Abstract
PURPOSE
Effective management of fatigue in cancer patients requires a clear delineation of what constitutes nontrivial fatigue. We defined numeric cutpoints for fatigue severity based on functional interference and described fatigue’s prevalence and characteristics in cancer patients and survivors.
METHODS
In a multicenter study, outpatients with breast, prostate, colorectal, or lung cancer rated fatigue severity and symptom interference with functioning on the M. D. Anderson Symptom Inventory (MDASI) 0–10 scale. MDASI ratings of symptom interference guided selection of numeric rating cutpoints between mild, moderate, and severe fatigue levels. Regression analysis identified significant factors related to reporting moderate/severe fatigue.
RESULTS
The statistically optimal cutpoints were ≥4 for moderate fatigue and ≥7 for severe fatigue. Moderate/severe fatigue was reported by 45% (983/2177) of patients undergoing active treatment and was more likely to occur in patients taking strong opioids (odds ratio [OR], 3.00), had poor performance status (OR, 2.00), had >5% weight loss within 6 months (OR, 1.60), were taking >10 medications (OR, 1.58), had lung cancer (OR, 1.55), or had a history of depression (OR, 1.42). Among survivors (patients with complete remission or no evidence of disease, and no current cancer treatment), 29% (150/515) had moderate/severe fatigue that was associated with poor performance status (OR, 3.48) and a history of depression (OR, 2.21).
CONCLUSION
This study statistically defined fatigue-severity categories related to significantly increased symptom interference. The high prevalence of moderate/severe fatigue in both actively treated cancer patients and survivors warrants the promoting of routine assessment and management of patient-reported fatigue.
Keywords: fatigue, patient-reported outcome, practice guidelines, MDASI, survivorship
INTRODUCTION
Fatigue is reported by patients more frequently than are other symptoms during the course of cancer and its treatment, and it is typically the most-severe symptom reported.1–3 Fatigue is the most-bothersome symptom primarily because of its persistence and interference with many aspects of daily life,4 even in cancer survivors with no evidence of active disease.5,6 Despite substantially increased clinical and translational research on fatigue, the precipitating factors and underlying mechanisms remain unclear, and no targeted therapies have been developed to address it.7 Further, wide variability in the percentage of patients experiencing “significant fatigue”—from as few as 4% to as many as 99%—has been reported, depending on the sample and assessment method.8–10 This variation appears even with comparable patient cohorts and similar assessment scales.
A foundational challenge for those who study or treat fatigue is the lack of an empirically derived definition of nontrivial fatigue. Because fatigue is such a universal phenomenon, even in the general population (where it is a common complaint for about 10% of the populace and increases with age or comorbidities11), it is important to characterize nontrivial fatigue and its interference with daily life. These 2 dimensions have been recognized as critical for defining fatigue.
Fatigue is a subjective symptom; thus, patient-reported outcome measures are the standard approach for assessing its severity and interference with daily activities.12 Patient report informs several widely used fatigue-management guidelines, including those produced by the US National Comprehensive Cancer Network (NCCN)13 and the Canadian Association of Psychosocial Oncology (CAPO).14 Such guidelines recommend that fatigue be rated on a well-constructed scale that includes reportable scores with established cutpoints for moderate and severe levels, that fatigue management be initiated when patients rate fatigue as moderate or severe, and that a single screening question may be sufficient for assessing fatigue quickly in the clinic, as is commonly done for pain. Although ratings on a single item may not capture the complexity of the fatigue construct,15 grouping patient ratings into 3 intensity categories (mild, moderate, severe) can be useful for informing treatment decisions, interpreting study outcomes, and aiding policy or clinical-practice guideline development.16 However, despite broad adoption, the cutpoints set forth in the NCCN and CAPO fatigue-management guidelines were generally based on suggestions from expert panels rather than on empirical data.17
In 1995, Serlin and colleagues18 used a quantitative technique to establish cutpoints for mild, moderate, and severe cancer pain on a 0–10 scale by correlating pain intensity and functional interference. This method has been used for more than a decade to categorize cancer pain; consequently, symptom change can be better monitored in clinical practice, and epidemiological and clinical trial data has become comparable across studies.
The goals of the current study were to statistically establish fatigue-severity cutpoints along the 0–10 scale by applying Serlin’s method for deriving pain cutpoints, to generate epidemiological fatigue profiles for both cancer patients and survivors for 4 common cancers, and to promote clinical consensus on a definition for nontrivial fatigue. Data were derived from the Symptom Outcomes and Practice Patterns (SOAPP) study of the Eastern Cooperative Oncology Group (ECOG). Patient-reported fatigue severity and interference with functioning were reported on the M. D. Anderson Symptom Inventory (MDASI), a well-validated multisymptom assessment tool that uses a 0–10 numeric rating scale.19
METHODS
Study Sample
Patients participated in the ECOG E2Z02 SOAPP study, a multicenter study designed to describe the prevalence and severity of cancer-related symptoms and their interference with daily living. Between 2006 and 2008, 3123 patients with breast, colorectal, prostate, or lung cancer were accrued through 38 institutions (including 6 academic and 32 community institutions). Patients were either in treatment or were being followed in outpatient clinical settings. E2Z02 was approved by the institutional review boards at each registering institution. All patients provided written informed consent.
