Abstract
Phosphate signaling and acquisition are critical for the bacterial response to phosphate limitation, and bacteria express multiple factors to scavenge phosphate. We previously found that multidrug-resistant strains of Pseudomonas aeruginosa from critically ill patients can form unusual outer-surface appendages harboring PstS proteins. Here, we have expanded our investigation to DING proteins that like PstS belong to the family of high-affinity phosphate-binding proteins but have strong similarity with eukaryotic DING proteins. We demonstrate the localization of DING on PstS-containing outer-surface appendages in both multidrug-resistant strain MDR25 and the PA14 strain of P. aeruginosa. However, the number of cells producing appendages and the amount of appendages on each cell in PA14 were found to be negligible, unless overexpression of either PstS or DING was achieved by transformation with constructed plasmids. We further noticed that DING expression under low phosphate conditions was significantly higher in MDR25 compared to PA14 which may explain the greater abundance of appendages in MDR25. Our finding that DING proteins are localized on extracellular appendages provides an opportunity to study the interaction of bacterial DING with host proteins by mimicking the action of host DINGs.
Keywords: PstS/DING proteins, cellular appendages, Pseudomonas aeruginosa
Introduction
Inorganic phosphate is the ultimate limiting nutrient for bacteria. As such bacteria have developed systems to cope with its extracellular content that, when deficient, shifts them to express a more virulent phenotype (Lamarche et al., 2008;, Long et al., 2008; Zaborin et al., 2009, 2012; Zaborina et al., 2011; Bains et al., 2012). In host environments in which bacteria colonize, such as the intestinal tract, phosphate levels can become severely depleted in response to extreme physiologic stress (Long et al., 2008; Geerse et al., 2010).
Under phosphate-limiting conditions, Pseudomonas aeruginosa has been found to express PstS and DING both of which belong to the superfamily of high-affinity phosphate-binding proteins (Berna et al., 2002, 2008, 2009; Ahn et al., 2007). While PstS proteins are present in bacteria only, DING proteins are ubiquitous in animal and plants and microorganisms (Adams et al., 2002; Berna et al., 2009). In bacteria, they are mainly found in Pseudomonas spp. (Berna et al., 2008) and have 70–80% sequence identity when compared to eukaryotic DING proteins (Ahn et al., 2007; Zaborina et al., 2008).
We have previously shown that during conditions under which phosphate availability becomes limited, such as occurs in the human intestine following major surgery, multidrug-resistant (MDR) P. aeruginosa strains form unusual appendages incorporating the PstS protein (Zaborina et al., 2008). These appendages are visually distinct from flagella and pili, and promote adhesion to intestinal epithelial cells. In addition to PstS, we also found DING proteins in sheared fractions of these appendages when these strains grew on Pseudomonas isolation agar (PIA) (Zaborina et al., 2008). Whether DING proteins are also present in PstS-containing appendages is unknown. In the present work, we sought to determine whether DING, like PstS, can be localized to the extracellular appendages of MDR strain of P. aeruginosa and whether other strains such as the well-studied P. aeruginosa PA14 also are capable of forming PstS/DING appendages.
Materials and methods
Bacterial strains and plasmids used in the study
Pseudomonas aeruginosa strains PA14 and derivative mutants were obtained from a PA14 mutant library (Liberati et al., 2006), and P. aeruginosa MDR25 was previously isolated by our group (Zaborina et al., 2006). Escherichia coli TOP10F' was used as a recipient strain for gene cloning and then as a donor for conjugative transfer into Pseudomonas cells. A summary of the other strains and plasmids used in the present study is provided in Table1. Pseudomonas aeruginosa and E. coli strains were routinely grown in tryptic soy broth (TSB) at 37 °C. When required, antibiotics were added at the following concentrations (μg mL−1): E. coli, ampicillin (Amp, 100), kanamycin (Km, 50), tetracycline (Tc, 10), or gentamicin (Gm, 10); For P. aeruginosa PA14: for routine maintenance of PA14 mutants, Gm, 15; for PA14 wild type overexpression strains, Gm, 100 or Tc, 50; for PA14 mutants with gene complementation and/or overexpression, Tc, 50.
Table 1.
