Abstract
Suberoylanilidehydroxamic acid (SAHA), or vorinostat, is a histone deacetylase inhibitor approved for use as chemotherapy for lymphoma in humans. The goal of this study was to establish pharmacological parameters of SAHA in cats. Our interest in treating cats with SAHA is two-fold: as an anti-cancer chemotherapeutic and as anti-latency therapy for feline retroviral infections. Relying solely on data from studies in other animals would be inappropriate as SAHA is partially metabolized by glucuronidation, which is absent in feline metabolism. SAHA was administered to cats intravenously (2 mg/kg) or orally (250 mg/m2, ~17 mg/kg) in a cross-over study design. Clinically, SAHA was well tolerated at these dosages as no abnormalities were noted following administration. The pharmacokinetics of SAHA in cats was found to be similar to that of dogs, but the overall serum drug exposure was much less than that of humans at an equivalent dose. The pharmacodynamic effect of an increase in acetylated histone proteins in blood was detected after both routes of administration. An increased oral dose of 60 mg SAHA/kg administered to one animal resulted in a surprisingly modest increase in peak drug concentration, suggesting possible saturation of absorption kinetics. This study provides a foundation for future studies of the clinical efficacy of SAHA in treating feline disease.
Keywords: SAHA, vorinostat, feline/cat, pharmacokinetics, pharmacodymanics
Short Communication
Suberoylanilidehydroxamic acid (SAHA), or vorinostat, is a pan-histone deacetylase inhibitor (HDACi) approved by the FDA for the treatment of cutaneous T-cell lymphoma in humans(Mann et al., 2007). Treatment with SAHA results in histone hyperacetylation, activating tumor-suppressor and pro-apoptotic genes in neoplastic cells(Kavanaugh et al., 2010; Boumber & Issa, 2011). Histone hyperacetylation has also been shown to reactivate latent human immunodeficiency virus(HIV) in in vivo-derived T-cell reservoirs(Richman et al., 2009; Margolis, 2011). Consequently, SAHA is currently under investigation as a treatment for many different human cancers and as anti-latency therapy for HIV infection (Lane & Chabner, 2009; Archin et al., 2012). SAHA has similarly been shown in our laboratory to induce histone hyperacetylation in feline leukocytes, and is able to reactivate latent feline immunodeficiency virus(FIV)in vitro(McDonnel et al., 2012) (1). Lymphoma is the most common malignancy diagnosed in domestic cats, accounting for 30% of all feline tumors (Ettinger, 2003). Therefore, the interest in treating cats with SAHA is two-fold: as an anti-cancer chemotherapeutic and as anti-latency therapy for the reactivation of latent feline immunodeficiency virus infection.
SAHA is partially metabolized by glucuronidation in humans and other animals(Sandhu et al., 2007; Balliet et al., 2009; Wong et al., 2011). As a result, pharmacokinetic and safety profiles for this drug are not directly translatable to cats, which lack the enzyme UDP glucuronosyl transferase responsible for glucuronidation. Before proceeding with in vivo investigation or clinical trials for feline cancer or FIV, the pharmacokinetic (PK) and pharmacodynamic (PD) properties of SAHA in domestic cats must be established.
All animal procedures conformed to the requirements of the Animal Welfare Act and protocols were approved by the University of California Davis IACUC. SAHA (>98% pure, Cayman Chemical) was administered to 6 adult (3 female and 3 male, aged 10 to 18 months) specific pathogen free cats from the UC Davis Feline Nutrition and Pet Care Center in a crossover design. Animals were randomized to first receive 2 mg/kg intravenous (IV) SAHA (solvated at 20 mg/mL in DMSO) or 250 mg/m2 oral (PO) SAHA in gelatin capsules, followed by the opposite route after a 1-week washout period (cross-over study design). Blood was collected from peripheral veins at 0, 5, 7.5, 10, 20, 30, 45, 60, 120, and 1440 min post IV administration, and at 0, 10, 15, 30, 45, 60, 120, 240, 360, and 1440min post PO administration. To monitor for adverse effects, physical examinations, complete blood counts(CBC), and serum biochemistry values were assessed 1 week prior (as baseline), 24 hours post, and 1 week post SAHA administration. The only significant findings on physical examination were moderate tachypnea in 4/6 cats and open-mouth breathing in 2/6 cats immediately following IV injection, which may have been attributable to handling stress or the DMSO used to solvate the SAHA. Moderate bleeding (up to 5 minutes) from venipuncture sites was also noted in 2/6 cats after IV administration of the SAHA/DMSO mixture. No clinically significant alterations from baseline were detected in any of the 14 CBC parameters or 16 serum biochemistry values assessed at either 24 hr or 7 days post IV or PO dosage (Supplementary Figure 1). In addition, daily food intakes were monitored for 1 week before and 1 week after each dosage. No significant change in food intake following drug administration was observed (Figure 1). Taken together, these data suggest that SAHA is well tolerated at these dosages and routes of administration in domestic cats.
