Abstract
At present it is unknown whether the higher prevalence of human papillomavirus (HPV) infection among smokers in men is attributed to a higher probability of acquiring an infection or because of longer infection persistence. Thus, we investigated the role of smoking on the (acquisition) and clearance (persistence) of genital HPV infections among 4,026 men in The HPV in Men (HIM) Study, a multinational prospective study of the natural history of genital HPV infection in men. Genital HPV infections were grouped any-, oncogenic-, and non-oncogenic HPV infections and smoking status was categorized as current-, former, and never smokers. The incidence of any-, oncogenic-, and non-oncogenic HPV infections was significantly higher among current smokers compared to former- and never smokers (P < 0.01). In multivariable analyses adjusting for sexual behavior and potential confounders, when compared to never smokers, current smokers exhibited significantly higher probability of acquiring any- (Hazard Ratio [HR] = 1.23; 95% confidence interval [CI] 1.02 – 1.50) and non-oncogenic (HR = 1.21; 95% CI 1.00 – 1.45) infections and a borderline significant probability for oncogenic infections (HR = 1.18; 95% CI 0.98 – 1.41). Although the median duration of HPV infection was generally longer among current smokers, we found no statistically significant associations in the multivariable analyses. Overall, these results demonstrated that current smoking exhibited the highest incidence and highest probability of acquiring genital HPV infections.
Keywords: HPV, epidemiology, incidence, smoking
Introduction
Human papillomavirus (HPV) is one of the most common sexually transmitted infections, with more than 14 million new infections occurring annually in the United States.1 More than 120 different HPV types have been identified of which more than 30 are transmitted through sexual activity. Of the approximately 5 to 15% of cancer cases that are attributed to infections nearly half are attributable to HPV.2 HPV is associated with multiple cancers in both men and women, the virus is readily transmitted from person to person, and thus can affect disease risk in both genders.3-5 However, most HPV infections are transient and subclinical, are usually self-cleared, and do not result in clinical disease. Studies on the health consequences of HPV infections in men have been lagging and to date there are limited published data documenting the factors associated with HPV acquisition, persistence, and duration, the latter of which is the obligate step in the progression to malignancy.
The prevalence of HPV infection among men has been estimated to be as high as 73%6 and numerous factors have been shown to modify HPV prevalence including age,7 race,8 sexual orientation,7 and circumcision,9 to name a few. Revealing factors that may have an influence on HPV incidence (acquisition) and clearance (persistence) may reveal new underpinnings of this infection and lead to strategies to reduce HPV-related disease burden. At present little is known about the relationship between smoking and HPV natural history among men. Since smoking10 and HPV are both important and prevalent risk factors among men, it is important to determine the potential influence of smoking on the natural history of HPV infection in men. Among women, cigarette smoking is associated with higher HPV prevalence,11-13 incidence,14,15 and persistence,16,17 and in a previous report among men18 we found current smoking was associated with an increased detection of any prevalent HPV infection (odds ratio [OR]=1.19; 95% confidence interval [CI]: 1.01–1.41) and prevalent oncogenic HPV infection (OR=1.24; 95% CI: 1.05–1.47). Presently it is unclear whether the higher prevalence of HPV infection among smokers in men is attributed to higher probability of acquisition of an infection or a longer duration of an infection. Thus, the purpose of this analysis is to assess the role of smoking on the incidence and clearance of HPV infections among men in The HPV in Men (HIM) Study
Materials and Methods
Study population
The HIM Study is a multinational prospective study of the natural history of genital HPV infection in men ages 18 to 70 years residing in Brazil, Mexico, and the USA. Men were eligible for participation if they were residents of southern Florida, USA, São Paulo, Brazil, or Cuernavaca, Mexico; reported no previous diagnosis of penile or anal cancers; reported no previous diagnosis of genital or anal warts; had not participated in an HPV vaccine study; reported no previous diagnosis of HIV; reported no current penile discharge or burning during urination; were not being treated for sexually transmitted infections; had not been imprisoned or homeless during the past 6 months; had not received drug treatment during the past 6 months; had no plans to relocate in the next 4 years; and were willing to comply with ten scheduled visits every 6 months for 4 years. All study subjects in this analysis completed at least two visits. The median number of clinic visits completed was four visits and the median interval between visits 6.23 months.
Men were recruited according to three age groups (18 to 30 years, 31 to 44 years, and 45 to 70 years) from the general population, universities, and organized health-care systems. Specifically, in Brazil, men were recruited from a large clinic in São Paulo that was providing genitourinary services, including tests for HIV and sexually transmitted infections, and the general population through television, radio, and newspaper advertisements. In Mexico, men were recruited in Cuernavaca and Morelos, through a large health plan, from factories and the military. In the USA, men were mainly recruited from the University of South Florida and the general community in Tampa, FL. A full description of cohort procedures, HPV prevalence, and factors associated with prevalent infections has already been reported elsewhere.16 Human-subjects' committees of the University of South Florida, FL, USA, the Ludwig Institute for Cancer Research, São Paulo, Brazil, the Centro de Referencia e Tratamento de Doencas Sexualmente Transmissiveis e AIDS, São Paulo, Brazil, and the National Institute of Public Health of Mexico, Cuernavaca, Mexico, approved all study procedures. Men who provided consent had a clinical examination two weeks prior to enrollment visit and every 6 months thereafter. Only men who returned for the enrollment visit were included in this study.
Risk Factor Questionnaire
An extensive 88-item computer-assisted self-interview sexual history and health questionnaire were given at enrollment to assess sociodemographic characteristics and risk factors. The questionnaire required approximately 20 minutes to complete, has been previously shown to elicit reliable responses,19 and was written in the region's primary language (Portuguese, Spanish, or English). Never smokers were defined as men who had smoked less than 100 cigarettes in their lifetime. Former smokers were defined as men who had smoked at least 100 cigarettes in their lifetime but quit smoking at least 1 year before the baseline interview. Current smokers were defined as men who smoked at least 100 cigarettes in their lifetime and were currently smoking at the time of the visit. Pack-years smoked were calculated using the average number of cigarette packs smoked per day and the numbers of years smoked.
