Abstract
The tobacco-specific nitrosamines NNN and NNK are potent carcinogens for the rat esophagus and lung, respectively. Consistent with the animal carcinogenicity data, we previously reported a remarkably strong association between prospectively measured urinary total NNN, a biomarker of human NNN intake, and the risk of developing esophageal cancer among smokers in the Shanghai Cohort Study. We also demonstrated that urinary total 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL), a biomarker of exposure to the lung carcinogen NNK, is strongly associated with the risk of lung, but not esophageal cancer in smokers. In this study, we investigated the potential relationship between NNN intake and lung cancer risk in the same cohort. The prospectively collected urine samples from lung cancer cases and matching controls selected for this study, all current smokers, have been previously analyzed for total NNAL, cotinine (a biomarker of nicotine intake), and phenanthrene tetraol (PheT) (a biomarker of exposure to polycyclic aromatic hydrocarbons). Urinary levels of total NNN were not associated with the risk of lung cancer: odds ratios (95% confidence intervals) associated with the second and third tertiles of total NNN, relative to the lowest tertile, were 0.82 (0.36–1.88) and 1.02 (0.39–2.89), respectively (P for trend = 0.959), after adjustment for self-reported smoking history, urinary cotinine, and PheT. The results of this study reaffirm the previously reported specificity of urinary total NNN and total NNAL as predictors of esophageal and lung cancer risks, respectively, in smokers, and demonstrate remarkable coherence between rat target tissues of these carcinogens and susceptibility to cancer in smokers.
Keywords: biomarkers, N'-nitrosonornicotine, lung cancer
INTRODUCTION
N'-nitrosonornicotine (NNN) is present it tobacco and cigarette smoke and is a potent esophageal and oral cavity carcinogen in rats.1,2 The related tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) is a powerful systemic lung carcinogen in laboratory animals.1 Both NNN and NNK are formed via the nitrosation of alkaloids specific to tobacco plant,3,4 therefore, human exposure to these carcinogens can occur only by contact with tobacco- or nicotine-containing products. Available laboratory animal, mechanistic, and epidemiological evidence, along with the relative abundance of these nitrosamines in unburned processed tobacco and cigarette smoke, support the role of NNN as a causative agent for esophageal and oral cancers in tobacco users, and NNK as a major contributor to lung cancer in smokers.1,5,6 Based on the accumulated evidence, the International Agency for Research on Cancer has classified NNN and NNK as carcinogenic to humans (3).
Use of tobacco products is estimated to be responsible for 22% of all cancer death worldwide.7,8 The quantitative relationships between human intake of specific cigarette smoke carcinogens and the risk of cancer are critical for the understanding of individual cancer susceptibility in smokers, and for the development of preventive measures in general. However, investigation of these relationships is generally challenging due to the chemical complexity of cigarette smoke, the lack of specificity of many constituents to cigarette smoke, and the known variations in smoking topography and resulting constituent intake by individual smokers.9 Biomarkers of exposure offer a quantitative measure of specific constituent intake from tobacco products and therefore represent a valuable tool to overcome these challenges. Exposure to NNN can be measured by urinary total NNN, which is the sum of unchanged NNN and its glucuronide.10 Exposure to NNK can be assessed by measuring total NNAL, which is the sum of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) and its glucuronides.11
We previously demonstrated an exceptionally strong association between urinary total NNN and esophageal cancer risk in the prospective Shanghai Cohort Study: odds ratios of esophageal cancer for the 2nd and 3rd tertiles of urinary total NNN were 3.99 and 17.0, respectively, compared to the 1st tertile after adjustment for urinary total NNAL, total cotinine (the sum of cotinine and its glucuronide, a biomarker of nicotine intake), and smoking intensity and duration.12 The results of that study provided evidence for a significant and unique role of NNN in esophageal cancer in smokers, which is remarkably consistent with NNN carcinogenicity in rats. Also, consistent with the lack of esophageal carcinogenic potency of NNK in laboratory animals, urinary total NNAL was not associated with esophageal cancer risk in that study.12 On the other hand, we demonstrated a strong association between NNK intake, as measured by urinary or serum NNAL, and lung cancer risk in smokers in the Shanghai Cohort Study, the Singapore Chinese Health Study,13 and the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial in the U.S.,14 all three studies being based on prospective cohorts. These findings demonstrated an outstanding coherence between the effect of NNK intake in humans and the results of animal experiments.
