The rate of autism spectrum disorders (ASD) is increasing with recent prevalence estimates reaching 1 in 88 in the United States (Baio, 2012). Although sensory features have been listed as commonly associated features [Diagnostic and Statistical Manual-IV-TR; American Psychiatric Association (APA), 2000] the new ASD diagnostic criteria proposed in the DSM-5 includes sensory features as core features and specifically describes hypo- and hyper-reactivity to sensory input, unusual interests in sensory aspects of the environment, and restricted and repetitive interests in sensory based activities (DSM-5; APA, 2012). Assessments are needed that comprehensively characterize sensory features in children with ASD and facilitate accurate diagnostic practices. Few instruments measure sensory features specific to ASD, thereby limiting large scale studies of prevalence and heterogeneity of sensory response patterns among children with ASD.
Sensory features are highly prevalent in ASD (Baranek, David, Poe, Stone, & Watson, 2006; Ben-Sasson et al., 2009; O’Donnell, Deitz, Kartin, Nalty, & Dawson, 2012), with studies consistently showing that children with ASD present with higher rates of sensory features than typically developing children (Dunn, Myles, & Orr, 2002; Ermer & Dunn, 1998; Schoen, Miller, Brett-Green, & Nielsen, 2009; Watling, Deitz, & White, 2001) as well as those with other developmental disabilities (DD) (Baranek et al., 2006; Baranek et al., 2013s;; Rogers, Hepburn, & Wehner, 2003).
The literature suggests that sensory features among children with ASD constellate into four distinct behavioral categories, or sensory response patterns -- hyporesponsiveness (HYPO), hyperresponsiveness (HYPER), sensory interests, repetitions and seeking behaviors (SIRS) and enhanced perception (EP). HYPO is considered a lack of or delayed response to sensory stimuli (e.g., a lack of orienting to loud sounds, slow to react to pain) (e.g., Ben-Sasson et al., 2009; Watson et al., 2011). HYPER is defined by an exaggerated or avoidant response to sensory stimuli (e.g., discomfort to grooming activities, covering ears in response to sounds) (e.g., Baranek, Boyd, Poe, David, & Watson, 2007; Schoen, Miller, & Green, 2008). SIRS is characterized by a fascination with or craving of sensory stimulation which is intense and may be repetitive in nature (e.g., fascination with flickering lights or rubbing textures) (e.g., Ben-Sasson et al., 2007; Liss et al., 2006). Although previous research has focused much on the prevalence of HYPO, HYPER, and specific aspects of SIRS among children with ASD (Ben-Sasson et al., 2009), EP has emerged as a fourth pattern of sensory response possibly unique to individuals with ASD (Happé & Frith, 2006; Mottron, Dawson, Soulieres, Hubert, & Burack, 2006; Mottron, Dawson, & Soulières, 2009). EP is characterized by superior acuity in the awareness of specific sensory stimuli and focuses on specific perceptual elements (e.g., recognizing perfect pitch, superior ability to recognize minor changes in visual appearance) (Mottron, et al., 2006). Recent neuropsychological theories suggest that EP is likely characterized by strengths in locally oriented visual and auditory perception and enhanced low-level discrimination (Mottron et al., 2006; Mottron et al., 2009), and may be related to hypersensitivity (e.g., lower threshold detection) and hyper-systemizing cognitive styles (Baron-Cohen, Ashwin, Tavassoli & Chakrabarti, 2009).
While these four constructs are uniquely defined, research suggests certain patterns may co-occur within individuals creating significant phenotypic heterogeneity across the population (Baranek et al., 2006; Ben-Sasson et al., 2007; Ben-Sasson et al., 2009; Hilton, Graver, & LaVesser, 2007; Lane, Young, Baker, & Angley, 2010; Liss, Saulnier, Fein, & Kinsbourne, 2006). HYPO and HYPER have been found to co-occur in children with ASD (Baranek et al., 2006; Ben-Sasson et al., 2007), SIRS has been associated with both HYPO and HYPER among children with ASD (Boyd et al., 2010; Gabriels et al., 2008), and HYPER and EP have also been associated (Mottron et al., 2006). Although patterns of sensory response do co-occur, some research suggests that HYPO better discriminates between children with ASD when compared with children with DD or typical development (Baranek et al., 2013; Ben-Sasson et al., 2009; Rogers & Ozonoff, 2005). While others have suggested HYPER, may differentiate children with ASD (Joosten & Bundy, 2010). Further, due to the core deficits of ASD, the impact of social context on sensory features is an important consideration in formulating conceptual models appropriate to this population. Studies have shown the presence of sensory features across both social and nonsocial contexts (Baranek et al., 2006; Hilton et al., 2007; Liss et al., 2006); however, findings vary depending upon assessment methods and specific sensory patterns studied.
Research is needed to develop refined sensory processing instruments based on current conceptual models, and validate their utility for the ASD population. Although a number of caregiver report measures are designed to assess sensory features in children, most instruments have the following limitations: (a) were not developed to measure unique sensory patterns among children with ASD (Sensory Profile; Dunn, 1999; Sensory Processing Measure; Parham, Ecker, Miller-Kuhananeck, Henry, & Glennon, 2007), and (b) were designed to assess only one sensory pattern, such as hyperresponsiveness (Sensory Sensitivity Questionnaire-Revised; Talay-Ongan & Wood, 2000) and/or (c) may conflate co-occurring sensory patterns within the same subscales (e.g., the Short Sensory Profile [McIntosh, Miller, & Shyu, 1999]; “Underresponsive/Seeks Sensation” subscale includes HYPO, HYPER, and SIRS items; “Auditory Filtering” subscale includes HYPER and HYPO items). Moreover, most were standardized on typical children or were validated with relatively small samples of children with ASD (Dunn, 1999; Glennon, Miller-Kuhaneck, Henry, Parham, & Ecker, 2007; McIntosh et al., 1999; Talay-Ongan & Wood, 2000).
The Sensory Experiences Questionnaire Version 3.0 (SEQ-3.0; Baranek, 2009) is a 105 item parent report tool designed specifically to measure behavioral responses to naturally occurring sensory stimuli in the context of everyday situations in children with ASD, ages 2 to 12 years. Previous research on earlier versions of the Sensory Experiences Questionnaire (Version 1.0, Version 2.1) has demonstrated the tool’s reliability and validity (Baranek et al., 2006; Little et al., 2011). However, emerging empirical research on the sensory response patterns among children with ASD has resulted in the need for the expansion of items and a confirmation of the proposed four-factor model designed to capture these features utilizing a sufficiently large sample which may provide researchers and clinicians with better ability to characterize the heterogeneity of sensory features specific to children with ASD.
