Abstract
Previous anti-inflammatory strategies against sepsis, a leading cause of death in hospitals, had limited efficacy in clinical trials, in part because they targeted single cytokines and the experimental models failed to mimic clinical settings1-3. Neuronal networks represent physiological mechanisms selected by evolution to control inflammation that can be exploited for the treatment of inflammatory and infectious disorders3. Here, we report that sciatic nerve activation with electroacupuncture controls systemic inflammation and rescues mice from polymicrobial peritonitis. Electroacupuncture at the sciatic nerve controls systemic inflammation by inducing a vagal activation of DOPA decarboxylase leading to the production of dopamine in the adrenal medulla. Experimental models with adrenolectomized animals mimic clinical adrenal insufficiency4, increase the susceptibility to sepsis, and prevent the anti-inflammatory potential of electroacupuncture. Dopamine inhibits cytokine production via dopaminergic type-1 receptors. Dopaminergic D1-agonists suppress systemic inflammation and rescue mice from polymicrobial peritonitis in animals with adrenal insufficiency. Our results suggest a novel anti-inflammatory mechanism mediated by the sciatic and the vagus nerves modulating the production of catecholamines in the adrenal glands. From a pharmacological perspective, selective dopaminergic agonists mimic the anti-inflammatory potential of electroacupuncture and can provide therapeutic advantages to control inflammation in infectious and inflammatory disorders.
Sepsis is the leading cause of mortality in non-coronary Intensive Care Units killing over 250,000 patients annually and accounting for 9.3% of overall deaths in the USA1-3. Infection, hemorrhage, resuscitation, shock, trauma, and cancer contribute to severe sepsis, which is characterized by overwhelming inflammatory responses that cause multiple organ failure2,5-8. New antibiotics are efficient in controlling the infection, but they do not control inflammation. Currently, there is no treatment approved by the FDA for severe sepsis, and most of the therapies are largely supportive. Despite the promising results inhibiting single inflammatory cytokines such as Tumor Necrosis Factor (TNF) or High Mobility Group Box (HMGB)1 in experimental models of sepsis2,7,8, these strategies have failed in clinical trials9. One explanation is that sepsis is not produced by a single cytokine and, thus a successful treatment for sepsis may require inhibiting multiple cytokines. Recent studies indicate that the vagus nerve controls inflammation10 and prevents lethal experimental sepsis11,12. Despite its recent identification, multiple investigators have already reported that the vagus nerve controls systemic inflammation in experimental ischemia and reperfusion13-15, hemorrhage and resuscitation15, pancreatitis16, colitis17, endotoxemia10,11, septic shock and severe sepsis18,19. However, the clinical implications of the vagus nerve are limited by the anesthetics and the surgery required for the direct nerve stimulation. We hypothesized that electroacupuncture can represent an alternative strategy for vagal stimulation. Although the use of electroacupuncture is endorsed by the National Institute of Health and the World Health Organization, and there is growing evidence supporting its effects in postoperative and stroke rehabilitation20-23, its mechanism to control inflammation remains unknown24,25.
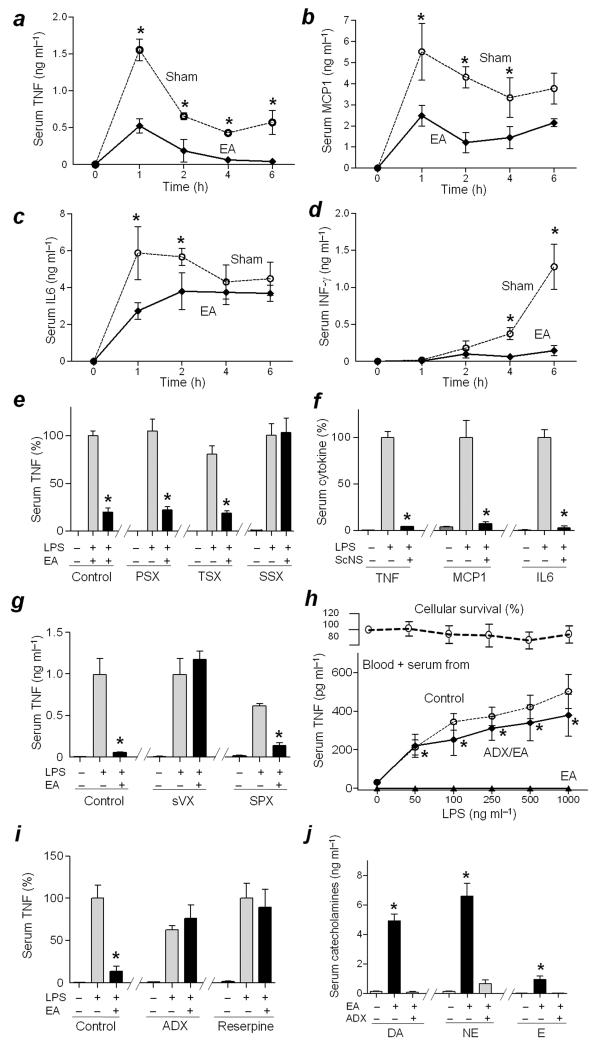
Electroacupuncture at the ST36 Zusanli acupoint reduced the Lipopolysaccharide (LPS)-induced serum levels of all the cytokines analyzed, including TNF, Monocyte Chemotactic Protein-1 (MCP1), Interleukin-6 (IL6), and Interferon-γ (INF-γ) (Fig.1a–d). These results indicated that electroacupuncture inhibited and did not merely delay cytokine production. The anti-inflammatory potential of electroacupuncture is voltage-dependent and electroacupuncture with a wood toothpick or stimulation of a non-acupuncture point did not inhibit cytokine levels (Supplementary Fig.1a–c). Local sensory signals were analyzed using capsaicin and selective neurectomies. Capsaicin, an agonist for the Transient Receptor Potential Vanilloid member 1 that interferes with nociceptive and voltage-dependent neuronal pathways, abolished the anti-inflammatory effect of electroacupuncture (Supplementary Fig.1d). Surgical sectioning of the sciatic, but not the common peroneal or tibial nerve, abolished the anti-inflammatory potential of electroacupuncture (Fig.1e). These results suggest that both the common peroneal and the tibial nerves contribute to the anti-inflammatory potential of electroacupuncture by activating the sciatic nerve. Conversely, direct electrical stimulation of the sciatic nerve mimicked the anti-inflammatory effects of electroacupuncture (Fig.1f) in a voltage-dependent manner demonstrating for the first time the ability of the sciatic nerve to control systemic inflammation in sepsis (Supplementary Fig.1e).