Bacterial strains and plasmids
| Strain, plasmid | Relevant properties and genotype | Source/reference |
|---|---|---|
| E. coli TOP10F' | Recipient strain for cloning and conjugative transfer | Invitrogen |
| P. aeruginosa PA14 | Wild type isolated from burn patients | Liberati et al. (2006) |
| P. aeruginosa MDR 25 | Multidrug-resistant strain of P. aeruginosa isolated from tracheal aspirate of ICU patient. | Zaborina et al. (2006) |
| PA14 ΔDING mutant ID 34094 | PA14 ΔDING, PA14 with a deletion of DING, Gmr | Liberati et al. (2006) |
| PA14 ΔpstS mutant ID 31354 | PA14 ΔpstS, PA14 with a deletion of pstS, Gmr | Liberati et al. (2006) |
| PA14/DING | PA14 complemented with pBBR1MCS-5 containing PA14_55410 gene, Gmr | This work |
| PA14/pstS | PA14 complemented with pME6032 containing PA14_70860 gene, Tcr | This work |
| PA14/DING/pstS | PA14 complemented with pBBR1MCS-5 containing PA14_55410 gene and pME6032 containing PA14_70860, Gmr, Tcr | This work |
| Plasmids | ||
| pRK2013 | Helper plasmid, Tra+, Kmr | Gifted by Xue Xian Zhang |
| pBBR1MCS-5 | E. coli – Pseudomonas shuttle vector, inducible with IPTG | Gifted by Mark Ambrose |
| pME6032 | E. coli – Pseudomonas shuttle vector, inducible with IPTG | Gifted by Xue Xian Zhang |
| pGEMT | TA cloning vector | Promega |
Construction of pBBR1MCS-5-DING and pME6032-pstS
The PA14_55410 (DING) gene was amplified using PA14 genomic DNA and the primers (forward PA14F1-EcoR1: 5′-GCTCAGGAATTCATGTACAAGCGCTCTCTGA-3′ and reverse PA14R1-BamH1: 5′-GCTCATGGATCCTTAGAGCGGACGGCCGAT-3′). The PA14_70860 (pstS) gene was amplified using PA14 genomic DNA and the primers (forward pME6032_70860F-EcoR: 5′-GCATGAATTCA-TGAAACTCAAGCGTTTGAT-3′ and reverse pME60-32_70860WTR-XhoI: 5′-GCATCTCGAGTTACAGGCCCAGTT-CCTTGA-3′). The PCR products were first cloned into pGEMT vector (Promega), and sequence identity was confirmed by DNA sequencing. The cloned gene was retrieved and cloned into the E. coli- P. aeruginosa shuttle vector pBBR1MCS-5 (Kovach et al., 1995) using EcoR1 and BamH1 restriction sites to create pBBR1MCS-5/PA14_55410 where PA14_55410 is expressed from the lac promoter, or E. coli – P. aeruginosa shuttle vector pME6032 (Zhang & Rainey, 2008) using EcoR1 and XhoI restriction sites to create pME6032-5/PA14_70860 where PA14_70860 is expressed from the Ptac promoter.
Construction of PA14/pBBR1MCS-5-DING and PA14/pME6032-pstS
The resulting plasmids pBBR1MCS-5-DING and pME6032-pstS were mobilized into P. aeruginosa PA14 wild type by a general procedure of plasmid conjugation also known as triparental mating with the help of the helper plasmid pRK2013 (Mob+, Tra+) (Ditta et al., 1980). Transconjugants were selected on PIA (to counter-select E. coli) and either Gm or Tc. The correct integration and the expression of plasmid were checked using SDS/PAGE and Western blotting after inducing the plasmid gene expression using 1 mM IPTG.
Threshold of recognition of phosphate limitation
In Pi limitation studies, P. aeruginosa strains were grown in TSB broth overnight at 37 °C with shaking at 200 r.p.m. The next day, overnight cultures were seeded in fresh TSB media at 1 : 100 dilution and the cultures were grown till the OD600 nm reached 0.5. The bacterial cells were isolated by centrifuging at 4500 g, 6 min, following by washing and resuspension in defined citrate medium (DCM) that contained citric acid (4.0 g L−1), (NH4)2SO4 (1.0 g L−1), and MgSO4·7H2O (0.2 g L−1) supplemented with varying concentrations of phosphate: 0, 25, 100, and 300 μM using potassium phosphate buffer, pH 6.0. The DCM cultures were grown for 24 h at 37 °C, 200 r.p.m. The bacteria were centrifuged at 13 000 g, and the supernatants were lyophilized and reconstituted using 200 μL of phosphate-buffered saline (PBS). The pellet was lysed using Bug Buster Master Mix (Novagen). The samples were then used for immunoblot analysis. Three biologic replicates in each group were used for the analysis.
