Abstract
Impaired affective expression, including social smiling, is common in children with autism spectrum disorder (ASD), and may represent an early marker for ASD in their infant siblings (Sibs-ASD). Social smiling and its component behaviors (eye contact and non-social smiling) were examined at 15 months in Sibs-ASD who demonstrated later ASD symptomatology (Sibs-ASD/AS), those who did not (Sibs-ASD/NS), and low-risk controls (Sibs-TD). Both Sibs-ASD subgroups demonstrated lower levels of social smiling than Sibs-TD, suggesting that early social smiling may reflect elevated genetic vulnerability rather than a specific marker for ASD. Only the Sibs-ASD/AS demonstrated less eye contact and non-social smiling than Sibs-TD, suggesting that different processes, threshold effects, or protective factors may underlie social smiling development in the two Sibs-ASD subgroups.
Keywords: Autism, High-risk siblings, Infants, Social smiling
Early social interactions between infants and adults are thought to underlie advances in social cognition that lead to the development of later, more complex social interactions (Bakeman & Adamson, 1984; Bruner, 1975; Striano & Rochat, 1999; Tronick, 1982; Vygotsky, 1978). In typical development, smiling is one of the most commonly observed social behaviors during the first 6 months of life (Kaye & Fogel, 1980; Weinberg & Tronick, 1994; Yale, Messinger, Cobo-Lewis, & Delgado, 2003). Social smiling emerges between 2 and 3 months of age (Adamson & Bakeman, 1985; Messinger & Fogel, 2007) and becomes increasingly intentional, referential, and communicative in the latter half of the first year (Carpenter, Nagell, & Tomasello, 1998; Venezia, Messinger, Thorp, & Mundy, 2004). As infants’ social understanding increases, their intentional coordination of smiling with gaze directed to a social partner becomes more prominent (Messinger & Fogel, 2007).
At a behavioral level, social smiling involves the temporal integration of two components: the facial expression of positive affect and the orientation of eye gaze toward another person. Impairments in both the affective and attentional components of social smiling have been found in children with autism spectrum disorders (ASD; Dawson, Hill, Spencer, Galpert, & Watson, 1990; Kasari, Sigman, & Yirmiya, 1993; Swettenham et al., 1998). However, there is evidence that impairments in the integration of eye gaze and positive affect (i.e., social smiling) may be particularly salient in children with ASD relative to those with other developmental disorders or typical development (TD; Dawson et al., 1990; Joseph & Tager-Flusberg, 1997; Kasari, Sigman, Mundy, & Yirmiya, 1990). For example, Dawson et al. (1990) found comparable rates of eye contact and positive affect in children with ASD and those with TD, but children with ASD were less likely to integrate the two behaviors during social bids. In addition, lower levels of social smiling have also been found in infants who later develop ASD, using both retrospective (e.g., Adrien et al., 1993) and prospective approaches (Barbaro & Dissanayake, 2012). Thus, deficits in social smiling have been identified as a possible early marker of later emergence of the ASD phenotype.
Many recent prospective studies searching for early behavioral markers of ASD have focused on infant siblings of children with ASD (Sibs-ASD), due to their elevated genetic risk for ASD (i.e., 18.7%; Ozonoff et al., 2011), and for ASD-related behaviors associated with the broader autism phenotype (BAP; Piven, Palmer, Jacobi, Childress, & Arndt, 1997; Zwaigenbaum et al., 2007). In general, studies comparing groups of infant Sibs-ASD to infant siblings of children with typical development (Sibs-TD) tend to find a broad range of behavioral differences (for review, see Rogers, 2009); however, the meaning of these group differences is difficult to interpret. Specifically, before infants’ diagnostic outcomes are clear, it cannot be said whether differences observed in infant Sibs-ASD are characteristic of that group as a whole, or are driven instead by the subset of yet-unidentified infants who themselves will eventually receive an ASD diagnosis. To date, research has focused primarily on detecting early behaviors that characterize the subset of infant Sibs-ASD who will later receive ASD diagnosis or display BAP symptoms, while less attention has been paid to the early social communication profiles of infant Sibs-ASD who ultimately have typical outcomes.
Studies that have followed infant Sibs-ASD to diagnostic outcome have revealed that social smiling differentiates the subset of Sibs-ASD who develop ASD from low-risk controls as young as 12 months of age (Brian et al., 2008; Ozonoff et al. 2010; Zwaigenbaum et al., 2005). However, one study also found lower levels of social smiling in infant Sibs-ASD who did not receive a later ASD diagnosis, again compared with low-risk controls (Brian et al., 2008). Though this study suggests that reduced social smiling may be associated more with elevated genetic risk than with an ASD outcome specifically, Sibs-ASD with BAP outcomes were not excluded from the non-ASD outcome group. The one infant sibling study to examine social smiling in separate BAP and non-ASD outcome subgroups (Landa, Holman, & Garrett-Mayer, 2007) did not directly compare the performance of these subgroups to that of the low-risk controls. Thus, the early expression of social smiling in high-risk infant siblings with symptom-free outcomes is not yet known.