Symptom Measurement and Clinical Data
Upon enrollment, patients used the MDASI to rate “fatigue (tiredness) at its worst” and other symptom items, along with 6 items measuring symptom interference with aspects of daily living (general activity, mood, work, walking, relations with others, and enjoyment of life). Symptoms were rated at their worst in the previous 24 hours on a 0–10 numeric scale, with 0 representing “not present/did not interfere” and 10 representing “as bad as you can imagine/interfered completely.” On a separate, single-item questionnaire, patients rated overall quality of life as excellent, good, fair, poor, or very poor.
From the patient’s medical record, research staff collected demographic information, ECOG performance status (PS), cancer type and disease status, previous and current cancer treatments, current medications, and change in weight in the past 6 months. Patients also reported any history of depression.
Statistical Analysis
For the cutpoint analyses, fatigue severity was categorized as mild (from 1 to the lower cutpoint), moderate (from the lower cutpoint +1 to the upper cutpoint), or severe (from the upper cutpoint +1 to 10). Patients who reported no fatigue (rated 0) were excluded from the cutpoint analyses. Serlin’s criteria,18 in which the optimal cutpoints are based on the largest F ratio from multivariate analysis of variance (MANOVA), were used to determine optimal boundaries for categorizing fatigue severity. The 6 interference variables were used as dependent (continuous) variables and the potential cutpoints for moderate or severe fatigue as independent (categorical) variables.
We performed MANOVAs and compared results from Hotelling’s trace, Wilks lambda, and Pillai’s trace tests to determine if the derived cutpoints were consistent regardless of test criteria. The largest ratio represented the optimal cutpoints. Further, to maximally discriminate among cutpoints for fatigue severity but minimally discriminate among disease sites, ratios of F (fatigue severity) to F (fatigue by disease site) were computed for the cutpoints of the 3 largest ratios from the mixed sample. To ensure that the derived cutpoints based on interference would be relevant to other clinical outcomes, we used the Fisher exact test to compare the identified optimal cutpoints with patient-reported quality of life, clinician-rated ECOG PS, and disease status.
On the basis of the derived cutpoints, the proportion of patients with moderate-to-severe fatigue was calculated by cancer type and patient group (undergoing treatment vs survivors). We defined “survivors” with strict criteria: receiving no cancer treatment at time of study and having either no evidence of disease or tumor response documented as complete remission. The significance of the difference in fatigue prevalence between patient categories was evaluated with the Fisher exact test.
Logistic regression models were fitted to identify potential risk factors for moderate-to-severe fatigue in patients undergoing active therapy and in survivors. Robust standard errors (ie, cluster sandwich estimators) were used in all regression models to account for the clustering effect of institutions (ie, patients enrolled in the same institution might not be independent). A separate category for missing data was generated for categorical covariates if the proportion of missingness was ≥5%. For categorical covariates with <5% missing data and for continuous covariates, patients with missing data were excluded from the logistic regression models. STATA 11.020 was used for all data analysis. All P values were 2-sided, with P < .05 considered statistically significant.
RESULTS
Table 1 shows patient and disease characteristics for the sample. Of 3123 patients accrued to the E2Z02 study, 3032 responded to the MDASI fatigue (tiredness) item and were included in our analyses. The median time from initial disease diagnosis to study registration was 13 months (range, 0–627 months) for actively treated patients and 27 months (range 0–454 months) for survivors.
TABLE 1.
Patient Characteristics (N = 3032)
| Characteristic | Value | n | % |
|---|---|---|---|
| Age | Mean (SD) | 3032 | 61.1 (12.4) |
| Median (range) | 61 (18–93) | ||
| Sex | Women | 2126 | 70.1 |
| Men | 906 | 29.9 | |
| Race (n = 37 missing data) | White | 2579 | 86.1 |
| Black | 359 | 12.0 | |
| Others | 57 | 1.9 | |
| Primary cancer site | Breast | 1509 | 49.8 |