Immunoblot analysis
For immunoblot analysis, 20 μg proteins per well was separated on a 12% SDS-PAGE gel and electro-transferred to PVDF membrane (Immobilon-P, Millipore). The protein was primed with either anti-DING antibodies at 1 : 1000 dilution or anti-PstS antibodies at 1 : 500 dilution. Anti-rabbit IgG conjugated with horseradish peroxidase (Sigma) at 1 : 5000 dilution was used as secondary antibody. Detection was performed using 3,3' diaminobenzamidine hydrochloride (Sigma; DAB) with metal enhancer tablets (Sigma). The blots were quantitatively scanned using image j software. The antibodies used for DING and PstS protein identification have been previously described (Scott & Wu, 2005; Zaborina et al., 2008). The antibodies did not show any cross reactivity toward the PstS or the DING protein (Supporting Information, Fig. S1). At least three biologic replicates in each group were used for the analysis.
Transmission- and immuno-electron microscopy (TEM and IEM)
TEM and IEM were performed as previously described (Zaborina et al., 2008). For IEM 1 : 10 dilution of anti-PA5369 (anti-PstS) and 1 : 10 dilution of anti-DING, antibodies were used. Goat anti-rabbit IgG conjugated with 15 nm gold particles (TED PELLA) at 1 : 10 dilution was used as secondary antibody. Independent experiments were performed at least in triplicate visualizing at least 500 cells on each grid.
QRT- PCR
Strains PA14 and MDR25 were grown overnight on PIA plates. RNA was isolated from overnight-grown plates with high colony density (90–95% confluence). Three individual plates per group were included in the experiment. The colonies were collected directly in the RNA protect buffer (Qiagen), and RNA isolation, DNA degradation, and cDNA synthesis were performed as previously described (Zaborin et al., 2012). Real-time PCR was performed on the ABI 7900HT System using SYBR Green qPCR SuperMix-UDG (Invitrogen), cDNA, and respective primers: for PA4748 gene, forward primer PA4748-F 5′ AACAAGCAAGGCGGCATCACA 3′ and reverse primer PA4748-R 5′ TGCACGGTACGCATTCCAGTGT 3′; for PstS PA5369 and PstS MDR25, forward primer PstS-F 5′ CTGCCGGAATATCAGAAAGC 3′ and reverse primer PstS-R 5′ GTCATCAGGTTGGCCAGAGT 3′; for MDR25 DING gene, forward primer MDR25-DING-F 5′ CCGGCA CTACCAACAAGAAC 3′ and reverse primer MDR25-DING-R 5′ TGAGATAGGGGTTGGTTTCG 3′, and for PA14 DING gene, forward primer PA14-DING-F 5′ CCGGCACCACCAACAAGAAC 3′ and reverse primer PA14-DING-R 5′ TGAGATAGGGGTTGGTTTCG 3′. RNA concentration was measured with the Qubit 2.0 Fluorimeter (Invitrogen) using Qubit RNA assay kit Q32855, and 10 ng of RNA was used for cDNA synthesis with high-capacity RNA-to-cDNA kit (Applied Biosynthesis). Control samples (-RT) confirming the absence of DNA were obtained from 10 ng of RNA with the same RNA-to-cDNA kit without including RT Enzyme mix. One microliter of cDNA (or control -RT) was used in qRT-PCR assay in total 10 μL reaction mixture containing 5 μL SYBR green (Platinum SYBR Green qPCR Super Mix-UDG with ROX, Invitrogen, Cat# 11744-100) and 0.2 μM of each primer. The quantitative PCR (qPCR) was performed using 7900HT Fast Real-Time PCR System (Applied Biosystems). The program for amplification had an initial heat step at 50 °C for 2 min, followed by the denaturation step at 95 °C for 15 s, and then followed by 40 cycles of 95 °C for 15 s and 60 °C for 1 min. The specificity of the reaction was monitored by melt-curve analysis following the real-time program. Each sample was run in triplicate, and mean values were used in calculations. Expression levels were calculated based on differences in Ct levels. Statistical analysis was performed by Student's t-test, with P < 0.05 accepted as the criterion for a statistically significant value.
