Abstract
Non-specific presentation and normal examination findings in early disease often result in tracheal obstruction being overlooked as a diagnosis until patients present acutely. Once diagnosed, surgical options should be considered, but often patient co-morbidity necessitates other interventional options. Non-resectable tracheal stenosis can be successfully managed by interventional bronchoscopy, with therapeutic options including airway dilatation, local tissue destruction and airway stenting. There are common aspects to the management of tracheal obstruction, tracheomalacia and tracheal fistulae. This paper reviews the pathogenesis, presentation, investigation and management of tracheal disease, with a focus on tracheal obstruction and the role of endotracheal intervention in management.
KEYWORDS : Airway stent, laser therapy, rigid bronchoscopy, tracheal stenosis, large airway obstruction
Introduction
Large airway obstruction, particularly tracheal disease, is widely believed to be under-diagnosed and under-reported. Recognition of the precipitants to tracheal obstruction and of the common clinical manifestations and investigation findings will facilitate prompt and appropriate management. Rigid bronchoscopy is increasingly popular for the diagnosis and management of tracheal obstruction, and has a role in the delivery of interventional bronchoscopy. Surgical management is often definitive but patient selection and preparation is essential for surgical success.
This paper reviews the pathogenesis, presentation, investigation and management of tracheal disease, with a focus on tracheal obstruction and the role of endotracheal intervention in the management of non-resectable disease. It is intended that this paper will provide guidance for those involved in the care of patients with tracheal disease.
Tracheal anatomy and physiology
The trachea extends from the lower body of the cricoid cartilage to the carina and is normally between 110 and 130 mm in length. The tracheal diameter is typically up to 22 mm in males and up to 18 mm in females. Air is a fluid which flows down a pressure gradient, from higher to lower pressure. Airflow can be laminar or turbulent, with the flow rate defined as the amount of fluid moving per second. When flow is laminar, the airflow rate is directly proportional to the pressure gradient but when flow is turbulent, the airflow rate is proportional to the square root of the pressure gradient. Turbulence thus increases airflow resistance, necessitating a higher pressure difference to maintain a given flow rate. Reynolds number is a non-dimensional parameter that governs the change from laminar to turbulent air flow. It is calculated by multiplying mean fluid velocity, density and airway diameter then dividing by fluid viscosity. The diameter of the trachea affects airflow by this equation and any abrupt variation in tracheal diameter produces a transition point which also predisposes to turbulence.
Tracheal obstruction
Congenital or acquired tracheal narrowing may result from intrinsic tracheal stenosis and/or external compression, at any anatomical level. Variation in the tracheal diameter alters the airflow dynamics in the direction of increased airflow resistance. Tracheal stenosis often results in varying degrees of distortion along the vertical plane in addition to airway narrowing. When the trachea becomes pulled away from or twisted within its normal anatomical path, this further predisposes to airway turbulence and increased resistance.
Congenital
Tracheal stenosis is rarely congenital but may result from posterior fusion of the tracheal rings. By contrast, congenital tracheal webs are well recognised, with 75% occurring at the level of the glottis. Tracheal webs differ from tracheal stenosis due to the absence of a cartilaginous framework. Both may present in adult life.
Congenital cardiovascular anomalies can result in extrinsic compression of the trachea. Most commonly, early bifurcation of the innominate artery compresses the anterior tracheal wall, appearing pulsatile at bronchoscopy. Other causes include anomalies of the subclavian artery and vascular rings, such as congenital double aortic arch, which encircle the trachea causing circumferential compression.
Acquired
Trauma
The cartilaginous trachea has a natural tendency to narrow and fibrose in the face of injury. Tracheal trauma is the most common cause of benign tracheal stenosis and is a feared complication of prolonged endotracheal intubation or tracheostomy tube placement. The presence of tracheal stenosis can necessitate re-intubation and delay respiratory weaning in intensive care unit patients or can present many years later.
The reported incidence of tracheal stenosis following endotracheal intubation ranges from 6-21% and following tracheostomy ranges from 0.6-21% (1-3). With the trend towards early tracheostomy as an aid to respiratory weaning, and increasing numbers of successful discharges from intensive care units, the incidence of tracheal complications is rising.
Stenosis occurs when pressure and friction on the mucosal surface stimulates inflammation and pressure necrosis. Granulation tissue formation is followed by fibroblast proliferation, scarring and contracture. Stenoses can develop after as little as 36 hours of endotracheal intubation but the risk of stenosis rises with duration of intubation. Most strictures occur at the site of the tube cuff, with reduced incidence following the introduction of compliant, large volume, low pressure cuffs (4).
After tracheostomy, stenosis most commonly occurs at the stomal site (3,5). Wound sepsis is a predisposing factor (6). Pre-existing chronic lung disease and airway infection are also associated with tracheal stenosis post endotracheal intubation or tracheostomy (7).
Trauma may also arise from thermal or chemical burns (including chemical warfare agents), resulting in localised stenosis.
Infection
Airway infection alone can result in the development of tracheal stenosis. Tuberculosis is the most common cause of post-infective stenosis but diphtheria, syphilis and fungal infection (e.g., histoplasmosis, blastomycosis) are also recognised causes.
Non-infectious inflammation
Non-infectious inflammatory conditions causing tracheal stenosis include collagen vascular disorders (e.g., Wegener’s granulomatosis), sarcoidosis, amyloidosis and chronic atrophic polychondritis. Diffuse inflammatory and infective processes often result in multi-level tracheobronchial stenoses.
Neoplastic
Airway obstruction develops in 20-30% of lung cancer patients (8), however, tracheal compromise occurs in less than 1% of all malignancies (9). Direct tumour invasion of the trachea by a bronchogenic malignancy is more common than metastatic involvement of the trachea. Primary benign tumours of the trachea such as chondromas, fibromas, hemangiomas, and squamous papillomas are rare causes of tracheal stenosis. Extrinsic compression of the trachea can occur from malignant lymphadenopathy, thyroid and mediastinal tumours.
Iatrogenic
The insertion of a tracheal stent (e.g., for tracheobronchomalacia) can, paradoxically, lead to stenosis due to tracheal irritation and the formation of granulation tissue at either end of the stent. Cervico-mediastinal radiotherapy is another recognised cause of stenosis.
Other
Tracheopathia osteochondroplastica is a rare, but increasingly recognised condition in which there is the idiopathic development of focal or diffuse, osseous and/or cartilaginous nodules in the submucosa of the trachea and bronchial walls. The posterior membranous portions of the trachea are characteristically spared. Significant tracheal stenosis and/tracheomalacia can result.
Superior mediastinal pathology can cause extrinsic tracheal compression. Most frequently this arises from lymphadenopathy secondary to infection, inflammation or neoplasia, but abnormalities of the aortic arch such as dissection or aneurysm can also compress the trachea (10). Thyroid goitre may also cause extrinsic compression, particularly if there is retrosternal extension.