The main purpose of this study is to provide empirical validation for the Sensory Experiences Questionnaire Version 3.0 (SEQ-3.0) with children with ASD ages 2–12 years. The following research questions were addressed:
Is there an empirical validation for a four factor model of sensory features in children with ASD?
To what extent do sensory response patterns predict autism severity while controlling for associated child and family characteristics?
Methods
Participants
Caregivers (n=1407) of children with ASD (i.e., diagnosis of Autistic Disorder, Asperger’s Disorder, or Pervasive Developmental Disorder-Not Otherwise Specified [PDD-NOS]) (DSM-IV-TR, APA, 2000) ages 2 to 12 years were recruited from a national registry augmented by national and local autism advocacy organizations methods to participate in this survey (see Study Procedures for further details of demographic and diagnostic information). Primary caregivers (mothers [95.1%], fathers [3.3%], other adults [1.6%] such as grandparents that lived in the home) completed an SEQ 3.0 for each study participant. All caregivers provided informed consent as approved by the Institutional Review Board of the University of North Carolina at Chapel Hill. Table 1 summarizes demographic data.
Table 1.
Child and Family Characteristics
| Primary Diagnosis (allowed to select more than one) (n=1307) (%)
| |
| Autism/Autistic Disorder | 63.0 |
| Asperger’s Disorder | 22.1 |
| PDD-NOS | 24.4 |
|
| |
| Gender (n=1307) | |
|
| |
| % Male | 82.3 |
|
| |
| SRS/SRS-P Total Score (SD) (n=1241) | 107.1 (27.3) |
|
| |
| Chronological Age (SD) (n=1307) | 7.7 (2.7) years |
|
| |
| IQ Proxy (SD) (n=1124) | 81.4 (28.8) |
|
| |
| Maternal College Education (n=1299) (%) | |
|
| |
| Partial High School or Lower | 0.8 |
| High School or GED | 15.6 |
| Associates Degree/Partial College | 29.1 |
| Bachelor or Master Degree | 50.4 |
| Advanced Degree such as doctorate | 4.2 |
|
| |
| Annual Household Income (n=1209) (%) | |
|
| |
| Less than $20,000 | 8.3 |
| $20,000 to $39,999 | 17.2 |
| $40,000 to $59,999 | 19.3 |
| $60,000 to $79,999 | 16.9 |
| $80,000 to $99,999 | 14.3 |
| $100,000 or more | 24.0 |
|
| |
| Race/Ethnicity (allowed to select more than one) (n=1307) (%) | |
|
| |
| African-American | 5.0 |
| American Indian/Alaskan Native | 3.5 |
| Asian | 3.9 |
| Native Hawaiian/Pacific Islander | 0.3 |
| Other | 3.4 |
| White | 93.0 |
| Hispanic or Latino Origin | 9.9 |
Exclusion Criteria
Exclusionary criteria for the study included the following: co-morbid conditions of ASD such as fragile X syndrome and tuberous sclerosis, genetic disorder or syndrome associated with a developmental disability (e.g., Down syndrome), severe physical impairment (e.g., cerebral palsy), significant visual or hearing impairment (e.g., blindness or deafness), traumatic brain injury or brain malformation, psychotic disorder such as bipolar disorder or schizophrenia, and seizure activity within the last 12 months. The final sample included 1307 children, as 100 participants were excluded on the basis of these criteria.
Study Instruments
The Sensory Experiences Questionnaire Version 3.0 (SEQ-3.0; Baranek, 2009) is a caregiver report instrument designed to characterize sensory features in children ages 2–12 years with ASD and/or DD in social and non-social contexts. The SEQ framework considers whether sensory experiences occur in a predominantly social context (e.g., experiencing contact with people), or a nonsocial context (e.g., experiencing loud sounds or textured objects). Deficits in social cognition and communication may impact a child’s ability to understand other people’s intentions (e.g., Baron-Cohen, 2002) as well as create difficulties in expressing sensory preferences and needs appropriately (e.g., Wetherby, 2006), which adds variability in responses to particular types of sensory experiences.
The SEQ-3.0 has been revised from its earlier version to better meet research needs and an evolving conceptual model. In particular, recent research on enhanced perception among individuals with ASD, and potential contributions to hyperresponsiveness (e.g., Baron-Cohen et al., 2009; Mottron et al., 2006), the SEQ-3.0 has been expanded to include items that tap this construct. Additional revisions included refining items on the basis of previous psychometric studies, expanding the total number of items to provide more balance across the sensory response patterns, sensory modalities, and contexts (social and nonsocial), and adding control items. The items based on sensory features included in the SEQ-3.0 were developed from a review of existing literature on sensory features in children with ASD, including empirical studies, parental report studies, expert clinical reports, conceptual models of sensory processing, and neuropsychological theories of ASD describing core and associated features. Through a consensus process, a team of experts grouped the items into the sensory response patterns, modality categories, and contexts (social or non-social), consistent with the conceptual framework of the SEQ.
The SEQ-3.0 has 105 items that measure the frequency of sensory behaviors across four sensory response patterns (i.e., HYPO, HYPER, SIRS, EP), five modality categories (i.e., auditory, visual, tactile, gustatory/olfactory, vestibular/proprioceptive), and two contexts (i.e., social and non-social). The first 97 items measure the frequency using a 5-point Likert scale ranging from 1 (never/almost never) to 5 (always/almost always) with a higher score indicating more sensory symptoms. The measure also includes eight items which address broader issues related to the children’s sensory behaviors and allow caregivers to elaborate with a qualitative response. The questionnaire takes approximately 15–20 minutes to complete.
Autism symptom severity was assessed using the Social Responsiveness Scale (Constantino & Gruber, 2005) for children ages 4 to 18 years, and Social Responsiveness Scale- Preschool Version (SRS-P; Pine, Luby, Abbacchi, & Constantino, 2006) for children ages 35 to 48 months. The SRS and SRS-P consist of 65 items and takes 15–20 minutes to complete, and both measures were designed as quantitative trait assessments of children’s autism symptoms in social settings with higher scores indicating more autistic symptoms (Constantino, 2003). Total scores on the SRS and SRS-P, versus T-scores, were used in analysis to allow for a direct comparison of the measures, as the SRS-P is in prepublication.