NNN was shown to induce some respiratory tract tumors in mice and hamsters. However, unlike NNK, it does not cause lung cancer in rats.1 Furthermore, the respiratory carcinogenic potency of NNN in mice and hamsters is significantly lower than that of NNK.1 Given the coherence between the rat lung-specific carcinogenicity data and human cancer risk observed for NNK, it was important to investigate whether NNN intake results in similar organ-specific carcinogenicity in smokers. Therefore, this study examined the relationship between urinary total NNN and lung cancer risk in smokers from Shanghai Cohort Study. The relationship between urinary total NNN and the risk of lung cancer development in this study was analyzed before and after adjustment for urinary total NNAL, total cotinine, smoking intensity and duration, and urinary phenanthrene tetraol (PheT) – a metabolite of phenanthrene, a polycyclic aromatic hydrocarbon (PAH) structurally related to carcinogenic PAH15 (Figure 1). We previously examined the association between urinary total NNAL and the risk of lung cancer, as well as the relationship of urinary total NNN and total NNAL to the risk of esophageal cancer, in the same cohort. Thus the present study not only fills a knowledge gap regarding the relationship between NNN exposure and the development of lung cancer in smokers, but also provides a complete set of data that elucidates the organ-specific roles of NNN and NNK in human cancer.
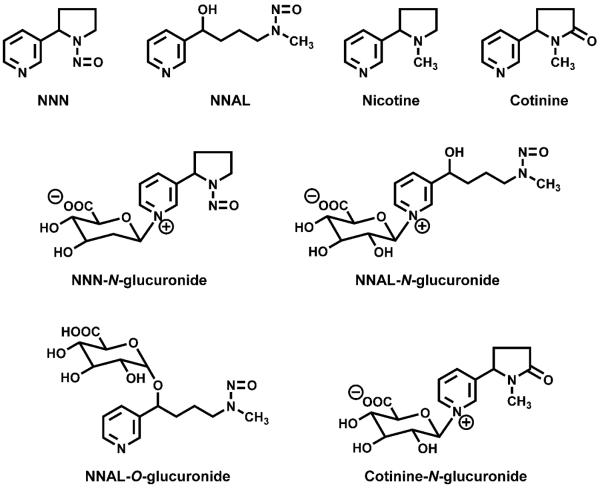
Figure 1.
Structures of biomarkers discussed in the text.
MATERIALS AND METHODS
Subjects
The Shanghai Cohort Study enrolled 18,244 men between 45 and 64 years of age from January 1, 1986 through September 30, 1989. This cohort has been approved by the Institutional Review Boards at the University of Minnesota, the Shanghai Cancer Institute, and the University of Pittsburgh. The details of the Shanghai Cohort Study have been described in previous publications.16,17
Identification of incident lung cancer cases and matching criteria for cancer-free controls were described in our previous study.13 We randomly selected 100 lung cancer cases and one matched control per case for whom urinary NNAL, cotinine, and PheT were available from our previous study of current smokers. The control subject was matched to the index case by age at enrollment (±2 years), the year and month of biospecimen collection (±1 month), and neighborhood of residence at recruitment. Urine samples were depleted on either cases or controls of seven matched case-control pairs. The remaining 93 matched case-control pairs were tested for urinary total NNN. All subjects analyzed here were current smokers.
Biomarker analyses
Two urine sample aliquots within a given matched case-control set (1 case and 1 control) were arranged in random order, identified only by unique codes, and were assayed in the same batch for total NNN by laboratory personnel who had no knowledge of the case/control status of the test samples. Total NNN was analyzed by our validated analytical procedure based on the use of liquid chromatography-tandem mass spectrometry, as previously described.12,18 The descriptions of urinary total NNAL, total cotinine, PheT, and creatinine analyses in these samples were published previously.13,19
Statistical analysis
Measured urinary concentrations of total NNN were expressed as fmol/mg creatinine (Cr), to correct for varying water contents of individual spot urine samples. The levels of total cotinine (nmol/mg Cr), total NNAL (pmol/mg Cr), and PheT (pmol/mg Cr) were available from our previous studies.13,19 To correct for skewed distributions of biomarker levels, formal statistical tests were performed on logarithmically transformed values, and geometric means are presented. Chi-square test or t test statistics were used to assess the statistical differences in distributions of frequency for categorical variables or continuous variables, respectively, between cases and controls.