Idiopathic
Idiopathic tracheal stenosis is rare, representing 3-5% of cases. Most commonly these stenoses develop at the level of the cricoid cartilage and are restricted to young women (11). Pathologically there is extensive keloidal fibrosis and mucus glands dilation which may represent a form of fibromatosis (12).
Tracheal fistulae
Any aggressive tracheal pathology can disturb the integrity of the tracheal wall resulting in communication with the mediastinum. Iatrogenic, traumatic and malignant cases are the most prevalent. Infection as an aetiological factor (tuberculosis, HIV infection, mediastinitis) has reduced in recent years. Communication may also be established between the tracheobronchial tree and the oesophagus, resulting in tracheo-oesophageal (or bronchial-oesophageal) fistulae. Acquired tracheo-oesophageal fistulae are frequently the result of mediastinal malignancy. Tumours arising from the oesophagus, trachea, lungs, larynx, thyroid and lymph glands have all been reported to cause fistula formation. Tracheo-oesophageal fistulae can also be congenital. These typically present in the neonatal period but may rarely present in adulthood.
Tracheomalacia
Tracheomalacia is characterised by flaccidity of the tracheal cartilage, leading to airway collapse during expiration. The condition may extend to involve the bronchi (tracheobronchomalacia). Significant airway malacia is defined as a greater than 60% reduction in luminal diameter.
Congenital tracheomalacia results from a developmental defect in the cartilage of the tracheal wall. Tracheomalacia may also develop in the context of congenital conditions such as cystic fibrosis, Mounier-Kuhn syndrome, Marfan syndrome, Ehlers-Danlos syndrome, and congenital trachea-oesophageal fistulae.
Acquired tracheomalacia is associated with prolonged endotracheal intubation and tracheostomy, trauma, head and neck surgery, radiotherapy, and inflammatory conditions such as polychondritis. Intrinsic tracheal disease such as tracheal stenosis and previous tracheal stenting may also contribute to weakening of the airway support and malacia. When tracheomalacia occurs in the absence of a clear pathophysiology, these patients are often obese, with smoking related lung disease or recurrent/chronic airways infection.
Diagnosis of tracheal disease
The diagnosis of tracheal disease is a recognised challenge because of the broad range of aetiologies and the non-specific nature of presentation, which often has an insidious onset at first. Thus, a detailed patient history and examination is imperative to guide further investigation and management. Sometimes when the patient presents in extremis this is not possible or appropriate, and rapid intervention in a controlled environment with appropriately skilled personnel is central to a successful outcome.
History and examination
Symptoms depend on the location and degree of airway narrowing, additional airway distortion and concurrent thoracic pathology. Most commonly, patients report shortness of breath on exertion, which may progress to dyspnoea at rest. Symptoms occur on exertion when the tracheal diameter is significantly reduced to 8 mm (13). Cough and wheeze are common. Airway obstruction may lead to difficulty with sputum clearance and recurrent infection. The combination of exertional dyspnoea and wheeze is frequently mistaken for chronic bronchitis or asthma. Failure to respond to bronchodilators should not be overlooked.
It is not uncommon for patients with tracheal disease to present with acute respiratory distress, even in benign disease. These presentations are usually triggered by the partial or complete occlusion of the abnormal airway by sputum or haemorrhage.
History taking should focus on potential tracheal insults such as intensive care admission, hoarseness after general anaesthesia (suggestive of traumatic injury), or respiratory tract infections. A full systems enquiry may also reveal information relevant to the underlying diagnosis.
Respiratory examination is often normal until there is severe tracheal stenosis or secondary airway occlusion due to sputum or haemorrhage. Stridor occurs when the tracheal diameter is less than 5 mm (13). Examination should explore the underlying diagnosis, looking carefully for signs such as a tracheostomy scar, goitre, lymphadenopathy or the classical nasal changes of Wegener’s granulomatosis.
Investigation
When tracheal disease is suspected, first line investigations should include targeted blood tests to look for the underlying diagnosis (e.g., inflammatory markers, autoimmune screen), pulse oximetry and/or arterial blood gas analysis and standard chest radiography. These investigations are often normal.
Lung function testing
When spirometry results are interpreted correctly, ensuring technical requirements are met, they can be the first investigation to suggest the diagnosis of tracheal obstruction. Flow volume diagrams provide an indication of the severity of airflow obstruction and the location of airway obstruction, i.e., intrathoracic or extrathoracic (14).
The pressure surrounding the intrathoracic airway approximates to pleural pressure, which changes during the respiratory cycle. During inspiration, negative intrapleural pressure causes the intrathoracic airway to be splinted open. During expiration, positive intrapleural pressure compresses the intrathoracic airway. Therefore, in intrathoracic airway obstruction (for example in lower tracheal stenosis) typically there is upper airway collapse during expiration and flattened expiratory flow volume curves, but the inspiratory flow volume curve remains normal.
The reverse is true in the fairly compliant extrathoracic airway that is not exposed to intrapleural pressure. Inspiration results in collapse of the extrathoracic upper airway, as the airflow acceleration into the lungs reduces intraluminal pressure. Extrathoracic airways obstruction (upper or mid tracheal obstruction) therefore typically causes airway collapse during inspiration, with flattening of the inspiratory flow volume curve. The force of expiration opens the extrathoracic airway usually resulting in a normal expiratory curve. The expiratory curve may become flattened when there is significant extrathoracic obstruction, resulting in reduced peak airflow rates. Schematics of the classical flow-volume diagrams are displayed in Figure 1.
Figure 1.
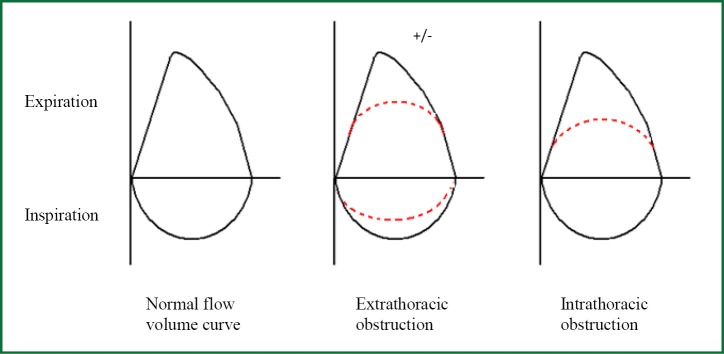
Flow volume curves showing upper airway obstruction.
Radiological imaging
Computed tomography (CT) is the radiological modality most often used to image the trachea. Dedicated tracheal protocols allow the acquisition of thin slices through the upper airways. With standard chest protocols, tracheal disease is easily underestimated. “Virtual endoscopy” procedures can be performed using CT images constructed during post-processing, with no additional radiation burden (Figure 2). The advantages of virtual endoscopy include the capability to view non-traditional perspectives, to provide volumetric analyses and to apply automatic feature recognition software (15).
Figure 2.