A Background Information Questionnaire (BIQ; unpublished questionnaire) was developed specifically for this study to obtain information about the child and family in four domains: family characteristics, child characteristics, child’s functioning level, and services the child receives. The BIQ is a parent-report instrument that takes approximately 10–15 minutes to complete. The following demographic and developmental variables were derived from the BIQ to provide descriptive data regarding key child and family characteristics: Chronological age (CA) was calculated from the child’s birth date. Autism diagnosis included Autism/Autistic Disorder, Asperger’s Disorder, and PDD-NOS. Diagnosis was obtained per caregiver report for all participants; a subset included previously authenticated diagnoses through a registry (see below in Study Procedures). Household income was reported in increments of $20,000, with categories ranging from <$20,000 to >$100,000, and the analysis used the floor for each income category (e.g., $20,001–$40,000 was recoded as $20,000). Maternal College Education indicated whether or not the child’s mother completed at least a bachelor’s degree. Race was categorized as either white or non-white due to the small number of participants in non-white categories (see Table 1). Parents’ Estimated Developmental Age (PEDA) was measured in 6 month increments between <12 months to 3 years and 12 month increments from 3 to 19 years. Specifically, caregivers were asked to estimate their child’s “overall level of cognitive functioning”. An IQ proxy was then calculated based on this developmental estimate using the following formula: [(PEDA/CA)*100]. This was done to obtain a standardized metric for all participants that would not be correlated with CA to use as a covariate in the analyses. A subset of 316 participants had reported previous IQ testing scores which showed a positive correlation with the calculated IQ proxy (r=.67).
Study Procedures
The IAN Research Database at the Kennedy Krieger Institute and Johns Hopkins Medicine-Baltimore (sponsored by Autism Speaks), the University of North Carolina at Chapel Hill Research Registry, and other online autism organizations were used to recruit a demographically diverse sample of families in the United States to participate in a longitudinal study on the sensory features of children with ASD. Approximately 50% of the sample was recruited through the Interactive Autism Network (IAN), a web-based autism registry, which recently authenticated the parent-report ASD diagnosis in their registry (Daniels et al., 2012). The remainder of participants was recruited through online autism advocacy and parent support groups.
Recruitment occurred in phases and was done solely through online recruitment material. Participants were screened for inclusion/exclusion before initiation of the survey. Once determined eligible, they were sent an electronic invitation to participate, followed by up to three electronic contacts to complete the survey if needed. All questionnaires were converted to an electronic format using Qualtrics software (Qualtrics Labs, 2011) and the online versions of the surveys were approved by the publisher or author, as appropriate, before administration. The surveys took 45–60 minutes to complete, and families were offered a $5.00 gift card upon completion.
Data Analysis
To address research question 1, the proposed structure of the SEQ-3.0 was tested with a confirmatory factor analysis (CFA). Factor analysis offers substantial benefits in assessing the relationships between item-level data and underlying latent variables (factors). In these models, responses to items are viewed as arising from underlying, unobserved latent variables. That is, the latent variables are the source of item covariance. In this case, the unobserved latent variables are the four sensory patterns of the SEQ-3.0. In CFA, a specific latent structure is proposed to explain covariance between manifest variables (item-level data).
Four distinct factors (HYPO, HYPER, SIRS, EP) were tested, which characterize the sensory response patterns in our conceptual model. Given the literature describing co-occurrence of these sensory features in children with ASD, correlations among the four constructs were expected (Baranek et al., 2006; Ben-Sasson et al., 2007; Ben-Sasson et al., 2009; Hilton et al., 2007; Lane et al., 2010; Liss et al., 2006). The five sensory modality categories (i.e., auditory, visual, tactile, gustatory/olfactory, vestibular/proprioceptive) presented additional sources of item covariance beyond the sensory response patterns. In any scale, some items may be related to one another for reasons outside the factors of interest (HYPO, HYPER, SIRS, EP); measurement error associated with one item is correlated with that of another item. In these data, it was anticipated that correlated errors would result from the superficial similarity of items in a given sensory modality (e.g., visual or auditory items). To account for the covariance, each sensory modality was included as a measurement factor that would act as latent measures of the error covariance (see Kenny & Kashy, 1992 for comprehensive discussion). This covariance should be unrelated to the constructs of interest in the model (e.g. sensory response pattern) and so the correlations of these measurement factors to each other and to the sensory response pattern factors were fixed to zero. There were several items on the scale regarding behaviors that occur in a social context. We believed this provided another source of error variance similar to that from the sensory modalities. A sixth measurement factor was proposed to account for shared variance among items occurring in the social context. Like all of the measurement factors, the social factor was fixed to be uncorrelated with all other factors in the model.
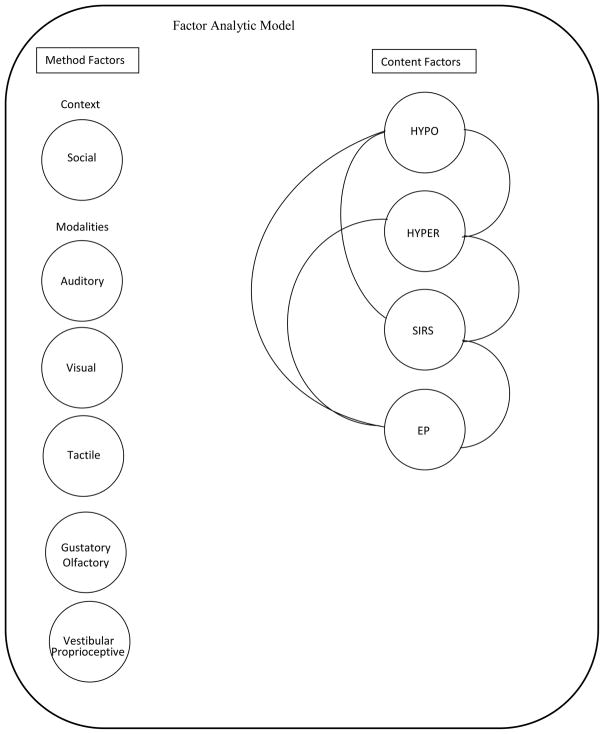
In sum, the model had a total of 10 latent variables: four sensory response pattern variables, the social context measurement variable, and the five sensory modality measurement variables, with 97 measured variables (items) from the SEQ-3.0. See Figure 1 for the factor analytic model. The model was estimated in Mplus (Muthén & Muthén, 2010) under maximum likelihood estimation. Factor means were fixed to zero and factor variances to one, with all hypothesized loadings and between factor correlations estimated freely. The 97 measured variables (items) were allowed to have 2–3 cross loadings on the latent variables depending on the item content (e.g. sensory pattern, sensory modality, social context). However, any one measured variable was only allowed to load on one sensory pattern variable, one sensory modality variable, and the social variable if appropriate.