The purpose of the present study was to extend our understanding of the construct of social smiling and its relation to outcomes in infant Sibs-ASD in two ways. First, our primary focus was to examine social smiling in infant Sibs-ASD without ASD symptomatology at outcome. The early development of infant Sibs-ASD without ASD symptoms remains relatively unknown, and may provide valuable information about the range of outcomes and potential protective factors for children at elevated genetic risk. Specifically, it is not yet known whether infant Sibs-ASD who demonstrate no ASD symptoms at outcome (i.e., neither ASD diagnoses nor BAP profiles) have early levels of social smiling that are comparable to those of low-risk infants, or whether, like the subset of Sibs-ASD who do later display ASD symptoms, they too exhibit less social smiling relative to low-risk peers. The answer to this question may have implications for the interpretation of early behavioral differences, the mutability of early challenges, and the significance of early affective expression in this subgroup of Sibs-ASD with optimal outcomes. Toward this end, we excluded Sibs-ASD with BAP outcomes from the non-ASD outcome group.
Our second goal was to measure social smiling and its component behaviors (i.e., social attention [eye contact] and positive affect [non-social smiling]) simultaneously, and as mutually exclusive categories. To our knowledge, no previous study has examined these behaviors concurrently, using the same behavior sample and metrics, to identify the extent to which eye contact and/or non-social smiling might individually limit (or facilitate) the expression of social smiling in infant Sibs-ASD. In the current study, we sought to determine whether particular components of social smiling differentiate Sibs-ASD with optimal outcomes from those who later express ASD symptoms, and to assess the extent to which affected and/or unaffected Sibs-ASD are distinguishable from low-risk peers with regard to these behaviors. A better understanding of the early social behaviors of Sibs-ASD who go on to have typical outcomes will be helpful in interpreting differences observed in infancy, and in making important clinical decisions with regard to when, and for whom, prevention and intervention strategies for high-risk infants are warranted.
Method
Participants
Participants were part of a larger sample of infant siblings in a prospective, longitudinal study examining the early development of ASD. The sample comprised two groups: (1) younger siblings of children with autism spectrum disorders (Sibs-ASD), and (2) younger siblings of children with typical development (Sibs-TD). Informed consent was obtained from parents prior to initiation of research procedures. Children were enrolled between 12 and 23 months of age, and participated in a total of five clinic visits across an 18-month period (see [reference deleted for blind review] for a description of eligibility and recruitment procedures). For the current study, inclusion requirements were: (1) available data from the initial visit and final (diagnostic) visit in the original study (hereafter referred to as Time 1 and Time 2, respectively); and (2) codeable videos of the Screening Tool for Autism in Toddlers (STAT; Stone, Coonrod, Turner, & Pozdol, 2004) at Time 1. From the original sample reported in [reference deleted for blind review], 24 Sibs-ASD and 17 Sibs-TD were excluded from the current study based on these criteria.
Outcome classifications
The final sample comprised 42 Sibs-ASD and 25 Sibs-TD. The presence or absence of ASD symptomatology at Time 2 was based on diagnostic evaluations conducted through the original study by licensed psychologists experienced in the diagnosis of young children with ASD, using the Autism Diagnostic Observation Schedule (ADOS; Lord et al., 2000) and a DSM-IV-based parent interview (American Psychiatric Association, 2000). The ASD classification was used for children with diagnoses of Autistic Disorder, Pervasive Developmental Disorder-Not Otherwise Specified (PDD-NOS), or Asperger’s Disorder. The BAP classification was used when three conditions were met: (1) the child did not meet clinical criteria for an ASD diagnosis at T2; (2) the psychologist at T2 had clinical concerns about the child’s social functioning; and (3) the child either obtained an ADOS Reciprocal Social Interaction algorithm score above the ASD cutoff at T2, or had received a previous ASD diagnosis through the research project but no longer met full criteria. None of the 25 Sibs-TD met criteria for either ASD or BAP. Among Sibs-ASD, children meeting criteria for either ASD or BAP at Time 2 were considered to have ASD symptomatology (Sibs-ASD/AS). Children not classified as ASD or BAP were considered to have no ASD symptomatology (Sibs-ASD/NS), reflecting optimal outcome at an age at which diagnosis has been shown to become stable (i.e., after 30 months of age; Turner & Stone, 2007; Wiggins et al., 2012).
Fifteen children (all from the Sibs-ASD group) comprised the Sibs-ASD/AS group: 6 had an outcome classification of ASD and 9 had an outcome classification of BAP. The remaining 27 Sibs-ASD comprised the Sibs-ASD/NS group. Demographic information for the sample at both time points is provided in Table 1. One-way ANOVAs revealed no significant differences between Sibs-ASD/AS, Sibs-ASD/NS, and Sibs-TD for Time 1 chronological age (CA), Time 2 CA, or number of months between Time 1 and Time 2 visits, all ps >.34. Significant differences were found for Time 1 mental age (MA), F(2, 64)=3.48, p < .05, and Time 2 MA, F(2, 63)=9.37, p < .001. Post-hoc tests for Time 1 MA revealed significantly lower MA scores for Sibs-ASD/AS than Sibs-TD, p < .05, but no significant differences between the other groups, ps >.12. Post-hoc tests for Time 2 MA revealed significantly lower MA scores for Sibs-ASD/AS compared with both Sibs-ASD/NS, p < .01, and Sibs-TD, p < .01, but no significant differences between the latter two groups, p = 1.00. Chi-square tests revealed no group differences for race or maternal education, ps > .15, but a significant difference for gender, χ2(2)= 6.59, p < .05. There was a 4:1 ratio of males to females in the Sibs-ASD/AS group and more equal distributions of males and females in the Sibs-ASD/NS and Sibs-TD groups.