Figure 1. Electroacupuncture controls systemic inflammation in sepsis via the sciatic, the vagus nerves, and catecholamines from the adrenal glands.
(a–d) Adult C57BL/6 male mice were treated with LPS and sham or electroacupuncture (EA). Serum cytokines levels of TNF (a), MCP1 (b), IL6 (c) and INFγ (d) were analyzed at the indicated time points post-LPS. (e) Mice underwent sham (Control) or surgical neurectomy of the common peroneal (PSX), tibial (TSX), or sciatic nerve (SSX) 72 h before the LPS challenge and the sham or electroacupuncture (EA). *P<0.01 vs LPS (n=3/group; Student’s t–test) (f) Mice underwent sham or sciatic nerve stimulation (ScNS) for 15 min before the LPS challenge. (g) Mice underwent sham (Control), subdiaphragmatic vagotomy (sVX) 72 h, or splenectomy (SPX) 3 days before the LPS challenge and the subsequent sham or electroacupuncture (EA). (h) Blood from untreated animals (Blood) was supplemented with 50% serum from donor animals that underwent sham (Control) or electroacupuncture in sham (EA) or adrenalectomized (ADX/EA) mice. Upper panels represent cell survival as determined by MTT assay. (t-test EA vs. ADX/EA). (i) Mice underwent control, surgical adrenalectomy (ADX), or treatment with Reserpine before electroacupuncture (EA). (j) Dopamine (DA), norepinephrine (NE) or epinephrine (E) were analyzed in the serum of control or adrenalectomized (ADX) mice with sham or electroacupuncture (EA). TNF was analyzed at 90 min in the serum or 180 min in the conditioned media post-LPS. *P<0.01 (n=4/group; One-way ANOVA with Bonferroni’s corrections).
Given that the vagus nerve inhibits cytokine production in the spleen11,26, and prevents systemic inflammation in endotoxemia11,26, we analyzed its contribution to the anti-inflammatory effects of electroacupuncture. Both cervical (Supplementary Fig.2a) and subdiaphragmatic (Fig.1g) vagotomy abolished the anti-inflammatory potential of electroacupuncture, whereas splenectomy did not prevent this effect (Fig.1g). These results reveal a novel mechanism for vagal modulation of systemic inflammation. To define this mechanism, blood from animals with sham or electroacupuncture was treated in vitro with endotoxin. Endotoxin induced TNF production in sham blood but not in that from animals with electroacupuncture (Supplementary Fig.2b). Likewise, the serum from the animals with electroacupuncture attenuated in vitro TNF production in untreated blood (Fig.1h). This anti-inflammatory potential of the serum with animals with electroacupuncture was prevented by adrenolectomy (Fig.1h) but not by splenectomy (Supplementary Fig.2c). Conversely, electroacupuncture lowered serum TNF levels in sham, but not in adrenalectomized animals (Fig.1i). The potential role of catecholamines was confirmed by using reserpine, a classical inhibitor of the vesicular monoamine transporter26, that abolishes the anti-inflammatory effects of electroacupuncture (Fig.1i, Supplementary Fig.2d). Conversely, electroacupuncture increased serum levels of all the three catecholamines, but predominantly dopamine and norepinephrine (Fig.1j). This mechanism was mediated by the vagus nerve and the adrenal glands because either adrenalectomy (Fig.1j), cervical (Supplementary Fig.2e) or subdiaphragmatic (Fig.2a) vagotomy abolished the production of catecholamines. Conversely, direct electrical stimulation of the vagus nerve mimicked the production of dopamine and norepinephrine induced by electroacupuncture (Fig.2a). These results establish a novel mechanism for vagal modulation of inflammation mediated by the adrenal glands and independent of the spleen. Both Kollmann (1860) and Teitelbaum (1933) demonstrated the direct vagal innervations of the adrenal glands by tracing the posterior vagus cord into the subdiaphragmatic region into the adrenal glands. The vagal innervation of the adrenal medulla has been recently reported by retrograde tracers27. Although sympathetic and parasympathetic systems are considered to act in opposition to maintain physiological homeostasis, both the preganglionic sympathetic neurons and vagus nerve converge in the production of acetylcholine in the adrenal medulla. Given that acetylcholine is the principal neurotransmitter of the vagus nerve and that vagal immune modulation requires the alpha7 acetylcholine receptors (α7nAChRs)28,29, we analyzed whether this receptor is required for electroacupuncture. Electroacupuncture did not require the α7nAChR to either inhibit serum TNF levels or induce dopamine or norepinephrine (Fig.2a,b; Supplementary Fig.3a).
Figure 2. Electroacupuncture induces the expression of DOPA decarboxylase and dopamine.
(a) Catecholamines were analyzed in the serum C57BL/6 mice with electroacupuncture in animals with subdiaphragmatic vagotomy (EA+sVX), vagus nerve stimulation (VNS), or electroacupuncture in α7nAChR-knockout (EA-7KO) mice. ND=Not Detected (b) Serum TNF levels were analyzed in C57BL/6 mice (Control), α7nAChR-knockout (α7KO) mice, β2adrenoceptor-knockout (β2KO) mice, or C57BL/6 mice treated with fusaric acid (40mg kg−1; i.p.) and electroacupuncture (EA). (c) Blood from wild-type (WT) or β2adrenoceptor-knockout (β2KO) mice was treated in vitro with LPS and the indicated concentrations of norepinephrine (NE). (d) Mice received fusaric acid (40mg kg−1; i.p.), and sham or electroacupuncture (EA) and serum levels of catecholamines were analyzed after 15 min post-EA. (e) The adrenal glands from adult C57BL/6 male mice that received sham (S) or electroacupuncture (EA) were collected and analyzed by Western-blot for DOPA decarboxylase (DDC) and Dopamine β–hydroxylase (DβH) expression. β–Actin was used to normalize protein loading. The graph depicts the relative values of the densitometry of the experimental samples as compared with the control. In all experiments *P<0.01 vs Sham (S) (n=3/group; Student’s t-test). (f) The adrenal glands from mice with sham or electroacupuncture (EA) were analyzed for immunostaining for DOPA decarboxylase (DDC) or Dopamine β–hydroxylase (DβH). (g) The adrenal glands from mice subjected to control or electroacupuncture (EA) were stained for phosphorylated neurofilaments (NF-P; green) or double stained for phosphorylated neurofilaments (NF-P; green) and DOPA decarboxylase (DDC; red). The figures are representative of three different experiments. Bars represent 100 μm.