| Colorectal | 700 | 23.1 | |
| Prostate | 307 | 10.1 | |
| Lung | 516 | 17.0 | |
| Current disease status (n = 17 missing data) | Complete response | 1135 | 37.7 |
| Partial response | 141 | 4.7 | |
| Stable disease | 1307 | 43.4 | |
| Progressive disease | 432 | 14.3 | |
| Current stage of disease (n = 10 missing data) | Nonadvanced | 1877 | 62.1 |
| No evidence of disease | 1306 | 43.2 | |
| Local/regional | 571 | 18.9 | |
| Advanced | 1145 | 37.9 | |
| Metastatic | 937 | 31.0 | |
| Local/regional + metastatic | 208 | 6.9 | |
| Duration of cancer (years) (n = 47 missing data) | Mean (SD) | 2985 | 2.9 (4.2) |
| Median (range) | 1.2 (0–52) | ||
| Prior chemo/immuno/hormonal therapy (n = 1 missing data) | Yes | 1871 | 61.7 |
| Prior radiotherapy (n = 26 missing data) | Yes | 1269 | 41.8 |
| Current chemo/immuno/hormonal therapy | Yes | 2249 | 74.2 |
| Current radiation therapy (n = 32 missing data) | Yes | 180 | 6.0 |
| History of depression | Yes | 881 | 29.1 |
| Currently using antidepressants | Yes | 586 | 19.3 |
| Currently using analgesics | None | 1472 | 48.6 |
| Nonopioids | 683 | 22.5 | |
| Weak opioids | 429 | 14.2 | |
| Strong opioids | 399 | 13.2 | |
| Number of medications currently taking | 0–4 | 882 | 32.3 |
| 5–9 | 1180 | 43.2 | |
| ≥10 | 668 | 24.5 | |
| ECOG PS (n = 14 missing data) | 0 | 1718 | 56.9 |
| 1 | 1077 | 35.7 | |
| 2–4 | 223 | 7.4 | |
| Overall quality of life (n = 9 missing data) | Excellent | 631 | 20.9 |
| Good | 1495 | 49.5 | |
| Fair | 724 | 24.0 | |
| Poor | 152 | 5.0 | |
| Very poor | 21 | 0.7 | |
| Weight loss in the previous 6 months (n = 34 missing data) | <5% | 2571 | 85.8 |
| 5–10% | 270 | 9.0 | |
| 10–20% | 132 | 4.4 | |
| ≥20% | 25 | 0.8 |
Abbreviations: SD, standard deviation; ECOG PS, Eastern Cooperative Oncology Group performance status.
Optimal Cutpoints for Fatigue Severity
Fatigue was reported (based on a score of 1–10) by 2341 patients. MANOVA results for these patients indicated that, among 16 combinations of lower and upper cutpoints, cutpoints 3 and 6 had the largest F statistics (Wilks lambda = 99.3, Pillai’s trace = 87.1, Hotelling’s trace = 111.9), indicating optimal cutpoints at 3 distinct levels of fatigue severity: ratings of 1–3 as mild, 4–6 as moderate, and 7–10 as severe (Table 2). Table 3 presents the fatigue over fatigue-disease site interactions for the 3 cutpoint combinations with the largest F ratios (3/6, 3/7, 4/7). The 3/6 cutpoint combination remained the optimal option. Fisher exact test analysis found significant differences in patient-reported quality of life (P < .001), ECOG PS (P < .001), and disease status (P < .001) among mild (1–3), moderate (4–6), and severe (7–10) fatigue categories.
TABLE 2.
Optimal Cutpoint Analysis using 3 Criteria (N = 2341 Rating Fatigue Severity as 1–10)
| Cutpoint Pairsa | Wilks lambda | Pillai’s trace | Hotelling’s trace |
|---|---|---|---|
| 2/4 | 80.8 | 72.7 | 89.0 |
| 3/4 | 79.4 | 71.8 | 87.2 |
| 3/5 | 94.5 | 83.3 | 105.9 |
| 3/6b | 99.3 | 87.1 | 111.9 |
| 3/7b | 98.7 | 86.7 | 111.0 |
| 3/8 | 89.0 | 79.4 | 98.8 |
| 4/5 | 88.6 | 78.7 | 98.7 |
| 4/6 | 94.8 | 83.6 | 106.2 |
| 4/7b | 96.5 | 85.1 | 108.2 |
| 4/8 | 90.9 | 80.9 | 101.1 |
| 5/6 | 80.3 | 72.3 | 88.5 |
| 5/7 | 84.2 | 75.5 | 93.1 |
| 5/8 | 83.9 | 75.3 | 92.6 |
| 6/7 | 72.2 | 65.7 | 78.8 |
| 6/8 | 73.9 | 67.2 | 80.7 |
| 7/8 | 59.5 | 55.2 | 63.7 |
The analyses categorize fatigue into 3 groups: mild (from 1 to lower cutpoint), moderate (from lower cutpoint +1 to upper cutpoint) and severe fatigue (from upper cutpoint +1 to 10).
Best model fit.
TABLE 3.
Ratio of F (Fatigue Level) over F (Interaction) for 3 Separate MANOVAs using 3 Different Criteria (N = 2341 Rating Fatigue Severity as 1–10)
| Criterion | ||||
|---|---|---|---|---|
| Cutpoint Pair | Wilks Lambda | Pillai’s Trace | Hotelling’s Trace | |
| 3/6 | F (Fatigue level) | 72.7 | 66.1 | 79.5 |
| F (Fatigue by disease site) | 1.3 | 1.3 | 1.3 | |
| Ratio | 57.7 | 52.5 | 63.1 | |
| 3/7 | F (Fatigue level) | 73.4 | 66.7 | 80.2 |
| F (Fatigue by disease site) | 1.4 | 1.4 | 1.4 | |
| Ratio | 51.7 | 47.3 | 56.4 | |
| 4/7 | F (Fatigue level) | 72.7 | 66.2 | 79.3 |
| F (Fatigue by disease site) | 1.5 | 1.5 | 1.5 | |
| Ratio | 47.5 | 43.6 | 52.5 | |
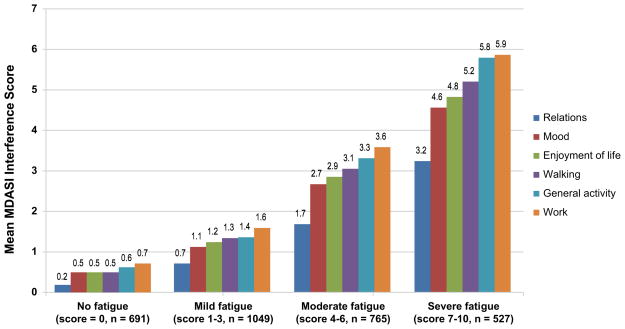
When fatigue was rated as none, mild, moderate, or severe with the identified optimal cutpoints (0, 1–3, 4–6, 7–10), interference levels were well differentiated (Figure 1).