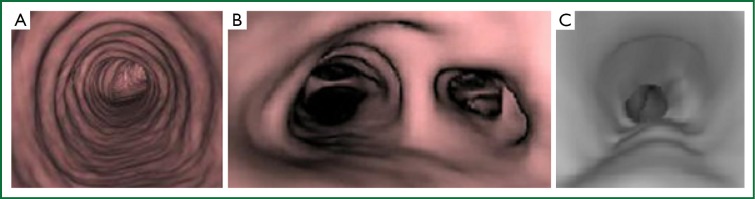
Virtual bronchoscopy images of the normal proximal (A) and distal (B) trachea and tracheal stenosis (C).
CT is useful for diagnosing tracheal disease, identifying the precise anatomical location, the characteristics of the lesion and the extent of disease, including distal airway patency and local vascular anatomy. When tracheal lesions are visualised in cross section, it is possible to assess whether they are circumferential or incomplete, in a single plane (web like) or in three dimensions, like a cork-screw. CT windows also include the wider chest and may provide supportive evidence of an underlying diagnosis.
The combination of axial imaging, multiplanar reformating, and 3-dimensional rendering is useful prior to tracheal intervention, especially when there is significant anatomical distortion or airway narrowing (16).
Bronchoscopy
Flexible bronchoscopy is often performed in the diagnostic work up for symptoms that are subsequently identified as tracheal in origin. Flexible bronchoscopy is however best avoided due to the risk of precipitating acute, complete airway obstruction or proximal haemorrhage. Rigid bronchoscopy is preferred for evaluating stenotic lesions in the trachea and the advantages over flexible bronchoscopy for diagnosis and therapy which will be discussed further below.
Management
Non-acute airway obstruction
In the non-acute setting, initial management should target ongoing tracheal insults such as inflammation or infection, to retard disease progression. Inflammatory conditions such as collagen vascular disease may respond to steroid or immunomodulatory therapies. Airway infection can be difficult to control and identification of the pathogenic organism is key. Recurrent pathogen isolation may prompt long term antibiotic prophylaxis as oral or nebulised therapy. Airway clearance is crucial and can be enhanced by the use of mucolytic agents such as carbocysteine, nebulised therapy with saline and/or N Acetyl Cysteine and chest physiotherapy.
Acute airway obstruction
When patients present acutely with significant upper airways obstruction, supportive measures may be necessary and include the commencement of an inspired Helium-oxygen (Heliox) mixture. Heliox is less dense than oxygen and nitrogen. In accordance with Reynold’s equation, reducing the density of the inspired gas has the effect of predisposing to laminar flow and this can be used to improve airway dynamics in the short term.
Definitive management
Most significant tracheal stenoses necessitate interventional bronchoscopy or surgical resection. Definitive management should be planned with the input of the multi-disciplinary team. It is the nature of patients with tracheal pathology that their underlying disease or history of intensive care admission may make them high risk surgical candidates; thus, endotracheal intervention is often preferable (17). All patients should, however, be considered for tracheal surgery.
Interventional bronchoscopy does not preclude future surgery in most cases and may optimise potential surgical candidates. Lower surgical success rates are evident if the patient has had previous tracheal surgery, but previous laser therapy does not affect surgical outcome (3).
The most frequent complication of tracheal resection and reconstruction is granulation tissue formation at the anastomotic site. Since the introduction of novel suture materials in 1978, the complication rate has fallen to 1.6% (18). It is possible to treat granulation tissue at the anastomotic site with endotracheal therapy.
Interventional bronchoscopy
Background
Interventional bronchoscopy should ideally be performed in specialist centres, with the support of experienced, consistent multi-disciplinary teams. Globally, interventional bronchoscopy is most commonly performed using intravenous awake sedation, local anaesthesia and the flexible bronchoscope. Rigid bronchoscopy under general anaesthesia has increased in popularity over the last two decades (19,20) but widespread adoption of the technique is limited by relative operator inexperience and a lack of available training. Rarely, if a tracheal stenosis is high, in close proximity to the vocal cords, a laryngeal mask and flexible bronchoscopy is indicated to visualise and treat the trachea.
Strengths and potential limitations of rigid bronchoscopy
Unlike flexible bronchoscopy, which relies upon the patient’s own, potentially unstable airway and ventilation, rigid bronchoscopy offers a controlled, ventilated airway under general anaesthetic, with the support of a cardiothoracic anaesthetist (21). Rigid bronchoscopy is therefore preferable for patients with severe respiratory disease who can be poorly tolerant of flexible bronchoscopy. The use of general anaesthesia also has the benefit of creating an immobile field, free from cough, allowing intervention to be performed more safely (22).
Biopsy or airway intervention during flexible bronchoscopy risks airway haemorrhage with potential compromise of both lungs, rendering the patient hypoxic. Rigid bronchoscopy offers a potentially safer means of obtaining a tissue diagnosis (23). Using the rigid bronchoscope it is possible to apply direct pressure to bleeding lesions, and to apply adrenaline soaked gauze using rigid forceps to tamponade the bleeding source, if direct application of adrenaline solution is not sufficient. Single lung isolation with the rigid bronchoscope can also be used to protect the non-bleeding lung if significant haemorrhage occurs.
The bronchoscope barrel can be used to dissect tissue or dilate tracheal stenoses directly, with excellent access for instrumentation with dilators or stents under direct vision. Rigid bronchoscopy minimises procedure times for endotracheal intervention. The median time to stent deployment is 12 minutes at our institution using rigid bronchoscopy (24).
In a specialist centre with a highly trained and experienced team, low complication rates are seen with rigid bronchoscopy. Potential complications include dental trauma, vocal cord trauma/inflammation and airway haemorrhage. Pneumothorax is a risk due to tracheal instrumentation and positive pressure ventilation but our local experience suggests rates of pneumothorax are less than 1% (based on review of >500 rigid bronchoscopy procedures) (24).
Performing rigid bronchoscopy
The rigid bronchoscope is a hollow, tapered metal tube, with distal side-holes along the body for optimal ventilation. The patient is positioned supine with their neck extended. The pharynx, larynx and trachea are aligned in order to insert the rigid tube, taking care to protect the teeth and vocal cords from trauma.
The lumen of the rigid bronchoscope is used for direct vision. Intervention is performed using rigid instruments passed through the rigid bronchoscope. A flexible bronchoscope is passed through the lumen of the rigid bronchoscope to better visualise segmental airways or to see beyond a narrowed trachea. Some centres use special thin flexible bronchoscopes for this purpose (25). The flexible bronchoscope is also utilised for laser therapy.
Endotracheal intervention
There is an overlap between the techniques used to treat tracheal and bronchial obstruction (26). Options include airway dilatation, tissue destruction and stent insertion, each of which is detailed below.
Airway dilatation
Dilatation is achieved with lubricated bougies of increasing diameter applying radial pressure circumferentially to the narrowed airway. Balloon dilatation is an alternative method. The flexible then rigid bronchoscope can also be used to perform blunt dissection and dilatation of stenosed areas under direct vision.
With all dilating techniques, it is imperative to identify the path of the true airway lumen. It is easy, especially when the trachea is distorted, to lose sight of the true lumen, risking airway perforation. Pre-operative imaging is useful to define patient anatomy.