The specific contribution of catecholamines was analyzed using β2-adrenoceptors (β2AdrR) knockout mice. β2-adrenoceptors are critical and necessary receptors for the anti-inflammatory effects of norepinephrine26. In vivo, electroacupuncture inhibited serum TNF levels in both control and β2AdrR-knockout mice (Fig.2b). However, norepinephrine inhibited TNF production in the blood from wild-type but not in that from the β2AdrR-knockout mice (Fig.2c) indicating that norepinephrine requires β2AdrR to inhibit TNF production in the blood. Together, these results indicate that electroacupuncture does not require norepinephrine to inhibit TNF production in the blood. This hypothesis was confirmed by using fusaric acid, a conventional inhibitor of the dopamine beta -hydroxylase that abolishes norepinephrine production. Fusaric acid did not prevent the anti-inflammatory effects of electroacupuncture (Fig.2b), but it specifically inhibited electroacupuncture-induced production of norepinephrine without affecting dopamine levels (Fig.2d). By contrast, reserpine inhibited the production of both dopamine and norepinephrine and prevented the anti-inflammatory potential of electroacupuncture (Fig.1i). These results suggest that dopamine plays a critical role in the anti-inflammatory potential of electroacupuncture. These results also provided a new role for dopamine, which is conventionally viewed in critical care just as the biological precursor of norepinephrine. In order to determine how electroacupuncture induces dopamine, we analyzed the regulation of DOPA decarboxylase (that catalyzes the formation of dopamine from L-DOPA) and Dopamine beta-hydroxylase (that catalyzes the degradation of dopamine to norepinephrine) in the adrenal gland. Electroacupuncture increased the levels of DOPA decarboxylase in the adrenal medulla by 4-fold without significantly affecting the levels of Dopamine beta-hydroxylase (Fig.2e,f). Electroacupuncture activated the neuronal network of the adrenal medulla in the proximity of the chromaffin cells overexpressing DOPA decarboxylase (Fig.2g).
Electroacupuncture started right after the septic challenge reduced mortality, morbidity, and weight loss in endotoxemic mice (Fig.3a–c) and improved survival in polymicrobial peritonitis induced by cecal ligation and puncture (Fig.3d). Furthermore, electroacupuncture also “rescued” the mice from lethal polymicrobial peritonitis even when the treatment was started 24 h after the cecal ligation and puncture (Fig.3e). Although these results suggest that electroacupuncture could be used in a clinically relevant time frame, we wondered the clinical implications of these results according to recent studies reporting adrenal insufficiency in septic patients4. To mimic the adrenal insufficiency, we studied sepsis and electroacupuncture in adrenalectomized animals. Adrenalectomy rendered the animals more susceptible to polymicrobial peritonitis (Supplementary Fig.3b), and prevented the potential of electroacupuncture to improve survival in polymicrobial peritonitis (Fig.3f).
Figure 3. Electroacupuncture “rescues” mice from established polymicrobial peritonitis.
(a-c) Adult C57BL/6 male mice underwent sham or electroacupuncture (EA) started right after the endotoxin challenge (LPS; 12 mg kg−1; i.p.). (a) Survival was represented in a Kaplan-Meier analysis (n=20/group, *P<0.01 Survival Logrank test vs. control). (b) Morbidity and (c) weight modifications were represented in mean ± SE. *P<0.01 (n=6/group; One-way ANOVA with Bonferroni’s corrections). (d-e) All mice underwent CLP and sham or electroacupuncture (EA) started (d) 15 min before or (e) 24 h after the CLP. In addition to the first treatment, all animals received sham or electroacupuncture for 15 min once a day for the next two days. Survival was represented in a Kaplan-Meier analysis (n=15/group, *P<0.05 Survival Logrank test vs. control). (f) Mice underwent surgical adrenalectomy (ADX) before CLP and subsequent sham or electroacupuncture (EA, given once a day for three days). Survival is represented in a Kaplan-Meier analysis (n=20/group, *P<0.05 Survival Logrank test vs. control).