HPV Penile and Scrotal Sampling
Samples were obtained from the external genitalia at each visit by use of Dacron (Digene, Gaithersburg, MD, USA) swabs prewetted with saline. Three separate samples were obtained: corona of glans penis (1 sample), penile shaft (1 sample), and scrotum (1 sample). The samples were placed in 450 μL of Specimen Transport Medium and then combined into one sample before DNA extraction (described below). The specimens were stored at -70°C until PCR analyses and HPV genotyping was performed. We have previously shown the validity of these three anatomical sites in the assessment of HPV status, and high sampling reproducibility for the detection of HPV DNA by use of this method.20
DNA extraction and HPV genotyping
DNA extraction was conducted with QIAamp DNA Mini Kit (Qiagen, Valencia, CA, USA) on a robotic system according to the manufacturer's instructions and DNA was stored at 4°C until use. The extracted DNA samples were tested for the presence of HPV types by amplification of 30 ng of DNA with the PGMY09/11 L1 consensus primer system.21 HPV genotyping was performed with the linear array method on all samples irrespective of the HPV PCR result (Roche Molecular Diagnostics, Alameda, CA, USA). Only samples that tested positive for beta-globin (99% at enrollment) were judged to be adequate and included in the analysis. Before genotyping, the amplification products were run on 2% agarose gels to visualize a 450 base pair band corresponding to HPV amplification for identification of samples that might have an HPV type other than the 37 types analyzed in the genotyping assay. Samples for which HPV was amplified on PCR but did not hybridize with a specific HPV type during the genotyping assay (e.g., unclassified infections) were classified as HPV negative. Across the ten visits the frequency of these unclassified infections that were classified as HPV negative ranged from 1.25% to 4.0%. The HPV types that were classified as oncogenic were 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 6822 and non-oncogenic types were 6, 11, 26, 40, 42, 53, 54, 55, 61, 62, 64, 66, 67, 69, 70, 71, 72, 73, 81, 82, 83, 84, 89, and IS39. The classification of ‘any HPV’ was defined as a positive test result for at least one of 37 HPV genotypes. HPV infections with single or multiple oncogenic virus types were classified as ‘oncogenic’. Similarly, HPV infections with at least one non-oncogenic HPV type were classified as ‘non-oncogenic’.
Statistical analysis
Smoking status was categorized by current-, former, and never smokers and then smoking status was analyzed with two different approaches: 1) by baseline only status and 2) among men who reported the same smoking status at both the baseline and their last follow-up (LFU). Sociodemographic and sexual behavioral characteristics by smoking status (never, former, and current) were compared using the Monte Carlo estimation of exact Pearson chi-square test for categorical variables. For each individual time a newly acquired HPV infection was estimated by use of the time from study entry to the date of the first HPV detected. For estimates of any- or type-specific HPV incidence, only those men at enrollment who were free of any- or a specific HPV type, respectively, were included.
For the incidence analyses (i.e., acquisition) the analytical unit is an individual. For the grouped analysis any HPV incidence was defined as a positive test result for at least one of 37 HPV genotypes, oncogenic HPV incidence was defined as testing positive for at least one oncogenic HPV type, and non-oncogenic was defined as a positivity for at least one non-oncogenic HPV type. For the type-specific and grouped HPV incidence analyses, only the first acquired infection was considered and thus the analytical unit is an individual. Cumulative risk of HPV incidence was estimated using the Kaplan-Meier method and the log-rank test was used to identify differences by smoking status. Twelve-month HPV incidence was also estimated using the Kaplan-Meier method and the association between smoking status and HPV incidence was assessed with multivariable Cox proportional hazards regression adjusting for age, race, ethnicity, country, circumcision, total number of female partners in the last 3 months, total number of female partners, and total number of male sex partners.
HPV clearance (i.e., persistence) was defined as a participant who tested HPV negative at two consecutive visits after testing positive, excluding infections detected for the first time at a participant's final visit. Men with HPV infections, regardless of HPV status at baseline, were included in the clearance analyses. The analytical unit for the clearance analyses was an infection with each individual genotype considered as a separate infection. Thus, since men could have been infected with multiple HPV types within a defined group (e.g., HPV16 and HPV18 are both oncogenic), we adjusted for within-subject correlation in the grouped HPV clearance analyses. The median time to HPV clearance among all men with an incident infection was estimated using the clustered Kaplan-Meier method and men whose infections did not clear were censored in the analysis. We utilized multivariable Cox proportional hazards regression with the robust covariance matrix estimator to model the association between smoking status and HPV clearance. Since HPV infection status at baseline had significant impact on HPV clearance, we included this covariate in the multivariable models of HPV clearance in addition to the factors that were adjusted for in the incidence analyses. All analyses were conducted with SAS (version 9.3) and tests were two-sided with a significance level of 0.05.
Results
The total sample sizes with complete available data were 4,026 for the baseline only smoking status analyses and 3,548 for the men who were concordant with smoking status at baseline and last follow-up. Statistically significant differences were observed for the distribution of the study population characteristics by smoking status for the baseline only smoking status (Table 1) and among men who reported the same smoking status at baseline and last follow-up (Supplemental Table 1). The concordance of men who reported the same smoking status at both baseline and last follow-up was 83.4%, 83.3%, and 89.2% for current-, former, and never smokers, respectively.