Figure 1.
Factor analytic model
The SEQ-3.0 is intended for use across a relatively wide population, so measurement invariance was tested for in the factor loading across gender and age. The sample was split into two age groups, preschool or younger (<5 years old) and school aged (>5 to <13 years old) for the age model. Invariance indicates that the model parameters are the same across the two age groups. Testing typically proceeds in a series of tests each requiring more stringent levels of invariance (Bollen, 1989; Horn & McArdle, 1992).
To address research question 2, first we computed a correlation matrix to look at associations among sensory response patterns (HYPO, HYPER, SIRS, EP), autism severity (SRS), and various child (i.e., CA, PEDA, IQ Proxy, gender, race) and family (income, maternal education) characteristics. Next, a mixed model regression in SAS 9.2 (SAS Institute, 2010) was used to predict autism severity (SRS) from the four sensory response patterns, controlling for key child and family characteristics found to be associated with the sensory patterns and/or autism severity. The presence of siblings (69 cases) from 33 families who had more than one child with ASD in the sample gave rise to non-independence between these observations. Mixed model regression, which allows for nesting of observations within child and within family, manages this non-independence through the inclusion of random effects which provide separate parameter estimates for each observation, in addition to the fixed effects estimates provided in general linear models (see Bryk & Raudenbush, 1992; Burchinal, Nelson, & Poe, 2006 for complete discussions). Finally, graphic depictions were used to demonstrate how mean sensory response pattern factor scores differed by ASD diagnostic categories (i.e., Autism/Autistic Disorder, Asperger’s Disorder, PDD-NOS).
Results
Factor Analysis
Model fit was good using standard fit measures. See Table 2 for indices. Chi-square is sensitive to both sample size and the number of parameters in the model (Schumacker & Lomax, 2004), and in this case both were large. Jorskog (1969) suggested the use of a normed chi-square (chi-square/degrees of freedom) provides some protection from this sensitivity. In these data, the normed chi-square of 4.35 indicates reasonable model fit (Bollen, 1989). Both the RMSEA and the SRMR fall within common guidelines for good model fit (Kline, 2010). Post hoc assessment suggested some small modifications to the model, but model fit did not significantly change when they were included and so the initial model was retained.
Table 2.
Model Fit Indices
| Statistic | Values |
|---|---|
| Chi-Square | 16, 724.18 (3984)** |
| RMSEA | .051 (.050 to 0.052) |
| SRMR | .07 |
The factor loadings for the latent sensory factors were generally strong and all significant (p < .001); all were greater than .20 and the vast majority were .40 or greater. Factor variances were set to one. This was considered strong support for the existence of our distinct hypothesized constructs. As expected, between factor correlations were positive and significant, (p < .001). With the exception of EP and HYPO (r=.22), the correlations were moderate to large ranging from .44 to .74. See Table 3 for sensory factor correlations.
Table 3.
Between Factor Correlations for Sensory Patterns
| HYPO | HYPER | SIRS | EP | |
|---|---|---|---|---|
| HYPO | 1.00 | |||
| HYPER | 0.49 | 1.00 | ||
| SIRS | 0.64 | 0.44 | 1.00 | |
| EP | 0.22 | 0.74 | 0.51 | 1.00 |
Invariance testing confirmed the SEQ-3.0 can be used across the different gender and age groups. Model fit for each of the separate groups, boys vs. girls and preschool vs. school-age was essentially unchanged from the overall model. Further, comparison of the configural models to the strong invariance models where factor loadings and item intercepts are constrained to be equal, indicated no significant difference either between boys and girls, χ2 (92) = 95.89, ns, or between younger and older children, χ2 (92) = 105.71, ns.
Correlation Matrix