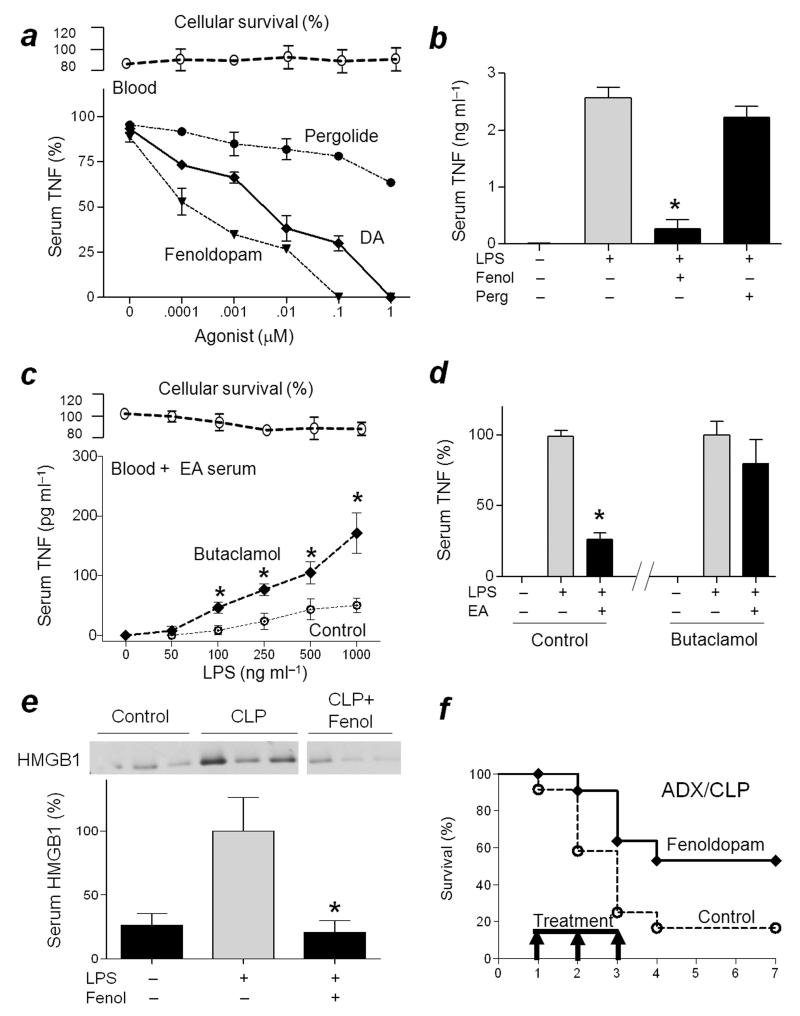
Given that the anti-inflammatory effects of electroacupuncture are mediated by dopamine, we reasoned that dopaminergic agonists may mimic electroacupuncture and control inflammation in septic patients with adrenal insufficiency. We analyzed the anti-inflammatory potential of dopamine, fenoldopam, a highly selective agonist for peripheral D1-receptors, and pergolide, a highly selective agonist for D2-like receptors30,31. Fenoldopam was more efficient than dopamine and pergolide at inhibiting LPS-induced TNF production both ex vivo and in vivo (Fig.4a,b). The role of D1-receptors was confirmed by using butaclamol, a standard D1-receptor antagonist32,33 that inhibited the anti-inflammatory effects of electroacupuncture (Fig.4c,d; Supplementary Fig.3c). One dose of fenoldopam right after the induction of sepsis prevented mortality in polymicrobial peritonitis (Supplementary Fig.3d), and inhibited serum HMGB1 levels (Fig.4e). Since serum HMGB1 levels peak around 24 h after cecal ligation and puncture18, we reasoned that treatment with Fenoldopam could be started after the onset of sepsis to provide a clinically relevant therapeutic time window. Treatment with fenoldopam, started 24 h after the cecal ligation and puncture, “rescued” mice with adrenal insufficiency from established polymicrobial peritonitis (Fig.4f). Survival was recorded for three weeks and no late deaths were observed, suggesting that fenoldopam induced a lasting protection and did not merely delay the inflammatory responses. Our results concur with a randomized, double-blind, placebo-controlled pilot trial indicating that prophylactic fenoldopam can protect renal function in septic patients34. Furthermore, the present results indicate that fenoldopam can improve organ function and survival even when the treatment is started after the onset of sepsis. Together, these results suggest that dopaminergic D1-agonists mimic the anti-inflammatory potential of electroacupuncture and can provide pharmacological advantages to control sepsis in patients with adrenal insufficiency and in a clinically relevant time frame (Supplementary Fig.3e).
Figure 4. D1R-agonist “rescues” mice from established polymicrobial peritonitis with adrenal insufficiency.
(a) Blood was treated with Dopamine (DA), Fenoldopam (Fenol) or Pergolide (Perg) 30 min before the LPS challenge. TNF levels were analyzed at 3 h post-LPS. Upper panels represent cell survival as determined by MTT assay. (b) Mice received Fenoldopam (10 mg kg−1 per dose; i.p.) or Pergolide (10 mg kg−1 per dose; i.p.) before the LPS challenge. Serum TNF levels were analyzed at 90 min post-LPS. *P<0.01 vs LPS (n=3/group; One-way ANOVA with Bonferroni’s corrections). (c) Untreated blood (Blood) was given vehicle (control) or Butaclamol 30 min before supplemented with 50% mice serum with electroacupuncture (EA Serum) and treated with LPS. TNF concentrations were analyzed 3 h later. (d) Mice were treated with butaclamol (12mg kg−1 per dose; i.p.) 60 min before the LPS challenge, and received sham or electroacupuncture (EA). Serum TNF levels were analyzed 90 min post-LPS. (e) Mice underwent sham (control) or CLP with vehicle or Fenoldopam (Fenol). Serum HMGB1 levels were analyzed at 24 h post-CLP. The graph below depicts the relative percent of serum HMGB1 levels as compared to CLP *P<0.01 (n=3/group; One-way ANOVA with Bonferroni’s corrections). (f) Mice underwent adrenalectomy (ADX) before CLP. Arrows represent the three doses of vehicle (control) or Fenoldopam (10mg kg−1 per dose; i.p.) started 1 day after the CLP, and given every 12 h for three days. Survival was recorded and represented in a Kaplan-Meier analysis (n=20/group, *P<0.01 Survival Logrank test vs. control).
On Line Methods
Chemicals and reagents
LPS (E. coli 0111:B4), capsaicin, reserpine, fusaric acid, dopamine, fenoldopam, pergolide and butaclamol were all purchased from Sigma-Aldrich® (Saint Louis, MO) and dissolved in sterile, pyrogen-free PBS (Gibco®, Life Technologies, Grand Island, NY). Capsaicin (5 mg kg−1 i.m.; dissolved in PBS with 10% ethyl alcohol) was administered at 72 h and 48 h before the LPS challenge. Reserpine (5 mg kg−1; i.p.; dissolved in 25% DMSO-PBS) was injected at 24 h before the lethal endotoxemia. Fusaric acid (40 mg kg−1 per dose; i.p.) was administered at 8 h and 4 h before the LPS challenge. Fenoldopam (10 mg kg−1 per dose; i.p.) was injected at 30 min and 5 min before LPS administration. Pergolide (10 mg kg−1 per dose; i.p.; dissolved in 25% DMSO-PBS) was given at 12 h and 5 min before the LPS challenge. Butaclamol (12 mg kg−1, i.p) was given 60 min before the endotoxemic challenge. In vitro; dopamine, fenoldopam, pergolide and butaclamol were used at 5 min before the LPS challenge at the concentration indicated in the figures.