Dilatation alone is very rarely a definitive therapy and re-stenosis usually occurs. Dilatation may be used in combination with other therapeutic techniques such as laser ablation and stent insertion, and can be repeated as necessary (NICE guideline IP938).
Tissue destruction
Once the true airway lumen has been identified, it is usually preferable to destroy and physically remove diseased tissue (Figure 3). The most rudimentary method of tissue destruction uses forceps to mechanically remove tissue from the trachea. Techniques used to effect tissue destruction include laser therapy, argon plasma, brachytherapy, electrocautery and cryotherapy. Most centres prefer laser therapy, of which the neodymium: yttrium-aluminum-garnet (Nd Yag; Nd: Y3Al5O12) laser is the most commonly used (19,27-29).
Figure 3.
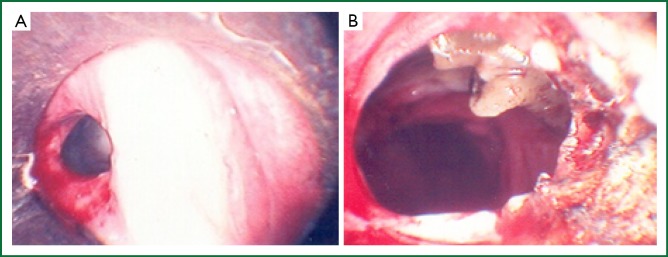
The endoscopic images of tracheal stenosis before (A) and after (B) Nd Yag laser therapy.
Nd-Yag laser energy is delivered via fibres inserted into the working channel of the flexible bronchoscope, using the rigid bronchoscope as a stable airway. The fibres can either be contact or non-contact and are used to devitalise or resect diseased tissue whilst assisting with haemostasis. Nd-Yag laser has a wavelength of 1,064 nm, which is in the invisible photo spectrum. A red light is therefore used to direct application. The bronchoscopist should always apply laser energy parallel to the central airway to avoid unintended trauma to local structures. Energy should be applied in a circumferential motion (Figure 4), using 1-5 seconds laser pulses. A circumferential as opposed to radial approach is preferred to open the airway in malignant and benign disease, to ensure good visualisation of the distal airway whilst improving airflow. The lowest possible power is recommended. Our recommended practice is to use a power of 15-20 watts in the trachea, and lower power distally.
Figure 4.
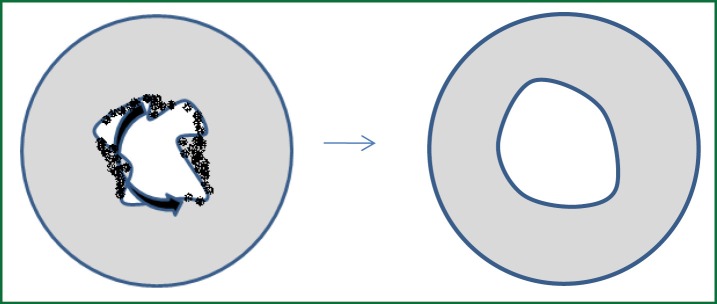
The technique of Nd Yag laser therapy. The laser fibre is moved in a circumferential fashion, starting from the centre of the airway.
Following laser treatment, the airway lumen may still appear narrowed. The effects of the treatment continue for days to weeks after the initial application. The bronchoscopist must therefore refrain from being too aggressive with laser therapy. During and after laser treatment, it is important to clear devitalised tissue from the trachea and distal airways. Aspiration is usually sufficient but manual forceps can also be used. The patient will cough up any remaining or further tissue that sloughs away over the coming days.
Personnel should be trained in the use of laser and a committee responsible for laser usage, maintenance and safety should be established and meet regularly. All staff within the potential laser field should wear protective eyewear. The operating room should be adapted for laser therapy with protective curtains, barriers and warning signs at all entry points, and a laser fume extraction device used (Figure 5). Inspired oxygen concentration should be less than 40 percent and ventilation should be ceased during laser pulses to reduce the risk of airway fire. If a laryngeal mask is used during laser therapy this should be inflated with saline rather than air, to reduce fire risk.
Figure 5.
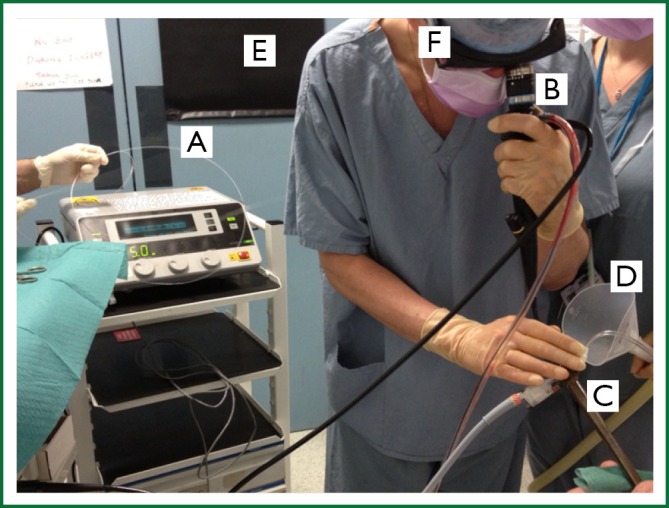
Nd Yag laser therapy. Laser fibre (A) to be inserted through the working channel of the flexible bronchoscope (B), passed through the lumen of the rigid bronchoscope (C). Laser fume extraction device (D), protective curtain (E) and eye wear (F) can be seen.
Reported complications of laser treatment include haemorrhage, airway perforation and airway fire. However, published case series report overall complication rates below 1% in approximately 7,000 treatments (27). With safety measures in place, laser therapy is an excellent and reliable way of treating tracheal stenosis.
There is no limitation to the amount of times laser therapy can be performed. Nearly all patients require more than one endotracheal treatment to achieve long term airway patency. Tissue regrowth can be significantly slowed or halted by serial treatments, with the chance of success increasing after each treatment (30).
Endotracheal stenting
Endotracheal stents are used to provide structural support to the airway and to maintain airway patency. They are, however, foreign bodies in the airway and disrupt mucociliary clearance. The complications of stent placement are listed in Table 1 and stent fracture is displayed in Figure 6.
Table 1. Potential complications of endotracheal stent insertion (27,31-39).
| Potential complications of endotracheal stent insertion |
|---|
| Mucous plugging |
| Stent migration |
| Halitosis |
| Cough |
| Obstructing granulation tissue formation |
| Stent fracture (Figure 6) |
| Bacterial colonisation/recurrent infection |
| Fistula formation |
| Airway malacia (after removal) |
Figure 6.