Figure 1.
Interaction of Symptom Interference and Fatigue Severity Using the Identified Optimal Cutpoints. MDASI indicates M. D. Anderson Symptom Inventory.
Moderate-to-Severe Fatigue by Patient or Survivor Group
Using the identified optimal cutpoints in the entire sample, we found that 23% (691/3032) of both cancer patients and survivors reported no fatigue (rated 0), 35% (1049/3032) had mild fatigue (1–3), 25% (765/3032) had moderate fatigue (4–6), and 17% (527/3032) had severe fatigue (7–10).
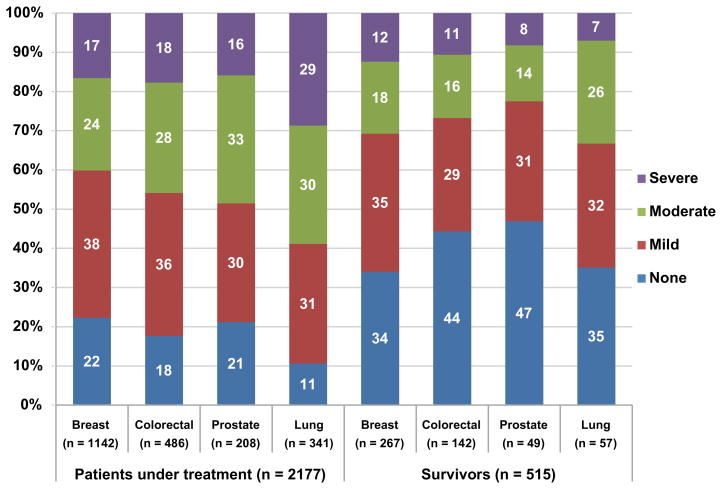
Figure 2 presents the prevalence of fatigue in the 4 severity categories (none, mild, moderate, and severe) by specific cancer type for patients undergoing treatment at enrollment (n = 2177; 72/2249 who started therapy after enrolling on study were excluded) and for survivors within (n = 373) and beyond (n = 136) 5 years. Among patients undergoing active treatment, 55% (1194/2177) reported no or mild fatigue and 45% (983/2177) reported moderate-to-severe fatigue (breast 40%, colorectal 46%, prostate 49%, lung 59%). Significantly more patients with lung cancer had moderate-to-severe fatigue (P < .05 for all pairwise comparisons).
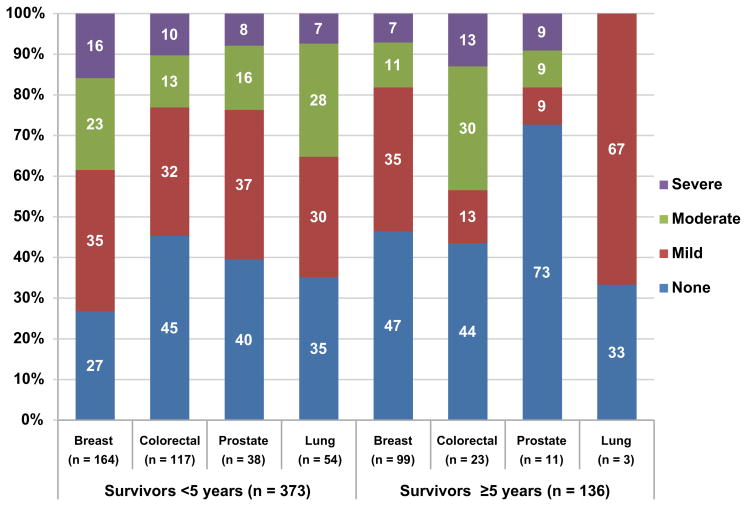
Figure 2.
Prevalence of fatigue by cancer type (M. D. Anderson Symptom Inventory ratings)
A: Patients Undergoing Treatment vs Survivors
Significant differences were found between patients undergoing treatment and survivors in terms of the percentages reporting moderate-to-severe fatigue: P = .005 for breast cancer (40% of treated patients vs 31% of survivors), P < .001 for colorectal cancer (46% vs 27%), P = .001 for prostate cancer (49% vs 22%), and P < .001 for lung cancer (59% vs 33%), using the Fisher exact test.
