Abstract
Radiation therapy (RT) continues to be a cornerstone in the treatment for many cancers. Unfortunately, not all individuals respond effectively to RT resulting clinically in two groups consisting of non-responders (progressive disease) and responders (tumor control/cure). The mechanisms that govern the outcome of radiotherapy are poorly understood. Interestingly, a new paradigm has emerged demonstrating that the immune system mediates many of the anti-tumor effects of RT. Therefore, we hypothesized that the immune response following RT may dictate the efficacy of treatment. To examine this, we developed a tumor model that mirrors this clinically relevant phenomenon in which mice bearing Colon38, a colon adenocarcinoma, were treated locally with 15Gy RT resulting in both non-responders and responders. More importantly, we were able to determine responders from non-responders as early as four days post-RT allowing for the unique opportunity to identify critical events that ultimately determined the effectiveness of therapy. Intratumoral immune cells and IFNγ were increased in responsive tumors and licensed CD8 T cells to exhibit lytic activity against tumor cells, a response that was diminished in tumors refractory to RT. Combinatorial treatment with RT and the immunomodulatory cytokine IL-12 resulted in complete remission of cancer in 100% of cases compared to a cure rate of only 12% with RT alone. Similar data were obtained when IL-12 was delivered by microspheres. Therefore, the efficacy of RT may depend on the strength of the immune response induced after radiotherapy. Additionally, immunotherapy that further stimulates the immune cells may enhance the effectiveness of RT.
Keywords: Cancer Radiotherapy, Responders, Cytotoxic T cells, Interferon-gamma, Interleukin-12
Introduction
Radiation therapy (RT) is an effective treatment for many primary cancers, however not all individuals respond equally to therapy 1-4. Clinically, cancer patients that received radiotherapy can often be divided into two groups: 1) responders, where radiation significantly controls or cures tumors and 2) non-responders in which radiotherapy has little to no efficacy. Despite the fact that it is widely accepted that RT results in these two groups, the underlying basis for these differences is incompletely understood. As a result, much emphasis has been devoted to understanding the responder/non-responder phenomena in the hope of skewing the ratio in favor of responders. The majority of the work addressing this issue focuses on predictive factors present in tumors before treatment 2, 4-6. Consequently, a list of genes that may discriminate responders versus non-responders has been identified and includes genes associated with cell cycle, apoptosis, DNA repair, and survival 2, 3, 5-7. However, little work has addressed whether changes occur directly within responder or non-responder tumors shortly after RT, and if these modifications correlate with outcome. In other words, what is lacking is a thorough understanding of the mechanism that governs either a positive or negative outcome in response to radiotherapy. Here, we describe a murine model of radiotherapy in which early intratumoral responses to RT (e.g. days after RT) ultimately dictate the long-term efficacy of this treatment.
Recent data suggests that the immune system mediates many of the anti-tumor effects of radiotherapy 8-15. In particular, our work and that of others demonstrated the importance of dendritic cells, which engulf tumor debris (antigen) released by RT, and ultimately initiate an immune response following RT 8, 12. Not surprisingly, the adaptive arm of the immune system, in particular, T cells, were shown to be pivotal in promoting the effectiveness of radiotherapy 9-11. RT not only enhanced the cytotoxicity of CD8+ T cells, but also induced the secretion of interferon-gamma (IFNγ); an essential cytokine that mediated the anti-tumor effects of RT in a murine adenocarcinoma model 9. Since immune involvement appears vital in promoting an effective RT outcome, we explored whether the immune response following RT may govern responder/non-responder fate.
In this report, we describe a Colon38 murine tumor model in which mice with irradiated tumors divide almost equally into responders and non-responders. More importantly, we could determine the responder/non-responder fate as early as four days after RT, which allowed for the unique opportunity to identify early differences to the tumor microenvironment between groups. We demonstrate that responder tumors have increased numbers of immune cells as early as four days after RT. Additionally, elevated levels of intratumoral IFNγ and CD8+ T cells, which have an enhanced ability to kill tumors, were observed specifically in responder tumors. In essence, non-responder tumors presented very similar to control unirradiated tumors. As a result of these data, we explored if immunotherapy could enhance the efficacy of radiotherapy. Indeed, tumor cells transfected with IL-12, a cytokine with potent immunostimulatory functions especially for cytotoxic T cells 16, 17, resulted in a significant conversion of non-responders to responders and a complete cure rate. Alternately, IL-12 microspheres were administered either before or after radiotherapy to simulate a more clinical setting. Interestingly, treatment with IL-12 microspheres enhanced the effectiveness of RT when given after RT. These data demonstrate that the immune response, which is apparent as early as a few days after RT, may dictate whether a positive (responder) or negative (non-responder) response to radiotherapy can be achieved. Consequently, modulating the immune response after RT can enhance the efficacy of treatment.
Materials and Methods
Tumor cell lines and Mice
Colon38, a murine adenocarcinoma 18, was transfected with pmIL-12-neo.1 as previously described 19 to generate Colon38/IL-12. IL-12 production was measured by culturing 2×105 Colon38/IL-12 cells in a 12 well plate in 2 mls media for 48hrs and examining the concentration of IL-12 in supernatant by ELISA (Peprotech, Rocky Hill, NJ). Cells routinely produced an average of 8 ng/ml/1×106 cells IL-12. All cells were maintained in MAT/P medium (US patent 4.816.401) supplemented with 100 U/mL penicillin, 100 micrograms/mL streptomycin, and 2% fetal calf serum. C57BL/6J mice were purchased from the Jackson Laboratory (Bar Harbor, ME) and were treated in accordance to guidelines approved by the University Committee on Animal Resources.
Tumor inoculation and radiotherapy
1×105 parental Colon38 or Colon38/IL-12 cells were injected intramuscularly (i.m.) in the left leg of mice. A 3200 Curie sealed 137Cesium source that operates at roughly 1.90 Gy/min was used for radiation treatment of mice. The source and collimators are calibrated periodically to ensure equal distribution of radiation. Specially constructed jigs were designed to locally treat the tumor bearing leg with 15 Gy radiation seven days after injection as previously described 9, 12. Tumor growth was measured using calipers.