Table 1.
Participant Demographics
| Demographic | Sibs-ASD/AS (n = 15) | Sibs-ASD/NS (n = 27) | Sibs-TD (n = 25) |
|---|---|---|---|
| Time 1 CA | |||
| M (SD) | 14.40 (3.18) | 15.52 (3.07) | 15.44 (3.34) |
| Range | 12–21 | 12–23 | 12–23 |
| Time 2 CA | |||
| M (SD) | 32.80 (3.21) | 34.48 (3.31) | 34.08 (4.12) |
| Range | 30–41 | 30–42 | 29–44 |
| # Months between Time 1 and Time 2 | |||
| M (SD) | 18.40 (1.68) | 18.96 (1.58) | 18.64 (1.60) |
| Range | 14–22 | 18–25 | 16–23 |
| Time 1 MA | |||
| M (SD) | 13.78 (3.37) | 16.16 (3.19) | 16.75 (3.94) |
| Range | 10.5–24.0 | 11.5–25.75 | 11.5–27.0 |
| Time 2 MA | |||
| M (SD) | 28.60 (7.44) | 37.05 (5.50)a | 36.90 (7.07) |
| Range | 12.50–41.25 | 29.75–48.25 | 28.25–55.25 |
| # (%) Male | 12 (80%) | 11 (41%) | 16 (64%) |
| # (%) Caucasian | 14 (93%) | 24 (89%) | 22 (88%) |
| # (%) Mothers with College Degrees or Higher | 12 (80%) | 20 (74%) | 23 (92%) |
Notes. Sibs-ASD/AS = Sibs-ASD with later ASD symptomatology. Sibs-ASD/NS = Sibs-ASD without later ASD symptomatology.
Cognitive score unavailable for one child.
Measures
Mullen Scales of Early Learning (MSEL; Mullen, 1995)
The MSEL is a measure of cognitive functioning for children from birth through 68 months of age. Subscales measure Visual Reception (nonverbal problem solving), Fine Motor, Receptive Language, and Expressive Language skills. For this study, subscale age equivalents were averaged and used descriptively to estimate MA.
Autism Diagnostic Observation Schedule (ADOS; Lord et al., 2000)
The ADOS is a diagnostic assessment for ASD involving semi-structured observations of play, social interaction, and communication skills. All Sibs-ASD received either Module 1 or 2 of the ADOS at Time 2. Sibs-TD were to receive the ADOS only if they scored above 25 on the Childhood Autism Rating Scale (CARS; Schopler, Reichler, & Renner, 1986); however, none exceeded this cutoff.
Screening Tool for Autism in Toddlers (STAT; Stone, Coonrod, & Ousley, 2000; Stone et al., 2004)
The STAT was administered at Time 1 and provided the context within which social smiling and component behaviors were coded. The STAT is a 20-minute interactive assessment comprising 12 items in the domains of play, requesting, directing attention, and motor imitation. Originally developed for 24–36 month olds, its utility as a screener has been demonstrated in children as young as 14 months (Stone, McMahon, & Henderson, 2008), and it has been used successfully as a context for coding social and communication behaviors (e.g., McDuffie, Yoder, & Stone, 2005).
Coded Variables
STAT videotapes were coded by research staff who were independent from the original study and blind to participant group and outcome diagnosis. Procoder, a coding system for behavioral research using videotape (Tapp & Walden, 1993), was used to code mutually exclusive instances of social smiling, eye contact, and non-social smiling. Because the duration of the STAT varied somewhat across participants, the frequencies of the coded variables were converted to rates per minute.
Social smiling was coded when the child laughed or smiled within 1/10 of a second (either before or after) a look to the examiner’s face. Thus, social smiling was coded when smiling and eye contact were temporally coordinated, and the social smiling code took precedence over the eye contact and non-social smiling codes. Social smiling was coded only if: (1) the “smile” could be distinguished from the child’s overall expressions surrounding the act in question, and (2) the child’s expression was judged to reflect positive feelings (e.g., as distinguished from upturned lips while chewing). Non-social smiling was coded when smiling (as described above) occurred in the absence of coordinated eye contact to the examiner. Eye contact was coded when the child directed his or her face and/or eye gaze toward the examiner’s face (i.e., such that some part of the examiner’s face was in the child’s direct line of vision) but did not display a smile within 1/10 of a second. A detailed coding manual is available from the first author.
Twenty-five STAT videos (37%) were selected randomly for reliability coding. Intraclass correlation coefficients indicated high interrater reliability for all coded variables: Social Smiling=.94, Non-Social Smiling=.82, Eye Contact=.94.
Results
Preliminary Analyses
Descriptive statistics were run for each of the dependent variables (social smiling, non-social smiling, and eye contact) to assess for violations of assumptions (e.g., non-normal distribution). The social smiling variable violated the assumption of a normal distribution, and was corrected to a normal distribution using a square root transformation. Accordingly, eye contact and non-social smiling were transformed in the same manner and all analyses used the transformed variables.
Correlations between Time 1 demographic variables and the dependent variables (social smiling, non-social smiling, and eye contact) were conducted in the full sample to determine whether any participant characteristics might affect the dependent variables and need to be included in later analyses as covariates. CA, MA, and race were not correlated with any dependent variables, and thus were not included as covariates in subsequent analyses. Gender was correlated with non-social smiling, r = .25, p < .05, with males exhibiting higher rates of non-social smiling. Maternal education was negatively correlated with social smiling, r = −.25, p < .05, and non-social smiling, r = −.24, p < .05; lower levels of maternal education were associated with higher rates of both social and non-social smiling. Based on these analyses, gender and maternal education were included as covariates in subsequent analyses examining group differences as appropriate. Table 2 provides the raw means and standard deviations for the coded variables.