In patients undergoing treatment, significant differences were found in the percentages of those reporting moderate-to-severe fatigue in pairwise comparisons between lung cancer and other cancer types: P < .001 for breast cancer (59% lung vs 40% breast), P < .001 for colorectal cancer (59% vs 46%), and P = .018 for prostate cancer (59% vs 49%), using the Chi square test.
Fisher exact test results were not significant for pairwise comparisons of moderate-to-severe fatigue between lung cancer and other cancer types in survivors (P > .05 for all comparisons).
B: Short-Term Survivors vs Long-Term Survivors
For comparisons between the percentages of short-term survivors and long-term survivors reporting moderate-to-severe fatigue, P = .001 for breast cancer (38% short-term vs 18% long-term), P = .067 for colorectal cancer (23% vs 43%), no significance for prostate cancer (23% vs 18%), and for lung cancer (35% vs 0%), using the Fisher exact test.
Six patients had a missing value for time since cancer diagnosis and were not included in this analysis.
Of 783 patients not receiving treatment, 515 met our criteria defining a survivor. Of these, 71% (365/515) reported no or mild fatigue and 29% (150/515) reported moderate-to-severe fatigue (breast 31%, colorectal 27%, prostate 22%, lung 33%). The prevalence of moderate-to-severe fatigue was similar for patients with breast or prostate cancer who had survived longer than five years (both 18%). In breast cancer patients, the proportion of moderate-to-severe fatigue was lower in patients who had survived ≥5 years compared with those who had survived <5 years (18% vs 38%, P = .001). Moderate-to-severe fatigue was more prevalent in patients receiving treatment than in survivors (P < .01 for all 4 disease groups).
Multiple factors were significantly related to moderate-to-severe fatigue in the multivariable regression analyses (Table 4). For patients receiving treatment (n = 2045 contributing sufficient covariate data), the 5 most-important contributors ordered by strength of odds ratios [OR] were opioid use, poor ECOG PS (≥1), more than 5% weight loss in the previous 6 months, currently taking 10 or more medications, and a diagnosis of lung cancer. For survivors (n = 489 contributing sufficient covariate data), poor ECOG PS (≥1) and a history of depression were associated with moderate-to-severe fatigue. Sex was not a significant factor for moderate-to-severe fatigue in the colorectal or lung cancer patients (the breast and prostate groups were single-gender).
TABLE 4.
Multivariable Logistic Regression for Risk Factors of Moderate-to-Severe Fatigue in Cancer Patients and Survivors (N = 2534 Contributing Covariate Data)
| Patients in Current Treatment (n = 2045) | Survivors (n = 489) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Level | ORa | 95% CI | P | ORa | 95% CI | P | ||
| Disease site | Colorectal vs breast | 1.11 | 0.86 | 1.42 | .421 | 0.87 | 0.50 | 1.50 | .613 |
| Prostate vs breast | 1.29 | 0.88 | 1.89 | .192 | 0.91 | 0.43 | 1.96 | .819 | |
| Lung vs breast | 1.55 | 1.13 | 2.12 | .006 | 0.80 | 0.39 | 1.65 | .555 | |
| Age (years) | Per year | 0.99 | 0.98 | 1.00 | .038 | 0.98 | 0.96 | 1.00 | .104 |
| Duration of disease (years) | Per year | 1.01 | 0.98 | 1.04 | .459 | 0.99 | 0.93 | 1.06 | .801 |
| Race | Black vs white | 1.54 | 1.07 | 2.21 | .019 | 1.48 | 0.78 | 2.82 | .229 |
| Others vs white | 1.34 | 0.51 | 3.52 | .556 | 2.36 | 0.78 | 7.13 | .127 | |
| ECOG PS | ≥1 vs 0 | 2.00 | 1.66 | 2.41 | < .001 | 3.48 | 1.78 | 6.78 | < .001 |
| Weight loss | >5% vs ≤5% | 1.60 | 1.19 | 2.14 | .002 | 1.93 | 0.61 | 6.13 | .267 |
| Prior chemo/immune/hormonal therapy | Yes vs no | 0.91 | 0.74 | 1.14 | .418 | 1.33 | 0.72 | 2.45 | .369 |
| Prior radiotherapy | Yes vs no | 0.92 | 0.76 | 1.11 | .365 | 1.09 | 0.73 | 1.64 | .666 |
| Number of medications currently taken | 5–9 vs 0–4 | 1.19 | 0.93 | 1.51 | .159 | 1.09 | 0.61 | 1.93 | .778 |
| ≥10 vs 0–4 | 1.58 | 1.21 | 2.06 | .001 | 1.09 | 0.48 | 2.46 | .834 | |
| Missing vs 0–4 | 0.94 | 0.62 | 1.42 | .767 | 0.49 | 0.24 | 1.00 | .049 | |
| History of depression | Yes vs no | 1.42 | 1.10 | 1.84 | .007 | 2.21 | 1.34 | 3.66 | .002 |
| Current antidepressant use | Yes vs no | 1.53 | 1.18 | 1.97 | .001 | 1.08 | 0.62 | 1.86 | .789 |
| Current analgesic use | Nonopioid vs none | 1.42 | 1.07 | 1.88 | .016 | 1.27 | 0.73 | 2.22 | .396 |
| Weak opioid vs none | 1.69 | 1.21 | 2.36 | .002 | 2.42 | 0.98 | 5.98 | .055 | |
| Strong opioid vs none | 3.00 | 2.17 | 4.15 | < .0001 | 1.73 | 0.53 | 5.63 | .364 | |
Boldface = statistically significant.