Surface shearing procedure
The shearing of bacterial surface for MDR strains as well as overexpression and gene-complemented strains of PA14 was adapted from a previously described procedure (Zaborina et al., 2008) with slight modification. Briefly, overnight-grown bacteria were harvested from PIA plates into PBS, washed once, and then suspended in protease inhibitor cocktail (Sigma) to an optical density at 600 nm (OD600 nm) of 5.0. The suspension was vortexed at 2500 g for 2 min and then centrifuged at 13 000 g for 10 min at 4 °C to separate the extracellular-appendage-enriched supernatant. The supernatant was centrifuged again to eliminate residual bacterial cells and then was used for immunoblot analysis as described previously (Zaborina et al., 2008).
Results
DING proteins localize on PstS appendages of P. aeruginosa MDR25
To confirm the presence of DING proteins on the PstS-containing appendages, we performed immuno-gold electron microscopy (IEM) of P. aeruginosa MDR25 isolated from a tracheal aspirate of a critically ill patient (Zaborina et al., 2006) that was previously demonstrated to form these appendages (Zaborina et al., 2008). Whole cells of MDR25 were directly harvested from PIA plates and subjected to IEM using the rabbit anti-DING antibodies followed by gold-labeled goat anti-rabbit antibody. We also performed IEM using anti-PstS antibodies to confirm the presence of PstS proteins. As seen by the dense spots localized on the cell surface structures (Fig.1), both DING and PstS proteins localize predominantly to these outer-surface structures in MDR25. Some gold spots were found outside of the appendages that may label secreted DING and PstS proteins, or disintegrated parts of the appendages, as we previously noticed the fragility of their structures. Gold spots were not found in negative controls performed in the absence of primary antibody (Fig.1).
Figure 1.
IEM images of PstS/DING appendages in Pseudomonas aeruginosa MDR25. (a) Staining with rabbit anti-DING antibodies. (b) Staining with anti-PstS antibodies. Arrows show gold spots on secondary goat anti-rabbit antibodies to locate DING and PstS. (c) Negative control of IEM of PstS/DING appendages in P. aeruginosa MDR25 performed in the absence of primary antibodies. The images are taken at magnification of 25 000 × 1.4.
Formation of PstS/DING-containing appendages by P. aeruginosa PA14
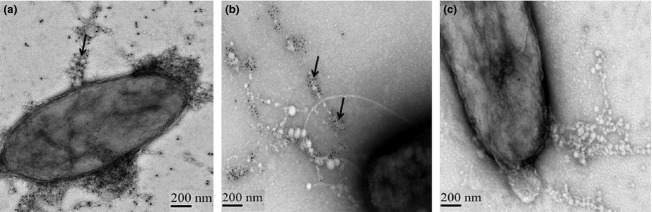
We next used the well-characterized virulent strain PA14 that harbors pstS and DING genes to determine whether appendages were formed. Using IEM, we were able to detect outer-surface appendages containing PstS and DING (Fig.2); however, fewer than 1% of PA14 cells produced these appendages compared with 50% of MDR25 cells.
Figure 2.

TEM and IEM images of Pseudomonas aeruginosa PA14 producing PstS/DING appendages. (a) TEM image. Appendages are shown by arrow. (b and c) IEM images to localize PstS (b) and DING (c) seen as gold spots pointed by arrows. The images are taken at magnification of 25 000 × 1.4.