Conditional logistic regression models were used to calculate odds ratios (ORs) and their corresponding 95% confidence intervals (CIs) and P values. Study subjects were grouped into tertiles according to the distribution of total NNN and total NNAL among all control subjects. The linear trend test for the association between levels of urinary total NNN and lung cancer risk was based on ordinal values. Multivariate logistic regression models were used to assess the independent effect of total NNN after adjusting for number of cigarettes/day, number of years of smoking, urinary total cotinine, total NNAL and PheT.
All statistical analyses were carried out using SAS software version 9.1 (SAS Institute, Cary, NC). All P-values reported are two-sided.
RESULTS
Of the 93 cases of lung cancer, 60 were histopathologically confirmed; there were 29 squamous cell cancers, 22 adenocarcinomas, and 9 other cell types. The remaining 33 cases were based on clinical diagnosis including radiography or computer-assisted tomography.
The baseline demographic and lifestyle characteristics of case patients and matching controls are summarized in Table 1. Age at recruitment, level of education, and alcohol drinking were comparable for lung cancer cases and controls. Patients with lung cancer had slightly lower body mass index (20.9 ± 2.3 kg/m2) than control subjects (22.0 ± 3.4 kg/m2) (P = 0.007). Individuals who developed lung cancer smoked higher numbers of cigarettes per day and had more total years of smoking than those who remained cancer-free, and these differences were statistically significant (Table 1).
Table 1.
Baseline demographic and lifestyle characteristics of current smokers who developed lung cancer (Cases) and those who remained cancer-free (Controls) in the Shanghai Cohort Study, 1986–2007
| Cases | Controls | P * | |
|---|---|---|---|
| Number of subjects | 93 | 93 | … |
| Age (years), mean±SD | 55.3±5.2 | 55.3±5.1 | 0.932 |
| Body mass index (kg/m2), mean±SD | 20.9±2.3 | 22.0±3.4 | 0.007 |
| Level of education, % | |||
| No formal education | 9.6% | 7.5% | 0.850 |
| Primary (1–6 years) | 28.0% | 30.1% | |
| Secondary & above | 62.4% | 62.4% | |
| No. cigarettes/day, mean±SD | 18.4±7.8 | 15.8±6.9 | 0.015 |
| No. years of smoking, mean±SD | 33.9±8.7 | 30.1±10.5 | 0.008 |
| No. pack-years of cigarettes, mean±SD | 32.0±16.7 | 24.1±14.0 | <0.001 |
| Alcohol drinking, % | |||
| Nondrinkers | 44.1% | 43.0% | 0.882 |
| Regular drinkers | 55.9% | 57.0% | |
| No. of drinks/day, mean±SD | 2.9±2.2† | 2.6±2.1† | 0.503 |
| Urinary biomarkers, geometric mean (95% CI) | |||
| Total cotinine (nmol/mg Cr) | 12.72 (10.62–15.26) | 7.80 (6.52–9.36) | <0.001 |
| Total NNN (fmol/mg Cr) | 67.62 (58.16–78.66) | 54.98 (47.28–63.94) | 0.0605 |
| Total NNAL (pmol/mg Cr) | 0.30 (0.26–0.34) | 0.22 (0.18–0.26) | 0.006 |
| PheT (pmol/mg Cr) | 30.64 (27.62–34.00) | 26.46 (23.86–29.36) | 0.0531 |
Two-sided Ps were based on t test for continuous variables or chi-square test for categorical variables.
Among alcohol drinkers only.
The geometric mean of urinary total NNN in lung cancer cases was 67.62 fmol/mg Cr, which was higher than in controls (54.98 fmol/mg Cr); however this difference was not statistically significant (P = 0.0605). Urinary total cotinine and total NNAL levels were also higher for cases than controls (P < 0.001 and P = 0.006, respectively), while the difference in urinary PheT was statistically borderline significant (P = 0.053) in this reduced dataset. Urinary total NNN correlated with urinary total NNAL (r = 0.67, P <0.001) and total cotinine (r = 0.36, P <0.001).
Urinary levels of total NNN were not significantly associated with increased risk of developing lung cancer (Table 2). Compared with the lowest tertile, unadjusted matched ORs (95% CIs) of lung cancer for the 2nd and 3rd tertiles of total NNN were 1.19 (0.59–2.37) and 1.99 (0.91–4.35), respectively (P for trend = 0.087). After adjustment for urinary total cotinine, urinary PheT, and smoking intensity and duration, the multivariate-adjusted ORs (95% CIs) for the 2nd and 3rd tertiles of total NNN were 0.82 (0.36–1.88) and 1.02 (0.39–2.69), respectively, compared to the lowest tertile (P for trend = 0.958). Additional adjustment for total NNAL further diminished the association between urinary total NNN and lung cancer risk (P for trend = 0.276).