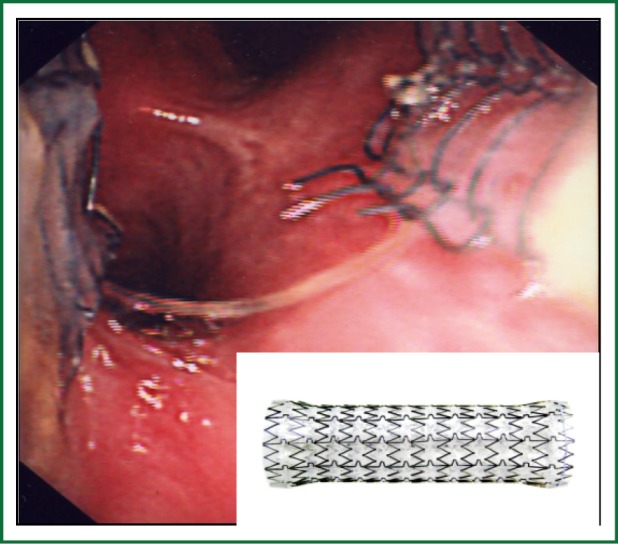
Tracheal metal stent fracture. Endotracheal view of a fractured covered metal tracheal stent and inset picture demonstrating an expanded metal airway stent.
Metal and silicone stents are available. Metal stents come with or without a silastic or polyurethane covering which is used to minimise tissue growth when intrinsic tracheal disease is present. The covering is purposefully absent at either end of the stent to allow the stent to anchor to the mucosa and reduce stent migration but re-growth or new tissue growth may occur in these areas.
Historically, metal stents expanded in an unpredictable and uneven manner, resulting in local airway ischaemia, granulation tissue formation, airway perforation and stent migration. The titanium stents in current use are lighter, easier to insert and demonstrate more uniform self-expansion. The application of a more consistent radial force to the airway means perforation, ischaemia and migration are less common and stronger forces can be withstood. There is also a greater availability of stent sizes.
Silicone stents result in a lesser local inflammatory response than metal stents, reducing granulation tissue formation. Silicone stents are therefore easier to remove but have a high risk of stent migration which limits their use (40,41).
When selecting a tracheal stent, it is desirable to use the greatest diameter stent possible. Selection will depend on patient size and disease extent after optimal airway remodelling. In general, airway stents deployed in tracheal disease are between 40-120 mm in length and 14-24 mm wide.
A stent should not be placed when there is active infection as this will promote granulation tissue formation. Treatment of bacterial colonisation in long term airway stents appears useful (42). Our centre routinely offers five days prophylactic oral antibiotics post stent placement.
Stent migration, especially in proximal lesions approaching the vocal cords, can acutely threaten the airway. External fixation of silicone stents has been trialled but with limited success and use, mainly due to cumbersome techniques (43,44). With careful prospective surveillance of metal tracheal stents there is usually minimal risk of migration and no requirement for stent fixation.
Concurrent tracheal and bronchial disease can be managed using Y-shaped silicone stents. The use of Y-shaped stents is limited by recurrent stent obstruction and infection. It is believed the stent structure results in excessive airway friction and mucociliary clearance disruption, with granulation tissue overgrowth and mucus impaction (45). Simultaneous stenting of the trachea and bronchi can be performed with metal stents, when necessary.
Following stent insertion, surveillance bronchoscopy is indicated in both malignant and benign disease (46). This facilitates early identification and management of complications. Relying on history and examination alone for surveillance is potentially hazardous due to the paucity of symptoms and signs before severe tracheal disease development. Treatment of peri-stent granulation tissue is most commonly addressed by laser therapy (20,31). Timely identification and treatment of airway infection is also crucial.
Indications for stent insertion
In malignant tracheal disease, stenting (with a covered stent) is used to reduce the occurrence of rapid, life threatening disease progression (47). Stenting is also indicated in malignant tracheal fistulae, even if there is no luminal compromise due to tumour bulk. Stents are used to physically obstruct the fistulae, palliating symptoms and protecting the large airway. Stenting for benign tracheal fistulae may be performed in non-operable disease.
Indications for tracheal stent placement in benign disease are less clear than for malignant disease, with varying practices seen worldwide. This is due to the better long term prognosis of individuals with benign pathology, the difficulty removing airway stents, and the reported complications of their use. Endotracheal stent insertion for benign disease should only be considered after airway remodelling by tracheal dilatation and/or tissue destruction has failed to effectively sustain airway patency.
The US Food and Drug Administration recommended in 2005 that metal stents should not be used for benign disease unless absolutely necessary (48). A major concern raised was turning operable cases into inoperable cases (36). Nevertheless, stenting does have a role as a bridge to surgery, enabling optimisation of a patient’s functional and physical state prior to surgical intervention.
Tracheal stents for airway malacia should only be considered when patients are symptomatic and airway collapse is greater than 60%. The dynamic radial forces in malacia lead to higher stent complication rates, including metal fracture (49). A further problem with stenting these patients is recognising where to stent, as often long segments are involved. Extensive airway stenting risks higher occlusion rates due to widespread disruption in mucociliary clearance. When a stent is too short for the involved segment this risks displacing airway collapse to the distal unsupported airway, failing to improve or worsening airway dynamics and symptoms.
Stent removal
Metal stents should be considered permanent as they remodel into the airway by granulation tissue growth and epithelialisation (9,32,50). The longer a stent remains in situ, the lower the chance of successful removal. Nevertheless, stent removal has been performed successfully in tracheobronchial disease using both rigid bronchoscopy (51-53) and flexible bronchoscopy (54).
Silicone stents are more easily removed than metal stents and so may lend themselves to short term placement if planned, despite their high migration rates. Research is on-going to produce a fully degradable tracheal stent which can remain in situ (55).
Airway management with a tracheal stent in situ
Great care should be taken to avoid damaging any tracheal stent if intubation is necessary. It is recommended to use a flexible bronchoscope to ensure that the endotracheal tube is sited above or within the stent lumen (56).
Strong consideration should be given to using rigid bronchoscopy to guide placement of percutaneous tracheostomy in complex tracheal disease (including tracheal stenosis or when a tracheal stent is in situ). Percutaneous tracheostomy using rigid bronchoscopy has been previously described (57,58) including where the endotracheal tube is removed and replaced by the rigid bronchoscope (59). The benefits to this approach include better visibility of the complex airway and/or stent, guide wire location, reduced risk of cuff rupture during cannulation and ease of haemostatic control.
Long term tracheostomy
Long term tracheostomy may become necessary for patients with complex tracheal disease. Commonly a Montgomery T tube is placed through a tracheostomy which serves as both a tracheal stent and tracheostomy tube. Tracheostomy is usually reserved for non-surgical candidates, after endotracheal therapy has become complicated and/or requires too frequent procedures, produces suboptimal clinical response or is anatomically too complex to perform safely. Tracheostomy can also be used as a bridge to tracheal surgery or as an adjunct to surgery. Our practice suggests patients are disinclined to tracheostomy, mainly due to negative cosmetic effects, and consider this a last resort.
