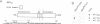Abstract
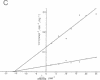
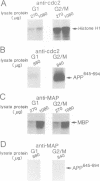
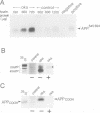
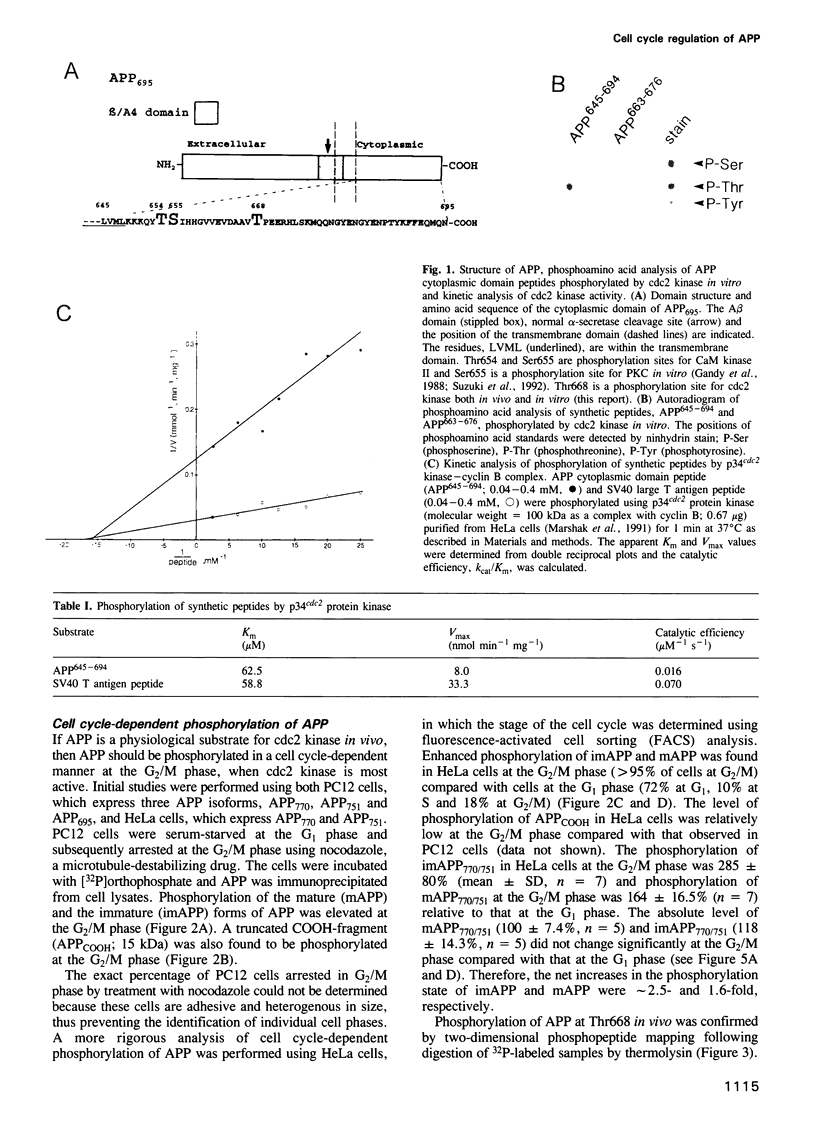
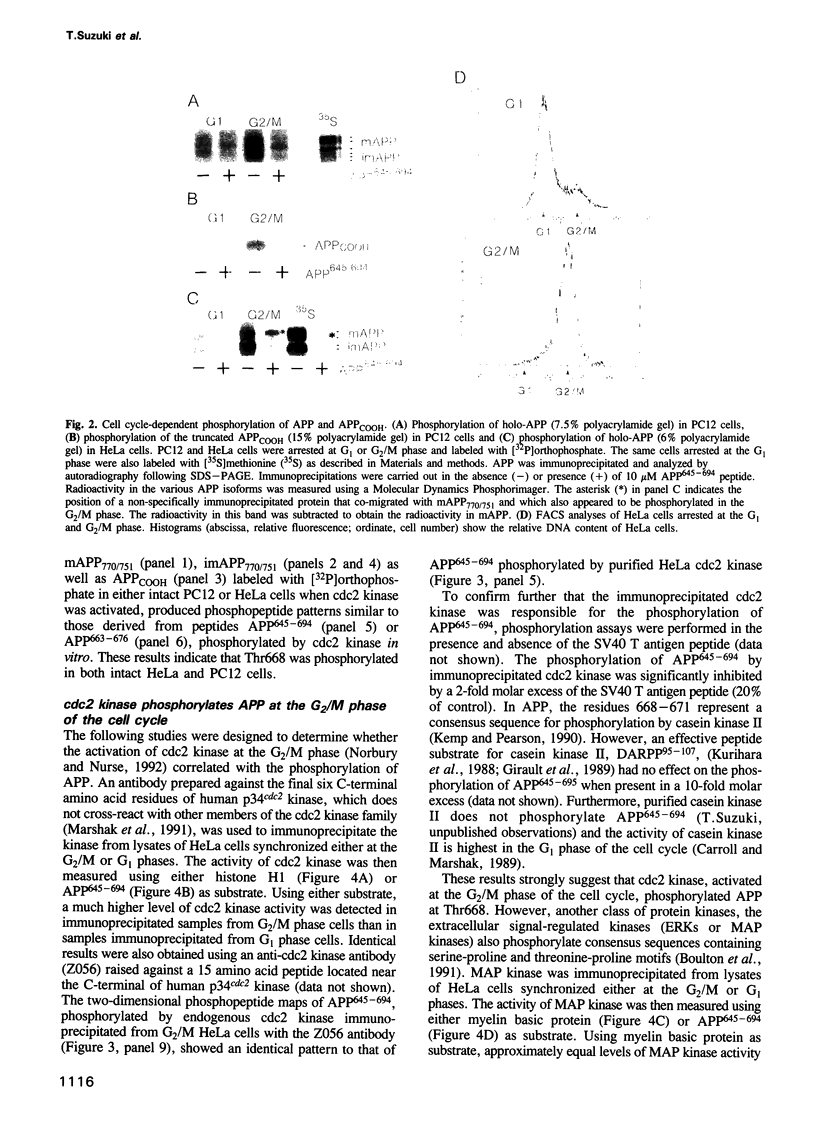
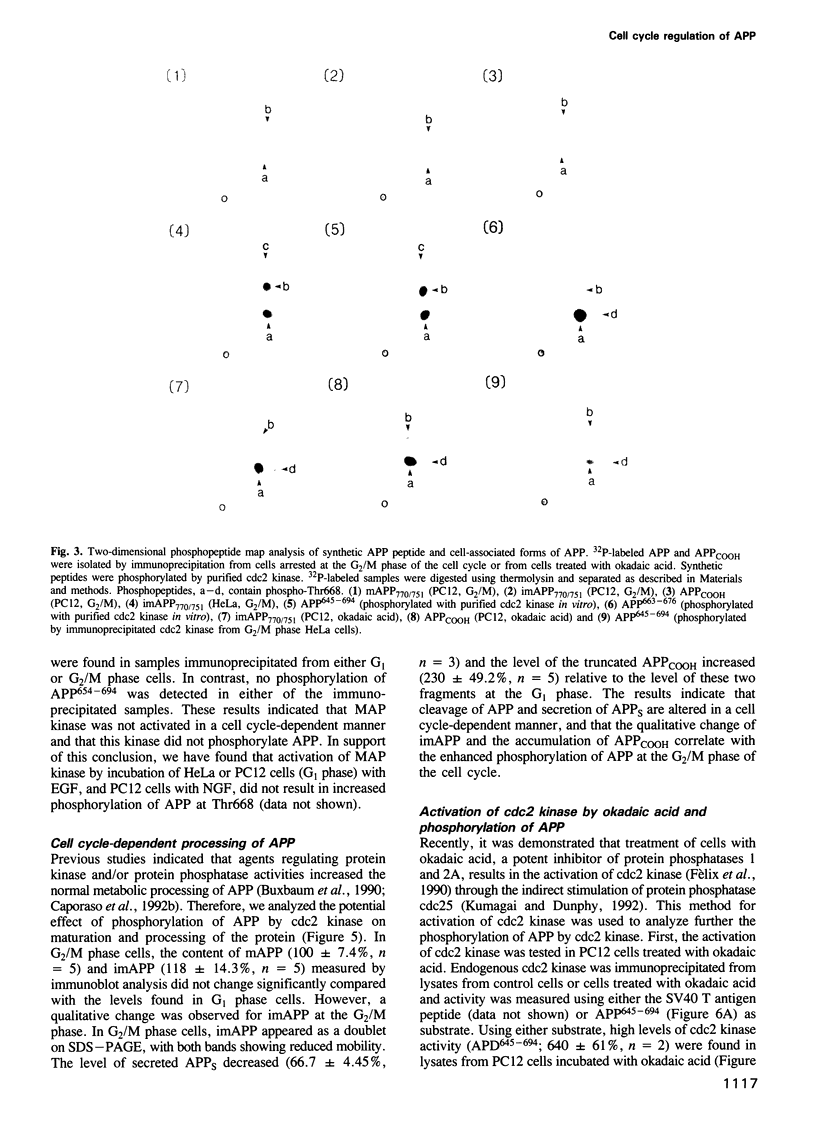
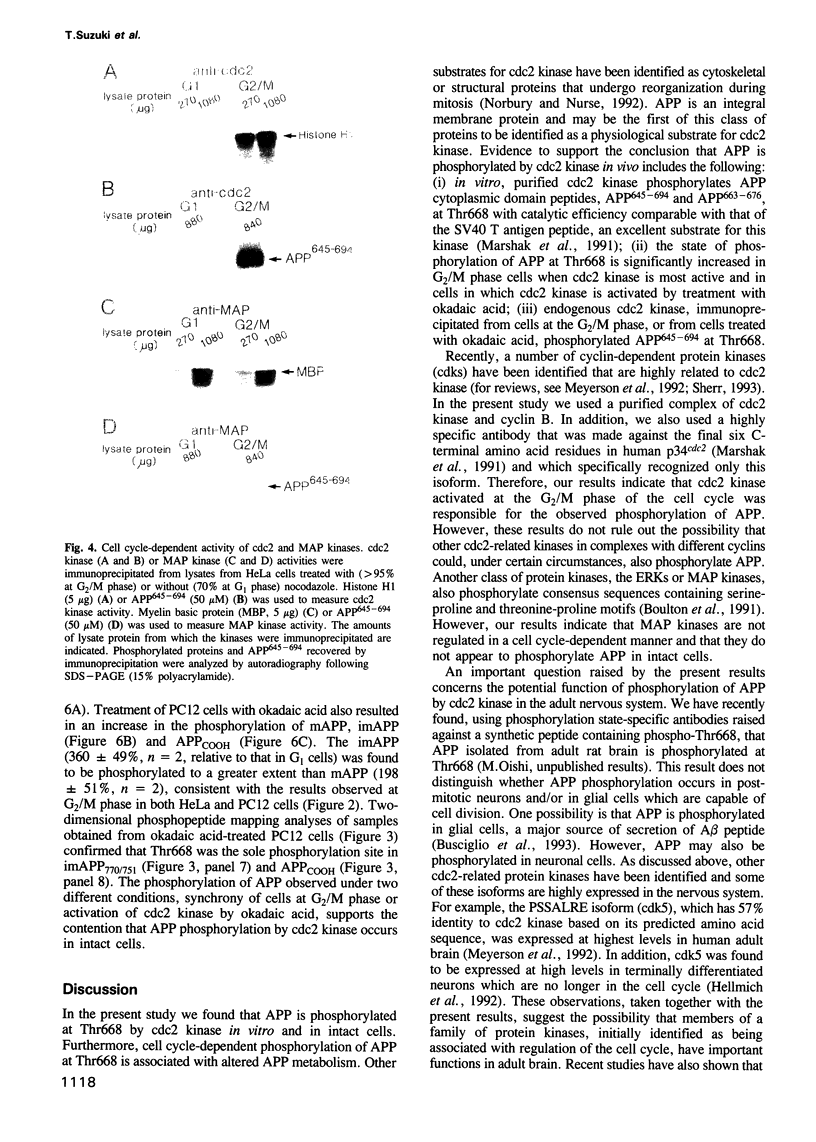
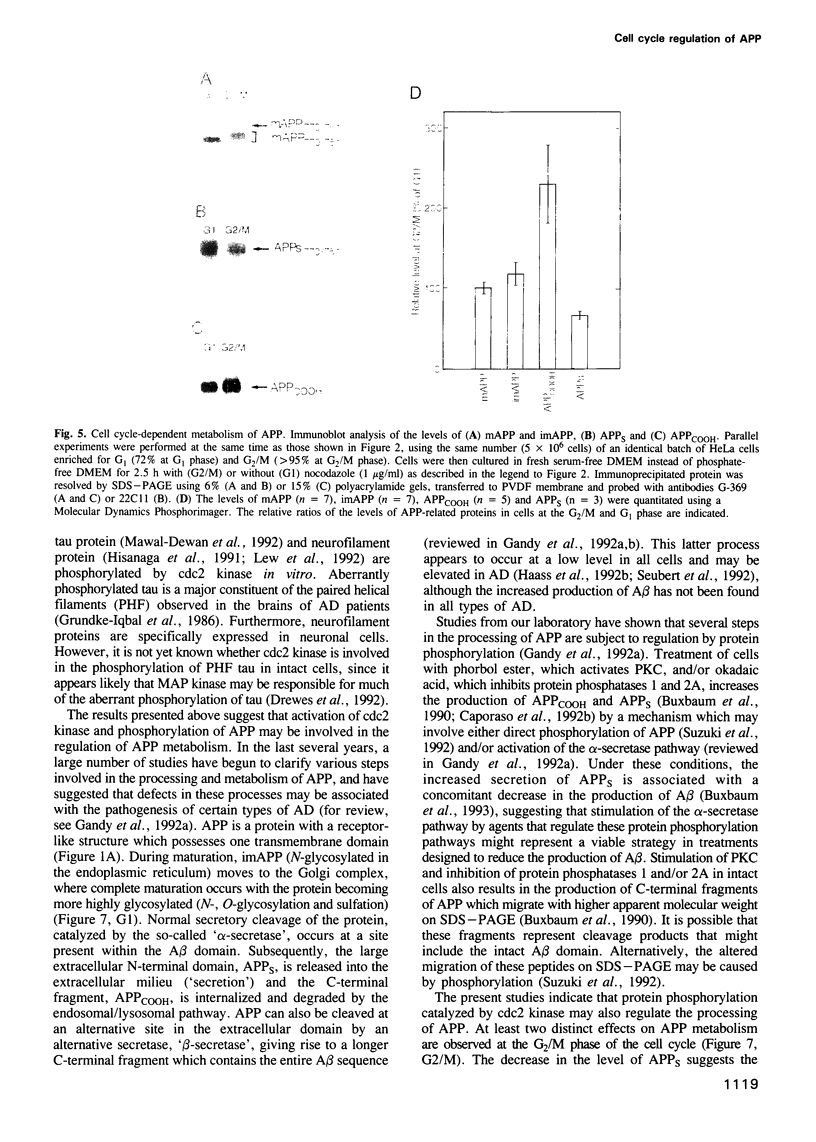
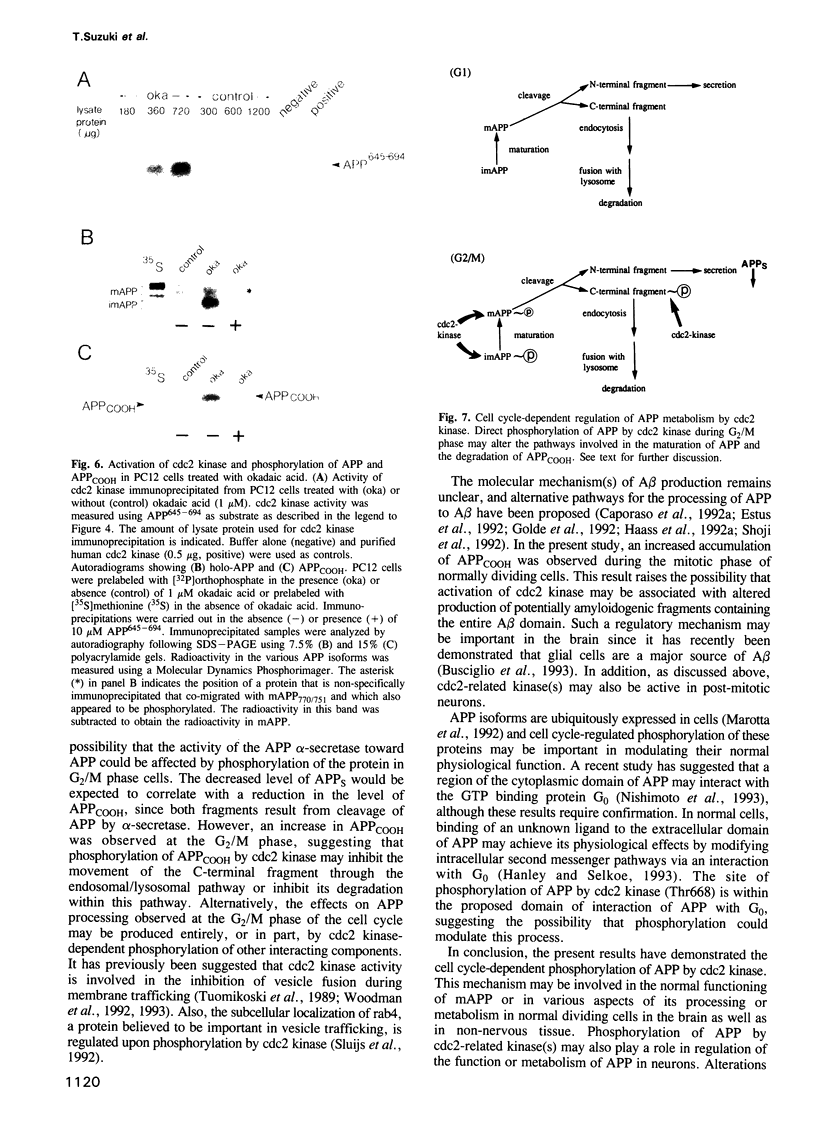
Accumulation of the amyloid A beta peptide, which is derived from a larger precursor protein (APP), and the formation of plaques, are major events believed to be involved in the etiology of Alzheimer's disease. Abnormal regulation of the metabolism of APP may contribute to the deposition of plaques. APP is an integral membrane protein containing several putative phosphorylation sites within its cytoplasmic domain. We report here that APP is phosphorylated at Thr668 by p34cdc2 protein kinase (cdc2 kinase) in vitro, and in a cell cycle-dependent manner in vivo. At the G2/M phase of the cell cycle, when APP phosphorylation is maximal, the levels of mature APP (mAPP) and immature APP (imAPP) do not change significantly. However, imAPP is altered qualitatively. Furthermore, the level of the secreted extracellular N-terminal domain (APPS) is decreased and that of the truncated intracellular C-terminal fragment (APPCOOH) is increased. These findings suggest the possibility that phosphorylation-dependent events occurring during the cell cycle affect the metabolism of APP. Alterations in these events might play a role in the pathogenesis of Alzheimer's disease.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boulton T. G., Nye S. H., Robbins D. J., Ip N. Y., Radziejewska E., Morgenbesser S. D., DePinho R. A., Panayotatos N., Cobb M. H., Yancopoulos G. D. ERKs: a family of protein-serine/threonine kinases that are activated and tyrosine phosphorylated in response to insulin and NGF. Cell. 1991 May 17;65(4):663–675. doi: 10.1016/0092-8674(91)90098-j. [DOI] [PubMed] [Google Scholar]