Table 4 presents the inter-correlations between the four sensory response patterns (HYPO, HYPER, SIRS, EP), autism severity (SRS), and child (i.e., CA, PEDA, IQ Proxy, gender) and family characteristics (i.e., income, maternal education). Although statistically significant, most correlations between sensory response patterns and child and family characteristics were small to medium (r= −.34 to .18) (Cohen, 1988). Income and maternal education were moderately correlated at r=.32, but of these two variables, income had a slightly stronger negative correlation with the sensory response patterns, likely due to the restricted binary nature of the maternal education variable. Small, but statistically significant, correlations were found between sensory response patterns and both CA and PEDA (−.21 to .11). Autism severity was significantly positively associated with all sensory response patterns (.33 to.57) as well as to maternal education, income, IQ proxy, and gender. Moderate to high inter-correlations were detected among maturational variables, particularly between PEDA and CA (r=.39) as well as PEDA and IQ (r=.70).
Table 4.
Correlations Among Sensory Response Patterns, Autism Severity, and Child and Family Characteristics
| Income | College (yes) | Race (white) | Gender (Male) | CA | PEDA | IQ Proxy | SRS Total | |
|---|---|---|---|---|---|---|---|---|
| Income | 1.00 | |||||||
| 1209 | ||||||||
| College (yes) | 0.32*** | 1.00 | ||||||
| 1203 | 1299 | |||||||
| Race (white) | 0.04 | 0.06* | 1.00 | |||||
| 1209 | 1299 | 1307 | ||||||
| Gender (Male) | 0.02 | 0.01 | −0.01 | 1.00 | ||||
| 1209 | 1299 | 1307 | 1307 | |||||
| CAa | 0.10*** | 0.07* | 0.03 | 0.05 | 1.00 | |||
| 1209 | 1299 | 1307 | 1307 | 1307 | ||||
| PEDAb | 0.05 | 0.03 | 0.02 | 0.00 | 0.39*** | 1.00 | ||
| 1209 | 1299 | 1307 | 1307 | 1307 | 1307 | |||
| IQ Proxy | 0.15*** | 0.13*** | 0.10*** | −0.09** | 0.04 | 0.70*** | 1.00 | |
| 1044 | 1117 | 1124 | 1124 | 1124 | 1124 | 1124 | ||
| SRSc Total | −0.19*** | −0.14*** | 0.00 | 0.15*** | 0.00 | −0.06* | −0.24*** | 1.00 |
| 1157 | 1243 | 1251 | 1251 | 1251 | 1251 | 1080 | 1251 | |
| HYPO | −0.27*** | −0.14*** | −0.06* | 0.03 | −0.17*** | −0.13*** | −0.24*** | 0.57*** |
| 1209 | 1299 | 1307 | 1307 | 1307 | 1307 | 1124 | 1251 | |
| HYPER | −0.18*** | −0.08** | 0.03 | −0.01 | 0.11*** | 0.11*** | 0.11*** | 0.50*** |
| 1209 | 1299 | 1307 | 1307 | 1307 | 1307 | 1124 | 1251 | |
| SIRS | −0.28*** | −0.14*** | −0.08** | 0.02 | −0.21*** | −0.16*** | −0.24*** | 0.50*** |
| 1209 | 1299 | 1307 | 1307 | 1307 | 1307 | 1124 | 1251 | |
| EP | −0.15*** | −0.07* | −0.01 | −0.02 | 0.14*** | 0.11*** | 0.15*** | 0.33*** |
| 1209 | 1299 | 1307 | 1307 | 1307 | 1307 | 1124 | 1251 |
<.05
<.01
<.001,
Chronological Age,
Parents’ Estimated Developmental Age,
Social Responsiveness Scale/Preschool
Mixed Model Regression Analysis
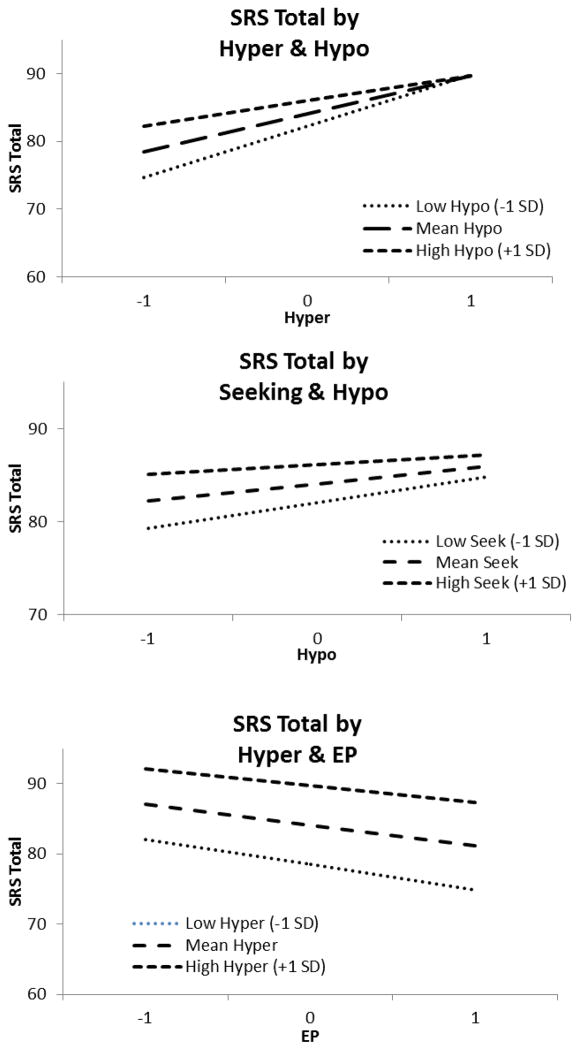
Based on previous literature and the pattern of inter-correlations in the above analyses, the following child and family characteristics were selected as the key covariates for inclusion in the mixed model regression analysis: CA, IQ Proxy, gender, and income. In the mixed model regression analysis (Table 5), all sensory response patterns were found to significantly predict autism severity. As expected, both CA and IQ Proxy were negatively related with SRS, such that increases in either were associated with lower autism severity. Boys tended to be about 2 points lower on the SRS than girls (mean difference = 2.03, p = .002). A number of significant two-way interactions were present (see Figure 2). Three and four way interactions were tested, but were not significant. The test of random effects for the intercept was significant, z = 2.37, p = .009, indicating significant between-subject variance.
Table 5.
Mixed Model Predicting SRS/SRS-P Score from Sensory Patterns (n=1156)
| SRS Total | |
|---|---|
|
| |
| Parameter (SE) | |
| Seek | 2.03 (0.50)*** |
| Hypo | 1.88 (0.52)*** |
| Hyper | 5.63 (0.59)*** |
| EP | −2.98 (0.65)*** |
| Seek*Hypo | −0.84 (0.30)** |
| Hypo*Hyper | −1.91 (0.33)*** |
| Hyper*EP | 0.65 (0.28)** |
| Male | −2.03 (0.61)** |
| CA | −0.35 (0.10)** |
| Income | −0.01 (.01) |
| IQ Proxy | −0.06 (.009)*** |
Figure 2.
Two way interactions predicting SRS/SRS-P score
Sensory Response Pattern Scores by ASD Diagnosis
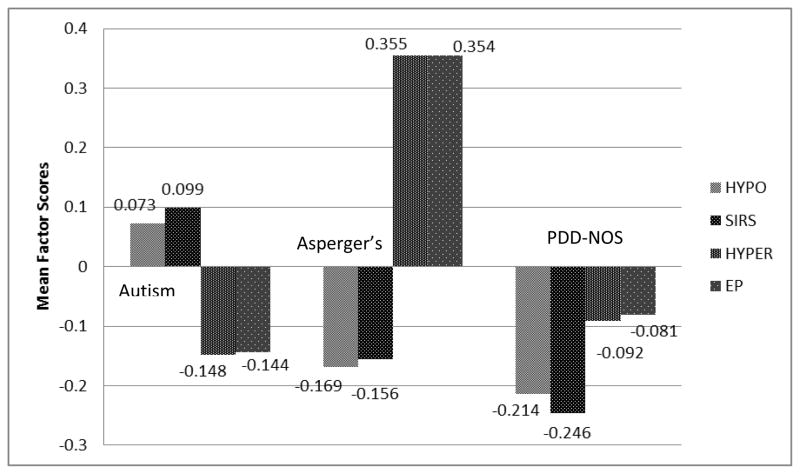
Figure 3 depicts the mean factor scores for each of the sensory response patterns by ASD diagnosis (Autism/Autistic Disorder, Asperger’s Disorder, PDD-NOS). Each diagnostic group showed a unique representation of the four sensory response patterns (HYPO, HYPER, SIRS, EP) as follows: Children with a PDD-NOS diagnosis, on average, had scores below the mean on all four sensory response patterns, indicating fewer sensory features. Children with Autism/Autistic Disorder and Asperger’s Disorder presented with a split profile: HYPO and SIRS were above the mean for children with Autism/Autistic Disorder, while HYPER and EP were above the mean for children with Asperger’s Disorder.
Figure 3.