Animal Experiments
Animal procedures were approved by the Institutional Animal Care & Use Committee of the New Jersey Medical School of the Rutgers University. All animal experiments were performed in 6–8 week old (≈25±5g) male mice without any exclusion criteria. Wild-type C57BL/6J male mice were obtained from The Jackson Laboratory (Bar Harbor, ME). α7nAChR-knockout (Chrna7tm1Bay) mice and wild-type littermates29 were obtained from Prof. Michael Marks. β2AdrR-knockout (Adrb2tm1Bkk) mice and wild-type littermates were obtained from Prof. Brian Kobilka26. α7nAChR- and β2AdrR-knockout mice were bred and genotyped by PCR using genomic DNA from mouse-tails and the Extract-N-Amp™ Tissue PCR kit (Sigma Chemical, Saint Louis, MO) as we described32,35. Animals were randomly distributed to ensure same age and sample size in different groups for experimental treatment. The investigators were blinded to the experimental treatments. Animals were maintained on 12-h light/dark cycle, with free access to food and water (ad libitum).
Experimental sepsis
Endotoxemia and cecal ligation and puncture were performed as we previously described in Nat Med18. Endotoxemia: Endotoxin (E. coli LPS 0111:B4; Sigma Chemical, Saint Louis, MO) was dissolved in sterile, pyrogen-free PBS (Gibco®: Life Technologies, Grand Island, NY), and sonicated for 30 min immediately before use. Animals received a LD50 dose of LPS (6 mg kg−1, i.p.). LPS was added to the whole blood to a final concentration of 250 ng ml−1 for the in vitro procedures. Cecal ligation and puncture (CLP): Cecal ligation and puncture is the most clinically relevant experimental model of sepsis because the inflammatory responses are induced by both polymicrobial peritonitis caused by the cecal puncture, and the necrotic tissue produced by the cecal ligation. Animals were anesthetized with ketamine (60 mg kg−1, i.p. Fort Dodge, Fort Dodge, IA) and xylazine (5 mg kg−1; i.p.; Boehringer Ingelheim, St. Joseph, MO) and subjected to a standard CLP procedure with 50% average mortality rate as we previously described in Nat Med18. An abdominal incision, of approximately 1.0 cm, was performed to expose and ligate the cecum at 5.0 mm from the cecal tip away from the ileocecal valve. The ligated cecal stump was punctured only once with a 22-gauge needle, and the stool was extruded (approx. 1.0 mm) to ascertain patency of puncture. The abdominal wound was closed in two layers, peritoneum and fascia separately, to prevent leakage of fluid. All animals received antibiotic (Enrofloxacine 0.9mg kg−1, s.c.; Baytril®, Bayer Health Care™, Swanee Mission, KA) dissolved in 0.9% normal saline immediately after surgery and every 12 h for 3 days.
Selective neurectomies and electrical stimulations
All selective neurectomies and electrical stimulations were performed in animals anesthetized with ketamine (60 mg kg−1, i.p. Fort Dodge, Fort Dodge, IA) and xylazine (5 mg kg−1; i.p.; Boehringer Ingelheim, St. Joseph, MO). The electrical stimulation in electroacupuncture, and direct nerve stimulation (sciatic and vagus nerve) was performed with a continuous mode stimulation for 15 min with a electrical potential difference of 4 V, an electric current of 40 mA, a pulse width of 50 μs, and a frequency of 10 Hz using the electrostimulator (STM 150, Biopac Systems, Goleta, CA) controlled with the AcqKnowledge Lab Assistant GLP software (ACK100W-G, Biopac Systems, Goleta, CA). The data was processed with the MP150 Data Acquisition System (MP150WSW, Biopac Systems, Goleta, CA). Control animals underwent a sham surgery, with the same surgical procedure but without the electrical stimulation. Electroacupuncture (EA): Electroacupuncture was performed similar to that described by Goldman et al.24. Electroacupuncture was performed by stimulating both limbs at the ST36 Zusanli acupoint by inserting each 12 mm unipolar stainless steel needle electrode (EL452, Biopac Systems, Goleta, CA) about 3 mm depth in each acupoint. The ST36 Zusanli acupoint is located 2 mm lateral to the anterior tubercle of the tibia in the anterior tibial muscle and 4 mm distal to the knee joint lower point. The ST36 Zusanli acupoint is located in the proximity of the common peroneal and tibial branches of the sciatic nerve34,35. Control treatments included the same procedure but using a non-electrical wood “toothpick” instead of the electrodes. Additional control treatments were performed by stimulating a distal non-acupoint (NAcuP). This non-acupoint is aprox 3 cm distal from the ST36 acupoint toward the tail and opposite to the knee joint. It is located over the semitendinosus muscle at 5 mm from the tail base. This non-acupoint is neither referred in the acupoint map of rodents nor close to any major nerve. Peroneal or Tibial neurectomy: The dissection of the peroneal or tibial nerve was done as previously described36. A thigh incision was performed on its external face to expose the artery parallel to the femur. The quadriceps muscles were dissected to expose the common peroneal and the tibial branches of the sciatic nerve. The selective neurectomy of the common peroneal or the tibial branches was performed by exposing the nerve, stabilizing it with nylon thread, and subsequent surgical sectioning of the nerve. The nerves were dissected 72 h before the electroacupuncture. In control sham-operated animals, the respective nerves were exposed and isolated from the surrounding tissue, but not transected. Sciatic neurectomy before electroacupuncture: A dorsal incision of aprox 1 cm was performed next to the posterior median line between the second and the fifth lumbar vertebrae. The latissimus dorsi muscle between the third and fifth lumbar vertebrae was dissected to expose the sciatic nerve branches on both sides of the dorsal root. The sciatic nerve branches were stabilized with nylon thread and cut just distal to the dorsal root ganglions. The sciatic nerve was sectioned 72 h before the electroacupuncture. Sciatic Nerve Stimulation: The sciatic nerve was exposed as described above, the nerve was not transected, and the bipolar electrode was placed at the ischeal notch for the electrical activation. Cervical vagotomy: was performed as we described in J immunol29. Animals were subjected to an anterior incision on the neck to access the sternocleidomastoid muscle. The sternocleidomastoid muscle was dissected to visualize the carotid artery and the vagus nerve. The vagus nerve branches on both sides of the neck were stabilized with nylon thread and cut. Subdiaphragmatic vagotomy was performed as we described in J Exp Med11. Animals underwent an abdominal incision covering the epigastrium and mesogastrium. The esophagus was exposed at the juncture to the stomach. Subsequently, the two vagal branches on both sides of the dorsal part of the esophagus were exposed by gently pulling down and twisting the stomach and stabilized with nylon thread. Both vagal branches were then cut 72 h before the experimental procedure. Vagus Nerve stimulation (VNS) was performed similar as we described J Exp Med11. The subdiaphragmatic vagal branches were exposed as described above, and the two vagal branches on both sides of the dorsal part of the esophagus were exposed by gently pulling down and twisting the stomach. Both vagal branches were stabilized with nylon thread, transected 72 h before the stimulation, and the bipolar electrode was placed across the base of the esophagus to the distal tips of the vagal nerves.