- Brizuela L., Draetta G., Beach D. Activation of human CDC2 protein as a histone H1 kinase is associated with complex formation with the p62 subunit. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4362–4366. doi: 10.1073/pnas.86.12.4362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busciglio J., Gabuzda D. H., Matsudaira P., Yankner B. A. Generation of beta-amyloid in the secretory pathway in neuronal and nonneuronal cells. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):2092–2096. doi: 10.1073/pnas.90.5.2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxbaum J. D., Gandy S. E., Cicchetti P., Ehrlich M. E., Czernik A. J., Fracasso R. P., Ramabhadran T. V., Unterbeck A. J., Greengard P. Processing of Alzheimer beta/A4 amyloid precursor protein: modulation by agents that regulate protein phosphorylation. Proc Natl Acad Sci U S A. 1990 Aug;87(15):6003–6006. doi: 10.1073/pnas.87.15.6003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxbaum J. D., Koo E. H., Greengard P. Protein phosphorylation inhibits production of Alzheimer amyloid beta/A4 peptide. Proc Natl Acad Sci U S A. 1993 Oct 1;90(19):9195–9198. doi: 10.1073/pnas.90.19.9195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso G. L., Gandy S. E., Buxbaum J. D., Greengard P. Chloroquine inhibits intracellular degradation but not secretion of Alzheimer beta/A4 amyloid precursor protein. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2252–2256. doi: 10.1073/pnas.89.6.2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso G. L., Gandy S. E., Buxbaum J. D., Ramabhadran T. V., Greengard P. Protein phosphorylation regulates secretion of Alzheimer beta/A4 amyloid precursor protein. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):3055–3059. doi: 10.1073/pnas.89.7.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll D., Marshak D. R. Serum-stimulated cell growth causes oscillations in casein kinase II activity. J Biol Chem. 1989 May 5;264(13):7345–7348. [PubMed] [Google Scholar]
- Drewes G., Lichtenberg-Kraag B., Döring F., Mandelkow E. M., Biernat J., Goris J., Dorée M., Mandelkow E. Mitogen activated protein (MAP) kinase transforms tau protein into an Alzheimer-like state. EMBO J. 1992 Jun;11(6):2131–2138. doi: 10.1002/j.1460-2075.1992.tb05272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esch F. S., Keim P. S., Beattie E. C., Blacher R. W., Culwell A. R., Oltersdorf T., McClure D., Ward P. J. Cleavage of amyloid beta peptide during constitutive processing of its precursor. Science. 1990 Jun 1;248(4959):1122–1124. doi: 10.1126/science.2111583. [DOI] [PubMed] [Google Scholar]
- Estus S., Golde T. E., Kunishita T., Blades D., Lowery D., Eisen M., Usiak M., Qu X. M., Tabira T., Greenberg B. D. Potentially amyloidogenic, carboxyl-terminal derivatives of the amyloid protein precursor. Science. 1992 Feb 7;255(5045):726–728. doi: 10.1126/science.1738846. [DOI] [PubMed] [Google Scholar]
- Félix M. A., Cohen P., Karsenti E. Cdc2 H1 kinase is negatively regulated by a type 2A phosphatase in the Xenopus early embryonic cell cycle: evidence from the effects of okadaic acid. EMBO J. 1990 Mar;9(3):675–683. doi: 10.1002/j.1460-2075.1990.tb08159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandy S. E., Caporaso G. L., Ramabhadran T. V., Suzuki T., Buxbaum J. D., Nordstedt C., Iverfeldt K., Czernik A. J., Nairn A. C., Greengard P. Characterization of alternative routes for processing of the Alzheimer beta/A4-amyloid precursor protein. Differential effects of phorbol esters and chloroquine. Ann N Y Acad Sci. 1992 Dec 31;674:203–217. doi: 10.1111/j.1749-6632.1992.tb27489.x. [DOI] [PubMed] [Google Scholar]