Abbreviations: OR, odds ratio; 95% CI, 95% confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status.
DISCUSSION
This large, nationwide study of cancer patients and survivors provides evidence that the best model fit for categorizing fatigue severity on the MDASI’s 0–10 scale is mild (1–3), moderate (4–6), and severe (7–10). According to these cutpoints, moderate-to-severe (nontrivial) CRF (rated ≥4 on a 0–10 scale) was experienced by 45% of patients undergoing treatment and 29% of survivors. Moderate-to-severe fatigue was significantly more prevalent in patients undergoing treatment than in survivors.
The presence of nontrivial fatigue in 45% of patients undergoing treatment indicates that nearly half will require fatigue management or else pay a high price for their cure. One reason for this is the well-recognized lack of gold-standard, mechanism-driven prevention or intervention strategies for nontrivial fatigue during acute therapy,21 which suggests that assessment of fatigue severity is not typically confounded by effective fatigue reduction.
We observed that, overall, impaired performance status and a history of depression were related to moderate-to-severe fatigue in the entire sample. Given the age of our sample, these factors could have been driven by other clinical conditions, with or without an impact from cancer or its treatments. More significant contributors to nontrivial fatigue were found for patients receiving anticancer treatment than for survivors. During treatment, taking strong opioids was the strongest factor associated with moderate-to-severe fatigue (OR, 3.00), followed by taking weak opioids, weight loss ≥5% within the past 6 months, taking more than 10 medications, and a lung cancer diagnosis.
Although many studies have shown fatigue in breast cancer survivors,22 the current study demonstrates that fatigue is also prevalent in survivors of lung, colorectal, or prostate cancer. In a side-by-side comparison of moderate-to-severe fatigue in survivors, we found no impact on fatigue categories from breast cancer compared with the other cancer types (Table 4). We observed a comparable prevalence of moderate-to-severe fatigue in short-term (<5 years) breast cancer survivors (38%) and lung cancer survivors (35%) (compared with 23% in colorectal cancer survivors and 24% in prostate cancer survivors), and a similar prevalence in breast and prostate long-term (≥5 years) survivors (both 18%, compared with 43% for colorectal survivors and 0% for lung cancer survivors) (Figure 2B).
Our findings demonstrate an increase in MDASI symptom interference when fatigue was moderate to severe, consistent with pain-research findings demonstrating increased symptom interference as pain worsened18 and with the NCCN definition of fatigue as “a persistent subjective sense of tiredness related to cancer or cancer treatment that interferes with usual functioning.”13 The validity of this categorization of fatigue severity is further demonstrated by significant differences seen in clinical outcomes, quality of life, performance status, and disease status by fatigue category.
The statistically derived cutpoints in this study support the cutpoints (≥4 for moderate and ≥7 for severe fatigue) used in various fatigue-management guidelines and are consistent with the Brief Fatigue Inventory validation study4 cutpoint results based on the “fatigue worst” item and fatigue interference. We found that the second-best cutpoint pair was 4/8 (Table 2), suggesting that the optimal clinical cutpoint for the severe category might vary. Further, our results were not consistent with the 2/5 MDASI cutpoint pair reported by Given et al.23 Trials with a single clinical condition or conducted in a single institution may have inherent limitations in finding optimal boundaries; in contrast, this study, with its larger, more-diverse patient sample, robustly addresses the fatigue-severity cutpoint question.
As an outcome measure, fatigue can be an important component of drug trials. A reduction in patient-reported fatigue could represent reduced treatment toxicity, improved palliation for advanced disease, increased quality assurance and patient satisfaction with medical care, or improved functional and health status for cancer survivors. However, clinicians are likely to rate a patient’s fatigue as being less severe and less frequent than the patient would report.24 Thus, adequate assessment by patient report and accurate interpretation of patient-reported fatigue data using valid cutpoints are essential to good fatigue management in the clinic. The manner in which nontrivial fatigue is defined in a given study or in practice could be a clinically meaningful indicator of whether fatigue intervention is needed and whether such an intervention is effective. For example, studies of long-acting methylphenidate and modafinil suggest that such interventions are effective in the subset of patients for whom fatigue is most severe, even when the cutpoints delineating moderate to severe fatigue were not consistent.25,26
This study had limitations. (1) Our approach used statistical criteria to optimize selection of cutpoints based on the relationship of patient-reported fatigue to patient report of the functional interference caused by that fatigue. It is important to confirm these cutpoints against those derived directly from expert clinical classification or from patients themselves, and it would be interesting to know how nontrivial fatigue on the 0–10 scale would correspond with other scales and with toxicity grading. (2) Our sample represented only 4 primary cancer sites. (3) Hemoglobin levels, psychotherapy received, objective sleep-quality metrics, and comorbidities, which could have furthered our understanding of treatable causes, were not collected. (4) The population in this study was 86% white, higher than the 76% reported in national census data.27 However, this proportion is consistent with the population of white patients who are being seen for cancer care at ECOG-affiliated institutions. (5) We utilized a method from pain research with a consideration of nonlinear correspondence between severity and interference; other methods in other patient cohorts might produce different “optimal” cutpoints for fatigue.28 Variability in optimal cutpoints is to be expected,29 and even the use of the categories “mild,” moderate,” and “severe,” although familiar, is arbitrary.