Table 2.
Urinary levels of total NNN and total NNAL in relation to risk of lung cancer, The Shanghai Cohort Study 1986–2007
| 1st tertile | 2nd tertile | 3rd tertile | P for trend | |
|---|---|---|---|---|
| Total NNN (fmol/mg Cr) | <40.9 | 40.9–70.7 | >70.7 | |
| Cases/controls | 24/31 | 29/33 | 40/29 | |
| Matched OR (95% CI)* | 1.00 | 1.19 (0.59–2.37) | 1.99 (0.91–4.35) | 0.087 |
| Adjusted OR (95% CI)† | 1.00 | 0.82 (0.36–1.88) | 1.02 (0.39–2.69) | 0.958 |
| NNAL-adjusted OR (95% CI)‡ | 1.00 | 0.50 (0.19–1.35) | 0.47 (0.13–1.64) | 0.271 |
| Total NNAL (pmol/mg Cr) | <0.14 | 0.14–0.29 | >0.29 | |
| Cases/controls | 15/31 | 34/33 | 44/29 | |
| Matched OR (95% CI)* | 1.00 | 2.44 (1.05–5.67) | 4.23 (1.66–10.80) | 0.003 |
| NNN-adjusted OR (95% CI)§ | 1.00 | 1.95 (0.63–6.07) | 4.29 (0.96–19.23) | 0.053 |
Matched odds ratios were derived from conditional logistic regression models that controlled for age, year and month of sample collection, and neighborhood of residence at enrollment.
Adjusted for number of cigarettes per day, number of years of smoking, urinary total cotinine, and urinary PheT.
Adjusted for number of cigarettes per day, number of years of smoking, urinary total cotinine, urinary PheT, and urinary total NNAL.
Adjusted for number of cigarettes per day, number of years of smoking, urinary total cotinine, urinary PheT, and urinary total NNN.
Urinary levels of total NNAL in this subset of previously reported study remained strongly associated with the risk of lung cancer.12 The ORs (95% CIs) for the 2nd and 3rd tertiles of total NNAL were 1.95 (0.63–6.07) and 4.29 (0.96–19.23), respectively, compared to the lowest tertile (P for trend = 0.053) after adjustment for urinary total NNN and other smoking related factors (Table 2), the same magnitude for the total NNAL-lung cancer risk association as we reported previously in a larger dataset.20
DISCUSSION
The results of this study reiterate the strength and importance of our previous findings that urinary total NNAL is a predictor of lung cancer risk,13,20 while urinary total NNN is a powerful predictor for esophageal cancer risk in smokers in the Shanghai cohort.12 We demonstrate that the levels of urinary total NNN measured in prospectively collected samples are not associated with the risk of developing lung cancer in smokers. Importantly, this study provides additional strong support for the coherence of NNN and NNK carcinogenicity data in rats with the effects of exposure to these carcinogens in smokers.
Biomarkers of exposure not only reflect the intake of specific tobacco carcinogens, but also account for individual differences in carcinogen uptake, metabolism, and excretion rates.21 These factors may affect individual susceptibility of smokers to carcinogenic effects of tobacco constituents. Therefore, biomarkers of exposure can potentially be used, along with other markers of susceptibility, in screening approaches to identify those smokers who are at higher risk of developing cancer.
In our previous study, urinary total NNN almost completely accounted for the observed associations of esophageal cancer risk with smoking history and intensity.12 The significant and strong association of urinary total NNN with esophageal cancer risk demonstrated the remarkable predictive power of this biomarker. In the same study, urinary total NNAL was not associated with the risk of esophageal cancer after adjustment for total NNN. Therefore, the lack of a relationship between urinary total NNN and the risk of lung cancer in the same cohort, as shown in this study, further validates the unique role of urinary total NNN as a predictor of esophageal cancer risk in smokers. Furthermore, the findings of the present study also reaffirmed our previous finding of a strong association between the levels of urinary total NNAL and risk of lung cancer in the Shanghai Cohort Study.13,20
The findings of this study demonstrate remarkable coherence between target tissues in F-344 rats treated with NNN or NNK, and the biomarker results in the Shanghai Cohort study. The esophagus is the main target tissue for carcinogenicity in rats treated with NNN in the drinking water; lung tumors have never been observed.1 In contrast, lung tumors are always induced by treatment of rats with NNK, but esophageal tumors have never been reported. The biomarker results reported here and in our previous studies are completely consistent with these results. Urinary total NNN is strongly related to esophageal cancer but not lung cancer, while urinary total NNAL is strongly related to lung cancer but not esophageal cancer. These results support the use of F-344 rat studies to elucidate the carcinogenic effects in smokers of tobacco-specific nitrosamines and possibly other tobacco smoke constituents.