Ablative Surgeries
All animals were anesthetized with ketamine (60 mg kg−1, i.p. Fort Dodge, Fort Dodge, Iowa) and xylazine (5 mg kg−1; i.p.; Boehringer Ingelheim, St. Joseph, MO) before the surgical procedures. Splenectomy: Splenectomy was performed as we described in J Exp Med11. Anesthetized animals were subjected to an abdominal incision on the epigastrium and mesogastrium. The spleen was exposed by gentle retraction of the stomach to the side. The three main branches of the spleen artery were stabilized with nylon thread, ligated and cut. The spleen was removed and the wound was closed with sutures; catgut for the abdominal wall, and nylon thread for the skin. Animals were splenectomized 3 days before the experimental procedure. Adrenalectomy: Adrenalectomy was performed as described29. A dorsal incision from the first to the third lumbar vertebrae was performed on anesthetized animals. The latissimus dorsi muscle was dissected and pulled away on both sides until the kidneys were visible. Both adrenal glands and their adipose tissue were removed. Adrenalectomized animals were given drinking water with 0.9% NaCl for 10 days before the experimental procedure.
Morbidity
The score is based on the specific endpoints previously described37. Characteristic clinical manifestations of endotoxemia (piloerection, exudates around the eyes and nostrils, lethargy, diarrhea and diminished locomotor activity) were scored by three independent researchers blinded to the treatment. A 4-points score was used for lethargy and diminished locomotor activity (Low, Low-moderate, moderate, severe) and a 2-points score (absent-present) for the evaluation of hypothermia, exudates around the eyes/nostrils, diarrhea, and piloerection.
Cytokine Analyses
For in vivo serum analyses, blood was collected at the indicated time points, allowed to clot for 2 h at room temperature, and centrifuged at 2,000 g for 15 min at 4°C. In vivo, serum TNF concentrations were analyzed in the serum of the endotoxemic mice at 90 min (or the specific time points indicated in Fig.1) post-LPS using the TNF ELISA Kit (eBioscience Inc. San Diego, CA). IL 6, MCP-1 and INFγ were analyzed at the indicated time points with Cytometric Bead Array Mouse Inflammation Kit (BD Bioscences™, San Jose, CA). Blood catecholamines were determined by ELISA (Rocky Mountain Diagnostics, Colorado Springs, CO) at 15 min post-stimulation as we described26,29. For the in vitro experiments, blood was collected by cardiac puncture in heparin microtainer, and placed in 96 well cell culture plate (Greiner bio-one, CELLSTAR®, BioExpress, Kaysville, UT), and incubated with equal volume of RPMI medium (Gibco®, Life Technologies, Grand Island, NY) or experimental serum for 15 min before the LPS-challenge. Then, the samples were centrifuged (800 g for 5 min at room temperature) and the cytokines were analyzed in the conditioned supernatant. In vitro, TNF was analyzed in the conditioned supernatant at 3 h after the LPS challenge. Cytokine analyses in the conditioned media were performed using the same reagents as reported above.
Cytotoxicity
Cell cytoxicity was analyzed using MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, a yellow tetrazole) colorimetric assay. Cells were incubated for 2 h with 10% of the 5 mg/ml MTT solution dissolved in PBS. Then cells were treated with 200 μl of solubilizer solution (isopropanol, HCl, and Triton X100) to dissolve the formazan crystals. The absorbance at 540 nm was determined.
Immunofluorescence
Immunofluorescences were performed as previously described38. The adrenal glands from animals treated with fusaric acid were collected, fixed overnight in 4% paraformaldehyde solution in PBS (USB®, Affimetrix, Santa Clara, CA), and embedded in paraffin wax. Sections were cut at a thickness of 5 μm with the microtome (Leica RM2235, Leica Microsystems Inc, Buffalo Grove, IL). Sections were dewaxed in xylene and rehydrated by a series of ethanol-water washes. Antigen retrieval was performed by incubating the sections in retrieval buffer (10 mM Tris Base, 1 mM EDTA Solution, 0.05% Tween 20, pH 9.0) at 100°C for 20 min. Sections were blocked in a solution of 10% FBS (Fetal Bovine Serum) with 1% BSA (Sigma Chemical, Saint Louis, MO) for 2h at room temperature. Then, the sections were incubated with antibody to DOPA decarboxylase (1:1000, ab3905, Abcam, Cambridge, MA), antibody to Dopamine β-hydroxylase (1:1000, ab43868 Abcam, Cambridge, MA) or SMI310 (1:1000, ab24570 Abcam, Cambridge, MA) primary antibody diluted in 1% BSA-TBST (0.1% Tween 20, Tris-buffered saline pH 7.4) for 1h at room temperature. Subsequently, the sections were washed four times in TBST for 5 min each wash and incubated with the goat antibody to mouse IgG (H+L) FITC Conjugated (1:250, AP124F, Millipore, Temecula, CA) or Alexa Fluor® 568 goat antibody to Rabbit IgG (H+L) (1:500, A-11011, Molecular Probes®, Life Technologies, Grand Island, NY) secondary antibody diluted in 1% BSA-TBST for 1h at room temperature in the dark. The slides were washed four times in TBST for 5 min each wash and mounted with Vectashield® Mounting Media (Vector Laboratories, Burlingame, CA). Then, the slides were analyzed with the Nikon A1R-A1 Confocal Microscope System® (Nikon Instruments Inc., Melville, NY) providing a wavelength of 488 nm and 561 nm to excite the FITC and Alexa Fluor® 568 labeled antibodies, respectively.