Mean factor scores by ASD diagnosis
Discussion
This study aimed to empirically validate the factor structure of the SEQ, and test the effect of sensory response patterns on autism severity while accounting for key child and family characteristics in a large national sample of children with ASD ages 2–12 years. A complex factor analytic model was confirmed for the specified factor structure, including four sensory response patterns (HYPO, HYPER, SIRS, EP), and one social context and five modality constructs as measurement variables. The model fit and factor loadings provided validation for the SEQ 3.0 conceptual model with four distinct sensory patterns among children with ASD. Inclusion of social context and modality constructs as part of the measurement structure of the model allowed for a more precise estimation of the individual sensory features that contributed to each sensory pattern, by removing the error variance or statistical noise associated with the modalities and social latent variables.
The limited number of necessary instrument modifications provided further evidence of a sound theoretical model. All measured variables representing the 97 Likert scale items from the SEQ-3.0 were included in the model, and all items made significant contributions to at least one of the latent variables. Within each sensory pattern construct, the high factor loadings provided strong evidence for the underlying latent concepts. For example, in the SIRS construct, items such as “seems fascinated with specific textures”, “fascination with specific visual effects”, and “movement such as jumping up and down or spinning in circles” are all examples of the types of items that were driving the SIRS construct with high factor loadings between .57 to .68. The item analysis of the 97 measured variables had no strong indications of missing pathways between SEQ-3.0 items and sensory factors, further supporting distinct sensory response patterns.
Despite clear distinctions between sensory patterns, findings from the present study include the estimation of between sensory factor correlations to characterize how sensory patterns are related to one another. The moderate correlations between sensory patterns are consistent with the literature that sensory patterns often co-exist in children with ASD (Baranek et al., 2006). However, stronger associations emerged specifically between HYPER and EP as well as between HYPO and SIRS, which adds to our understanding of these features in this sample of children with ASD. These findings also suggest a potential co-occurrence of these patterns in ASD. Furthermore, the mixed model regression analysis and the mean distribution of sensory patterns by ASD diagnosis provided further support for this co-occurrence.
While sensory features have long been described as common symptoms in ASD, there is little information about how they impact autism severity. The current findings suggested that sensory patterns contributed to autism severity even when controlling for key child (i.e., CA, IQ Proxy, gender) and family (i.e., income) characteristics. Furthermore, novel findings from this study demonstrated that each of the four sensory response patterns contributed to autism severity through two-way interactions rather than through simple additive effects. For example, as the effect of HYPER on autism severity score increases, the effect of HYPO decreases. By examining sensory response pattern mean scores by diagnosis, clear combinations of sensory response patterns emerged for each ASD diagnosis (i.e., Autism/Autistic Disorder, Asperger’s Disorder, PDD-NOS). Such differential distributions may provide insight into the co-occurrence of sensory response patterns and specific characteristics associated with various types or functioning levels of children with ASD. For instance, children with Asperger’s Disorder are often described as having increased IQ as well as speech and language skills (APA, 2000; Green et al., 2006). In this study, the distribution of sensory patterns among children with Asperger’s Disorder was driven by higher means of HYPER and EP, which was also related to higher IQ Proxy. Although the DSM-5 no longer differentiates on the basis of these diagnostic subcategories, the current findings have implications for parsing out the heterogeneity in this population on the basis of sensory response patterns, and uncovering sensory phenotypes that may differentially predict developmental outcomes.
A unique contribution of this study was the inclusion of enhanced perception in the four factor model. The validation of this construct expands current conceptualizations in the literature, and provides support for addressing enhanced perception in the comprehensive clinical assessment of sensory features and diagnostic practices for children with ASD. This may be particularly important as various combinations of sensory features are differentially associated with adaptive and maladaptive outcomes (Boyd et al., 2010; Green, Ben-Sasson, Soto, Carter, 2012; Lane et al., 2010; Watson et al., 2011), and moreover, enhanced perception reflects some areas of strength in sensory-perceptual functions (Baron-Cohen et al., 2009; Mottron, et al., 2006) as opposed to deficits commonly attributed to other sensory response patterns. Intriguing patterns of concomitant strengths and deficits are found to be characteristic of individuals with autism across various developmental domains (Wallace, Happe & Giedd, 2009) and these patterns deserve further study in issues related to sensory processing as well.
Limitations and Future Directions
While the study procedures allowed for testing of a large heterogeneous sample of children with ASD, online recruitment and survey administration presented with some limitations. Specifically, the findings rely on parent-report and we were not able to validate sensory features or other child characteristics (e.g., ASD diagnostic category, IQ) through observational methods in this study for such an extensive sample. Online methods provide limited access to people without computers and internet access, resulting in less racial and socioeconomic diversity. A more stratified sample in the future could explore whether or not these demographic variables may differentially affect parents’ perceptions of their children’s sensory features Other studies have shown modest correlations between parent reported sensory features and clinician observed lab measures (Miller et al., 1999) and may be an underestimate compared to self-reports (Parush, Doryon, & Katz, 2006). Nonetheless, the factor structure confirmed by this method was consistent with previous work using multi-trait, multi-method procedures for children with ASD in this age group using a similar conceptual model (e.g., Watson et al., 2011). Future studies and instruments are needed to test the extent to which this conceptual model is applicable to individuals with ASD older than 12 years.
Currently, sensory features in children with ASD are often the target of a wide range of interventions. The development of psychometrically sound assessment tools such as the SEQ-3.0 should lead to increased diagnostic accuracy, which may aid in designing more targeted treatment interventions for sensory features in ASD. Additionally, future research could further validate these sensory response patterns, as well as their inter-correlations with specific behavioral and physiological measures to deepen our understanding of neurobiological mechanisms that may give rise to such features. Understanding the vast heterogeneity in ASD, including how sensory features contribute to this heterogeneity, is an ongoing quest for researchers and clinicians in this field; the SEQ-3.0 may be one useful tool toward this goal.
Acknowledgments
Thank you to the families that participated in the study as well as the research team at the Sensory Experiences Project. Thank you also to the Interactive Autism Network Research Database at the Kennedy Krieger Institute and John Hopkins Medicine-Baltimore (sponsored by Autism Speaks), the University of North Carolina at Chapel Hill Research Registry, and the multiple other autism organizations who assisted in recruitment. This study was supported by the National Institute of Child Health and Human Development/National Institutes of Health; ARRA Supplement A10-0589 (R01-HD042168). Recruitment was partially supported by the Intellectual and Developmental Disabilities Research Center; P30HD03110.
References
- American Psychiatric Association. Proposed Autism spectrum disorder. 2012 Retrieved from http://www.dsm5.org/proposedrevisions/pages/proposedrevision.aspx?rid=94.