Overexpression of DING and PstS from transformed plasmids enhances the production of DING/PstS appendages by P. aeruginosa PA14
We then constructed PA14-derivative strains with overexpression of either DING (PA14/pBBR1MCS-5-DING), or PstS (PA14/pME6032-pstS), or PstS and DING simultaneously (PA14/pBBR1MCS-5-DING/pME6032-pstS). Overexpression of either DING or PstS or both resulted in less than fivefold higher number of cells displaying appendages as counted by TEM. The number of appendages per cell was also increased (Fig.3a–c). Immunoblot analysis to quantify the protein in sheared appendage fractions confirmed the higher amount of PstS and DING in constructed strains (Fig.3d and e). The band density corresponding to PstS protein was increased in PA14/pME6032-pstS and further increased in PA14/pBBR-1MCS-5-DING/pME6032-pstS. The density of the band corresponding to DING protein was increased in PA14/pBBR1MCS-5-DING but was not however further increased during double overexpression, suggesting that PstS and DING use the same mechanism of secretion or extrusion, resulting in competitive inhibition when both are overexpressed.
Figure 3.

Overexpression of PstS and DING in constructed plasmids enhances the production of PstS/DING appendages in PA14. (a–c) TEM images of (a) PA14/pBBR1MCS-5-DING, (b) PA14/pME6032-pstS, (c) PA14/pBBR1MCS-5-DING/pME6032- pstS with overproduction of PstS and DING. The images are taken at magnification of 25 000 × 1.4. (d and e) Immunoassay analysis of sheared fractions using anti-PstS (d) and anti-DING (e) antibodies.
Either PstS or DING alone can contribute to appendage formation
In order to determine whether PstS-DING cointeraction is necessary for appendage formation, we first performed TEM analysis of the PA14 mutants, ΔDING, and ΔPstS. We were able to detect ΔDING and ΔPstS cells producing appendages, suggesting that either PstS or DING alone can lead to appendage formation (Fig.4a and b). Plasmid transformation was used to overproduce DING and PstS in ΔDING and ΔPstS mutants of PA14. Using immunoblot analysis, we found DING proteins in ΔPstS and PstS proteins in ΔDING sheared fractions (Fig.4c and d). The amounts of PstS and DING proteins were greatly increased in plasmid-bearing mutant strains (Fig.4c and d) suggesting that PstS or DING alone can contribute to appendage formation. These data were confirmed using IEM analysis with DING and PstS antibodies (Fig.4e and f). To further confirm that PstS and DING are the main proteins contributing to the appendage formation, we performed TEM analysis of PA14/pBBR1MCS-5-DING and PA14/pME6032-pstS grown on phosphate-rich agar plates (Pi, 15 mM). This approach relies on the ability of inorganic phosphate to suppress the expression of pstS and DING and to suppress the appendage formation. However, we found that these strains constructed to overexpress DING and PstS under IPTG induction produced appendages even in phosphate-rich media (Fig. S2). These data were confirmed by Western blot analysis of PstS and DING production in liquid TSB (Fig. S2). Additionally, reiterative experiments were performed by growing the cultures in defined citrate media supplemented with 60 mM Pi. Similarly to the above experiment, DING and PstS expression was only found in plasmid-bearing constructed strains where the expression of these genes was induced by IPTG (data not shown).
Figure 4.
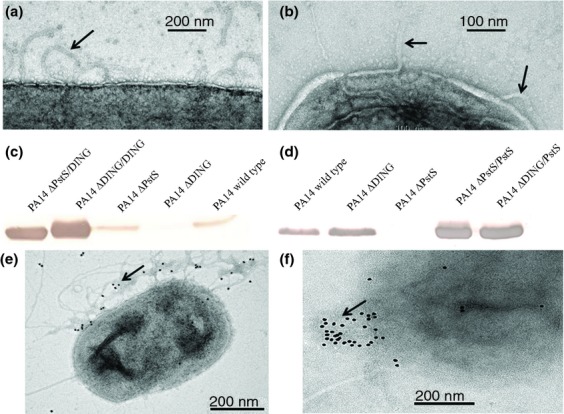
Either PstS or DING alone can contribute to the appendages formation by PA14 that is further increased due to overproduction. (a and b) TEM images of (a) ΔDING, (b) ΔPstS isolated from PIA plates at magnification of 25 000 × 1.4. Arrows show the appendages. (c and d) Immunoblot analysis of sheared fractions using anti-DING (c) and anti-PstS (d) antibodies. (e and f) IEM images of (e) ΔPstS/DING stained with anti-DING antibodies and (f) ΔDING/PstS/stained with anti-PstS antibodies. Arrows indicate localization of gold spots.