Western Blot
Western Blot assay was performed similarly as we described39 The adrenal glands were homogenized (1:20 w/v) in lysis buffer with protease inhibitor (CelLytic™MT and Protease Inhibitor Cocktail P8340; 1:100 v/v, both from Sigma-Aldrich®, Saint Louis, MO) and centrifuged at 16,000 g for 15 min at 4°C. Protein concentration in the supernatant was analyzed using the Bio-Rad Protein Assay (Bio-Rad™, Life Science Research, Hercules, CA). 30 μg of protein sample was loaded per lane for sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) analyses. Samples were run on the NuPAGE® Bis-Tris Mini Gels (Novex®, Invitrogen™, Life Technologies, Grand Island, NY) at 200V for 50 min. The samples were transferred onto the PVDF membranes (Hybond-P, Amersham, GE Healthcare, Pittsburg, PA) by using the iBlot® Gel Transfer Device (Invitrogen™,Life Technologies, Grand Island, NY). The membranes were blocked with 5% BSA-TBST for 1 hour at 4°C, and incubated with antibody to DOPA decarboxylase (1:1000, ab3905, Abcam, Cambridge, MA), antibody to Dopamine β-hydroxylase (1:1000, ab43868, Abcam, Cambridge, MA) or anti-β-actin (1:1000, 13E5, Cell Signaling, Danvers, MA) primary antibody overnight at 4°C. The membranes were washed 5 times for 5 min with TBST and incubated with the HRP-linked antibody to rabbit IgG (1:1000, #7074, Cell Signaling, Danvers, MA) secondary antibody at room temperature for 1 hour. The membranes were washed again 5 times for 5 min in TBST and processed using the SuperSignal™ West Femto Chemiluminescent Substrate kit (Thermo Fisher Scientific Inc, Waltham, MA). The X-ray films were scanned, and the signal density was quantified using the ImageJ software (NIH Image program, Bethesda, MD).
Statistical Analyses
All tests were performed using the GraphPad Prism Software® (GraphPad Software, La Jolla, CA). Sample size was determined using standard deviation values and power analyses of our previous studies on the vagus nerve stimulation11,29. All data in the figures and text are expressed as mean ± standard error (SE). Statistical analyses were performed using the one way ANOVA with multiple pair-wise comparisons with the Bonferroni’s adjustment for multiple hypothesis testing. Normality and homogeneity of variance were confirmed using the the Kolmogorov-Smirnov analysis. ANOVA was used to compare all treatments and specific pair-wise comparisons as stated in the experiments. The student’s t-test (Mann–Whitney U test) was used to compare mean values between two experimental groups. Statistical analyses of survival were determined using the Logrank test. P values <0.05 were considered statistically significant.
Supplementary Material
Acknowledgements
We greatly appreciate the cooperation of Drs. P. Morcillo, JM Inclan-Rico & JR Berlin for their comments and suggestions, and Drs. M. Marks (University of Colorado, Denver, CO) & B. Kobilka (Stanford University, Stanford, CA) for their reagents. R.T.R was supported by the University Autonoma Benito Juarez from Oaxaca (UABJO). M.R.T.B. was supported by the Mexican National Council for Science and Technology (CONACyT). L.U. is supported by the faculty program of the Department of Surgery of the New Jersey Medical School, the UMDNJ foundation and the United States National Institute of Health (NIH) RO1-GM084125.
Footnotes
Author contributions R.T.R, G.Y., G.P., P.M., M.R.T.B. performed the experiments, prepared the figures and revised the article. M.A.M.E., L.A.A.P., A.I. contributed in the design of the study and revised the article. L.U. designed, directed the study, and wrote the article.
Competing financial interests The authors declare no competing financial interests.
References
- 1.Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med. 2013;369:840–851. doi: 10.1056/NEJMra1208623. [DOI] [PubMed] [Google Scholar]
- 2.Ulloa L, Tracey KJ. The "cytokine profile": a code for sepsis. Trends Mol Med. 2005;11:56–63. doi: 10.1016/j.molmed.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 3.Ulloa L. The vagus nerve and the nicotinic anti-inflammatory pathway. Nat Rev Drug Discov. 2005;4:673–684. doi: 10.1038/nrd1797. [DOI] [PubMed] [Google Scholar]
- 4.Annane D. Adrenal insufficiency in sepsis. Curr Pharm Des. 2008;14:1882–1886. doi: 10.2174/138161208784980626. [DOI] [PubMed] [Google Scholar]
- 5.Tracey KJ, et al. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature. 1987;330:662–664. doi: 10.1038/330662a0. [DOI] [PubMed] [Google Scholar]
- 6.Riedemann NC, Guo RF, Ward PA. Novel strategies for the treatment of sepsis. Nature medicine. 2003;9:517–524. doi: 10.1038/nm0503-517. [DOI] [PubMed] [Google Scholar]
- 7.Ulloa L, Brunner M, Ramos L, Deitch EA. Scientific and clinical challenges in sepsis. Curr Pharm Des. 2009;15:1918–1935. doi: 10.2174/138161209788453248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nathan C. Points of control in inflammation. Nature. 2002;420:846–852. doi: 10.1038/nature01320. [DOI] [PubMed] [Google Scholar]
- 9.Abraham E, et al. Lenercept (p55 tumor necrosis factor receptor fusion protein) in severe sepsis and early septic shock: a randomized, double-blind, placebo-controlled, multicenter phase III trial with 1,342 patients. Critical care medicine. 2001;29:503–510. doi: 10.1097/00003246-200103000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Borovikova LV, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–462. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 11.Huston JM, et al. Splenectomy inactivates the cholinergic antiinflammatory pathway during lethal endotoxemia and polymicrobial sepsis. J Exp Med. 2006;203:1623–1628. doi: 10.1084/jem.20052362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peña G, et al. Cholinergic regulatory lymphocytes re-establish neuromodulation of innate immune responses in sepsis. Journal of immunology. 2011;187:718–725. doi: 10.4049/jimmunol.1100013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bernik TR, et al. Cholinergic antiinflammatory pathway inhibition of tumor necrosis factor during ischemia reperfusion. J Vasc Surg. 2002;36:1231–1236. doi: 10.1067/mva.2002.129643. [DOI] [PubMed] [Google Scholar]