Whole mount histology
Tumors were examined by whole mount histology as previously described 19. Briefly, tumor pieces were stained with anti-CD8-PerCP to label CD8+ T cells and anti-CD31-PE to label blood vessels in PBS/1% BSA/0.1% azide for 2 hours at 4 degrees C. Samples were washed in 3 mls PBS/1% BSA/0.1% azide and whole mounted on a slide to be examined by conventional fluorescence microscopy. Images of the same field of view were taken for each marker and presented as an overlay.
Flow cytometry
Tumors were removed on various days and single cell suspensions were obtained as previously described 9. 1×106 cells were blocked with Fc Block (clone 2.4G2) followed by staining with directly conjugated primary antibodies (CD45 clone 30-F11; CD8 clone 53-6.7) for 30 minutes. Samples were washed once in 1 ml of PBS/1% BSA/0.1% azide and further analyzed using a FACSCanto Flow Cytometer (BD Biosciences, San Jose, CA) and FlowJo software (Tree Star, Ashland, OR). The immune cell gate was drawn based on CD45+ staining. Tumor cells were gated as CD45- cells. Additionally, GFP+ tumor cells were used previously to determine the location of tumor cells in flow plots and indicated that over 90% of cells which were included in the tumor gate were indeed tumor cells (data not shown). Data are normalized per gram of tumor where indicated.
Cytotoxicity assay
A standard 51chromium release assay was performed as previously described 9. Briefly, tumors were dissociated with collagenase D (Sigma Aldrich) as previously described 9 and intratumoral lymphocytes were magnetically isolated using anti-Thy1.1 mab (clone T24/40.7) conjugated beads. Purified lymphocytes were incubated with 51chromium-labeled Colon38 tumor cells at selected effector (lymphocytes) to target (tumor cells) ratios for 6 hours. The amount of 51chromium released into the culture supernatant was quantified as a measure of the ability of lymphocytes to lyse Colon38 tumor cells thereby releasing 51chromium. Data is plotted as percent specific lysis defined as (experimental lysis − spontaneous lysis)/(maximum lysis − spontaneous lysis) × 100.
Measurement of intratumoral IFNγ
Tumors were homogenized as previously described 9 and amounts of IFNγ were determined by ELISA according to manufacturer’s instructions (PeproTech). Samples were normalized to total protein as determined by a BCA assay (Pierce Biotechnology, Rockford, IL).
CD8+ T cell depletion
Mice were treated with 200 ug anti-CD8 (53-6.7) diluted in BSS (Sigma) intraperitoneally (i.p.) one day before tumor challenge and than every 4 days until sacrifice to deplete CD8+ T cells. Rat IgG diluted in BSS served as a vehicle control.
Treatment of tumors with microspheres
IL-12-encapsulated poly-lactic acid microspheres with a cytokine loading of 0.025% (weight/weight) were prepared using the phase inversion nanoencapsulation method as described previously 20. 2 mg (50ul) of either empty or IL-12 containing microspheres (equivalent to 0.5 ug of IL-12) were injected directly into the tumor either 1 day before (Day 6 post tumor injection) or 1 day after (Day 8) radiotherapy using a tuberculin syringe.
Statistical analysis
Data are presented as means +/- SE from at least 3 replicates. Significance was determined by one-way analysis of variance (ANOVA) followed by a Bonferroni post-hoc test or a non-paired student’s t-test where appropriate.
Results
Radiotherapy of Colon38 tumors results in two outcomes: Responders and non-responders
Mice were injected with Colon38 cells i.m. and tumors were allowed to establish for seven days followed by 15 Gy local radiotherapy. Unirradiated tumors grew progressively (Figure 1a), however irradiated tumors could be divided into two distinct groups; a group of tumors that did not respond well to RT resulting initially in only minimal inhibition of tumor growth followed by rapid outgrowth of tumors (Figure 1b, solid lines and Figure 1c, mouse farthest to the right), and a group of tumors that responded well to therapy that not only exhibited slowed tumor growth but also a substantial loss of tumor burden (Figure 1b, dashed lines and Figure 1c, middle mouse). Both groups of irradiated tumors continued to grow progressively two days after RT (day 9), however only the responder tumors demonstrated a reduction of tumor size by day 11, whereas non-responder tumors were increased in size at day 11. Therefore, these criteria could be used to define responder tumors (day 11 tumor size < day 9) and non-responders (day 11 tumor size > day 9) throughout the rest of this manuscript. Additionally, day 11 (4 days post RT) also represents the earliest timepoint in which we could determine whether a tumor would respond or not respond to radiotherapy. Therefore, day 11 will be the main timepoint examined with regards to particular immune parameters. As a whole, out of 42 irradiated tumors, 57% could be classified as non-responders and 43% as responders (data not shown).
Figure 1. Radiotherapy of Colon38 tumors results in responders and non-responders.
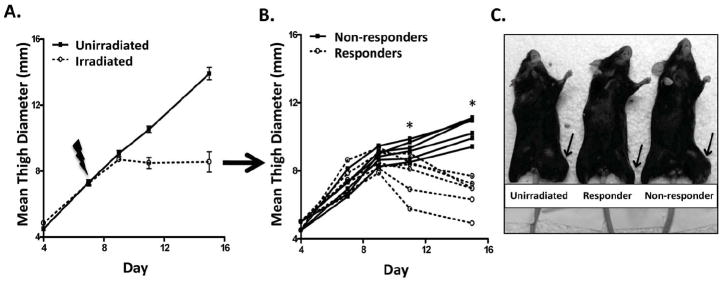
1×105 Colon38 tumor cells were injected i.m. in the left thigh and either treated with 15 Gy radiation seven days later or left unirradiated. Tumor size was measured with calipers and presented as means +/- SE (A) or individual tumor measurement from irradiated animals only (B). A digital picture showing representative tumor burdens is illustrated in (C). The tumor bearing leg is designated with an arrow. Mice that responded to radiation had tumor measurements at day 11 that were smaller than day 9 tumor measurements and vice-versa for non-responder tumors. * denotes a significant difference between non-responder and responder tumor size based on t-test (p < 0.05) at that particular timepoint. n=6 for unirradiated tumors and 12 for irradiated tumors (6 responders and 6 non-responders).