In summary, we investigated the relationship between a prospectively measured urinary biomarker of exposure to the potent tobacco carcinogen NNN and the risk of lung cancer among smokers from the Shanghai Cohort Study. There was no association between NNN exposure and lung cancer risk in these smokers. This study reaffirms our previous findings that urinary total NNN and total NNAL are specific predictors of esophageal and lung cancers, respectively, in smokers, demonstrating exceptional coherence between human and rat target tissues of these carcinogens.
Novelty and Impact: Laboratory animal data demonstrate that the tobacco-specific nitrosamines NNN and NNK are highly carcinogenic for the rat esophagus and lung, respectively. This study provides strong support for the coherence of the NNN and NNK carcinogenicity data in rats with the effects of exposure to these carcinogens in smokers. These results support the use of F-344 rat studies to elucidate the carcinogenic effects in smokers of tobacco-specific nitrosamines and possibly other tobacco smoke constituents.
Acknowledgements
We thank Ms. Xue-Li Wang of the Shanghai Cancer Institute for supervising the field work of the Shanghai Cohort Study. We also thank the Shanghai Cancer Registry for assistance with identification of cancer outcomes in the Shanghai Cohort Study. We also thank Bob Carlson for editorial assistance. This study was supported by grants no. CA-129534, CA-144034, and CA-81301 from the National Cancer Institute.
Reference List
- 1.Hecht SS. Biochemistry, biology, and carcinogenicity of tobacco-specific N-nitrosamines. Chem Res Toxicol. 1998;11:559–603. doi: 10.1021/tx980005y. [DOI] [PubMed] [Google Scholar]
- 2.Balbo S, James-Yi S, O'Sullivan G, Stepanov I, Wang M, Zhang S, Kassie F, Carmella S, Wettlaufer C, Hohol K, Knezevich A, Upadhyaya P, et al. (S)-N'-Nitrosonornicotine, a constituent of smokeless tobacco, is a potent oral tumorigen in rats. Abstracts, AACR Annual Meeting, March 31-April 4, 2012; Chicago, IL. 2013 Jun 12; 2012. Epub ahead of print. [Google Scholar]
- 3.Hoffmann D, Hecht SS, Ornaf RM, Wynder EL, Tso TC. Nitrosonornicotine: presence in tobacco, formation and carcinogenicity. In: Walker EA, Bogovski P, Griciute L, editors. Environmental N-Nitroso Compounds: Analysis and Formation. International Agency for Research on Cancer; Lyon, France: 1976. pp. 307–320. [Google Scholar]
- 4.Hecht SS, Chen CB, Dong M, Ornaf RM, Hoffmann D, Tso TC. Studies on non-volatile nitrosamines in tobacco. Beitr Tabakforsch Int. 1977;9:1–6. [Google Scholar]
- 5.International Agency for Research on Cancer . IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. vol. 83. IARC; Lyon, FR: 2004. Tobacco Smoke and Involuntary Smoking. [PMC free article] [PubMed] [Google Scholar]
- 6.International Agency for Research on Cancer . IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. v. 89. IARC; Lyon, FR: 2007. Smokeless tobacco and tobacco-specific nitrosamines. [PMC free article] [PubMed] [Google Scholar]
- 7.American Cancer Society . Cancer Facts and Figures 2009. 2009. [Google Scholar]
- 8.Secretan B, Straif K, Baan R, Grosse Y, El Ghissassi F, Bouvard V, Benbrahim-Tallaa L, Guha N, Freeman C, Galichet L, Cogliano V. A review of human carcinogens - Part E: tobacco, areca nut, alcohol, coal smoke, and salted fish. Lancet Oncol. 2009;10:1033–34. doi: 10.1016/s1470-2045(09)70326-2. [DOI] [PubMed] [Google Scholar]
- 9.Djordjevic MV, Stellman SD, Zang E. Doses of nicotine and lung carcinogens delivered to cigarette smokers. J Natl Cancer Inst. 2000;92(2):106–11. doi: 10.1093/jnci/92.2.106. [DOI] [PubMed] [Google Scholar]