Table 2.
Raw Means for Dependent Variables (Rate/Minute)
| Variable | Sibs-ASD/AS (n = 15) | Sibs-ASD/NS (n = 27) | Sibs-TD (n = 25) |
|---|---|---|---|
| Social Smiling | |||
| M (SD) | .25 (.28)a | .36 (.39)a | .53 (.47)b |
| Range | .00–.83 | .00–1.74 | .00–1.76 |
| Non-Social Smiling | |||
| M (SD) | .81 (.81)a | .81 (.69)a,b | 1.13 (.73)b |
| Range | .00–2.43 | .00–2.37 | .00–2.43 |
| Eye Contact | |||
| M (SD) | 1.67 (.99)a | 2.84 (1.71)a,b | 2.88 (1.41)b |
| Range | .58–3.31 | .56–6.48 | .72–5.71 |
Notes. Means with different superscripts were significantly different from each other; means with both superscripts were not statistically significant from any other mean.
Sibs-ASD/AS = Sibs-ASD with later ASD symptomatology. Sibs-ASD/NS = Sibs-ASD without later ASD symptomatology.
Group comparisons
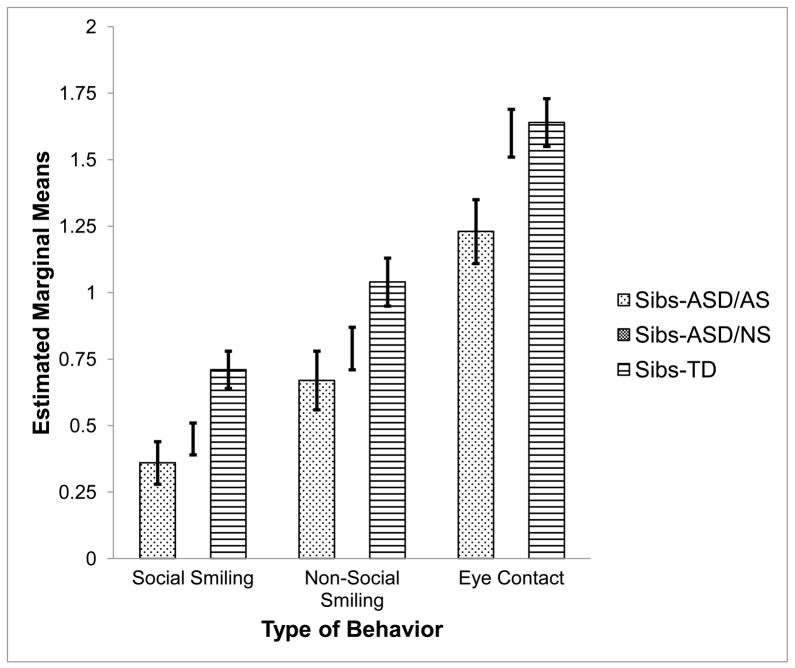
Two ANCOVAs and one ANOVA were used to examine whether rates of social smiling, non-social smiling, and eye contact differed between Sibs-ASD/AS, Sibs-ASD/NS, and Sibs-TD. Analyses were conducted separately for each dependent variable due to inclusion of different demographic covariates, as described above. Post-hoc analyses used Bonferroni’s adjustment to account for the multiple comparisons between groups. Means and standard deviations are reported in Table 2 and estimated marginal means and standard errors are displayed in Figure 1. Notably, within the Sibs-ASD/AS group, means did not differ between those children with ASD diagnoses and those with BAP on any of the dependent variables, ps > .36, supporting our decision to combine these children into a single “symptomatic” outcome group.
Fig 1.
Estimated marginal means of transformed Social Smiling, Non-Social Smiling, and Eye Contact variables by outcome group. Sibs-ASD/AS = infant siblings of children with ASD who later demonstrated ASD symptomatology. Sibs-ASD/NS = infant siblings of children with ASD who later demonstrated no ASD symptomatology. Sibs-TD = infant siblings of typically children with typical development (none with later ASD symptomatology). Standard error bars depict +/− 1 SE.
Social Smiling
Results revealed a significant main effect for group, F(2, 63) = 5.95, p < .01, ηp2= .16. Post-hoc analyses indicated that both Sibs-ASD/AS and Sibs-ASD/NS demonstrated significantly lower rates of social smiling than Sibs-TD (p < .01 and p < .05, respectively). No significant differences were found between the Sibs-ASD/AS and the Sibs-ASD/NS groups, p = 1.00.
Non-Social Smiling
Results revealed a significant main effect for group, F(2, 62) = 4.21, p < .05, ηp2= .12. Post-hoc analyses indicated that Sibs-ASD/AS showed a significantly lower rate of non-social smiling than Sibs-TD, p < .05, but no significant differences between the other groups were found, ps >.13.
Eye Contact
Results revealed a significant main effect for group, F(2, 64) = 4.04, p <.05, ηp2= .11. Post-hoc analyses indicated that Sibs-ASD/AS showed a significantly lower rate of eye contact than Sibs-TD, p < .05. Sibs-ASD/NS did not differ significantly from Sibs-TD, p = 1.00, but tended to have a higher rate of eye contact than Sibs-ASD/NS, p =.054.