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: American Psychiatric Publishing, Inc; 2000. text rev. [Google Scholar]
- Baio J. Prevalence of autism spectrum disorders–autism and developmental disabilities monitoring network, 14 sites, United States, 2008. Centers for Disease Control and Prevention Surveillance Summaries. 2012;61(3):1–19. Retrieved from http://www.cdc.gov/mmwr/pdf/ss/ss6103.pdf. [PubMed] [Google Scholar]
- Baranek GT. Sensory experiences questionnaire version 3.0. 2009. Unpublished manuscript. [Google Scholar]
- Baranek GT, David FJ, Poe MD, Stone WL, Watson LR. Sensory experiences questionnaire: Discriminating sensory features in young children with autism, developmental delays, and typical development. Journal of Child Psychology and Psychiatry. 2006;47(6):591–601. doi: 10.1111/j.1469-7610.2005.01546.x. [DOI] [PubMed] [Google Scholar]
- Baranek GT, Watson LR, Boyd BA, Poe MD, David FJ, McGuire L. Hyporesponsiveness to social and nonsocial sensory stimuli in children with autism, children with developmental delays, and typically developing children. Development and Psychopathology. 2013;25(02):307–320. doi: 10.1017/S0954579412001071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Cohen S. The extreme male brain theory of autism. Trends in cognitive sciences. 2002;6(6):248–254. doi: 10.1016/s1364-6613(02)01904-6. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Ashwin E, Ashwin C, Tavassoli T, Chakrabarti B. Talent in autism: hyper-systemizing, hyper-attention to detail and sensory hypersensitivity. Philosophical Transactions of the Royal Society. 2009;364:1377–1383. doi: 10.1098/rstb.2008.0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Sasson A, Cermak SA, Orsmond GI, Tager-Flusberg H, Carter AS, Kadlec MB, Dunn W. Extreme sensory modulation behaviors in toddlers with autism spectrum disorders. American Journal of Occupational Therapy. 2007;61(5):584–592. doi: 10.5014/ajot.61.5.584. [DOI] [PubMed] [Google Scholar]
- Ben-Sasson A, Hen L, Fluss R, Cermak SA, Engel-Yeger B, Gal E. A meta-analysis of sensory modulation symptoms in individuals with autism spectrum disorders. Journal of Autism and Developmental Disorders. 2009;39(1):1–11. doi: 10.1007/s10803-008-0593-3. [DOI] [PubMed] [Google Scholar]
- Bollen KA. Structural equations with latent variables. New York, NY: Wiley; 1989. [Google Scholar]
- Boyd BA, Baranek GT, Sideris J, Poe MD, Watson LR, Patten E, Miller H. Sensory features and repetitive behaviors in children with autism and developmental delays. Autism Research. 2010;3(2):78–87. doi: 10.1002/aur.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryk A, Raudenbush S. Hierarchical Linear Models for Social and Behavioral Research: Applications and Data Analysis Methods. Newbury Park, CA: Sage; 1992. [Google Scholar]
- Burchinal MR, Nelson L, Poe M. Growth curve analysis: An introduction to various methods for analyzing longitudinal data. Monographs of the Society for Research in Child Development. 2006;71(3):65–87. doi: 10.1111/j.1540-5834.2006.00405.x. [DOI] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Social Sciences. 2. New York: Psychology Press; 1988. pp. 79–80. [Google Scholar]
- Constantino JN, Gruber CP. Social responsiveness scale manual. Los Angeles, CA: Western Psychological Services; 2005. [Google Scholar]
- Daniels AM, Rosenberg RE, Anderson C, Law JK, Marvin AR, Law PA. Verification of parent-report of child autism spectrum disorder diagnosis to a web-based autism registry. Journal of Autism and Developmental Disorders. 2012;42(2):257–265. doi: 10.1007/s10803-011-1236-7. [DOI] [PubMed] [Google Scholar]
- Dunn W. The sensory profile: Examiner’s manual. San Antonio, TX: The Psychological Corporation; 1999. [Google Scholar]
- Dunn W, Myles BS, Orr S. Sensory processing issues associated with Asperger syndrome: A preliminary investigation. The American Journal of Occupational Therapy. 2002;56(1):97–102. doi: 10.5014/ajot.56.1.97. [DOI] [PubMed] [Google Scholar]
- Ermer J, Dunn W. The sensory profile: A discriminant analysis of children with and without disabilities. The American Journal of Occupational Therapy. 1998;52(4):283–290. doi: 10.5014/ajot.52.4.283. [DOI] [PubMed] [Google Scholar]
- Gabriels RL, Agnew JA, Miller LJ, Gralla J, Pan Z, Goldson E, Hooks E. Is there a relationship between restricted, repetitive, stereotyped behaviors and interests and abnormal sensory response in children with autism spectrum disorders? Research in Autism Spectrum Disorders. 2008;2(4):660–670. doi: 10.1016/j.rasd.2008.02.002. [DOI] [Google Scholar]
- Glennon TJ, Miller-Kuhaneck H, Henry DA, Parham LD, Ecker C. Sensory processing measure manual. Los Angeles: Western Psychological Services; 2007. [Google Scholar]
- Green SA, Ben-Sasson A, Soto TW, Carter AS. Anxiety and sensory over-responsivity in toddlers with autism spectrum disorders: bidirectional effects across time. Journal of Autism and Developmental Disorders. 2012;42(6):1112–9. doi: 10.1007/s10803-011-1361-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green VA, Pituch KA, Itchon J, Choi A, O’Reilly M, Sigafoos J. Internet survey of treatments used by parents of children with autism. Research in Developmental Disabilities. 2006;27:70–84. doi: 10.1016/j.ridd.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Happé F, Frith U. The weak coherence account: Detail-focused cognitive style in autism spectrum disorders. Journal of Autism and Developmental Disorders. 2006;36(1):5–25. doi: 10.1007/s10803-005-0039-0. [DOI] [PubMed] [Google Scholar]
- Hilton C, Graver K, LaVesser P. Relationship between social competence and sensory processing in children with high functioning autism spectrum disorders. Research in Autism Spectrum Disorders. 2007;1(2):164–173. doi: 10.1016/j.rasd.2006.10.002. [DOI] [Google Scholar]
- Horn JL, McArdle JJ. A practical and theoretical guide to measurement invariance in aging research. Experimental Aging Research. 1992;18(3):117–144. doi: 10.1080/03610739208253916. [DOI] [PubMed] [Google Scholar]
- Joosten AV, Bundy AC. Sensory Processing and stereotypical and repetitive behavior in children with autism and intellectual disability. Australian occupational therapy journal. 2010;57(6):366–372. doi: 10.1111/j.1440-1630.2009.00835.x. [DOI] [PubMed] [Google Scholar]