Responder tumors exhibit a greater number of intratumoral immune cells when compared to non-responder tumors
We assessed the number of immune cells in unirradiated control tumors and in both responder and non-responder tumors at day 11 by flow cytometry as described in the materials and methods. Dot plots were gated for tumor cells (CD45 negative) and CD45+ immune cells and expressed as a percentage of the total cells (Figure 2a). Unirradiated tumors contained more tumor cells (~60%) when compared to immune cells (~40%) (Figure 2a-representative dot plot and Figure 2b-cumlative data). Interestingly, responder tumors demonstrated a potent increase of immune cells (85%) compared to tumor cells (15%), whereas non-responder tumors had far fewer intratumoral immune cells and looked similar to unirradiated tumors (Figure 2a and 2b). Similar differences between groups, with regards to immune to tumor cell ratio, were also observed when total immune and tumor cells per gram of tumor were calculated (Figure 2c). Collectively, these data illustrate that responder tumors demonstrate an increase of intratumoral immune cells, which far outnumber the remaining tumor cells.
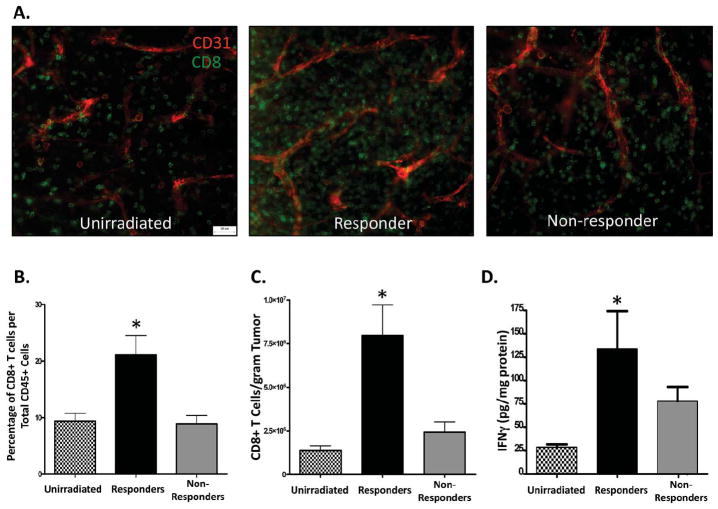
Figure 2. Immune cells are increased in responder tumors.
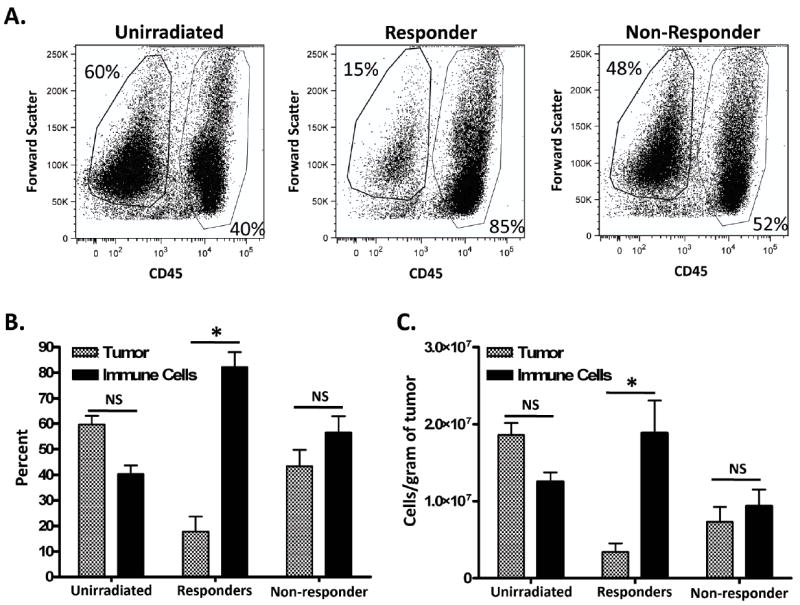
Tumors were characterized as responders or non-responders to radiotherapy (RT on day 7), removed on day 11, dissociated into a single cell suspension and examined by flow cytometry. (A) Representative dot plots of samples that were gated for immune cells (CD45+) or tumor cells (CD45-). Percentage (B) or standardized number (C) of tumor cells compared to immune cells between groups. * represents significance (p < 0.05) as determined by ANOVA and Bonferroni post-hoc test. n=7 for unirradiated; 6 for responders; 5 for non-responders.
CD8+ T cells and intratumoral IFNγ are increased in responder tumors
We previously demonstrated that CD8+ T cells and IFNγ are important to mediate the anti-tumor effects of radiotherapy9. Therefore, we examined whether CD8+ T cells were enhanced in tumors that respond to RT. Day 11 tumor pieces from unirradiated, responder, and non-responder tumors were stained with anti-CD8 to label CD8+ T cells and anti-CD31 to label blood vessels and analyzed by whole mount microscopy as described in the materials and methods (Figure 3a). Responder tumors illustrated an increase of CD8+ T cells when compared to the other groups (Figure 3a-middle panel). To quantify changes in CD8+ T cells, tumors were processed as described in Figure 2 and examined by flow cytometry. Intratumoral CD45+ immune cells were further gated for CD8+ T cells. Responder tumors had a significant increase in both percentage (Figure 3b) and number (Figure 3c) of CD8+ T cells when compared to both unirradiated and non-responder tumors. Additionally, even though non-responder tumors were treated with RT, CD8+ T cell numbers remained similar to those of unirradiated control tumors. IFNγ is not only secreted by activated CD8+ T cells but can also promote enhanced function by these cells. Therefore, we assessed the level of intratumoral IFNγ from tumor homogenates by ELISA. Responder tumors demonstrated a significant increase of intratumoral IFNγ when compared to unirradiated and non-responder tumors (Figure 3d). In all, tumors that respond to radiotherapy have a unique phenotype consisting of marked increases in both CD8+ T cells and IFNγ when compared to non-responding tumors.