Table 1. Distribution of select baseline characteristics of men in the HIM study by smoking status.
| Characteristic | Baseline only1 | ||||
|---|---|---|---|---|---|
|
| |||||
| Overall | Current Smokers | Former Smokers | Never Smokers | P-value2 | |
| Age | < 0.001 | ||||
| 18 to 30 | 1960 (48.7%) | 463 (48.8%) | 249 (33.2%) | 1248 (53.7%) | |
| 31 to 44 | 1544 (38.4%) | 375 (39.6%) | 317 (42.2%) | 852 (36.6%) | |
| 45 to 70 | 521 (12.9%) | 110 (11.6%) | 185 (24.6%) | 226 (9.7%) | |
| Total | 4025 (100%) | 948 (100%) | 751 (100%) | 2326 (100%) | |
| Race | < 0.001 | ||||
| White | 1803 (45.4%) | 373 (39.9%) | 341 (46.0%) | 1089 (47.5%) | |
| Black | 628 (15.8%) | 129 (13.8%) | 77 (10.4%) | 422 (18.4%) | |
| Asian/PI | 110 (2.8%) | 12 (1.3%) | 8 (1.1%) | 90 (3.9%) | |
| Other | 1428 (36.0%) | 422 (45.1%) | 315 (42.5%) | 691 (30.1%) | |
| Total | 3969 (100%) | 936 (100%) | 741 (100%) | 2292 (100%) | |
| Ethnicity | < 0.001 | ||||
| Hispanic | 1818 (45.6%) | 519 (55.2%) | 375 (50.2%) | 924 (40.1%) | |
| Non – Hispanic | 2173 (54.4%) | 421 (44.8%) | 372 (49.8%) | 1380 (59.9%) | |
| Total | 3991 (100%) | 940 (100%) | 747 (100%) | 2304 (100%) | |
| Clinic Site/Country | < 0.001 | ||||
| United States | 1312 (32.6%) | 269 (28.4%) | 207 (27.6%) | 836 (35.9%) | |
| Brazil | 1396 (34.7%) | 259 (27.3%) | 252 (33.6%) | 885 (38.0%) | |
| Mexico | 1318 (32.7%) | 420 (44.3%) | 292 (38.9%) | 606 (26.0%) | |
| Total | 4026 (100%) | 948 (100%) | 751 (100%) | 2327 (100%) | |
| Circumcision status | < 0.001 | ||||
| Not Circumcised | 2558 (63.6%) | 628 (66.2%) | 483 (64.3%) | 1447 (62.2%) | |
| Circumcised | 1467 (36.4%) | 320 (33.8%) | 268 (35.7%) | 879 (37.8%) | |
| Total | 4025 (100%) | 948 (100%) | 751 ((100%) | 2326 (100%) | |
| Lifetime No. of Female Partners | < 0. 001 | ||||
| 0 to 1 | 711 (17.7%) | 116 (12.2%) | 71 (9.5%) | 524 (22.5%) | |
| 2 to 9 | 1606 (39.9%) | 347 (36.6%) | 292 (38.9%) | 967 (41.6%) | |
| 10 to 49 | 1263 (31.4%) | 348 (36.7%) | 294 (39.1%) | 621 (26.7%) | |
| 50+ | 225 (5.6%) | 67 (7.1%) | 50 (6.7%) | 108 (4.6%) | |
| Refused | 220 (5.5%) | 70 (7.4%) | 44 (5.9%) | 106 (4.6%) | |
| Total | 4025 (100%) | 948 (100%) | 751 (100%) | 2326 (100%) | |
| No. of Female Partners in Past 3 or 6 Months | < 0.001 | ||||
| None | 836 (20.8%) | 204 (21.5%) | 192 (25.6%) | 440 (18.9%) | |
| 1 | 1636 (40.6%) | 368 (38.8%) | 333 (44.3%) | 935 (40.2%) | |
| 2 | 515 (12.8%) | 128 (13.5%) | 86 (11.5%) | 301 (12.9%) | |
| 3+ | 525 (13.0%) | 139 (14.7%) | 80 (10.7%) | 306 (13.2%) | |
| Refused | 513 (12.7%) | 109 (11.5%) | 60 (8.0%) | 344 (14.8%) | |
| Total | 4025 (100%) | 948 (23.6%) | 751 (18.7%) | 2326 (57.8%) | |
| Lifetime No. of Male Partners | 0.136 | ||||
| 0 to 1 | 3607 (90.3%) | 832 (88.6%) | 673 (90.2%) | 2102 (91.0%) | |
| 2 to 9 | 228 (5.7%) | 58 (6.2%) | 48 (6.4%) | 122 (5.3%) | |
| 10+ | 160 (4.0%) | 49 (5.2%) | 25 (3.4%) | 86 (3.7%) | |
| Total | 3995 (100%) | 939 (23.5%) | 746 (18.7%) | 2310 (57.8%) | |
The demographic features were analyzed by baseline smoking status
P-value Pearson's chi-square test comparing demographics by smoking status for Baseline only
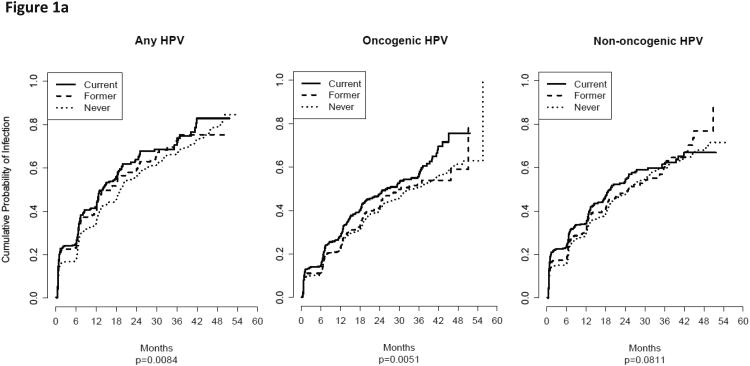
The twelve-month incidence, the rate of a man acquiring an infection during within twelve months in the HIM study, for any-, oncogenic-, and non-oncogenic HPV infections was significantly higher among current smokers compared to former and never smokers (Table 2). Consistent findings were generally observed when examining the baseline smoking status only and the baseline and last follow-up. When we analyzed the entire available follow-up data (Figure 1a), current smoking was also associated with a statistically significantly higher probability of acquiring any- (P = 0.0084) and oncogenic (P = 0.0051) HPV infections. Current smoking was associated with borderline significant higher probability of acquiring a non-oncogenic infection (P = 0.0811).
Table 2. Twelve-incidence rate of HPV infection by smoking status among men in the HIM study.
| HPV Status | Smoking Status3 | Twelve-Month Incidence1 | Log Rank p Value2 | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Overall | Current Smokers | Former Smokers | Never Smokers | |||
| Any HPV | Baseline | 37.3% (35.0% – 39.8%) | 42.1% (36.8% – 47.9%) | 40.2% (34.8% – 46.2%) | 34.9% (32.0% – 38.0%) | 0.008 |
| Any HPV | Baseline & last follow-up | 37.4% (34.9% – 40.0%) | 44.7% (38.6% – 51.3%) | 40.1% (34.2% – 46.5%) | 34.6% (31.5% – 37.8%) | 0.003 |
| Non-oncogenic | Baseline | 31.1% (29.1% – 33.2%) | 34.4% (30.0% – 39.3%) | 30.9% (26.4% – 35.9%) | 30.0% (27.5% – 32.7%) | 0.079 |
| Non-oncogenic | Baseline & last follow-up | 31.1% (29.0% – 33.3%) | 36.0% (31.0% – 41.5%) | 30.5% (25.7% – 36.0%) | 29.7% (27.1% – 32.5%) | 0.030 |
| Oncogenic | Baseline | 23.5% (21.9% – 25.3%) | 28.1% (24.2% – 32.4%) | 23.0% (19.3% – 27.3%) | 22.2% (20.1% – 24.4%) | 0.005 |
| Oncogenic | Baseline & last follow-up | 23.3% (21.5% – 25.3%) | 28.8% (24.4% – 33.7%) | 22.9% (18.8% – 27.7%) | 21.9% (19.7% – 24.3%) | 0.009 |
| HPV6 | Baseline | 4.8% (4.2% – 5.6%) | 6.3% (4.7% – 8.4%) | 5.5% (4.0% – 7.6%) | 4.1% (3.3% – 5.1%) | 0.448 |
| HPV6 | Baseline & last follow-up | 4.8% (4.1% – 5.7%) | 6.2% (4.5% – 8.6%) | 5.2% (3.6% – 7.5%) | 4.3% (3.4% – 5.3%) | 0.361 |
| HPV11 | Baseline | 1.1% (0.8% – 1.6%) | 1.7% (1.0% – 3.0%) | 1.0% (0.4% – 2.1%) | 1.0% (0.6% – 1.5%) | 0.901 |
| HPV11 | Baseline & last follow-up | 1.1% (0.8% – 1.6%) | 2.0% (1.1% – 3.6%) | 0.7% (0.3% – 1.9%) | 1.0% (0.6% – 1.5%) | 0.873 |
| HPV16 | Baseline | 5.5% (4.8% – 6.4%) | 6.9% (5.2% – 9.1%) | 6.0% (4.3% – 8.2%) | 4.9% (4.0% – 6.0%) | 0.655 |
| HPV16 | Baseline & last follow-up | 5.5% (4.7% – 6.4%) | 7.2% (5.3% – 9.8%) | 5.8% (4.1% – 8.3%) | 4.9% (3.9% – 6.0%) | 0.485 |
| HPV18 | Baseline | 2.4% (1.9% – 2.9%) | 2.7% (1.7% – 4.2%) | 1.9% (1.1% – 3.4%) | 2.4% (1.8% – 3.2%) | 0.045 |
| HPV18 | Baseline & last follow-up | 2.4% (1.9% – 3.1%) | 2.9% (1.8% – 4.7%) | 2.4% (1.3% – 4.2%) | 2.3% (1.7% – 3.1%) | 0.034 |
Twelve-month incidence is the rate of male acquiring an infection during any given twelve month period (i.e., year) in the HIM study
P-value from log-rank test for HPV incidence by smoking status
Smoking status was analyzed by two different approaches: 1) among baseline only status and 2) among men who reported the same smoking status at both the baseline and their last follow-up (LFU)
Figure 1.