Figure 1.
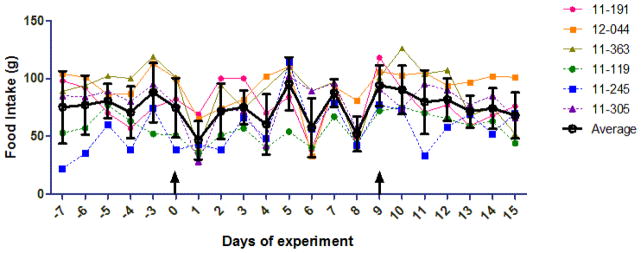
Food intake before and after suberoylanilidehydroxamic acid (SAHA) administration to cats. Cat numbers are shown on the right, and the average and standard deviation for each day are superimposed on the plot (black lines and circles). The arrow at day 0 indicates the first dosage of SAHA (PO for 11-191, 12-044, and 11-363; IV for 11-119, 11-245, 11-305), and the second arrow at day 9 indicates the cross-over dosage. Data are unavailable for days -1 and -2 before the experiment. A one-way ANOVA followed by Kruskal-Wallis post-test found no significant difference between any two pairs of days.
For pharmacokinetic analysis, serum was collected at the time-points listed above in blood tubes lacking an anticoagulant and frozen at −70 °C (up to one week) until analysis by liquid chromatography tandem mass spectrometry (LC-MS/MS) as described previously (Patel et al., 2008).Briefly, deuterated SAHA (d5, Toronto Research Chemicals) was added to serum at a final concentration of 1000 ng/mL as an internal standard (IS), and following a brief incubation, protein was extracted with 3 volumes of acetonitrile. Compounds were separated on a Hypersil BDS C18 (100L x 3.0mm I.D., Phenomenex) and 3 μm particle size protected by a HyPURITY C18 (10L x 3.0mm I.D., Phenomenex) and 3 μm particle size guard cartridge. The Waters ultra-high pressure liquid chromatography (UPLC) system was coupled to a Thermo TSQ Quantum Discovery Max tandem quadrupole mass spectrometer with a heated electrospray ionization source operating in the positive ion mode. Concentrations of 0, 1, 10, 50, 100, 250, 500, and 1000 ng/mL SAHA with added IS (1000 ng/mL SAHA-d5) were freshly prepared in water as a standard curve for each run. Quality control (QC) samples were prepared in SPF feline serum at 0, 10, 50, and 500 ng/mL SAHA with added IS. The peak area ratios of SAHA/SAHA-d5 were used for all quantitation and to construct calibration curves. The limit of detection and limit of quantitation using this method were 0.935 and 2.26 ng/mL respectively. Serum [SAHA] at the time points analyzed following IV and PO administration is displayed in Figure 2. Pharmacokinetic parameters (Table 1) were calculated using a commercial software program (WinNonlin version 5.2) with non-compartmental analysis.
Figure 2.