In summary, our study confirmed statistical categories for mild, moderate, and severe fatigue on a 0–10 scale that have been clinically accepted in NCCN and other practice guidelines. Our results also demonstrate high prevalence of moderate-to-severe fatigue among 4 major types of cancer, not only in cancer patients undergoing active treatment, but in survivors as well.
Acknowledgments
Funding: Supported in part by grants from the National Cancer Institute of the National Institutes of Health, including U10 CA37403 and U10 CA17145 to the Eastern Cooperative Oncology Group, R01 CA026582 to C.S.C, and MD Anderson Cancer Center Support Grant P30 CA016672 to R. A. DePinho. The contents of this report are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
The authors acknowledge Jeanie F. Woodruff, BS, ELS, for editorial support.
Footnotes
Financial Disclosures/Conflicts of Interest: None.
References
- 1.Yanez B, Pearman T, Lis CG, Beaumont JL, Cella D. The FACT-G7: a rapid version of the functional assessment of cancer therapy-general (FACT-G) for monitoring symptoms and concerns in oncology practice and research. Ann Oncol. 2013;24(4):1073–1078. doi: 10.1093/annonc/mds539. [DOI] [PubMed] [Google Scholar]
- 2.Wang XS, Cleeland CS, Mendoza TR, et al. Impact of cultural and linguistic factors on symptom reporting by patients with cancer. J Natl Cancer Inst. 2010;102(10):732–738. doi: 10.1093/jnci/djq097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Minton O, Strasser F, Radbruch L, Stone P. Identification of factors associated with fatigue in advanced cancer: a subset analysis of the European palliative care research collaborative computerized symptom assessment data set. J Pain Symptom Manage. 2012;43(2):226–235. doi: 10.1016/j.jpainsymman.2011.03.025. [DOI] [PubMed] [Google Scholar]
- 4.Mendoza TR, Wang XS, Cleeland CS, et al. The rapid assessment of fatigue severity in cancer patients: use of the Brief Fatigue Inventory. Cancer. 1999;85(5):1186–1196. doi: 10.1002/(sici)1097-0142(19990301)85:5<1186::aid-cncr24>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 5.Servaes P, Gielissen MF, Verhagen S, Bleijenberg G. The course of severe fatigue in disease-free breast cancer patients: a longitudinal study. Psychooncology. 2007;16(9):787–795. doi: 10.1002/pon.1120. [DOI] [PubMed] [Google Scholar]
- 6.Pachman DR, Barton DL, Swetz KM, Loprinzi CL. Troublesome symptoms in cancer survivors: fatigue, insomnia, neuropathy, and pain. J Clin Oncol. 2012;30(30):3687–3696. doi: 10.1200/JCO.2012.41.7238. [DOI] [PubMed] [Google Scholar]
- 7.Minton O, Richardson A, Sharpe M, Hotopf M, Stone P. A systematic review and meta-analysis of the pharmacological treatment of cancer-related fatigue. J Natl Cancer Inst. 2008;100(16):1155–1166. doi: 10.1093/jnci/djn250. [DOI] [PubMed] [Google Scholar]
- 8.Hofman M, Morrow GR, Roscoe JA, et al. Cancer patients’ expectations of experiencing treatment-related side effects: a University of Rochester Cancer Center--Community Clinical Oncology Program study of 938 patients from community practices. Cancer. 2004;101(4):851–857. doi: 10.1002/cncr.20423. [DOI] [PubMed] [Google Scholar]
- 9.Patrick DL, Ferketich SL, Frame PS, et al. National Institutes of Health State-of-the-Science Conference Statement: Symptom management in cancer: pain, depression, and fatigue, July 15–17, 2002. J Natl Cancer Inst Monogr. 2004;(32):9–16. doi: 10.1093/jncimonographs/djg014. [DOI] [PubMed] [Google Scholar]
- 10.Cella D, Davis K, Breitbart W, Curt G. Cancer-related fatigue: prevalence of proposed diagnostic criteria in a United States sample of cancer survivors. J Clin Oncol. 2001;19(14):3385–3391. doi: 10.1200/JCO.2001.19.14.3385. [DOI] [PubMed] [Google Scholar]
- 11.Cella D, Lai JS, Chang CH, Peterman A, Slavin M. Fatigue in cancer patients compared with fatigue in the general United States population. Cancer. 2002;94(2):528–538. doi: 10.1002/cncr.10245. [DOI] [PubMed] [Google Scholar]
- 12.US Food and Drug Administration. [accessed July 31, 2012];Guidance for industry. Patient-reported outcome measures: use in medical product development to support labeling claims. doi: 10.1186/1477-7525-4-79. Available from URL: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM071975.pdf. [DOI] [PMC free article] [PubMed]
- 13.Berger AM, Abernethy AP, Atkinson A, et al. [accessed July 17, 2013];NCCN Practice Guidelines in Oncology: Cancer-Related Fatigue. Available from URL: http://www.nccn.org/professionals/physician_gls/PDF/fatigue.pdf.