- Gandy S., Czernik A. J., Greengard P. Phosphorylation of Alzheimer disease amyloid precursor peptide by protein kinase C and Ca2+/calmodulin-dependent protein kinase II. Proc Natl Acad Sci U S A. 1988 Aug;85(16):6218–6221. doi: 10.1073/pnas.85.16.6218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girault J. A., Hemmings H. C., Jr, Williams K. R., Nairn A. C., Greengard P. Phosphorylation of DARPP-32, a dopamine- and cAMP-regulated phosphoprotein, by casein kinase II. J Biol Chem. 1989 Dec 25;264(36):21748–21759. [PubMed] [Google Scholar]
- Golde T. E., Estus S., Younkin L. H., Selkoe D. J., Younkin S. G. Processing of the amyloid protein precursor to potentially amyloidogenic derivatives. Science. 1992 Feb 7;255(5045):728–730. doi: 10.1126/science.1738847. [DOI] [PubMed] [Google Scholar]
- Goldgaber D., Lerman M. I., McBride O. W., Saffiotti U., Gajdusek D. C. Characterization and chromosomal localization of a cDNA encoding brain amyloid of Alzheimer's disease. Science. 1987 Feb 20;235(4791):877–880. doi: 10.1126/science.3810169. [DOI] [PubMed] [Google Scholar]
- Grundke-Iqbal I., Iqbal K., Tung Y. C., Quinlan M., Wisniewski H. M., Binder L. I. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4913–4917. doi: 10.1073/pnas.83.13.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haass C., Koo E. H., Mellon A., Hung A. Y., Selkoe D. J. Targeting of cell-surface beta-amyloid precursor protein to lysosomes: alternative processing into amyloid-bearing fragments. Nature. 1992 Jun 11;357(6378):500–503. doi: 10.1038/357500a0. [DOI] [PubMed] [Google Scholar]