- 10.Stepanov I, Hecht SS. Tobacco-specific nitrosamines and their N-glucuronides in the urine of smokers and smokeless tobacco users. Cancer Epidemiol Biomarkers & Prev. 2005;14:885–91. doi: 10.1158/1055-9965.EPI-04-0753. [DOI] [PubMed] [Google Scholar]
- 11.Carmella SG, Akerkar S, Hecht SS. Metabolites of the tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in smokers' urine. Cancer Res. 1993;53:721–24. [PubMed] [Google Scholar]
- 12.Yuan JM, Knezevich AD, Wang R, Gao YT, Hecht SS, Stepanov I. Urinary levels of the tobacco-specific carcinogen N'-nitrosonornicotine and its glucuronide are strongly associated with esophageal cancer risk in smokers. Carcinogenesis. 2011;32(9):1366–71. doi: 10.1093/carcin/bgr125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yuan JM, Koh WP, Murphy SE, Fan Y, Wang R, Carmella SG, Han S, Wickham KM, Gao YT, Yu MC, Hecht SS. Urinary levels of tobacco-specific nitrosamine metabolites in relation to lung cancer development in two prospective cohorts of cigarette smokers. Cancer Res. 2009;69(7):2990–2995. doi: 10.1158/0008-5472.CAN-08-4330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Church TR, Anderson KE, Caporaso NE, Geisser MS, Le C, Zhang Y, Benoit A, Carmella SG, Hecht SS. A prospectively measured serum biomarker for a tobacco-specific carcinogen and lung cancer in smokers. Cancer Epidemiol Biomarkers & Prev. 2009;19:260–266. doi: 10.1158/1055-9965.EPI-08-0718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hecht SS, Carmella SG, Villalta PW, Hochalter JB. Analysis of phenanthrene and benzo[a]pyrene tetraol enantiomers in human urine: relevance to the bay region diol epoxide hypothesis of benzo[a]pyrene carcinogenesis and to biomarker studies. Chem Res Toxicol. 2010;23:900–908. doi: 10.1021/tx9004538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ross RK, Yuan JM, Yu MC, Wogan GN, Qian GS, Tu JT, Groopman JD, Gao YT, Henderson BE. Urinary aflatoxin biomarkers and risk of hepatocellular carcinoma. The Lancet. 1992;339:943–46. doi: 10.1016/0140-6736(92)91528-g. [DOI] [PubMed] [Google Scholar]
- 17.Yuan JM, Ross RK, Wang XL, Gao YT, Henderson BE, Yu MC. Morbidity and mortality in relation to cigarette smoking in Shanghai, China. A prospective male cohort study. JAMA. 1996;275:1646–50. [PubMed] [Google Scholar]
- 18.Stepanov I, Carmella SG, Briggs A, Hertsgaard L, Lindgren B, Hatsukami D, Hecht SS. Presence of the carcinogen N'-nitrosonornicotine in the urine of some users of oral nicotine replacement therapy products. Cancer Res. 2009;69(21):8236–40. doi: 10.1158/0008-5472.CAN-09-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hecht SS, Murphy SE, Stepanov I, Nelson HH, Yuan JM. Tobacco smoke biomarkers and cancer risk among male smokers in the Shanghai Cohort Study. Cancer Lett. 2012;334:34–38. doi: 10.1016/j.canlet.2012.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yuan JM, Gao YT, Murphy SE, Carmella SG, Wang R, Zhong Y, Moy KA, Davis AB, Tao L, Chen M, Han S, Nelson HH, et al. Urinary levels of cigarette smoke constituent metabolites are prospectively associated with lung cancer development in smokers. Cancer Res. 2011;71(21):6749–57. doi: 10.1158/0008-5472.CAN-11-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hecht SS, Yuan JM, Hatsukami D. Applying tobacco carcinogen and toxicant biomarkers in product regulation and cancer prevention. Chem Res Toxicol. 2010;23:1001–8. doi: 10.1021/tx100056m. [DOI] [PMC free article] [PubMed] [Google Scholar]