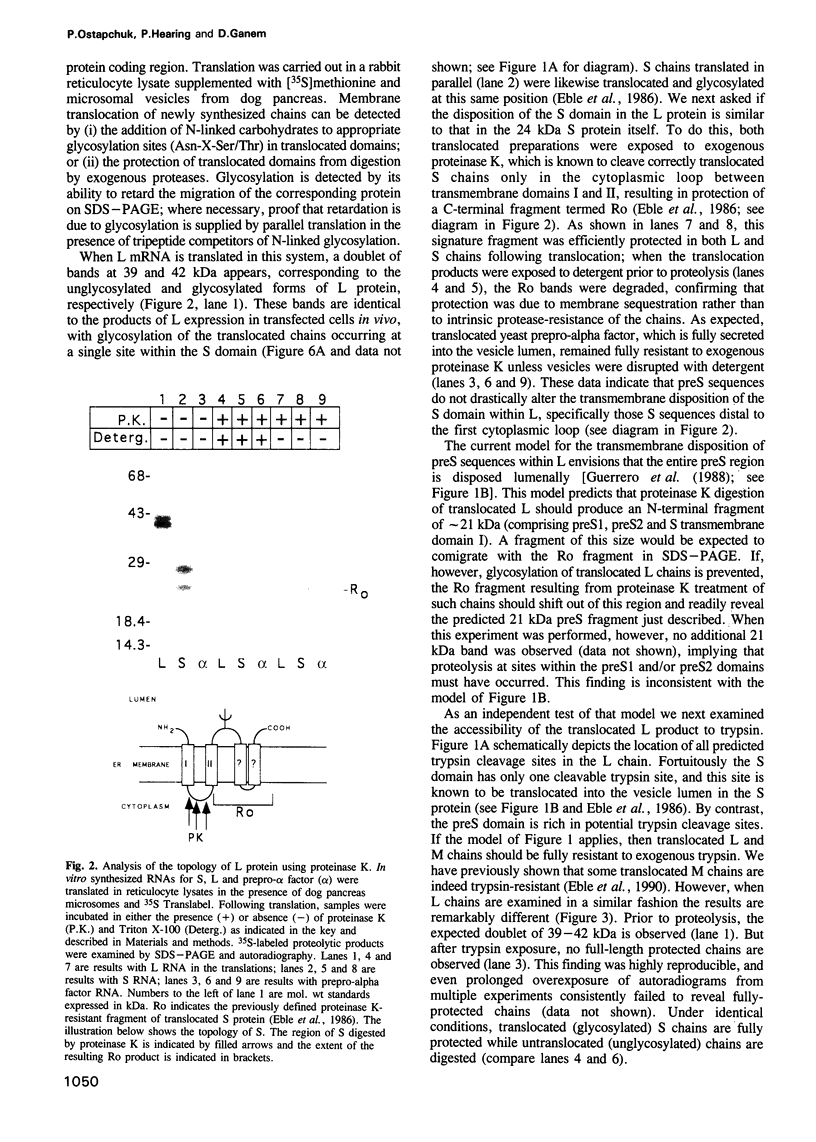
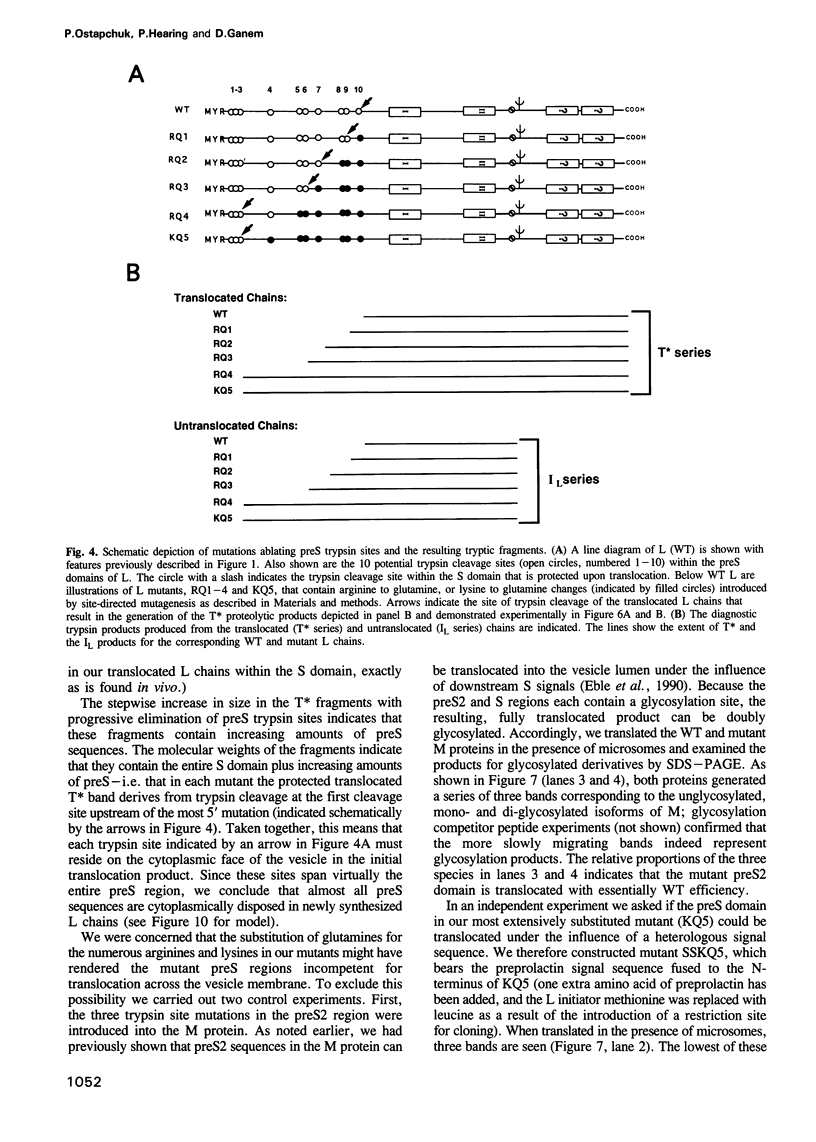
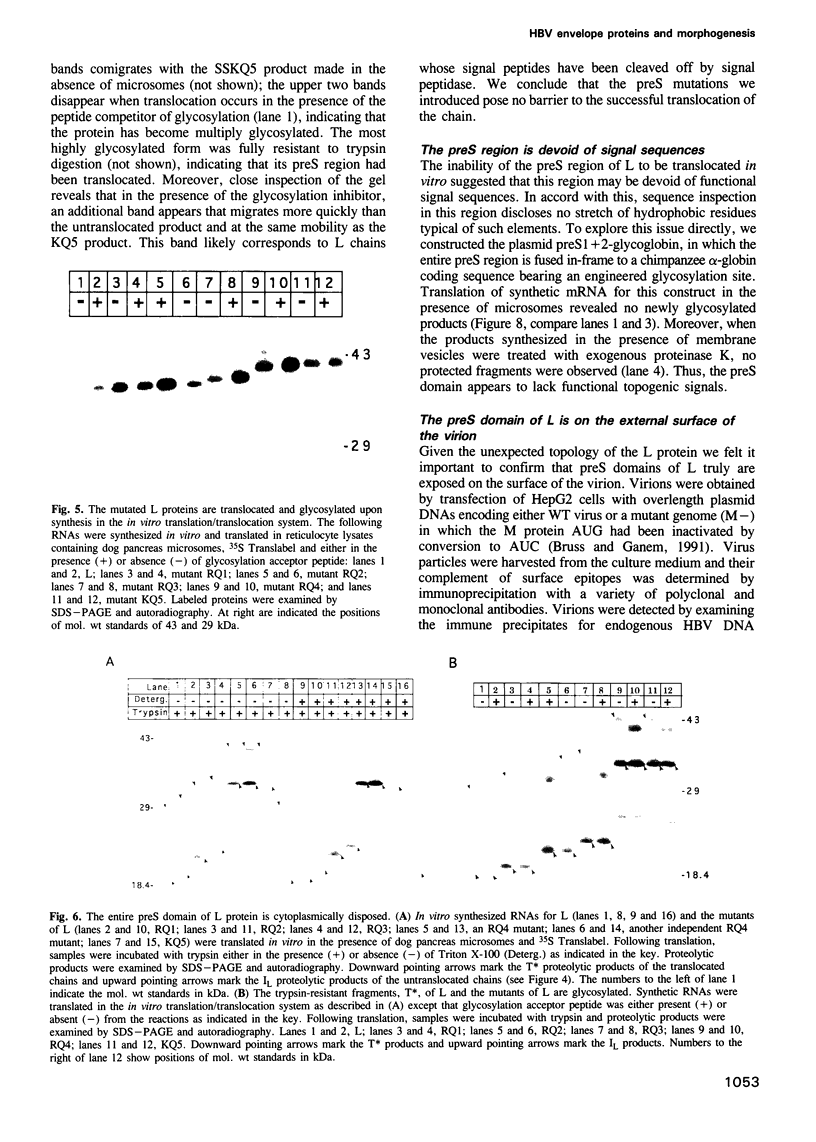
The envelope of hepatitis B virus contains three related glycoproteins (termed L, M and S) produced by alternative translation initiation in a single coding region. The smallest of these, the S protein, is a 24 kDa glycoprotein with multiple transmembrane domains. The M and L proteins contain the entire S domain at their C-termini, but harbor at their N-terminal additional (preS) domains of 55 or 174 amino acids, respectively. Most of these preS residues are displayed on the surface of mature virions and hence would be expected to be translocated into the endoplasmic reticulum (ER) lumen during biosynthesis. Using a coupled, in vitro translation/translocation system we now demonstrate that, contrary to expectation, virtually all preS residues of the L protein are cytoplasmically disposed in the initial translocation product. This includes some preS sequences which in the M protein are indeed translocated into the ER lumen. Since preS sequences are found on the external surface of the virion envelope, our results indicate that during or following budding a dramatic reorganization of either the envelope proteins or the lipid bilayer (or both components) must occur to allow surface display of these sequences. These findings imply that some membrane budding events can have remarkable and previously unsuspected topological consequences.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bruss V., Ganem D. The role of envelope proteins in hepatitis B virus assembly. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):1059–1063. doi: 10.1073/pnas.88.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dane D. S., Cameron C. H., Briggs M. Virus-like particles in serum of patients with Australia-antigen-associated hepatitis. Lancet. 1970 Apr 4;1(7649):695–698. doi: 10.1016/s0140-6736(70)90926-8. [DOI] [PubMed] [Google Scholar]
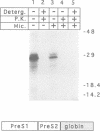
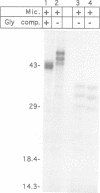
- Eble B. E., Lingappa V. R., Ganem D. Hepatitis B surface antigen: an unusual secreted protein initially synthesized as a transmembrane polypeptide. Mol Cell Biol. 1986 May;6(5):1454–1463. doi: 10.1128/mcb.6.5.1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eble B. E., Lingappa V. R., Ganem D. The N-terminal (pre-S2) domain of a hepatitis B virus surface glycoprotein is translocated across membranes by downstream signal sequences. J Virol. 1990 Mar;64(3):1414–1419. doi: 10.1128/jvi.64.3.1414-1419.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eble B. E., MacRae D. R., Lingappa V. R., Ganem D. Multiple topogenic sequences determine the transmembrane orientation of the hepatitis B surface antigen. Mol Cell Biol. 1987 Oct;7(10):3591–3601. doi: 10.1128/mcb.7.10.3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller S. D. The T=4 envelope of Sindbis virus is organized by interactions with a complementary T=3 capsid. Cell. 1987 Mar 27;48(6):923–934. doi: 10.1016/0092-8674(87)90701-x. [DOI] [PubMed] [Google Scholar]
- Ganem D., Varmus H. E. The molecular biology of the hepatitis B viruses. Annu Rev Biochem. 1987;56:651–693. doi: 10.1146/annurev.bi.56.070187.003251. [DOI] [PubMed] [Google Scholar]