- Haass C., Schlossmacher M. G., Hung A. Y., Vigo-Pelfrey C., Mellon A., Ostaszewski B. L., Lieberburg I., Koo E. H., Schenk D., Teplow D. B. Amyloid beta-peptide is produced by cultured cells during normal metabolism. Nature. 1992 Sep 24;359(6393):322–325. doi: 10.1038/359322a0. [DOI] [PubMed] [Google Scholar]
- Hanley M. R., Selkoe D. J. Alzheimer's disease. Amyloid precursor on the G(o) Nature. 1993 Mar 4;362(6415):14–15. doi: 10.1038/362014a0. [DOI] [PubMed] [Google Scholar]
- Hellmich M. R., Pant H. C., Wada E., Battey J. F. Neuronal cdc2-like kinase: a cdc2-related protein kinase with predominantly neuronal expression. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):10867–10871. doi: 10.1073/pnas.89.22.10867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisanaga S., Kusubata M., Okumura E., Kishimoto T. Phosphorylation of neurofilament H subunit at the tail domain by CDC2 kinase dissociates the association to microtubules. J Biol Chem. 1991 Nov 15;266(32):21798–21803. [PubMed] [Google Scholar]
- Kang J., Lemaire H. G., Unterbeck A., Salbaum J. M., Masters C. L., Grzeschik K. H., Multhaup G., Beyreuther K., Müller-Hill B. The precursor of Alzheimer's disease amyloid A4 protein resembles a cell-surface receptor. Nature. 1987 Feb 19;325(6106):733–736. doi: 10.1038/325733a0. [DOI] [PubMed] [Google Scholar]
- Kemp B. E., Benjamini E., Krebs E. G. Synthetic hexapeptide substrates and inhibitors of 3':5'-cyclic AMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1038–1042. doi: 10.1073/pnas.73.4.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp B. E., Pearson R. B. Protein kinase recognition sequence motifs. Trends Biochem Sci. 1990 Sep;15(9):342–346. doi: 10.1016/0968-0004(90)90073-k. [DOI] [PubMed] [Google Scholar]