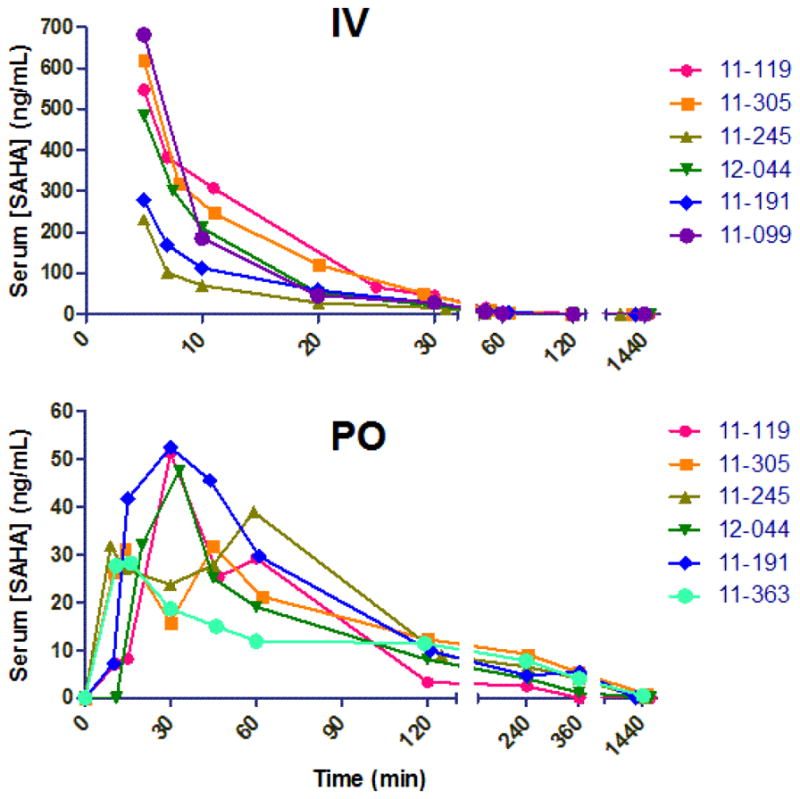
Suberoylanilidehydroxamic acid (SAHA) concentration profiles versus time for 6 cats administered 2 mg/kg IV (A) or 250 mg/m2orally (PO) (B). Individual cat numbers are shown on the right, and time 0 indicates the time of administration. Serum samples were analyzed by UPLC coupled to tandem mass spectrometry using deutered SAHA as an internal standard. Axes are broken to visualize all time points analyzed.
Table 1.
Calculated mean ± standard deviation non-compartmental pharmacokinetic parameters for serum suberoylanilidehydroxamic acid (SAHA) concentration data after IV and PO administration to adult cats (n=6). Doses and administration routes were 2 mg/kg intravenous (IV) SAHA (solvated at 20 mg/mL in DMSO) or 250 mg/m2 oral (PO) SAHA in gelatin capsules.
| Parameter | IV | PO |
|---|---|---|
| Dose | 2 mg/kg | 250 mg/m2 = 17±2 mg/kg |
| T1/2λz (hr) | 0.15 ± 0.03 | 2 ±1 |
| Clobs (mL/hr/kg) | 14,000 ± 5,000 | - |
| VDss,obs (mL/kg) | 1600 ± 800 | - |
| Cmaxobs (ng/mL) | - | 40 ± 10 |
| Tmaxobs (hr) | - | 0.6 ± 0.3 |
| AUC0 → τ (hr*ng/mL) | 150 ± 40 | 70 ± 10 |
| AUC0 → ∞ (hr*ng/mL) | 150 ± 50 | 80 ± 20 |
| Percent Extrapolated (%) | 0.6 ± 0.2 | 17 ± 7 |
| F (%), n=5 | - | 8 ± 4 |
T1/2λz, elimination half-life, CLobs: Observed clearance, VDss,obs: Observed volume of distribution at steady state, Cmax(obs), observed maximum serum concentration; Tmax(obs), time to observed maximal serum concentration; AUC0 → τ area under the serum concentration vs. time curve to the last value; AUC0 → ∞ area under the serum concentration vs. time curve extrapolated to infinity; F(%): oral bioavailability
In this study, the serum terminal elimination half-life (T1/2λz) after 2 mg/kg IV SAHA administration to cats was relatively rapid (9 min), but was similar to that for both dogs and rats (12 min) at the same dosage (Sandhu et al., 2007).The maximum concentration (Cmax) observed after oral administration of 250 mg/m2(~16.8 mg/kg) SAHA was 41.7 ng/mL (160 nM), with an overall serum exposure (AUC0-τ) of 70 hr*ng/mL (266hr*nM).In previous studies, dogs administered SAHA at a slightly higher dose (20mg/kg), achieved an average Cmax of 260 nM and AUC0-τ of 420 hr*nM(Sandhu et al., 2007). However, there is increased oral bioavailability in the cat (8%) relative to the dog (1.8%). Because SAHA is rarely administered intravenously to humans, very few studies report bioavailability. However, it has been estimated at 43% in one report of patients with advanced cancer(Kelly et al., 2005). Most human studies report a Cmax of 1–1.5 μM at similar dosages to those used here on a body surface area basis (400 mg/day or ~250 mg/m2)(Rubin et al., 2006; Ramalingam et al., 2010; Doi et al., 2013). Surprisingly, the half-life of SAHA in cats following IV administration is much less than that of humans (21–58 min) (Kelly et al., 2003; Kelly et al., 2005), despite feline deficiencies inglucuronidation. To summarize, the pharmacokinetics of SAHA in cats is similar to that of dogs, but the overall serum drug exposure is much less than that of humans at an equivalent dose.