Discussion
This study compared the early expression of social smiling and its behavioral components (i.e., eye contact and non-social smiling) in three groups of infant siblings: infants at elevated risk for ASD who showed later ASD symptomatology (Sibs-ASD/AS); infants at elevated risk for ASD who did not show later ASD symptomatology (Sibs-ASD/NS); and infant siblings of typically developing children (Sibs-TD). The primary aim of this study was to characterize early behavioral features of Sibs-ASD with optimal outcomes, and to determine the extent to which their early social behaviors compared and contrasted with the social behaviors of Sibs-ASD who developed later ASD symptoms, and with those of low-risk Sibs-TD. Thus, unlike most previous studies, the Sibs-ASD/NS group in this study excluded those high-risk siblings who met criteria for the broader autism phenotype (BAP), in addition to those who received formal ASD diagnoses, providing a relatively “clean” sample of unaffected siblings whose early development could be examined. Results revealed that, similar to Sibs-ASD with ASD symptoms at outcome, Sibs-ASD with no autism symptoms at outcome showed lower rates of social smiling in infancy relative to Sibs-TD. However, only Sibs-ASD/AS exhibited lower rates of eye contact and non-social smiling than Sibs-TD. Sibs-ASD/NS did not differ from Sibs-TD in these component behaviors.
These results replicate previous findings of lower rates of social smiling for Sibs-ASD with ASD outcomes compared with Sibs-TD (Brian et al., 2008; Landa et al., 2007; Ozonoff et al., 2010), and extend them by suggesting that this finding holds true even when the ASD outcome group is defined more broadly to include children with BAP symptoms. In fact, despite different contexts and coding systems, the mean rates of social smiling and eye contact for the Sibs-ASD/AS and Sibs-TD in the present study were remarkably similar to those reported by Ozonoff et al. (2010) for their samples at comparable ages. However, no previous studies have examined social smiling in an unimpaired Sibs-ASD outcome group (i.e., excluding children with BAP); thus, ours may be the first to identify a relative deficit in social smiling in symptom-free Sibs-ASD. The lack of group differences in social smiling between the Sibs-ASD/NS and the Sibs-ASD/AS in the current study replicates the results of Brian et al. (2008), providing further evidence that lower levels of social smiling may be associated more generally with elevated genetic risk for ASD than with an ASD outcome per se.
To our knowledge, this study is the first to examine social smiling and its two component behaviors, non-social smiling and eye contact, as non-overlapping categories within the same behavior sample, to determine the extent to which component behaviors might be contributing to group differences in social smiling. Despite comparable levels of social smiling for the Sibs-ASD/NS and Sibs-ASD/AS, different patterns for the component skills relative to Sibs-TD emerged: (1) Sibs-ASD/NS had lower rates of social smiling, but average rates of non-social smiling and eye contact compared to Sibs-TD; and (2) Sibs-ASD/AS had lower rates of social smiling, non-social smiling, and eye contact compared to Sibs-TD. Thus, even though Sibs-ASD/NS were less likely to integrate smiles and eye contact to produce social smiling, they did not smile less frequently in response to their own activities or make less eye contact with the examiner relative to Sibs-TD. In contrast, relative to Sibs-TD, Sibs-ASD/AS were less likely to both smile and make eye contact, either integrated or in isolation. Thus, results of the present study suggest that lower rates of social smiling alone do not represent a specific risk factor for later ASD symptoms, whereas lower rates of social smiling as well as reduced eye contact and non-social smiling may confer increased risk.
Although the nature of the data collected through this study is not sufficient to draw conclusions about the specific developmental mechanisms underlying these findings, it may be of heuristic value to speculate about potential explanations. One possibility may be that different underlying processes account for the lower rates of social smiling and lead to the differences in outcome seen in Sibs-ASD/NS versus Sibs-ASD/AS. For example, for Sibs-ASD/NS, lower rates of social smiling in the presence of average rates of non-social smiling and eye contact may reflect challenges in the temporal integration of the different behaviors, as have been described previously for children with ASD (Joseph & Tager-Flusberg, 1997; Kasari et al., 1990). In contrast, the lower level of social smiling and component behaviors demonstrated by the Sibs-ASD/AS, but not the Sibs-ASD/NS, may reflect alterations in more basic developmental mechanisms – such as impairments in social reward systems (Schultz, 2005; Schumann, Barnes, Lord, & Courchesne, 2009) and/or higher levels of arousal in social contexts (Watson, Roberts, Baranek, Mandulak, & Dalton, 2012) – that ultimately contribute to less optimal outcomes for this subgroup. Another possible interpretation of these findings is that the expression of ASD symptoms may reflect the cumulative effects of multiple developmental perturbations in infancy. Thus, ASD symptoms may develop only when the number of affected developmental domains, or the severity of suboptimal skill development, surpasses a critical threshold. This interpretation is consistent with previous research reporting a continuous distribution of ASD-related social deficits in sibling samples (Constantino & Todd, 2003).