- Kumagai A., Dunphy W. G. Regulation of the cdc25 protein during the cell cycle in Xenopus extracts. Cell. 1992 Jul 10;70(1):139–151. doi: 10.1016/0092-8674(92)90540-s. [DOI] [PubMed] [Google Scholar]
- Kurihara T., Lewis R. M., Eisler J., Greengard P. Cloning of cDNA for DARPP-32, a dopamine- and cyclic AMP-regulated neuronal phosphoprotein. J Neurosci. 1988 Feb;8(2):508–517. doi: 10.1523/JNEUROSCI.08-02-00508.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lew J., Winkfein R. J., Paudel H. K., Wang J. H. Brain proline-directed protein kinase is a neurofilament kinase which displays high sequence homology to p34cdc2. J Biol Chem. 1992 Dec 25;267(36):25922–25926. [PubMed] [Google Scholar]
- Marotta C. A., Majocha R. E., Tate B. Molecular and cellular biology of Alzheimer amyloid. J Mol Neurosci. 1992;3(3):111–125. doi: 10.1007/BF02919403. [DOI] [PubMed] [Google Scholar]
- Marshak D. R., Vandenberg M. T., Bae Y. S., Yu I. J. Characterization of synthetic peptide substrates for p34cdc2 protein kinase. J Cell Biochem. 1991 Apr;45(4):391–400. doi: 10.1002/jcb.240450413. [DOI] [PubMed] [Google Scholar]
- Mawal-Dewan M., Sen P. C., Abdel-Ghany M., Shalloway D., Racker E. Phosphorylation of tau protein by purified p34cdc28 and a related protein kinase from neurofilaments. J Biol Chem. 1992 Sep 25;267(27):19705–19709. [PubMed] [Google Scholar]
- Meyerson M., Enders G. H., Wu C. L., Su L. K., Gorka C., Nelson C., Harlow E., Tsai L. H. A family of human cdc2-related protein kinases. EMBO J. 1992 Aug;11(8):2909–2917. doi: 10.1002/j.1460-2075.1992.tb05360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimoto I., Okamoto T., Matsuura Y., Takahashi S., Okamoto T., Murayama Y., Ogata E. Alzheimer amyloid protein precursor complexes with brain GTP-binding protein G(o) Nature. 1993 Mar 4;362(6415):75–79. doi: 10.1038/362075a0. [DOI] [PubMed] [Google Scholar]