For pharmacodynamic analysis, ~0.5 mL blood was collected in EDTA-containing tubes from 4 of the cats at 0, 30, 45, 60, 120, and 1440 min post IV and 0, 30, 60, 120, 360, and 1440 min post PO administration. RBC were lysed and leukocyte pellets were snap frozen for later analysis by Western blot as previously described (McDonnel et al., 2012) (2). Blots were probed with anti-acetylated histone (H3K9,14, Millipore) or anti-total histone (H3, Cell Signaling Technologies), followed by quantitative analysis using densitometry (Figure 3). An increase in acetylated histone protein was detected after both IV and PO administration of SAHA to cats, which returned to baseline after approximately 2 hours.
Figure 3.
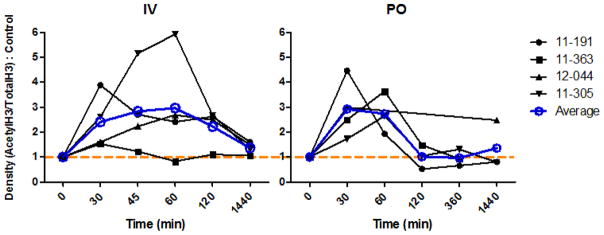
Pharmacodynamic profiles of SAHA in 4 cats administered 2 mg/kg IV (A) or 250 mg/m2 orally (PO) (B). Leukocytes collected at various time points after SAHA administration were analyzed by Western blotting for both acetylated and total histone H3. Densitometry ratios of acetyl H3/total H3 were normalized to pre-dose controls (time 0). Values above the dashed orange line (1) have a relative increase in histone H3 acetylation. Cat numbers are shown on the right, and the average for each time point is superimposed on the plot (blue lines and circles). X-axis time scale is not proportional.
In an attempt to increase Cmax and serum SAHA exposure, a single pilot animal (#11–245) was administered an increased oral dose of 60 mg/kg in gelatin capsules, more than 3x the original dose. This dose was chosen because it was the no observed adverse effects level (NOAEL) in dogs (Kerr et al., 2010).Serum was collected at a limited number of time points(0, 30, 45, 60, 120, and 1440 min), and analyzed by LC-MS/MS as above. Even at this elevated dosage, the observed Cmax in this animal increased by a modest 29% (from 39.0 to 50.1 ng/ml, Fig. 4a), suggesting the possibility of saturation of absorption kinetics, though additional animals would be required to draw conclusions. Peripheral blood mononuclear cells collected at 0, 60, 120, and 1440 min were analyzed by Western blotting as above, and demonstrated a pharmacodynamic effect which roughly correlated with pharmacokinetics (Fig. 4b).
Figure 4.
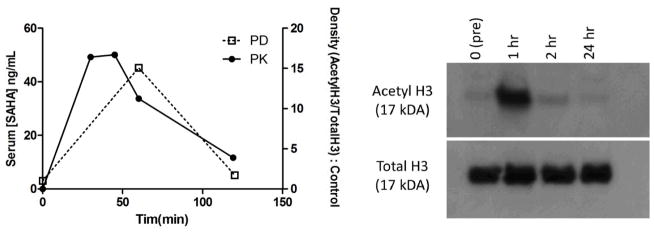
Pharmacokinetics (PK) and pharmacodynamics (PD) of SAHA administered orally at 60 mg/m2 to cat #11-245. Serum [SAHA] (solid line, left y-axis) was analyzed as in Fig. 2, and is superimposed with histone acetylation data (dashed line, right y-axis) in leukocytes analyzed as in Fig. 3 (A). The corresponding Western blot images are shown in (B).