- Gavilanes F., Gonzalez-Ros J. M., Peterson D. L. Structure of hepatitis B surface antigen. Characterization of the lipid components and their association with the viral proteins. J Biol Chem. 1982 Jul 10;257(13):7770–7777. [PubMed] [Google Scholar]
- Heermann K. H., Goldmann U., Schwartz W., Seyffarth T., Baumgarten H., Gerlich W. H. Large surface proteins of hepatitis B virus containing the pre-s sequence. J Virol. 1984 Nov;52(2):396–402. doi: 10.1128/jvi.52.2.396-402.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huovila A. P., Eder A. M., Fuller S. D. Hepatitis B surface antigen assembles in a post-ER, pre-Golgi compartment. J Cell Biol. 1992 Sep;118(6):1305–1320. doi: 10.1083/jcb.118.6.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroki K., Floreani M., Mimms L. T., Ganem D. Epitope mapping of the PreS1 domain of the hepatitis B virus large surface protein. Virology. 1990 Jun;176(2):620–624. doi: 10.1016/0042-6822(90)90032-m. [DOI] [PubMed] [Google Scholar]
- Liu C. C., Yansura D., Levinson A. D. Direct expression of hepatitis B surface antigen in monkey cells from an SV40 vector. DNA. 1982;1(3):213–221. doi: 10.1089/dna.1.1982.1.213. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimms L. T., Floreani M., Tyner J., Whitters E., Rosenlof R., Wray L., Goetze A., Sarin V., Eble K. Discrimination of hepatitis B virus (HBV) subtypes using monoclonal antibodies to the PreS1 and PreS2 domains of the viral envelope. Virology. 1990 Jun;176(2):604–619. doi: 10.1016/0042-6822(90)90031-l. [DOI] [PubMed] [Google Scholar]