Abundant DING expression in MDR25
We hypothesized that the abundance of appendages in MDR25 compared with PA14 may be related to differential expression of PstS and/or DING. To test this hypothesis, we first sequenced the MDR25 DING gene (deposited in GenBank: JX500097.1). Amino acid sequence alignment revealed 95–99% of identity with hypothetical proteins NCGM2_1352 (P. aeruginosa NCGM2.S1), PA39016_000050001 (P. aeruginosa 39016), and the DING proteins PA14_55410 (P. aeruginosa PA14), PSPA7_4822 (P. aeruginosa PA7), and P. aeruginosa MDR1 (Zaborina et al., 2008). Comparative transcriptional analysis of pstS and DING expression in PA14 and MDR25 under equivalent growth conditions suitable for appendage formation was performed. RNA was isolated from cells grown overnight on PIA plates with a colony density of 90–95% confluence. Gene expression was estimated relative to the expression of housekeeping gene PA4748, encoding triose phosphate isomerase (Zaborin et al., 2009). There was no significant difference in pstS expression between PA14 and MDR25 strains; however, the expression of DING in MDR25 was close to 25-fold higher compared with PA14 (Fig.5a). We tested for a difference in copy numbers between PA14 and MDR25. The quantitative PCR analysis was performed using 1 ng of DNA as a template, and Ct levels of PA4748, pstS, and DING gene amplification were compared. As Fig.5b shows, there was no apparent difference in DING copy number, suggesting that changes in the mechanism of regulation of DING expression rather than gene copy number are responsible for the highly enhanced DING expression in MDR25.
Figure 5.
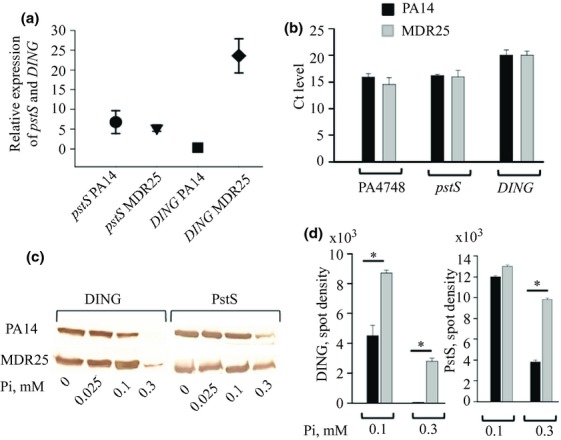
Comparative analysis of PstS and DING expression in PA14 and MDR25. (a) QRT-PCR analysis demonstrating > 20 higher expression of DING in MDR25 compared with PA14. The expression of pstS and DING was normalized to PA4748. (b) Ct levels of PA4748, pstS, and DING determined by quantitative PCR using 1 ng DNA as a template demonstrating no differences in gene copy numbers. (c) Immunoblot analysis of PstS and DING production by PA14 and MDR25 grown in citrate defined media varying in phosphate concentrations demonstrating that MDR25 has higher threshold sensitivity to phosphate level. (d) Quantitative evaluation of immunoblot analysis. n = 3/group, *P < 0.01.
Higher concentrations of inorganic phosphate are required to suppress the expression of PstS and DING in MDR25 compared with PA14
We next decided to check whether pstS and DING expression differentially respond to varying phosphate concentrations in PA14 and MDR25. We performed the experiment in liquid DCM, complemented with different concentrations of potassium phosphate to determine the threshold concentration of phosphate at which the expression of PstS and DING is suppressed. Results demonstrated that 100 μM of inorganic phosphate significantly suppressed the DING expression and 300 μM suppressed the PstS expression in PA14, but not in MDR25 (Fig.5c and d). These data demonstrate that MDR25 expresses the two proteins at phosphate levels which repress expression in PA14.
Discussion
The effect of stressful environmental conditions on microbial behaviors, such as those that occur during nutrient limitation, is an important aspect of infection pathogenesis. Prolonged exposure to such conditions, as often occurs when MDR strains colonize epithelial surfaces of critically ill humans, can result in the selection of strains that express novel virulence properties. Studying clinical strains exposed to these complex conditions offers an opportunity to discover emergent properties in pathogens that may lead to novel treatment strategies.