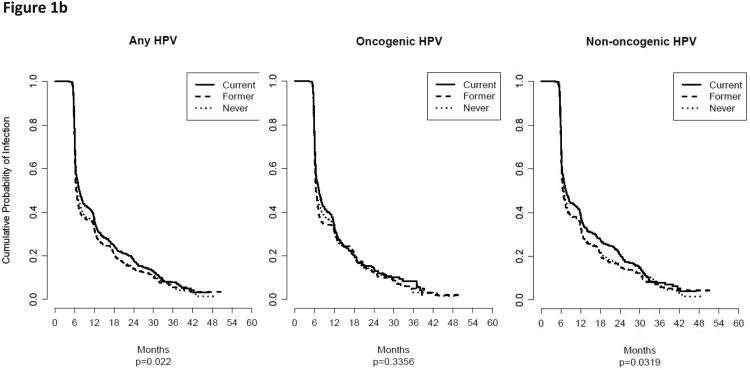
A) The cumulative probability for acquiring (i.e., incidence) an HPV infection for baseline only smoking status among men in the HIM study. Current smoking was associated with a statistically significantly higher probability of acquiring any- and oncogenic HPV infections and borderline significant for non-oncogenic infections (P = 0.0811). B) The cumulative probability for clearing an HPV infection for baseline only smoking status among men in the HIM study. Current smoking was associated with a statistically significantly lower probability of clearing any- and non-oncogenic HPV infections.
Multivariable Cox models (Table 3) were used to adjust for potential confounding factors which revealed that current smoking exhibited significantly increased risks for any- (HR = 1.23; 95% CI 1.02-1.50) and non-oncogenic (HR = 1.21; 95% CI 1.00-1.45) incident HPV infections. When the baseline only smoking status was examined, a statistically significant association among current smokers was observed for incident oncogenic HPV infections (HR = 1.17%; 95% CI 1.00 – 1.38). Current smoking was also significantly associated with an increased incidence of HPV11 infection (HR = 4.90; 95% CI 1.40 – 17.2). No other individual type specific associations were statistically significant.
Table 3. Hazard ratios for HPV incidence by smoking status among men in the HIM study1.
| HPV Status | Smoking Status | Baseline | Baseline & Last Follow-up |
|---|---|---|---|
|
| |||
| mHR (95% CI)2 | mHR (95% CI)2 | ||
| Any HPV | Never | 1.00 | 1.00 |
| Former | 1.09 (0.91 – 1.32) | 1.12 (0.92 – 1.37) | |
| Current | 1.13 (0.95 – 1.34) | 1.23 (1.02 – 1.50) | |
| Oncogenic | Never | 1.00 | 1.00 |
| Former | 1.05 (0.88 – 1.27) | 1.08 (0.88 – 1.32) | |
| Current | 1.17 (1.00 – 1.38) | 1.18 (0.98 – 1.42) | |
| Non–oncogenic | Never | 1.00 | 1.00 |
| Former | 0.94 (0.81 – 1.09) | 1.01 (0.83 – 1.22) | |
| Current | 0.88 (0.71 – 1.08) | 1.21 (1.00 – 1.45) | |
| HPV6 | Never | 1.00 | 1.00 |
| Former | 1.10 (0.8 – 1.51) | 1.18 (0.79 – 1.75) | |
| Current | 1.12 (0.84 – 1.49) | 0.99 (0.57 – 1.71) | |
| HPV11 | Never | 1.00 | 1.00 |
| Former | 0.95 (0.51 – 1.77) | 1.48 (0.67 – 3.27) | |
| Current | 0.89 (0.51 – 1.56) | 4.90 (1.40 – 17.2) | |
| HPV16 | Never | 1.00 | 1.00 |
| Former | 1.06 (0.78 – 1.42) | 0.89 (0.62 – 1.27) | |
| Current | 0.99 (0.75 – 1.31) | 1.31 (0.74 – 2.31) | |
| HPV18 | Never | 1.00 | 1.00 |
| Former | 0.75 (0.45 – 1.23) | 0.89 (0.54 – 1.49) | |
| Current | 1.17 (0.80 – 1.69) | 1.19 (0.50 – 2.83) | |
Smoking status was analyzed by two different approaches: 1) among baseline only status and 2) among men who reported the same smoking status at both the baseline and their last follow-up (LFU)
Multivariable hazard ratio (mHR) adjusted for age, race, ethnicity, country, circumcision, total number of female partners in the last 3 months, total number of female partners, and total number of male sex partners
The median duration of infection (months) was generally longer among current smokers for any-, oncogenic-, and non-oncogenic HPV infections (Table 4); however, current smoking was only significantly longer for any- and non-oncogenic infections for the baseline only data. When we analyzed the entire available follow-up data (Figure 1b), current smoking was associated with a statistically significantly lower probability of clearing any- (P = 0.022) and non-oncogenic (P = 0.0319) HPV infections. In the multivariable analyses none of the hazard ratios were statistically significant for the clearance (Table 5). We also explored stratified multivariable analyses by number of lifetime female partners and pack-years smoked but these analyses did not reveal any appreciable differences in the point estimates (data not shown).