Interventional outcomes in tracheal obstruction
Malignant disease
There is no randomised controlled trial evidence regarding the use of tracheal intervention in malignant disease due to the ethical challenges in patients requiring life-saving intervention or palliation. The impact of tracheal intervention on survival cannot, therefore, be quoted accurately. Studies have, however, consistently demonstrated that stenting can improve symptoms of breathlessness, quality of life and lung function in malignant disease. Data supports the use of metal covered stents to achieve success rates from 82-97% in these parameters (9,34,40,46,50,60-64). Importantly, improvements in performance status following stenting can open avenues to other therapies for malignancy, potentially improving outcomes further.
Benign disease
Successful short and long term outcomes using a combination of controlled dilatation and/or Nd-Yag laser therapy to destroy endotracheal tissue in tracheal stenosis have been published (28,30,65-68). Despite concerns regarding the use of tracheal stents in benign disease, there are a number of supportive case series and reports in the literature (31,32,35,68,69).
Early studies suggested that endotracheal treatment was less effective for circumferential disease and for stenoses greater than 1 cm in length (70). Recent studies have demonstrated that involvement of the cricoid cartilage and stenoses over 3 cm are associated with a reduced chance of success (71). Time from tracheal stenosis development to first intervention is also important. One study in post intubation tracheal stenosis established that 90% of patients who had intervention within six months of extubation had a positive outcome compared to 61% of those with a longer delay before intervention (72).
Galluccio et al. proposed the classification of tracheal stenosis into simple and complex, with simple stenoses defined as those less than 1 cm in length with no associated tracheomalacia or loss of cartilaginous support. Using this classification, silicone stent insertion as part of an endoscopic approach achieved airway patency in 96% of simple lesions at two years follow up but only 69% of complex lesions (30). When considering the removal of short term silicone stents, higher success rates have been seen when sizeable air pockets (longer than 1 cm) between the stent and tracheal wall are visualised at CT (in post tuberculosis tracheobronchial stenosis) (73).
Although our centre strongly advocates the use of rigid bronchoscopy for endotracheal intervention, other centres have described using flexible bronchoscopy to intubate, dilate and stent patients with tracheal stenosis without complication, in limited patient series (74).
Patient selection for airway stenting in tracheobronchomalacia is crucial as there must be limited disease and a strong enough, supported airway distal to the stent to avoid collapse. As discussed the complication rates of stent insertion in tracheomalacia are higher and when there is malacia from loss of cartilaginous support in tracheal stenosis this reduces the chance of a successful outcome (30,70). However, when patients are carefully selected, studies have demonstrated that patients can achieve relief from breathlessness and an improved quality of life (75,76).
Benign tracheal stenosis has been successfully treated with tracheostomy at long term follow up, including tracheostomy tube placement through tracheal stents remodelled into the airway (36,77).
Summary
Tracheal disease resulting in upper airways obstruction can be life threatening and is an important diagnosis to consider early. A thorough history to identify predisposition to tracheal disease is necessary, with a high clinical index of suspicion directing comprehensive investigation. Prompt treatment of concurrent airway infection is crucial.
Due to the aetiology of tracheal obstruction, the patients are often poor surgical candidates and patients seldom wish to pursue long term tracheostomy. Fortunately, non-resectable tracheal disease can be successfully treated with interventional rigid bronchoscopy to restore airway patency using debulking and/or dilatation techniques. Tracheal stenting is often performed in malignant disease to protect the airway but should be carefully considered in benign disease as stent removal can be difficult. We advocate the use of covered metal stents when stenting is required for tracheal stenosis, due to their infrequent migration ahead of silicone stents. With regular follow up, including surveillance repeat bronchoscopy, endotracheal intervention can achieve long term success for patients with tracheal disease.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- 1.Whited RE. A prospective study of laryngotracheal sequelae in long-term intubation. Laryngoscope 1984;94:367-77 [DOI] [PubMed] [Google Scholar]
- 2.Andrews MJ, Pearson FG. Incidence and pathogenesis of tracheal injury following cuffed tube tracheostomy with assisted ventilation: analysis of a two-year prospective study. Ann Surg 1971;173:249-63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anand VK, Alemar G, Warren ET. Surgical considerations in tracheal stenosis. Laryngoscope 1992;102:237-43 [DOI] [PubMed] [Google Scholar]
- 4.Grillo HC, Cooper JD, Geffin B, et al. A low-pressure cuff for tracheostomy tubes to minimize tracheal injury. A comparative clinical trial. J Thorac Cardiovasc Surg 1971;62:898-907 [PubMed] [Google Scholar]
- 5.Pearson FG, Andrews MJ. Detection and management of tracheal stenosis following cuffed tube tracheostomy. Ann Thorac Surg 1971;12:359-74 [DOI] [PubMed] [Google Scholar]
- 6.Sarper A, Ayten A, Eser I, et al. Tracheal stenosis aftertracheostomy or intubation: review with special regard to cause and management. Tex Heart Inst J 2005;32:154-8 [PMC free article] [PubMed] [Google Scholar]
- 7.Pearson FG, Fairley HB. Tracheal stenosis complicating tracheostomy with cuffed tubes. Int Anesthesiol Clin 1970;8:889-905 [DOI] [PubMed] [Google Scholar]
- 8.Ginsberg RJ, Vokes EE, Ruben A. Non-small cell lung cancer. In: DeVita VT, Hellman S, Rosenberg SA, et al. eds. Cancer principles and practice of oncology, 5th ed. Philadelphia: Lippincott-Raven, 1997:858-911. [Google Scholar]
- 9.Gaafar AH, Shaaban AY, Elhadidi MS. The use of metallic expandable tracheal stents in the management of inoperable malignant tracheal obstruction. Eur Arch Otorhinolaryngol 2012;269:247-53 [DOI] [PubMed] [Google Scholar]
- 10.Rowlands RG, Adam EJ, Madden BP. Tracheal stenosis due to brachiocephalic artery aneurysm successfully treated with stenting. Monaldi Arch Chest Dis 2001;56:318-9 [PubMed] [Google Scholar]
- 11.Perotin JM, Jeanfaivre T, Thibout Y, et al. Endoscopic management of idiopathic tracheal stenosis. Ann Thorac Surg 2011;92:297-301 [DOI] [PubMed] [Google Scholar]
- 12.Mark EJ, Meng F, Kradin RL, et al. Idiopathic tracheal stenosis: a clinicopathologic study of 63 cases and comparison of the pathology with chondromalacia. Am J Surg Pathol 2008;32:1138-43 [DOI] [PubMed] [Google Scholar]
- 13.Geffin B, Grillo HC, Cooper JD, et al. Stenosis following tracheostomy for respiratory care. JAMA 1971;216:1984-8 [PubMed] [Google Scholar]
- 14.Wilde M, Nair S, Madden B. Pulmonary function tests: a review. Care Crit lll 2007;23:173-7.