To date, among studies of infant siblings of children with ASD, considerable emphasis has been placed on identifying specific early features that indicate risk for a later diagnosis of ASD, so that intervention or preventative strategies can be implemented as needed. However, the role of potential protective factors should also be considered and studied. For example, higher rates of eye contact may serve a protective role for high-risk siblings who do not develop later ASD symptoms, as eye contact levels in Sibs-ASD/NS were more comparable to those of Sibs-TD than were those of Sibs-ASD/AS. Eye contact with others provides opportunities not only for interpersonal engagement, but also for the social, emotional, and language inputs and feedback (i.e., learning) it engenders (Mundy, Sullivan, & Mastergeorge, 2009). As such, increased eye contact may result in more optimal short- and long-term outcomes for a subset of Sibs-ASD. Just as future neurobiological and genetic research can help identify the specific mechanisms underlying behavior differences in social smiling in individuals with ASD and those at elevated genetic risk, a better understanding of both risk and protective factors associated with outcomes in high-risk infant sibling samples will enhance our ability to identify the type, timing, and necessity of intervention (or prevention) strategies.
There are several limitations of this study, many of which have been noted in previous research on high-risk siblings. The first relates to sample size. Out of the 42 infants in the high-risk group, only 15 had ASD symptoms at outcome, and only six received formal ASD diagnoses. The small size of the group of children with ASD symptoms calls for cautious interpretation of these findings, and emphasizes the need for further examination in larger samples. Second, the sample was primarily Caucasian and of relatively high socio-economic status, which may limit the generalizability of the findings. Finally, our “optimal outcome” determination for the Sibs-ASD was based on diagnostic evaluations between the ages of 30 and 42 months; their development beyond this age is not yet known. Although 30 months has been identified as an age at which ASD diagnoses become stable (Turner & Stone, 2007; Wiggins et al., 2012), a longer follow-up period would be preferable. Previous research has demonstrated that siblings and other biological relatives of individuals with ASD who do not themselves meet clinical diagnostic criteria for ASD remain at risk for other features of the broader autism phenotype (BAP), such as executive functioning or language-related deficits (for review, see Bailey, Palferman, Heavey, and Le Couteur, 1998). While our study removed individuals identified with BAP from the sample of “unaffected” siblings (Sibs-ASD/NS), we defined BAP in terms of ASD-specific features, with an emphasis on social symptoms. However, it is unknown whether Sibs-ASD/NS are at increased risk for other behavioral or neurocognitive differences that may set them apart from their low-risk peers longer term, or whether early deficits in social smiling in Sibs-ASD/NS might presage such risks.
These limitations notwithstanding, the results of this study have added to our knowledge about early social development in siblings of children with ASD in several ways. First, lower rates of social smiling were found even in those high-risk siblings who did not show later ASD symptomatology, which: (1) suggests that this early behavior does not represent a specific risk factor for ASD; and (2) provides additional evidence that early behavioral differences between high-risk and low-risk infants do not necessarily translate into later impairments (cf., Malesa et al., 2012; Warren et al., 2012; Young, Merin, Rogers, & Ozonoff, 2009). Second, the different patterns found for high-risk siblings with and without later ASD symptoms, with respect to the component behaviors of social smiling (i.e., eye contact and non-social smiling), suggest the possibility that different developmental processes, or threshold effects, may be contributing to lower rates of social smiling and differential outcome in the two Sib-ASD groups. Continued investigation of the development of social smiling, the neurocognitive processes underlying social smiling and its component behaviors, and the long-term implications of early differences in social smiling will be critical for furthering our knowledge about the early social development of children at elevated risk for ASD and our ability to design targeted, individualized prevention and intervention strategies for optimizing their outcomes.
Acknowledgments
This research was supported by NICHD grants R01HD043292 and T32HD07226. This manuscript was developed from data collected through Caitlin McMahon Nichols’ dissertation project. The authors would like to thank Hannah Benneyworth and the TRIAD graduate students and research assistants for their tireless efforts in coding and data entry. We also extend our sincere gratitude to the many families whose participation made this project possible
References
- Adamson L, Bakeman R. Affect and attention: Infants observed with mothers and peers. Child Development. 1985;56:582–593. [Google Scholar]
- Adrien JL, Lenoir P, Martineau J, Perrot A, Hameury L, Larmande C, Sauvage D. Blind ratings of early symptoms of autism based upon family home movies. Journal of the American Academy of Child and Adolescent Psychiatry. 1993;32:617–626. doi: 10.1097/00004583-199305000-00019. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: American Psychiatric Association; 2000. text revision. [Google Scholar]