Table 4. Median duration of HPV Infection by smoking status among men in the HIM Study.
| HPV Status | Smoking Status1 | Median Duration of Infection (Months) | p Value2 | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Overall | Current Smokers | Former Smokers | Never Smokers | |||
| Any HPV | Baseline | 6.8 (6.6 – 7.0) | 7.4 (6.9 – 8.2) | 6.5 (6.3 – 6.9) | 6.7 (6.5 – 7.0) | 0.023 |
| Any HPV | Baseline & last follow-up | 6.7 (6.5 – 6.9) | 7.2 (6.7 – 7.9) | 6.6 (6.3 – 6.9) | 6.6 (6.4 – 6.9) | 0.109 |
| Non-oncogenic | Baseline | 6.9 (6.6 – 7.2) | 7.6 (6.9 – 8.5) | 6.7 (6.4 – 7.1) | 6.8 (6.5 – 7.1) | 0.033 |
| Non-oncogenic | Baseline & last follow-up | 6.8 (6.6 – 7.1) | 7.3 (6.8 – 8.4) | 6.7 (6.3 – 7.1) | 6.7 (6.5 – 7.2) | 0.154 |
| Oncogenic | Baseline | 6.6 (6.4 – 6.9) | 7.4 (6.6 – 8.1) | 6.4 (6.2 – 6.8) | 6.5 (6.3 – 6.9) | 0.335 |
| Oncogenic | Baseline & last follow-up | 6.5 (6.4 – 6.7) | 7.1 (6.4 – 8.0) | 6.4 (6.2 – 6.9) | 6.5 (6.3 – 6.7) | 0.551 |
| HPV6 | Baseline | 6.4 (6.2 – 6.8) | 6.2 (6.1 – 10.5) | 6.4 (6.0 – 10.6) | 6.3 (6.1 – 6.8) | 0.348 |
| HPV6 | Baseline & last follow-up | 6.3 (6.1 – 6.8) | 6.2 (6.0 – 11.9) | 6.4 (5.9 – 12.2) | 6.3 (6.1 – 6.8) | 0.368 |
| HPV11 | Baseline | 6.9 (6.2 – 11.8) | 11.8 (5.7 – 18.0) | 6.2 (5.8 – 18.1) | 7.1 (6.2 – 11.8) | 0.884 |
| HPV11 | Baseline & last follow-up | 6.6 (6.0 – 8.5) | 11.8 (5.7 – 17.1) | 6.3 (5.9 – 18.1) | 6.6 (6.0 – 8.5) | 0.981 |
| HPV16 | Baseline | 7.4 (6.5 – 10.3) | 8.0 (6.3 – 14.0) | 7.2 (6.1 – 12.2) | 7.8 (6.5 – 11.5) | 0.769 |
| HPV16 | Baseline & last follow-up | 7.4 (6.4 – 10.3) | 7.8 (6.3 – 14.7) | 7.2 (6.0 – 12.2) | 7.8 (6.3 – 11.9) | 0.706 |
| HPV18 | Baseline | 6.4 (6.2 – 6.9) | 6.5 (6.2 – 11.2) | 6.6 (5.9 – 7.0) | 6.2 (6.0 – 7.8) | 0.849 |
| HPV18 | Baseline & last follow-up | 6.4 (6.2 – 6.9) | 6.4 (6.1 – 11.2) | 6.6 (5.9 – 7.0) | 6.2 (6.0 – 7.8) | 0.992 |
Smoking status was analyzed by two different approaches: 1) among baseline only status and 2) among men who reported the same smoking status at both the baseline and their last follow-up (LFU)
p-value of the univariate Cox model with the robust covariance matrix estimator for HPV clearance across entire follow-up period by smoking status
Table 5. Hazard ratios for clearance of HPV Infection by smoking status among men in the HIM Study.
| HPV Status | Smoking Status1 | Baseline | Baseline & Last Follow–up |
|---|---|---|---|
|
| |||
| mHR (95% CI)1 | mHR (95% CI)2 | ||
| Any HPV | Never | 1.00 | 1.00 |
| Former | 0.94 (0.87 – 1.01) | 0.95 (0.87 – 1.03) | |
| Current | 0.93 (0.86 – 1.01) | 0.93 (0.85 – 1.01) | |
| Oncogenic | Never | 1.00 | 1.00 |
| Former | 0.96 (0.86 – 1.07) | 0.95 (0.85 – 1.06) | |
| Current | 0.94 (0.85 – 1.05) | 0.94 (0.83 – 1.06) | |
| Non–oncogenic | Never | 1.00 | 1.00 |
| Former | 0.93 (0.85 – 1.02) | 0.95 (0.85 – 1.06) | |
| Current | 0.93 (0.84 – 1.02) | 0.94 (0.83 – 1.06) | |
| HPV6 | Never | 1.00 | 1.00 |
| Former | 0.93 (0.70 – 1.23) | 0.95 (0.85 – 1.06) | |
| Current | 0.91 (0.70 – 1.19) | 0.94 (0.83 – 1.06) | |
| HPV11 | Never | 1.00 | 1.00 |
| Former | 1.02 (0.54 – 1.94) | 1.00 (0.50 – 1.97) | |
| Current | 0.83 (0.49 – 1.43) | 0.80 (0.43 – 1.46) | |
| HPV16 | Never | 1.00 | 1.00 |
| Former | 0.98 (0.74 – 1.29) | 0.96 (0.71 – 1.30) | |
| Current | 0.92 (0.71 – 1.20) | 0.86 (0.64 – 1.14) | |
| HPV18 | Never | 1.00 | 1.00 |
| Former | 0.80 (0.49 – 1.30) | 0.68 (0.40 – 1.18) | |
| Current | 0.82 (0.54 – 1.25) | 0.87 (0.55 – 1.36) | |
Smoking status was analyzed by two different approaches: 1) among baseline only status and 2) among men who reported the same smoking status at both the baseline and their last follow-up (LFU)
Multivariable hazard ratio (mHR) adjusted for HPV status at baseline, age, race, ethnicity, country, circumcision, total number of female partners in the last 3 months, total number of female partners, and total number of male sex partners.
Discussion
In this multinational cohort study of HPV in men, our analyses revealed that current smoking was associated with a statistically significantly higher incidence of any-, oncogenic-, and non-oncogenic HPV infections. We also found that for current smokers the median duration of any- and non-oncogenic infections infection was generally longer and associated with a lower probability of clearing of any- and non-oncogenic infections. However, none of the multivariable point estimates for clearance for current smoking were statistically significant.
In a previous report18 we found that current smoking was associated with an increased risk of any prevalent HPV infection and prevalent oncogenic HPV infection and that these associations were restricted to men who self-reported the fewest lifetime female sexual partners. Additionally, Nielson et al.23 reported an increased risk of prevalent HPV infection among men who were current smokers and among those who smoked ≥ 10 cigarettes per day. To the best of our knowledge this analysis is the first to investigate the role of smoking on the incidence and clearance of genital HPV infections among men. Our results are generally consistent with prior findings in women that showed smoking is associated with a higher probability of incident infections.14,15 As observed in previously published studies of the cervix,16,17 the median duration of infection was generally longer among current smokers (Table 4); however, when we adjusted for potential confounding factors (Table 5) none of the point estimates were statistically significant for clearance.