- 14.Altavilla D, et al. Activation of the cholinergic anti-inflammatory pathway reduces NF-kappab activation, blunts TNF-alpha production, and protects againts splanchic artery occlusion shock. Shock. 2006;25:500–506. doi: 10.1097/01.shk.0000209539.91553.82. [DOI] [PubMed] [Google Scholar]
- 15.Cai B, et al. Alpha7 cholinergic-agonist prevents systemic inflammation and improves survival during resuscitation. J Cell Mol Med. 2009;13:3774–3785. doi: 10.1111/j.1582-4934.2008.00550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Westerloo DJ, et al. The vagus nerve and nicotinic receptors modulate experimental pancreatitis severity in mice. Gastroenterology. 2006;130:1822–1830. doi: 10.1053/j.gastro.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 17.Pullan RD, et al. Transdermal nicotine for active ulcerative colitis. N Engl J Med. 1994;330:811–815. doi: 10.1056/NEJM199403243301202. [DOI] [PubMed] [Google Scholar]
- 18.Wang H, et al. Cholinergic agonists inhibit HMGB1 release and improve survival in experimental sepsis. Nature medicine. 2004;10:1216–1221. doi: 10.1038/nm1124. [DOI] [PubMed] [Google Scholar]
- 19.van Westerloo DJ, et al. The cholinergic anti-inflammatory pathway regulates the host response during septic peritonitis. J Infect Dis. 2005;191:2138–2148. doi: 10.1086/430323. [DOI] [PubMed] [Google Scholar]
- 20.Vickers AJ, et al. Acupuncture for chronic pain: individual patient data meta-analysis. Arch Intern Med. 2012;172:1444–1453. doi: 10.1001/archinternmed.2012.3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee A, Done ML. Stimulation of the wrist acupuncture point P6 for preventing postoperative nausea and vomiting. Cochrane Database Syst Rev. 2004:CD003281. doi: 10.1002/14651858.CD003281.pub2. [DOI] [PubMed] [Google Scholar]
- 22.Napadow V, Kaptchuk TJ. Patient characteristics for outpatient acupuncture in Beijing, China. J Altern Complement Med. 2004;10:565–572. doi: 10.1089/1075553041323849. [DOI] [PubMed] [Google Scholar]
- 23.Wu HM, et al. Acupuncture for stroke rehabilitation. Cochrane Database Syst Rev. 2006:CD004131. doi: 10.1002/14651858.CD004131.pub2. [DOI] [PubMed] [Google Scholar]
- 24.Goldman N, et al. Adenosine A1 receptors mediate local anti-nociceptive effects of acupuncture. Nat Neurosci. 2010;13:883–888. doi: 10.1038/nn.2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ernst E, Lee MS, Choi TY. Acupuncture: does it alleviate pain and are there serious risks? A review of reviews. Pain. 2011;152:755–764. doi: 10.1016/j.pain.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 26.Vida G, et al. β2-Adrenoreceptors of regulatory lymphocytes are essential for vagal neuromodulation of the innate immune system. FASEB J. 2011;25:4476–4485. doi: 10.1096/fj.11-191007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coupland RE, Parker TL, Kesse WK, Mohamed AA. The innervation of the adrenal gland. III. Vagal innervation. J Anat. 1989;163:173–181. [PMC free article] [PubMed] [Google Scholar]
- 28.Wang H, et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003;421:384–388. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- 29.Vida G, Peña G, Deitch EA, Ulloa L. Alpha7-nicotinic receptor mediates vagal induction of splenic norepinephrine. Journal of immunology. 2011;186:4340–4346. doi: 10.4049/jimmunol.1003722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grenader A, Healy DP. Fenoldopam is a partial agonist at dopamine-1 (DA1) receptors in LLC-PK1 cells. J Pharmacol Exp Ther. 1991;258:193–198. [PubMed] [Google Scholar]
- 31.Weber RR, et al. Pharmacokinetic and pharmacodynamic properties of intravenous fenoldopam, a dopamine1-receptor agonist, in hypertensive patients. Br J Clin Pharmacol. 1998;25:17–21. doi: 10.1111/j.1365-2125.1988.tb03276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Denef C, Manet D, Dewals R. Dopaminergic stimulation of prolactin release. Nature. 1980;285:243–246. doi: 10.1038/285243a0. [DOI] [PubMed] [Google Scholar]
- 33.Gorissen H, Laduron P. Solubilisation of high-affinity dopamine receptors. Nature. 1979;279:72–74. doi: 10.1038/279072a0. [DOI] [PubMed] [Google Scholar]
- 34.Kagitani F, Uchida S, Hotta H, Aikawa Y. Manual acupuncture needle stimulation of the rat hindlimb activates groups I, II, III and IV single afferent nerve fibers in the dorsal spinal roots. Jpn J Physiol. 2005;55:149–155. doi: 10.2170/jjphysiol.R2120. [DOI] [PubMed] [Google Scholar]
- 35.Sorkin LS, Yaksh TL. Behavioral models of pain states evoked by physical injury to the peripheral nerve. Neurotherapeutics. 2009;6:609–619. doi: 10.1016/j.nurt.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Richner M, Bjerrum OJ, Nykjaer A, Vaegter CB. The spared nerve injury (SNI) model of induced mechanical allodynia in mice. Journal of visualized experiments : JoVE. 2011 doi: 10.3791/3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kadl A, Pontiller J, Exner M, Leitinger N. Single bolus injection of bilirubin improves the clinical outcome in a mouse model of endotoxemia. Shock. 2007;28:582–588. doi: 10.1097/shk.0b013e31804d41dd. [DOI] [PubMed] [Google Scholar]
- 38.Panayotis N, et al. Morphological and functional alterations in the substantia nigra pars compacta of the Mecp2-null mouse. Neurobiol Dis. 2011;41:385–397. doi: 10.1016/j.nbd.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 39.Ulloa L, Doody J, Massague J. Inhibition of transforming growth factor-beta/SMAD signalling by the interferon-gamma/STAT pathway. Nature. 1999;397:710–713. doi: 10.1038/17826. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.