- 15.Kligerman S, Sharma A.Radiologic evaluation of the trachea. Semin Thorac Cardiovasc Surg 2009;21:246-54 [DOI] [PubMed] [Google Scholar]
- 16.Morshed K, Trojanowska A, Szymański M, et al. Evaluation of tracheal stenosis: comparison between computed tomography virtual tracheobronchoscopy with multiplanar reformatting, flexible tracheofiberoscopy and intra-operative findings. Eur Arch Otorhinolaryngol 2011;268:591-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Madden B.Management of large airway problems. Medicine 2008;36:168-71 [Google Scholar]
- 18.Grillo HC, Donahue DM, Mathisen DJ, et al. Postintubation tracheal stenosis. Treatment and results. J Thorac Cardiovasc Surg 1995;109:486-92; discussion 492-3 [DOI] [PubMed] [Google Scholar]
- 19.Wahidi MM, Herth FJ, Ernst A. State of the art: interventional pulmonology. Chest 2007;131:261-74 [DOI] [PubMed] [Google Scholar]
- 20.Madden BP, Datta S, Charokopos N. Experience with Ultraflex expandable metallic stents in the management of endobronchial pathology. Ann Thorac Surg 2002;73:938-44 [DOI] [PubMed] [Google Scholar]
- 21.Crerar-Gilbert A, Madden BP. The use of rigid bronchoscopy for bronchial stenting in patients with tracheal stenosis. J Cardiothorac Vasc Anesth 2007;21:320. [DOI] [PubMed] [Google Scholar]
- 22.Jones C, Crerar-Gilbert AJ, Madden BP. Anaesthesia for endobronchial intervention and tracheobronchial stents. Curr Anaesth Crit Care 2009;20:160-3 [Google Scholar]
- 23.Nimako K, Smith K, Ranu H, et al. Performing biopsies of proximal airway lesions: Flexible versus rigid bronchoscopy. American Journal of Respiratory and Critical Care Medicine 2010 Thoracic Society International Conference, ATS;Conference: Ameran. [Google Scholar]
- 24.Bacon JL, Leaver SK, Madden BP. Six year experience with rigid bronchoscopy: complications, indications and changing referral patterns. Thorax 2012;67:Suppl 3A151-A152 [Google Scholar]
- 25.Oki M, Saka H.Thin bronchoscope for evaluating stenotic airways during stenting procedures. Respiration 2011;82:509-14 [DOI] [PubMed] [Google Scholar]
- 26.Bacon JL, Wilde MP, Walker ME, et al. The Diagnosis of Large Airway Pathology and the Role of Rigid Bronchoscopy. Curr Respir Med Rev 2013;9:11-25 [Google Scholar]
- 27.Ernst A, Feller-Kopman D, Becker HD, et al. Central airway obstruction. Am J Respir Crit Care Med 2004;169:1278-97 [DOI] [PubMed] [Google Scholar]
- 28.Madden BP, Datta S, McAnulty G. Tracheal granulation tissue after percutaneous tracheostomy treated with Nd:Yag laser: three cases. J Laryngol Otol 2001;115:743-4 [DOI] [PubMed] [Google Scholar]
- 29.Madden BP, Kumar P, Sayer R, et al. Successful resection of obstructing airway granulation tissue following lung transplantation using endobronchial laser (Nd:YAG) therapy. Eur J Cardiothorac Surg 1997;12:480-5 [DOI] [PubMed] [Google Scholar]
- 30.Galluccio G, Lucantoni G, Battistoni P, et al. Interventional endoscopy in the management of benign tracheal stenoses: definitive treatment at long-term follow-up. Eur J Cardiothorac Surg 2009;35:429-33; discussion 933-4 [DOI] [PubMed] [Google Scholar]
- 31.Madden BP, Stamenkovic SA, Mitchell P. Covered expandable tracheal stents in the management of benign tracheal granulation tissue formation. Ann Thorac Surg 2000;70:1191-3 [DOI] [PubMed] [Google Scholar]
- 32.Madden BP, Loke TK, Sheth AC. Do expandable metallic airway stents have a role in the management of patients with benign tracheobronchial disease? Ann Thorac Surg 2006;82:274-8 [DOI] [PubMed] [Google Scholar]
- 33.Hind CR, Donnelly RJ. Expandable metal stents for tracheal obstruction: permanent or temporary? A cautionary tale. Thorax 1992;47:757-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hosokawa Y, Tsujino I, Syoda T, et al. Examination of expandable metallic stent removed at autopsy. Respirology 2003;8:522-4 [DOI] [PubMed] [Google Scholar]
- 35.Thornton RH, Gordon RL, Kerlan RK, et al. Outcomes of tracheobronchial stent placement for benign disease. Radiology 2006;240:273-82 [DOI] [PubMed] [Google Scholar]
- 36.Gaissert HA, Grillo HC, Wright CD, et al. Complication of benign tracheobronchial strictures by self-expanding metal stents. J Thorac Cardiovasc Surg 2003;126:744-7 [DOI] [PubMed] [Google Scholar]
- 37.Bolliger CT, Mathur PN, Beamis JF, et al. ERS/ATS statement on interventional pulmonology. European Respiratory Society/American Thoracic Society. Eur Respir J 2002;19:356-73 [DOI] [PubMed] [Google Scholar]
- 38.Noppen M, Piérard D, Meysman M, et al. Bacterial colonization of central airways after stenting. Am J Respir Crit Care Med 1999;160:672-7 [DOI] [PubMed] [Google Scholar]
- 39.Chen W, Ruan Y.Late complications of nickel-titanium alloy stent in tracheal stenosis. Laryngoscope 2012;122:817-20 [DOI] [PubMed] [Google Scholar]
- 40.Bolliger CT, Probst R, Tschopp K, et al. Silicone stents in the management of inoperable tracheobronchial stenoses. Indications and limitations. Chest 1993;104:1653-9 [DOI] [PubMed] [Google Scholar]
- 41.Wood DE, Liu YH, Vallières E, et al. Airway stenting for malignant and benign tracheobronchial stenosis. Ann Thorac Surg 2003;76:167-72; discussion 173-4 [DOI] [PubMed] [Google Scholar]
- 42.Holden EL, Jaafar M, Madden BP. Bacterial colonisation of endobronchial stents: a precursor to granulation tissue formation? A retrospective review from one stenting centre.Am J Respir Crit Care Med 2011;183.1:A1561
- 43.Miwa K, Takamori S, Hayashi A, et al. Fixation of silicone stents in the subglottic trachea: preventing stent migration using a fixation apparatus. Ann Thorac Surg 2004;78:2188-90 [DOI] [PubMed] [Google Scholar]
- 44.Majid A, Fernandez-Bussy S, Kent M, et al. External fixation of proximal tracheal airway stents: a modified technique. Ann Thorac Surg 2012;93:e167-9 [DOI] [PubMed] [Google Scholar]
- 45.Yarmus L, Gilbert C, Akulian J, et al. Novel use of the GlideScope for rigid bronchoscopic placement of a Dynamic (Y) Stent. Ann Thorac Surg 2012;94:308-10 [DOI] [PubMed] [Google Scholar]
- 46.Madden BP, Park JE, Sheth A. Medium-term follow-up after deployment of ultraflex expandable metallic stents to manage endobronchial pathology. Ann Thorac Surg 2004;78:1898-902 [DOI] [PubMed] [Google Scholar]
- 47.Ranu H, Madden BP. Endobronchial stenting in the management of large airway pathology. Postgrad Med J 2009;85:682-7 [DOI] [PubMed] [Google Scholar]
- 48.Shultz D. FDA public health notification: complications from metallic tracheal stents in patients with benign airway disorders. Open URL: Services DHH, 2005.