At present, it is unclear how smoking influences HPV infection in men, but smoking has many systemic affects that could impact HPV incidence. For instance, laboratory studies have shown that smoking increases cellular proliferation and metaplasia in various tissues and cell types24-28, which in turn could result in an increase in replication or production of HPV due to smoking-induced cell proliferation. Specific compounds in cigarette smoke have also been shown to modify the function of immune cells, affect neutrophil function,29 cause DNA damage,30 and suppress resistance to infections.31 Any one of these features could result in an increased susceptibility to the acquisition of HPV. Further, studies have demonstrated that smoking has deleterious effects on both systemic and local immunity,24,26,32,33 which are important in the detection, elimination, and immunity to viruses. Experimental evidence suggests that smoking acts as an immunosuppressant for the function of immune cells and causes the release of a variety of inflammatory factors including cytokines, oxygen radicals and proteases which can ultimately alter the function of immune cells.34 Additionally, nicotine, the addictive component of tobacco responsible for the dependence-forming properties of smoking, has been shown to be immunosuppressive.35,36 Thus, the increased incidence of HPV infection associated with current smoking in our analysis could be due an attenuated host immune response due to the deleterious effects attributed to smoking.
Since current smoking has potent and immediate effects on systemic and local immune function compared to those who quit smoking or never smoked, we assessed smoking status by using the baseline smoking status as well as by those individuals who self-reported the same smoking status at both baseline and the last available follow-up. We included the latter method in order to avoid misclassification of current smokers at baseline who quit smoking during follow-up and to avoid misclassification for former and never smokers who began smoking after baseline. We were concerned that current smokers at baseline who quit smoking during follow-up would be misclassified and ultimately dilute any potential smoking-related associations. Likewise, former- and never smokers who started smoking after the baseline visit would be misclassified since the long-term systemic effects of smoking could be attenuated compared to consistent current smokers. Although the concordance of men who reported the same smoking status at both baseline and last available follow-up was quite high (83.4%, 83.3%, and 89.2% for current-, former-, and never smokers, respectively), we do acknowledge the possibility that men could have changed smoking status several times. Thus there still may be some misclassification, but this misclassification is likely very minor and by reporting the results among those men who reported the same smoking status at baseline and last follow-up, we are likely more conservative with our interpretation. Although the twelve-month incidence and median duration data were generally consistent by the two approaches we employed, the P-values were not always consistent which is likely attributed to small incremental differences in the values between the two approaches and reduced statistical power because of smaller sample size in the more restrictive analysis.
There are many strengths and some limitations of this analysis that should be noted. First, the HIM Study is a unique resource since it is the only multi-national prospective study of the natural history of HPV infection in men. This international cohort is well-characterized,8,18,37-41 includes men of a wide age range and extensive and previously validated participant information.42 Another strength of the study is the large sample size with follow-up; although we do acknowledge that stratification by smoking and sexual behavior did result in smaller subgroups. We acknowledge that we cannot account for bias due to unmeasured or unknown confounding. Sexual behavior is potentially an important confounder in the association between smoking and HPV infection. Although we accounted for potential confounding by adjusting for and stratifying by self-reported sexual behavior, residual confounding still may exist which could potentially inflate the observed point estimates. Another possible limitation is that there may be concordance of smoking and HPV among partners of the men in this study;43,44 however, we do not have data on the partners. We also acknowledge that the men in the HIM cohort may not be a representative of the general male populations of the participating countries which may limit the generalizability of our findings. Thus, we caution overinterpretation of our results, but these data are important and novel as there is limited information on the association between smoking and HPV in men. Furthermore, since smoking is a modifiable risk factor, these data have public health implications for reducing HPV incidence.
Overall, these results demonstrated that current smokers exhibited the highest incidence and highest probability of acquiring genital HPV infections. Although the median duration of HPV infection was generally longer among current smokers, we found no statistically significant associations in the multivariable analyses. The biological role that smoking plays in HPV infection in men remains understudied, and limited epidemiology data exist on the association between smoking and HPV infection. To the best of our knowledge these are the first epidemiologic data to demonstrate an association between current smoking and incidence of genital HPV infections among men.
Supplementary Material
Supplemental Table 1. Distribution of select characteristics of men in the HIM study by smoking status
Novelty and Impact.
HPV infection can cause cancer in men and women. Revealing factors that have an influence on HPV incidence (acquisition) and clearance (persistence) may reveal new underpinnings of this infection. These results demonstrated that current smoking was associated with the highest probability of acquiring genital HPV infections in men. To the best of our knowledge these are the first epidemiologic data to demonstrate an association between current smoking and incidence of genital HPV infections among men.
Acknowledgments
Funding information: This work was supported by the National Cancer Institute at the National Institutes of Health Grant (CA R01CA098803).
Thank you: The authors thank the following staff members for their dedication in recruiting, examining, and maintaining data on cohort participants, as well as conducting HPV DNA laboratory analyses: Kathy Eyring, CCRP; Christine Gage, ARNP; Nadia Lambermont, ARNP; Kim Isaacs, BA; Andrea M. Bobanic, BA;, Kayoko Kennedy, BA;, and the Tissue Core staff of the Moffitt Cancer Center for their help managing biological samples from the United States site; M Luiza Baggio, Roberto Silva, Lenice Galan, Elimar Gomes, Ricardo Cintra, Viviane Relvas, Filomena Cernicchiaro, Raquel Hessel, Sandra Araujo, Graça Ribeiro, Rosária Otero, Roberta Bocalon, Juliana Antunes, Rossana Terreri, Fernanda Silva, Rubens Matsuo, Ricardo Cunha, Vera Souza, Elisa Brito, Birgit Fietzek, from the Brazil site; Verónica Chávez, Aurelio Cruz, María Griselda Díaz, Rossana del Carmen González, Pilar Hernández, Ana Laura Landa, Alicia Rodríguez, and Oscar Rojas from the Mexico site. The authors also thank the Digene Corporation for kindly providing STM and collection vials at no charge to the study.
Abbreviations
- HPV
human papillomavirus
- HIM
The HPV in Men Study
- HR
Hazard Ratio
- CI
confidence interval
- LFU
last follow-up
Footnotes
Disclosure of potential conflicts of interest: Luisa L. Villa is a consultant for Merck, Sharp, Dohme, BD, Roche, and Qiagen.