In conclusion, SAHA is a member of a novel class of drugs, HDACi, which may provide valuable therapeutics for both neoplastic and infectious diseases of cats. Here we evaluated the pharmacokinetic and pharmacodynamic properties of SAHA in cats. The serum half-life was unexpectedly rapid, indicating that other metabolic or excretory pathways may be compensating for the lack of glucuronidation in cats. As in the dog, oral bioavailability was generally poor, and lower drug concentrations were achieved in cats compared with humans administered similar dosages. In a single pilot animal, increasing the oral dose three-fold had little effect on serum SAHA exposure. However, the serum drug concentrations observed here (~160 nM), may still be within therapeutic range (McDonnel et al., 2012) (1).It is worth noting that pure (>98%) SAHA was used for this study, rather than pharmaceutical compound with the excipients microcrystalline cellulose, sodium croscarmellose and magnesium stearate (package insert, Zolinza®, Merck&Co INC). So while it is possible that the pharmaceutical compound may result in increased bioavailability in cats, similarities between PK parameters found here and those in the Merck study in dogs suggest that the excipients may not make a significant difference. Although food intake has not been shown to substantially increase absorption in humans (Kelly et al., 2005; Rubin et al., 2006), administration of SAHA to cats in the fed stateis an avenue of further exploration as food may have a different effect in the more acidic gastrointestinal system of a carnivore. An alternative route of administration such as subcutaneous or constant rate IV infusion may provide more optimal drug concentrations in cats. This study demonstrates that both oral and IV-administered SAHA are well tolerated in cats and produce the predicted pharmacodynamic effect of histone acetylation, thereby laying the foundation for future studies of the clinical efficacy of SAHA in treating feline disease.
Supplementary Material
Acknowledgments
The authors are grateful for the research support provided by Scott Wetzlich, Andrea Fascetti, VMD, PhD, DACVIM, DACVN, Laurie Brignolo, DVM, DACLAM, and Alan Buckpitt, PhD, and the excellent animal care provided by Deborah Bee and the staff at the University of California Davis Nutrition and Pet Care center. Funding for this study was provided by the Center for Companion Animal Health, School of Veterinary Medicine, University of California, Davis, and NIH T32 training grants #5TL1RR024145 and #5T32AI060555.
References
- Archin NM, Liberty AL, Kashuba AD, Choudhary SK, Kuruc JD, Crooks AM, Parker DC, Anderson EM, Kearney MF, Strain MC, Richman DD, Hudgens MG, Bosch RJ, Coffin JM, Eron JJ, Hazuda DJ, Margolis DM. Administration of vorinostat disrupts HIV-1 latency in patients on antiretroviral therapy. Nature. 2012;487(7408):482–485. doi: 10.1038/nature11286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balliet RM, Chen G, Gallagher CJ, Dellinger RW, Sun D, Lazarus P. Characterization of UGTs active against SAHA and association between SAHA glucuronidation activity phenotype with UGT genotype. Cancer Res. 2009;69(7):2981–2989. doi: 10.1158/0008-5472.CAN-08-4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boumber Y, Issa JP. Epigenetics in cancer: what’s the future? Oncology (Williston Park) 2011;25(3):220–226. 228. [PubMed] [Google Scholar]
- Doi T, Hamaguchi T, Shirao K, Chin K, Hatake K, Noguchi K, Otsuki T, Mehta A, Ohtsu A. Evaluation of safety, pharmacokinetics, and efficacy of vorinostat, a histone deacetylase inhibitor, in the treatment of gastrointestinal (GI) cancer in a phase I clinical trial. Int J Clin Oncol. 2013;18(1):87–95. doi: 10.1007/s10147-011-0348-6. [DOI] [PubMed] [Google Scholar]
- Ettinger SN. Principles of treatment for feline lymphoma. Clin Tech Small Anim Pract. 2003;18(2):98–102. doi: 10.1053/svms.2003.36623. [DOI] [PubMed] [Google Scholar]
- Kavanaugh SM, White LA, Kolesar JM. Vorinostat: A novel therapy for the treatment of cutaneous T-cell lymphoma. Am J Health Syst Pharm. 2010;67(10):793–797. doi: 10.2146/ajhp090247. [DOI] [PubMed] [Google Scholar]