- 14.Howell D, Keller-Olaman S, Oliver TK, et al. [accessed August 21, 2013];A pan-Canadian practice guideline: screening, assessment and care of cancer-related fatigue in adults with cancer. doi: 10.3747/co.20.1302. Available from URL: http://www.capo.ca/Fatigue_Guideline.pdf. [DOI] [PMC free article] [PubMed]
- 15.Barsevick AM, Cleeland CS, Manning DC, et al. ASCPRO recommendations for the assessment of fatigue as an outcome in clinical trials. Journal of Pain and Symptom Management. 2010;39(6):1086–1099. doi: 10.1016/j.jpainsymman.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anderson KO. Role of cutpoints: why grade pain intensity? Pain. 2005;113(1–2):5–6. doi: 10.1016/j.pain.2004.10.024. [DOI] [PubMed] [Google Scholar]
- 17.Oldenmenger WH, de Raaf PJ, de Klerk C, van der Rijt CC. Cut points on 0–10 numeric rating scales for symptoms included in the Edmonton Symptom Assessment Scale in cancer patients: a systematic review. J Pain Symptom Manage. 2013;45(6):1083–1093. doi: 10.1016/j.jpainsymman.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 18.Serlin RC, Mendoza TR, Nakamura Y, Edwards KR, Cleeland CS. When is cancer pain mild, moderate or severe? Grading pain severity by its interference with function. Pain. 1995;61(2):277–284. doi: 10.1016/0304-3959(94)00178-H. [DOI] [PubMed] [Google Scholar]
- 19.Cleeland CS, Mendoza TR, Wang XS, et al. Assessing symptom distress in cancer patients: the M.D. Anderson Symptom Inventory. Cancer. 2000;89(7):1634–1646. doi: 10.1002/1097-0142(20001001)89:7<1634::aid-cncr29>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 20.StataCorp LP. Stata Statistical Software, version 11 [computer program] College Station TX: StataCorp LP; 2009. [Google Scholar]
- 21.Wang XS. Cancer-related fatigue. In: Berger AM, Shuster JL, von Roenn JH, editors. Principles and Practice of Palliative Care and Supportive Oncology. 4. Philadelphia PA: Wolters Kluwer/Lippincott Williams & Wilkins; 2013. pp. 84–95. [Google Scholar]
- 22.Bower JE, Ganz PA, Desmond KA, Rowland JH, Meyerowitz BE, Belin TR. Fatigue in breast cancer survivors: occurrence, correlates, and impact on quality of life. J Clin Oncol. 2000;18(4):743–753. doi: 10.1200/JCO.2000.18.4.743. [DOI] [PubMed] [Google Scholar]
- 23.Given B, Given CW, Sikorskii A, et al. Establishing mild, moderate, and severe scores for cancer-related symptoms: how consistent and clinically meaningful are interference-based severity cut-points? J Pain Symptom Manage. 2008;35(2):126–135. doi: 10.1016/j.jpainsymman.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Basch E. The missing voice of patients in drug-safety reporting. N Engl J Med. 2010;362(10):865–869. doi: 10.1056/NEJMp0911494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jean-Pierre P, Morrow GR, Roscoe JA, et al. A phase 3 randomized, placebo-controlled, double-blind, clinical trial of the effect of modafinil on cancer-related fatigue among 631 patients receiving chemotherapy: a University of Rochester Cancer Center Community Clinical Oncology Program Research base study. Cancer. 2010;116(14):3513–3520. doi: 10.1002/cncr.25083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moraska AR, Sood A, Dakhil SR, et al. Phase III, randomized, double-blind, placebo-controlled study of long-acting methylphenidate for cancer-related fatigue: North Central Cancer Treatment Group NCCTG-N05C7 trial. J Clin Oncol. 2010;28(23):3673–3679. doi: 10.1200/JCO.2010.28.1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.US Census Bureau. [accessed August 21, 2013];ACS demographic and housing estimates: 2010 American Community Survey 1-year estimates. Available from URL: http://factfinder2.census.gov/faces/tableservices/jsf/pages/productview.xhtml.
- 28.Farrar JT. Cut-points for the measurement of pain: the choice depends on what you want to study. Pain. 2010;149(2):163–164. doi: 10.1016/j.pain.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 29.Hirschfeld G, Zernikow B. Variability of “optimal” cut points for mild, moderate, and severe pain: Neglected problems when comparing groups. Pain. 2013;154(1):154–159. doi: 10.1016/j.pain.2012.10.008. [DOI] [PubMed] [Google Scholar]