- Bailey A, Palferman S, Heavey L, Le Couteur A. Autism: The phenotype in relatives. Journal of Autism and Developmental Disorders. 1998;28(5):369–392. doi: 10.1023/a:1026048320785. [DOI] [PubMed] [Google Scholar]
- Bakeman R, Adamson L. Coordinating attention of people and objects in mother-infant and peer-infant interaction. Child Development. 1984;55:1278–1289. [PubMed] [Google Scholar]
- Barbaro J, Dissanayake C. Developmental profiles of infants and toddlers with autism spectrum disorders identified prospectively in a community based setting. Journal of Autism and Developmental Disorders. 2012;42(9):1939–1948. doi: 10.1007/s10803-012-1441-z. [DOI] [PubMed] [Google Scholar]
- Brian J, Bryson SE, Garon N, Roberts W, Smith IM, Szatmari P, Zwaigenbaum L. Clinical assessment of autism in high-risk 18-month-olds. Autism. 2008;12:433–456. doi: 10.1177/1362361308094500. [DOI] [PubMed] [Google Scholar]
- Bruner JS. From communication to language: A psychological perspective. Cognition. 1975;3:255–287. [Google Scholar]
- Carpenter M, Nagell K, Tomasello M. Social cognition, joint attention, and communicative competence from 9 to 15 months of age. Monographs of the Society for Research in Child Development. 1998;63:1–143. [PubMed] [Google Scholar]
- Constantino JN, Todd RD. Autistic trains in the general population: A twin study. Archives of General Psychiatry. 2003;60:524–530. doi: 10.1001/archpsyc.60.5.524. [DOI] [PubMed] [Google Scholar]
- Dawson G, Hill D, Spencer A, Galpert L, Watson L. Affective exchanges between young autistic children and their mothers. Journal of Abnormal Child Psychology. 1990;18:335–345. doi: 10.1007/BF00916569. [DOI] [PubMed] [Google Scholar]
- Joseph R, Tager-Flusberg H. An investigation of attention and affect in children with autism and Down syndrome. Journal of Autism and Developmental Disorders. 1997;27:385–396. doi: 10.1023/a:1025853321118. [DOI] [PubMed] [Google Scholar]
- Kasari C, Sigman M, Mundy P, Yirmiya N. Affective sharing in the context of joint attention interactions of normal, autistic, and mentally retarded children. Journal of Autism and Developmental Disorders. 1990;20:87–100. doi: 10.1007/BF02206859. [DOI] [PubMed] [Google Scholar]
- Kasari C, Sigman M, Yirmiya N. Focused and social attention of autistic children in interactions with familiar and unfamiliar adults: A comparison of autistic, mentally retarded, and normal children. Development and Psychopathology. 1993;5:403–414. [Google Scholar]
- Kaye K, Fogel A. The temporal structure of face-to-face communication between mothers and infants. Developmental Psychology. 1980;16:454–464. [Google Scholar]
- Landa R, Holman KC, Garrett-Mayer E. Social and communication development in toddlers with early and later diagnosis of autism spectrum disorders. Archives of General Psychiatry. 2007;64:853–864. doi: 10.1001/archpsyc.64.7.853. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Jr, Leventhal BL, DiLavore PC, Pickles A, Rutter M. The Autism Diagnostic Observation Schedule-Generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30:205–223. [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism diagnostic interview-revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Malesa E, Foss-Feig J, Yoder PJ, Warren ZE, Walden T, Stone WL. Autism. 2012. Predicting language and social outcomes at age 5 for later-born siblings of children with autism spectrum disorders. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDuffie A, Yoder PJ, Stone WL. Prelinguistic predictors of vocabulary in young children with autism spectrum disorders. Journal of Speech, Language, and Hearing Research. 2005;48:1080–1097. doi: 10.1044/1092-4388(2005/075). [DOI] [PubMed] [Google Scholar]
- Messinger D, Fogel A. The interactive development of social smiling. In: Kail R, editor. Advances in child development and behavior. Oxford, UK: Elsevier; 2007. pp. 327–366. [DOI] [PubMed] [Google Scholar]
- Messinger D, Fogel A. Give and take: The development of conventional infant gestures. Merrill Palmer Quarterly. 1998;44:566–590. [Google Scholar]
- Mullen EM. Mullen Scales of Early Learning. Circle Pines, MN: American Guidance Service Inc; 1995. (AGS ed.) [Google Scholar]
- Mundy P, Sullivan L, Mastergeorge AM. A parallel and distributed-processing model of joint attention, social cognition and autism. Autism Research. 2009;2:2–21. doi: 10.1002/aur.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy P, Sigman M, Ungerer J, Sherman T. Defining the social deficits of autism: The contribution of nonverbal communication measures. Journal of Child Psychology and Psychiatry. 1986;27:657–669. doi: 10.1111/j.1469-7610.1986.tb00190.x. [DOI] [PubMed] [Google Scholar]
- Ozonoff S, Iosif AM, Baguio F, Cook IC, Hill MM, Hutman T, et al. A prospective study of the emergence of early behavioral signs of autism. Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49:256–266. [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Young GS, Carter A, Messinger D, Yirmiya N, Zwaigenbaum L, Bryson S, Carver LJ, Constantino JN, Dobkins K, Hutman T, Iverson JM, Landa R, Rogers SJ, Sigman M, Stone WL. Recurrence risk for autism spectrum disorders: A Baby Siblings Research Consortium study. Pediatrics. 2011;128:488–495. doi: 10.1542/peds.2010-2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piven J, Palmer P, Jacobi D, Childress D, Arndt S. Broader autism phenotype: Evidence from a family history study of multiple-incidence autism families. American Journal of Psychiatry. 1997;154:185–190. doi: 10.1176/ajp.154.2.185. [DOI] [PubMed] [Google Scholar]