Figure 3. Responder tumors have enhanced numbers of CD8+ T cells and amounts of intratumoral IFNγ.
Day 11 tumor pieces were analyzed by whole mount histology for CD8+ T cells (green) and CD31+ blood vessels (red). Representative images of unirradiated, responder, and non-responder tumors are presented as overlays and illustrated in (A). Tumors were examined by flow cytometry on day 11 as described in figure 2. Immune cells were first gated for CD45+ cells followed by gating on CD8+ cells and presented as percentage (B) or standardized number (C). (D) Tumors were homogenized and intratumoral IFNγ was examined by ELISA and normalized to total protein. * represents significance (p < 0.05) as determined by ANOVA and Bonferroni post-hoc test. n=7 for unirradiated; 6 for responders; 5 for non-responders.
T cells from responder tumors demonstrate enhanced cytolytic activity
The combination of CD8+ T cells and IFNγ within the tumor microenvironment may promote enhanced effector cell function. To test this, T cells were isolated from each group of tumors and immediately (no antigen restimulation) assayed for the ability to lyse tumor targets in a standard 51Cr release assay as described in the materials and methods. As shown in Figure 4, T cells isolated from responder tumors exhibited potent lytic activity against tumor targets when compared to T cells from unirradiated and non-responder tumors. Although T cells from non-responder tumors demonstrated slightly higher specific lysis over T cells from unirradiated tumors, these values failed to reach significance. Incubation with anti-CD8 antibodies abrogated all cytolytic activity (data not shown). Taken together, these results suggest that CD8+ T cells from responder tumors have a greater functional capacity to kill tumor cells, and may suggest why radiotherapy is effective in controlling tumor growth in this particular group.
Figure 4. T cells from responder tumors exhibit increased ability to lyse tumor cells.
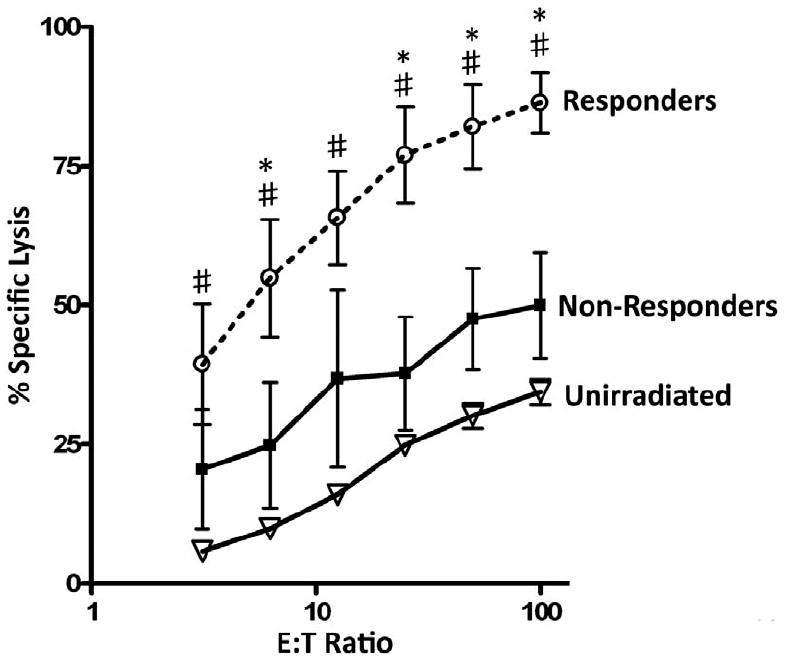
Tumors were injected and irradiated as described in figure 1 and processed into a single cell suspension. T cells were isolated as described in the materials and methods and examined for the ability to lyse 51Cr labeled Colon38 tumor cells in a standard 6-hour assay. Specific lysis was determined from various effector to target ratios. * represents significance (p < 0.05) compared to non-responders as determined by ANOVA and Bonferroni post-hoc test. # represents significance (p < 0.05) compared to T cells from unirradiated tumors as determined by ANOVA and Bonferroni post-hoc test. n=4 for unirradiated and 3 for both responders and non-responders.
Delivery of intratumoral IL-12 combined with radiation promotes the complete conversion of non-responders to responders
Based on the data described above, the efficacy of radiation therapy coincided with a strong anti-tumor immune response. Therefore, we hypothesized that a combination of radiation treatment along with immunotherapy would promote a stronger anti-tumor effect. We utilized IL-12 as an immunotherapeutic agent due to its ability to enhance the levels of IFNγ and augment the function of effector T cells. Colon38 cells were stably transfected with an IL-12 expression vector and the transfected cells produce an average of 8 ng/ml/106 cells of cytokine over 48 hours when assayed in vitro by ELISA. Identical numbers of parental Colon38 and Colon38/IL-12 cells were injected i.m., irradiated on day 7, and tumor growth was monitored. Although both the parental and the IL-12 expressing tumor lines that did not receive RT grew progressively in mice, Colon38/IL-12 did demonstrate slowed growth kinetics when compared to parental tumors (Figure 5a, solid lines). This is not surprising, as IL-12 is known to elicit anti-tumor properties basally 19, 21. As expected, parental Colon38 tumors treated with RT were divided into responders and non-responders, however, all Colon38/IL-12 tumors treated with RT demonstrated a reduction of tumor burden and therefore could all be classified as responders (Figure 5a, dashed lines). Long-term survival was also monitored and is illustrated using a Kaplan-Meier plot (Figure 5b). All mice with untreated parental Colon38 tumors needed to be euthanized as a result of tumor burden by day 13, whereas mice with untreated Colon38/IL- 12 tumors survived longer; some as long as day 32 (Figure 5b, solid lines). However, all mice with untreated tumors (parental or IL-12 expressing) eventually succumbed to illness. Irradiation of parental Colon38 tumors significantly extended survival when compared to untreated parental tumors, however only 12% of mice completely rejected the tumors and the remaining mice had to be sacrificed as a result of large malignancies. Importantly, 100% of mice with Colon38/IL-12 tumors treated with RT demonstrated complete tumor rejection (Figure 5b, dashed lines). To determine whether CD8+ T cells were mediating the enhanced IL-12-induced anti-tumor effect, we treated mice with antibody to deplete the CD8+ T cells. Elimination of CD8+ T cells abrogated the anti-tumor effect elicited by IL-12 both basally and following radiotherapy (Figure 5c). Unexpectedly, 3 out of 7 unirradiated tumors demonstrated a spontaneous loss of burden after i.p. rat IgG administration. Although, the nature of this response is unclear, no spontaneous loss of burden or tumor control resulting from radiotherapy was observed when CD8 T cells were eliminated. Collectively, these data identify IL-12 as an immunotherapeutic agent that may greatly enhance the efficacy of radiotherapy.