- Norbury C., Nurse P. Animal cell cycles and their control. Annu Rev Biochem. 1992;61:441–470. doi: 10.1146/annurev.bi.61.070192.002301. [DOI] [PubMed] [Google Scholar]
- Oltersdorf T., Ward P. J., Henriksson T., Beattie E. C., Neve R., Lieberburg I., Fritz L. C. The Alzheimer amyloid precursor protein. Identification of a stable intermediate in the biosynthetic/degradative pathway. J Biol Chem. 1990 Mar 15;265(8):4492–4497. [PubMed] [Google Scholar]
- Robakis N. K., Ramakrishna N., Wolfe G., Wisniewski H. M. Molecular cloning and characterization of a cDNA encoding the cerebrovascular and the neuritic plaque amyloid peptides. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4190–4194. doi: 10.1073/pnas.84.12.4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seubert P., Vigo-Pelfrey C., Esch F., Lee M., Dovey H., Davis D., Sinha S., Schlossmacher M., Whaley J., Swindlehurst C. Isolation and quantification of soluble Alzheimer's beta-peptide from biological fluids. Nature. 1992 Sep 24;359(6393):325–327. doi: 10.1038/359325a0. [DOI] [PubMed] [Google Scholar]
- Sherr C. J. Mammalian G1 cyclins. Cell. 1993 Jun 18;73(6):1059–1065. doi: 10.1016/0092-8674(93)90636-5. [DOI] [PubMed] [Google Scholar]
- Shoji M., Golde T. E., Ghiso J., Cheung T. T., Estus S., Shaffer L. M., Cai X. D., McKay D. M., Tintner R., Frangione B. Production of the Alzheimer amyloid beta protein by normal proteolytic processing. Science. 1992 Oct 2;258(5079):126–129. doi: 10.1126/science.1439760. [DOI] [PubMed] [Google Scholar]
- Sisodia S. S., Koo E. H., Beyreuther K., Unterbeck A., Price D. L. Evidence that beta-amyloid protein in Alzheimer's disease is not derived by normal processing. Science. 1990 Apr 27;248(4954):492–495. doi: 10.1126/science.1691865. [DOI] [PubMed] [Google Scholar]