Results from the current experiments have extended our knowledge regarding the role of the identified novel adhesive appendages discovered on some MDR strains of Pseudomonas aeruginosa that appear during low phosphate conditions. Two phosphate-binding proteins, PstS and DING, coded by genes of the pho regulon and that are known to be induced at low phosphate concentrations, were localized to these nonflagellar appendages. PstS/DING appendages are much rarer on PA14 cells, but can be increased by recombinant overexpression of either DING or PstS. The comparative expression of these proteins in PA14 and MDR25 revealed DING protein expression is c. 25-fold higher in MDR25 and that a higher concentration of phosphate is required to suppress its expression. Taken together, it is plausible that PstS/DING appendages can be evolved among clinical MDR strains as a result of the unprecedented conditions to which they are exposure during extreme medical interventions, most notably phosphate limitation. They may have developed to ensure their ability to acquire phosphate under such environmentally harsh conditions.
The function of bacterial DING proteins is still not known. Their proposed role in phosphate scavenging has not yet been supported by direct experimental evidence. Given that bacterial DING proteins share very high amino acid sequence similarity with mammalian DING proteins, it is possible that they may be involved in the modulation of host cell signaling. Several examples exist indicating that eukaryotic DING proteins act in this way (Berna et al., 2009), and there is also evidence that bacterial DING proteins influence gene expression and proliferation of human cells (Ahn et al., 2007; Suh et al., 2013). Work by Suh et al. (2013) is particularly interesting in this regard, in that it demonstrates transcriptional regulation when P. aeruginosa DING proteins are transiently expressed in human cells. However, transient expression of PstS displays no such activity indicating a clear functional difference between these two proteins, which may also be relevant to bacterial pathogenesis.
Acknowledgments
We thank Yimei Chen, Electron Microscopy Core Facility for technical assistance at University of Chicago and Adrian Turner, Electron Microscopy Core Facility for technical assistance at University of Auckland, and P. aeruginosa PA14 transposon insertion mutant library for providing all requested mutants. This study was funded by NIH RO1 5R01GMO62344 (J.C.A.), University of Auckland PReSS account and Contestable Travel grant (M.S.) and Maurice and Phyllis Paykel Trust (K.S.). The authors have no conflict of interest to declare.
Authors' contribution
J.C.A, K.S., and O.Z. are senior co-authors.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Immunoblot analysis of sheared appendages of MDR25 grown on PIA demonstrating no cross-reaction between anti-PstS and anti-DING antibodies.
PA14/pBBR1MCS-5-DING and PA14/pME6032-pstS in which expression of DING and pstS is induced by IPTG, produce appendages in phosphate-rich media.
References
- Adams L, Davey S. Scott K. The DING protein: an autocrine growth-stimulatory protein related to the human synovial stimulatory protein. Biochim Biophys Acta. 2002;1586:254–264. doi: 10.1016/s0925-4439(01)00104-1. [DOI] [PubMed] [Google Scholar]
- Ahn S, Moniot S, Elias M, Chabriere E, Kim D. Scott K. Structure-function relationships in a bacterial DING protein. FEBS Lett. 2007;581:3455–3460. doi: 10.1016/j.febslet.2007.06.050. [DOI] [PubMed] [Google Scholar]
- Bains M, Fernandez L. Hancock RE. Phosphate starvation promotes swarming motility and cytotoxicity of Pseudomonas aeruginosa. Appl Environ Microbiol. 2012;78:6762–6768. doi: 10.1128/AEM.01015-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berna A, Bernier F, Scott K. Stuhlmuller B. Ring up the curtain on DING proteins. FEBS Lett. 2002;524:6–10. doi: 10.1016/s0014-5793(02)03053-3. [DOI] [PubMed] [Google Scholar]