Figure 5. IL-12 enhances the anti-tumor effect of radiotherapy.
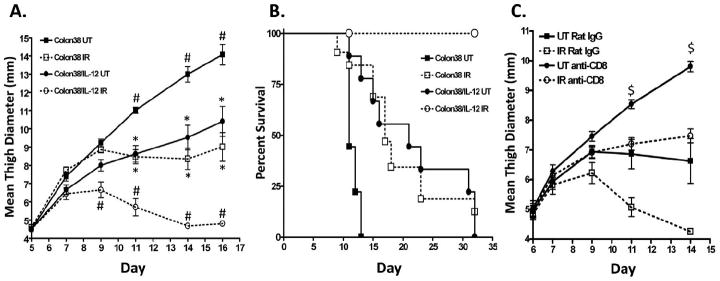
1×105 Colon38 or Colon38/IL-12 cells were injected i.m. and the tumors either left untreated or irradiated with 15 Gy seven days post inoculation. (A) Tumor size was measured over time. Significance was determined by ANOVA and Bonferroni post-hoc test (p < 0.05), # represents significance to all 3 groups; * represents significance to Colon38 UT and Colon38/IL-12 RT. (n=5 for Colon38 UT; 10 for Colon38 RT; 8 for Colon38/IL-12 UT; 12 for Colon38/IL-12 RT). (B) A Kaplan-Meier plot to demonstrate survival is shown where endpoint was defined when tumors reached 10mm in size (n=9 for Colon38 UT; 32 for Colon38 RT; 9 for Colon38/IL-12 UT; 13 for Colon38/IL-12 RT). (C) Mice were treated with anti- CD8 or control rat IgG as described in the materials and methods followed by injection of 1×105 Colon38/IL-12 cells i.m. Tumors were irradiated seven days post inoculation and tumor growth monitored. $ represents significance (p < 0.05) in all groups except between RT anti-CD8 and UT rat IgG as determined by a one-way ANOVA followed by a Bonferroni post-hoc test. (n=7).
Treatment of tumors with IL-12 microspheres enhances the efficacy of radiotherapy
The previous experiment featured IL-12 delivery by transfected tumor cells. We sought to deliver IL-12 in a more clinically applicable setting. We utilized IL-12 microspheres, which provide a local and sustained release of IL-12 to the tumor microenvironment 20, 22, 23. Mice were injected with parental Colon38 and tumors were treated with 15 Gy radiation seven days later. To gain insight as to the most effective schedule for administration of microspheres, empty microspheres (control) or IL-12 microspheres (equaling 0.5 ug/ml of IL-12) were directly injected into the tumor either one-day before (day 6) or one-day after (day 8) radiotherapy and tumor growth was monitored. Although radiation was still able to significantly control tumor growth, no significant differences were observed after treatment with IL-12 loaded microspheres in either the unirradiated or irradiated groups when microspheres were administered before RT therapy (Figure 6a). However, if microspheres were administered after radiotherapy, tumor burden was significantly decreased in RT treated groups given IL-12 microspheres from day 13-21 when compared to empty microsphere treatment (Figure 6b). IL-12 microspheres did not significantly alter tumor growth in unirradiated groups. We further examined differences between administration of treatment either before or after radiotherapy by plotting the time to endpoint (11.5 mm tumor burden). Similar to the data above, only IL-12 microspheres administered after RT resulted in a significant delay in tumor growth when compared to irradiated tumors treated with empty microspheres at the same time (Figure 6c). Time to endpoint differences failed to reach significance between IL-12 and empty microsphere treated RT tumors when given before RT. These data demonstrate that the administration of IL-12 in a more clinical fashion (e.g. microspheres) enhances radiotherapy especially if given after RT treatment.
Figure 6. IL-12 microspheres enhance the efficacy of radiotherapy when administered after RT.
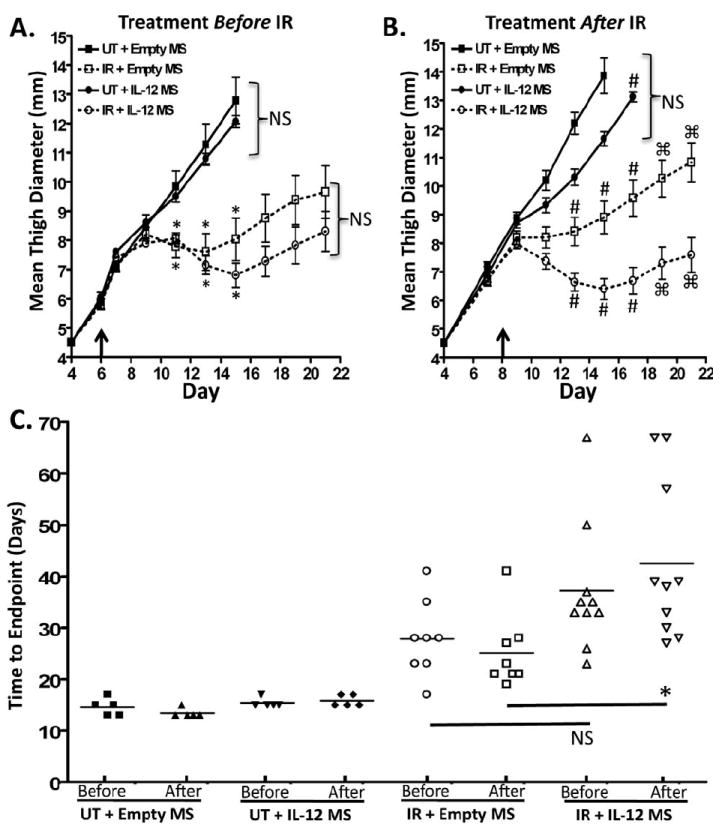
Mice were injected i.m. with 1×105 Colon38 tumor cells and tumors were directly injected with empty or IL-12 loaded microspheres (MS) either 1 day before (day 6-arrow) RT (A) or 1 day after (day 8-arrow) RT (B). Radiation treatment occurred seven days post inoculation. (C) The length of time until tumors reached 11.5 mm (endpoint) was plotted among all groups. In (A), * represents significance to both unirradiated groups as determined by a one-way ANOVA followed by a Bonferroni post-hoc test. NS represents non-significant. In (B), # represents significance to all groups as determined by a one-way ANOVA followed by a Bonferroni post-hoc test and □ represents significance as determined by a student’s t-test. In (C), * represents significance as determined by a one-way ANOVA followed by a Bonferroni post-hoc test. (n=5 for all unirradiated (UT) groups; n=8 for all RT + empty MS groups; n=10 for all RT + IL-12 MS groups).