- Jöreskog KG. A general approach to confirmatory maximum likelihood factor analysis. Psychometrika. 1969;34(2):183–202. doi: 10.1007/BF02289343. [DOI] [Google Scholar]
- Kenny DA, Kashy DA. Analysis of the multitrait-multimethod matrix by confirmatory factor analysis. Psychological Bulletin. 1992;112(1):165–172. doi: 10.1037/0033-2909.112.1.165. [DOI] [Google Scholar]
- Kline RB. Principles and practice of structural equation modeling. New York, NY: Guilford Press; 2010. [Google Scholar]
- Lane AE, Young RL, Baker AEZ, Angley MT. Sensory processing subtypes in autism: Association with adaptive behavior. Journal of Autism and Developmental Disorders. 2010;40(1):112–122. doi: 10.1007/s10803-009-0840-2. [DOI] [PubMed] [Google Scholar]
- Liss M, Saulnier C, Fein D, Kinsbourne M. Sensory and attention abnormalities in autistic spectrum disorders. Autism. 2006;10(2):155–172. doi: 10.1177/1362361306062021. [DOI] [PubMed] [Google Scholar]
- Little LM, Freuler AC, Houser MB, Guckian L, Carbine K, David FJ, Baranek GT. Psychometric validation of the sensory experiences questionnaire. The American Journal of Occupational Therapy. 2011;65(2):207–210. doi: 10.5014/ajot.2011.000844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh DN, Miller LJ, Shyu V. Development and validation of the short sensory profile. In: Dunn W, editor. Sensory profile manual. San Antonio, TX: Psychological Corporation; 1999. pp. 59–73. [Google Scholar]
- Miller LJ, McIntosh DN, McGrath J, Shyu V, Lampe M, Taylor AK, Tassone F, Neitzel K, Stackhouse T, Hagerman RJ. Electrodermal responses to sensory stimuli in individuals with fragile X syndrome: A preliminary report. American Journal of Medical Genetics. 1999;83:268–279. doi: 10.1002/(SICI)1096-8628(19990402)83:4<268::AID-AJMG7>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Mottron L, Dawson M, Soulières I. Enhanced perception in savant syndrome: Patterns, structure and creativity. Philosophical Transactions of the Royal Society B: Biological Sciences. 2009;364(1522):1385–1391. doi: 10.1098/rstb.2008.0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottron L, Dawson M, Soulieres I, Hubert B, Burack J. Enhanced perceptual functioning in autism: An update, and eight principles of autistic perception. Journal of Autism and Developmental Disorders. 2006;36(1):27–43. doi: 10.1007/s10803-005-0040-7. [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus user’s guide. 6. Los Angeles: Muthén & Muthén; 2010. [Google Scholar]
- O’Donnell S, Deitz J, Kartin D, Nalty T, Dawson G. Sensory processing, problem behavior, adaptive behavior, and cognition in preschool children with autism spectrum disorders. AJOT: American Journal of Occupational Therapy. 2012;66:586–594. doi: 10.5014/ajot.2012.004168. [DOI] [PubMed] [Google Scholar]
- Parham LD, Ecker C, Miller-Kuhananeck H, Henry DA, Glennon T. Los Angeles: Western Psychological Services, editor. Sensory processing measure (SPM) manual. Los Angeles: Western Psychological Services; 2007. [Google Scholar]
- Parush S, Doryon YD, Katz N. A comparison of self-report and informant report of tactile defensiveness amongst children in Israel. Occupational Therapy International. 2006;3(4):274–283. doi: 10.1002/oti.41. [DOI] [Google Scholar]
- Pine E, Luby J, Abbacchi A, Constantino JN. Quantitative assessment of autistic symptomatology in preschoolers. Autism. 2006;10(4):344–352. doi: 10.1177/1362361306064434. [DOI] [PubMed] [Google Scholar]
- Qualtrics Labs, Inc. software. Version 21269 of the Qualtrics Research Suite. Provo, UT: Qualtrics Labs, Inc; 2011. http://www.qualtrics.com. [Google Scholar]
- Rogers SJ, Hepburn S, Wehner E. Parent reports of sensory symptoms in toddlers with autism and those with other developmental disorders. Journal of Autism and Developmental Disorders. 2003;33(6):631–642. doi: 10.1023/B:JADD.0000006000.38991.a7. [DOI] [PubMed] [Google Scholar]
- Rogers SJ, Ozonoff S. Annotation: What do we know about sensory dysfunction in autism? A critical review of the empirical evidence. Journal of Child Psychology and Psychiatry. 2005;46(12):1255–1268. doi: 10.1111/j.1469-7610.2005.01431.x. [DOI] [PubMed] [Google Scholar]
- Schoen SA, Miller LJ, Brett-Green BA, Nielsen DM. Physiological and behavioral differences in sensory processing: A comparison of children with autism spectrum disorder and sensory modulation disorder. Frontiers in Integrative Neuroscience. 2009;3:29–40. doi: 10.3389/neuro.07.029.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacker RE, Lomax RG. A beginner’s guide to structural equation modeling. Lawrence Erlbaum; 2004. [Google Scholar]
- Stata Corp. Stata statistical software. 12. College Station, TX: Stata Corp, LP; 2011. [Google Scholar]
- Talay-Ongan A, Wood K. Unusual sensory sensitivities in autism: A possible crossroads. International Journal of Disability, Development and Education. 2000;47(2):201–212. doi: 10.1080/713671112. [DOI] [Google Scholar]
- Wallace GL, Happe F, Giedd JN. A case study of a multiply talented savant with an autism spectrum disorder: Neuropsychological functioning and brain morphometry. Philosophsical Transactions of the Royal Society: Biological Sciences. 2009;364(1522):1425–1432. doi: 10.1098/rstb.2008.0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watling RL, Deitz J, White O. Comparison of sensory profile scores of young children with and without autism spectrum disorders. The American Journal of Occupational Therapy. 2001;55(4):416–423. doi: 10.5014/ajot.55.4.416. [DOI] [PubMed] [Google Scholar]
- Watson LR, Patten E, Baranek GT, Poe M, Boyd BA, Freuler A, Lorenzi J. Differential associations between sensory response patterns and language, social, and communication measures in children with autism or other developmental disabilities. Journal of Speech, Language, and Hearing Research. 2011;54(6):1562–1576. doi: 10.1044/1092-4388(2011/10-0029). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherby A. Social & communication development in autism spectrum disorders: Early identification, diagnosis, & intervention. New York, NY US: Guilford Press; 2006. Understanding and Measuring Social Communication in Children with Autism Spectrum Disorders; pp. 3–34. [Google Scholar]