- Kelly WK, O’Connor OA, Krug LM, Chiao JH, Heaney M, Curley T, MacGregore-Cortelli B, Tong W, Secrist JP, Schwartz L, Richardson S, Chu E, Olgac S, Marks PA, Scher H, Richon VM. Phase I study of an oral histone deacetylase inhibitor, suberoylanilide hydroxamic acid, in patients with advanced cancer. J Clin Oncol. 2005;23(17):3923–3931. doi: 10.1200/JCO.2005.14.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly WK, Richon VM, O’Connor O, Curley T, MacGregor-Curtelli B, Tong W, Klang M, Schwartz L, Richardson S, Rosa E, Drobnjak M, Cordon-Cordo C, Chiao JH, Rifkind R, Marks PA, Scher H. Phase I clinical trial of histone deacetylase inhibitor: suberoylanilide hydroxamic acid administered intravenously. Clin Cancer Res. 2003;9(10 Pt 1):3578–3588. [PubMed] [Google Scholar]
- Kerr JS, Galloway S, Lagrutta A, Armstrong M, Miller T, Richon VM, Andrews PA. Nonclinical safety assessment of the histone deacetylase inhibitor vorinostat. Int J Toxicol. 2010;29(1):3–19. doi: 10.1177/1091581809352111. [DOI] [PubMed] [Google Scholar]
- Lane AA, Chabner BA. Histone deacetylase inhibitors in cancer therapy. J Clin Oncol. 2009;27(32):5459–5468. doi: 10.1200/JCO.2009.22.1291. [DOI] [PubMed] [Google Scholar]
- Mann BS, Johnson JR, Cohen MH, Justice R, Pazdur R. FDA approval summary: vorinostat for treatment of advanced primary cutaneous T-cell lymphoma. Oncologist. 2007;12(10):1247–1252. doi: 10.1634/theoncologist.12-10-1247. [DOI] [PubMed] [Google Scholar]
- Margolis DM. Histone deacetylase inhibitors and HIV latency. Curr Opin HIV AIDS. 2011;6(1):25–29. doi: 10.1097/COH.0b013e328341242d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnel SJ, Sparger EE, Luciw PA, Murphy BG. Pharmacologic reactivation of latent feline immunodeficiency virus ex vivo in peripheral CD4+ T-lymphocytes. Virus Res. 2012;170(1–2):174–179. doi: 10.1016/j.virusres.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnel SJ, Sparger EE, Luciw PA, Murphy BG. Transcriptional Regulation of Latent Feline Immunodeficiency Virus in Peripheral CD4+ T-lymphocytes. Viruses. 2012;4(5):878–888. doi: 10.3390/v4050878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel K, Guichard SM, Jodrell DI. Simultaneous determination of decitabine and vorinostat (Suberoylanalide hydroxamic acid, SAHA) by liquid chromatography tandem mass spectrometry for clinical studies. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;863(1):19–25. doi: 10.1016/j.jchromb.2007.12.014. [DOI] [PubMed] [Google Scholar]
- Ramalingam SS, Kummar S, Sarantopoulos J, Shibata S, LoRusso P, Yerk M, Holleran J, Lin Y, Beumer JH, Harvey RD, Ivy SP, Belani CP, Egorin MJ. Phase I study of vorinostat in patients with advanced solid tumors and hepatic dysfunction: a National Cancer Institute Organ Dysfunction Working Group study. J Clin Oncol. 2010;28(29):4507–4512. doi: 10.1200/JCO.2010.30.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richman DD, Margolis DM, Delaney M, Greene WC, Hazuda D, Pomerantz RJ. The challenge of finding a cure for HIV infection. Science. 2009;323(5919):1304–1307. doi: 10.1126/science.1165706. [DOI] [PubMed] [Google Scholar]
- Rubin EH, Agrawal NG, Friedman EJ, Scott P, Mazina KE, Sun L, Du L, Ricker JL, Frankel SR, Gottesdiener KM, Wagner JA, Iwamoto M. A study to determine the effects of food and multiple dosing on the pharmacokinetics of vorinostat given orally to patients with advanced cancer. Clin Cancer Res. 2006;12(23):7039–7045. doi: 10.1158/1078-0432.CCR-06-1802. [DOI] [PubMed] [Google Scholar]
- Sandhu P, Andrews PA, Baker MP, Koeplinger KA, Soli ED, Miller T, Baillie TA. Disposition of vorinostat, a novel histone deacetylase inhibitor and anticancer agent, in preclinical species. Drug Metab Lett. 2007;1(2):153–161. doi: 10.2174/187231207780363642. [DOI] [PubMed] [Google Scholar]
- Wong NS, Seah E, Wang LZ, Yeo WL, Yap HL, Chuah B, Lim YW, Ang PC, Tai BC, Lim R, Goh BC, Lee SC. Impact of UDP-gluconoryltransferase 2B17 genotype on vorinostat metabolism and clinical outcomes in Asian women with breast cancer. Pharmacogenet Genomics. 2011;21(11):760–768. doi: 10.1097/FPC.0b013e32834a8639. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