Discussion
The field of radiation oncology has witnessed the emergence of a new concept suggesting that the immune system may mediate many of the anti-tumor effects of radiotherapy 8-10, 15. To that end, it is not surprising that the immune system is intimately involved in and may help explain some of the more poorly understood phenomena associated with radiation biology such as the existence of both responder and non-responders after RT or the induction of radiation-induced tumor dormancy. In this report, we demonstrate that a positive response to radiation therapy (e.g. responders) coincides with potent increases of intratumoral immune cells resulting in exacerbated levels of IFNγ and cytotoxic T cells. This response is very early in nature occurring only days after RT. Non-responding tumors have limited increases of both immune cells and IFNγ, and have muted CTL responses. An elegant report from Liang et al. also illustrates immune involvement but rather in radiation-induced dormancy 11. This report argued that dormancy is induced by a balance of tumor proliferation and immune cell killing. Elimination of the immune component abolished the dormant state and promoted tumor relapse. Therefore, both reports highlight a critical need of an immune response for successful radiation therapy but at two different time points following RT: Liang et al demonstrating the need for long-term immune involvement to establish dormancy, and the current report illustrating an immediate immunological requirement after RT that may ultimately dictate efficacy.
RT should optimally potentiate/modulate an existing immune response to be successful. In other words, radiation, through various aspects of tumor cell killing, may act synergistically with the immune system, each making the other more efficient. However, it is still unclear why a potent immune response is evident in some tumors (responders), but not in others (non-responders). We examined whether differences in tumor size at the time of radiotherapy could dictate responder/non-responder fate, however there were no significant changes in tumor size on day 7 between both groups (responder: 7.4 mm +/- .3; non-responder: 7.1 mm +/- .2) arguing that small variations in tumor burden do not play an important role in this model. A contributing factor could be the radiosensitive or radioresistant nature of the tumor cells 1, 3. Following RT, we believe DCs, either present in the draining lymph node (DLN) or in the tumor, are activated by danger-associated molecular patterns (DAMPs) released by tumor cells destroyed from radiation 24. Subsequently, these DCs have the ability to engulf tumor antigen, then process and present it to T cells thereby further stimulating a potent anti-tumor immune response 12. Since the initiating factor in this scenario is the destruction of tumor cells by radiation, it is likely that radioresistant tumor cells will not release DAMPs and tumor antigen and therefore lack the ability to initiate DC-mediated immune responses following RT. Radioresistance is commonly associated with radiotherapy and impedes effectiveness 3, 25. Factors within the tumor microenvironment may contribute to the induction of radioresistance in malignant cells. For example, since the presence of oxygen increases the killing efficiency of RT, oxygen status within the tumor is an important consideration 26. Tumors that are hypoxic tend to respond poorly to radiotherapy 25-27. Our preliminary data suggests that non-responder tumors have a significant increase of hypoxia-inducible factor-1α (HIF-1α), when compared to responder tumors (unpublished observations). These data may indicate a more hypoxic environment within this subset of tumors however further analysis is required. Additionally, a potential hypoxic environment may signify the presence of vessels that lack the functional ability to not only deliver oxygen, but also immune cells into the tumor. We have shown that RT induces vascular cell adhesion molecule (VCAM-1) on tumor blood vessels in a B16 melanoma model, and this molecule may potentiate the infiltration of tumor reactive immune cells 13. We have observed a similar, albeit slight, increase of VCAM-1 on the vasculature within irradiated Colon38 tumors compared to unirradiated controls (unpublished observations). Perhaps changes in vascular phenotype following RT may dictate the rate of infiltration of immune cells into tumors and ultimately responder or non-responder fate. This concept is currently a focus of investigation in our lab. Finally, the immunogenicity of the tumor cells is likely to play an important role in determining the strength of the immune response following RT. Colon38 cells are capable of stimulating a productive immune response. For example, immunization with lethally irradiated Colon38 cells completely protected mice from tumors following challenge with Colon38 28. Additionally, mice that completely rejected parental Colon38 tumor following 15 Gy RT, demonstrated significantly delayed tumor growth when re-challenged with Colon38 in the opposite leg (data not shown). Overall, responder/non-responder fate is likely dictated by how multiple factors within the tumor microenvironment respond to radiation.