- 49.Filler RM, Forte V, Chait P. Tracheobronchial stenting for the treatment of airway obstruction. J Pediatr Surg 1998;33:304-11 [DOI] [PubMed] [Google Scholar]
- 50.Monnier P, Mudry A, Stanzel F, et al. The use of the covered Wallstent for the palliative treatment of inoperable tracheobronchial cancers. A prospective, multicenter study. Chest 1996;110:1161-8 [DOI] [PubMed] [Google Scholar]
- 51.Ranu H, Evans J, Sheth A, et al. Removal of long-term tracheal stents with excellent tracheal healing. Ann Thorac Surg 2010;89:598-9 [DOI] [PubMed] [Google Scholar]
- 52.Ose N, Inoue M, Minami M, et al. Successful removal of expandable metallic stent in a patient with lung cancer. Asian Cardiovasc Thorac Ann 2012;20:202-4 [DOI] [PubMed] [Google Scholar]
- 53.Noppen M, Stratakos G, D’Haese J, et al. Removal of covered self-expandable metallic airway stents in benign disorders: indications, technique, and outcomes. Chest 2005;127:482-7 [DOI] [PubMed] [Google Scholar]
- 54.Fruchter O, Raviv Y, Fox BD, et al. Removal of metallic tracheobronchial stents in lung transplantation with flexible bronchoscopy. J Cardiothorac Surg 2010;5:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ng AH, Ng NS, Zhu GH, et al. A fully degradable tracheal stent: in vitro and in vivo characterization of material degradation. J Biomed Mater Res B Appl Biomater 2012;100:693-9 [DOI] [PubMed] [Google Scholar]
- 56.Davis N, Madden BP, Sheth A, et al. Airway management of patients with tracheobronchial stents. Br J Anaesth 2006;96:132-5 [DOI] [PubMed] [Google Scholar]
- 57.Cavaliere S, Venuta F, Foccoli P, et al. Endoscopic treatment of malignant airway obstructions in 2,008 patients. Chest 1996;110:1536-42 [DOI] [PubMed] [Google Scholar]
- 58.Grigo AS, Hall ND, Crerar-Gilbert AJ, et al. Rigid bronchoscopy-guided percutaneous tracheostomy. Br J Anaesth 2005;95:417-9 [DOI] [PubMed] [Google Scholar]
- 59.Madden BP, Sheth A. An approach to tracheostomy in a patient with an expandable metallic tracheal stent. J Laryngol Otol 2005;119:731-2 [DOI] [PubMed] [Google Scholar]
- 60.Saad CP, Murthy S, Krizmanich G, et al. Self-expandable metallic airway stents and flexible bronchoscopy: long-term outcomes analysis. Chest 2003;124:1993-9 [DOI] [PubMed] [Google Scholar]
- 61.Dasgupta A, Dolmatch BL, Abi-Saleh WJ, et al. Self-expandable metallic airway stent insertion employing flexible bronchoscopy: preliminary results. Chest 1998;114:106-9 [DOI] [PubMed] [Google Scholar]
- 62.Miyazawa T, Yamakido M, Ikeda S, et al. Implantation of ultraflex nitinol stents in malignant tracheobronchial stenoses. Chest 2000;118:959-65 [DOI] [PubMed] [Google Scholar]
- 63.Bolliger CT. Introduction to different approaches to intrabronchial treatment. Monaldi Arch Chest Dis 1996;51:316-24 [PubMed] [Google Scholar]
- 64.Madden BP, Sheth A, Walters N. Does large airway intervention for patients with malignant disease result in early clinical benefit? Am J Respir Crit Care Med 2007;175:A622 [Google Scholar]
- 65.Dumon JF, Reboud E, Garbe L, et al. Treatment of tracheobronchial lesions by laser photoresection. Chest 1982;81:278-84 [DOI] [PubMed] [Google Scholar]
- 66.Shapshay SM, Beamis JF, Jr, Hybels RL, et al. Endoscopic treatment of subglottic and tracheal stenosis by radial laser incision and dilation. Ann Otol Rhinol Laryngol 1987;96:661-4 [DOI] [PubMed] [Google Scholar]
- 67.Cavaliere S, Foccoli P, Toninelli C.Curative bronchoscopic laser therapy for surgically resectable tracheobronchial tumors: personal experience. J Bronchol 2002;9:90-5 [Google Scholar]
- 68.Jeong BH, Um SW, Suh GY, et al. Results of interventional bronchoscopy in the management of postoperative tracheobronchial stenosis. J Thorac Cardiovasc Surg 2012;144:217-22 [DOI] [PubMed] [Google Scholar]
- 69.Brichet A, Tavernier JY, Ramon P, et al. Post intubation tracheal stenosis. Med Hyg 2003;61:647-9 [Google Scholar]
- 70.Simpson GT, Strong MS, Healy GB, et al. Predictive factors of success or failure in the endoscopic management of laryngeal and tracheal stenosis. Ann Otol Rhinol Laryngol 1982;91:384-8 [DOI] [PubMed] [Google Scholar]
- 71.Schweinfurth JM. Endoscopic treatment of tracheal stenosis. Oper Tech Otolaryngol Head Neck Surg 2012;23:188-91 [Google Scholar]
- 72.Lim SY, Kim H, Jeon K, et al. Prognostic factors for endotracheal silicone stenting in the management of inoperable post-intubation tracheal stenosis. Yonsei Med J 2012;53:565-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Verma A, Park HY, Lim SY, et al. Posttuberculosis tracheobronchial stenosis: use of CT to optimize the time of silicone stent removal. Radiology 2012;263:562-8 [DOI] [PubMed] [Google Scholar]
- 74.Li WT, Xiao YB, Liu GN, et al. Management of benign tracheal stenosis by intubation dilatation under flexible bronchoscopic guidance. Zhonghua Yi Xue Za Zhi 2011;91:2995-8 [PubMed] [Google Scholar]
- 75.Hramiec JE, Haasler GB. Tracheal wire stent complications in malacia: implications of position and design. Ann Thorac Surg 1997;63:209-12; discussion 213 [DOI] [PubMed] [Google Scholar]
- 76.Hautmann H, Huber RM. Stent flexibility: an essential feature in the treatment of dynamic airway collapse. Eur Respir J 1996;9:609-11 [DOI] [PubMed] [Google Scholar]
- 77.Matsuoka K, Kuroda A, Kang A, et al. Tracheal stenosis after metal stent insertion treated successfully with a T-tube. Ann Thorac Surg 2012;93:1291-2 [DOI] [PubMed] [Google Scholar]