- Suzuki T., Nairn A. C., Gandy S. E., Greengard P. Phosphorylation of Alzheimer amyloid precursor protein by protein kinase C. Neuroscience. 1992 Jun;48(4):755–761. doi: 10.1016/0306-4522(92)90264-3. [DOI] [PubMed] [Google Scholar]
- Tanzi R. E., Gusella J. F., Watkins P. C., Bruns G. A., St George-Hyslop P., Van Keuren M. L., Patterson D., Pagan S., Kurnit D. M., Neve R. L. Amyloid beta protein gene: cDNA, mRNA distribution, and genetic linkage near the Alzheimer locus. Science. 1987 Feb 20;235(4791):880–884. doi: 10.1126/science.2949367. [DOI] [PubMed] [Google Scholar]
- Tuomikoski T., Felix M. A., Dorée M., Gruenberg J. Inhibition of endocytic vesicle fusion in vitro by the cell-cycle control protein kinase cdc2. Nature. 1989 Dec 21;342(6252):942–945. doi: 10.1038/342942a0. [DOI] [PubMed] [Google Scholar]
- Weidemann A., König G., Bunke D., Fischer P., Salbaum J. M., Masters C. L., Beyreuther K. Identification, biogenesis, and localization of precursors of Alzheimer's disease A4 amyloid protein. Cell. 1989 Apr 7;57(1):115–126. doi: 10.1016/0092-8674(89)90177-3. [DOI] [PubMed] [Google Scholar]
- Woodman P. G., Adamczewski J. P., Hunt T., Warren G. In vitro fusion of endocytic vesicles is inhibited by cyclin A-cdc2 kinase. Mol Biol Cell. 1993 May;4(5):541–553. doi: 10.1091/mbc.4.5.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodman P. G., Mundy D. I., Cohen P., Warren G. Cell-free fusion of endocytic vesicles is regulated by phosphorylation. J Cell Biol. 1992 Jan;116(2):331–338. doi: 10.1083/jcb.116.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Sluijs P., Hull M., Huber L. A., Mâle P., Goud B., Mellman I. Reversible phosphorylation--dephosphorylation determines the localization of rab4 during the cell cycle. EMBO J. 1992 Dec;11(12):4379–4389. doi: 10.1002/j.1460-2075.1992.tb05538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]