Our data strongly implicate CD8+ T cells as the predominate effector population required to mediate the anti-tumor effects of RT. CD8+ T cells not only make the bulk of the essential cytokine IFNγ, but also directly kill Colon38 tumor cells 9. Interestingly, non-responder tumors have reduced CD8+ T cells and consequently less intratumoral IFNγ, which we speculate translates into reduced cytolytic T-cell function. Therefore, we chose IL-12, as an immunomodulatory cytokine known to enhance the cytolytic ability of T cells through the induction of Th1 responses 29, 30, as a means of increasing the percentage of tumors responsive to RT. Indeed, IL-12 delivered intratumorally by transfection greatly enhanced radiotherapy resulting in complete cures. This response to IL-12 was primarily mediated by CD8+ T cells as knockdown of this cell type partially abrogated the effects of RT (Figure 5c). This contrasts with data in parental tumors in which elimination of CD8+ T cells completely abrogates the anti-tumor response of RT 9. It is possible that IL-12 is additionally directly acting on host cells (or indirectly through IFNγ), such as blood vessels, in an anti-angiogenic nature thereby inhibiting/altering vessel formation 19, and this effect is exacerbated after RT. With that being said, we believe that IL-12 therapy is promoting a strong immune response as mice that cured Colon38/IL-12 tumors (after RT) were challenged in the opposite leg with parental Colon38. Most of these mice completely rejected the challenged tumor and the few that grew were greatly delayed (data not shown). These results suggest the generation of an effective immune (memory) response.
Utilization of IL-12 loaded microspheres allowed us to simulate combinational therapy (RT + immunotherapy) in a clinical setting. For example, microspheres were injected into “established” tumors, which more closely represents a clinical scenario. This is in contrast to IL-12 transfected tumors, which develop in the presence of this cytokine from the start (not necessary reflecting the clinical scenario). More importantly, we were able to address a physiologically relevant question of whether it is more efficacious to enhance radiotherapy with immunotherapy administered before or after RT. Although the differences were small, only administration of IL-12 microspheres post-RT resulted in significant delays in tumor growth. This may attest to the radiosensitive nature of immune cells, such as lymphocytes 31. For example, it is likely that IL-12 given before RT could initiate the infiltration of and/or the activation of tumor reactive lymphocytes, however these cells would be particularly sensitive to radiation and subsequently be eliminated upon RT. In contrast, IL-12 given after RT would perpetuate an already inflammatory microenvironment induced by RT and further promote the generation of anti-tumor effector T cells. Obviously, a detailed examination of dose scheduling is warranted, (e.g. multiple doses of IL-12 microspheres), however it is clear that modulating the immune system in conjunction with RT enhances radiotherapy efficacy.
Overall, this model has allowed us to uncover changes within the tumor microenvironment shortly after RT. In particular, we observed differences in the immune cell populations within the tumors and thus, employed immunotherapy to promote a greater percentage of responders. However, much more can be learned using this model such as early changes in apoptosis, alterations of vasculature, genetic profile, or a multitude of other factors associated with radiation biology, which could influence the outcome of therapy. Deciphering these mechanisms could lead to therapeutic interventions aimed at enhancing radiotherapy efficacy and conversion of non-responders to responders.
Acknowledgments
This work was supported by a grant from the National Institutes of Health (R01CA28332).
References
- 1.Ogawa K, Murayama S, Mori M. Predicting the tumor response to radiotherapy using microarray analysis (Review) Oncol Rep. 2007;18:1243–8. [PubMed] [Google Scholar]
- 2.Watanabe T, Komuro Y, Kiyomatsu T, Kanazawa T, Kazama Y, Tanaka J, Tanaka T, Yamamoto Y, Shirane M, Muto T, Nagawa H. Prediction of sensitivity of rectal cancer cells in response to preoperative radiotherapy by DNA microarray analysis of gene expression profiles. Cancer Res. 2006;66:3370–4. doi: 10.1158/0008-5472.CAN-05-3834. [DOI] [PubMed] [Google Scholar]
- 3.Farnebo L, Jerhammar F, Ceder R, Grafstrom RC, Vainikka L, Thunell L, Grenman R, Johansson AC, Roberg K. Combining factors on protein and gene level to predict radioresponse in head and neck cancer cell lines. J Oral Pathol Med. 2011;40:739–46. doi: 10.1111/j.1600-0714.2011.01036.x. [DOI] [PubMed] [Google Scholar]
- 4.Farnebo L, Tiefenbock K, Ansell A, Thunell LK, Garvin S, Roberg K. Strong expression of survivin is associated with positive response to radiotherapy and improved overall survival in head and neck squamous cell carcinoma patients. Int J Cancer. 2013 doi: 10.1002/ijc.28200. [DOI] [PubMed] [Google Scholar]
- 5.Kurt A, Yanar F, Asoglu O, Balik E, Olgac V, Karanlik H, Kucuk ST, Ademoglu E, Yegen G, Bugra D. Low Mmp 9 and VEGF levels predict good oncologic outcome in mid and low rectal cancer patients with neoadjuvant chemoradiation. BMC Clin Pathol. 2012;12:27. doi: 10.1186/1472-6890-12-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sobajima J, Kumamoto K, Haga N, Tamaru J, Takahashi T, Miyazaki T, Ishida H. Early evaluation of the apoptotic index ratio is useful in predicting the efficacy of chemoradiotherapy in esophageal squamous cell carcinoma. Oncol Lett. 2012;3:287–92. doi: 10.3892/ol.2011.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–60. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 8.Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, Mignot G, Maiuri MC, Ullrich E, Saulnier P, Yang H, Amigorena S, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050–9. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 9.Gerber SA, Sedlacek AL, Cron KR, Murphy SP, Frelinger JG, Lord EM. IFN-gamma Mediates the Antitumor Effects of Radiation Therapy in a Murine Colon Tumor. Am J Pathol. 2013;182:2345–54. doi: 10.1016/j.ajpath.2013.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee Y, Auh SL, Wang Y, Burnette B, Meng Y, Beckett M, Sharma R, Chin R, Tu T, Weichselbaum RR, Fu YX. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood. 2009;114:589–95. doi: 10.1182/blood-2009-02-206870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liang H, Deng L, Chmura S, Burnette B, Liadis N, Darga T, Beckett MA, Lingen MW, Witt M, Weichselbaum RR, Fu YX. Radiation-induced equilibrium is a balance between tumor cell proliferation and T cell-mediated killing. J Immunol. 2013;190:5874–81. doi: 10.4049/jimmunol.1202612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lugade AA, Moran JP, Gerber SA, Rose RC, Frelinger JG, Lord EM. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J Immunol. 2005;174:7516–23. doi: 10.4049/jimmunol.174.12.7516. [DOI] [PubMed] [Google Scholar]
- 13.Lugade AA, Sorensen EW, Gerber SA, Moran JP, Frelinger JG, Lord EM. Radiation-induced IFN-gamma production within the tumor microenvironment influences antitumor immunity. J Immunol. 2008;180:3132–9. doi: 10.4049/jimmunol.180.5.3132. [DOI] [PubMed] [Google Scholar]
- 14.Meng Y, Beckett MA, Liang H, Mauceri HJ, van Rooijen N, Cohen KS, Weichselbaum RR. Blockade of tumor necrosis factor alpha signaling in tumor-associated macrophages as a radiosensitizing strategy. Cancer Res. 2010;70:1534–43. doi: 10.1158/0008-5472.CAN-09-2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takeshima T, Chamoto K, Wakita D, Ohkuri T, Togashi Y, Shirato H, Kitamura H, Nishimura T. Local radiation therapy inhibits tumor growth through the generation of tumor-specific CTL: its potentiation by combination with Th1 cell therapy. Cancer Res. 2010;70:2697–706. doi: 10.1158/0008-5472.CAN-09-2982. [DOI] [PubMed] [Google Scholar]
- 16.Vignali DA, Kuchroo VK. IL-12 family cytokines: immunological playmakers. Nat Immunol. 2012;13:722–8. doi: 10.1038/ni.2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trinchieri G, Scott P. Interleukin-12: basic principles and clinical applications. Curr Top Microbiol Immunol. 1999;238:57–78. doi: 10.1007/978-3-662-09709-0_4. [DOI] [PubMed] [Google Scholar]
- 18.Corbett TH, Griswold DP, Jr, Roberts BJ, Peckham JC, Schabel FM., Jr Tumor induction relationships in development of transplantable cancers of the colon in mice for chemotherapy assays, with a note on carcinogen structure. Cancer Res. 1975;35:2434–9. [PubMed] [Google Scholar]
- 19.Gerber SA, Moran JP, Frelinger JG, Frelinger JA, Fenton BM, Lord EM. Mechanism of IL-12 mediated alterations in tumour blood vessel morphology: analysis using whole-tissue mounts. Br J Cancer. 2003;88:1453–61. doi: 10.1038/sj.bjc.6600907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Egilmez NK, Jong YS, Mathiowitz E, Bankert RB. Tumor vaccination with cytokine-encapsulated microspheres. Methods Mol Med. 2003;75:687–96. doi: 10.1385/1-59259-324-0:687. [DOI] [PubMed] [Google Scholar]
- 21.Kilinc MO, Aulakh KS, Nair RE, Jones SA, Alard P, Kosiewicz MM, Egilmez NK. Reversing tumor immune suppression with intratumoral IL-12: activation of tumor-associated T effector/memory cells, induction of T suppressor apoptosis, and infiltration of CD8+ T effectors. J Immunol. 2006;177:6962–73. doi: 10.4049/jimmunol.177.10.6962. [DOI] [PubMed] [Google Scholar]
- 22.Egilmez NK, Jong YS, Sabel MS, Jacob JS, Mathiowitz E, Bankert RB. In situ tumor vaccination with interleukin-12-encapsulated biodegradable microspheres: induction of tumor regression and potent antitumor immunity. Cancer Res. 2000;60:3832–7. [PubMed] [Google Scholar]
- 23.Harden JL, Gu T, Kilinc MO, Rowswell-Turner RB, Virtuoso LP, Egilmez NK. Dichotomous effects of IFN-gamma on dendritic cell function determine the extent of IL-12-driven antitumor T cell immunity. J Immunol. 2011;187:126–32. doi: 10.4049/jimmunol.1100168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McBride WH, Chiang CS, Olson JL, Wang CC, Hong JH, Pajonk F, Dougherty GJ, Iwamoto KS, Pervan M, Liao YP. A sense of danger from radiation. Radiat Res. 2004;162:1–19. doi: 10.1667/rr3196. [DOI] [PubMed] [Google Scholar]
- 25.Meijer TW, Kaanders JH, Span PN, Bussink J. Targeting hypoxia, HIF-1, and tumor glucose metabolism to improve radiotherapy efficacy. Clin Cancer Res. 2012;18:5585–94. doi: 10.1158/1078-0432.CCR-12-0858. [DOI] [PubMed] [Google Scholar]
- 26.Horsman MR, Mortensen LS, Petersen JB, Busk M, Overgaard J. Imaging hypoxia to improve radiotherapy outcome. Nat Rev Clin Oncol. 2012;9:674–87. doi: 10.1038/nrclinonc.2012.171. [DOI] [PubMed] [Google Scholar]
- 27.Sovik A, Malinen E, Bruland OS, Bentzen SM, Olsen DR. Optimization of tumour control probability in hypoxic tumours by radiation dose redistribution: a modelling study. Phys Med Biol. 2007;52:499–513. doi: 10.1088/0031-9155/52/2/013. [DOI] [PubMed] [Google Scholar]
- 28.Sedlacek AL, Gerber SA, Randall TD, van Rooijen N, Frelinger JG, Lord EM. Generation of a Dual-Functioning Antitumor Immune Response in the Peritoneal Cavity. Am J Pathol. 2013 doi: 10.1016/j.ajpath.2013.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gee K, Guzzo C, Che Mat NF, Ma W, Kumar A. The IL-12 family of cytokines in infection, inflammation and autoimmune disorders. Inflamm Allergy Drug Targets. 2009;8:40–52. doi: 10.2174/187152809787582507. [DOI] [PubMed] [Google Scholar]
- 30.Del Vecchio M, Bajetta E, Canova S, Lotze MT, Wesa A, Parmiani G, Anichini A. Interleukin-12: biological properties and clinical application. Clin Cancer Res. 2007;13:4677–85. doi: 10.1158/1078-0432.CCR-07-0776. [DOI] [PubMed] [Google Scholar]
- 31.Belka C, Marini P, Budach W, Schulze-Osthoff K, Lang F, Gulbins E, Bamberg M. Radiation-induced apoptosis in human lymphocytes and lymphoma cells critically relies on the up-regulation of CD95/Fas/APO-1 ligand. Radiat Res. 1998;149:588–95. [PubMed] [Google Scholar]