References
- 1.Satterwhite CL, Torrone E, Meites E, Dunne EF, Mahajan R, Ocfemia MC, Su J, Xu F, Weinstock H. Sexually transmitted infections among US women and men: prevalence and incidence estimates, 2008. Sexually transmitted diseases. 2013;40:187–193. doi: 10.1097/OLQ.0b013e318286bb53. [DOI] [PubMed] [Google Scholar]
- 2.Parkin DM, Bray F. Chapter 2: The burden of HPV-related cancers. Vaccine. 2006;24(3):S3/11–25. doi: 10.1016/j.vaccine.2006.05.111. [DOI] [PubMed] [Google Scholar]
- 3.Luthra UK, Prabhakar AK, Seth P, Agarwal SS, Murthy NS, Bhatnagar P, Das DK, Sharma BK. Natural history of precancerous and early cancerous lesions of the uterine cervix. Acta Cytol. 1987;31:226–234. [PubMed] [Google Scholar]
- 4.Zunzunegui MV, King MC, Coria CF, Charlet J. Male influences on cervical cancer risk. Am J Epidemiol. 1986;123:302–307. doi: 10.1093/oxfordjournals.aje.a114238. [DOI] [PubMed] [Google Scholar]
- 5.Buckley JD, Harris RW, Doll R, Vessey MP, Williams PT. Case-control study of the husbands of women with dysplasia or carcinoma of the cervix uteri. Lancet. 1981;2:1010–1015. doi: 10.1016/s0140-6736(81)91215-0. [DOI] [PubMed] [Google Scholar]
- 6.Dunne EF, Nielson CM, Stone KM, Markowitz LE, Giuliano AR. Prevalence of HPV infection among men: A systematic review of the literature. J Infect Dis. 2006;194:1044–1057. doi: 10.1086/507432. [DOI] [PubMed] [Google Scholar]
- 7.Nyitray AG, da Silva RJ, Baggio ML, Lu B, Smith D, Abrahamsen M, Papenfuss M, Quiterio M, Villa LL, Giuliano AR. The prevalence of genital HPV and factors associated with oncogenic HPV among men having sex with men and men having sex with women and men: the HIM study. Sex Transm Dis. 2011;38:932–940. doi: 10.1097/OLQ.0b013e31822154f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akogbe GO, Ajidahun A, Sirak B, Anic GM, Papenfuss MR, Fulp WJ, Lin HY, Abrahamsen M, Villa LL, Lazcano-Ponce E, Quiterio M, Smith D, et al. Race and prevalence of human papillomavirus infection among men residing in Brazil, Mexico and the United States. Int J Cancer. 2012;131:E282–291. doi: 10.1002/ijc.27397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Albero G, Castellsague X, Giuliano AR, Bosch FX. Male circumcision and genital human papillomavirus: a systematic review and meta-analysis. Sex Transm Dis. 2012;39:104–113. doi: 10.1097/OLQ.0b013e3182387abd. [DOI] [PubMed] [Google Scholar]
- 10.Warner KE, Mackay J. The global tobacco disease pandemic: nature, causes, and cures. Glob Public Health. 2006;1:65–86. doi: 10.1080/17441690500430771. [DOI] [PubMed] [Google Scholar]
- 11.Bauer HM, Hildesheim A, Schiffman MH, Glass AG, Rush BB, Scott DR, Cadell DM, Kurman RJ, Manos MM. Determinants of genital human papillomavirus infection in low-risk women in Portland, Oregon. Sex Transm Dis. 1993;20:274–278. doi: 10.1097/00007435-199309000-00007. [DOI] [PubMed] [Google Scholar]
- 12.Sellors JW, Karwalajtys TL, Kaczorowski J, Mahony JB, Lytwyn A, Chong S, Sparrow J, Lorincz A. Incidence, clearance and predictors of human papillomavirus infection in women. CMAJ. 2003;168:421–425. [PMC free article] [PubMed] [Google Scholar]
- 13.Sellors JW, Mahony JB, Kaczorowski J, Lytwyn A, Bangura H, Chong S, Lorincz A, Dalby DM, Janjusevic V, Keller JL. Prevalence and predictors of human papillomavirus infection in women in Ontario, Canada. Survey of HPV in Ontario Women (SHOW) Group. CMAJ. 2000;163:503–508. [PMC free article] [PubMed] [Google Scholar]
- 14.Minkoff H, Feldman JG, Strickler HD, Watts DH, Bacon MC, Levine A, Palefsky JM, Burk R, Cohen MH, Anastos K. Relationship between smoking and human papillomavirus infections in HIV-infected and -uninfected women. J Infect Dis. 2004;189:1821–1828. doi: 10.1086/383479. [DOI] [PubMed] [Google Scholar]
- 15.Winer RL, Lee SK, Hughes JP, Adam DE, Kiviat NB, Koutsky LA. Genital human papillomavirus infection: incidence and risk factors in a cohort of female university students. Am J Epidemiol. 2003;157:218–226. doi: 10.1093/aje/kwf180. [DOI] [PubMed] [Google Scholar]
- 16.Giuliano AR, Sedjo RL, Roe DJ, Harri R, Baldwi S, Papenfuss MR, Abrahamsen M, Inserra P. Clearance of oncogenic human papillomavirus (HPV) infection: effect of smoking (United States) Cancer causes & control: CCC. 2002;13:839–846. doi: 10.1023/a:1020668232219. [DOI] [PubMed] [Google Scholar]
- 17.Koshiol J, Schroeder J, Jamieson DJ, Marshall SW, Duerr A, Heilig CM, Shah KV, Klein RS, Cu-Uvin S, Schuman P, Celentano D, Smith JS. Smoking and time to clearance of human papillomavirus infection in HIV-seropositive and HIV-seronegative women. Am J Epidemiol. 2006;164:176–183. doi: 10.1093/aje/kwj165. [DOI] [PubMed] [Google Scholar]
- 18.Schabath MB, Villa LL, Lazcano-Ponce E, Salmeron J, Quiterio M, Giuliano AR. Smoking and human papillomavirus (HPV) infection in the HPV in Men (HIM) study. Cancer Epidemiol Biomarkers Prev. 2012;21:102–110. doi: 10.1158/1055-9965.EPI-11-0591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nyitray AG, Kim J, Hsu CH, Papenfuss M, Villa L, Lazcano-Ponce E, Giuliano AR. Test-retest reliability of a sexual behavior interview for men residing in Brazil, Mexico, and the United States: the HPV in Men (HIM) Study. Am J Epidemiol. 2009;170:965–974. doi: 10.1093/aje/kwp225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Flores R, Abalos AT, Nielson CM, Abrahamsen M, Harris RB, Giuliano AR. Reliability of sample collection and laboratory testing for HPV detection in men. J Virol Methods. 2008;149:136–143. doi: 10.1016/j.jviromet.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 21.Gravitt PE, Peyton CL, Alessi TQ, Wheeler CM, Coutlee F, Hildesheim A, Schiffman MH, Scott DR, Apple RJ. Improved amplification of genital human papillomaviruses. J Clin Microbiol. 2000;38:357–361. doi: 10.1128/jcm.38.1.357-361.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cogliano V, Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F. Carcinogenicity of human papillomaviruses. The lancet oncology. 2005;6:204. doi: 10.1016/s1470-2045(05)70086-3. [DOI] [PubMed] [Google Scholar]