- Berna A, Bernier F, Chabriere E, Perera T. Scott K. DING proteins; novel members of a prokaryotic phosphate-binding protein superfamily which extends into the eukaryotic kingdom. Int J Biochem Cell Biol. 2008;40:170–175. doi: 10.1016/j.biocel.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Berna A, Bernier F, Chabriere E, Elias M, Scott K. Suh A. For whom the bell tolls? DING proteins in health and disease. Cell Mol Life Sci. 2009;66:2205–2218. doi: 10.1007/s00018-009-0006-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditta G, Stanfield S, Corbin D. Helinski DR. Broad host range DNA cloning system for gram-negative bacteria: construction of a gene bank of Rhizobium meliloti. P Natl Acad Sci USA. 1980;77:7347–7351. doi: 10.1073/pnas.77.12.7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geerse DA, Bindels AJ, Kuiper MA, Roos AN, Spronk PE. Schultz MJ. Treatment of hypophosphatemia in the intensive care unit: a review. Crit Care. 2010;14:R147. doi: 10.1186/cc9215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovach ME, Elzer PH, Hill DS, Robertson GT, Farris MA, Roop RM., 2nd Peterson KM. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene. 1995;166:175–176. doi: 10.1016/0378-1119(95)00584-1. [DOI] [PubMed] [Google Scholar]
- Lamarche MG, Wanner BL, Crepin S. Harel J. The phosphate regulon and bacterial virulence: a regulatory network connecting phosphate homeostasis and pathogenesis. FEMS Microbiol Rev. 2008;32:461–473. doi: 10.1111/j.1574-6976.2008.00101.x. [DOI] [PubMed] [Google Scholar]
- Liberati NT, Urbach JM, Miyata S, et al. An ordered, nonredundant library of Pseudomonas aeruginosa strain PA14 transposon insertion mutants. P Natl Acad Sci USA. 2006;103:2833–2838. doi: 10.1073/pnas.0511100103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long J, Zaborina O, Holbrook C, Zaborin A. Alverdy J. Depletion of intestinal phosphate after operative injury activates the virulence of P. aeruginosa causing lethal gut-derived sepsis. Surgery. 2008;144:189–197. doi: 10.1016/j.surg.2008.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott K. Wu L. Functional properties of a recombinant bacterial DING protein: comparison with a homologous human protein. Biochim Biophys Acta. 2005;1744:234–244. doi: 10.1016/j.bbamcr.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Suh A, Le Douce V, Rohr O, Schwartz C. Scott K. Pseudomonas DING proteins as human transcriptional regulators and HIV-1 antagonists. Virol J. 2013;10:234. doi: 10.1186/1743-422X-10-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaborin A, Romanowski K, Gerdes S, et al. Red death in Caenorhabditis elegans caused by Pseudomonas aeruginosa PAO1. P Natl Acad Sci USA. 2009;106:6327–6332. doi: 10.1073/pnas.0813199106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaborin A, Gerdes S, Holbrook C, Liu DC, Zaborina OY. Alverdy JC. Pseudomonas aeruginosa overrides the virulence inducing effect of opioids when it senses an abundance of phosphate. PLoS One. 2012;7:e34883. doi: 10.1371/journal.pone.0034883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaborina O, Kohler JE, Wang Y, et al. Identification of multi-drug resistant Pseudomonas aeruginosa clinical isolates that are highly disruptive to the intestinal epithelial barrier. Ann Clin Microbiol Antimicrob. 2006;5:14. doi: 10.1186/1476-0711-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaborina O, Holbrook C, Chen Y, et al. Structure-function aspects of PstS in multi-drug-resistant Pseudomonas aeruginosa. PLoS Pathog. 2008;4:e43. doi: 10.1371/journal.ppat.0040043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaborina O, Zaborin A, Romanowski K, Babrowski T. Alverdy J. Host stress and virulence expression in intestinal pathogens: development of therapeutic strategies using mice and C. elegans. Curr Pharm Des. 2011;17:1254–1260. doi: 10.2174/138161211795703771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XX. Rainey PB. Dual involvement of CbrAB and NtrBC in the regulation of histidine utilization in Pseudomonas fluorescens SBW25. Genetics. 2008;178:185–195. doi: 10.1534/genetics.107.081984. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Immunoblot analysis of sheared appendages of MDR25 grown on PIA demonstrating no cross-reaction between anti-PstS and anti-DING antibodies.
PA14/pBBR1MCS-5-DING and PA14/pME6032-pstS in which expression of DING and pstS is induced by IPTG, produce appendages in phosphate-rich media.