- Rogers SJ. What are infant siblings teaching us about autism in infancy? Autism Research. 2009;2(3):125–137. doi: 10.1002/aur.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schopler E, Reichler R, Renner B. The Childhood Autism Rating Scale. Los Angeles, CA: Western Psychological Services; 1986. [Google Scholar]
- Schultz RT. Developmental deficits in social perception in autism: The role of the amygdala and fusiform face area. International Journal of Developmental Neuroscience. 2005;23:125–141. doi: 10.1016/j.ijdevneu.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Schumann CM, Barnes CC, Lord C, Courchesne E. Amygdala enlargement in toddlers with autism related to severity of social and communication impairments. Biological Psychiatry. 2009;66:942–949. doi: 10.1016/j.biopsych.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone WL, Coonrod E, Ousley O. Screening Tool for Autism Two-Year-Olds (STAT): Development and preliminary data. Journal of Autism and Developmental Disorders. 2000;30:607–612. doi: 10.1023/a:1005647629002. [DOI] [PubMed] [Google Scholar]
- Stone WL, Coonrod EE, Turner LM, Pozdol SL. Psychometric properties of the STAT for early autism screening. Journal of Autism and Developmental Disorders. 2004;34:691–701. doi: 10.1007/s10803-004-5289-8. [DOI] [PubMed] [Google Scholar]
- Stone WL, McMahon CR, Henderson LM. Use of the Screening Tool for Autism in Two-year-olds (STAT) for children under 24 months: An exploratory study. Autism. 2008;12:573–589. doi: 10.1177/1362361308096403. [DOI] [PubMed] [Google Scholar]
- Stone WL, McMahon CR, Yoder PJ, Walden TA. Early social–communicative and cognitive development of younger siblings of children with autism spectrum disorders. Archives of Pediatric & Adolescent Medicine. 2007;161:384–390. doi: 10.1001/archpedi.161.4.384. [DOI] [PubMed] [Google Scholar]
- Striano T, Rochat P. Developmental link between dyadic and triadic social competence in infancy. British Journal of Developmental Psychology. 1999;17:551–562. [Google Scholar]
- Swettenham J, Baron-Cohen S, Charman T, Cox A, Baird G, Drew A, Rees L, Wheelwright S. The frequency and distribution of spontaneous attention shifts between social and nonsocial stimuli in autistic, typically developing, and nonautistic developmentally delayed infants. Journal of Child Psychology and Psychiatry. 1998;39:747–753. [PubMed] [Google Scholar]
- Tapp J, Walden T. Procoder: A professional tape control, coding, and analysis system for behavioral research using videotape. Behavior Research Methods, Instruments, & Computers. 1993;25:53–56. [Google Scholar]
- Tronick EZ. Affectivity and sharing. In: Tronick E, editor. Social interchange in infancy: Affect, cognition, and communication. Baltimore, MD: University Park Press; 1982. pp. 1–6. [Google Scholar]
- Turner LM, Stone WL. Variability in outcome for children with an ASD diagnosis at age 2. Journal of Child Psychology & Psychiatry. 2007;48:793–802. doi: 10.1111/j.1469-7610.2007.01744.x. [DOI] [PubMed] [Google Scholar]
- Venezia M, Messinger DS, Thorp D, Mundy P. The development of anticipatory smiling. Infancy. 2004;6:397–406. doi: 10.1207/s15327078in0603_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vygotsky L. Mind in society: The development of higher mental function. Cambridge, MA: Harvard University Press; 1978. [Google Scholar]
- Warren ZE, Foss-Feig J, Malesa E, Lee EB, Lounds Taylor J, Newsom CR, Crittendon J, Stone WL. Neurocognitive and behavioral outcomes of young siblings of children with autism spectrum disorders at age 5. Journal of Autism and Developmental Disorders. 2012;43:409–418. doi: 10.1007/s10803-011-1263-4. [DOI] [PubMed] [Google Scholar]
- Watson LR, Roberts JE, Baranek GT, Mandulak KC, Dalton JC. Behavioral and physiological changes to child-directed speech of children with autism spectrum disorders or typical development. Journal of Autism and Developmental Disorders. 2012;42:1616–1629. doi: 10.1007/s10803-011-1401-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg MK, Tronick EZ. Beyond the face: An empirical study of infant affective configurations of facial, vocal, gestural, and regulatory behaviors. Child Development. 1994;65:1503–1515. doi: 10.1111/j.1467-8624.1994.tb00832.x. [DOI] [PubMed] [Google Scholar]
- Wiggins LD, Baio J, Schieve L, Lee L, Nicholas J, Rice C. Retention of autism spectrum diagnoses by community professionals: findings from the autism and developmental disabilities monitoring network, 2000 and 2006. Journal of Developmental and Behavioral Pediatrics. 2012;33:387–395. doi: 10.1097/DBP.0b013e3182560b2f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yale M, Messinger D, Cobo-Lewis A, Delgado C. The temporal coordination of early infant communication. Developmental Psychology. 2003;39:815–824. doi: 10.1037/0012-1649.39.5.815. [DOI] [PubMed] [Google Scholar]
- Young GS, Merin N, Rogers SJ, Ozonoff S. Gaze behavior and affect at 6 months: predicting clinical outcomes and language development in typically developing infants and infants at risk for autism. Developmental Science. 2009;12:798–814. doi: 10.1111/j.1467-7687.2009.00833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwaigenbaum L, Bryson S, Rogers T, Roberts W, Brian J, Szatmari P. Behavioral manifestations of autism in the first year of life. International Journal of Developmental Neuroscience. 2005;23:143–152. doi: 10.1016/j.ijdevneu.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Zwaigenbaum L, Thurm A, Stone W, Baranek G, Bryson S, Iverson J, et al. Studying the emergence of autism spectrum disorders in high-risk infants: Methodological and practical issues. Journal of Autism and Developmental Disorders. 2007;37:466–480. doi: 10.1007/s10803-006-0179-x. [DOI] [PubMed] [Google Scholar]