- 23.Nielson CM, Harris RB, Dunne EF, Abrahamsen M, Papenfuss MR, Flores R, Markowitz LE, Giuliano AR. Risk factors for anogenital human papillomavirus infection in men. J Infect Dis. 2007;196:1137–1145. doi: 10.1086/521632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kalra R, Singh SP, Savage SM, Finch GL, Sopori ML. Effects of cigarette smoke on immune response: chronic exposure to cigarette smoke impairs antigen-mediated signaling in T cells and depletes IP3-sensitive Ca(2+) stores. J Pharmacol Exp Ther. 2000;293:166–171. [PubMed] [Google Scholar]
- 25.Peters EJ, Morice R, Benner SE, Lippman S, Lukeman J, Lee JS, Ro JY, Hong WK. Squamous metaplasia of the bronchial mucosa and its relationship to smoking. Chest. 1993;103:1429–1432. doi: 10.1378/chest.103.5.1429. [DOI] [PubMed] [Google Scholar]
- 26.Sekhon HS, Wright JL, Churg A. Cigarette smoke causes rapid cell proliferation in small airways and associated pulmonary arteries. Am J Physiol. 1994;267:L557–563. doi: 10.1152/ajplung.1994.267.5.L557. [DOI] [PubMed] [Google Scholar]
- 27.Wright JL, Lawson LM, Pare PD, Wiggs BJ, Kennedy S, Hogg JC. Morphology of peripheral airways in current smokers and ex-smokers. Am Rev Respir Dis. 1983;127:474–477. doi: 10.1164/arrd.1983.127.4.474. [DOI] [PubMed] [Google Scholar]
- 28.Wright JL, Jeng AY, Battistini B. Effect of ECE and NEP inhibition on cigarette smoke-induced cell proliferation in the rat lung. Inhal Toxicol. 2001;13:497–511. doi: 10.1080/08958370117619. [DOI] [PubMed] [Google Scholar]
- 29.Finkelstein EI, Nardini M, van der Vliet A. Inhibition of neutrophil apoptosis by acrolein: a mechanism of tobacco-related lung disease? Am J Physiol Lung Cell Mol Physiol. 2001;281:L732–739. doi: 10.1152/ajplung.2001.281.3.L732. [DOI] [PubMed] [Google Scholar]
- 30.Feng ZH, Hu WW, Hu Y, Tang MS. Acrolein is a major cigarette-related lung cancer agent: Preferential binding at p53 mutational hotspots and inhibition of DNA repair. P Natl Acad Sci USA. 2006;103:15404–15409. doi: 10.1073/pnas.0607031103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li L, Holian A. Acrolein: a respiratory toxin that suppresses pulmonary host defense. Rev Environ Health. 1998;13:99–108. [PubMed] [Google Scholar]
- 32.Barton SE, Maddox PH, Jenkins D, Edwards R, Cuzick J, Singer A. Effect of cigarette smoking on cervical epithelial immunity: a mechanism for neoplastic change? Lancet. 1988;2:652–654. doi: 10.1016/s0140-6736(88)90469-2. [DOI] [PubMed] [Google Scholar]
- 33.Poppe WA, Ide PS, Drijkoningen MP, Lauweryns JM, Van Assche FA. Tobacco smoking impairs the local immunosurveillance in the uterine cervix. An immunohistochemical study. Gynecol Obstet Invest. 1995;39:34–38. doi: 10.1159/000292372. [DOI] [PubMed] [Google Scholar]
- 34.Mehta H, Nazzal K, Sadikot RT. Cigarette smoking and innate immunity. Inflamm Res. 2008;57:497–503. doi: 10.1007/s00011-008-8078-6. [DOI] [PubMed] [Google Scholar]
- 35.Geng Y, Savage SM, Razani-Boroujerdi S, Sopori ML. Effects of nicotine on the immune response. II. Chronic nicotine treatment induces T cell anergy. J Immunol. 1996;156:2384–2390. [PubMed] [Google Scholar]
- 36.Guslandi M. Long-term effects of a single course of nicotine treatment in acute ulcerative colitis: remission maintenance in a 12-month follow-up study. Int J Colorectal Dis. 1999;14:261–262. doi: 10.1007/s003840050221. [DOI] [PubMed] [Google Scholar]
- 37.Albero G, Villa LL, Lazcano-Ponce E, Fulp W, Papenfuss MR, Nyitray AG, Lu B, Castellsague X, Abrahamsen M, Smith D, Bosch FX, Salmeron J, et al. Male circumcision and prevalence of genital human papillomavirus infection in men: a multinational study. BMC Infect Dis. 2013;13:18. doi: 10.1186/1471-2334-13-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Giuliano AR, Lee JH, Fulp W, Villa LL, Lazcano E, Papenfuss MR, Abrahamsen M, Salmeron J, Anic GM, Rollison DE, Smith D. Incidence and clearance of genital human papillomavirus infection in men (HIM): a cohort study. Lancet. 2011;377:932–940. doi: 10.1016/S0140-6736(10)62342-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anic GM, Lee JH, Stockwell H, Rollison DE, Wu Y, Papenfuss MR, Villa LL, Lazcano-Ponce E, Gage C, Silva RJ, Baggio ML, Quiterio M, et al. Incidence and human papillomavirus (HPV) type distribution of genital warts in a multinational cohort of men: the HPV in men study. J Infect Dis. 2011;204:1886–1892. doi: 10.1093/infdis/jir652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vaccarella S, Plummer M, Franceschi S, Gravitt P, Papenfuss M, Smith D, Villa L, Ponce EL, Giuliano AR. Clustering of human papillomavirus (HPV) types in the male genital tract: the HPV in men (HIM) study. J Infect Dis. 2011;204:1500–1504. doi: 10.1093/infdis/jir595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nielson CM, Harris RB, Flores R, Abrahamsen M, Papenfuss MR, Dunne EF, Markowitz LE, Giuliano AR. Multiple-type human papillomavirus infection in male anogenital sites: prevalence and associated factors. Cancer Epidemiol Biomarkers Prev. 2009;18:1077–1083. doi: 10.1158/1055-9965.EPI-08-0447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nyitray AG, Kim J, Hsu CH, Papenfuss M, Villa L, Lazcano-Ponce E, Giuliano AR. Test-retest reliability of a sexual behavior interview for men residing in Brazil, Mexico, and the United States: the HPV in Men (HIM) Study. American journal of epidemiology. 2009;170:965–974. doi: 10.1093/aje/kwp225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Venters MH, Jacobs DR, Jr, Luepker RV, Maiman LA, Gillum RF. Spouse concordance of smoking patterns: the Minnesota Heart Survey. Am J Epidemiol. 1984;120:608–616. doi: 10.1093/oxfordjournals.aje.a113922. [DOI] [PubMed] [Google Scholar]
- 44.Kuo PH, Wood P, Morley KI, Madden P, Martin NG, Heath AC. Cohort trends in prevalence and spousal concordance for smoking. Drug Alcohol Depend. 2007;88:122–129. doi: 10.1016/j.drugalcdep.2006.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. Distribution of select characteristics of men in the HIM study by smoking status