- Patzer E. J., Nakamura G. R., Simonsen C. C., Levinson A. D., Brands R. Intracellular assembly and packaging of hepatitis B surface antigen particles occur in the endoplasmic reticulum. J Virol. 1986 Jun;58(3):884–892. doi: 10.1128/jvi.58.3.884-892.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persing D. H., Varmus H. E., Ganem D. Inhibition of secretion of hepatitis B surface antigen by a related presurface polypeptide. Science. 1986 Dec 12;234(4782):1388–1391. doi: 10.1126/science.3787251. [DOI] [PubMed] [Google Scholar]
- Simon K., Lingappa V. R., Ganem D. Secreted hepatitis B surface antigen polypeptides are derived from a transmembrane precursor. J Cell Biol. 1988 Dec;107(6 Pt 1):2163–2168. doi: 10.1083/jcb.107.6.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K., Garoff H. The budding mechanisms of enveloped animal viruses. J Gen Virol. 1980 Sep;50(1):1–21. doi: 10.1099/0022-1317-50-1-1. [DOI] [PubMed] [Google Scholar]
- Stephens E. B., Compans R. W. Assembly of animal viruses at cellular membranes. Annu Rev Microbiol. 1988;42:489–516. doi: 10.1146/annurev.mi.42.100188.002421. [DOI] [PubMed] [Google Scholar]
- Ueda K., Tsurimoto T., Matsubara K. Three envelope proteins of hepatitis B virus: large S, middle S, and major S proteins needed for the formation of Dane particles. J Virol. 1991 Jul;65(7):3521–3529. doi: 10.1128/jvi.65.7.3521-3529.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigler M., Silverstein S., Lee L. S., Pellicer A., Cheng Y. c., Axel R. Transfer of purified herpes virus thymidine kinase gene to cultured mouse cells. Cell. 1977 May;11(1):223–232. doi: 10.1016/0092-8674(77)90333-6. [DOI] [PubMed] [Google Scholar]